
ebook
THE GUILFORD PRESS
HANDBOOK OF EMOTION REGULATION

Handbook of
EMOTION
REGUL ATION
SECOND EDITION
Edited by
James J. Gross
THE GUILFORD PRESS
New York London
© 2014 The Guilford Press
A Division of Guilford Publications, Inc.
72 Spring Street, New York, NY 10012
www.guilford.com
All rights reserved
No part of this book may be reproduced, translated, stored in a retrieval system, or transmitted,
in any form or by any means, electronic, mechanical, photocopying, microfilming, recording,
or otherwise, without written permission from the publisher.
Printed in the United States of America
This book is printed on acid-free paper.
Last digit is print number: 9 8 7 6 5 4 3 2 1
Library of Congress Cataloging-in-Publication Data is available from the Publisher
ISBN 978-1-4625-0350-6
To Paul, Mark, and Anne
vi
James J. Gross, PhD, is Professor of Psychology at Stanford University and Director
of the Stanford Psychophysiology Laboratory (http://spl.stanford.edu). He is a leading
figure in the areas of emotion and emotion regulation and is a recipient of early career
awards from the American Psychological Association, the Western Psychological Asso-
ciation, and the Society for Psychophysiological Research. A Bass University Fellow
in Undergraduate Education and Director of the Stanford Psychology One Teaching
Program, Dr. Gross has won numerous awards for his teaching, including the Dean’s
Award for Distinguished Teaching, the Phi Beta Kappa Teaching Prize, the Stanford
Postdoctoral Mentoring Award, and the Walter J. Gores Award for Excellence in Teach-
ing. He has an extensive program of investigator-initiated research, with grants from
the National Institutes of Health, the National Science Foundation, and the Institute
of Education Sciences. He is the author of over 250 publications and is a Fellow in the
Association for Psychological Science and the American Psychological Association.
About the Editor
vii
Dustin Albert, PhD, Center for Child and Family Policy, Duke University,
Durham, North Carolina
Adam K. Anderson, PhD, Department of Human Development, College of Human Ecology,
Cornell University, Ithaca, New York
Allison A. Appleton, ScD, Geisel School of Medicine at Dartmouth College,
Hanover, New Hampshire
Ozlem Ayduk, PhD, Department of Psychology, University of California, Berkeley,
Berkeley, California
David H. Barlow, PhD, Center for Anxiety and Related Disorders, Boston University,
Boston, Massachusetts
Lisa Feldman Barrett, PhD, Department of Psychology, Northeastern University,
Boston, Massachusetts
Lawrence W. Barsalou, PhD, Department of Psychology, Emory University, Atlanta, Georgia
Matthias Berking, PhD, Department of Clinical Psychology and Psychotherapy,
University of Marburg, Marburg, Germany
Lian Bloch, PhD, Department of Psychology, Stanford University, Stanford, California
Martin Bohus, MD, Central Institute of Mental Health, University of Heidelberg,
Mannheim, Germany
Laura Campbell-Sills, PhD, Department of Psychiatry, University of California, San Diego,
La Jolla, California
Laura L. Carstensen, PhD, Department of Psychology, Stanford University, Stanford, California
Susan Turk Charles, PhD, Department of Psychology and Social Behavior,
University of California, Irvine, Irvine, California
Edith Chen, PhD, Department of Psychology, Northwestern University, Evanston, Illinois
James A. Coan, PhD, Department of Psychology, University of Virginia,
Charlottesville, Virginia
Steven W. Cole, PhD, Geffen School of Medicine, University of California, Los Angeles,
Los Angeles, California
William A. Cunningham, PhD, Department of Psychology, University of Toronto,
Toronto, Ontario, Canada
Contributors
viii Contributors
Jozefien De Leersnyder, MA, Center for Social and Cultural Psychology,
Katholieke
Universiteit
Leuven, Leuven,
Belgium
Jonathan P. Dunning, PhD, Department of Psychology, Nevada State College,
Henderson, Nevada
Nancy Eisenberg, PhD, Department of Psychology, Arizona State University, Tempe, Arizona
Kristen K. Ellard, PhD, Department of Psychology, Massachusetts General Hospital/
Harvard Medical School, Boston, Massachusetts
Joshua Eng, PhD, Department of
Psychology, University of California, Berkeley,
Berkeley,
California
Amit Etkin, MD, PhD, Department of
Psychiatry and Behavioral Sciences, Stanford University
School of
Medicine, Stanford, California
Norman A. S. Farb, PhD, Rotman Research Institute, Toronto, Ontario,
Canada
Daniel Foti, PhD, Department of
Psychology, Stony Brook University, Stony Brook, New York
David M. Fresco, PhD, Department of
Psychology, Kent State University, Kent, Ohio
Dina Gohar, MA, Department of
Psychology and Neuroscience, Duke University,
Durham,
North
Carolina
Ben Grafton, MA, Centre for
the Advancement of Research on Emotion, School of Psychology,
University of
Western Australia, Crawley, Australia; School of Psychology,
Babes‑Bolyai
University, Cluj
‑N
apoca,
Romania
Alessandro Grecucci, PhD, Department of
Cognitive Science, University of Trento,
Rovereto,
Italy
James J. Gross, PhD, Department of
Psychology, Stanford University, Stanford, California
Anett Gyurak, PhD, Department of
Psychology, Stanford University, Stanford, California
Claudia M. Haase, PhD, Department of
Psychology, University of California, Berkeley,
Berkeley,
California
Todd F. Heatherton, PhD, Department of
Psychological and Brain Sciences,
Dartmouth College, Hanover,
New
Hampshire
Claire Hofer, PhD, Department of
Psychology, Charles de Gaulle University,
Villeneuve
d’Ascq,
France
Wilhelm Hofmann, PhD, Center for
Decision Research, University of Chicago
Booth
School of Business, Chicago, Illinois
Sarah R. Holley, PhD, Psychology Department, San Francisco State University,
San
Francisco, California
Julie A. Irving, PhD, Mood and Anxiety Disorders Program, Centre for
Addiction
and
Mental Health, Toronto, Ontario, Canada
Oliver P. John, PhD, Department of
Psychology and Institute of Personality
and
Social Research, University of California, Berkeley, Berkeley, California
Tom Johnstone, PhD, School of
Psychology, Centre for Integrative Neuroscience
and
Neurodynamics, University of Reading, Reading, United Kingdom
Christopher R. Jones, PhD, Annenberg School for
Communication, University of Pennsylvania,
Philadelphia,
Pennsylvania
Jutta Joormann, PhD, Department of
Psychology, Northwestern University, Evanston, Illinois
Bokyung Kim, MA, Department of
Psychology, Stanford University, Stanford, California
Contributors ix
Tabitha Kirkland, MA, Department of Psychology, The Ohio State University, Columbus, Ohio
Kathrin Klipker, PhD, Max Planck Research Group “Affect Across the Lifespan,” Max Planck
Institute for Human Development, Berlin, Germany
Hedy Kober, PhD, Interdepartmental Neuroscience Program, Yale University,
New Haven, Connecticut
Hiroki P. Kotabe, BA, Center for Decision Research, University of Chicago Booth School
of Business, Chicago, Illinois
Laura D. Kubzansky, PhD, Department of
Social and Behavioral Sciences,
Harvard
School of Public Health, Boston, Massachusetts
Mark R. Leary, PhD, Department of
Psychology and Neuroscience, Duke University,
Durham,
North
Carolina
Robert W. Levenson, PhD, Department of
Psychology, University of California, Berkeley,
Berkeley,
California
Marsha M. Linehan, PhD, Department of
Psychology, University of Washington,
Seattle,
Washington
Anna Luerssen, PhD, Department of
Psychology, Lehman College, City University
of
New York, New York, New York
Colin MacLeod, PhD, School of
Psychology, University of Western Australia, Perth, Australia
Erin L. Maresh, MA, Department of
Psychology, University of Virginia,
Charlottesville,
Virginia
Iris B. Mauss, PhD, Department of
Psychology, University of California, Berkeley,
Berkeley,
California
Samuel M. McClure, PhD, Department of
Psychology, Stanford University, Stanford, California
Douglas S. Mennin, PhD, Department of
Psychology, Hunter College, and The
Graduate
Center, City University of New York, New York, New York
Batja Mesquita, PhD, Department of
Psychology, Katholieke Universiteit Leuven,
Leuven,
Belgium
Mario Mikulincer, PhD, Interdisciplinary Center, New
School of Psychology, Herzliya, Israel
Eric M. Miller, PhD, Department of
Psychology, Stanford University, Stanford, California
Gregory E. Miller, PhD, Department of
Psychology, University of British Columbia, Vancouver,
British Columbia,
Canada
Andrada D. Neacsiu, PhD, Department of
Psychiatry and Behavioral Sciences, Duke University
Medical Center, Durham,
North Carolina
Kevin N. Ochsner, PhD, Department of
Psychology, Columbia University,
New
York, New York
Michael I. Posner, PhD, Department of
Psychology, University of Oregon, Eugene, Oregon
Greg Hajcak Proudfit, PhD, Department of
Psychology, Stony Brook University,
Stony
Brook, New York
Michaela Riediger, PhD, Max Planck Research Group “Affect Across the
Lifespan,”
Max
Planck Institute for Human Development, Berlin, Germany
Christian Rodriguez, BA, Department of
Psychology, Stanford University, Stanford, California
Mary K. Rothbart, PhD, Distinguished Professor Emerita, Department of
Psychology,
University of
Oregon, Eugene,
Oregon
x Contributors
Alan G. Sanfey, PhD, Centre for Cognitive Neuroimaging, Donders Institute for Brain,
Cognition, and Behavior, Nijmegen, The Netherlands
Jeanine Schwarz, MA, Department of Clinical Psychology and Psychotherapy,
University of Marburg, Marburg, Germany
Zindel V. Segal, PhD, Centre for Addiction and Mental Health, University of Toronto,
Toronto, Ontario, Canada
Benjamin H. Seider, PhD, Marin County Department of Health and Human Services,
San Rafael, California
Phillip R. Shaver, PhD, Department of Psychology, University of California, Davis,
Davis, California
Brad E. Sheese, PhD, Department of Psychology, Illinois Wesleyan University,
Bloomington, Illinois
Gal Sheppes, PhD, The School of Psychological Sciences, Tel Aviv University, Tel Aviv, Israel
Matthias Siemer, PhD, Department of Psychology, University of Miami, Coral Gables, Florida
Tracy L. Spinrad, PhD, School of Social and Family Dynamics, Arizona State University,
Tempe, Arizona
Michael J. Sulik, MA, Department of Psychology, Arizona State University, Tempe, Arizona
Maya Tamir, PhD, Department of Psychology, The Hebrew University, Jerusalem, Israel
Ross A. Thompson, PhD, Department of Psychology, University of California, Davis,
Davis, California
Dylan D. Wagner, PhD, Department of Psychological and Brain Sciences, Dartmouth College,
Hanover, New Hampshire
Henrik Walter, MD, PhD, Department of Psychiatry and Psychotherapy,
Charité Universitätsmedizin, Berlin, Germany
Anna Weinberg, MA, Department of Psychology, Stony Brook University,
Stony Brook, New York
Christine D. Wilson-Mendenhall, MA, Department of Psychology, Northeastern University,
Boston, Massachusetts
xi
By any measure, the field of emotion regulation is thriving. Books, articles, special jour-
nal issues, and conferences related to emotion regulation seem to be everywhere. This
high (and growing) level of interest in emotion regulation is reflected in citation trends.
Until the early 1990s, there were just a few citations each year containing the term
emotion regulation. By 2007—the year in which the first edition of this handbook was
published—there were more than 3,000 Google Scholar citations for that year alone. In
2012, the citation count passed 8,000 for that year alone. Doing the math, it turns out
that many more papers have been published on emotion regulation in the years since
the first edition of this handbook was published than in all years up to—and includ-
ing—2007. Hence, this second edition of the Handbook of Emotion Regulation.
The goal of this edition is to provide an authoritative and up-to-date account of
findings in this field that will (1) encourage cumulative science by drawing together and
integrating the specialized literatures on emotion regulation that exist in each of the
subareas of psychology; and (2) facilitate cross-disciplinary dialogue about one of the
most fascinating puzzles regarding the human condition, namely, that we are at once
governed by—and governors of—our emotions. Of the 36 chapters in this new edition,
two-thirds are brand new chapters for this edition, and the remaining chapters have
been thoroughly updated. To reflect exciting new developments in the field, three new
sections have been added in this edition: Psychopathology, Interventions, and Health
Implications.
The Handbook begins with a section on foundations, in which I provide a concep-
tual and empirical orientation to the field. To this end, I first set emotion in the context
of other affective processes. Next, I relate emotion regulation to other forms of affect
regulation. I then selectively review what is known about emotion regulation goals,
strategies, and outcomes. In the final part of the chapter, I highlight several fundamen-
tal questions and associated directions for future research on emotion and emotion
regulation.
The second section considers biological bases of emotion regulation. Ochsner and I
draw upon the human neuroimaging literature to present an integrated framework that
links emotion and emotion regulation to other important forms of valuation, including
affective learning, affective decision making, and expectancies, beliefs, and placebo
effects. Proudfit, Dunning, Foti, and Weinberg review electroencephalographic studies
of the temporal dynamics of emotion generation and emotion regulation, demonstrating
that one particular event-related brain potential—the late positive potential—is sen-
sitive to attentional and cognitive emotion regulation manipulations. Johnstone and
Preface
xii Preface
Walter describe the network of prefrontal and subcortical brain structures that are cru-
cial for optimal health and show how this network is dysfunctional in clinical disorders.
Finally, Gyurak and Etkin present a process-oriented neurobiological framework that
seeks to integrate a wide range of implicit and explicit emotion regulation processes that
are crucial to understanding mental health and illness.
The third section examines cognitive aspects of emotion regulation. Miller, Rodri-
guez, Kim, and McClure present a multiple systems account of delay discounting, and
argue that delay discounting provides a framework for studying important instances
of emotion regulation, including responses to temptations, procrastination, willpower,
and addictions. Luerssen and Ayduk review the literature on delay of gratification, with
an emphasis on identifying precursors, assessing the role of current affective state, link-
ing delay of gratification to emotion regulation, and discussing insights from neuroim-
aging. Sheppes introduces the notion of emotion regulation choice; explains why it is
crucial for healthy functioning; and explores the emotional, cognitive, and motivational
determinants of emotion regulation choice. Finally, Grecucci and Sanfey review the
literature on emotion and decision making, and make the case that emotion regulation
influences our decision making in a number of important ways.
The fourth section focuses on developmental considerations, ranging from child-
hood through old age. Eisenberg, Hofer, Sulik, and Spinrad examine the development
of effortful control in childhood and assess its impact on socioemotional, academic,
and moral development. Thompson reviews the literature on the socialization of emo-
tion and emotion regulation in the family, framing the socialization process in terms
of an interaction between bottom-up and top-down influences. Riediger and Klipker
consider emotion regulation in adolescence, with particular attention to the acquisition
of emotion regulation skills, the motivation to regulate, and emotion regulation effec-
tiveness. Finally, Charles and Carstensen take a lifespan perspective, and show how bio-
logical and motivational changes interact to shape the trajectory of emotion regulation
throughout adulthood and into older age.
The fifth section considers social aspects of emotion regulation. Coan and Maresh
present social baseline theory, which they use to explain how and why each of us uses
other people to help regulate our emotions. Shaver and Mikulincer apply attachment
theory to adolescents and adults, and show how this theory illuminates basic processes
and individual differences in interpersonal emotion regulation. Jones, Kirkland, and
Cunningham link the literature on attitudes and evaluation to emotion regulation,
with particular attention to their iterative reprocessing model. Levenson, Haase, Bloch,
Holley, and Seider consider emotion regulation in couples, with particular attention to
the nature, development, and consequences of this form of social emotion regulation.
Finally, Mesquita, De Leersnyder, and Albert present a cultural perspective on emo-
tion regulation and show that regulatory patterns are aligned with cultural ideas and
practices.
The sixth section examines personality processes and individual differences. Roth-
bart, Sheese, and Posner present a temperament systems approach, which provides a
context for understanding individual differences, as well as the development of emo-
tion regulation. John and Eng consider individual differences in emotion regulation,
with particular attention to individual differences in coping, emotional competence,
and specific regulatory processes such as reappraisal and suppression. Hofmann and
Kotabe argue that desires share many similarities with emotion and show how an emo-
tion regulation perspective can be used to organize findings relative to the regulation
Preface xiii
of desire. Mauss and Tamir argue that emotion goals are critical to emotion regula-
tion, and consider their content, hierarchical structure, and modes of operation. Finally,
Leary and Gohar analyze the many ways self-awareness influences emotion regulation.
The seventh section focuses on emotion regulation and psychopathology.
Campbell-Sills, Ellard, and Barlow review behavioral and neurobiological findings rela-
tive to the role of emotion regulation in the development, phenomenology, and treatment
of anxiety disorders. Joormann and Siemer apply an emotion regulation perspective to
mood disorders, with an eye toward understanding the factors that govern the onset
and maintenance of these disorders. Kober discusses the role of emotion regulation in
substance use disorders, featuring both the ways drugs regulate emotions and the ways
in which emotion dysregulation is at once a cause for and a consequence of drug use.
Finally, Barrett, Wilson-Mendenhall, and Barsalou examine emotion regulation from
the perspective of situated conceptualizations and show how this perspective sheds new
light on emotion dysregulation.
The eighth section considers clinical interventions designed to change emotion
regulation. Mennin and Fresco present emotion regulation therapy, and show how this
integrative intervention can be applied to generalized anxiety disorder and major depres-
sion. Neacsiu, Bohus, and Linehan describe dialectical behavior therapy, a treatment
designed for individuals with severe and pervasive disorders of emotion regulation, such
as borderline personality disorder. MacLeod and Grafton review research on atten-
tional bias modification procedures and show how these procedures help individuals
with conditions involving deficient emotion regulation. Berking and Schwarz describe
the development of affect regulation training, which seeks to enhance emotional com-
petence by training specific affect regulation skills. Finally, Farb, Anderson, Irving,
and Segal review the literature on mindfulness meditation, showing how mindfulness
meditation engages basic emotion regulation processes.
The ninth section of this handbook focuses on emotion regulation and health. Cole
draws upon research on social genomics to show how emotional suppression might
affect the molecular underpinnings of disease. Chen and Miller document how socio-
economic status predicts health outcomes, and argue that one reason for this associa-
tion may be that low-socioeconomic-status children fail to learn adaptive forms of emo-
tion regulation. Appleton and Kubzansky employ an emotion regulation framework to
explain the association between negative emotions and cardiovascular disease. Finally,
Wagner and Heatherton show how negative affect—and its misregulation—can lead to
self-control failures ranging from binge eating to aggression.
Although this handbook is divided into sections, one of its major goals is break-
ing down barriers to cross-area communication. For this reason, there are considerably
more cross-chapter links and citations than is typical in a handbook of this kind. There
are also many points at which an author in one section will present material that makes
contact with ideas, methods, and evidence from another section (e.g., developmental
considerations in the health section; neuroscience in the cognitive section; analyses of
individual differences in the social section). My hope is that these carefully assembled
chapters—written by leading scholars in the field—will bring the field of emotion regu-
lation together in a way that will be productive and new.
A large number of wonderful people helped to bring this handbook into being. I
am grateful to Seymour Weingarten, Editor-in-Chief at The Guilford Press, for convinc-
ing me that the time was right for this handbook, and to Robert Levenson, with whom
my work on emotion regulation began. I am also grateful to my many friends and col-
xiv Preface
leagues who have helped shape my thinking about emotion regulation, and I would like
to particularly thank the generous reviewers who provided helpful feedback on each of
these chapters. Finally, I would like to acknowledge the contributions of the members
of the Stanford Psychophysiology Laboratory, who help make Stanford such a fun place
to be.
James J. Gross
xv
Part I. Foundations
1. Emotion Regulation: Conceptual and Empirical Foundations 3
James J. Gross
Part II. Biological Bases
2. The Neural Bases of Emotion and Emotion Regulation: 23
A Valuation Perspective
Kevin N. Ochsner and James J. Gross
3. Temporal Dynamics of Emotion Regulation 43
Greg Hajcak Proudfit, Jonathan P. Dunning, Daniel Foti, and Anna Weinberg
4. The Neural Basis of Emotion Dysregulation 58
Tom Johnstone and Henrik Walter
5. A Neurobiological Model of Implicit and Explicit Emotion Regulation 76
Anett Gyurak and Amit Etkin
Part III. Cognitive Approaches
6. Delay Discounting: A Two‑Systems Perspective 93
Eric M. Miller, Christian Rodriguez, Bokyung Kim, and Samuel M. McClure
7. The Role of Emotion and Emotion Regulation 111
in the Ability to Delay Gratification
Anna Luerssen and Ozlem Ayduk
Contents
xvi Contents
8. Emotion Regulation Choice: Theory and Findings 126
Gal Sheppes
9. Emotion Regulation and Decision Making 140
Alessandro Grecucci and Alan G. Sanfey
Part IV. Developmental Considerations
10. Self-Regulation, Effortful Control, and Their Socioemotional Correlates 157
Nancy Eisenberg, Claire Hofer, Michael J. Sulik, and Tracy L. Spinrad
11. Socialization of Emotion and Emotion Regulation in the Family 173
Ross A. Thompson
12. Emotion Regulation in Adolescence 187
Michaela Riediger and Kathrin Klipker
13. Emotion Regulation and Aging 203
Susan Turk Charles and Laura L. Carstensen
Part V. Social Aspects
14. Social Baseline Theory and the Social Regulation of Emotion 221
James A. Coan and Erin L. Maresh
15. Adult Attachment and Emotion Regulation 237
Phillip R. Shaver and Mario Mikulincer
16. Attitudes, Evaluation, and Emotion Regulation 251
Christopher R. Jones, Tabitha Kirkland, and William A. Cunningham
17. Emotion Regulation in Couples 267
Robert W. Levenson, Claudia M. Haase, Lian Bloch, Sarah R. Holley,
and Benjamin H. Seider
18. The Cultural Regulation of Emotions 284
Batja Mesquita, Jozefien De Leersnyder, and Dustin Albert
Part VI. Personality Processes and Individual Differences
19. Temperament and Emotion Regulation 305
Mary K. Rothbart, Brad E. Sheese, and Michael I. Posner
20. Three Approaches to Individual Differences in Affect Regulation: 321
Conceptualizations, Measures, and Findings
Oliver P. John and Joshua Eng

Contents xvii
21. Desire and Desire Regulation: Basic Processes 346
and Individual Differences
Wilhelm Hofmann and Hiroki P. Kotabe
22. Emotion Goals: How Their Content, Structure, and Operation 361
Shape Emotion Regulation
Iris B. Mauss and Maya Tamir
23. Self-Awareness and Self-Relevant Thought in the Experience 376
and Regulation of Emotion
Mark R. Leary and Dina Gohar
Part VII. Psychopathology
24. Emotion Regulation in Anxiety Disorders 393
Laura Campbell-Sills, Kristen K. Ellard, and David H. Barlow
25. Emotion Regulation in Mood Disorders 413
Jutta Joormann and Matthias Siemer
26. Emotion Regulation in Substance Use Disorders 428
Hedy Kober
27. A Psychological Construction Account of Emotion Regulation 447
and Dysregulation: The Role of Situated Conceptualizations
Lisa Feldman Barrett, Christine D. Wilson-Mendenhall,
and Lawrence W. Barsalou
Part VIII. Interventions
28. Emotion Regulation Therapy 469
Douglas S. Mennin and David M. Fresco
29. Dialectical Behavior Therapy: An Intervention 491
for Emotion Dysregulation
Andrada D. Neacsiu, Martin Bohus, and Marsha M. Linehan
30. Regulation of Emotion through Modification of Attention 508
Colin MacLeod and Ben Grafton
31. Affect Regulation Training 529
Matthias Berking and Jeanine Schwarz
32. Mindfulness Interventions and Emotion Regulation 548
Norman A. S. Farb, Adam K. Anderson, Julie A. Irving,
and Zindel V. Segal

xviii Contents
Part IX. Health Implications
33. Emotion Regulation and Gene Expression 571
Steven W. Cole
34. Early-Life Socioeconomic Status, Emotion Regulation, 586
and the Biological Mechanisms of Disease across the Lifespan
Edith Chen and Gregory E. Miller
35. Emotion Regulation and Cardiovascular Disease Risk 596
Allison A. Appleton and Laura D. Kubzansky
36. Emotion and Self-Regulation Failure 613
Dylan D. Wagner and Todd F. Heatherton
Author Index 629
Subject Index 657

PART I
FOUNDATIONS

3
Emotions often are wonderfully helpful.
They can direct attention to key features of
the environment, optimize sensory intake,
tune decision making, ready behavioral
responses, facilitate social interactions, and
enhance episodic memory. However, emo-
tions can harm as well as help, particularly
when they are of the wrong type, intensity,
or duration for a given situation.
At such moments, we may try to regulate
our emotions. This fundamental insight—
that emotions can and should be regulated
in certain situations— is well represented
over the centuries in each of the major world
traditions (for a more detailed historical
overview of the field, see Gross, 1999).
In the past century, psychological inves-
tigations of emotion regulation have
focused on psychological defenses (Freud,
1926/1959), stress and coping (Lazarus,
1966), attachment (Bowlby, 1969), and self-
regulation (Mischel, 1996). However, until
the early 1990s, there were relatively few
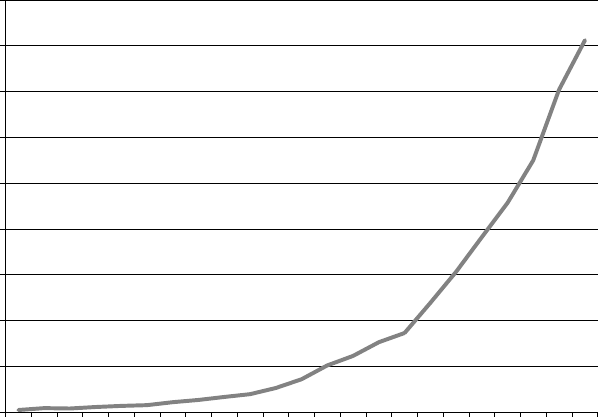
papers each year containing the term emo-
tion regulation (see Figure 1.1). Now there
are thousands of new publications each year,
making emotion regulation one of the fastest
growing areas within the field of psychology
(Koole, 2009; Tamir, 2011).
What is needed is a framework for orga-
nizing this bewildering array of findings. My
aim in this chapter is to provide such a con-
ceptual and empirical framework. Because
a discussion of emotion regulation presup-
poses an understanding of what emotion is,
in the first section I present the modal model
of emotion and relate emotion to other
affective processes. In the second section, I
describe the process model of emotion regu-
lation and distinguish emotion regulation
from other forms of self- regulation. This pre-
pares the way for the third section, in which
I discuss key findings regarding emotion
regulation goals, strategies, and outcomes.
In the final section, I highlight three of the
biggest challenges— and opportunities— for
those interested in emotion regulation.
Emotions and Related Processes
One of the toughest questions in the field
of affective science is one of the simplest,
namely: What is an emotion? Theorists have
tried to address this question by posing two
other questions: What attributes are shared
by all emotions (necessary conditions)?
What attributes— if present— guarantee that
something is an emotion (sufficient condi-
tions)? Unfortunately, efforts to derive this
kind of tidy classical definition of emotion
are thwarted by the fact that emotion refers
to an astonishing array of responses, from the
mild to the intense, the brief to the extended,
CHAPTER 1
Emotion Regulation:
Conceptual and Empirical Foundations
James J. Gross
4 FOUNDATIONS
the simple to the complex, and the private to
the public. Disgust at a prejudiced comment
counts as an emotion. So does amusement
at a funny mishap, anger at social injustice,
joy at the prospect of receiving a promotion,
surprise at a friend’s “new look,” grief at the
death of a spouse, and embarrassment at a
child’s misbehavior. What are the core fea-
tures of these diverse emotions?
Core Features of Emotion
The first core feature of emotion has to do
with when it occurs. According to appraisal
theory, emotions arise when an individual
attends to and evaluates (appraises) a situ-
ation as being relevant to a particular type
of currently active goal (Lazarus, 1991;
Scherer, Schorr, & Johnstone, 2001). The
goals that underlie this evaluation may be
enduring (staying alive) or transient (want-
ing another piece of cake). They may be con-
scious and complicated (aspiring to become
a professor) or unconscious and simple (try-
ing to avoid stepping in puddles). They may
be widely shared (having close friends) or
highly idiosyncratic (finding a new way of
tying one’s shoes). Whatever the goal, and
whatever meaning the situation has in light
of the goal, it is this meaning that gives rise
to emotion. As this meaning changes over
time—due either to changes in the situation
itself or changes in the meaning the situation
holds for the individual— the emotion will
also change.
The second core feature of emotion has to
do with its multifaceted nature. Emotions
are whole-body phenomena that involve
loosely coupled changes in the domains of
subjective experience, behavior, and central
and peripheral physiology (Mauss, Leven-
son, McCarter, Wilhelm, & Gross, 2005).
The subjective aspect of emotion is so cen-
tral to many instances of emotion that the
terms “emotion” and “feeling” often are
used interchangeably. But emotions not
only make us feel, they also incline us to
act (Frijda, 1986). These impulses to act in
certain ways (and not act in others) include
changes in facial behavior and body posture,
as well as situation- specific instrumental
actions such as staring, hitting, or running.
0
1000
2000
3000
4000
5000
6000
7000
8000
9000
1990
1991
1992
1993
1994
1995
1996
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
FIGURE 1.1. Number of publications containing the exact term emotion regulation in Google Scholar
each year from 1990 to 2012 (Gross, 2013). Note that this is not a cumulative plot; each point repre-
sents 1 year’s citations.

Conceptual and Empirical Foundations 5
These changes in experience and behavior
are associated with autonomic and neuro-
endocrine responses that both anticipate
emotion- related behaviors (thereby provid-
ing metabolic support for the action) and
follow them, often as a consequence of the
motor activity associated with the emotional
response (Lang & Bradley, 2010). As func-
tionalist accounts of emotion make clear,
the multifaceted responses that comprise
emotion often (but not always) are useful in
helping to achieve the goals that gave rise to
emotions in the first place (Levenson, 1999).
The Modal Model of Emotion
These core features constitute what has
been referred to as the modal model of
emotion— so called because these features
are evident in many different approaches to
emotion (Barrett, Ochsner, & Gross, 2007;
Gross, 1998a). According to this model,
emotions involve person– situation transac-
tions that compel attention, have meaning
to an individual in light of currently active
goals, and give rise to coordinated yet flex-
ible multisystem responses that modify the
ongoing person– situation transaction in
crucial ways. The modal model lies at the
heart of lay intuitions about emotion and
also represents key points of convergence
among researchers and theoreticians con-
cerned with emotion.
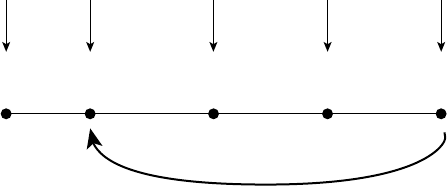
In Figure 1.2, I present the situation–
attention– appraisal– response sequence speci-
fied by the modal model of emotion in
highly abstracted and simplified form. This
sequence begins with a psychologically rel-
evant situation. Often, this is a situation that
can be specified by referring to features of
the external environment (e.g., the snake
slithering into my tent). However, psycho-
logically relevant “situations” can also be
internal (e.g., the sneaking suspicion that
I’ll never amount to anything). Whether
external or internal, situations are attended
to in various ways, giving rise to appraisals
that constitute the individual’s assessment
of what the situation means in light of rel-
evant goals (Ellsworth & Scherer, 2003).
The emotional responses generated by these
unfolding appraisals involve changes in
experiential, behavioral, and neurobiologi-
cal response systems.
Like many other responses we make,
emotional responses often change the situ-
ation that gave rise to the response in the
first place. Figure 1.2 depicts this aspect of
emotion by showing the response looping
back to (and modifying) the situation that
gave rise to the emotion. To make this idea
more concrete, imagine a husband and wife
heatedly disagreeing about whether house-
hold chores are being fairly divided. Several
minutes into the discussion, the husband
starts to cry (yes, the husband). This emo-
tional response dramatically alters the inter-
personal situation. This new situation now
gives rise to a new response from the wife—
now no longer angry but instead feeling
compassion. This compassionate response
itself further changes the interpersonal situ-
ation, giving rise to other emotions in each
of them. The key idea here is that emotional
responses often lead to changes in the envi-
ronment that alter the probability of subse-
quent instances of that and other emotions
(for a more detailed discussion of this point,
see Gross & Thompson, 2007).
Emotions and Other
Affective
Processes
One thing that makes the emotion literature
challenging is that many different terms are
used to refer to emotion- related processes,
including affect, emotion, stress, and mood
(Davidson, 1994). Unfortunately, these
terms are used in different ways by differ-
ent researchers, leading at times to some
degree of “conceptual and definitional
chaos” (Buck, 1990, p. 330). To organize
this chaotic landscape, I find it useful to
view affect as the umbrella term for states
that involve relatively quick good–bad dis-
criminations (Scherer, 1984). These affec-
tive states include (1) emotions such as anger
and sadness, (2) stress responses to circum-
stances that exceed an individual’s ability to FIGURE 1.2. The modal model of emotion.
Situation Attention Appraisal Response

6 FOUNDATIONS
cope, and (3) moods such as depression and
euphoria.
How are these various affective processes
distinguished? Although both emotion and
stress involve whole-body responses to sig-
nificant events, “stress” typically refers to
negative (but otherwise unspecified) affec-
tive responses, whereas “emotion” refers to
both negative and positive affective states
(Lazarus, 1993). Emotions also may be
distinguished from moods (Parkinson, Tot-
terdell, Briner, & Reynolds, 1996). Moods
often last longer than emotions, and com-
pared to moods, emotions are typically
elicited by specific objects and give rise to
behavioral response tendencies relevant to
these objects. By contrast, moods are more
diffuse, and although they may give rise to
broad action tendencies such as approach or
withdrawal (Lang, 1995), moods bias cog-
nition more than they bias action (Siemer,
2001).
Lest these distinctions seem academic,
consider the term affect. From my perspec-
tive, affect belongs at the top of the hier-
archy, as the superordinate term in this set
of emotion- related terms. However, others
take a different view. For example, some use
the terms affect and emotion interchange-
ably (Zajonc, 1984). For others, affect refers
to the experiential component of emotion
(Buck, 1993; MacLean, 1990). Still others
use affect to refer to the behavioral compo-
nent of emotion (American Psychiatric Asso-
ciation, 2013; Kaplan & Sadock, 1991). As
these observations suggest, clarity regarding
how each of these constructs is being used
is a necessary prerequisite for an analysis of
how these various processes are (or are not)
regulated (Gross, 2010).
Emotion Regulation
and Related Processes
Emotion regulation refers to shaping which
emotions one has, when one has them, and
how one experiences or expresses these emo-
tions (Gross, 1998b). Thus, emotion regula-
tion is concerned with how emotions them-
selves are regulated (regulation of emotions),
rather than how emotions regulate some-
thing else (regulation by emotions). Defined
in this way, many different activities count
as emotion regulatory. These include pound-
ing your pillow when you’re angry at a boss,
imagining your audience naked when you’re
nervous about performing in a piano recital,
picking up the phone to call a friend when
you’re feeling sad, telling a child who is hav-
ing a tantrum not to act like such a baby,
anticipating going to a fun party on the
weekend to reenergize yourself midweek,
going for a run after an upsetting fight
with a friend, playing calming music after
a long day at work, leaving a tense meeting
early to cool down, going to a club to have
a drink, and watching It’s a Wonderful Life
for the 600th time. Because there seems to
be no limit to the activities that may qualify
as emotion regulatory, what is needed— as
with emotion— is a description of its core
features.
Core Features of Emotion Regulation
The first core feature of emotion regula-
tion is the activation of a goal to modify the
emotion- generative process (Gross, Sheppes,
& Urry, 2011). This goal may be activated
either in oneself or in someone else. To
mark this distinction, it is useful to refer
to intrinsic emotion regulation in the first
case (James regulates his own emotions:
emotion regulation in self) and to extrin-
sic emotion regulation in the second case
(James regulates Sarah’s emotions: emotion
regulation in another). Researchers who
work with adults typically focus on intrinsic
emotion regulation (Gross, 1998b; but see
Levenson, Haase, Bloch, Holley, & Seider,
this volume). By contrast, researchers who
work with infants and children typically
focus on extrinsic emotion regulation (e.g.,
Cole, Martin, & Dennis, 2004). Although
this distinction is often helpful, it is worth
noting that in some situations intrinsic and
extrinsic emotion regulation co-occur, such
as when James regulates Sarah’s emotions
(extrinsic regulation) in order to calm him-
self down (intrinsic regulation).
The second core feature of emotion reg-
ulation is the engagement of the processes
that are responsible for altering the emotion
trajectory. Many different processes can be
recruited to regulate emotions, and these
vary considerably in the degree to which
they are explicit versus implicit. Many pro-
totypical instances of emotion regulation are
explicit, and thus conscious, such as when
Conceptual and Empirical Foundations 7
we try hard to look calm even though we are
very anxious before a talk, or when we try to
look on the bright side of a bad outcome to
cheer ourselves up. However, emotion regu-
latory activity can also be implicit and take
place without conscious awareness. Exam-
ples include hiding the affection one feels for
another person due to a fear that one will
be rejected, or quickly turning one’s atten-
tion away from potentially upsetting mate-
rial. Previous discussions have distinguished
categorically between explicit and implicit
processes (Masters, 1991). However, it may
be more useful to think of a continuum of
emotion regulation possibilities that range
from explicit, conscious, effortful, and con-
trolled regulation to implicit, unconscious,
effortless, and automatic regulation (Gyurak
& Etkin, this volume; Gyurak, Gross, &
Etkin, 2011).
The third core feature of emotion regu-
lation is its impact on emotion dynamics
(Thompson, 1990), or the latency, rise time,
magnitude, duration, and offset of responses
in experiential, behavioral, or physiologi-
cal domains. Depending on the individual’s
goals, emotion regulation may increase or
decrease the latency, rise time, magnitude,
duration, or offset of the emotional response
(compared to the emotional response that
would have occurred in the absence of
emotion regulation) (Gross, 1998b). Emo-
tion regulation also may change the degree
to which emotion response components
cohere as the emotion unfolds, such as when
changes in emotion experience and physi-
ological responding occur in the absence
of facial behavior (Dan- Glauser & Gross,
2013).
These three core features of emotion
regulation— the activation of a regulatory
goal, the engagement of regulatory pro-
cesses, and the modulation of the emotion
trajectory— are common features of many
diverse types of emotion regulation. In a
later section, I turn to a more complete dis-
cussion of each of these three core features,
as I review what is known about emotion
regulation goals, emotion regulation strat-
egies, and emotion regulation outcomes.
Before elaborating upon each of these core
features, however, we need to consider what
makes different forms of emotion regulation
so different from one another.
The Process Model
of Emotion Regulation
One framework that has proven useful for
addressing this question is the process model
of emotion regulation (Gross, 1998b). This
information- processing model takes as
its starting point the modal model (Figure
1.2), which—as we have seen— specifies the
sequence of processes involved in emotion
generation. The process model of emotion
regulation builds on the modal model, and
treats each step in the emotion- generative
process that is described in the modal model
as a potential target for regulation. In Fig-
ure 1.3, I present the process model, which
highlights five points at which individuals
can regulate their emotions.
These five points represent five families
of emotion regulation processes: situation
selection, situation modification, atten-
tional deployment, cognitive change, and
response modulation (Gross, 1998b). These
FIGURE 1.3. The process model of emotion regulation.
Situation Attention Appraisal Response
Response
Modulation
Cognitive
Change
Attentional
Deployment
Situation
Modification
Situation
Selection

8 FOUNDATIONS
families are distinguished by the point in the
emotion- generative process at which they
have their primary impact. Movement from
left to right in Figure 1.3 represents move-
ment through time: A particular situation is
selected, modified, attended to, appraised,
and yields a particular set of emotional
responses. However, as emphasized in Fig-
ure 1.2, emotion generation is an ongoing
process, extending beyond a single episode.
This dynamic aspect of emotion and emotion
regulation is signaled by the feedback arrow
in Figure 1.3 from the emotional response
back to the situation (there may in fact be
many such points of feedback). I describe
these five families of regulatory strategies in
more detail below.
Emotion Regulation
and Related Constructs
Before considering emotion regulation pro-
cesses in greater detail, however, it is impor-
tant to note in passing that— paralleling the
distinctions drawn among members of the
affective family presented earlier— emotion
regulation can be seen as subordinate to
the broader construct of affect regulation.
Under this broad heading fall all manner of
efforts to influence our valenced responses
(Westen, 1994), including (1) emotion regu-
lation, (2) coping, and (3) mood regulation.
Because virtually all goal- directed behavior
can be construed as maximizing pleasure or
minimizing pain—and thus as affect regula-
tory in a broad sense—it is frequently useful
to sharpen the focus by examining one or
more of these three second- level families of
processes.
Coping is distinguished from emotion
regulation both by its predominant focus on
decreasing negative affect and its emphasis
on much larger periods of time (e.g., cop-
ing with bereavement). As noted earlier,
moods are typically of longer duration and
are less likely to involve responses to spe-
cific “objects” than are emotions (Parkinson
et al., 1996). In part due to their less well-
defined behavioral response tendencies, in
comparison with emotion regulation, mood
regulation and mood repair are more con-
cerned with altering emotion experience
than emotion behavior (Larsen, 2000). It
is not yet known whether the regulation of
emotion, stress responses, and moods are
more similar than different, more different
than similar, or somewhere in between. It
therefore is usually a good idea to pay close
attention to the type of affect targeted for
regulation.
Emotion Regulation Goals,
Strategies, and Outcomes
As we have seen, emotion regulation has
three core features. The first—the emotion
regulation goal—is what people are trying to
accomplish. The second— the emotion regu-
lation strategy—is the particular processes
that are engaged in order to achieve that
goal. The third—the outcome—refers to the
consequences of trying to achieve that par-
ticular emotion regulation goal using that
particular strategy. In the following sections,
I review each of these three core features of
emotion regulation in turn, selectively high-
lighting what we know about each.
Emotion Regulation Goals
If asked about times they have tried to regu-
late their emotions, people often describe
efforts to down- regulate negative emotions
(i.e., diminish their intensity or duration),
especially anger, sadness, and anxiety, with
a particular focus on decreasing the expe-
riential and behavioral aspects of negative
emotions (Gross, Richards, & John, 2006).
People also report trying to up- regulate pos-
itive emotions (i.e., increase their intensity or
duration), especially love, interest, and joy,
often by sharing their positive experiences
with others (Quoidbach, Berry, Hansenne,
& Mikolajczak, 2010). These reports of
everyday emotion regulation are consistent
with traditional hedonic accounts of affect
regulation, which assume that individuals
are motivated to decrease negative emo-
tional states and increase positive emotional
states (Larsen, 2000).
It turns out, however, that there is more
to emotion regulation than this. Indeed,
the down- regulation of negative emotions
and the up- regulation of positive emotions
can be seen as just two cells in the 2 × 2
matrix shown in Figure 1.4. It may seem
odd to imagine people wanting more of a
“bad” thing or less of a “good” thing, but
there are many reasons people might want
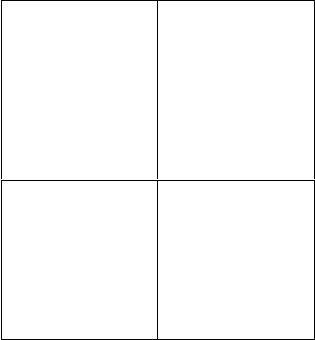
Conceptual and Empirical Foundations 9
to up- regulate negative emotions or down-
regulate positive emotions, as Parrott (1993)
has observed in the context of mood regu-
lation. Motives for up- regulating negative
emotions include promoting a focused, ana-
lytic mindset; fostering an empathic stance;
and influencing others’ actions. Motives for
down- regulating positive emotions include
maintaining a realistic mindset; being mind-
ful of social conventions; and concealing
one’s feelings from others.
This broader view of emotion regulation
goals suggests something important about
what people are trying to accomplish when
they regulate their emotions. Sometimes—
and perhaps often— people are motivated
by “hedonic considerations,” or the wish to
increase short-term pleasure and decrease
short-term pain. At other times, however,
people are motivated by “instrumental con-
siderations”; that is, they are motivated to
change their emotions in order to achieve
some other, nonemotional outcome (Mauss
& Tamir, this volume; Tamir, 2009). Some-
times, these instrumental goals may be
related to specific work demands, such as
appearing relaxed and upbeat for nervous
airline passengers (Hochschild, 1983), seem-
ing calm yet empathic for nervous medical
patients (Larson & Yao, 2005), showing
high levels of interest in students (R. E. Sut-
ton, 2004), or sounding angry when trying
to collect payment on debts (R. I. Sutton,
1991). At other times, these instrumental
goals are related to broader cultural impera-
tives (Mesquita, De Leersnyder, & Albert,
this volume); these may dictate that people
show (Szczurek, Monin, & Gross, 2012) or
feel (Tsai, 2007) particular emotions and
not others in a given situation.
Emotion Regulation Strategies
Whatever emotion regulation goals people
may have, they can do many different things
to achieve them. They can even do many dif-
ferent things at once—or at least in quick
succession. For example, after a stressful
day, some people might turn off their cell
phone, have a beer, and watch an entertain-
ing program on television while holding
hands with their partner. This kind of mix-
ing of regulation strategies is probably com-
mon in everyday life. For analytic purposes,
however, the process model distinguishes
five families of regulatory processes.
The most forward- looking approach to
emotion regulation is situation selection.
This type of emotion regulation involves
taking actions that make it more (or less)
likely that one will end up in a situation
that one expects will give rise to desirable
(or undesirable) emotions. Examples include
avoiding a grumpy neighbor, arranging a
play date for a child, or seeking out a friend
with whom one can have a good cry. Despite
the commonness of situation selection, it is
hard to tell how one will feel in different sit-
uations (in the case of intrinsic regulation),
and harder still to be sure how another per-
son will feel in various situations (in the case
of extrinsic regulation).
Situation modification refers to directly
modifying a situation so as to alter its emo-
tional impact. When one’s parents visit at
college, situation modification may take
the form of hiding piles of dirty laundry or
questionable artwork. Parents also engage
in their share of situation modification,
which ranges from helping with frustrating
math problems to suggesting games to play
on a rainy day. Because efforts to modify a
Decrease Increase
Negative
emotion
Trying to calm
oneself down when
angry (Int)
Helping a tearful
child untangle his
kite (Ext)
Firing oneself up
before a big game
(Int)
Reframing a friend’s
“little fight” with a
spouse as serious
(Ext)
Positive
emotion
Wiping a smile
off one’s face at a
funeral (Int)
Helping giggling
girls calm down at
bedtime (Ext)
Sharing great news
with close friends
(Int)
Telling someone a
joke to cheer her up
(Ext)
FIGURE 1.4. Emotion regulation goals can
include efforts to decrease or increase either the
magnitude or duration of negative or positive
emotion. Decreasing negative emotion appears
to be the most common regulation goal in every-
day life, followed by increasing positive emotion.
Emotion regulation may be either intrinsic (Int)
or extrinsic (Ext).
10 FOUNDATIONS
situation may effectively call a new situa-
tion into being, it is sometimes difficult to
distinguish between situation selection and
situation modification. Also, although “situ-
ations” can be external or internal, situation
modification— as I mean it here—has to do
with modifying external, physical environ-
ments. Modifying “internal” environments
(i.e., thoughts) will be considered later, in
the context of cognitive change.
Attentional deployment refers to direct-
ing attention within a given situation in
order to influence one’s emotions. Atten-
tional deployment is one of the first emotion
regulatory processes to appear during devel-
opment (Rothbart, Ziaie, & O’Boyle, 1992),
and it is used from cradle to grave, particu-
larly when it is not possible to modify one’s
situation. One of the most common forms of
attentional deployment is distraction, which
focuses attention on other aspects of the situ-
ation or moves attention away from the situ-
ation altogether; distraction also may involve
changing internal focus, such as when some-
one calls to mind thoughts or memories that
help to instantiate the desired emotional state
(Thiruchselvam, Hajcak, & Gross, 2012).
Cognitive change refers to modifying
how one appraises a situation so as to alter
its emotional significance, either by chang-
ing how one thinks about the situation or
about one’s capacity to manage the demands
it poses. Sometimes, cognitive change is
applied to an external situation (e.g., “This
interview is a chance for me to learn more
about the company”). At other times, cogni-
tive change is applied to an internal situation
(e.g., “I’m not anxious— I’m getting ‘pumped
up’ for a game, and this will help me play my
best”). One particularly well- studied form
of cognitive change is reappraisal; this form
of cognitive change is often used to decrease
negative emotions, but it can also be used
to increase or decrease negative or positive
emotions (Samson & Gross, 2012).
The fifth family of emotion regulatory
processes, response modulation, occurs
late in the emotion- generative process, after
response tendencies have already been initi-
ated, and refers to directly influencing expe-
riential, behavioral, or physiological com-
ponents of the emotional response. Physical
exercise and deep- breathing relaxation tech-
niques can be used to decrease experiential
and physiological aspects of negative emo-
tions, and alcohol, cigarettes, drugs, and
even food also may be used to modify emo-
tion experience. Another common form of
response modulation involves regulating
emotion- expressive behavior. One well-
researched example of response modulation
is expressive suppression, in which a person
tries to inhibit ongoing negative or positive
emotion- expressive behavior.
Emotion Regulation Outcomes
At the heart of the process model is the intu-
ition that different forms of emotion regula-
tion might have different consequences, both
immediately and over the long term. This
prediction flows from the idea that if emo-
tions develop over time, then intervening at
different points in the emotion- generative
process should lead to different outcomes.
To test this idea, researchers have used
both experimental and correlational
approaches to investigate the affective, cog-
nitive, and social consequences of different
types of emotion regulation. This work is
yielding a rich and nuanced understanding
of how specific forms of emotion regulation
affect both the people who are doing the
regulating and the people around them.
To illustrate this rapidly growing body
of work, I focus on one of the most well-
researched contrasts in the field, namely, the
contrast between reappraisal (from the cog-
nitive change family) and suppression (from
the response modulation family). This con-
trast is an interesting one because although
both suppression and reappraisal are com-
monly employed to down- regulate emotion,
suppression is a behaviorally oriented form
of emotion regulation in which a person
decreases emotion- expressive behavior while
emotionally aroused, whereas reappraisal is
a cognitively oriented form of emotion regu-
lation in which a person tries to think about
a situation in a way that alters the emotional
response (for a more comprehensive review
of the effects of different emotion regula-
tion strategies, see Webb, Miles, & Sheeran,
2012).
Affectively, experimental studies have
shown that suppression leads to decreased
positive but not negative emotion experi-
ence (Gross, 1998a; Gross & Levenson,
1993, 1997; Stepper & Strack, 1993; Strack,
Martin, & Stepper, 1988), increased sympa-
Conceptual and Empirical Foundations 11
thetic nervous system responses (Demaree
et al., 2006; Gross, 1998a; Gross & Lev-
enson, 1993, 1997; Harris, 2001; Richards
& Gross, 2000), and greater activation in
emotion- generative brain regions such as
the amygdala (Goldin, McRae, Ramel, &
Gross, 2008). Correlational studies are
largely congruent with these experimental
findings and, if anything, suggest a more
negative profile of affective consequences
for suppression, in that compared to people
who do not report using suppression, people
who report using suppression experience less
positive emotion and more negative emo-
tion, including painful feelings of inauthen-
ticity as well as depressive symptoms (Gross
& John, 2003; Moore, Zoellner, & Mollen-
holt, 2008; Nezlek & Kuppens, 2008).
By contrast, experimental studies have
shown that reappraisal leads to decreased
levels of negative emotion experience and
increased positive emotion experience
(Gross, 1998a; Feinberg, Willer, Antonenko,
& John, 2012; Lieberman, Inagaki, Tabib-
nia, & Crockett, 2011; Ray, McRae, Och-
sner, & Gross, 2010; Szasz, Szentagotai, &
Hofmann, 2011; Wolgast, Lundh, & Viborg,
2011), has no impact on or even decreases
sympathetic nervous system responses
(Gross, 1998a; Kim & Hamann, 2012;
Stemmler, 1997; Shiota & Levenson, 2012;
Wolgast et al., 2011), and leads to lesser acti-
vation in emotion- generative brain regions
such as the amygdala (Goldin et al., 2008;
Kanske, Heissler, Schonfelder, Bongers,
& Wessa, 2011; Ochsner & Gross, 2008;
Ochsner et al., 2004) and ventral striatum
(Staudinger, Erk, Abler, & Walter, 2009).
Correlational studies suggest that compared
to people who do not use reappraisal, people
who use reappraisal experience and express
more positive emotion and less negative
emotion, including fewer depressive symp-
toms (Gross & John, 2003; Nezlek & Kup-
pens, 2008). Reappraisers’ reports of less
negative emotion are corroborated by func-
tional imaging studies that show less activa-
tion in emotion- related regions such as the
amygdala (Drabant et al., 2009).
Cognitively, experimental studies have
shown that suppression leads to worse mem-
ory (Johns, Inzlicht, & Schmader, 2008;
Richards, Butler, & Gross, 2003; Richards
& Gross, 1999, 2000, 2006). Correlational
findings support these conclusions: Indi-
viduals who typically use suppression have
worse memory for emotional interactions
than do individuals who use suppression less
frequently (Richards & Gross, 2000).
By contrast, experimental studies have
found that reappraisal either has no impact
on subsequent memory or actually improves
it (Richards & Gross, 2000; Hayes et al.,
2011), and can enhance performance on
standardized exams (Jamieson, Mendes,
Blackstock, & Schmader, 2010). Correla-
tional studies bear out these findings, show-
ing that individuals who typically reappraise
have comparable or even enhanced memory
compared to others (Richards & Gross,
2000).
Socially, experimental studies have
reported that suppression leads to less lik-
ing from social interaction partners, and to
an increase in partners’ blood pressure lev-
els (Butler et al., 2003). Correlational stud-
ies support these laboratory findings. Indi-
viduals who typically use suppression report
avoiding close relationships and having less
positive relations with others; this dove-
tails with peers’ reports that suppressors
have relationships with others that are less
emotionally close (English, John, & Gross,
2013; Gross & John, 2003; Srivastava,
Tamir, McGonigal, John, & Gross, 2009).
Reappraisal, by contrast, has no detectable
adverse consequences for social affiliation in
a laboratory context (Butler et al., 2003).
Correlational studies support these findings:
Individuals who typically use reappraisal
are more likely to share their emotions—
both positive and negative— and report hav-
ing closer relationships with friends, which
matches their peers’ reports of greater liking
(Gross & John, 2003; Mauss et al., 2011).
Across these three outcome domains,
reappraisal seems preferable to suppression.
However, caution is required here, because
the effects of emotion regulation vary by con-
text. Thus, the adverse social consequences
of suppression are not evident in individu-
als with bicultural European– Asian values
(Butler, Lee, & Gross, 2007; Soto, Perez,
Kim, Lee, & Minnick, 2011). Similarly,
some of the benefits of reappraisal are mod-
erated by context. For example, if emotional
intensity is already high when reappraisal is
engaged, it no longer has the experiential or
physiological benefits seen in other contexts
(Sheppes, Catran, & Meiran, 2009).

12 FOUNDATIONS
The context specificity of the effects of sup-
pression and reappraisal (and, presumably,
other forms of emotion regulation) means
that global conclusions about one strategy
being “better” than another are likely to be
misleading. Indeed, any given emotion regu-
lation strategy may be used to make things
either better or worse, depending on whose
point of view is adopted, on the outcome
of interest, and on details regarding the
context. For example, cognitive strategies
that dampen negative emotions may help a
medical professional operate efficiently in
stressful circumstances, but they also may
neutralize negative emotions associated
with empathy and thereby decrease helping.
It also bears emphasizing that regulatory
strategies may accomplish one person’s goals
at the expense of another’s. For example, a
mother may accomplish her goals when she
stops a child from crying for candy in the
supermarket, but this success may come at
the expense of the child’s failure to achieve
his or her goal of getting candy.
Fundamental Questions
and Directions for Future Research
As is the case with any new and vital area
of science, the study of emotion regulation
has generated many more questions than
answers (Gross, 2013). In the following
sections, I describe three of the questions I
think are particularly important to the field
of emotion regulation.
How Separable Are Emotion
and Emotion Regulation?
One of the most intuitively compelling dis-
tinctions in the field of emotion research is
that between emotion and emotion regula-
tion. We feel angry, and try not to show it. A
child cries, and we comfort her. We are dis-
couraged, and try to find hope. In each case,
it seems utterly obvious that one set of psy-
chological processes governs the emergence
of an emotion, and another governs whether
and how we manage these emotions.
However, the closer one looks, the harder
it is to draw a bright line between emotion
and emotion regulation (Gross et al., 2011).
Many situations seem to call forth both
emotion and emotion regulation (Campos,
Frankel, & Camras, 2004), and many of the
brain systems that give rise to emotion are
also engaged by emotion regulation (Ochsner
et al., 2009). This has led some commenta-
tors to argue that the two sets of processes
are so intertwined that no clear distinction
can be made between them (Kappas, 2011;
Thompson, 2011).
Part of the problem here is that there are
many different ways to define emotion, each
of which suggests a different take on how
(and whether) emotion and emotion regu-
lation should be distinguished (Gross &
Barrett, 2011). From my perspective, the
crucial distinction between emotion and
emotion regulation is a functional one. As
we have seen, an emotion arises when a
person attends to a situation that he or she
evaluates as relevant to a particular type of
goal. For example, I may feel angry at others
when they throw garbage from their cars. I
may even have an emotion about my anger
response. For example, I may feel proud that
I feel anger at others who are degrading the
environment. Emotion regulation may be
said to occur when (1) an emotional response
itself is subject to valuation as good or bad,
and (2) this valuation leads to the activation
of a goal to change that particular emotion
response trajectory. To continue the earlier
example, if I find myself getting so annoyed
at others who pollute the environment that I
snap at my children, I may negatively value
my anger and feel upset that I am snapping
at my children. If this is all that happens,
there’s no emotion regulation— just two
overlapping instances of emotion (anger at
polluters, and upset at myself for snapping at
my children). But if this feeling of upset leads
me to try to curb my anger, then this would
be an instance of emotion regulation.
A more general way of putting this idea is
to say that emotion regulation involves the
valuation of a valuation. That is, an emo-
tional response is itself judged to be good or
bad—hence leading to an affective response
about the target emotional response— and
this second affective response motivates an
effort to modify the first affective response.
This perspective on emotion regulation is
functional in that it doesn’t define a priori
what should “count” as emotion versus
emotion regulation; instead, the question of
separability hinges on whether a goal has
been activated to influence the emotion-
Conceptual and Empirical Foundations 13
generative process itself (for a more detailed
exposition of this valuation perspective on
emotion regulation, see Ochsner & Gross,
this volume).
Why Do People Regulate Their
Emotions as They Do?
Anyone who has ever seen two grown men
step out of their cars to fight over who is the
bigger idiot has likely wondered why on earth
the two can’t manage to regulate their emo-
tions in more productive ways. This puzzle-
ment points to a more general question about
why people regulate (or fail to regulate) their
emotions as they do. Answering this ques-
tion requires a more complete analysis of
the emotion regulatory process than I have
provided so far, and one of the most press-
ing challenges for researchers in this area is
to contribute to this analysis. In answering
this overarching question, a number of more
specific questions must be addressed:
1. What leads people to activate a goal to
regulate emotion?
2. What determines the fate of this regula-
tory goal?
3. Which strategy is employed to achieve a
given emotion regulatory goal?
Why do some people activate a goal to
regulate emotions when others do not? One
reason may be differences in awareness of
the person’s own ongoing (or anticipated)
emotional responses— in the case of intrin-
sic emotion regulation— or in awareness of
another person’s ongoing (or anticipated)
emotional response— in the case of extrinsic
emotion regulation. People differ substan-
tially in their ability to track subtle emotion
dynamics and represent these in a differen-
tiated fashion; some do this very well, but
others (e.g., those who have alexithymia
or low levels of emotion awareness) have
little or no awareness of ongoing emotional
responses (Salovey & Mayer, 1990; Taylor,
1994). Emotional awareness appears to be
a crucial rate- limiting factor in successfully
regulating emotions (Barrett, Gross, Con-
ner, & Benvenuto, 2001; Samson, Huber,
& Gross, 2012), but much remains to be
learned about the precise role of emotional
awareness in activation of the goal to engage
in emotion regulation.
Even after a person has become aware of
an emotion and activated a goal to regulate
that emotion, there remains the question:
What determines how this regulatory goal
will fare in its competition with other cur-
rently active goals? As discussed earlier,
people have both hedonic and instrumen-
tal goals, but it is far from clear how these
various goals interact. What is known sug-
gests that emotion regulation often involves
tradeoffs between hedonic and instrumental
motives. Avoidance that may bring short-
term relief (“If I skip the cocktail party, I
can avoid feeling anxious”) may have a sub-
stantial long-term price tag (“If I skip the
cocktail party, I may miss out on develop-
ing helpful professional contacts”). Over the
course of development, it appears that the
balance of motives shifts repeatedly, first
from hedonic to instrumental goals, then,
later in life, away from instrumental motives
and toward hedonic motives, reflecting an
awareness of the reduced value of long-
term investments as one moves toward the
end of one’s life (Carstensen, Isaacowitz, &
Charles, 1999; Charles & Carstensen, this
volume). Just how people flexibly manage
competing regulatory goals is likely to be an
important determinant of healthy adapta-
tion.
Once an emotion regulatory goal has
been activated and has survived a com-
petition with other currently active goals,
there remains the question: Which emotion
regulatory strategy (or strategies) will be
selected in order to achieve that particular
emotion regulatory goal? Part of the answer
may hinge on context- specific factors, such
as the type and intensity of emotion that
needs regulating. For example, people pre-
fer reappraisal to distraction when emotion
intensity is low, but prefer distraction to
reappraisal when emotion intensity is high,
because at high- intensity levels, reappraisal
is often no longer effective (Sheppes, this
volume; Sheppes, Scheibe, Suri, & Gross,
2011). Another important context- specific
factor may be a person’s perceptions of his
or her currently available social and/or psy-
chological resources (Coan & Maresh, this
volume; Opitz, Gross, & Urry, 2012). Other
factors that govern strategy selection may be
more stable across situations. For example,
some people have incremental beliefs about
emotion, and see emotions as the kinds of
14 FOUNDATIONS
things that can be changed. Others have
entity beliefs about emotion, and see emo-
tions as relatively immutable. Perhaps not
surprisingly, those with incremental beliefs
seem to be more adept at emotion regula-
tion than those with entity beliefs (Mauss
& Tamir, this volume; Tamir, John, Srivas-
tava, & Gross, 2007). Another important
kind of belief has to do with whether one
believes one is able to engage in a particular
form of emotion regulation when one wishes
to do so. This type of belief is referred to
as emotion regulation self- efficacy, and
self- efficacy beliefs can be modified. For
example, in the context of generalized social
anxiety disorder, patients who received
cognitive- behavioral therapy (vs. those
randomized to a wait-list group) showed
increased reappraisal self- efficacy, and these
changes in self- efficacy mediated the effects
of therapy on clinical improvement (Goldin
et al., 2012; John & Eng, this volume).
It is evident that these three questions—
regarding the activation of a regulatory goal,
the relative dominance of that regulation
goal compared to other goals, and the emo-
tion regulation strategy that is selected—
represent a small subset of the many ques-
tions that need to be answered before we
fully understand whether (and how) a par-
ticular person will regulate emotion in a
particular situation, and whether he or she
will do so successfully. Other determinants
include person- based factors, such as work-
ing memory capacity, as well as situation-
based factors that make some forms of emo-
tion regulation easier to implement than
others. One pressing challenge for future
research is to clarify the rules that govern
the skillful application of emotion regula-
tion. This work is important, because it
will create a framework for understanding
individual and group differences in emotion
regulation, and suggest strategies for inter-
vention when regulation is deficient.
How Can We Use What We Know
to Make the World a Better Place?
Although we still have much to learn about
the psychological processes that are neces-
sary for skillful and flexible emotion regula-
tion, we now know enough to begin think-
ing about how to use what we know about
emotion regulation to make the world a
better place. Efforts in this direction are jus-
tified by not only their ends but also what
they can teach us about basic processes as
we apply what we think we know to real-
world situations.
One type of application— and perhaps the
most obvious— is individual- level interven-
tions designed to teach healthier patterns of
emotion regulation (Gross & Munoz, 1995).
Such interventions might take the form of
crafting instructional materials, teacher
workshops, classroom- based interventions,
and parenting classes designed to increase
awareness of the importance of emotion and
skillful emotion regulation. Interventions
may target individuals at heightened risk
of adverse outcomes, such as daughters of
depressed mothers, children who live in abu-
sive families, members of underrepresented
minorities in work or academic contexts,
or those with high temperamental levels of
negative emotion. More specific interven-
tions will target individuals who have clini-
cal diagnoses (Barrett, Wilson- Mendenhall,
& Barsalou, this volume; Campbell- Sills,
Ellard, & Barlow, this volume; Joormann
& Siemer, this volume; Kober, this volume).
These are the inventions that come to mind
most easily, and many of our pharmacologi-
cal and psychosocial interventions for psy-
chiatric disorders have an emotion regula-
tion component, although much remains to
be learned about exactly how each type of
intervention influences particular aspects
of emotion regulation (Berking & Schwarz,
this volume; Neacsiu, Bohus, & Linehan,
this volume; MacLeod & Grafton, this vol-
ume; Mennin & Fresco, this volume; Farb,
Anderson, Irving, & Segal, this volume).
A second type of application involves
making larger changes in the physical and
social worlds in which we live. An example
of this class of interventions comes from
applying an emotion regulation perspec-
tive to seemingly intractable global con-
flicts (Halperin, 2013). These conflicts are
characterized by high levels of negative
emotions that powerfully shape attitudes
and behaviors of each of the parties to the
conflict. In particular, negative intergroup
emotions— emotions that arise as a result
of belonging to a certain group—can lead
to the commencement and maintenance of
hostilities, then block progress toward a
peaceful solution to the ongoing conflict. To

Conceptual and Empirical Foundations 15
assess the role of emotion regulation in one
such conflict, namely, the ongoing Israeli–
Palestinian conflict, a nationwide survey of
Jewish- Israeli adults was conducted during
the Gaza War between Israelis and Palestin-
ians. This survey assessed both reappraisal
use and attitudes toward providing humani-
tarian aid to Palestinian citizens. Findings
indicated that Israelis who regulated their
negative emotions during the war by using
reappraisal were more supportive of pro-
viding humanitarian aid than Israelis who
did not use reappraisal (Halperin & Gross,
2011). Building on this foundation, a sec-
ond study randomized Israeli participants to
either a reappraisal training condition or a
control condition just before the Palestinian
United Nations (UN) bid in 2011. Findings
indicated that a week following the training,
participants who had been trained to use
reappraisal showed greater support for con-
ciliatory policies and less support for aggres-
sive policies toward Palestinians. These
effects persisted when assessed 5 months
after training, and at each time point, nega-
tive emotion mediated the effects of reap-
praisal on conflict- related attitudes (Hal-
perin, Porat, Tamir, & Gross, 2012). These
findings hint at the broader, real-world rel-
evance of an emotion regulation perspective,
and in future work it will be interesting to
investigate how such a perspective might be
applied in other arenas.
Acknowledgments
I would like to thank Gal Sheppes, Heather Urry,
and members of the Stanford Psychophysiology
Laboratory for comments on a prior version of
this chapter.
References
American Psychiatric Association. (2013). Diag-
nostic and statistical manual of mental disor-
ders (5th ed.). Arlington, VA: Author.
Barrett, L. F., Wilson- Mendenhall, C. D., &
Barsalou, L. W. (this volume, 2014). A psy-
chological construction account of emotion
dysregulation. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 447–465).
New York: Guilford Press.
Barrett, L. F., Gross, J. J., Conner, T., & Benve-
nuto, M. (2001). Knowing what you’re feeling
and knowing what to do about it: Mapping the
relation between emotion differentiation and
emotion regulation. Cognition and Emotion,
15, 713–724.
Barrett, L. F., Ochsner, K. N., & Gross, J. J.
(2007). On the automaticity of emotion. In J.
Bargh (Ed.), Social psychology and the uncon-
scious: The automaticity of higher mental
processes (pp. 173–217). New York: Psychol-
ogy Press.
Berking, M., & Schwarz, J. (this volume, 2014).
Affect regulation training. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 529–547). New York: Guilford Press.
Bowlby, J. (1969). Attachment and loss: Attach-
ment. New York: Basic Books.
Buck, R. (1990). Mood and emotion: A compari-
son of five contemporary views. Psychological
Inquiry, 1, 330–336.
Buck, R. (1993). What is this thing called sub-
jective experience?: Reflections on the neuro-
psychology of qualia. Neuropsychology, 7,
490–499.
Butler, E. A., Egloff, B., Wilhelm, F. W., Smith,
N. C., Erickson, E. A., & Gross, J. J. (2003).
The social consequences of expressive suppres-
sion. Emotion, 3, 48 – 67.
Butler, E. A., Lee, T. L., & Gross, J. J. (2007).
Emotion regulation and culture: Are the social
consequences of emotion suppression culture-
specific? Emotion, 7, 30–48.
Campbell- Sills, L., Ellard, K. K., & Barlow, D.
H. (this volume, 2014). Emotion regulation in
anxiety disorders. In J. J. Gross (Ed.), Hand-
book of emotion regulation (2nd ed., pp. 393–
412). New York: Guilford Press.
Campos, J. J., Frankel, C. B., & Camras, L.
(2004). On the nature of emotion regulation.
Child Development, 75, 377–394.
Carstensen, L. L., Isaacowitz, D. M., & Charles,
S. T. (1999). Taking time seriously. American
Psychologist, 54, 165–181.
Charles, S. T., & Carstensen, L. L. (this volume,
2014). Emotion regulation and aging. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 203–218). New York: Guilford
Press.
Coan, J. A., & Maresh, E. L. (this volume, 2014).
Social baseline theory and the social regula-
tion of emotion. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 221–236).
New York: Guilford Press.
Cole, P., Martin, S., & Dennis, T. (2004). Emo-
tion regulation as a scientific construct: Meth-
16 FOUNDATIONS
odological challenges and directions for child
development research. Child Development,
75, 317–333.
Dan- Glauser, E. S., & Gross, J. J. (2013). Emo-
tion regulation and emotion coherence: Evi-
dence for strategy- specific effects. Emotion.
Davidson, R. J. (1994). On emotion, mood, and
related affective constructs. In P. Ekman &
R. J. Davidson (Eds.), The nature of emotion:
Fundamental questions (pp. 51–55). New
York: Oxford University Press.
Demaree, H. A., Schmeichel, B. J., Robinson,
J. L., Pu, J., Everhart, D., & Berntson, G. G.
(2006). Up- and down- regulating facial dis-
gust: Affective, vagal, sympathetic, and respi-
ratory consequences. Biological Psychology,
71, 90 –99.
Drabant, E. M., McRae, K., Manuck, S. B.,
Hariri, A. R., & Gross, J. J. (2009). Individual
differences in typical reappraisal use predict
amygdala and prefrontal responses. Biological
Psychiatry, 65, 367–373.
Ellsworth, P. C., & Scherer, K. R. (2003).
Appraisal processes in emotion. In R. J. David-
son, K. R., Scherer, & H. H. Goldsmith (Eds.),
Handbook of affective sciences (pp. 572–595).
New York: Oxford University Press.
English, T., John, O. P., & Gross, J. J. (2013).
Emotion regulation in close relationships. In J.
A. Simpson & L. Campbell (Eds.), The Oxford
handbook of close relationships (pp. 500–
513). New York: Oxford University Press.
Farb, N. A. S., Anderson, A. K., & Segal, Z. V.
(this volume, 2014). Mindfulness interven-
tions and emotion regulation. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp. 548–568). New York: Guilford Press.
Feinberg, M., Willer, R., Antonenko, O., &
John, O. P. (2012). Liberating reason from the
passions. Psychological Science, 23, 788–795.
Freud, S. (1959). Inhibitions, symptoms, anxiety
(A. Strachey, Trans.; J. Strachey, Ed.). New
York: Norton. (Original work published 1926)
Frijda, N. H. (1986). The emotions. Cambridge,
UK: Cambridge University Press.
Goldin, P. R., McRae, K., Ramel, W., & Gross,
J. J. (2008). The neural bases of emotion
regulation: Reappraisal and suppression of
negative emotion. Biological Psychiatry, 63,
577–586.
Goldin, P., Ziv, M., Jazaieri, H., Werner, K.,
Kraemer, H., Heimberg, R. G., et al. (2012).
Cognitive reappraisal self- efficacy mediates
the effects of individual cognitive- behavioral
therapy for social anxiety disorder. Journal
of Consulting and Clinical Psychology, 80,
1034–1040.
Gross, J. J. (1998a). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Gross, J. J. (1998b). The emerging field of emo-
tion regulation: An integrative review. Review
of General Psychology, 2, 271–299.
Gross, J. J. (1999). Emotion regulation: Past,
present, future. Cognition and Emotion, 13,
551–573.
Gross, J. J. (2010). The future’s so bright, I gotta
wear shades. Emotion Review, 2, 212–216.
Gross, J. J. (2013). Emotion regulation: Tak-
ing stock and moving forward. Emotion, 13,
359–365.
Gross, J. J. & Barrett, L. F. (2011). Emotion
generation and emotion regulation: One or
two depends on your point of view. Emotion
Review, 3, 8–16.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., & Levenson, R. W. (1993). Emo-
tional suppression: Physiology, self- report,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64, 970–986.
Gross, J. J., & Levenson, R. W. (1997). Hiding
feelings: The acute effects of inhibiting posi-
tive and negative emotions. Journal of Abnor-
mal Psychology, 106, 95–103.
Gross, J. J., & Munoz, R. F. (1995). Emotion
regulation and mental health. Clinical Psy-
chology: Science and Practice, 2, 151–164.
Gross, J. J., Richards, J. M., & John, O. P.
(2006). Emotion regulation in everyday life. In
D. K. Snyder, J. A. Simpson, & J. N. Hughes
(Eds.), Emotion regulation in couples and
families: Pathways to dysfunction and health.
Washington, DC: American Psychological
Association.
Gross, J. J., Sheppes, G., & Urry, H. L. (2011).
Emotion generation and emotion regulation: A
distinction we should make (carefully). Cogni-
tion and Emotion, 25, 765–781.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 3–24). New York: Guilford Press.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
Conceptual and Empirical Foundations 17
emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Gyurak, A., Gross, J. J., & Etkin, A. (2011).
Explicit and implicit emotion regulation: A
dual-
process framework. Cognition and Emo-
tion, 25, 400– 412.
Halperin, E. (2013). Current and potential con-
tributions of the study of emotion and emotion
regulation to the study and practice of conflict
resolution. Emotion Review.
Halperin, E., & Gross, J. J. (2011). Emotion reg-
ulation in violent conflict: Reappraisal, hope,
and support for humanitarian aid to the oppo-
nent in war time. Cognition and Emotion, 25,
1228–1236.
Halperin, E., Porat, R., Tamir, M., & Gross, J. J.
(2012). Can emotion regulation change politi-
cal attitudes in intractable conflict?: From the
laboratory to the field. Psychological Science,
24(1), 106–111.
Harris, C. R. (2001). Cardiovascular responses
of embarrassment and effects of emotional
suppression in a social setting. Journal of Per-
sonality and Social Psychology, 81, 886– 897.
Hayes, J. P., Morey, R. A., Petty, C. M., Seth,
S., Smoski, M. J., McCarthy, G., et al. (2011).
Staying cool when things get hot: Emotion
regulation modulates neural mechanisms of
memory encoding. Frontiers in Human Neu-
roscience, 4, 1–10.
Hochschild, A. R. (1983). The managed heart.
Berkeley: University of California Press.
Jamieson, J. P., Mendes, W. B., Blackstock, E.,
& Schmader, T. (2010). Turning the knots in
your stomach into bows: Reappraising arousal
improves performance on the GRE. Journal
of Experimental Social Psychology, 46, 208–
212.
John, O. P., & Eng, J. (this volume, 2014). Indi-
vidual differences in emotion regulation: Con-
ceptualization, measures, and findings. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp.
321–345). New York: Guilford
Press.
Johns, M. J., Inzlicht, M., & Schmader, T.
(2008). Stereotype threat and executive
resource depletion: Examining the influence
of emotion regulation. Journal of Experimen-
tal Psychology: General, 137, 691–705.
Joormann, J., & Siemer, M. (this volume, 2014).
Emotion regulation in mood disorders. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 413–427). New York: Guilford
Press.
Kanske, P., Heissler, J., Schonfelder, S., Bongers,
A., & Wessa, M. (2011). How to regulate emo-
tion?: Neural networks for reappraisal and
distraction. Cerebral Cortex, 21, 1379–1388.
Kaplan, H. I., & Sadock, B. J. (1991). Synopsis
of psychiatry (6th ed.). Baltimore: Williams &
Wilkins.
Kappas, A. (2011). Emotion and regulation are
one! Emotion Review, 3, 17–25.
Kim, S. H., & Hamann, S. (2012). The effect of
cognitive reappraisal on physiological reac-
tivity and emotional memory. International
Journal of Psychophysiology, 83, 348–56.
Kober, H. (this volume, 2014). Emotion regula-
tion in substance use disorders. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp.
428–446). New York: Guilford Press.
Koole, S. L. (2009). The psychology of emotion
regulation: An integrative review. Cognition
and Emotion, 23, 4– 41.
Lang, P. J. (1995). The emotion probe: Studies of
motivation and attention. American Psycholo-
gist, 50, 372–385.
Lang, P. J., & Bradley, M. M. (2010). Emotion
and the motivational brain. Biological Psy-
chology, 84, 437–450.
Larsen, R. J. (2000). Toward a science of mood
regulation. Psychological Inquiry, 11, 129–
141.
Larson, E. B., & Yao, X. (2005). Clinical empa-
thy as emotional labor in the patient–
ph
ysician
relationship. Journal of the American Medical
Association, 293, 1100–1106.
Lazarus, R. S. (1966). Psychological stress and
the coping process. New York: McGraw-Hill.
Lazarus, R. S. (1991). Emotion and adaptation.
Oxford, UK: Oxford University Press.
Lazarus, R. S. (1993). From psychological stress
to the emotions: A history of changing out-
looks. Annual Review of Psychology, 44,
1–21.
Levenson, R. W. (1999). The intrapersonal func-
tions of emotion. Cognition and Emotion, 13,
481–504.
Levenson, R. W., Haase, C. M., Bloch, L., Hol-
ley, S. R., & Seider, H. (this volume, 2014).
Emotion regulation in couples. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp.
267–283). New York: Guilford Press.
Lieberman, M. D., Inagaki, T. K., Tabibnia, G.,
& Crockett, M. J. (2011). Subjective responses
to emotional stimuli during labeling, reap-
praisal, and distraction. Emotion, 11, 468–80.
Masters, J. C. (1991). Strategies and mechanisms
for the personal and social control of emotion.
18 FOUNDATIONS
In J. Garber & K. A. Dodge (Eds.), The devel-
opment of emotion regulation and dysregu-
lation (pp. 182–207). Cambridge, UK: Cam-
bridge University Press.
MacLean, P. D. (1990). The triune brain in evo-
lution: Role in paleocerebral functions. New
York: Plenum.
MacLeod, C., & Grafton, B. (this volume, 2014).
Regulation of emotion through modification
of attention. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 508–528).
New York: Guilford Press.
Mauss, I. B., Levenson, R. W., McCarter, L.,
Wilhelm, F. H., & Gross, J. J. (2005). The tie
that binds?: Coherence among emotion expe-
rience, behavior, and physiology. Emotion, 5,
175–190.
Mauss, I. B., & Tamir, M. (this volume, 2014).
Emotion goals: How their content, structure,
and operation shape emotion regulation. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp.
361–375). New York: Guil-
ford Press.
Mauss, I. B., Shallcross, A. J., Troy, A. S., John,
O. P., Ferrer, E., Wilhelm, F. H., et al. (2011).
Don’t hide your happiness!: Positive emotion
dissociation, social connectedness, and psy-
chological functioning. Journal of Personality
and Social Psychology, 100, 738–748.
Mennin, D. S., & Fresco, D. M. (this volume,
2014). Emotion regulation therapy. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp.
469–490). New York: Guilford
Press.
Mesquita, B., De Leersnyder, J., & Albert, D.
(this volume, 2014). The cultural regulation
of emotions. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp.
2
84–302).
New York: Guilford Press.
Mischel, W. (1996). From good intentions to
willpower. In P. Gollwitzer & J. Bargh (Eds.),
The psychology of action (pp.
197–218). New
York: Guilford Press.
Moore, S. A., Zoellner, L. A., & Mollenholt, N.
(2008). Are expressive suppression and cogni-
tive reappraisal associated with stress-
r
elated
symptoms? Behaviour Research and Therapy,
46, 993–1000.
Neacsiu, A. D., Bohus, M., & Linehan, M. (this
volume, 2014). Dialectical behavior therapy:
An intervention for emotion dysregulation.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp.
491–507). New York:
Guilford Press.
Nezlek, J. B., & Kuppens, P. (2008). Regulating
positive and negative emotions in daily life.
Journal of Personality, 76, 561–579.
Ochsner, K. N., & Gross, J. J. (2008). Cogni-
tive emotion regulation: Insights from social
cognitive and affective neuroscience. Current
Directions in Psychological Science, 17, 153–
158.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp.
23–42). New York: Guilford
Press.
Ochsner, K. N., Ray, R. R., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D. E.,
et al. (2004). For better or for worse: Neural
systems supporting the cognitive down- and
up-
r
egulation of negative emotion. NeuroIm-
age, 23, 483–499.
Ochsner, K. N., Ray, R. R., Hughes, B., McRae,
K., Cooper, J. C., Weber, J., et al. (2009).
Bottom-up and top-down processes in emo-
tion generation: Common and distinct neu-
ral mechanisms. Psychological Science, 20,
1322–1331.
Opitz, P., Gross, J. J., & Urry, H. L. (2012).
Selection, optimization, and compensation
in the domain of emotion regulation: Appli-
cations to adolescence, older age, and major
depressive disorder. Social and Personality
Psychology Compass, 6, 142–155.
Parkinson, B., Totterdell, P., Briner, R. B., &
Reynolds, S. (1996). Changing moods: The
psychology of mood and mood regulation.
London: Longman.
Parrott, W. G. (1993). Beyond hedonism: Motives
for inhibiting good moods and for maintain-
ing bad moods. In D. M. Wegner & J. W. Pen-
nebaker (Eds.), Handbook of mental control
(pp.
278–305). Englewood Cliffs, NJ: Prentice
Hall.
Quoidbach, J., Berry, E. V., Hansenne, M., &
Mikolajczak, M. (2010). Positive emotion reg-
ulation and well-being: Comparing the impact
of eight savoring and dampening strategies.
Personality and Individual Differences, 49,
368–373.
Ray, R. D., McRae, K., Ochsner, K. N., & Gross,
J. J. (2010). Cognitive reappraisal of negative
affect: Converging evidence from EMG and
self-
report. Emotion, 10, 587–592.
Richards, J. M., Butler, E., & Gross, J. J. (2003).
Emotion regulation in romantic relationships:
Conceptual and Empirical Foundations 19
The cognitive consequences of concealing feel-
ings. Journal of Personal and Social Relation-
ships, 20, 599–620.
Richards, J. M., & Gross, J. J. (1999). Compo-
sure at any cost?: The cognitive consequences
of emotion suppression. Personality and Social
Psychology Bulletin, 25, 1033–1044.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79, 410–424.
Richards, J. M., & Gross, J. J. (2006). Person-
ality and emotional memory: How regulat-
ing emotion impairs memory for emotional
events. Journal of Research in Personality, 40,
631–651.
Rothbart, M. K., Ziaie, H., & O’Boyle, C. G.
(1992). Self- regulation and emotion in infancy.
In N. Eisenberg & R. A. Fabes (Eds.), Emo-
tion and its regulation in early development
(pp. 7–23). San Francisco: Jossey-Bass.
Salovey, P., & Mayer, J. D. (1990). Emotional
intelligence. Imagination, Cognition and Per-
sonality, 9, 185–211.
Samson, A., & Gross, J. J. (2012). Humor as
emotion regulation: The differential conse-
quences of negative versus positive humor.
Cognition and Emotion, 26, 375–384.
Samson, A. C., Huber, O., & Gross, J. J. (2012).
Emotion regulation in Asperger’s syndrome
and high functioning autism. Emotion, 12(4),
659–665.
Scherer, K. R. (1984). On the nature and func-
tion of emotion: A component process
approach. In K. R. Scherer & P. E. Ekman
(Eds.), Approaches to emotion (pp. 293–317).
Hillsdale, NJ: Erlbaum.
Scherer, K. R., Schorr, A., & Johnstone, T.
(2001). Appraisal processes in emotion: The-
ory, methods, research. New York: Oxford
University Press.
Sheppes, G. (this volume, 2014). Emotion reg-
ulation choice: Theory and findings. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 126–139). New York: Guilford
Press.
Sheppes, G., Catran, E., & Meiran, N. (2009).
Reappraisal (but not distraction) is going to
make you sweat: Physiological evidence for
self- control effort. International Journal of
Psychophysiology, 71, 91–96.
Sheppes, G., Scheibe, S., Suri, G., & Gross, J. J.
(2011). Emotion regulation choice. Psycholog-
ical Science, 22, 1391–1396.
Shiota, M. N., & Levenson, R. W. (2012). Turn
down the volume or change the channel?:
Emotional effects of detached versus positive
reappraisal. Journal of Personality and Social
Psychology, 103(3), 416–429.
Siemer, M. (2001). Mood- specific effects on
appraisal and emotion judgements. Cognition
and Emotion, 15, 453–485.
Soto, J. A., Perez, C. R., Kim, Y.-H., Lee, E.
A., & Minnick, M. R. (2011). Is expressive
suppression always associated with poorer
psychological functioning?: A cross- cultural
comparison between European Americans
and Hong Kong Chinese. Emotion, 11, 1450 –
1455.
Srivastava, S., Tamir, M., McGonigal, K. M.,
John, O. P., & Gross, J. J. (2009). The social
costs of emotional suppression: A prospective
study of the transition to college. Journal of
Personality and Social Psychology, 96, 883–
897.
Staudinger, M. R., Erk, S., Abler, B., & Walter,
H. (2009). Cognitive reappraisal modulates
expected value and prediction error encod-
ing in the ventral striatum. NeuroImage, 47,
713–721.
Stemmler, G. (1997). Selective activation of
traits: Boundary conditions for the activation
of anger. Personality and Individual Differ-
ences, 22, 213–233.
Stepper, S., & Strack, F. (1993). Proprioceptive
determinants of emotional and nonemotional
feelings. Journal of Personality and Social
Psychology, 64, 211–220.
Strack, F., Martin, L. L., & Stepper, S. (1988).
Inhibiting and facilitating conditions of the
human smile: A nonobtrusive test of the facial
feedback hypothesis. Journal of Personality
and Social Psychology, 54, 768–777.
Sutton, R. E. (2004). Emotional regulation goals
and strategies of teachers. Social Psychology
of Education, 7, 379–398.
Sutton, R. I. (1991). Maintaining norms about
expressed emotions: The case of bill collec-
tors. Administrative Science Quarterly, 36,
245–268.
Szasz, P. L., Szentagotai, A., & Hofmann, S. G.
(2011). The effect of emotion regulation strat-
egies on anger. Behaviour Research and Ther-
apy, 49, 114–119.
Szczurek, L., Monin, B., & Gross, J. J. (2012).
The stranger effect: The rejection of affec-
tive deviants. Psychological Science, 23(10),
1105–1111.
20 FOUNDATIONS
Tamir, M. (2009). What do people want to feel
and why?: Pleasure and utility in emotion reg-
ulation. Current Directions in Psychological
Science, 18, 101–105.
Tamir, M. (2011). The maturing field of emotion
regulation. Emotion Review, 3, 3 –7.
Tamir, M., John, O. P., Srivastava, S., & Gross, J.
J. (2007). Implicit theories of emotion: Affec-
tive and social outcomes across a major life
transition. Journal of Personality and Social
Psychology, 92, 731–744.
Taylor, G. J. (1994). The alexithymia construct:
Conceptualization, validation, and relation-
ship with basic dimensions of personality.
New Trends in Experimental and Clinical
Psychiatry, 10, 61–74.
Thiruchselvam, R., Hajcak, G., & Gross, J. J.
(2012). Looking inwards: Shifting attention
within working memory representations alters
emotional responses. Psychological Science,
23(12), 1461–1466.
Thompson, R. A. (1990). Emotion and self-
regulation. In Socioemotional development:
Nebraska Symposium on Motivation (Vol. 36,
pp. 367–467). Lincoln: University of Nebraska
Press.
Thompson, R. (2011). Emotion and emotion
regulation: Two sides of the developing coin.
Emotion Review, 3, 53–61.
Tsai, J. L. (2007). Ideal affect: Cultural causes
and behavioral consequences. Perspectives on
Psychological Science, 2, 242–259.
Webb, T. L., Miles, E., & Sheeran, P. (2012).
Dealing with feeling: A meta- analysis of the
effectiveness of strategies derived from the
process model of emotion regulation. Psycho-
logical Bulletin, 138(4), 775–808.
Westen, D. (1994). Toward an integrative model
of affect regulation: Applications to social-
psychological research. Journal of Personal-
ity, 62, 641– 667.
Wolgast, M., Lundh, L.-G., & Viborg, G.
(2011). Cognitive reappraisal and acceptance:
An experimental comparison of two emotion
regulation strategies. Behaviour Research and
Therapy, 49, 858–866.
Zajonc, R. B. (1984). On the primacy of affect.
American Psychologist, 39, 117–123.

PART II
BIOLOGICAL BASES

23
The observation that emotions can be pow-
erful forces for good and for ill has moti-
vated researchers’ efforts to understand how
emotions arise and how they are regulated
(Gross, 2007; Kalisch, 2009; Ochsner &
Gross, 2005, 2008; Phillips, Ladouceur,
& Drevets, 2008; Quirk & Beer, 2006). In
particular, neuroscience research recently
has made great strides in describing the
neural systems that give rise to emotional
responses and that permit their regulation.
At the same time, parallel progress has been
made in delineating the neural bases of
related abilities, including affective learning,
affective decision making, and expectancies,
beliefs, and placebo effects (Cunningham
& Zelazo, 2007; Hartley & Phelps, 2010;
Murray, O’Doherty, & Schoenbaum, 2007;
Pessoa, 2008; Rangel, Camerer, & Mon-
tague, 2008). It is becoming evident that the
neural systems implicated across these vari-
ous literatures— including those concerned
with emotion and emotion regulation— are
strikingly similar. This suggests that any
account of the neural bases of emotion and
its regulation— or related abilities— should
be informed by these similarities. Such an
integrated framework would be both more
robust and more translatable to multiple
basic and clinical contexts. Our goal in this
chapter is to provide such a framework.
Our starting point is the assertion that
goal- directed (motivated) behavior may be
defined as behavior that aims to decrease the
probability of states of either our bodies or
of the world that have negative value for us
(e.g., putting on a sweater when we are cold;
picking up trash in the park), or increase
the probability of states that have positive
value for us (e.g., opening a can of soup to
eat when we are hungry; arranging to have
coffee with a friend).
The determination of value occurs
dynamically at many levels of the brain,
at different time scales, and with respect
to many features in the environment (Lev-
enthal, 1984; Rangel et al., 2008; Scherer,
2001). A sudden loss of blood pressure may
occasion a valuation, as may the smell of
dinner being prepared, an aggressive driver
cutting one off, or a new way of thinking
about a poem. If valuations are assessments
of what is bad for me (negative value) or
good for me (positive value), computed for
many different objects, then different types
of valuation might be expected to give rise
to different types of responses, and indeed,
they do (Ortony, Clore, & Collins, 1988;
CHAPTER 2
The Neural Bases of Emotion
and Emotion Regulation:
A Valuation Perspective
Kevin N. Ochsner
James J. Gross

24 BIOLOGICAL BASES
Scherer, Schorr, & Johnstone, 2001). One
particularly important type of valuation is
emotion, which arises when a situation is
evaluated as relevant to an individual’s goals,
thereby triggering a loosely coordinated set
of experiential, behavioral, and peripheral
physiological responses (Mauss, Levenson,
McCarter, Wilhelm, & Gross, 2005). Emo-
tional responses such as joy, anger, and dis-
gust all have at their core an evaluation of
whether something is good for me or bad for
me.
Given the major role that emotions play
in shaping how we feel about and respond
to the world around us, it is no surprise that
emotions themselves can become the tar-
get of valuation. For example, we may feel
angry at a child’s impolite behavior, and
judge our anger as either inappropriate (hav-
ing negative value) or appropriate (having
positive value), depending on the age of the
child. In this and in similar cases, we assign
negative or positive value to the valuation
process itself, thereby energizing processes
that tend to make the emotion in question
either less or more likely to occur, depend-
ing on whether the valuation is negative or
positive. When individuals influence their
emotions in this way, they are engaging in
emotion regulation.
Our framework therefore holds that emo-
tions arise via the valuation of internal or
external stimuli, and that emotion regula-
tion arises via the valuation of the emotion
itself. From this perspective, emotion and
emotion regulation both have valuation at
their core.
This three-part chapter uses a valuation
perspective to integrate diverse findings
in current neuroscience research regard-
ing emotion, emotion regulation, affective
learning, decision making, and expectancy.
In the first part, we propose a multilevel
framework that analyzes emotion and emo-
tion regulation in terms of valuation. In
the second part we use this framework to
understand current research on emotion
and its regulation. Finally, in the third part
we explore the general utility of this frame-
work by giving examples of how it can be
extended to account for current research
on related phenomena, including affective
learning, decision making, and expectancy
effects.
The Functional Architecture
of Valuation
According to the framework we propose
here, valuation can be schematized as the
three-stage processing cycle outlined in
Figure 2.1A. As detailed below, a percep-
tion stage takes various kinds of stimuli
as inputs; a valuation stage dynamically
appraises the value of these stimuli given
current goals, context, and prior experience
with similar stimuli; and an action stage
comprises valuation- appropriate responses
ranging from covert adjustments of low-level
sensory (e.g., increased pupil dilation) or
higher- level cognitive processes (e.g., shifts
in effortful attention) to overt adjustments of
a wide range of response systems (e.g., facial
behavior, postural adjustments, sympathetic
nervous system activation). This perception–
valuation– action (PVA) sequence repeats as
the new state of the world, resulting from the
action, becomes the input for the next PVA
sequence, thus setting in motion a new PVA
cycle. Because multiple PVA cycles are typi-
cally running at any given time, these cycles
interact, and it is these processing dynamics
that give rise to behavior.
PVA Components:
The Perception Stage
A PVA sequence is initiated by an external
or internal stimulus that can vary in com-
plexity from low-level perceptual features
(like eye whites or low spatial frequencies) or
physiological responses (e.g., a racing heart)
to organized perceptual exemplars (e.g.,
objects or scenes) to abstract constructs
such as the self. In this initial perception
stage of the sequence, sensory systems (e.g.,
thalamus plus primary and secondary sen-
sory cortices) encode these types of sensory
inputs and pass them along to systems for
computing value (Kravitz, Saleem, Baker, &
Mishkin, 2011).
PVA Components: The Valuation Stage
Valuations are subserved by an overlapping
set of interacting brain systems that com-
pute the badness or goodness of perceptual
inputs (Hamann, Ely, Hoffman, & Kilts,
2002; Ochsner & Barrett, 2001; Rangel et
Valuation and Emotion Regulation 25
al., 2008; Rolls, 1999), thereby providing
a common currency for comparing vari-
ous objects and events (Levy & Glimcher,
2011). In this chapter we use valuation as an
umbrella term to connote the same kinds of
underlying processes that emotion theorists
would describe using the term appraisal and
attitude researchers would describe using the
term evaluation. Targets of valuation range
from primary reinforcers— objects that are
innately seen as “bad” or “good,” such as
a sweet drink, to secondary reinforcers—
objects that derive their negative or positive
value from their association with primary
reinforcers, such as an A+ written at the top
of one’s term paper (Rangel et al., 2008;
Rolls, 1999). Figure 2.2A shows the brain
regions associated with valuation processes.
Multiple valuations are computed for a
given stimulus, and these vary along a con-
tinuum of representational complexity, with
more complex valuations typically taking
B. Regulation
W2 P2 V2
A2
W1 P1 V1
A1
Present
Future
A. Valuation
FIGURE 2.1A. The perception– valuation– action (PVA) processing cycle that comprises the fundamen-
tal building block of emotion and other types of valuation. As described in the text, multiple PVA cycles
can operate at once, here represented by subscripts 1 to n. Each cycle involves individual PVA sequences
that iteratively feed into one another across time (shown here progressively spiraling into the future),
thereby comprising a PVA cycle. For each cycle, some set of internal and external stimuli comprising
an initial state of the world (W) is represented perceptually (P), values are placed upon the stimuli (V),
and associated action links (A) that are activated result in a new state of the world that feeds into the
next iteration of the PVA processing cycle. Note that here we place emphasis on the three-stage (P-V-A)
processing cycle, and the world (W) is considered to be the result of a prior action and the input for the
next PVA sequence. PVAs can interact with one another, exciting or inhibiting each other’s activation
(schematically shown here by double- headed arrows between PVAs). Emotions are specific types of
PVA sequences that involve specific types of perceptions, valuations, and actions. The neural systems
supporting valuation are shown in Figure 2.2A and elaborated in the text and in Figure 2.3.
FIGURE 2.1B. How PVA sequences instantiate regulation. To illustrate how PVA sequences enable
regulation, we zoom in on two PVA sequences, PVA
1
and PVA
2
. As shown here, emotion regulation
(or value regulation more generally) is a functional relationship between two PVA sequences in which
one (PVA
1
) is “generating emotion” and the other (PVA
2
) is taking the first PVA as its “P,” valuing it
(negatively or positively), and targeting that first PVA for change via its “A.” The “A” of PVA
2
enacts
an emotion regulation strategy that influences one or more steps of PVA
1
. As described in the text, five
types of regulatory strategies may be distinguished. Each regulatory strategy depends on different com-
binations of cognitive control systems (see text, Figure 2.2B, and Figure 2.3) whose regulatory effects
can be understood in terms of the stage of the PVA sequence that is targeted for change. On this view,
emotion regulation is a type of valuation in which the valuation process is itself the target of valuation.
26 BIOLOGICAL BASES
longer to compute than less complex valua-
tions (Leventhal, 1984; Scherer, 2001).
At the lowest level of this continuum,
core valuations are made. These represent
relatively direct associations between per-
cepts and basic physiological and behav-
ioral responses at the action stage (e.g.,
snake → fear response). Core valuation
involves primarily subcortical and brain-
stem systems implicated in affective learning
and responding. While many of these sys-
tems, including the ventral striatum, amyg-
dala, and periaqueductal gray (PAG), receive
inputs from a variety of sensory inputs
(Keay & Bandler, 2001; Packard, 2009),
and as such can be involved in the valuation
of a variety of stimuli, there is evidence that
core valuations for pain sensations involve
dedicated pathways from the thalamus to
nociceptive regions of the midcingulate cor-
tex and anterior insula (Willis & Westlund,
1997). In addition, while the ventral stria-
tum and amygdala are typically linked with
positive/appetitive and negative/aversive
valuations, respectively, both human and
animal studies suggest that subregions of
each structure may play roles in both kinds
of valuations (Delgado, Jou, LeDoux, &
Phelps, 2009; Holland & Gallagher, 2004;
Wager, Barrett, et al., 2008). The associa-
tions underlying core valuations can be (but
are not always) activated automatically and
B. Regulation/Control SystemsA. Valuation Systems
FIGURE 2.2A. Neural systems supporting valuation. Medial (top), lateral (bottom left), and coronal
(bottom right) views of the brain showing systems implicated in different types of valuation processes
that can be arrayed along a continuum from core level valuations (amygdala, ventral striatum) that
consist of links between stimuli and reinforcers, to contextual level valuations (vmPFC/OFC) that
place these S-R links in their historical, social, and motivational context, to conceptual level valua-
tions that represent the value of stimuli in belief– desire terms (rostral and dorsal medial PFC) that may
be verbalizable and consciously reportable. The insula, implicated in the representation of body states,
may play a role in representing the body states associated with all three types of valuation. See text
and Figure 2.3 for examples of how these systems play roles in specific types of valuation and emotion.
FIGURE 2.2B. Neural systems supporting regulation. Medial (left) and lateral (right) views of the
brain showing systems implicated in regulatory strategies that can be used to regulate valuation in
general, and emotion in particular Regions include: the dorsal anterior cingulate cortex (dACC), impli-
cated in monitoring conflicts between desired and actual actions, posterior and dlPFC and inferior
parietal cortex, implicated in holding control strategies and goals in mind and directing attention to
relevant perceptual inputs, and vlPFC, implicated in selecting context- appropriate responses and inhib-
iting context- inappropriate responses. See text and Figure 2.3 for examples of how these systems play
roles in specific types of emotion regulation and related phenomena.
Valuation and Emotion Regulation 27
without conscious intent, and are implicit
insofar as they are not directly accessible
to awareness, although one can be aware of
the actions they trigger, and thereby become
aware of them indirectly. Core valuations
typically are linked to stereotyped action
impulses (Kober et al., 2008; LeDoux, 2000;
Rolls, 1999; Russell & Barrett, 1999), and
as such, can provide the basis for stimulus–
response (S-R) links of the sort that under-
lie Pavlovian conditioning and other basic
forms of affective responding that involve
pleasure and pain (Rangel et al., 2008).
At an intermediate level, contextual valu-
ations evaluate inputs that represent combi-
nations of S-R links and at least three types
of contextual information: the historical as
well as current social and motivational con-
texts of the person (for an illustrative exam-
ple, see the section “Emotion as a Type of
PVA Sequence”). This computational step
involves at least three regions. The first com-
prises the orbitofrontal cortex (OFC) and
ventromedial prefrontal cortex (vmPFC)
(Ongur, Ferry, & Price, 2003; Price, 1999),
whose inputs include the output of both the
core valuation level and the medial tempo-
ral lobe (MTL) and the cortical associative
memory systems, which provide temporal
and spatial context (Davachi, 2006; Murray
et al., 2007). Second is the superior tempo-
ral sulcus/temporoparietal junction (STS/
TPJ), which itself is a multisensory zone that
integrates expectancies with feedback, and
reorients attention accordingly, including
when expectations must be adjusted about
the beliefs, actions, and intentions of others
(Saxe, 2006; Young, Camprodon, Hauser,
Pascual- Leone, & Saxe, 2010). Third is the
anterior insula (AI), which integrates and
makes available to awareness information
about current body states, especially as they
pertain to one’s current affective state (Craig,
2003; Harrison, Gray, Gianaros, & Critch-
ley, 2010; Kurth, Zilles, Fox, Laird, & Eick-
hoff, 2010; Zaki, Davis, & Ochsner, 2012).
Contextual valuations indicate whether an
object is good or bad in the present context,
and therefore whether it should be sought or
avoided at the present time. One commonly
studied form of contextual valuation is fear
extinction, in which an organism learns that
a stimulus that previously predicted an aver-
sive outcome (and was therefore negatively
valued) no longer does so (and in the present
temporal context, can be valued less nega-
tively; Quirk & Beer, 2006). More gener-
ally, contextual valuations play key roles in
other forms of affective learning, in deter-
mining whether the value of stimuli change
across contexts, and in subjective awareness
of one’s affective states (Craig, 2009; Cun-
ningham, Raye, & Johnson, 2004; Holland
& Gallagher, 2004; Lieberman, Jarcho, &
Satpute, 2004; Rangel et al., 2008; Schoen-
baum, Saddoris, & Stalnaker, 2007). Con-
textual valuations influence behavior either
by activating action impulses themselves
or—as detailed below in the section on emo-
tion regulation— by influencing which core
valuations are expressed via actions (Och-
sner, Ray, et al., 2009).
At the highest level of this continuum,
conceptual valuations represent apprais-
als of stimuli that are abstract and often
verbalizable. By this we mean representa-
tions of evaluations and affective states that
abstracted across exemplars and contexts
and are accessible to awareness in the form
of “belief– desire” language. For example, a
conceptual valuation of a snake may involve
activation of a conceptual representation of
“fear,” which one can verbalize using that
word.
We propose that this level involves at least
four regions. First, the rostromedial (rmPFC)
and dorsomedial (dmPFC) prefrontal regions
implicated in attending to and explicitly
judging the value of stimuli and use of cat-
egories and belief– desire language to elabo-
rate semantically the affective value of a
wide range of stimuli, from simple objects to
the self (Cato et al., 2004; Lindquist & Bar-
rett, 2008; Lindquist, Wager, Kober, Bliss-
Moreau, & Barrett, 2012; Mitchell, 2009;
Olsson & Ochsner, 2008; Zysset, Huber,
Ferstl, & von Cramon, 2002). An unre-
solved question about medial PFC (mPFC)
is whether different subregions are involved
in making judgments (whether evaluative or
not) about others, the self, and/or stimuli in
general (Denny, Kober, Wager, & Ochsner,
2012; Ferstl & von Cramon, 2002; Zys-
set, Huber, Samson, Ferstl, & von Cramon,
2003). For our present purposes, we con-
sider mPFC to be critical for using concep-
tual information to elaborate the affective
meaning of stimuli, whether the stimulus
triggering the valuation and emotion is the
self, another person, or some other object/
28 BIOLOGICAL BASES
event/situation. A third region involved in
conceptual valuation is the ventrolateral
prefrontal cortex (vlPFC), which helps select
desired and inhibit undesired value represen-
tations (Aron, Robbins, & Poldrack, 2004;
Badre & Wagner, 2007; Barrett, 2006; Gal-
lagher & Frith, 2003; Lieberman et al.,
2007; Lindquist & Barrett, 2008; Mitchell,
2009; Olsson & Ochsner, 2008; Thompson-
Schill, Bedny, & Goldberg, 2005). Finally,
regions of the anterior insula that support
introspective awareness of body states also
may be integral to awareness of body states
and use of conceptual knowledge about
them to make judgments about one’s cur-
rent affective states (Craig, 2009; Damasio,
Damasio, & Tranel, 2013; Gray et al., 2012;
Harrison et al., 2010; Zaki et al., 2012).
Conceptual valuations influence behavior
either by activating action impulses them-
selves or—as detailed below in the section
on regulation— influencing which contex-
tual and core valuations are expressed via
actions (Ochsner, Ray, et al., 2009). We
propose that conceptual valuations play key
roles in introspection about and self- reports
of affective states, in mental state attribu-
tion, and in judgments about the values of
stimuli and actions that involve conscious
reasoning about their value (Kalisch, Wiech,
Critchley, & Dolan, 2006; Mitchell, 2009;
Olsson & Ochsner, 2008).
PVA Components: The Action Stage
At any given level of valuation, the action
impulses associated with a PVA sequence can
be either mental (e.g., retrieving information
from memory, forming a mental image, or
introspecting about one’s mood) or physical
(e.g., including overt behaviors such as shifts
of gaze or starting to run, and autonomic/
physiological responses such as heart rate
increases or the release of stress hormones;
Levenson, 1999). Although elaborating the
brain systems supporting the action stage is
not the focus of this chapter, it likely involves
subcortical and cortical regions involved in
selecting motor actions, as well as initiating
autonomic responses (e.g., the PAG, primary
motor and supplementary motor areas, cin-
gulate motor regions, and insula) (Buhle et
al., 2012; Critchley, 2005; Dum, Levinthal,
& Strick, 2009; Mobbs et al., 2009).
PVA Operating Principles:
Processing Dynamics
As multiple valuations are computed at dif-
ferent levels and time scales— each with
its own associated action impulses— only
a subset of the possible actions associated
with a percept and its valuations can be
enacted. What determines which actions are
expressed, whether mental (e.g., thoughts
and feelings) or physical (e.g., smiling and
hugging)?
One factor is the existing structure of the
PVA sequences an individual possesses at
any given moment in time. This factor has
been addressed primarily in psychologi-
cal and computational models of associa-
tive memory networks that suggest the P’s,
V’s, and A’s of all currently activated PVAs
mutually excite and/or inhibit one another
in such a way that the most activated action
tendency or (a set of equally activated) ten-
dencies “win” and are manifested as mental
and/or physical actions (Desimone & Dun-
can, 1995; Barrett, Ochsner, & Gross, 2007;
Maas, 2000; Miller & Cohen, 2001). The
schematic PVA sequences of Figure 2.1A
illustrate the possible kinds of links that may
exist between P, V, and A nodes.
A second factor is the multiple contextual
and historical considerations that determine
the level of activation for each PVA sequence
and whether they have inhibitory or excit-
atory links with other PVAs— including
the stimuli that are (or have been) present
as inputs, their activation history (which
determines the strength of links within and
across PVAs, and hence their relative ease of
activation [Anderson, 1983; Neely, 1991]),
and whether they are in the focus of atten-
tion (which enhances activity, especially at
the perception stage [Pessoa, Kastner, &
Ungerleider, 2003; Polk, Drake, Jonides,
Smith, & Smith, 2008]).
At any given moment, an individual’s
affective response comprises the profile of
activation across all PVAs—at all levels—
that may combine or cancel one another
depending on the nature of their connections
and levels of activation (Barrett, Mesquita,
Ochsner, & Gross, 2007; Scherer, 2001). As
described below, depending on the circum-
stances, core, contextual, and/or conceptual
PVAs may be activated most strongly and
lead to action.

Valuation and Emotion Regulation 29
PVA Operating Principles:
Interacting Networks
As our process- level description makes clear,
the PVA sequence is continually unfolding
in real time for multiple stimuli as multi-
ple levels of analysis. This means that net-
works of interacting brain systems underlie
each stage of the PVA sequence, as well as
the interactions among stages. This follows
from the fact that an individual’s affective
response comprises the profile of activation
across all PVAs, at all levels, which in turn
follows from the idea that the P, V, and A
stages all involve multiple brain systems
working together to compute the perceptual,
evaluative, and action components of one’s
response to a given stimulus.
Thus, the totality of one’s valuation of a
stimulus cannot be understood in terms of
the activation of a single brain system. That
said, most of what we know about the func-
tions of brain systems implicated in the P,
V, and A stages comes from studies employ-
ing analytic techniques (e.g., simple con-
trasts) designed to isolate the contributions
to behavior of single regions rather than
integrated networks. Increasingly, however,
various kinds of connectivity, network, and
multivoxel pattern analyses are being used
to describe the task- varying functional rela-
tionships among regions that define them as
critical for aspects of the P, V, and A stages
(Kober et al., 2008). For example, we and
others have used mediation and structural
equation modeling to describe the ways in
which prefrontal control regions regulate
emotional response via their impact on
subcortical regions that trigger affective
responses (Johnstone, van Reekum, Urry,
Kalin, & Davidson, 2007; Kober et al.,
2010; Urry et al., 2006; Wager, Barrett, et
al., 2008; for review, see Ochsner, Silvers,
& Buhle, 2012). As such analytic techniques
mature, we expect that our framework will
be able to describe more precisely the func-
tional interactions governing the P, V, and A
stages, as well as their interactions.
A Valuation Perspective on Emotion
and Emotion Regulation
Emotions are particular types of valuation
that (1) have a well- specified object (i.e.,
one is angry about something), (2) unfold
over seconds to minutes, and (3) involve
coordinated changes in subjective experi-
ence, behavior, and physiology (Barrett et
al., 2007; Mauss et al., 2005; Scherer et al.,
2001). In keeping with our overall goal of
showing how the valuation framework is
broadly applicable, in the sections that fol-
low we employ an expansive view of emo-
tion.
Emotion as a Type of PVA Sequence
Imagine you are a commuter in a crowded
New York subway car. Across from you sit
a sleepy- eyed old man, a muscular teen, and
an attractive woman. As the subway rattles
toward your stop, the teen removes a knife
from his pocket, shifting it from hand to
hand.
In our framework, emotional reactions to
the knife- wielding teen may be conceived of
as specific kinds of PVA sequences derived
from particular perceptions, valuations, and
associated action impulses (Ortony et al.,
1988; Scherer et al., 2001). Thus, an ini-
tial response may reflect a core-level valua-
tion of the teen and his knife as potentially
threatening by the amygdala and related
regions, which triggers corresponding action
impulses that mobilize you to avoid harm
(e.g., increased heart rate, behavioral readi-
ness to fight or flee; LeDoux, 2000; Phelps,
2006). At the contextual level, the action
outputs of core-level PVA sequences become
perceptual inputs that are integrated with
other inputs representing the historical (epi-
sodic) context of, for example, having prior
subway conversations with the teen (via
MTL inputs), the social context of multiple
other passengers being present (via STS/TPJ
inputs), and the motivational context of cur-
rent stress and bodily complaints (via AI and
other subcortical inputs). As time passes,
activation of the contextual- level PVAs,
which dictate other courses of action, can
begin to build, and the initial valuation may
evolve dynamically into valuations of the
teen as relatively innocuous or highly dan-
gerous, depending on whether the teen pre-
viously indicated he has a role as a thug in
a school play or is on medication for a delu-
sional disorder (historical context), whether
he elicits calm or anxious reactions from
30 BIOLOGICAL BASES
other passengers (social context), or whether
you are stressed from work or just had a
great day (motivational context). Then, the
action outputs of activated contextual- level
PVAs are taken as inputs to systems (rmPFC,
and/or vlPFC) that compute a valuation of
the teen in belief– desire terms that can—at
the action stage—be introspectively accessed
or reported to others as the thoughts and
feelings you attribute to yourself or others,
including, for example, the thoughts that
you yourself are brave, that the knife- wielder
looks aggressive, and that the old man and
young woman seem calm.
The order in which these valuation sys-
tems is activated, and their interplay, is not
fixed and depends on the circumstances of
your encounter with a stimulus. For exam-
ple, if you are sitting on the subway, and the
teen enters from the opposite end of the car
and does not pose an immediate threat, then
conceptual valuation systems might evaluate
his intentions (“Is he dangerous?”) and your
own level of fear (“I’m not scared— yet”). As
the teen moves closer, contextual systems
might be most active as you evaluate the
goodness or badness of potential courses of
action based on your changing motivational
state (increasing anxiety), history (the seat
next to you was just vacated, and the teen
moves toward this open seat) and the appar-
ent anxiety of your fellow passengers (who
look increasingly afraid). Finally, as the teen
moves even closer and the threat level is very
high, activation in core valuation systems
may escalate to promote defensive actions
such as freezing, escape or fighting (Mobbs
et al., 2007).
The key idea is that, taken together, all of
these PVAs, however they were activated,
and each with their associated mental and
physical action tendencies, comprise an
emotional response.
Emotion Regulation as a Type
of PVA Sequence
As noted earlier, emotions themselves are
sometimes the target of valuation. For
example, in the previous subway exam-
ple, we might wish to protect our view of
ourselves as brave and, as a consequence,
desire to decrease our fear responses. To
do this, we can take as objects of valuation
the action outputs of PVAs that comprise a
fear response. When we do this— thereby
activating a goal to influence the nature of
the emotional response— we are engaging in
emotion regulation. As described below, this
involves interactions among regions impli-
cated in cognitive control (i.e., regulation)
and/or valuation.
In our framework, emotion regulation is
initiated when a PVA cycle that gives rise
to emotion becomes the object of valua-
tion (see Figure 2.1B). We propose that this
typically happens across levels of valuation,
as a higher- level PVA places a good or bad
valuation on a lower-level PVA (although it
also can happen between PVAs at a single
level). It also can happen if there is a high
level of conflict between active PVAs, such
as whether the impulse to flee a poten-
tially dangerous situation conflicts with the
impulse to freeze, and a clear set of emo-
tional responses isn’t activated. We propose
that when this happens, the level of conflict
constitutes an input to the next PVA cycle,
and evaluation of that conflict triggers an
appropriate course of regulatory action.
One key feature of our framework is the
idea that some of the prefrontal systems that
support emotion regulation are involved in
the control of nonaffective forms of behav-
ior as well (Miller & Cohen, 2001; Ochsner
& Gross, 2005). These systems (see Figure
2.2B) include dorsal and ventrolateral pre-
frontal regions that support selective atten-
tion, working memory, and retrieval from
semantic memory; cingulate regions that
monitor conflicts between competing As
and the need for continued control; medial
regions that support mental state attribu-
tion; and ventromedial prefrontal regions
that place contextual constraints on the
expression of core-level PVAs (Miller, 2000;
Ochsner & Gross, 2005; Olsson & Ochsner,
2008; Wager, Jonides, & Reading, 2004;
Wager & Smith, 2003). As detailed below,
the regulatory actions supported by these
systems comprise different types of “A’s” in
PVA sequences that place a value on one’s
current affective state.
Distinguishing among Emotion
Regulation Processes
We have previously argued that emotion
regulation processes can be differentiated
into five families according to which stage
Valuation and Emotion Regulation 31
of the emotion generation sequence they
target. In the context of the present frame-
work, this idea is expressed by suggesting
that emotion regulatory processes differ
in the stage of the PVA sequence at which
they have their primary impact (see Figure
2.3). Some strategies influence the situation-
dependent perceptual inputs (situation selec-
tion, situation modification, and attention
deployment). Others influence the valua-
tion step itself (cognitive change). Still oth-
ers influence the response output associated
with activated action sequences (response
modulation). By impacting different states
of the PVA cycle, different strategies impact
emotional responding in different ways, as
detailed below.
Situation selection refers to altering the
inputs to the PVA sequence through deci-
sions about whether to expose oneself to a
given situation/stimulus based on its pro-
jected affective impact. For example, call-
ing to mind the image of the subway might
lead to a negative evaluation, and a feeling
of fear. This feeling might motivate a higher-
level PVA that would trigger a decision to
take an alternative means of transportation
in order to decrease the probability of the
negative experiences that one associates with
taking the subway. More generally, situation
selection can take many forms, for example,
when a socially anxious individual avoids a
social event. To date, the neural bases of sit-
uation selection have been studied only with
avoidance conditioning tasks in which an
animal learns to select an action (e.g., run-
ning in a wheel when a light is illuminated
predicts an upcoming shock) that enables
it to avoid experiencing a noxious stimulus
(that prevents shock administration). Rodent
studies have shown this involves modulation
of two core valuation systems, the striatum
and amygdala (Everitt et al., 1999; LeDoux
& Gorman, 2001), and one human imaging
study (Delgado et al., 2009) indicates that
it also engages vlPFC and dorsolateral pre-
frontal cortex (dlPFC) regions involved in
cognitive control that presumably modulate
the core valuation systems.
Situation modification refers to alter-
ing the situation one is in, thereby modi-
fying inputs to the PVA sequence, and
changing the emotion (e.g., sitting further
away from the teen or exiting the subway).
We would expect that prefrontal systems
should be involved in the selection of escape
behaviors— especially in the kinds of emo-
tionally arousing situations humans face in
everyday life. Although this hypothesis has
not been tested, the involvement of prefron-
tal regions is suggested by behavioral stud-
ies in humans showing that emotion can be
regulated by deliberately changing situation-
dependent stimulus inputs in the service of
explicit regulatory goals. For example, either
physically or mentally, using visual imagery,
one can move closer to or further away from
an emotion- eliciting stimulus (e.g., making
oneself feel more positive by approaching a
pleasant stimulus or less negative by with-
drawing from an unpleasant one; Davis,
Gross, & Ochsner, 2011; Muhlberger, Neu-
mann, Wieser, & Pauli, 2008; Williams &
Bargh, 2008).
Attentional deployment refers to altering
the inputs to the PVA sequence by increasing
or decreasing attention to them (e.g., look-
ing away from the teen and at the man or
woman). While this can gate specific stim-
uli wholly into or out of the PVA stream,
thereby promoting or preventing responses
to them, we propose that more graded
changes in attention to stimuli may result
in correspondingly graded levels of acti-
vation of their associated PVA sequences.
This strategy involves interactions between
cognitive control systems and valuation sys-
tems, with particular involvement of dorsal
PFC and inferior parietal regions associated
with selective attention (Pessoa et al., 2003),
and in some cases rmPFC regions implicated
in attending to and explicitly judging the
value of stimuli (Bishop, 2007; Egner, Etkin,
Gale, & Hirsch, 2008; Etkin, Egner, Peraza,
Kandel, & Hirsch, 2006; Lane et al., 1998;
Ochsner, Hughes, Robertson, Cooper, &
Gabrieli, 2009). A growing but somewhat
inconsistent imaging literature shows that,
by and large, when a task manipulation
diminishes attention to an affectively arous-
ing stimulus, activation increases in PFC
regions implicated in cognitive control (sug-
gesting that cognitive control systems are
involved in manipulating attention), and
activity decreases in regions implicated in
core (e.g., amygdala, PAG), contextual (e.g.,
insula) or conceptual (e.g., mPFC) valuation
(e.g., Ochsner & Gross, 2005; Pessoa, 2009).
While it is clear that the specific valuation
systems modulated by attentional deploy-
32
Neural Systems
Emotion Regulation Strategies Related Phenomena
Region
Type of
System Processes
Attentional
Deployment
Cognitive
Change
(Reappraisal)
Response
Modulation
(e.g.,
Expressive
Suppression)
Affective
Learning
(e.g., Fear
Extinction)
Affective
Decisions
(e.g.,
Intertemporal
Choice)
Expectancies
(e.g., Placebo
Effects)
dorsolateral
PFC
Control
selective
attention/
working
memory
dorsal
posterior
mPFC
inferior parietal
dorsal ACC
performance
monitoring
ventrolateral
PFC
selection/
inhibiti
on
dorsal mPFC
Conceptual
Valuation
Contextual
Valuation
Core Valuation
conceptual/
categorical
valuations
rostral mPFC
attention to
valuation
ventromedial
PFC/OFC
value of
stimulus in
current context
? ?
ventral
striatum
reward/
reinforcement
value
amygdala
arousal (and
threat) value of
stimulus
insula Valuation
representation
and awareness
of body states
for all types of
valuation
PVA Cycle Illustrations
1 1 1 1
2 2 2 2
W P V A
W P V A
W
1
P
1
A
1
Vsafe
Vthreat
P
1
V
1
A
1
P
1
V
1
A
1
P
2
V
2
A
2
Option2
Option1
W
1
P
1
V
1
A
1
P
2
V
2
A
2
Placebo
Painful
Stimulus
1 1 1 1
2 2 2 2
W P V A
W P V A
1 1 1 1
1 2 2 2
W P V A
W P V A
FIGURE 2.3. Neural systems for valuation and control postulated by the valuation framework pre-
sented in the chapter (left-hand columns), as well as the roles these neural systems play in three kinds
of emotion regulation strategies (center columns, see text) and three kinds of related phenomena (right-
hand columns, see text). Up arrows indicate increased activation, down arrows indicate decreased
activation, and “?” indicates involvement in some (but not the majority) of the studies. The final row
diagrams, in PVA terms, how each emotion regulation strategy or related phenomenon might oper-
ate (see text for details). The three center columns show, for each emotion regulation strategy, how
control actions impact either attention paid to particular stimuli at the perception stage (attentional
deployment), how one values those stimuli (reappraisal), or what actions one takes as a consequence of
this valuation (response modulation). The three right-hand columns show for related phenomena how
initial valuations (e.g., threat) may be overridden if one learns new valuations (e.g., safe) for a stimulus
(extinction) one may select among choice options as a function of their relative valuations, with control
actions coming into play when the choice options are similarly valued and/or in conflict (intertemporal
choice), or a placebo may influence the valuation of a painful stimulus via the action of control pro-
cesses (placebo effects).

Valuation and Emotion Regulation 33
ment depend on the sensory qualities of the
stimulus, distraction from pain modulates
nociceptive regions of insula and cingulate
cortex (Frankenstein, Richter, McIntyre, &
Remy, 2001; Tracey et al., 2002), whereas
distraction from an aversive image modu-
lates the amygdala (McRae et al., 2010; Pes-
soa, 2009); for example, cross-study vari-
ability in attentional deployment strategies
and the lack of a common metric for deter-
mining how much any given strategy dimin-
ishes attention to a stimulus in one study
compared to others (see Ochsner & Gross,
2005, for a detailed review) have limited the
conclusions that can be drawn about when
and how specific cognitive control systems
are involved.
Cognitive change refers to altering the
subjective meaning and/or perceived self-
relevance of the present situation (e.g.,
thinking of the knife as a stage prop or that
knife- tossing is an innocent way of pass-
ing time). The framework suggests that this
strategy should involve interactions between
cognitive control systems that can be used
deliberately to change one’s interpretation of
a stimulus and valuation systems that trigger
an affective response. Of note here is the fact
that the framework predicts that conceptual
valuation systems can play a role on either
side of this regulatory equation: on the one
hand, being the target of cognitive control
systems that seek to change one’s high-level
conceptual valuation of a stimulus, and on
the other, assisting those cognitive control
systems in reformulating the attributions one
makes about the nature of one’s own beliefs,
desires, and feelings (e.g., “I’m feeling less
afraid now”)—or those expressed by others
(e.g., “The subway passengers are anxious
about the crowding, not the teen”)—as one
deliberately changes his or her interpretation
of an emotion- eliciting stimulus. Research
on cognitive change— referred to in the lit-
erature as “reappraisal”—consistently sup-
ports the predictions of the framework:
When engaging in a cognitive change strat-
egy, activation is observed in lPFC and cin-
gulate PFC regions associated with cognitive
control, as well as mPFC regions associated
with conceptual valuation (albeit primarily
when up- regulating emotional responses)
and at the same time increasing or decreas-
ing activity in core (e.g., amygdala, striatum)
and/or contextual (e.g., insula) valuation
systems (e.g., Kober et al., 2010; Ochsner et
al., 2004; Urry et al., 2006; Wager, David-
son, Hughes, Lindquist, & Ochsner, 2008;
reviewed in Kalisch, 2009; Ochsner &
Gross, 2005, 2008) in accordance with one’s
regulatory goals.
Finally, response modulation refers to tar-
geting behavioral manifestations of emotion
(e.g., playing it cool by not showing fear of
the knife- wielding teen). Human research
primarily has focused on one exemplar of
this strategy, expressive suppression, which
involves hiding behavioral manifestations of
emotion (Gross, 1998). Behaviorally, expres-
sive suppression effectively reduces facial
expressions of emotion, but the effort and
attention required to do so trigger autonomic
responses, impair memory for visual cues,
and can negatively impact social interactions
(Butler et al., 2003; Gross, 1998; Richards
& Gross, 2000). In keeping with these find-
ings, an initial imaging study showed that
suppressing the expression of disgust acti-
vated two key PFC regions associated with
cognitive control (dlPFC associated with
maintaining goals, and vlPFC associated
with response selection and inhibition more
generally; Aron et al., 2004; Badre & Wag-
ner, 2007; Thompson- Schill et al., 2005),
and increased activation in core (amgydala)
and contextual (insula) valuation regions
associated with detection of threats and
awareness of body states (Goldin, McRae,
Ramel, & Gross, 2008). This supports the
idea that expressive suppression, like other
strategies, depends on interactions between
cognitive control and valuation regions, and
may have neural bases similar to those sup-
porting response inhibition more generally
(Aron et al., 2004).
Applications of
the Valuation Perspective
The neural systems implicated in emotion
and emotion regulation play key roles in
other phenomena that involve valuation and
cognitive control (Hartley & Phelps, 2010;
Murray et al., 2007; Pessoa, 2008; Phillips
et al., 2008; Rangel et al., 2008). We believe
it is important that any account of emotion
and emotion regulation use terminology and
concepts that are broadly applicable to allied
phenomena as well.
34 BIOLOGICAL BASES
With this in mind, we illustrate in this
section the broad applicability of our valu-
ation perspective on emotion and emotion
regulation by showing how it can provide a
framework for describing the mechanisms
underlying three types of related phenomena
that traditionally are considered in relatively
separate literatures. This has the dual bene-
fits of broadening the framework to account
for aspects of related phenomena it was not
initially formulated to address, and in so
doing, making the framework more robust
and generally applicable.
Affective or Emotional Learning
As noted in the earlier section on the PVA
processing dynamics, our valuation perspec-
tive allows learning to occur by updating
the valuations placed on stimuli with each
iteration of the PVA sequence. To account
more broadly for various forms of affective
or emotional learning, we can elaborate the
way in which this updating process occurs.
When encountering a stimulus, one’s cur-
rent valuation of it sets an expectation for the
outcome states of the world that should fol-
low from execution of the associated action
impulse(s). These outcomes become inputs to
the next PVA, which evaluates discrepancies
between the expected and actual outcomes.
If this valuation is negative (i.e., when the
discrepancy is large and/or important in
light of currently active goals), this valuation
triggers learning and updating processes
that change links between a stimulus and its
valuation (P-V) or between a valuation and
an action (V-A)—or between separate PVA
sequences— so that future valuations are
more accurate (Delgado, Olsson, & Phelps,
2006; Rangel et al., 2008; Schultz, Dayan, &
Montague, 1997). Each change is small, so
that one’s value expectations for a stimulus
at a given moment in time are a function of
one’s prior experiences with it, biased more
heavily toward recent experiences. While
this value updating process typically is stud-
ied in the context of conditioning, reward,
and affective learning, it fits neatly within
our valuation framework as the way that
changes in the contingency between actions
and outcomes can adaptively alter the valua-
tions that drive the actions.
To illustrate this, we can use our subway
example to consider one of the most studied
examples of value updating, namely, extinc-
tion of a fear response. We discussed extinc-
tion earlier as an example of contextual
valuation in which an organism learns that
a previously feared stimulus need no longer
be feared in the current temporal context.
In that section, however, we did not explain
how the organism learns this contextual
association. Here we propose that value
updating is the learning mechanism.
The subway example can help make this
concrete. Recall that the knife- wielding teen
initially elicits a threat valuation and fear
response involving amygdala- mediated core
level PVAs. If the expected outcome does
not transpire (i.e., the teen takes no harm-
ful actions), however, then over time a new
contextual- level PVA is acquired by ventro-
medial/orbitomedial PFC systems indicating
the teen is not a threat. The longer the teen
takes no harmful action, the stronger this
PVA becomes. Ultimately, even though the
knife still connotes threat at the core level,
the contextual PVA wins out for expression
in behavior (see the section on PVA process-
ing dynamics). Because the core-level PVA
itself remains unchanged, a fear response
can be quickly reinstated in the future
should the teen become truly threatening
(Bouton, 2004; LeDoux, 1993). The frame-
work can be similarly applied to other cases
in which one learns, or already has learned,
that a given emotional impulse is inappro-
priate or unnecessary in the current context,
as in reversal of learned appetitive or aver-
sive associations (Bouton, 2004; Corcoran
& Quirk, 2007; Schoenbaum et al., 2007).
As this example makes clear, affective
learning and the types of regulatory strate-
gies reviewed earlier are not mutually exclu-
sive and may in many contexts work together.
For example, the act of reappraising can be
seen as a way of cognitively creating a dis-
crepancy between an expected internal out-
come (e.g., a fear response) associated with
a given percept (e.g., the knife- wielding teen)
and the response that actually occurs (e.g.,
calmness). This discrepancy could activate
learning processes that weaken core-level
PVA representations of the teen as threat-
ening, build new contextual- level represen-
tations of the teen as nonthreatening, and
strengthen conceptual- level PVAs of the teen
as an actor. In this way, reappraisal— and
by extension other regulatory strategies—
Valuation and Emotion Regulation 35
can be seen as providing top-down “teach-
ing” inputs to outcome- driven regulatory
processes that typically are triggered by
external cues (cf. Delgado, Gillis, & Phelps,
2008; Delgado, Nearing, LeDoux, & Phelps,
2008).
Affective Decision Making
Our valuation perspective also may be
applied to affect- laden decision making.
Affective decisions require a choice between
options that are associated with different
expected rewards or punishments. In our
framework, these expectations are reflected
in the values computed for choice options
at various levels of the valuation hierarchy.
To the extent that the play of activation
and inhibition across PVA sequences associ-
ated with choice options results in a core-,
contextual-, or conceptual- level valuation
determining the behavioral output, then the
choice option associated with that valuation
will be selected. However, in some cases, this
play of activation fails to determine clearly a
most highly valued selection, and cognitive
control processes may be engaged in order to
construct, hold in mind, and implement top-
down processes that influence PVAs associ-
ated with choice options. This commonly
happens when choice options are similarly
valued and/or conflict with one another,
but it also may happen when the valuation
process itself becomes a target of valuation
(e.g., when there is a negative valuation of an
attractive response option).
To illustrate (see Figure 2.3), consider
how the framework accounts for a com-
monly studied choice dilemma in behavioral
economics and neuroeconomics known
as “intertemporal choice” (or as delay of
gratification in the developmental litera-
ture; Mischel, Shoda, & Rodriguez, 1989).
This dilemma involves choosing between
a smaller reward available now or a larger
reward available at some point in the future.
In our framework, selection of the immedi-
ate reward would be promoted by core-level
(striatal) or contextual- level (medial/orbital
frontal) valuation systems that represent
the reward value of the currently available
stimulus. By contrast, picking the delayed
reward would require the use of lateral pre-
frontal cognitive control systems in order
to maintain a representation of the delayed
reward in working memory and inhibit acti-
vation of PVAs for the immediately avail-
able choice option (Figner et al., 2011). In
keeping with this account, human imaging
studies have shown greater ventral striatal
(VS) and/or vmPFC versus greater dlPFC
activity when participants select immediate
versus delayed rewards (McClure, Ericson,
Laibson, Loewenstein, & Cohen, 2007;
McClure, Laibson, Loewenstein, & Cohen,
2004), and a recent transcranial magnetic
stimulation study showed that disruption of
left—but not right—dlPFC led participants
to “impulsively” select immediate rewards
when they had shown a prior preference
for the delayed reward (Figner et al., 2011).
Strikingly, this result dovetails with the find-
ing that a pathway from left dlPFC to the VS
supports the use of reappraisal to diminish
craving for desired substances (e.g., sugary/
fattening foods) when participants think
about the negative long term (e.g., diabetes)
as opposed to the immediate (e.g., delicious
taste) consequences of consuming them
(Kober et al., 2010).
As these findings make clear, affective
decision making and the regulatory strate-
gies reviewed earlier may depend upon very
similar neural systems and, as such, the line
between them is not always clear. Indeed,
intertemporal choices— and other choices
that require selecting between options con-
sistent with long- versus short-term goals—
can be viewed as self- control tasks (Figner et
al., 2011; Hare, Camerer, & Rangel, 2009;
Wunderlich, Rangel, & O’Doherty, 2009) in
which the decision to select an option consis-
tent with a long-term goal is influenced by
attention deployment and cognitive change
strategies (Mischel et al., 1989). Our valua-
tion perspective can also be applied to other
types of choice dilemmas in which control
and valuation processes interact to deter-
mine choice, including risky decision making
(Gianotti et al., 2009), in which the choice is
to be fair toward or to punish others (Knoch
et al., 2008; Knoch, Pascual- Leone, Meyer,
Treyer, & Fehr, 2006), and when the act of
choice itself changes our valuations of stim-
uli via the value- updating process (Sharot,
De Martino, & Dolan, 2009; Sharot, Shiner,
& Dolan, 2010) as in cognitive dissonance
reduction (Lieberman, Ochsner, Gilbert,
& Schacter, 2001; Sharot et al., 2009; van
Veen, Krug, Schooler, & Carter, 2009).

36 BIOLOGICAL BASES
Expectancies, Beliefs,
and Placebo Effects
Our valuation framework also helps make
sense of the growing imaging literatures on
the ways in which expectancies and beliefs of
various sorts— including placebo effects—
influence responses to various kinds of affec-
tive stimuli (Wager, 2005). In these tasks,
participants are given one of two kinds of
explicit expectations. In studies of expectan-
cies or anticipation, participants are told that
an upcoming stimulus— whether a painful
sensation, an image, or something else—will
be of a particular intensity or kind. In pla-
cebo experiments, participants are told that
a drug (e.g., a cream or a pill) will increase
or decrease their subsequent responses to a
stimulus. In either case, these expectations
lead participants to experience the stimulus,
when presented, as subjectively more simi-
lar to what they expected than would have
been the case had they held no expectations
or beliefs about its nature or the protective
properties of a drug.
From the perspective of our framework,
these phenomena all involve the top-down
influence of cognitive control systems on
valuation systems or the influence of higher
level valuation systems on lower level valu-
ation systems. Our interpretation of these
effects is consistent with results of imaging
studies of expectancies and placebo effects
on pain responses. Such studies indicate
that expectancies and placebo beliefs about
pain are maintained in a combination of
lateral prefrontal/parietal working memory
systems and/or medial prefrontal systems
(Atlas, Bolger, Lindquist, & Wager, 2010;
Lieberman, Jarcho, Berman, et al., 2004;
Wager, 2005; Wager, Atlas, Leotti, & Rill-
ing, 2011) that in the framework could be
described as representing either conceptual-
level beliefs (e.g., “The cream on my forearm
should lessen the pain”) or contextual- level
expectations about the stimulus or placebo.
According to our framework, these systems
influence attention to and appraisal of the
value of stimuli in contextual- level and/
or core-level valuation systems (see Figure
2.3), modifying their levels of activation to
be consistent with top-down beliefs (e.g.,
lessening activation of pain- sensitive valu-
ations systems, including contextual- level
regions (e.g., cingulate and insular cortex)
and core-level regions (e.g., amygdala and
PAG) (Ploghaus, Becerra, Borras, & Bor-
sook, 2003; Wager, 2005).
Thus, from the viewpoint of the frame-
work, expectancies and beliefs operate much
like two of the emotion regulation strategies
described earlier— attention deployment
and cognitive change— in that they alter
lower-level inputs to PVA systems and/or the
valuation process.
Summary
One of the fundamental challenges faced by
any animal is computing and expressing the
value of stimuli in an accurate and timely
manner. This is difficult, because the ani-
mal’s internal state and external environment
change over time, and its information acqui-
sition, processing, and response resources
and capabilities are limited. To address these
challenges, humans (and other animals) have
developed a complex set of interacting valua-
tion systems, each of which can be described
in terms of a simplified P-V-A sequence, in
which a particular perceptual input is valued
(negatively or positively to a given degree),
leading to an impulse to alter ongoing behav-
ioral or cognitive responses. These P-V-A
sequences run in parallel at various levels in
the brain and compete for expression.
This process- oriented valuation frame-
work suggests a number of directions for
future research. One direction concerns
the valuation systems. While Figures 2.2A
and 2.3 feature three kinds of valuation
systems (core, contextual, and conceptual),
future work should clarify the number and
kind of valuation systems, as well as the
rules that govern their engagement in par-
ticular contexts. A second direction has to
do with how the often- competing action
impulses associated with different P-V-A
sequences are coordinated. We have empha-
sized the role of competitive activation and
inhibition, but how this and other processes
lead to coordinated and sustained adaptive
behavior rather than erratic and conflicting
behavior is not yet clear. A third direction
concerns the inputs and outputs of valuation
systems. We have suggested that the class
of P-V-A sequences whose inputs are other
P-V-A sequences, and outputs that include
the engagement of cognitive control pro-
cesses are fundamental to emotion regula-

Valuation and Emotion Regulation 37
tion and self- control more generally. That
said, the range of relevant inputs and out-
puts, and the malleability of input– output
relations requires further study. A fourth
direction concerns the efficacy of various
forms of value regulation and how they are
intermixed in everyday life. Which “pure”
or “hybrid” forms of value regulation are
most effective? A fifth direction concerns
translation of what we learn to illuminate
individual differences. In our framework,
a given emotional response and regulation
profile could involve individual differences
in (1) the initial valuations placed on spe-
cific classes of stimuli by systems at the core,
contextual, and/or conceptual levels; (2) the
speed with which these valuations are made;
(3) how quickly and easily one resolves con-
flicts between them to express emotional
responses; (4) how quickly and effectively
learning processes update these valuations
given that some emotional responses may be
more difficult to change than others; (5) the
knowledge of how and when to deploy emo-
tion regulatory strategies; and (6) the capac-
ity and ability to deploy top-down control
systems to implement these strategies. One
important direction for future research is
examining how each of these differences—
and others— may interact to produce vari-
ous forms of psychopathology.
Our goal in presenting this valuation
framework is to provide a common plat-
form for analyzing the neural systems that
are important for many different types of
valuation. The impetus for this framework
came from the observation that neural sys-
tems implicated in emotion generation and
emotion regulation overlapped in important
ways with neural systems implicated in other
literatures that typically are not considered
side by side (see Figure 2.3). Across all these
research domains an organism’s adaptive
capacity crucially hinges on the coordina-
tion of multiple valuation systems in real
time, and a key challenge for future research
is delineating these PVA interactions, ideally
with adequate specificity to permit more
explicitly computational approaches. We
believe that an explicitly integrative valu-
ation framework represents a step in this
direction, and holds out the possibility of
better coordinating hitherto unconnected
research literatures, while simultaneously
deepening our understanding of each one.
References
Anderson, J. R. (1983). A spreading activation
theory of memory. Journal of Verbal Learning
and Verbal Behavior, 22(3), 261–295.
Aron, A. R., Robbins, T. W., & Poldrack, R. A.
(2004). Inhibition and the right inferior fron-
tal cortex. Trends in Cognitive Sciences, 8(4),
170–177.
Atlas, L. Y., Bolger, N., Lindquist, M. A., &
Wager, T. D. (2010). Brain mediators of pre-
dictive cue effects on perceived pain. Journal
of Neuroscience, 30(39), 12964–12977.
Badre, D., & Wagner, A. D. (2007). Left ven-
trolateral prefrontal cortex and the cognitive
control of memory. Neuropsychologia, 45(13),
2883–2901.
Barrett, L. F. (2006). Solving the emotion para-
dox: Categorization and the experience of
emotion. Personality and Social Psychology
Review, 10(1), 20 – 46.
Barrett, L. F., Mesquita, B., Ochsner, K. N., &
Gross, J. J. (2007). The experience of emotion.
Annual Review of Psychology, 58, 373–403.
Barrett, L. F., Ochsner, K. N., & Gross, J. J.
(2007). Automaticity and emotion. In J.
A. Bargh (Ed.), Social Psychology and the
Unconscious (pp. 173–218). New York: Psy-
chology Press.
Bishop, S. J. (2007). Neurocognitive mechanisms
of anxiety: An integrative account. Trends in
Cognitive Sciences, 11(7), 307–316.
Bouton, M. E. (2004). Context and behavioral
processes in extinction. Learning and Mem-
ory, 11(5), 485–494.
Buhle, J. T., Kober, H., Ochsner, K. N., Mende-
Siedlecki, P., Weber, J., Hughes, B. L., et al.
(2012). Common representation of pain and
negative emotion in the midbrain periaque-
ductal gray. Social Cognitive and Affective
Neuroscience. [E-publication ahead of print]
Butler, E. A., Egloff, B., Wilhelm, F. H., Smith,
N. C., Erickson, E. A., & Gross, J. J. (2003).
The social consequences of expressive suppres-
sion. Emotion, 3(1), 48– 67.
Cato, M. A., Crosson, B., Gokcay, D., Soltysik,
D., Wierenga, C., Gopinath, K., et al. (2004).
Processing words with emotional connotation:
An fMRI study of time course and laterality
in rostral frontal and retrosplenial cortices.
Journal of Cognitive Neuroscience, 16(2),
167–177.
Corcoran, K. A., & Quirk, G. J. (2007). Recall-
ing safety: Cooperative functions of the ven-
tromedial prefrontal cortex and the hippo-
38 BIOLOGICAL BASES
campus in extinction. CNS Spectrums, 12(3),
200–206.
Craig, A. D. (2003). Interoception: The sense of
the physiological condition of the body. Cur-
rent Opinion in Neurobiology, 13(4), 500–
505.
Craig, A. D. (2009). How do you feel—now?:
The anterior insula and human awareness.
Nature Reviews Neuroscience, 10(1), 59–70.
Critchley, H. D. (2005). Neural mechanisms of
autonomic, affective, and cognitive integra-
tion. Journal of Comparative Neurology,
493(1), 154–166.
Cunningham, W. A., Raye, C. L., & Johnson,
M. K. (2004). Implicit and explicit evaluation:
fMRI correlates of valence, emotional inten-
sity, and control in the processing of attitudes.
Journal of Cognitive Neuroscience, 16(10),
1717–1729.
Cunningham, W. A., & Zelazo, P. D. (2007).
Attitudes and evaluations: A social cognitive
neuroscience perspective. Trends in Cognitive
Sciences, 11(3), 97–104.
Damasio, A., Damasio, H., & Tranel, D. (2013).
Persistence of feelings and sentience after
bilateral damage of the insula. Cerebral Cor-
tex, 23(4), 833–846.
Davachi, L. (2006). Item, context and relational
episodic encoding in humans. Current Opin-
ion in Neurobiology, 16(6), 693–700.
Davis, J. I., Gross, J. J., & Ochsner, K. N. (2011).
Psychological distance and emotional experi-
ence: What you see is what you get. Emotion,
11(2), 438–444.
Delgado, M. R., Gillis, M. M., & Phelps, E. A.
(2008). Regulating the expectation of reward
via cognitive strategies. Nature Neuroscience,
11(8), 880–881.
Delgado, M. R., Jou, R. L., LeDoux, J. E., &
Phelps, E. A. (2009). Avoiding negative out-
comes: Tracking the mechanisms of avoidance
learning in humans during fear conditioning.
Frontiers in Behavioral Neuroscience, 3, 33.
Delgado, M. R., Nearing, K. I., Ledoux, J. E.,
& Phelps, E. A. (2008). Neural circuitry
underlying the regulation of conditioned fear
and its relation to extinction. Neuron, 59(5),
829–838.
Delgado, M. R., Olsson, A., & Phelps, E. A.
(2006). Extending animal models of fear con-
ditioning to humans. Biological Psychology,
73(1), 39–48.
Denny, B. T., Kober, H., Wager, T. D., & Och-
sner, K. N. (2012). A meta- analysis of func-
tional neuroimaging studies of self- and other
judgments reveals a spatial gradient for men-
talizing in medial prefrontal cortex. Journal
of Cognitive Neuroscience, 24(8), 1742–1752.
Desimone, R., & Duncan, J. (1995). Neural
mechanisms of selective visual attention.
Annual Review of Neuroscience, 18, 193–
222.
Dum, R. P., Levinthal, D. J., & Strick, P. L.
(2009). The spinothalamic system targets
motor and sensory areas in the cerebral cortex
of monkeys. Journal of Neuroscience, 29(45),
14223–14235.
Egner, T., Etkin, A., Gale, S., & Hirsch, J. (2008).
Dissociable neural systems resolve conflict
from emotional versus nonemotional distract-
ers. Cerebral Cortex, 18(6), 1475–1484.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E.
R., & Hirsch, J. (2006). Resolving emotional
conflict: A role for the rostral anterior cingu-
late cortex in modulating activity in the amyg-
dala. Neuron, 51(6), 871–882.
Everitt, B. J., Parkinson, J. A., Olmstead, M. C.,
Arroyo, M., Robledo, P., & Robbins, T. W.
(1999). Associative processes in addiction and
reward: The role of amygdala– ventral striatal
subsystems. Annals of the New York Acad-
emy of Sciences, 877, 412–438.
Ferstl, E. C., & von Cramon, D. Y. (2002). What
does the frontomedian cortex contribute to
language processing: coherence or theory of
mind? NeuroImage, 17(3), 1599–1612.
Figner, B., Knoch, D., Johnson, E. J., Krosch, A.
R., Lisanby, S. H., Fehr, E., et al. (2011). Lat-
eral prefrontal cortex and self- control in inter-
temporal choice. Nature Neuroscience, 13(5),
538–539.
Frankenstein, U. N., Richter, W., McIntyre, M.
C., & Remy, F. (2001). Distraction modu-
lates anterior cingulate gyrus activations dur-
ing the cold pressor test. NeuroImage, 14(4),
827–836.
Gallagher, H. L., & Frith, C. D. (2003). Func-
tional imaging of “theory of mind.” Trends in
Cognitive Sciences, 7(2), 77–83.
Gianotti, L. R., Knoch, D., Faber, P. L., Lehm-
ann, D., Pascual- Marqui, R. D., Diezi, C., et
al. (2009). Tonic activity level in the right pre-
frontal cortex predicts individuals’ risk tak-
ing. Psychological Science, 20(1), 33–38.
Goldin, P. R., McRae, K., Ramel, W., & Gross,
J. J. (2008). The neural bases of emotion regu-
lation: Reappraisal and suppression of nega-
tive emotion. Biological Psychiatry, 63(6),
577–586.
Gray, M. A., Beacher, F. D., Minati, L., Nagai,
Valuation and Emotion Regulation 39
Y., Kemp, A. H., Harrison, N. A., et al.
(2012). Emotional appraisal is influenced by
cardiac afferent information. Emotion, 12(1),
180–191.
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74(1), 224–237.
Gross, J. J. (2007). The handbook of emotion
regulation. New York: Guilford Press.
Hamann, S. B., Ely, T. D., Hoffman, J. M., &
Kilts, C. D. (2002). Ecstasy and agony: Acti-
vation of the human amygdala in positive
and negative emotion. Psychological Science,
13(2), 135–141.
Hare, T. A., Camerer, C. F., & Rangel, A. (2009).
Self- control in decision- making involves mod-
ulation of the vmPFC valuation system. Sci-
ence, 324(5927), 646–648.
Harrison, N. A., Gray, M. A., Gianaros, P. J.,
& Critchley, H. D. (2010). The embodiment
of emotional feelings in the brain. Journal of
Neuroscience, 30(38), 12878–12884.
Hartley, C. A., & Phelps, E. A. (2010). Chang-
ing fear: The neurocircuitry of emotion regu-
lation. Neuropsychopharmacology, 35(1),
136–146.
Holland, P. C., & Gallagher, M. (2004).
Amygdala– frontal interactions and reward
expectancy. Current Opinion in Neurobiol-
ogy, 14(2), 148–155.
Johnstone, T., van Reekum, C. M., Urry, H. L.,
Kalin, N. H., & Davidson, R. J. (2007). Failure
to regulate: Counterproductive recruitment of
top-down prefrontal– subcortical circuitry in
major depression. Journal of Neuroscience,
27(33), 8877–8884.
Kalisch, R. (2009). The functional neuroanat-
omy of reappraisal: Time matters. Neurosci-
ence and Biobehavioral Reviews, 33(8), 1215–
1226.
Kalisch, R., Wiech, K., Critchley, H. D., &
Dolan, R. J. (2006). Levels of appraisal: A
medial prefrontal role in high-level appraisal
of emotional material. NeuroImage, 30(4),
1458–1466.
Keay, K. A., & Bandler, R. (2001). Parallel cir-
cuits mediating distinct emotional coping
reactions to different types of stress. Neuro-
science and Biobehavioral Reviews, 25(7–8),
669–678.
Knoch, D., Nitsche, M. A., Fischbacher, U.,
Eisenegger, C., Pascual- Leone, A., & Fehr, E.
(2008). Studying the neurobiology of social
interaction with transcranial direct current
stimulation— the example of punishing unfair-
ness. Cerebral Cortex, 18(9), 1987–1990.
Knoch, D., Pascual- Leone, A., Meyer, K., Treyer,
V., & Fehr, E. (2006). Diminishing reciprocal
fairness by disrupting the right prefrontal cor-
tex. Science, 314(5800), 829–832.
Kober, H., Barrett, L. F., Joseph, J., Bliss-
Moreau, E., Lindquist, K., & Wager, T. D.
(2008). Functional grouping and cortical–
subcortical interactions in emotion: A meta-
analysis of neuroimaging studies. NeuroIm-
age, 42(2), 998–1031.
Kober, H., Mende- Siedlecki, P., Kross, E. F.,
Weber, J., Mischel, W., Hart, C. L., et al.
(2010). Prefrontal– striatal pathway underlies
cognitive regulation of craving. Proceedings
of the National Academy of Sciences USA,
107(33), 14811–14816.
Kravitz, D. J., Saleem, K. S., Baker, C. I., &
Mishkin, M. (2011). A new neural framework
for visuospatial processing. Nature Reviews
Neuroscience, 12(4), 217–230.
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R.,
& Eickhoff, S. B. (2010). A link between the
systems: Functional differentiation and inte-
gration within the human insula revealed by
meta- analysis. Brain Structure and Function,
214(5–6), 519–534.
Lane, R. D., Reiman, E. M., Axelrod, B., Yun,
L. S., Holmes, A., & Schwartz, G. E. (1998).
Neural correlates of levels of emotional aware-
ness: Evidence of an interaction between emo-
tion and attention in the anterior cingulate
cortex. Journal of Cognitive Neuroscience,
10(4), 525–535.
LeDoux, J. E. (1993). Emotional memory: In
search of systems and synapses. Annals of the
New York Academy of Sciences, 702, 149–
157.
LeDoux, J. E. (2000). Emotion circuits in the
brain. Annual Review of Neuroscience, 23,
155–184.
LeDoux, J. E., & Gorman, J. M. (2001). A call
to action: Overcoming anxiety through active
coping. American Journal of Psychiatry,
158(12), 1953–1955.
Levenson, R. W. (1999). The intrapersonal func-
tions of emotion [Special issue]. Cognition and
Emotion, 13(5), 481–504.
Leventhal, H. (1984). A perceptual– motor the-
ory of emotion. Advances in Experimental
Social Psychology, 17, 117–182.
Levy, D. J., & Glimcher, P. W. (2011). Compar-
ing apples and oranges: Using reward- specific
40 BIOLOGICAL BASES
and reward- general subjective value represen-
tation in the brain. Journal of Neuroscience,
31(41), 14693–14707.
Lieberman, M. D., Eisenberger, N. I., Crockett,
M. J., Tom, S. M., Pfeifer, J. H., & Way, B.
M. (2007). Putting feelings into words: Affect
labeling disrupts amygdala activity in response
to affective stimuli. Psychological Science,
18(5), 421–428.
Lieberman, M. D., Jarcho, J. M., Berman, S.,
Naliboff, B. D., Suyenobu, B. Y., Mandelkern,
M., et al. (2004). The neural correlates of pla-
cebo effects: A disruption account. NeuroIm-
age, 22(1), 447– 455.
Lieberman, M. D., Jarcho, J. M., & Satpute, A.
B. (2004). Evidence- based and intuition- based
self- knowledge: An fMRI study. Journal of
Personality and Social Psychology, 87(4),
421–435.
Lieberman, M. D., Ochsner, K. N., Gilbert, D.
T., & Schacter, D. L. (2001). Do amnesics
exhibit cognitive dissonance reduction?: The
role of explicit memory and attention in atti-
tude change. Psychological Science, 12(2),
135–140.
Lindquist, K. A., & Barrett, L. F. (2008). Con-
structing emotion: The experience of fear as a
conceptual act. Psychological Science, 19(9),
898–903.
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-
Moreau, E., & Barrett, L. F. (2012). The brain
basis of emotion: A meta- analytic review.
Behavioral and Brain Sciences, 35(3), 121–
143.
Maas, W. (2000). On the computational power
of winner- take-all. Neural Computation,
12(11), 2519–2535.
Mauss, I. B., Levenson, R. W., McCarter, L.,
Wilhelm, F. H., & Gross, J. J. (2005). The tie
that binds?: Coherence among emotion experi-
ence, behavior, and physiology. Emotion, 5(2),
175–190.
McClure, S. M., Ericson, K. M., Laibson, D. I.,
Loewenstein, G., & Cohen, J. D. (2007). Time
discounting for primary rewards. Journal of
Neuroscience, 27(21), 5796–5804.
McClure, S. M., Laibson, D. I., Loewenstein, G.,
& Cohen, J. D. (2004). Separate neural sys-
tems value immediate and delayed monetary
rewards. Science, 306(5695), 503–507.
McRae, K., Hughes, B., Chopra, S., Gabrieli, J.
D., Gross, J. J., & Ochsner, K. N. (2010). The
neural bases of distraction and reappraisal.
Journal of Cognitive Neuroscience, 22(2),
248–262.
Miller, E. K. (2000). The prefrontal cortex and
cognitive control. Nature Reviews Neurosci-
ence, 1(1), 59–65.
Miller, E. K., & Cohen, J. D. (2001). An inte-
grative theory of prefrontal cortex function.
Annual Review of Neuroscience, 24, 167–
202.
Mischel, W., Shoda, Y., & Rodriguez, M. L.
(1989). Delay of gratification in children. Sci-
ence, 244(4907), 933–938.
Mitchell, J. P. (2009). Inferences about men-
tal states. Philosophical Transactions of the
Royal Society of London B: Biological Sci-
ences, 364(1521), 1309–1316.
Mobbs, D., Hassabis, D., Seymour, B., March-
ant, J. L., Weiskopf, N., Dolan, R. J., et al.
(2009). Choking on the money: Reward-based
performance decrements are associated with
midbrain activity. Psychological Science,
20(8), 955–962.
Mobbs, D., Petrovic, P., Marchant, J. L., Has-
sabis, D., Weiskopf, N., Seymour, B., et al.
(2007). When fear is near: Threat imminence
elicits prefrontal– periaqueductal gray shifts in
humans. Science, 317(5841), 1079–1083.
Muhlberger, A., Neumann, R., Wieser, M. J.,
& Pauli, P. (2008). The impact of changes in
spatial distance on emotional responses. Emo-
tion, 8(2), 192–198.
Murray, E. A., O’Doherty, J. P., & Schoenbaum,
G. (2007). What we know and do not know
about the functions of the orbitofrontal cortex
after 20 years of cross- species studies. Journal
of Neuroscience, 27(31), 8166–8169.
Neely, J. H. (1991). Semantic priming effects in
visual word recognition: A selective review of
current findings and theories. In D. Besner &
G. Humphreys (Eds.), Basic processes in read-
ing: Visual word recognition (pp. 264–336).
Hillsdale, NJ: Erlbaum.
Ochsner, K. N., & Barrett, L. F. (2001). A mul-
tiprocess perspective on the neuroscience of
emotion. In T. J. Mayne & G. A. Bonanno
(Eds.), Emotions: Currrent issues and future
directions (pp. 38–81). New York: Guilford
Press.
Ochsner, K. N., & Gross, J. J. (2005). The cogni-
tive control of emotion. Trends in Cognitive
Sciences, 9(5), 242–249.
Ochsner, K. N., & Gross, J. J. (2008). Cogni-
tive emotion regulation: Insights from social
cognitive and affective neuroscience. Currents
Directions in Psychological Science, 17(1),
153–158.
Ochsner, K. N., Hughes, B., Robertson, E. R.,
Valuation and Emotion Regulation 41
Cooper, J. C., & Gabrieli, J. D. (2009). Neural
systems supporting the control of affective and
cognitive conflicts. Journal of Cognitive Neu-
roscience, 21(9), 1842–1855.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D. E.,
et al. (2004). For better or for worse: Neural
systems supporting the cognitive down- and
up- regulation of negative emotion. NeuroIm-
age, 23(2), 483– 499.
Ochsner, K. N., Ray, R. R., Hughes, B., McRae,
K., Cooper, J. C., Weber, J., et al. (2009).
Bottom-up and top-down processes in emo-
tion generation: Common and distinct neural
mechanisms. Psychological Science, 20(11),
1322–1331.
Ochsner, K. N., Silvers, J. A., & Buhle, J. T.
(2012). Functional imaging studies of emo-
tion regulation: A synthetic review and evolv-
ing model of the cognitive control of emotion.
Annals of the New York Academy of Sciences,
1251, E1–E24.
Olsson, A., & Ochsner, K. N. (2008). The role of
social cognition in emotion. Trends in Cogni-
tive Sciences, 12(2), 65–71.
Ongur, D., Ferry, A. T., & Price, J. L. (2003).
Architectonic subdivision of the human orbital
and medial prefrontal cortex. Journal of Com-
parative Neurology, 460(3), 425–449.
Ortony, A., Clore, G. L., & Collins, A. (1988).
The cognitive structure of emotions. New
York: Cambridge University Press.
Packard, M. G. (2009). Anxiety, cognition, and
habit: A multiple memory systems perspective.
Brain Research, 1293, 121–128.
Pessoa, L. (2008). On the relationship between
emotion and cognition. Nature Reviews Neu-
roscience, 9(2), 148–158.
Pessoa, L. (2009). How do emotion and motiva-
tion direct executive control? Trends in Cogni-
tive Sciences, 13(4), 160–166.
Pessoa, L., Kastner, S., & Ungerleider, L. G.
(2003). Neuroimaging studies of attention:
From modulation of sensory processing to
top-down control. Journal of Neuroscience,
23(10), 3990–3998.
Phelps, E. A. (2006). Emotion and cognition:
Insights from studies of the human amygdala.
Annual Review of Psychology, 57, 27–53.
Phillips, M. L., Ladouceur, C. D., & Drevets, W.
C. (2008). A neural model of voluntary and
automatic emotion regulation: Implications
for understanding the pathophysiology and
neurodevelopment of bipolar disorder. Molec-
ular Psychiatry, 13(9), 829, 833–857.
Ploghaus, A., Becerra, L., Borras, C., & Bor-
sook, D. (2003). Neural circuitry underly-
ing pain modulation: Expectation, hypnosis,
placebo. Trends in Cognitive Sciences, 7(5),
197–200.
Polk, T. A., Drake, R. M., Jonides, J. J., Smith, M.
R., & Smith, E. E. (2008). Attention enhances
the neural processing of relevant features and
suppresses the processing of irrelevant features
in humans: A functional magnetic resonance
imaging study of the Stroop task. Journal of
Neuroscience, 28(51), 13786–13792.
Price, J. L. (1999). Prefrontal cortical networks
related to visceral function and mood. Annals
of the New York Academy of Sciences, 877,
383–396.
Quirk, G. J., & Beer, J. S. (2006). Prefron-
tal involvement in the regulation of emo-
tion: Convergence of rat and human studies.
Curentr Opinion in Neurobiology, 16(6),
723–727.
Rangel, A., Camerer, C., & Montague, P. R.
(2008). A framework for studying the neurobi-
ology of value-based decision making. Nature
Reviews Neuroscience, 9(7), 545–556.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79(3), 410–424.
Rolls, E. T. (1999). The brain and emotion.
Oxford, UK: Oxford University Press.
Russell, J. A., & Barrett, L. F. (1999). Core
affect, prototypical emotional episodes, and
other things called emotion: Dissecting the
elephant. Journal of Personality and Social
Psychology, 76(5), 805–819.
Saxe, R. (2006). Uniquely human social cog-
nition. Current Opinion in Neurobiology,
16(2), 235–239.
Scherer, K. R. (2001). Appraisal considered as a
process of multilevel sequential checking. In K.
R. Scherer & A. Schorr (Eds.), Appraisal pro-
cesses in emotion: Theory, methods, research
(pp. 92–120). New York: Oxford University
Press.
Scherer, K. R., Schorr, A., & Johnstone, T.
(Eds.). (2001). Appraisal processes in emo-
tion: Theory, methods, research. New York:
Oxford University Press.
Schoenbaum, G., Saddoris, M. P., & Stalnaker,
T. A. (2007). Reconciling the roles of orbi-
tofrontal cortex in reversal learning and the
encoding of outcome expectancies. Annals
of the New York Academy of Sciences, 1121,
320–335.
42 BIOLOGICAL BASES
Schultz, W., Dayan, P., & Montague, P. R.
(1997). A neural substrate of prediction and
reward. Science, 275(5306), 1593–1599.
Sharot, T., De Martino, B., & Dolan, R. J.
(2009). How choice reveals and shapes
expected hedonic outcome. Journal of Neuro-
science, 29(12), 3760–3765.
Sharot, T., Shiner, T., & Dolan, R. J. (2010).
Experience and choice shape expected aversive
outcomes. Journal of Neuroscience, 30(27),
9209–9215.
Thompson- Schill, S. L., Bedny, M., & Goldberg,
R. F. (2005). The frontal lobes and the regu-
lation of mental activity. Current Opinion in
Neurobiology, 15(2), 219–224.
Tracey, I., Ploghaus, A., Gati, J. S., Clare, S.,
Smith, S., Menon, R. S., et al. (2002). Imaging
attentional modulation of pain in the periaq-
ueductal gray in humans. Journal of Neurosci-
ence, 22(7), 2748–2752.
Urry, H. L., van Reekum, C. M., Johnstone, T.,
Kalin, N. H., Thurow, M. E., Schaefer, H.
S., et al. (2006). Amygdala and ventromedial
prefrontal cortex are inversely coupled during
regulation of negative affect and predict the
diurnal pattern of cortisol secretion among
older adults. Journal of Neuroscience, 26(16),
4415–4425.
van Veen, V., Krug, M. K., Schooler, J. W., &
Carter, C. S. (2009). Neural activity pre-
dicts attitude change in cognitive dissonance.
Nature Neuroscience, 12(11), 1469–1474.
Wager, T. D. (2005). The neural bases of placebo
effects in pain. Current Directions in Psycho-
logical Science, 14(4), 175–179.
Wager, T. D., Atlas, L. Y., Leotti, L. A., & Rill-
ing, J. K. (2011). Predicting individual differ-
ences in placebo analgesia: Contributions of
brain activity during anticipation and pain
experience. Journal of Neuroscience, 31(2),
439–452.
Wager, T. D., Barrett, L. F., Bliss- Moreau, E.,
Lindquist, K., Duncan, S., Kober, H., et al.
(2008). The neuroimaging of emotion. In M.
Lewis, J. M. Haviland- Jones, & L. F. Barrett
(Eds.), The handbook of emotion (3rd ed.,
pp. 249–271). New York: Guilford Press.
Wager, T. D., Davidson, M. L., Hughes, B. L.,
Lindquist, M. A., & Ochsner, K. N. (2008).
Prefrontal– subcortical pathways mediating
successful emotion regulation. Neuron, 59(6),
1037–1050.
Wager, T. D., Jonides, J., & Reading, S. (2004).
Neuroimaging studies of shifting attention:
A meta- analysis. NeuroImage, 22(4), 1679–
1693.
Wager, T. D., & Smith, E. E. (2003). Neuroim-
aging studies of working memory: A meta-
analysis. Cognitive, Affective, and Behavioral
Neuroscience, 3(4), 255–274.
Williams, L. E., & Bargh, J. A. (2008). Keeping
one’s distance: The influence of spatial dis-
tance cues on affect and evaluation. Psycho-
logical Science, 19(3), 302–308.
Willis, W. D., & Westlund, K. N. (1997). Neu-
roanatomy of the pain system and of the path-
ways that modulate pain. Journal of Clinical
Neurophysiology, 14(1), 2–31.
Wunderlich, K., Rangel, A., & O’Doherty, J.
P. (2009). Neural computations underlying
action- based decision making in the human
brain. Proceedings of the National Academy
of Sciences USA, 106(40), 17199–17204.
Young, L., Camprodon, J. A., Hauser, M.,
Pascual- Leone, A., & Saxe, R. (2010). Disrup-
tion of the right temporoparietal junction with
transcranial magnetic stimulation reduces the
role of beliefs in moral judgments. Proceedings
of the National Academy of Sciences USA,
107(15), 6753–6758.
Zaki, J., Davis, J. I., & Ochsner, K. N. (2012).
Overlapping activity in anterior insula during
interoception and emotional experience. Neu-
roImage, 62(1), 493–499.
Zysset, S., Huber, O., Ferstl, E., & von Cramon,
D. Y. (2002). The anterior frontomedian cor-
tex and evaluative judgment: An fMRI study.
NeuroImage, 15(4), 983–991.
Zysset, S., Huber, O., Samson, A., Ferstl, E. C.,
& von Cramon, D. Y. (2003). Functional spe-
cialization within the anterior medial prefron-
tal cortex: A functional magnetic resonance
imaging study with human subjects. Neurosci-
ence Letters, 335(3), 183–186.

43
Gross and colleagues have framed emotion
generation and regulation as a recursive
process involving attention and appraisal,
whereby an emotional response unfolds over
time as a function of both how attention is
allocated to an emotion- eliciting stimulus
or situation and the interpretation of (or
meaning attributed to) the stimulus or situ-
ation (see Gross, this volume). Based on this
model, both attentional deployment and
reappraisal are increasingly studied strate-
gies for emotion regulation— and both strat-
egies are effective means of altering emotion.
Functional magnetic resonance imaging
(fMRI) measures have been used in the con-
text of emotion regulation studies, provid-
ing a portrait of neural regions that are more
active during emotion regulation than con-
trol conditions— and these data suggest that
areas of the prefrontal cortex become more
active, and amygdala activity is decreased,
when emotion is down- regulated (see Och-
sner & Gross, this volume). Though we are
coming to know the circuitry of emotion
regulation, this is a static portrait: a fine-
grained but frozen map representing “more
activation here” and “less activation there.”
To be sure, more advanced analytic tech-
niques can augment traditional analyses of
fMRI data to indicate the temporal sequenc-
ing of these effects: Presumably, increases
in frontal activation precede decreases in
amygdala activity.
This chapter focuses on a different neu-
roimaging method that complements the
excellent spatial resolution of fMRI: We
review research that uses neural activity in
the electroencephalogram (EEG) to study
mechanisms and the time course of emo-
tion regulation. We believe that investiga-
tions of emotion regulation benefit enor-
mously from measures that are temporally
precise and can track processes unfolding—
and changing— over time. Much of our
work has focused on event- related poten-
tials (ERPs) derived from the EEG. ERPs
reflect the near- instantaneous activity of
underlying neuronal populations and are
well- suited for tracking rapidly changing
brain activity. For instance, ERPs can index
emotional reactivity and its regulation from
one second to the next—and can be used
to separately quantify reactivity and regu-
lation. Moreover, ERPs are relatively inex-
pensive and well- tolerated neural measures.
In this chapter, we focus mainly on the late
positive potential (LPP), an ERP compo-
nent that reflects the flexible and dynamic
deployment of motivated attention, and as
such represents an ideal vehicle for investi-
gating iterative and interactive processes as
they unfold over time.
CHAPTER 3
Temporal Dynamics of Emotion Regulation
Greg Hajcak Proudfit
Jonathan P. Dunning
Daniel Foti
Anna Weinberg
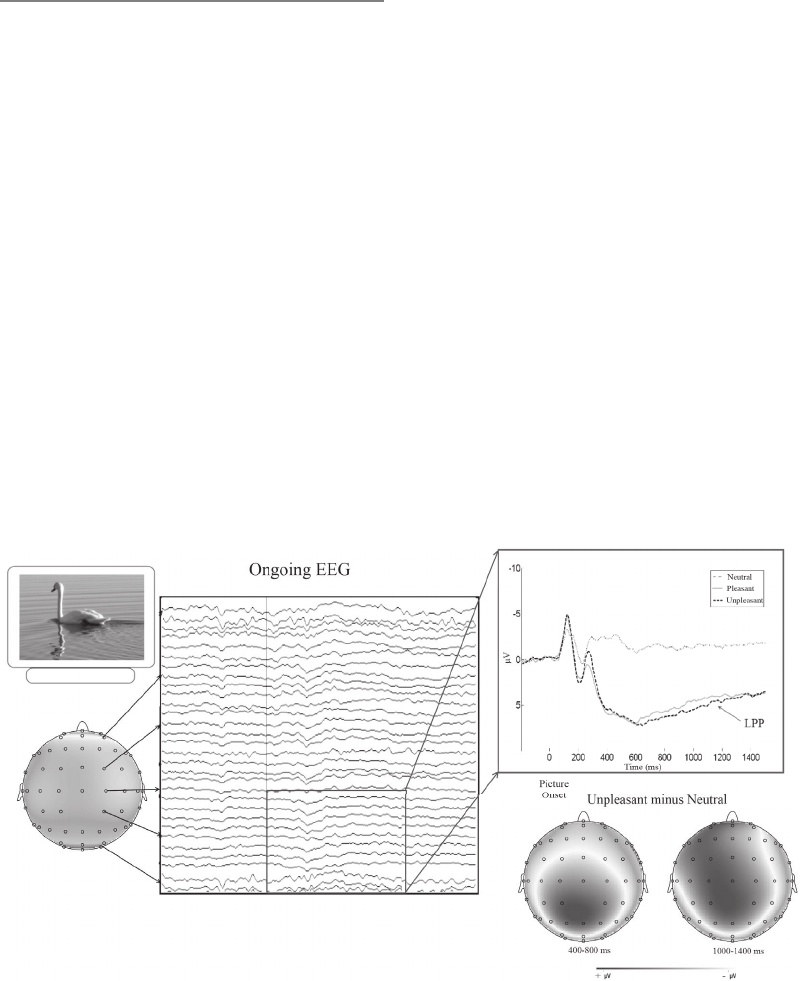
44 BIOLOGICAL BASES
ERPs and the LPP
What Is an ERP?
ERPs are a direct measure of neural activ-
ity. Scalp- recorded ERPs reflect the summed
activity of excitatory and inhibitory post-
synaptic potentials (PSPs) generated by large
populations of pyramidal cortical neurons
aligned perpendicular to the cortical sur-
face. Because these neurons are oriented in
the same direction, simultaneously occur-
ring or closely occurring signals typically
do not cancel each other out; moreover,
because PSPs tend to be rather sustained in
nature (compared to, for example, action
potentials), it is possible for this activity to
summate and propagate to the surface of the
scalp (Luck, 2005). This summated activity
is what is recorded in the ongoing EEG (see
Figure 3.1). ERPs then reflect the activity
of the EEG, time- locked to specific events
and averaged across many trials to increase
signal and reduce noise. In the studies we
discuss, the event of interest is typically the
presentation of a visual stimulus, frequently
images from the International Affective
Picture System (IAPS; Lang, Bradley, &
Cuthbert, 2008), a standardized database
containing hundreds of pictures that have
been normed on ratings of hedonic valence
and arousal. Neural activity elicited by emo-
tional compared to neutral pictures, and as a
function of emotion regulation instructions,
can then be averaged and compared within
and between subjects.
The LPP
The LPP is a sustained positive- going ERP
with a central– parietal scalp distribution;
the amplitude of the LPP is modulated by
emotional content, becoming more positive
as early as 200 milliseconds following the
presentation of both pleasant and unpleas-
ant compared to neutral stimuli (see Figure
3.1; Foti, Hajcak, & Dien, 2009; Hajcak
& Olvet, 2008; Hajcak, Weinberg, Mac-
Namara, & Foti, 2011). The LPP is larger
FIGURE 3.1. A participant views images as the ongoing electroencephalogram (EEG) is recorded via
sensors affixed to the scalp (left). Event-related potentials (ERPs) reflect the activity of the EEG time-
locked to an event of interest, in this case, the onset of an image. On the right, the LPP is presented as a
positive-going deflection in the waveform, which is enhanced for both pleasant and unpleasant images
compared to neutral (data from Weinberg & Hajcak, 2010). Additionally, while the early portion of
the LPP (400–800 milliseconds) has a parietal distribution—represented here by the positive difference
between unpleasant and neutral images, as indicated by the dark area on the scalp—the later LPP (after
1,000 milliseconds) has a broader and more frontal distribution.
Temporal Dynamics of Emotion Regulation 45
following the presentation of emotional
images (Cuthbert, Schupp, Bradley, Birbau-
mer, & Lang, 2000; Foti et al., 2009),
words (Kissler, Herbert, Winkler, & Jung-
hofer, 2009; Tacikowski & Nowicka, 2010),
and even hand gestures (Flaisch, Häcker,
Renner, & Schupp, 2011).
It has been argued that the LPP reflects
sustained attention to visual stimuli (Haj-
cak, MacNamara, & Olvet, 2010; Hajcak
& Olvet, 2008; Hajcak et al., 2011; Wein-
berg & Hajcak, 2011b). Furthermore, there
may be functional differences between
early compared to late portions of the LPP,
highlighting the utility of this component
in tracking multiple processes over time.
Relatively early portions of the LPP (i.e.,
300–600 milliseconds) appear to reflect
more obligatory processing of emotional
stimuli, whereas later time windows (i.e.,
600 milliseconds and beyond) might reflect
more sustained and elaborative engagement
with stimuli (Olofsson, Nordin, Sequeira, &
Polich, 2008; Weinberg & Hajcak, 2011b;
Weinberg, Hilgard, Bartholow, & Hajcak,
2012). For instance, in one study, larger LPPs
in response to task- irrelevant pictures pre-
dicted greater behavioral interference— in
both within- and between- subjects analyses
(Weinberg & Hajcak, 2011b). Moreover,
though the early LPP was modulated by
emotional stimuli, it was the later portion
of the LPP (i.e., just preceding target onset)
that uniquely predicted behavioral interfer-
ence: Only increased sustained attention just
prior to target presentation related to slower
reaction times.
During passive picture- viewing para-
digms, the emotional modulation of the LPP
is sustained throughout stimulus presenta-
tion (i.e., up to several seconds; Hajcak et
al., 2010), and can even be observed for as
long as 1,000 milliseconds after stimulus
offset. These data are consistent with the
notion that emotional stimuli not only cap-
ture but also hold attention (Vuilleumier,
2005)—and we have suggested that the LPP
is sensitive to the continued allocation of
attention to emotional stimuli. Consistent
with this view, it is possible to manipulate
the duration of the LPP by instructions
that encourage maintainance of previously
viewed images in working memory, again
suggesting that this later component may
reflect more volitional engagement with
emotional stimuli (Hajcak et al., 2010; Thi-
ruchselvam, Hajcak, & Gross, 2012).
The modulation of the LPP is thought to
reflect the motivational salience of the stim-
ulus being viewed. In the case of emotional
stimuli, salience is thought to be determined
by the content of the stimuli— and how rele-
vant the content is to basic biological imper-
atives (e.g., survival themes that include
defense and reproduction). Indeed, evidence
suggests that the LPP is most enhanced by
image content more directly related to these
imperatives, such as images of mutilation
and gore, or erotic images (Weinberg &
Hajcak, 2010). Additionally, food depriva-
tion compared to satiated states enhances
the magnitude of the LPP elicited by images
of food—a motivationally salient category
of images when one is hungry (Stock-
burger, Schmälzle, Flaisch, Bublatzky, &
Schupp, 2009). The LPP is also enhanced
by personally relevant stimuli (e.g., photo-
graphs of relatives, or one’s own name and
face; Grasso & Simons, 2010; Tacikowski
& Nowicka, 2010) compared to even very
familiar famous faces.
There is also evidence that extrinsic
manipulations of salience can impact the
LPP. It is possible to designate specific stim-
uli as “targets” by asking participants to
count or otherwise respond to them when
they are presented among other nontarget
stimuli. The LPP is enhanced for arbitrarily
designated target stimuli compared to non-
target stimuli (Ferrari, Bradley, Codispoti,
& Lang, 2010; Weinberg et al., 2012), even
when the same images serve as both targets
and nontargets (Weinberg et al., 2012). The
LPP is largest when emotional images are
also targets, compared to both neutral tar-
gets and emotional nontargets (Ferrari et al.,
2010; Weinberg et al., 2012). This suggests
that intrinsic and extrinsic manipulations of
salience are additive in their impact on the
magnitude of the LPP.
Work that combines EEG and fMRI sug-
gests that the LPP reflects widespread and
concurrent activity across the visual system
(Sabatinelli, Keil, Frank, & Lang, 2013),
including the visual cortex (Bradley et al.,
2003; Keil et al., 2002) as well as occipital,
parietal, and inferotemporal regions of the
brain (Sabatinelli, Lang, Keil, & Bradley,
2007). Rather than the activation of a sin-
gle anatomical node, it is possible that the
46 BIOLOGICAL BASES
LPP reflects fairly widespread neuromodu-
latory activity (de Rover et al., 2012; Haj-
cak et al., 2010), or communication among
diverse brain regions. This latter possibility
is supported by a recent study indicating
that the magnetic equivalent of the LPP was
generated in occipitoparietal and prefrontal
cortices— and that the emotional modula-
tion of the LPP might index the coordina-
tion of frontoparietal attention networks
(Moratti, Saugar, & Strange, 2011).
The involvement of frontal areas in the
generation of the LPP is consistent with stud-
ies indicating that both physical stimulation
(Hajcak, Anderson, et al., 2010) and func-
tional activation (based on working memory
load; MacNamara, Ferri, & Hajcak, 2011)
of the dorsolateral prefrontal cortex reduces
the amplitude of the LPP. Moreover, fron-
tal involvement in the LPP is also consistent
with our observation that the scalp distri-
bution of the emotional modulation of the
LPP during passive picture viewing becomes
evident at central and even frontal recording
sites after approximately 1,000 milliseconds
(see Figure 3.1; Foti et al., 2009; MacNa-
mara, Foti, & Hajcak, 2009).
A substantial literature indicates that the
LPP is a robust measure of neural activity
associated with emotional processing. In
contrast to the majority of peripheral and
central measures that are sensitive to emo-
tional stimuli, emotional modulation of the
LPP is highly stable. Whereas skin conduc-
tance, heart rate, facial muscle activity, and
neural activation indexed by fMRI habitu-
ate over repeated presentations of stimuli
(Codispoti & De Cesarei, 2007; Codispoti,
Ferrari, & Bradley, 2006; Phan, Liberzon,
Welsh, Britton, & Taylor, 2003), emotional
modulation of the LPP does not (Codispoti
et al., 2006; Codispoti, Ferrari, & Bradley,
2007; Olofsson & Polich, 2007).
The LPP and Individual Differences
The LPP has proven to be an effective
measure of attentional allocation to emo-
tional stimuli across multiple populations.
For example, enhancement of LPP ampli-
tude by emotional content appears cross-
culturally (Hot, Saito, Mandai, Kobayashi,
& Sequeira, 2006; Yen, Chen, & Liu, 2010).
The LPP has also been applied to the study
of emotional processing across the lifespan;
affective modulation of the LPP has been
observed in both very young children (Den-
nis & Hajcak, 2009; Kujawa, Hajcak, Tor-
pey, Kim, & Klein, 2012) and in adults as
old as 81 (Kisley, Wood, & Burrows, 2007;
Langeslag & Van Strien, 2010). Further-
more, the LPP has been used to test theo-
ries of aging, which posit that younger com-
pared to older participants are more reactive
to unpleasant stimuli, and less reactive to
pleasant stimuli (Kisley et al., 2007; Lang-
eslag & Van Strien, 2010).
Additionally, the LPP has been used effec-
tively to characterize the abnormal process-
ing of affective stimuli in anxiety, mood, and
substance use disorders (e.g., Dunning et al.,
2011; Foti, Olvet, Klein, & Hajcak, 2010;
Weinberg & Hajcak, 2011a). For instance,
consistent with the notion that depression
is characterized by motivational disengage-
ment from the environment, depressed indi-
viduals tend to show decreased emotional
modulation of the LPP compared to healthy
controls (Foti et al., 2010). Likewise, while
cocaine use disorders are associated with a
decreased LPP in response to normative emo-
tional stimuli, individuals with this diagno-
sis also show an increased LPP in response
to cocaine- related images compared to
healthy controls (Dunning et al., 2011). The
relationship of the LPP to anxiety appears
to be somewhat context- dependent. For
instance, anxiety has been associated with
an increased LPP when pictures are task-
irrelevant (MacNamara, Ferri, et al., 2011),
suggesting deficits in disengaging attention
from emotional stimuli. However, there is
also evidence from passive- viewing tasks—
in which the pictures are task- relevant— that
anxiety is instead characterized by initial
vigilance for threat images, as indicated by
enhancement of early ERP components, fol-
lowed by a failure to engage in sustained
processing of these images, as indicated by
an attenuation of the LPP (Weinberg & Haj-
cak, 2011a). These data indicate that the
LPP is a useful tool for quantifying abnor-
malities in the temporal dynamics of emo-
tional processing across different forms of
psychopathology. Below, we review findings
that have extended this literature to emotion
regulation, focusing in particular on atten-
tional deployment and reappraisal.
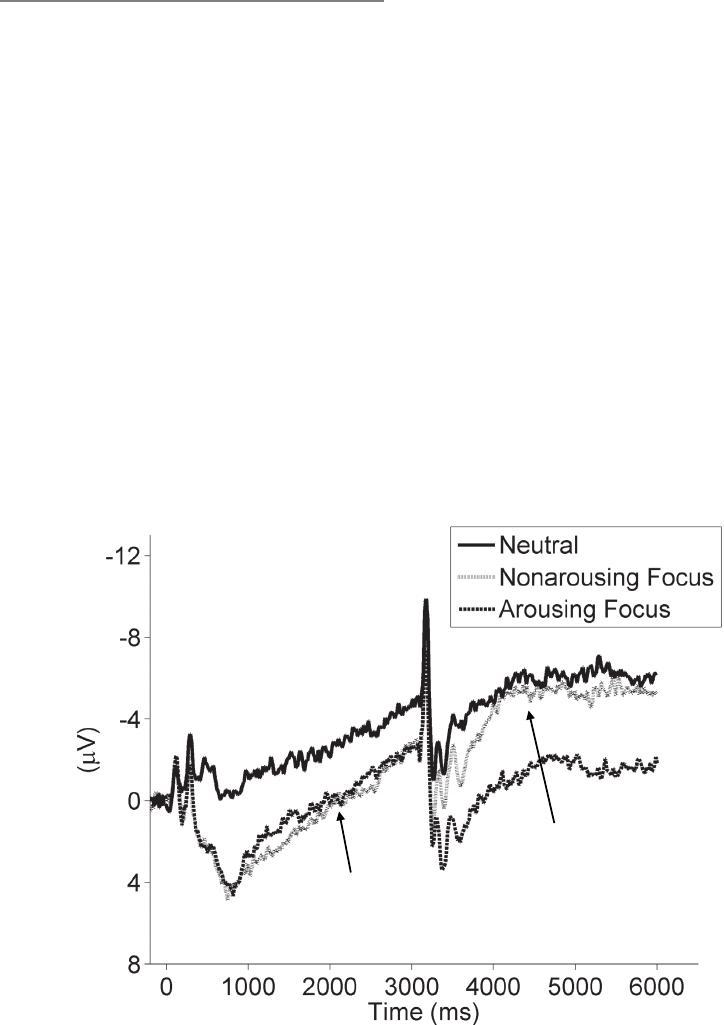
Temporal Dynamics of Emotion Regulation 47
The LPP and Emotion Regulation
Directed Attention
An emotional response to a stimulus unfolds
over time as a function of multiple pro-
cesses, including the allocation of attention
to the content of the stimulus, as well as the
meaning that is assigned to that stimulus
(Sheppes & Gross, 2011). With regard to
emotion regulation, distraction and other
attentional deployment strategies alter the
emotional response by directing attention
away from affective content. Spatial atten-
tion, in particular, plays a large role in
the electrocortical response to emotional
images (Eimer, Holmes, & McGlone, 2003;
Holmes, Vuilleumier, & Eimer, 2003; Keil,
Moratti, Sabatinelli, Bradley, & Lang, 2005)
and, consequently, emotion regulation pro-
cesses. Indeed, a growing body of work
demonstrates that directing attention, either
implicitly or explicitly, away from emotion-
ally salient features of a stimulus is an effec-
tive method of down- regulating emotional
response.
Online Regulation
Attempts to regulate an emotional response
after a stimulus has been encountered are
considered “online” regulation. Recent
work has examined whether the amplitude
of the LPP can be manipulated by explic-
itly directing participants’ visual attention
to various areas within unpleasant images.
This approach is considered online regula-
tion insofar as visual attention is manipu-
lated during the generation of the emotional
response. In one study, participants were
shown an unpleasant picture for 6 sec-
onds; after passively viewing the image for
3 seconds, participants’ attention was then
directed to either an arousing or nonarous-
ing area of the picture for the last 3 seconds
of each trial. As indicated in Figure 3.2,
directing attention to nonarousing com-
FIGURE 3.2. Grand averaged ERPs at electrode site Pz elicited by neutral pictures (solid black line;
always associated with an instruction to focus on a nonarousing area of the image) and unpleasant pic-
tures (associated with an instruction to attend to either a nonarousing [dotted line] or arousing [dashed
line area of the image]). Picture onset occurred at 0 milliseconds and the instruction tone occurred at
3,000 milliseconds. The figure is based on data collected by Hajcak, Dunning, and Foti (2009).
LPP to unpleasant
pictures during
passive viewing
Reduction in LPP when
focusing on nonarousing
areas of unpleasant
pictures
48 BIOLOGICAL BASES
pared to highly arousing areas of unpleasant
images resulted in a decreased LPP (Dun-
ning & Hajcak, 2009). This finding was
subsequently replicated with a focus on the
time course of LPP modulation: Sequential
significance testing indicated that reduction
of the LPP occurred 620 milliseconds after
signaling participants to attend to nonar-
ousing areas of unpleasant images (Hajcak,
Dunning, & Foti, 2009).
Directed attention also appears to impact
the emotional response to representations
in working memory during a post- stimulus
period. Thiruchselvam and colleagues
(2012) had participants view unpleasant and
neutral pictures, then maintain representa-
tions of each image in working memory
after picture offset. Participants were then
instructed to focus on arousing or nonarous-
ing aspects of their mental representation.
Results showed that focusing on nonarous-
ing compared to arousing aspects of pic-
tures held in working memory was related
to a reduction in both the LPP and self-
reported ratings of unpleasantness. These
results suggest that the emotional response
can be modulated, and indexed by the LPP,
even after images have been fully encoded in
working memory.
Convergence of Directed Attention
Findings across Neural Measures
This same pattern of LPP modulation via
directed spatial attention has also been rep-
licated and extended to steady- state visual
evoked potentials (ssVEP; Hajcak, MacNa-
mara, Foti, Ferri, & Keil, 2013). The ssVEP
can be used to index visual processing of
stimuli flickering at a particular frequency. It
is not an ERP component, but rather a mea-
sure of the degree to which the EEG signal
over occipital cortex is being driven at the
flicker frequency. For instance, suppose two
faces are simultaneously presented to the left
and right of fixation, flickered at 8 and 18
Hz, respectively. The ssVEP would be charac-
terized by increased activity at both frequen-
cies, which is typically maximal at occipital
electrodes. Furthermore, if participants were
instructed to attend to the left face, power at
8 Hz in the ssVEP would be increased (Hill-
yard et al., 1997). Related to this chapter, the
ssVEP is larger for emotional compared to
neutral flickering stimuli, and modulation of
this activity is linked to facilitated process-
ing in the visual cortex (Keil et al., 2003).
In a study combining the LPP and ssVEP
within a single paradigm, directing attention
to nonarousing areas of unpleasant pictures
reduced both of these electrocortical indices,
indicating that each neural measure tracks
the effective down- regulation of the emo-
tional response via attentional deployment
(Hajcak et al., 2013). Moreover, modula-
tions of the LPP and ssVEP were unrelated,
suggesting that these measures provide dis-
tinct sources of information regarding emo-
tional processing.
Cognitive Reappraisal
Unlike distraction and other attentional
deployment techniques, where attention is
directed away from the emotional content
of a stimulus, reappraisal involves attend-
ing directly to emotional content and alter-
ing the emotional response by reinterpret-
ing the meaning of the stimulus (Sheppes &
Gross, 2011). In this way, emotion regula-
tion is achieved while engaging directly with
emotionally arousing aspects of stimuli, an
approach that has been shown to be effec-
tive for reducing both subjective and periph-
eral physiological indicators of emotional
arousal (Urry, 2010). Of interest is how LPP
amplitude may complement these data as a
neurobiological index of effective emotion
regulation, clarifying the impact of cognitive
reappraisal on the time course of emotional
processing.
According to the process- specific tim-
ing hypothesis, online emotion regulation
likely requires significant effort (i.e., cogni-
tive resources) in order to modify existing
and incoming emotional information— and
that effort would be directly proportional
to the strength and intensity of the emotion
being experienced (Sheppes & Gross, 2011).
On the other hand, several studies have
presented emotion regulation instructions
to participants prior to the presentation of
emotional stimuli. In this way, regulation
goals might be established while emotional
intensity is low, in anticipation of the emo-
tional response to come. Anticipatory regu-
lation ought to impact the initial processing
of an affective stimulus, indicated by the
Temporal Dynamics of Emotion Regulation 49
affective modulation of the LPP elicited by
the first presentation of the stimulus.
In one of the first investigations of cogni-
tive reappraisal using the LPP, Hajcak and
Nieuwenhuis (2006) had participants view
a series of unpleasant IAPS images for 1
second; an instruction to “reinterpret” (i.e.,
reappraise the picture in order to reduce
one’s negative response) or “attend” (i.e.,
focus on one’s natural feelings about the
picture) was then presented for 4,500 mil-
liseconds, after which the same picture was
again presented for 2,000 milliseconds.
Compared to unpleasant pictures presented
after the attend instructions, those presented
after reappraisal instructions were associ-
ated with a reduced LPP, beginning 200 mil-
liseconds after picture onset—an effect that
lasted for the duration of picture presenta-
tion. Furthermore, greater reduction in the
LPP was related to greater reduction in self-
reported arousal ratings of the images. This
study was the first to demonstrate that the
magnitude of the LPP could be modulated
by cognitive reappraisal, down- regulating
the LPP by reinterpreting the meaning of
emotional stimuli.
Anticipatory Regulation
In the Hajcak and Nieuwenhuis (2006)
study, regulation instructions were pre-
sented after the first encounter with the
emotional stimulus, indicating that the LPP
is sensitive to cognitive reappraisal when it
is done online. Separate from this study, and
consistent with the notion of anticipatory
regulation, experimental manipulations that
vary how participants attend to affective
stimuli during the initial presentation have
also been shown to modulate the LPP. For
example, Hajcak, Moser, and Simons (2006)
had participants view pleasant and unpleas-
ant pictures, and either categorize the pic-
tures affectively (i.e., indicating whether
the picture was pleasant or unpleasant) or
nonaffectively (i.e., indicating how many
people were present in the picture). Results
indicated that the LPP elicited by both pleas-
ant and unpleasant pictures was reduced
when participants evaluated images— and,
presumably, interpreted the meaning of the
images— along a nonaffective compared to
an affective dimension.
In three additional studies, the impact of
cognitive reappraisal within an anticipatory
emotion regulation context was examined,
testing whether alteration of the manner
in which participants appraise stimuli dur-
ing the initial presentation would modulate
LPP amplitude. Participants were instructed
to use effortful cognitive reappraisal of
emotional scenes (Krompinger, Moser,
& Simons, 2008; Moser, Hajcak, Bukay,
& Simons, 2006) and angry faces (Blech-
ert, Sheppes, Di Tella, Williams, & Gross,
2012). Prior to viewing each stimulus, par-
ticipants were instructed to decrease the
intensity of their emotional response by rein-
terpreting the image. Compared to a passive
viewing condition, the LPP elicited by pleas-
ant and unpleasant images was blunted for
the reappraisal condition at an early latency
range, beginning approximately 300–600
milliseconds following picture presentation.
Together, these lines of research demonstrate
that LPP amplitude tracks the successful
down- regulation of an emotional response
using cognitive reappraisal, whether reap-
praisal is enacted online or in anticipation of
an affective stimulus.
Building on these lines of research, Thi-
ruchselvam, Blechert, Sheppes, Rydstrom,
and Gross (2011) compared the effects of
different anticipatory regulation strategies
on LPP amplitude at both the initial pre-
sentation of stimuli and a later reexposure.
Whereas the aforementioned studies com-
pared reappraisal to a passive viewing con-
dition, here the impact of reappraisal on the
temporal dynamics of LPP amplitude was
also contrasted with the impact of distrac-
tion instructions. Reappraisal was achieved
by instructing participants to reinterpret
affective stimuli in a more neutral man-
ner. Distraction, on the other hand, was
achieved by asking participants to generate
neutral thoughts unrelated to the stimulus,
such as imagining complex geometric pat-
terns. During a baseline session, the LPP in
response to unpleasant images was recorded
under conditions of passive viewing, effort-
ful reappraisal, and distraction; 30 minutes
later, participants passively viewed the same
images with no regulation instructions.
Effortful reappraisal, compared to passive
viewing, yielded a blunted LPP only within
a relatively later latency range (i.e., 1,500–
50 BIOLOGICAL BASES
1,700 milliseconds). The effect of distrac-
tion was stronger and apparent earlier, with
a significant and sustained reduction in LPP
amplitude as early as 300 milliseconds. A
very different pattern emerged during the
reexposure session: Images that had previ-
ously been processed under the reappraisal
condition again yielded a blunted LPP com-
pared to the passive viewing condition, from
800 to 1,400 milliseconds. Images that had
previously been processed under the dis-
traction condition, however, yielded a sus-
tained increase in LPP amplitude compared
to the passive viewing condition, beginning
at approximately 1,200 milliseconds. This
study highlights the fact that emotion reg-
ulation strategies likely have a differential
impact on initial compared to subsequent
encounters with emotional stimuli. Unlike
reappraisal, distraction may only be an
effective emotion regulation strategy in the
short-term, reducing LPP amplitude during
the initial stimulus exposure but resulting in
an enhanced LPP during reexposure.
Preappraisal
The previous studies indicate that LPP
amplitude is sensitive to the implementation
of anticipatory emotion regulation through
cognitive reappraisal, but they do not fully
address whether the down- regulation of the
LPP is due to a shift in stimulus appraisal/
meaning per se. An alternate explanation
is that the reduction in LPP amplitude is a
result of reappraisal being more difficult and
more cognitively taxing than passive view-
ing, an important possibility to rule out in
light of evidence that cognitive load results
in a reduced LPP (MacNamara, Ferri, et al.,
2011). To pursue this issue further, several
studies have used a guided version of cog-
nitive reappraisal, in which images are pre-
ceded by verbal descriptions that frame the
upcoming stimuli in either a more negative
or more neutral manner (Foti & Hajcak,
2008; MacNamara et al., 2009). In other
words, reappraisal frames were provided to
participants, a manipulation that might be
best described as preappraisal. For example,
an unpleasant image of a man pointing a
gun at his head might be preceded by either
“This man is about to commit suicide” or
“This man ends up not committing suicide”;
the latter description is designed to down-
regulate the initial emotional response elic-
ited by the image. On each trial, the descrip-
tion precedes the presentation of the image,
thereby shaping the first appraisal of the
stimulus during the initial iteration of the
emotion- generative cycle. Guiding the ini-
tial stimulus appraisal with verbal descrip-
tions removes the potential confound of task
difficulty, such that each condition simply
involves listening to descriptions and then
viewing pictures.
In an initial study using this paradigm with
an adult sample, modulation of LPP ampli-
tude by description type was evident as early
as 400 milliseconds following picture pre-
sentation, with neutral descriptions reducing
the subsequent LPP elicited by unpleasant
images (Foti & Hajcak, 2008). Compared
to neutral images— which in this study were
always preceded by a neutral description—
the LPP in response to unpleasant images
preceded by unpleasant descriptions was
increased throughout stimulus presentation,
from 400 to 3,000 milliseconds. The LPP in
response to unpleasant images was increased
only from 400 to 1,000 milliseconds when
preceded by neutral descriptions, and to a
lesser extent than when preceded by nega-
tive descriptions. This temporal pattern sug-
gests that unpleasant images preappraised
by a neutral description were associated with
reduced early, obligatory attentional capture
associated with emotion generation; more-
over, the sustained, elaborative processing
that is typically observed for highly arousing
affective stimuli was nearly absent. Unpleas-
ant images preceded by neutral descriptions
were also rated as less unpleasant and less
emotionally arousing than those preceded
by negative descriptions, providing fur-
ther evidence that manipulating the initial
appraisal of the stimuli effectively regulated
the subsequent emotional response.
Because neutral images were always pre-
ceded by neutral descriptions in this initial
study, an important alternative explanation
was that the reduced LPP observed for neu-
trally described unpleasant images was due
to the mismatch between the valence of the
description and the image. To address this
confound in a follow- up study on preap-
praisal, description and image type were
fully crossed: Neutral and negative descrip-
tions preceded both neutral and unpleas-
ant images (MacNamara et al., 2009). For
Temporal Dynamics of Emotion Regulation 51
instance, a neutral image of a drill might be
described as “This drill is used by a repair-
man” or “This drill was used in a grisly mur-
der,” with the latter description designed
to up- regulate the emotional response elic-
ited by the image. As before, the LPP was
reduced in response to unpleasant images
preceded by neutral descriptions compared
to those preceded by negative descriptions.
Importantly, though, the LPP was increased
in response to neutral pictures that were
preceded by negative compared to neutral
descriptions. Framing neutral images in a
more negative manner elicited affective mod-
ulation of the LPP that would not normally
be observed for low- arousal, neutral stimuli.
These data demonstrate that the LPP tracks
the emotionality of the preappraisal and not
the match between description and image. In
fact, at late latencies (>1,500 milliseconds),
affective modulation of LPP amplitude was
apparent only for images preceded by nega-
tive descriptions, regardless of whether the
image itself was unpleasant or neutral. Thus,
the later portion of the LPP was only sensi-
tive to meaning imbued by the preappraisals.
Altering the context in which stimuli are
initially appraised has a robust impact on
LPP amplitude during the first viewing of a
stimulus. A further question is how modify-
ing the initial appraisal may influence sub-
sequent iterations of the emotion- generative
process when the stimuli are encountered
again. To examine this, MacNamara, Och-
sner, and Hajcak (2011) first presented
images paired with negative or neutral
descriptions, then conducted a second view-
ing session 30 minutes later without the pre-
ceding descriptions. During the reexposure
session, images that had previously been pre-
appraised in a more neutral manner elicited
a blunted LPP in a middle latency range (i.e.,
700–1,400 milliseconds), and the images
were rated as being less unpleasant and less
emotionally arousing. This is a less pro-
tracted effect of preappraisal on LPP than
observed previously (Foti & Hajcak, 2008;
MacNamara et al., 2009), suggesting that
preappraisal effects may diminish over time.
However, modifying the initial appraisal of
an affective stimulus— whether by effortful
reappraisal (Thiruchselvam et al., 2011) or
preappraisal (MacNamara, Ochsner, et al.,
2011)—appears to yield down- regulation of
the LPP upon reexposure.
Although the aforementioned studies used
paradigms in which the stimuli were task-
relevant and the primary focus of attention,
modifying the initial stimulus appraisal
also appears to impact the LPP elicited by
task- irrelevant, distracting images. For
instance, during a task in which participants
were required to judge the spatial orienta-
tion of geometric shapes, mutilation and
neutral scenes were presented as distrac-
tors (Mocaiber et al., 2010). These images
were described beforehand as being either
real or fictitious, thereby shaping the initial
appraisal. In the “real” context, unpleasant
images elicited an increased LPP compared
to neutral images in an early latency range
(i.e., 300–600 milliseconds) and were asso-
ciated with significant reaction time slow-
ing, indicating attentional capture that inter-
fered with performance on the primary task.
In the “fictitious” context, however, there
was no affective modulation of the LPP and
no reaction time slowing, indicating that
the distractor images were less salient and
produced less behavioral interference. This
study is consistent with other research link-
ing the LPP to attentional capture and task
interference (Weinberg & Hajcak, 2011b),
and it demonstrates that the LPP elicited by
task- irrelevant stimuli can be modulated by
preappraisal.
Convergence of Cognitive Reappraisal
Findings across Neural Measures
The work reviewed thus far focused on iden-
tifying the consequences of regulation on
the emotion- generative process, as indexed
by electrocortical measures. In other words,
electrocortical measures were used to index
the downstream impact of emotion regu-
lation on neural activity. In addition to
this, it may be possible to measure neural
activation responsible for these regulation
effects. For instance, Parvaz, MacNamara,
Goldstein, and Hajcak (2012) examined
the LPP and alpha bandwidth power while
participants either passively viewed or reap-
praised unpleasant pictures. Consistent with
previous work, the amplitude of the LPP
was reduced when reappraising unpleas-
ant pictures. However, reappraisal was also
associated with increased left prefrontal
cortex activation, as evidenced by reduced
left frontal alpha band power (alpha power
52 BIOLOGICAL BASES
is inversely related to brain activity). LPP
amplitude and alpha power were not corre-
lated in this study, though, suggesting that
both of these measures contributed unique
information about cognitive reappraisal
processes. These data are consistent with
fMRI research indicating that reappraisal
is associated with increased activity in the
ventromedial and lateral prefrontal cor-
tex (Johnstone, van Reekum, Urry, Kalin,
& Davidson, 2007; Ochsner et al., 2004;
Wager, Davidson, Hughes, Lindquist, &
Ochsner, 2008), and suggests that EEG can
be used not only to index the downstream
consequences of emotion regulation but also
the neural activity that may be responsible
for regulatory effects.
Clinical Applications
Researchers have begun to apply the LPP to
the study of emotion regulation in clinical
contexts. For example, the effects of preap-
praisal have been studied in children, with
the purpose of identifying risk factors that
might aid in detection and treatment of emo-
tion regulation difficulties early in life. In
one study of children ages 7–10, the LPP was
reduced for unpleasant images preceded by a
neutral description, compared to unpleasant
images preceded by a negative description
(Dennis & Hajcak, 2009). This modulation
of the LPP by description type was associ-
ated with reduced symptoms of depression
and anxiety, suggesting that modulation of
LPP amplitude may serve as an early marker
for emotion regulation difficulties or mood
disruptions. In younger children ages 5–7,
however, preappraisal does not appear to
modulate LPP amplitude (Decicco, Solo-
mon, & Dennis, 2012; Dennis & Hajcak,
2009), suggesting important developmental
differences that warrant further investiga-
tion.
The LPP has also been used to track
emotion regulation processes related to the
treatment of psychological disorders. For
example, individuals with phobias are char-
acterized by an attentional pattern of vigi-
lance followed by avoidance: They rapidly
detect and attend to phobic objects initially
but quickly direct attention away from the
phobic object. This is a maladaptive emo-
tion regulation strategy that prevents habit-
uation and reinforces the anxiety response
over time through active attentional avoid-
ance. In one study of individuals with a
spider phobia, the LPP elicited by spider
stimuli was increased compared to other
negative stimuli during an early time win-
dow (340–770 milliseconds) but not a later
time window (800–1,500 milliseconds;
Leutgeb, Schafer, & Schienle, 2009)—con-
sistent with an attentional pattern of vigi-
lance followed by avoidance. Participants
were then treated with exposure therapy in
which they were trained to approach and
engage with a spider until their anxiety
diminished, rather than avoid the spider.
Compared to a wait-list control group, indi-
viduals in the exposure therapy condition
exhibited an increased and sustained LPP in
response to spider stimuli from 800 to 1,500
milliseconds at a follow- up testing session.
This effect of treatment on LPP amplitude
was specific to spider stimuli and was not
observed for other unpleasant stimuli. These
data suggest that successful treatment led to
increased attention and engagement with the
phobic object.
Other researchers have used the LPP to
clarify patterns of emotion regulation defi-
cits associated with the treatment of sub-
stance use disorders. Attentional biases
toward drug- related stimuli play a sig-
nificant role in drug abuse and addiction
(Field & Cox, 2008), and several studies
have found that larger LPPs are elicited by
drug- related compared to neutral pictures
in alcoholics (Herrmann et al., 2000; Nam-
koong, Lee, Lee, Lee, & An, 2004), cocaine-
addicted individuals (Dunning et al., 2011;
Franken et al., 2008), and smokers (Littel
& Franken, 2007, 2011a). Furthermore,
attentional bias toward drug cues and away
from other, intrinsically pleasant stimuli
has been related to risk for relapse. In one
smoking cessation study, the LPP elicited by
cigarette- related cues was increased among
all smokers, but a subgroup of individuals
also exhibited a blunted LPP to normative
pleasant stimuli that was associated with
higher rates of relapse over 6 months (Ver-
sace et al., 2012). This suggests that the LPP
could serve as a biomarker for identifying
smokers with a higher risk of relapse (Ver-
sace et al., 2012).
Given that drug craving has been shown
to be modulated by cognitive regulation
(Kober, Kross, Mischel, Hart, & Ochsner,

Temporal Dynamics of Emotion Regulation 53
2010), Littel and Franken (2011b) sought
to examine whether the LPP in response to
smoking- related images could be modulated
by emotion regulation strategies in smok-
ers. Indeed, both reappraisal (i.e., viewing
the scenes from an uninvolved, rational, and
detached perspective) and distraction (i.e.,
identifying the dominant color in the scene)
strategies reduced the amplitude of the LPP
to smoking- related pictures; this effect was
prevalent in both light and heavy smokers,
suggesting that regulation of drug- related
stimuli can be accomplished by smokers
with differing levels of dependency (Littel &
Franken, 2011b).
Future Research Directions
Although electrocortical activity has been
used for decades to study cognitive pro-
cesses that include attention, it has more
recently been used to measure attention to
emotional stimuli (Hajcak et al., 2011). We
have argued that the LPP is modulated by
sustained attention to motivationally salient
stimuli, possibly indexing the coordinated
increased activity of frontoparietal attention
networks. However, the precise mechanisms
of this sustained attention remain unclear.
Although evidence suggests that the LPP
reflects enhanced attention to emotional
stimuli, some have suggested that the LPP
might instead reflect the suppressed pro-
cessing of competing stimuli (Brown, van
Steenbergen, Band, de Rover, & Nieuwen-
huis, 2012; MacNamara, Ferri, et al., 2011;
Mocaiber et al., 2010; Weinberg & Hajcak,
2011b). Future work using fMRI might help
to clarify whether the LPP indexes facili-
tated attention to emotional stimuli. For
instance, given that the LPP is modulated
by both attentional deployment and cogni-
tive reappraisal, it would be informative
to know what neural circuits are similarly
increased or decreased during these emotion
regulation strategies. Moreover, it is impera-
tive to combine EEG and fMRI technologies
in future research. What neural regions and
networks support the emotional modula-
tion of the LPP and its reduction through
reappraisal and attentional deployment? Do
fMRI and EEG provide complementary or
redundant information within and between
subjects? In our view, these are crucial and
exciting questions that can be answered in
the years ahead.
Initial research indicated that the LPP
is reduced via reappraisal, and subsequent
studies confirm that changes in stimulus
meaning are sufficient to modulate the LPP.
Moreover, meaning- based changes that
characterize reappraisal appear to alter the
LPP elicited by subsequent encounters with
emotional stimuli— and more so than other
emotion regulation strategies (i.e., distrac-
tion). Both the LPP and ssVEPs are modu-
lated by attentional deployment: When
participants focus on less arousing aspects
of emotional stimuli, both metrics of neu-
ral activity are down- regulated— and these
effects become evident within about 200
milliseconds. Because of this temporal reso-
lution, electrocortical indices of emotion and
its regulation can be used to index the itera-
tive and recursive processes that determine
an emotional response. Recent work also
highlights the possibility that frontal acti-
vation in reappraisal can be indexed using
time- frequency decompositions of EEG data
(Parvaz et al., 2012).
Electrocortical measures of emotion and
its regulation have been fruitful, yet there
are many open avenues for further explora-
tion, particularly with regard to the study
of individual differences in emotion regula-
tion. What emotion strategies work best for
whom? In what ways does emotion regula-
tion go awry across psychological disorders?
There is an emerging clinical literature uti-
lizing the LPP to shed light on the temporal
dynamics of information processing abnor-
malities associated with psychopathology.
As discussed elsewhere in this volume (Parts
VII and VIII, Chapters 24–32), research on
emotion regulation has important implica-
tions for the treatment of a range of psy-
chological disorders, and bringing the LPP
to bear on this topic may be useful for both
quantifying impairment in emotion regula-
tion and clarifying mechanisms of change
over the course of treatment. As a neuro-
biological complement to self- report and
behavioral indices of emotion regulation
difficulties, LPP data may provide unique
information regarding the time course of
abnormal emotional information processing
within a specific patient population, which
may then become a target for subsequent
intervention.

54 BIOLOGICAL BASES
As evidenced by the chapters in this vol-
ume, emotion regulation is a topic that
figures heavily in both developmental and
clinical literatures. Although electrocortical
activity has been relatively underutilized in
these areas, EEG-based measures of neural
function might be particularly suitable for
emotion regulation studies in children and
clinical populations. The few and emerging
studies in these areas suggest a large and
positive potential.
References
Blechert, J., Sheppes, G., Di Tella, C., Williams,
H., & Gross, J. J. (2012). See what you think:
Reappraisal modulates behavioral and neural
responses to social stimuli. Psychological Sci-
ence, 23(4), 346–353.
Bradley, M., Sabatinelli, D., Lang, P., Fitzsim-
mons, J., King, W., & Desai, P. (2003). Activa-
tion of the visual cortex in motivated attention.
Behavioral Neuroscience, 117(2), 369–380.
Brown, S. B. R. E., van Steenbergen, H., Band,
G. P. H., de Rover, M., & Nieuwenhuis,
S. (2012). Functional significance of the
emotion- related late positive potential. Fron-
tiers in Human Neuroscience, 6. [E-publica-
tion ahead of print]
Codispoti, M., & De Cesarei, A. (2007). Arousal
and attention: Picture size and emotional reac-
tions. Psychophysiology, 44(5), 680–686.
Codispoti, M., Ferrari, V., & Bradley, M. (2006).
Repetitive picture processing: Autonomic and
cortical correlates. Brain Research, 1068(1),
213–220.
Codispoti, M., Ferrari, V., & Bradley, M. (2007).
Repetition and event- related potentials: Dis-
tinguishing early and late processes in affec-
tive picture perception. Journal of Cognitive
Neuroscience, 19(4), 577–586.
Codispoti, M., Mazzetti, M., & Bradley, M.
(2009). Unmasking emotion: Exposure dura-
tion and emotional engagement. Psychophysi-
ology, 46(4), 731–738.
Cuthbert, B., Schupp, H., Bradley, M., Birbau-
mer, N., & Lang, P. (2000). Brain potentials in
affective picture processing: Covariation with
autonomic arousal and affective report. Bio-
logical Psychology, 52(2), 95–111.
de Rover, M., Brown, S., Boot, N., Hajcak, G.,
van Noorden, M., van der Wee, N., & Nieu-
wenhuis, S. (2012). Beta receptor– mediated
modulation of the late positive potential in
humans. Psychopharmacology, 219(4), 971–
979.
Decicco, J. M., Solomon, B., & Dennis, T. A.
(2012). Neural Correlates of Cognitive Reap-
praisal in Children: An ERP study. Develop-
mental Cognitive Neuroscience, 2(1), 79–80.
Dennis, T., & Hajcak, G. (2009). The late posi-
tive potential: A neurophysiological marker
for emotion regulation in children. Journal
of Child Psychology and Psychiatry, 50(11),
1373–1383.
Dunning, J. P., & Hajcak, G. (2009). See no evil:
Directing visual attention within unpleasant
images modulates the electrocortical response.
Psychophysiology, 46(1), 28–33.
Dunning, J. P., Parvaz, M. A., Hajcak, G.,
Maloney, T., Alia-Klein, N., Woicik, P. A.,
et al. (2011). Motivated attention to cocaine
and emotional cues in abstinent and current
cocaine users—An ERP study. European Jour-
nal of Neuroscience, 33, 1716–1723.
Eimer, M., Holmes, A., & McGlone, F. (2003).
The role of spatial attention in the processing
of facial expression: An ERP study of rapid
brain responses to six basic emotions. Cogni-
tive, Affective, and Behavioral Neuroscience,
3(2), 97–110.
Ferrari, V., Bradley, M., Codispoti, M., & Lang,
P. (2010). Detecting novelty and significance.
Journal of Cognitive Neuroscience, 22(2),
404–411.
Field, M., & Cox, W. M. (2008). Attentional bias
in addictive behaviors: A review of its devel-
opment, causes, and consequences. Drug and
Alcohol Dependence, 97(1–2), 1–20.
Flaisch, T., Häcker, F., Renner, B., & Schupp, H.
(2011). Emotion and the processing of sym-
bolic gestures: An event- related brain poten-
tial study. Social Cognitive and Affective Neu-
roscience, 6(1), 109–118.
Foti, D., & Hajcak, G. (2008). Deconstructing
reappraisal: Descriptions preceding arous-
ing pictures modulate the subsequent neural
response. Journal of Cognitive Neuroscience,
20(6), 977–988.
Foti, D., Hajcak, G., & Dien, J. (2009). Differen-
tiating neural responses to emotional pictures:
Evidence from temporal– spatial PCA. Psycho-
physiology, 46, 521–530.
Foti, D., Olvet, D., Klein, D., & Hajcak, G.
(2010). Reduced electrocortical response to
threatening faces in major depressive disorder.
Depression and Anxiety, 27(9), 813–820.
Franken, I. H. A., Dietvorst, R. C., Hesselmans,
M., Franzek, E. J., Van De Wetering, B. J. M.,
Temporal Dynamics of Emotion Regulation 55
& Van Strien, J. W. (2008). Cocaine craving
is associated with electrophysiological brain
responses to cocaine- related stimuli. Addic-
tion Biology, 13, 386–392.
Grasso, D. J., & Simons, R. F. (2011). Perceived
parental support predicts enhanced late posi-
tive event- related brain potentials to parent
faces. Biological Psychology, 86(1), 26–30.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Hajcak, G., Anderson, B., Arana, A., Borckardt,
J., Takacs, I., George, M., et al. (2010). Dorso-
lateral prefrontal cortex stimulation modulates
electrocortical measures of visual attention:
Evidence from direct bilateral epidural cortical
stimulation in treatment- resistant mood disor-
der. Neuroscience, 170(1), 281–288.
Hajcak, G., Dunning, J. P., & Foti, D. (2009).
Motivated and controlled attention to emo-
tion: Time- course of the late positive poten-
tial. Clinical Neurophysiology, 120, 505–510.
Hajcak, G., MacNamara, A., Foti, D., Ferri, J.,
& Keil, A. (2013). The dynamic allocation of
attention to emotion: Simultaneous and inde-
pendent evidence from the late positive poten-
tial and steady state visual evoked potentials.
Biological Psychology, 92(3), 447–455.
Hajcak, G., MacNamara, A., & Olvet, D. M.
(2010). Event- related potentials, emotion, and
emotion regulation: An integrative review.
Developmental Neuropsychology, 35(2),
129–155.
Hajcak, G., Moser, J. S., & Simons, R. F. (2006).
Attending to affect: Appraisal strategies mod-
ulate the electrocortical response to arousing
pictures. Emotion, 6(3), 517–522.
Hajcak, G., & Nieuwenhuis, S. (2006). Reap-
praisal modulates the electrocortical response
to unpleasant pictures. Cognitive, Affective,
and Behavioral Neuroscience, 6, 291–297.
Hajcak, G., & Olvet, D. M. (2008). The persis-
tence of attention to emotion: Brain potentials
during and after picture presentation. Emo-
tion, 8(2), 250–255.
Hajcak, G., Weinberg, A., MacNamara, A., &
Foti, D. (2011). ERPs and the study of emo-
tion. In S. Luck & E. Kappenman (Eds.), The
Oxford handbook of event- related poten-
tial components (pp. 441–474). New York:
Oxford University Press.
Herrmann, M. J., Weijers, H., Wiesbeck, G.
A., Aranda, D., Böning, J., & Fallgatter, A.
J. (2000). Event- related potentials and cue-
reactivity in alcoholism. Alcoholism: Clinical
and Experimental Research, 24(11), 1724–
1729.
Hillyard, S. A., Hinrichs, H., Tempelmann, C.,
Morgan, S. T., Hansen, J. C., Scheich, H.,
et al. (1997). Combining steady- state visual
evoked potentials and fMRI to localize brain
activity during selective attention. Human
Brain Mapping, 5(4), 287–292.
Holmes, A., Vuilleumier, P., & Eimer, M. (2003).
The processing of emotional facial expression
is gated by spatial attention: Evidence from
event- related brain potentials. Cognitive Brain
Research, 16(2), 174–184.
Hot, P., Saito, Y., Mandai, O., Kobayashi, T.,
& Sequeira, H. (2006). An ERP investiga-
tion of emotional processing in European and
Japanese individuals. Brain Research, 1122(1),
171–178.
Johnstone, T., van Reekum, C. M., Urry, H. L.,
Kalin, N. H., & Davidson, R. J. (2007). Failure
to regulate: Counterproductive recruitment of
top-down prefrontal– subcortical circuitry in
major depression. Journal of Neuroscience,
27(33), 8877–8884.
Keil, A., Bradley, M., Hauk, O., Rockstroh, B.,
Elbert, T., & Lang, P. (2002). Large-scale neu-
ral correlates of affective picture processing.
Psychophysiology, 39(5), 641–649.
Keil, A., Gruber, T., Muller, M., Moratti, S., Sto-
larova, M., Bradley, M., & Lang, P. (2003).
Early modulation of visual perception by emo-
tional arousal: Evidence from steady- state
visual evoked brain potentials. Cognitive,
Affective, and Behavioral Neuroscience, 3(3),
195–206.
Keil, A., Moratti, S., Sabatinelli, D., Bradley, M.,
& Lang, P. (2005). Additive effects of emo-
tional content and spatial selective attention
on electrocortical facilitation. Cerebral Cor-
tex, 15(8), 1187–1197.
Kisley, M. A., Wood, S., & Burrows, C. L.
(2007). Looking at the sunny side of life. Psy-
chological Science, 18(9), 838–843.
Kissler, J., Herbert, C., Winkler, I., & Junghofer,
M. (2009). Emotion and attention in visual
word processing— An ERP study. Biological
Psychology, 80(1), 75–83.
Kober, H., Kross, E. F., Mischel, W., Hart, C. L.,
& Ochsner, K. N. (2010). Regulation of crav-
ing by cognitive strategies in cigarette smok-
ers. Drug and Alcohol Dependence, 106(1),
52–55.
Krompinger, J., Moser, J., & Simons, R. (2008).
56 BIOLOGICAL BASES
Modulations of the electrophysiological
response to pleasant stimuli by cognitive reap-
praisal. Emotion, 8(1), 132–137.
Kujawa, A., Hajcak, G., Torpey, D., Kim, J., &
Klein, D. (2012). Electrocortical reactivity to
emotional faces in young children and associa-
tions with maternal and paternal depression.
Journal of Child Psychology and Psychiatry,
53, 207–215.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N.
(2008). International Affective Picture Sys-
tem (IAPS): Affective ratings of pictures and
instructional manual (Technical Report A-8).
Gainesville: University of Florida.
Langeslag, S. J. E., & Van Strien, J. W. (2010).
Comparable modulation of the late positive
potential by emotion regulation in younger
and older adults. Journal of Psychophysiol-
ogy, 24(3), 186–197.
Leutgeb, V., Schafer, A., & Schienle, A. (2009).
An event- related potential study on exposure
therapy for patients suffering from spider pho-
bia. Biological Psychology, 82(3), 293–300.
Littel, M., & Franken, I. H. A. (2007). The
effects of prolonged abstinence on the process-
ing of smoking cues: An ERP study among
smokers, ex- smokers and never- smokers. Jour-
nal of Psychopharmacology, 21(8), 873–882.
Littel, M., & Franken, I. H. A. (2011a). Implicit
and explicit selective attention to smoking
cues in smokers indexed by brain potentials.
Journal of Psychopharmacology, 25(4), 503–
513.
Littel, M., & Franken, I. H. A. (2011b). Inten-
tional modulation of the late positive potential
in response to smoking cues by cognitive strat-
egies in smokers. PloS ONE, 6(11), e27519.
Luck, S. (2005). An introduction to the event-
related potential technique. Cambridge, MA:
MIT Press.
MacNamara, A., Ferri, J., & Hajcak, G. (2011).
Working memory load reduces the late posi-
tive potential and this effect is attenuated with
increasing anxiety. Cognitive, Affective, and
Behavioral Neuroscience, 11(3), 321–331.
MacNamara, A., Foti, D., & Hajcak, G. (2009).
Tell me about it: Neural activity elicited by
emotional stimuli and preceding descriptions.
Emotion, 9(4), 531–543.
MacNamara, A., Ochsner, K. N., & Hajcak, G.
(2011). Previously reappraised: The lasting
effect of description type on picture- elicited
electrocortical activity. Social Cognitive and
Affective Neuroscience, 6(3), 348–358.
Mocaiber, I., Pereira, M. G., Erthal, F. S.,
Machado- Pinheiro, W., David, I. A., Cagy,
M., et al. (2010). Fact or fiction?: An event-
related potential study of implicit emotion
regulation. Neuroscience Letters, 476(2),
84–88.
Moratti, S., Saugar, C., & Strange, B. A. (2011).
Prefrontal– occipitoparietal coupling under-
lies late latency human neuronal responses
to emotion. Journal of Neuroscience, 31(47),
17278–17286.
Moser, J., Hajcak, G., Bukay, E., & Simons, R.
(2006). Intentional modulation of emotional
responding to unpleasant pictures: An ERP
study. Psychophysiology, 43(3), 292–296.
Namkoong, K., Lee, E., Lee, C. H., Lee, B. O.,
& An, S. K. (2004). Increased P3 amplitudes
induced by alcohol- related pictures in patients
with alcohol dependence. Alcoholism: Clini-
cal and Experimental Research, 28(9), 1317–
1323.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp.23–42). New York: Guilford
Press.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D. E., et
al. (2004). For better or for worse: Neural sys-
tems supporting the cognitive down-and up-
regulation of negative emotion. NeuroImage,
23(2), 483–499.
Olofsson, J., Nordin, S., Sequeira, H., & Polich,
J. (2008). Affective picture processing: An
integrative review of ERP findings. Biological
Psychology, 77(3), 247–265.
Olofsson, J., & Polich, J. (2007). Affective visual
event- related potentials: Arousal, repetition,
and time-on-task. Biological Psychology,
75(1), 101–108.
Parvaz, M., MacNamara, A., Goldstein, R., &
Hajcak, G. (2012). Event- related frontal alpha
as a marker of lateral prefrontal cortex acti-
vation during cognitive reappraisal. Cogni-
tive, Affective, and Behavioral Neuroscience,
12(4), 730 –740.
Phan, K., Liberzon, I., Welsh, R., Britton, J., &
Taylor, S. (2003). Habituation of rostral ante-
rior cingulate cortex to repeated emotionally
salient pictures. Neuropsychopharmacology,
28(7), 1344–1350.
Sabatinelli, D., Keil, A., Frank, D., & Lang, P.
(2013). Emotional perception: Correspon-
Temporal Dynamics of Emotion Regulation 57
dence of early and late event- related potentials
with cortical and subcortical functional MRI.
Biological Psychology, 92(3), 513–519.
Sabatinelli, D., Lang, P., Keil, A., & Bradley, M.
(2007). Emotional perception: Correlation of
functional MRI and event- related potentials.
Cerebral Cortex, 17(5), 1085–1091.
Sheppes, G., & Gross, J. J. (2011). Is timing
everything?: Temporal considerations in emo-
tion regulation. Personality and Social Psy-
chology Review, 15(4), 319–331.
Stockburger, J., Schmälzle, R., Flaisch, T., Bub-
latzky, F., & Schupp, H. T. (2009). The impact
of hunger on food cue processing: An event-
related brain potential study. NeuroImage,
47(4), 1819–1829.
Tacikowski, P., & Nowicka, A. (2010). Alloca-
tion of attention to self-name and self-face:
An ERP study. Biological Psychology, 84(2),
318–324.
Thiruchselvam, R., Blechert, J., Sheppes, G.,
Rydstrom, A., & Gross, J. J. (2011). The tem-
poral dynamics of emotion regulation: An
EEG study of distraction and reappraisal. Bio-
logical Psychology, 87(1), 84–92.
Thiruchselvam, R., Hajcak, G., & Gross, J. J.
(2012). Looking inwards: Shifting attention
within working memory representations alters
emotional responses. Psychological Science,
23(12), 1461–1466.
Urry, H. L. (2010). Seeing, thinking, and feeling:
Emotion- regulating effects of gaze- directed
cognitive reappraisal. Emotion, 10(1), 125–
135.
Versace, F., Lam, C. Y., Engelmann, J. M., Rob-
inson, J. D., Minnix, J. A., Brown, V. L., et al.
(2012). Beyond cue reactivity: Blunted brain
responses to pleasant stimuli predict long-term
smoking abstinence. Addiction Biology, 17(6),
991–1000.
Vuilleumier, P. (2005). How brains beware: Neu-
ral mechanisms of emotional attention. Trends
in Cognitive Sciences, 9(12), 585–594.
Wager, T. D., Davidson, M. L., Hughes, B. L.,
Lindquist, M. A., & Ochsner, K. N. (2008).
Prefrontal- subcortical pathways mediating
successful emotion regulation. Neuron, 59(6),
1037–1050.
Weinberg, A., & Hajcak, G. (2010). Beyond
good and evil: The time- course of neural activ-
ity elicited by specific picture content. Emo-
tion, 10, 767–782.
Weinberg, A., & Hajcak, G. (2011a). Electrocor-
tical evidence for vigilance- avoidance in Gen-
eralized Anxiety Disorder. Psychophysiology,
48, 842–851.
Weinberg, A., & Hajcak, G. (2011b). The Late
Positive Potential predicts subsequent interfer-
ence with target processing. Journal of Cogni-
tive Neuroscience, 23, 2995 –3007.
Weinberg, A., Hilgard, J., Bartholow, B., & Haj-
cak, G. (2012). Emotional targets: Evaluative
categorization as a function of context and
content. International Journal of Psychophys-
iology, 84, 149–154.
Yen, N. S., Chen, K. H., & Liu, E. H. (2010).
Emotional modulation of the late positive
potential (LPP) generalizes to Chinese individ-
uals. International Journal of Psychophysiol-
ogy, 75(3), 319–325.

58
Humans have long had an ambivalent rela-
tionship with their emotions. Emotions have
been celebrated as the inspiration for pin-
nacles of human art, literature, and music.
Equally, however, emotions have often been
seen as “evil spirits” that misguide us and
tempt us to do things we should not— as
“primal” instincts that need to be conquered
by the rational human mind. Indeed, history
is replete with fables of the terrible conse-
quences of unchecked emotion, from the
tragedies of Shakespeare and his recurring
theme of “the fatalism of overmastering pas-
sion” (Corson, 1890, Preface) to the appall-
ing crowd violence that can occur anywhere,
from the streets of war- torn Baghdad to the
neighborhoods of London.
The uncertainty with which we view
our emotions reflects their function, both
through our evolutionary past and in day-
to- day life. Emotions allow us to respond to
important environmental changes quickly
and with a minimum of deliberation, and
provide us with the drive to seek out things
that are good for us and avoid things that
are harmful. Emotion and mood
1
can be
seen as arising from heuristic neural systems
that act upon a vast quantity of incoming
information and make speedy decisions,
guiding behavior in a way that is robust
against incomplete information or computa-
tional overload. Emotions are vital for quick,
adaptive responses in the face of complexity
that rules out a rational calculation of all
our options but afford us far more flexibility
than simple stimulus– response rules.
Emotion Regulation
and Dysregulation
Emotions are not always “correct,” based as
they are on probabilistic systems that have
evolved to ensure our survival across a wide
range of circumstances. One of the func-
tions emotions serve is to interrupt ongo-
ing behavior when an encountered event
or stimulus is highly personally significant
and deserves our attention or requires us
to prepare for action (Frijda, 1986; Gross,
this volume). Yet we cannot afford to be
constantly interrupted; emotions exist in
a balance with other, ongoing cognitive,
attentional, and behavioral processes. In
order for us to benefit, emotions need to be
appropriately managed depending on the
specific contexts in which they occur. Given
the importance of emotion regulation, it is
unsurprising that neural systems have been
identified that serve to manage emotions
and moods in such a way that, on the bal-
ance, they contribute to, rather than inter-
fere with, our daily lives (Ochsner & Gross,
this volume).
CHAPTER 4
The Neural Basis of Emotion Dysregulation
Tom Johnstone
Henrik Walter

The Neural Basis of Emotion Dysregulation 59
In this chapter we consider how the dys-
function of these neural systems might
underlie the inability to regulate emotions
appropriately. The outcome of such a fail-
ure to regulate can range from a short-
term inability to stop getting angry during
a debate with a colleague, all the way to
long- term psychopathology such as mood
and anxiety disorders. We limit discussion
to types of emotion dysregulation that are
in some sense not normative. Thus, we do
not discuss mechanisms that give rise to
panic behavior when a bomb explodes, even
though remaining calm might be the most
adaptive thing to do in the context, since
panic in such a situation would be considered
quite normal. We also take a look at how
knowledge of the neural systems involved in
emotion regulation, and their dysfunction in
psychopathology, is leading to novel, brain-
focused clinical treatments.
Emotion regulation processes can be
examined at multiple levels, from neu-
rotransmitters and inhibitory interneurons
to cortical and subcortical network feed-
back loops, to socially mediated regulation
of moods and emotional behaviors. All of
these types of regulation may be at least par-
tially automatic and subconscious (Gyurak
& Etkin, this volume), so limiting a discus-
sion of emotion regulation to that which is
“deliberate” or “conscious” is not particu-
larly useful. Gross (this volume) makes the
distinction between antecedent- focused and
response- focused emotion regulation. Neu-
robiologically, this conceptual distinction
can be elaborated by considering the neural
circuits involved in detecting and appraising
situations and contexts that call for certain
emotional responses (e.g., threat detection,
relevance detection, recognition of socially
rewarding stimuli, evaluation of possible
outcomes and their probabilities), as well
as those responsible for generating the neu-
ral, physiological, and behavioral responses
that make up emotions. Many of the brain
processes involved in emotion regulation
have thus been extensively studied in other
domains of psychology and cognitive neuro-
science, including (but not limited to) selec-
tive attention, cognitive control, working
memory, and response inhibition. Effective
emotion regulation involves the coordinated
recruitment of these different neural mecha-
nisms in specific contexts (Ochsner & Gross,
this volume). One important question is the
extent to which different manifestations of
emotion dysregulation can be understood in
terms of specific patterns of dysfunction in
one or more of these neural systems.
Prefrontal Cortex
and Emotion Regulation
Given the broad sweep of processes we
just outlined, an exhaustive review of the
role of different neural systems in emotion
regulation and dysregulation would require
an entire book. Instead, we focus here on
research demonstrating the involvement of
the prefrontal cortex (PFC) in the regulation
of “bottom- up” emotion- generating neural
circuits, which has provided the clearest
empirical distinction between generation
and regulation of emotions, and also poten-
tially has the greatest implications for emo-
tion regulation– based therapies for a variety
of affective disorders (see Figure 4.1; also see
Ochsner & Gross, this volume). Research on
prefrontal cortical regulation of emotion
has largely followed one of two approaches.
Studies of cognitive reappraisal have focused
on how the explicit cognitive reinterpreta-
tion of the emotional meaning or possible
outcome of a stimulus can be used to mod-
ify the resulting emotional response. On a
more automatic level, a range of studies has
examined the more automatic engagement
of prefrontal mechanisms that serve to regu-
late attention and the contents of working
memory, and to shield these processes from
emotional interference.
Cognitive Reappraisal
Based on cognitive appraisal theories of emo-
tion (Scherer, Schorr, & Johnstone, 2001),
the study of emotion regulation through
reappraisal has formed the core of cognitive
neuroscience research on emotion regulation
(Gross, this volume). Reappraising the affec-
tive meaning of negative emotional stimuli
can reduce the magnitude of felt negative
emotion, as well as physiological indicators,
such as potentiated eyeblink startle (Jackson,
Malmstadt, Larson, & Davidson, 2000).
Eyeblink startle increases in magnitude dur-
ing presentation of negative pictures, rela-
tive to neutral pictures, an effect thought to
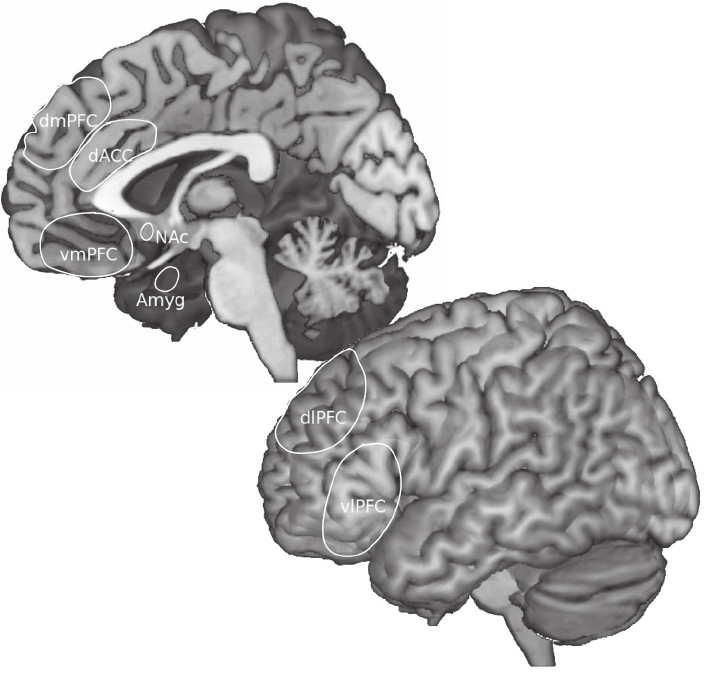
60 BIOLOGICAL BASES
be mediated by projections from the central
nucleus of the amygdala to the brainstem
(Davis, 2006). Evidence that reappraisal can
directly influence this amygdala circuitry
comes from consistent findings in positron
emission tomographic (PET) and functional
magnetic resonance imaging (fMRI) studies
of healthy individuals showing reappraisal-
dependent decreases in amygdala activation
in response to negative stimuli. Increased
dorsolateral and ventrolateral PFC activa-
tion is commonly measured in such reap-
praisal studies (Ochsner & Gross, this
volume), though discrepancies such as the
hemispheric lateralization of PFC activation
still exist, with some studies finding predom-
inantly right prefrontal activation and others
finding left prefrontal activation.
2
Spontaneous Regulation
and Cognitive Control
In daily life we need to regulate our emo-
tions continuously and automatically in
order to remain focused on current thoughts
and actions; we do not always have the
luxury of being able to reappraise con-
sciously the emotion- inducing events around
us. A number of studies have used tasks
in which regulating emotion is required
but not explicitly instructed. For example,
when cognitive tasks, such as those that
tax working memory, are performed under
an anxiety induction or in the presence of
emotional distractors, a drop in task perfor-
mance is observed. There is some evidence
that this interference is reduced when the
FIGURE 4.1. Depiction of prefrontal cortical and subcortical regions commonly linked to emotion
regulation. dmPFC, dorsomedial PFC; dACC, dorsal anterior cingulate cortex; vmPFC, ventromedial
PFC; NAc: nucleus accumbens; Amyg, amygdala; vlPFC, ventrolateral PFC; dlPFC, dorsolateral PFC.
The Neural Basis of Emotion Dysregulation 61
cognitive load of the task increases, possi-
bly through the automatic engagement of
top-down control mechanisms (Blair et al.,
2007; Clarke & Johnstone, 2013; Erthal et
al., 2005; Van Dillen, Heslenfeld, & Koole,
2009). The specific subregions of the PFC
that are involved in such automatic emo-
tion regulation may depend on properties
of the distractors, for example, the relative
involvement of ventrolateral or dorsal PFC
in regulating negative or positive distrac-
tors, respectively (Erk, Kleczar, & Walter,
2007). A number of the prefrontal regions
implicated in either spontaneous or explicit
emotion regulation, including the ventrolat-
eral PFC, dorsolateral PFC, and the dorsal
anterior cingulate, overlap with those com-
monly identified in studies of cognitive and
attentional control (Mitchell, 2011). The
dorsal anterior cingulate cortex (dACC) is
posited to be involved in performance moni-
toring and detecting when control is neces-
sary (Ridderinkhof, Ullsperger, Crone, &
Nieuwenhuis, 2004). Lateral regions of the
PFC, including the ventrolateral and dor-
solateral PFC, are involved in maintaining
and manipulating information in working
memory, and in implementing attentional,
cognitive, or behavioral adjustments (Rid-
derinkhof et al., 2004).
Prefrontal–Subcortical Connectivity
There are few direct neural connections
between dorsal and lateral PFC and the
amygdala (Ghashghaei, Hilgetag, & Barbas,
2007), raising the question of how these
lateral PFC brain regions might exert their
top-down regulatory influence. One possi-
bility is that the ventromedial PFC (vmPFC),
which has neural connections with lateral
PFC and the amygdala, might provide the
missing link. Ventral parts of the medial PFC
have been implicated in down- regulation
of amygdala function in nonhuman and
human studies of fear conditioning and
extinction. Extinction of conditioned fear
responses can be considered a form of emo-
tion regulation, since the fear association
itself is not destroyed (Bouton, 2004), but
rather a new association indicating safety is
created and inhibits or overcomes the origi-
nal conditioned response in specific con-
texts. In rodent studies, the infralimbic cor-
tex (homologous to the vmPFC in humans)
plays an important role in the creation and
maintenance and/or recall of extinction of
conditioned fear responses (Milad & Quirk,
2002, 2012). Consistent with these stud-
ies, activation in the vmPFC correlates with
recall of extinction in humans (Kalisch et
al., 2006; Milad et al., 2007; Phelps, Del-
gado, Nearing, & LeDoux, 2004). The role
of vmPFC– amygdala connectivity seems to
be under genetic control, pointing to a role
of dynorphins in human extinction learning
(Bilkei- Gorzo et al., 2012). Thus, based on
its role in fear conditioning and extinction
paradigms, the vmPFC would appear to be
a promising candidate as a region that can
mediate more lateral PFC regulatory effects
on the amygdala.
Evidence that this might be the case comes
from studies of spontaneous regulation, as
well as reappraisal- based down- regulation
of emotional responses to aversive pictures.
For example, Etkin, Egner, Peraza, Kandel,
and Hirsch (2006) used a variant of an emo-
tional Stroop task, in which participants
responded to the words fear and happy
superimposed on faces expressing either fear
or happiness. In this task, there is a need
to down- regulate automatic responses to
the emotional facial expressions when they
conflict with the word (e.g., the word happy
superimposed on a fearful face). Their
results indicated that while dorsal and lat-
eral PFC detect the need for top-down regu-
lation, rostral ACC (rACC) implements that
regulation. Specifically, rACC activation
was higher and amygdala activation was
lower during the down- regulation of emo-
tionally conflicting information, with a neg-
ative correlation between the two regions.
Two studies of reappraisal- based emotion
regulation, (Johnstone, van Reekum, Urry,
Kalin, & Davidson, 2007; Urry et al., 2006)
found that individual differences in activa-
tion of the vmPFC during down- regulation
correlated negatively with amygdala acti-
vation; the more activated the vmPFC dur-
ing down- regulation, the less activated the
amygdala. Furthermore, vmPFC activation
was found statistically to mediate the associ-
ation between dorsomedial PFC (Urry et al.,
2006) ventrolateral PFC (Johnstone et al.,
2007) or dorsolateral PFC (Erk et al., 2010)
and amygdala activation during the down-
regulation condition. It should be noted,
however, that these studies did not find cat-

62 BIOLOGICAL BASES
egorically increased activation of vmPFC
during down- regulation.
One study (Delgado, Nearing, LeDoux,
& Phelps, 2008) directly tested the con-
nection between reappraisal- based emo-
tion regulation and extinction. Participants
were instructed simply to attend to a colored
square that might give them a shock (the
conditioned stimulus) or to down- regulate
their conditioned response to the square by
reappraising the meaning of the color (e.g.,
by thinking of contexts in which the color
had a more calming influence, such as the
sky for a blue square). Greater activation in
the down- regulation relative to the attend
condition was measured in dorsolateral
PFC and vmPFC, with less activation in the
amygdala. Prefrontal activation correlated
negatively with skin conductance responses,
providing evidence of its role in regulating
emotional responses. Notably, the region of
activation in vmPFC overlapped with that
found in prior studies of extinction using the
same stimuli.
In summary, evidence points to a pre-
frontal regulatory network in which dorsal
and lateral regions of the PFC exert a top-
down influence on subcortical structures
involved in generating emotional responses.
Some evidence suggests that this regula-
tory effect is via the same region of vmPFC
involved in extinction, though whether this
is the primary pathway, or other important
regulatory pathways exist (Wager, David-
son, Hughes, Lindquist, & Ochsner, 2008)
is the topic of continuing investigation (e.g.
see Clarke & Johnstone [2013] for an exam-
ination of the role of dACC in inhibition of
amygdala output to PFC during spontane-
ous threat regulation). Identifying the exact
pathways involved in effective emotion regu-
lation will be vital in determining the extent
to which the dysregulation of emotion can
be explained in terms of a malfunction in
the prefrontal emotion regulation network.
Emotion Dysregulation
and Affective Disorders
Emotional disorders such as depression and
anxiety are estimated by the World Health
Organization to be the greatest cause of dis-
ability worldwide (Mathers, Lopez, & Mur-
ray, 2006). Efforts to understand the neu-
robiological causes of these conditions and
potential avenues for treatment are there-
fore of enormous importance to the general
population. Most brain research into mood
and anxiety disorders has focused on the
“bottom-up” determinants of emotions,
with a focus on subcortical and early sen-
sory processing that generates emotional
responses and biases our behavior in a fairly
automatic way. For example, much of the
focus in anxiety disorders has been on atten-
tional biases toward anxiety- relevant stimuli
(Campbell- Sills, Ellard, & Barlow, this vol-
ume; MacLeod, Mathews, & Tata, 1986),
generated either through low- level sensory
cortex or subcortical structures such as the
amygdala, known to play a role in early
threat detection. In depression, an extensive
literature exists on the role of the monoami-
nergic neurotransmitters serotonin, norepi-
nephrine, and dopamine in subcortical and
subcortical– cortical networks involved in
mood, though for all that research, there
have been no definitive breakthroughs in
understanding the causes of depression.
Although negative emotions such as anxi-
ety and sadness are a normal occurrence in
response to adversity in all individuals, one
feature that distinguishes those with mood
and anxiety disorders is their inability to
regulate certain negative emotions effec-
tively when they arise. Indeed, it might well
be argued that the defining feature of many
mood and anxiety disorders is the extended
duration of emotional episodes rather than
their intensity (phobias and panic disor-
ders are obvious exceptions, though even
in these cases, feelings of panic and anxi-
ety typically last well beyond the immediate
trigger of the episode). Cognitive neurosci-
entists have argued that depression should
not be equated with the “down” state itself,
but rather with the tendency to “enter and
get stuck in this state. Thus, the neurobiol-
ogy of depression should be that of mood
reaction and regulation rather than the
mood state per se” (Holtzheimer & May-
berg, 2011, p. 1). Importantly, it is not just
negative emotions that are dysregulated in
mood disorders. “Anhedonia,” a markedly
reduced interest and pleasure in normally
rewarding activities, is one of the three core
features of major depression that may be
partly explained by an inability to engage
cognitively in rewarding situations and sus-
The Neural Basis of Emotion Dysregulation 63
tain positive emotions, a different type of
emotion regulation.
Although abnormalities in the neural
circuitry supporting adaptive regulation of
certain emotions may play a decisive role in
determining vulnerability to mood disorders
(Davidson, Pizzagalli, Nitschke, & Putnam,
2002), only recently have researchers explic-
itly examined this possibility. Below we
discuss the findings separately for anxiety,
which is symptomatic of a variety of disor-
ders and phobias; the more general negative
mood characteristic of depression; and the
dysregulation of positive emotions in anhe-
donia.
Emotion Dysregulation and Anxiety
Substantial research has demonstrated
that high- anxious individuals show greater
attentional capture by threat- relevant infor-
mation, with slowed reaction times and an
increased number of errors when perform-
ing tasks such as the emotional Stroop or the
dot- probe task, than low- anxious individuals
(MacLeod et al., 1986). A number of mod-
els of anxiety- related attentional bias have
proposed that an overly sensitive preatten-
tive threat detection system is responsible for
drawing attention toward threatening stim-
uli (see Cisler & Koster, 2010, for a review).
In line with this, elevated amygdala activa-
tion in response to threat- relevant informa-
tion is often measured in high- anxiety indi-
viduals, suggesting that attentional biases
may have their roots in hyperactive or low-
threshold threat detection at a subcortical
level. Adults assessed as having an inhibited
temperament as infants show heightened
and sustained amygdala responses to novel
social stimuli (Schwartz & Rauch, 2004;
Schwartz, Wright, Shin, Kagan, & Rauch,
2003), suggesting that such amygdala hyper-
activity might be stable across development.
Heightened amygdala activation is not
always found in high- anxiety individuals,
however, in part because amygdala engage-
ment may depend on the specific amygdala
nucleus and properties of the threatening
stimulus (Etkin et al., 2004). There are
also other candidate subcortical regions
that might underlie overly sensitive threat
detection in anxious individuals, such as the
bed nucleus of the stria terminalis (BNST),
which plays an important role in sustained,
as distinct from transient, forms of vigilance
(Somerville et al., 2012; Walker, Toufexis,
& Davis, 2003).
Nonetheless, it seems likely that anxiety
disorders involve more than just hypersen-
sitivity in bottom-up threat detection sys-
tems. There is now a large body of evidence
that highly anxious individuals, in par-
ticular those with anxiety disorders, show
deficits in prefrontal circuits that regulate
attention to and responding to threaten-
ing stimuli. Over and above an attentional
bias toward threatening information, highly
anxious individuals have greater difficulty
in disengaging from threat- relevant infor-
mation when required. In Posner spatial
cuing tasks (Posner, Snyder, & Davidson,
1980), the participant focuses on a central
fixation, and a cue flashed up in one of
two locations on either side of the fixation
point is followed rapidly by a target in one
of the two locations. When the cue is pre-
sented in a different location from the fol-
lowing target (invalid trials), response to the
target is slowed. Highly anxious individu-
als show even greater slowing when the cue
is threat- related than when it is of neutral
valence, an effect that is reliable across mul-
tiple clinical anxiety disorders and high trait
anxiety populations, but not in nonanxious
individuals (Bar- Haim, Lamy, Pergamin,
Bakermans- Kranenburg, & van IJzendoorn,
2007). Results such as this indicate that
weakened top-down attentional control, a
core feature of spontaneous emotion regula-
tion, might underpin some features of high
trait anxiety and anxiety disorders (Cisler &
Koster, 2010). Deficits in the performance
of working memory tasks in the presence of
sustained emotional distractors or anxiety
induction in anxious individuals (Shackman
et al., 2006) also point to weakened top-
down control of emotion, potentially involv-
ing the prefrontal network for cognitive
control and spontaneous emotion regulation
discussed earlier.
Human brain imaging studies have found
reduced prefrontal recruitment in high-
anxious individuals relative to low- anxious
individuals when faced with threat or
threat- relevant stimuli. For example, using a
perceptual discrimination task in the pres-
ence of fearful versus neutral facial expres-
sions, Bishop, Duncan, Brett, and Lawrence
(2004) found lower rACC and ventrolateral
64 BIOLOGICAL BASES
PFC activation in high- anxious than in low-
anxious individuals, and the opposite pat-
tern in the amygdala. This result is consis-
tent with a model in which rACC detects the
need for control over incoming information
that conflicts with task demands, and ven-
trolateral PFC is subsequently involved in
regulating attention toward task- relevant
information and away from threatening,
task- irrelevant information. The lower rACC
and ventrolateral PFC activation and higher
amygdala activation seen in high- anxious
individuals presumably reflects reduced
prefrontal control. This deficit in top-down
attentional control of task- irrelevant distrac-
tors might not be specific to threatening or
even emotional distractors: In a later study,
Bishop (2008) reported that high trait anx-
ious individuals showed greater interference
from nonemotional distractors, along with
reduced activation in dorsolateral PFC. Thus
elevated trait anxiety is associated with gen-
erally reduced lateral PFC- mediated top-
down control of attention, resulting in less
efficient attentional regulation of distract-
ing emotional information. Coupled with a
hypersensitive bottom-up threat detection
system, the consequence would be hypervig-
ilance to and an inability to disengage from
potentially threatening stimuli.
The picture of dysfunctional prefrontal
regulation in anxiety disorders (as opposed
to high trait anxiety) is not as clear- cut as
one might presume from this model, how-
ever. The patterns of observed prefron-
tal hypoactivity and hyperactivity differ
across anxiety disorders (Etkin & Wager,
2007; Shin & Liberzon, 2010). Of these,
the most consistent neuroimaging findings
have been reported for posttraumatic stress
disorder (PTSD). A number of studies have
shown impaired extinction of conditioned
responses to aversive stimuli in PTSD, and
hypoactivation in vmPFC/rACC has been
reported in response to both trauma- related
and non- trauma- related aversive stimuli, as
well as during extinction, other emotional
and cognitive tasks, and resting baseline
(Shin & Liberzon, 2010). Activation in this
region also negatively correlates with PTSD
symptom severity. All of these results suggest
that PTSD is characterized by a disrupted
ability to regulate previously conditioned
fear responses through the engagement of
vmPFC extinction circuitry. In contrast
with PTSD, neuroimaging studies of other
anxiety disorders have shown inconsistent
results, with both hyper- and hypoactivation
in vmPFC, though there are fewer such stud-
ies than those examining PTSD, and they
have used a variety of different experimental
paradigms (Etkin & Wager, 2007).
Dysregulation of Emotion
in Negative Mood and Depression
Brain imaging studies of depressed individu-
als have demonstrated abnormal patterns of
activation in many of the same prefrontal
brain regions thought to contribute to the
regulation of mood and emotion, though the
findings have not been consistent. A num-
ber of PET studies have indicated reduced
metabolism in dorsolateral PFC and dACC
in both the resting state and during expo-
sure to negative stimuli (Fitzgerald, Laird,
Maller, & Daskalakis, 2008). These results
are consistent with the notion that dysregu-
lation of negative mood in depression might
be due to underrecruitment of dorsal pre-
frontal brain regions important for the regu-
lation of negative emotions, yet the fact that
these studies did not directly measure emo-
tion regulation limits the inferences that can
be made.
Some PET resting state studies have also
reported elevated metabolism in depressed
versus nondepressed individuals in ventral
regions of the PFC, including ventrolateral
and orbitofrontal cortex (Savitz & Drevets,
2009), which is more difficult to recon-
cile with a deficit in emotion regulation. It
should be noted though that this region of
the PFC serves a more general role in emo-
tion processing than its posited role in top-
down emotion regulation, including flexible
updating of stimulus value, as well as moni-
toring of the autonomic state (Rolls, 2004).
In addition to top-down projections to the
amygdala, it also receives excitatory input
from both the amygdala and sensory corti-
ces. In the absence of top-down regulatory
signals, then, hyperactivity in ventral PFC
in depressed individuals might reflect more
bottom-up- driven elaborative or ruminative
processing of emotional information.
A number of fMRI studies have mea-
sured prefrontal cortical activation dur-
ing instructed emotion regulation tasks.
Beauregard, Paquette, and Levesque (2006)
The Neural Basis of Emotion Dysregulation 65
reported increased dorsal ACC activation in
depressed compared with healthy individu-
als when they were decreasing emotional
responses to sad films. No group differences
were found in the more anterior and lateral
PFC regions found previously in studies of
reappraisal in nondepressed samples. In a
study of reappraisal of negatively valenced
emotional pictures, Johnstone et al. (2007)
found that healthy individuals showed left-
lateralized activation of ventrolateral PFC,
whereas depressed individuals showed bilat-
eral activation. In the healthy group, vmPFC
activation was negatively correlated with
amygdala activation and found statistically
to mediate a negative association between
ventrolateral PFC and the amygdala, con-
sistent with a top-down regulation path-
way from ventrolateral PFC via vmPFC to
the amygdala. However, for the depressed
group, a positive correlation was found
between vmPFC and amygdala activation,
with no correlation between lateral PFC and
amygdala. One possible explanation for this
finding is that in depressed individuals, the
lack of regulatory input from left dorsal or
lateral prefrontal regions results in sustained
vmPFC activation, under either greater
bottom-up or right lateral PFC influence.
Both explanations would be consistent with
the elevated vmPFC metabolism in depres-
sion seen in some PET studies, perhaps
reflecting greater self- referential processing.
Results consistent with a model of
reduced lateral PFC top-down regulation of
amygdala in depression have now been seen
in a number of emotion regulation stud-
ies, although lateralization of this reduced
PFC involvement has not been consistently
found across studies. In a study with par-
tially remitted depressed patients, Erk et
al. (2010) demonstrated that although
depressed patients are able to down- regulate
their amygdala using distancing, a form of
reappraisal, the degree of down- regulation
was diminished with increasing scores of
depression (i.e., the more depressed the
patients were, the less they were able to
down- regulate the amygdala). This could be
explained by reduced activation of the right
dorsolateral PFC, which also showed signif-
icantly reduced coupling with the amygdala
in depressed patients.
Erk et al. (2010) also showed differences
between healthy and depressed individuals
in sustained effects of emotion regulation.
In healthy controls, regulation effects on
the amygdala could still be demonstrated
after the emotion regulation task; negative
pictures that had been paired with a down-
regulation instruction invoked not only
reduced amygdala activation compared to
an attend condition during the initial emo-
tion regulation task but also reduced amyg-
dala activation in a simple picture- watching
task 15 minutes later. Furthermore, the
degree of reduction in the second task was
correlated with dorsolateral PFC activa-
tion in the first task. In contrast, depressed
patients had no such lasting effect of emo-
tion regulation; activation in response to
previously down- regulated pictures was no
different than that in response to nonregu-
lated pictures. Together, these findings sug-
gest that depression is characterized not by
the negative mood itself but by the tendency
to get stuck in that mood (Holtzheimer &
Mayberg, 2011).
Kanske, Heissler, Schönfelder, and Wessa
(2012) recently described how remitted
patients with a diagnosis of depression
show a deficit in down- regulation of the
amygdala when reappraising socially nega-
tive pictures but not when using a distrac-
tion strategy. Additionally, they found that
down- regulation of the amygdala was bet-
ter in those patients who habitually used
reappraisal strategies, as measured with the
Emotion Regulation Questionnaire (ERQ;
Gross & John, 2003). This finding extends
the work of Abler, Erk, Herwig, and Walter
(2007), whose examination of patients with
concurrent depression demonstrated that
those who had higher reappraisal scores, as
measured with the ERQ, showed less antici-
patory amygdala activation to negative stim-
uli. Kanske et al.’s study (2012) shows that
neurobiological deficits during reappraisal
extend into remission, pointing to the pos-
sibility that impaired emotion regulation of
negative stimuli might be a trait marker of
depression, as suggested previously in behav-
ioral studies (Ehring, Fischer, Schnülle,
Bösterling, & Tuschen- Caffier, 2008).
Dysregulation of Positive Emotion:
Anhedonia
The dysregulation of emotion in depression
is not limited to negative mood and emo-

66 BIOLOGICAL BASES
tions. One of the core features of depres-
sion is anhedonia, a deficit in the experi-
ence of positive emotions and the ability
to take pleasure from previously rewarding
stimuli. Anhedonia is a particularly debili-
tating condition, because it removes the
pleasurable experiences that motivate much
of what we do in life. The neural basis of
anhedonia is not well understood, but it is
likely that it involves the frontostriatal net-
work, which includes connected regions of
the striatum, particularly the ventral stria-
tum,
3
and the PFC. Based on extensive ani-
mal and human research, the frontostriatal
network is regarded as a core system for the
learning of reward signals, the experience
of pleasure, and the motivation of reward-
seeking behaviors. Despite its role in positive
emotion, however, research linking the fron-
tostriatal network to anhedonia has been
mixed, with some studies reporting reduced
activity in the striatum (Epstein et al., 2006;
Keedwell, Andrew, Williams, Brammer, &
Phillips, 2005) and others showing no such
effect (Knutson, Bhanji, Cooney, Atlas, &
Gotlib, 2008; Schaefer, Putnam, Benca, &
Davidson, 2006).
One explanation for these inconsisten-
cies might be the model of anhedonia being
tested. Anhedonia has often been consid-
ered to reflect a tonically dampened posi-
tive emotion system, in which the general
capacity to experience pleasure is reduced
(Meehl, 1975). An alternative hypothesis is
that an inability to prolong the duration of
positive emotion, or increase the intensity
of a rewarding experience— both aspects of
emotion regulation— might underlie at least
some anhedonic symptoms (Tomarken &
Keener, 1998). The ventral striatum has bidi-
rectional connections with a number of pre-
frontal cortical regions, and brain imaging
studies have shown top-down modulation of
ventral striatal activation in humans (Del-
gado, Gillis, & Phelps, 2008; Staudinger,
Erk, & Walter, 2011).
Compared with the regulation of emo-
tional responses to negative stimuli, there
have been very few studies of top-down
regulation of positive emotions. Kim and
Hamann (2007) showed that regulation of
positive emotion engaged prefrontal regions
partially overlapping those involved in regu-
lating negative emotions, and that subcor-
tical (e.g., amygdala) effects of regulating
positive emotions were more pronounced
than those for negative emotions. In a recent
study of up- regulation of positive emotion in
response to pleasant pictures, Heller et al.
(2009) found that, compared with controls,
patients with depression were unable to sus-
tain activation in the ventral striatum across
an experimental session, and that the degree
to which ventral striatal activity was sus-
tained correlated with self- reported positive
emotion, as well as functional connectivity
between the ventral striatum and a region
in lateral PFC. In a follow- up study on the
same patients after 8 weeks of antidepres-
sant treatment, Heller et al. (2013) found
that those who demonstrated the greatest
increase in their ability to sustain ventral
striatal activation and frontostriatal con-
nectivity while up- regulating positive emo-
tion also showed the largest increases in
positive emotion. It is worth noting that this
study only involved antidepressant treat-
ment, and it is unclear whether those show-
ing greatest symptom improvement did so
as a consequence of increased frontostriatal
engagement. Future studies that examine the
neural impact of cognitive- behavioral thera-
pies on regulation of positive emotions will
shed further light on the causes and possible
treatments for anhedonia. In an example of
neurally guided emotion regulation therapy,
a recently published proof of concept study,
Linden et al. (2012) used neurofeedback
with fMRI to target this problem in depres-
sion. Eight patients with depression learned
to increase activation in brain regions
thought to be related to positive emotions,
such as the insula and the vMPFC, during
four neurofeedback sessions. Their depres-
sive symptoms were reduced significantly,
whereas a control group that underwent a
training procedure with the same cognitive
strategies, but without neurofeedback, did
not show clinical improvement.
Genes, Development,
and Emotion Regulation
Although much has been learned in recent
years about the neural underpinnings of
disorders of emotion regulation, we are a
long way from understanding the enormous
individual differences in our ability to regu-
late our moods and emotions. What are the
The Neural Basis of Emotion Dysregulation 67
causes of these individual differences? To
what extent do they reflect innate biologi-
cal differences? Are there aspects of develop-
ment that render some individuals more at
risk to psychopathology related to emotion
dysregulation?
It is well known that individual differ-
ences in emotional experience and regula-
tion are modulated by differences in genetic
makeup. One prominent example is genetic
variation within the promoter region of the
serotonin transporter gene (5-HTTLPR) in
which the long variant has been associated
with anxiety and neuroticism, as well as
with depression, particularly in combination
with stressful life events (Karg, Burmeister,
Shedden, & Sen, 2011). Hariri, Mattay, Tes-
sitore, Fera, and Weinberger (2003) showed
that the short variant of the 5-HTTLPR is
also associated with increased activation of
the amygdala in reaction to aversive stimuli,
a finding that has been replicated quite con-
sistently (Caspi, Hariri, Holmes, Uher, &
Moffitt, 2010).
Indirect evidence suggesting that genetic
differences in 5-HTTLPR might be asso-
ciated with regulation of emotion came
from findings that short allele carriers
(s- carriers) show decreased connectivity of
amygdala and the perigenual cingulate (a
ventral prefrontal region previously impli-
cated in extinction of aversive conditioning),
and that the lower this connectivity was
in s- carriers, the higher their anxious tem-
perament (Pezawas et al., 2005). Heinz et
al. (2005) found greater vmPFC– amygdala
coupling in s- carriers, however, a result that
is difficult to reconcile with the Pezewas
et al. (2005) results, although the locus of
prefrontal activation was different. Another
study using a sad mood induction and arte-
rial spin- labeling brain imaging (which mea-
sures blood perfusion) showed increased
amygdala activation in s- carriers not during
sad mood induction but during recovery, in
which emotion regulatory processes can be
assumed (Gillihan et al., 2010).
More direct evidence of the possible links
between genetic variation in the 5-HTTLPR
and emotion regulation comes from stud-
ies that used cognitive reappraisal tasks.
Schardt et al. (2010) found that, consis-
tent with prior studies, amygdala activa-
tion during a passive viewing condition was
increased in s- carriers. However, during
active emotion regulation there were no dif-
ferences in amygdala activation. Moreover,
it was shown that subjects with an s- allele
showed higher coupling of right amygdala
with the vmPFC when they regulated their
emotions. Although this appears to be coun-
terintuitive, it has to be noted that prior
studies investigating amygdala reactivity in
s- carriers used passive viewing conditions.
It seems that although s- carriers have an
increased reactivity to passive stimulation,
they can compensate with active regulation,
possibly mediated by increased connectivity
of vmPFC and amygdala relative to long-
allele carriers (l- carriers). This is consistent
with a study in which Lemogne et al. (2011)
found decreased amygdala activation and
increased connectivity of right amygdala
and subgenual ACC in s- carriers when they
compared labeling of affective pictures (a
simple form of emotion regulation) with
self- referential processing of those pictures.
A study by Friedel et al. (2009) has dem-
onstrated increased prefrontal activation
and PFC– amygdala connectivity in healthy
s- carriers, but no such effects in s- carriers
with depression, indicating that the ability
to regulate negative emotion may be a pro-
tective factor against elevated risks associ-
ated with the short allele. The question then
arises as to what enables some individuals,
but not others, to compensate in this way.
As a cautionary note, it also should be men-
tioned that within the long allele there exists
a rare variant (with an A to G exchange) that
behaves like a short allele with respect to
transcription of 5-HTTLPR. This was only
taken into account by some researchers (e.g.,
Friedel et al., 2009), so that results of other
studies have to be taken as preliminary.
Whereas it is clear that some aspects of
emotional reactivity might be tied to innate
individual differences in temperament, our
ability to regulate emotions flexibly and
appropriately is something that develops
over time. Emotion regulation draws on a
range of cognitive and attentional skills and
strategies that have differing developmental
profiles. In addition, the prefrontal brain
regions that have consistently been associ-
ated with emotion regulation are among
the latest parts of the brain to mature fully.
Accumulating evidence from brain imaging
studies points to differential engagement of
prefrontal brain regions in children and ado-

68 BIOLOGICAL BASES
lescents compared to adults when required
to modulate attention to emotional stimuli
(Monk et al., 2003; Nelson et al., 2003)
or when using explicit strategies to regu-
late emotions (Levesque et al., 2004). Some
researchers point to the mismatch between
development of subcortical brain regions
involved in emotion generation and prefron-
tal regions exerting top-down control as key
to understanding emotion dysregulation in
children and adolescents (Casey, Totten-
ham, Liston, & Durston, 2005). Because the
PFC and its connections with subcortical
limbic structures continue to mature from
childhood through adolescence into early
adulthood, there is a particular risk that
maladaptive prefrontal– limbic connectivity
and associated dysregulation of emotion will
develop, rendering individuals vulnerable to
psychopathology later in life.
Very few researchers have addressed the
neurobiology of childhood or adolescent
emotion regulation. Perlman et al. (2012)
examined emotion regulation in 14 depressed
adolescents (ages 13–17 years). Compared
to an age- matched healthy control group,
the depressed group showed increased
amygdala activation and less connectivity
between amygdala and medial PFC/insula
during the control condition. Frontal–
amygdala connectivity was correlated with
psychosocial function, hinting that those
depressed adolescents with less intact PFC–
amygdala regulatory circuitry were more
severely affected. No differences between
the depressed and nondepressed groups were
seen during the active regulation condition,
however, which might indicate that active
emotion regulation via cognitive reappraisal
is able to overcome differences in baseline
emotion reactivity, at least in the short term,
when explicitly prompted.
In a study of adult patients with depres-
sion, Frodl, Reinhold, Koutsouleris, Rei-
ser, and Meisenzahl (2010) have shown
that previous childhood stress is associated
with reductions in prefrontal gray matter,
and that prefrontal gray matter volume and
childhood neglect interact to predict adult
depression duration. A recent study (Burghy
et al., 2012) showed that elevated cortisol
levels associated with early life stress was
predictive of reduced vmPFC- amygdala rest-
ing state functional connectivity in females.
Furthermore, vmPFC- amygdala resting
state connectivity was inversely correlated
with anxiety symptoms but positively cor-
related with depressive symptoms in female
adolescents, pointing to one possible factor
in the development of PFC- subcortical emo-
tion regulation circuitry. The developmental
neurobiological profile of individual differ-
ences in emotion regulation has barely been
studied, yet it is likely to have far- reaching
consequences for our understanding of emo-
tion dysregulation throughout life.
Cortical Brain Stimulation
as a Treatment for Depression
Up to now we have considered psychologi-
cal self- regulation of emotion. However, it
is also possible to regulate emotions using
technical devices that alter activity in brain
regions that are involved in emotion regu-
lation. These are particularly important
when dysregulation of emotion is present as
a pathological condition. Three techniques
of brain stimulation— repetitive transcra-
nial magnetic stimulation (rTMS), a closely
related technique transcranial direct current
stimulation (tDCS), and deep brain stimula-
tion (DBS)—have recently been proposed as
methods offering a viable treatment for oth-
erwise untreatable depression.
Repetitive Transcranial
Magnetic Stimulation
rTMS involves generating trains of brief
bursts of a strong magnetic field close to
the scalp. When the magnetic field is ori-
ented perpendicular to the cortical surface,
electric currents are induced in the underly-
ing neural tissue to a depth of several mil-
limeters (a consequence of Faraday’s law of
electromagnetic induction). While the exact
mechanism of action of rTMS on networks
of neurons is not yet fully understood, dif-
ferent frequencies of rTMS can have either
short- or long- term facilitatory or disruptive
effects on cortical processing (Fitzgerald,
Fountain, & Daskalakis, 2006). In general,
low- frequency (< 1 Hz) rTMS is thought to
inhibit cortical processing, whereas high-
frequency (> 5 Hz) is thought to enhance
cortical processing. Supporting evidence
for this model of rTMS effects on cortical
function comes from PET studies following
The Neural Basis of Emotion Dysregulation 69
rTMS to lateral PFC, which show greater
blood flow and glucose metabolism fol-
lowing high- frequency rTMS and reduced
metabolism following low- frequency rTMS
(Speer et al., 2000). It is also possible that
rTMS can affect the neurotransmitter sys-
tems that have been linked to depression. In
rodent models, altered dopaminergic neu-
rotransmission in the striatum and altered
serotoninergic neurotransmission the amyg-
dala and cortex have also been observed
following rTMS, though there are concerns
about the applicability of these results to
humans for methodological and anatomical
reasons (Padberg & George, 2009).
For treatment of depression, rTMS has
typically been applied to dorsolateral PFC—
sometimes as low- frequency rTMS to right
dorsolateral PFC to inhibit cortical process-
ing, but most often as high- frequency rTMS
to left dorsolateral PFC to enhance process-
ing. Although results have been mixed, three
recent meta- analytic reviews have concluded
that high- frequency rTMS applied to left
dorsolateral PFC is an effective treatment
for depression. Schutter (2009) analyzed
30 double- blind sham- controlled high-
frequency rTMS studies, and concluded that
rTMS was significantly better than sham
and produced effects comparable in reli-
ability and magnitude of response to anti-
depressant drugs. In a meta- analysis of 34
studies, Slotema, Blom, Hoek, and Sommer
(2010) also found rTMS to be superior to
sham TMS, although the authors concluded
that rTMS was not as effective as electrocon-
vulsive treatment (ECT). A meta- analysis by
Allan, Herrmann, and Ebmeier (2011) of
31 randomized trials of TMS versus a sham
control yielded the same conclusion. Some
caution is needed, however, in that most if
not all the studies have not been truly blind
(i.e., the experimenter applying the stimula-
tion or sham knows which condition is being
applied) and sham TMS is perceptually quite
different from actual TMS.
Transcranial Direct Current
Stimulation
Although first applied prior to TMS, tDCS
has only relatively recently received an
upsurge in interest as a treatment for depres-
sion. It is easy to apply and, compared to
TMS, very inexpensive. tDCS consists of
passing a small direct electrical current of
1–2 milliamps (mA) between two electrodes
placed on the scalp. Most typically in treat-
ment for depression, the anode (the electrode
from which electrons flow) is placed on the
left dorsolateral PFC, and the cathode (the
electrode to which electrons flow) is placed
on the right PFC. Stimulation is then applied
for 10–20 minutes, with repeated treatment
sessions daily or on alternate days. Com-
pared with TMS, relatively more is known
about how tDCS affects neural activity.
When current flows parallel to the axon–
dendrite axis of pyramidal cells in the cor-
tex, neurons under the anode become more
depolarized, which makes them more excit-
able and increases neural firing. The oppo-
site occurs under the cathode, with neurons
becoming hyperpolarized, which leads to
a reduction in firing. Thus, tDCS with an
anode placed over the left dorsolateral PFC
will tend to increase activity in that region
of the brain. The effects of tDCS on corti-
cal excitability can last for a few hours after
application, and there is some evidence that
tDCS can also produce longer term effects
through synaptic changes to glutamate
receptors and possible effects on long- term
potentiation, though evidence is scarce at
present.
Early efforts to use tDCS to treat depres-
sion were unconvincing, possibly due to the
much smaller currents that were used, as
well as the mode and site of electrode place-
ments typically used. More recent studies
with greater consistency of target region (left
dorsolateral PFC), stimulation strength, and
timing have led to more promising results,
with a meta- analysis reporting significant
improvements in depression symptoms fol-
lowing tDCS versus sham (Allan et al., 2011),
though many studies are small and there are
fewer overall studies than those using rTMS.
Most recently, in a double- blind study, 64
depressed patients showed improvement in
mood when tDCS versus sham was used,
though the overall percentage of responders
did not differ between the two conditions
(Loo et al., 2012), highlighting the tentative
nature of results to date.
Both rTMS and tDCS have achieved their
most promising results for treatment of
depression when applied over left dorsolat-
eral PFC in an excitatory mode (e.g., high
frequency for rTMS and anode placement

70 BIOLOGICAL BASES
for tDCS). Although the exact mechanism of
action for both brain stimulation treatments
remains unknown, it is noteworthy that the
dorsolateral PFC is consistently activated in
brain imaging studies of reappraisal, and
plays an important role in working mem-
ory and executive control. The implication
is that lack of engagement of dorsolateral
PFC, specifically left dorsolateral PFC, in
depressed patients compromises their ability
to regulate their emotions, and that stimu-
lating these areas leads to an improvement
in emotion regulation through reappraisal
or executive control. Until researchers spe-
cifically test the effects of dorsolateral PFC
stimulation (either tDCS or rTMS) on regu-
lation of emotion, this hypothesis remains
speculative. One possibility to enhance the
effect of rTMS or tDCS is to use neuroim-
aging to determine which hemisphere of the
PFC is dysfunctional to direct the stimula-
tion protocol according to these findings
(Schönfeldt- Lecuona et al., 2010), possibly
using an emotion regulation task. Given that
prefrontal tDCS has been shown to improve
learning in working memory tasks (Brasil-
Neto, 2012) it is conceivable that tDCS could
be applied during the training of psychologi-
cal emotion regulation in order to boost the
learning of emotion regulation strategies.
Deep Brain Stimulation
DBS involves the delivery of a small electric
current directly to parts of the brain through
implanted wire electrodes, with stimulation
delivered via an implantable pulse genera-
tor. As an invasive surgical procedure, it has
so far been investigated as a treatment for
the most intractable and debilitating cases
of major depressive disorder in a number
of small, open- label (i.e., nonblind) stud-
ies. The most common site for stimula-
tion is the white matter in the subcallosal
cingulate region, chosen on the basis of its
connectivity with brain regions thought to
be important for antidepressant response
(Holtzheimer & Mayberg, 2011; Lozano et
al., 2008; Mayberg et al., 2005). These stud-
ies have shown remission rates of 40–60%
over an extended period (up to several years)
in patients with severe depression who have
failed to respond to any other treatments.
Given the relatively small sample sizes, it is
difficult to pinpoint exactly how subcallo-
sal DBS is achieving these promising results,
although Holtzheimer and Mayberg (2011)
suggest that modulation of a mood regula-
tion network may be the underlying mecha-
nism.
Conclusions
Research on how the human brain creates
and regulates our emotions is still in its
infancy, but it has already given us remark-
able insights into the complexity of the brain
systems responsible for shaping our moods
and emotions. In many ways human brain
imaging studies have validated the notion
that the neural systems supporting flexible
emotion regulation are a key component of
mental health. Knowledge of the role of spe-
cific corticolimbic networks in emotion dys-
regulation and psychopathology is now lead-
ing to new experimental treatments involving
brain stimulation and neurofeedback. This
progress notwithstanding, there are many
outstanding questions to be resolved. One
central question is the exact role of different
subregions of PFC in different types of emo-
tion (dys)regulation. For example, although
there is consistent evidence for vmPFC
involvement in extinction recall, the exact
role of vmPFC in more explicit, cognitive
types of emotion regulation such as reap-
praisal remains unclear. It is possible that
this question will be answered not by rela-
tively crude fMRI measures of the amount
of vmPFC activation but with more nuanced
techniques that can capture changes to the
way vmPFC encodes the hedonic value of
stimuli, perhaps under “direction” from lat-
eral PFC. Multivariate pattern analysis (cf.
Davis & Poldrack, 2013), for example, can
probe not only how much a brain region is
engaged during a task but also the extent to
which it represents different types of infor-
mation. Combining such methods with mea-
sures of whole- brain effective and functional
connectivity should lead to testable models
of emotion regulation networks.
Another unresolved question is how dif-
ferent emotion regulation disturbances map
onto different symptoms. In this chapter, we
have rather simplistically discussed anxiety
disorders and depression separately, even
though there is a large amount of comorbid-
ity between these and other disorders. Are

The Neural Basis of Emotion Dysregulation 71
there common or distinct emotion regula-
tion processes involved? To what extent is
emotion dysregulation distinct, or does it
overlap with more general disturbances in
top-down control? To address these fun-
damental questions will require larger
scale studies (including meta- analyses) that
combine data from different samples and
experimental tasks (e.g., nonemotional tasks
involving attentional control, working mem-
ory, and response inhibition).
Further research on the impact of genetic
variation and early life environment on the
development of the brain systems that allow
us to regulate our emotions will also be
important if we are to develop useful predic-
tors of vulnerability to disorders of emotion
regulation. Along similar lines, we know
almost nothing about how age- related neu-
ral decline affects emotion regulation. Some
of the brain regions showing the greatest
age- related atrophy, such as lateral PFC, are
a key component of emotion regulation cir-
cuitry. Do older individuals show the same
sorts of age- related decline in emotion regu-
lation as they do in cognitive function? Early
evidence suggests that the picture might not
be so straightforward (van Reekum et al.,
2011; Winecoff, LaBar, Madden, Cabeza, &
Huettel, 2011). Far more cross- sectional and
longitudinal research relating age, measures
of cortical integrity, cognitive function, and
emotion regulation capability is required to
answer these important questions.
Notes
1. In this chapter we have adopted a liberal con-
sideration of emotion and mood, using the
terms interchangeably. Although these terms
might be considered more or less distinct by
emotion theorists, the evidence for a clear dis-
tinction in terms of neurobiology is lacking.
In addition, from a clinical standpoint, mood
dysregulation is at least as important as emo-
tion dysregulation.
2. Most studies have not explicitly tested the
hemispheric lateralization of prefrontal acti-
vation, so caution must be exercised in infer-
ring preferential involvement of one hemi-
sphere in reappraisal of emotional stimuli.
3. Although the term ventral striatum is some-
times used interchangeably with nucleus
accumbens, the latter refers to a specific
nucleus, and the former refers to a subregion
of the striatum, which might contain a more
varied collection of neurons.
References
Abler, B., Erk, S., Herwig, U., & Walter, H.
(2007). Anticipation of aversive stimuli acti-
vates extended amygdala in unipolar depres-
sion. Journal of Psychiatric Research, 41(6),
511–522.
Allan, C. L., Herrmann, L. L., & Ebmeier, K.
P. (2011). Transcranial magnetic stimulation
in the management of mood disorders. Neuro-
psychobiology, 64(3), 163–169.
Bar- Haim, Y., Lamy, D., Pergamin, L.,
Bakermans- Kranenburg, M. J., & van IJzen-
doorn, M. H. (2007). Threat- related atten-
tional bias in anxious and nonanxious indi-
viduals: A meta- analytic study. Psychological
Bulletin, 133(1), 1–24.
Beauregard, M., Paquette, V., & Levesque, J.
(2006). Dysfunction in the neural circuitry of
emotional self- regulation in major depressive
disorder. NeuroReport, 17(8), 843–846.
Bilkei- Gorzo, A., Erk, S., Schurmann, B., Mauer,
D., Michel, K., Boecker, H., et al. (2012). Dyn-
orphins regulate fear memory: From mice to
men. Journal of Neuroscience, 32(27), 9335–
9343.
Bishop, S. J. (2008). Trait anxiety and impover-
ished prefrontal control of attention. Nature
Neuroscience, 12(1), 92–98.
Bishop, S. J., Duncan, J., Brett, M., & Lawrence,
A. D. (2004). Prefrontal cortical function
and anxiety: Controlling attention to threat-
related stimuli. Nature Neuroscience, 7(2),
184–188.
Blair, K. S., Smith, B. W., Mitchell, D. G. V.,
Morton, J., Vythilingam, M., Pessoa, L., et al.
(2007). Modulation of emotion by cognition
and cognition by emotion. NeuroImage, 35(1),
430–440.
Bouton, M. E. (2004). Context and behavioral
processes in extinction. Learning and Mem-
ory, 11(5), 485–494.
Brasil- Neto, J. P. (2012). Learning, memory, and
transcranial direct current stimulation. Fron-
tiers in Neuropsychiatric Imaging and Stimu-
lation, 3, 80.
Burghy, C. A., Stodola, D. E., Ruttle, P. L.,
Molloy, E. K., Armstrong, J. M., Oler, J. A.,
et al. (2012). Developmental pathways to
amygdala– prefrontal function and internaliz-
72 BIOLOGICAL BASES
ing symptoms in adolescence. Nature Neuro-
science, 15(12), 1736–1741.
Campbell- Sills, L., Ellard, K., & Barlow, D. (this
volume, 2014). Emotion regulation in anxiety
disorders. In J. J. Gross (Ed.), Handbook of
emotion regulation (2nd ed., pp. 393–412).
New York: Guilford Press.
Casey, B. J., Tottenham, N., Liston, C., & Dur-
ston, S. (2005). Imaging the developing brain:
What have we learned about cognitive devel-
opment? Trends in Cognitive Sciences, 9(3),
104–110.
Caspi, A., Hariri, A. R., Holmes, A., Uher, R., &
Moffitt, T. E. (2010). Genetic sensitivity to the
environment: The case of the serotonin trans-
porter gene and its implications for studying
complex diseases and traits. American Journal
of Psychiatry, 167(5), 509–527.
Cisler, J. M., & Koster, E. H. W. (2010). Mecha-
nisms of attentional biases towards threat in
anxiety disorders: An integrative review. Clin-
ical Psychology Review, 30(2), 203–216.
Clarke, R. J., & Johnstone, T. (2013). Prefrontal
inhibition of threat processing protects work-
ing memory from interference. Frontiers in
Human Neuroscience, 7, 228.
Corson, H. (1890). An introduction to the study
of Shakespeare. Boston: Heath. Retrieved
from http://archive.org/details/introduction-
tost01cors.
Davidson, R. J., Pizzagalli, D., Nitschke, J. B., &
Putnam, K. (2002). Depression: Perspectives
from affective neuroscience. Annual Review
of Psychology, 53(1), 545–574.
Davis, M. (2006). Neural systems involved
in fear and anxiety measured with fear-
potentiated startle. American Psychologist,
61(8), 741–756.
Davis, T., & Poldrack, R. A. (2013). Measuring
neural representations with fMRI: Practices
and pitfalls. Annals of the New York Acad-
emy of Sciences.
Delgado, M. R., Gillis, M. M., & Phelps, E. A.
(2008). Regulating the expectation of reward
via cognitive strategies. Nature Neuroscience,
11(8), 880–881.
Delgado, M. R., Nearing, K. I., LeDoux, J.
E., & Phelps, E. A. (2008). Neural circuitry
underlying the regulation of conditioned fear
and its relation to extinction. Neuron, 59(5),
829–838.
Ehring, T., Fischer, S., Schnülle, J., Bösterling,
A., & Tuschen- Caffier, B. (2008). Charac-
teristics of emotion regulation in recovered
depressed versus never depressed individuals.
Personality and Individual Differences, 44(7),
1574–1584.
Epstein, J., Pan, H., Kocsis, J. H., Yang, Y.,
Butler, T., Chusid, J., et al. (2006). Lack of
ventral striatal response to positive stimuli in
depressed versus normal subjects. American
Journal of Psychiatry, 163(10), 1784–1790.
Erk, S., Kleczar, A., & Walter, H. (2007).
Valence- specific regulation effects in a work-
ing memory task with emotional context.
NeuroImage, 37(2), 623–632.
Erk, S., Mikschl, A., Stier, S., Ciaramidaro, A.,
Gapp, V., Weber, B., et al. (2010). Acute and
sustained effects of cognitive emotion regula-
tion in major depression. Journal of Neurosci-
ence, 30(47), 15726–15734.
Erthal, F., De Oliveira, L., Mocaiber, I., Pereira,
M., Machado- Pinheiro, W., Volchan, E., et al.
(2005). Load- dependent modulation of affec-
tive picture processing. Cognitive, Affective,
and Behavioral Neuroscience, 5(4), 388–395.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E.
R., & Hirsch, J. (2006). Resolving emotional
conflict: A role for the rostral anterior cingu-
late cortex in modulating activity in the amyg-
dala. Neuron, 51(6), 871–882.
Etkin, A., Klemenhagen, K. C., Dudman, J. T.,
Rogan, M. T., Hen, R., Kandel, E. R., et al.
(2004). Individual differences in trait anxiety
predict the response of the basolateral amyg-
dala to unconsciously processed fearful faces.
Neuron, 44(6), 1043–1055.
Etkin, A., & Wager, T. D. (2007). Functional
neuroimaging of anxiety: A meta- analysis of
emotional processing in PTSD, social anxiety
disorder, and specific phobia. American Jour-
nal of Psychiatry, 164(10), 1476–1488.
Fitzgerald, P. B., Fountain, S., & Daskalakis,
Z. J. (2006). A comprehensive review of the
effects of rTMS on motor cortical excitabil-
ity and inhibition. Clinical Neurophysiology,
117(12), 2584–2596.
Fitzgerald, P. B., Laird, A. R., Maller, J., & Das-
kalakis, Z. J. (2008). A meta- analytic study
of changes in brain activation in depression.
Human Brain Mapping, 29(6), 683–695.
Friedel, E., Schlagenhauf, F., Sterzer, P., Park, S.,
Bermpohl, F., Ströhle, A., et al. (2009). 5-HTT
genotype effect on prefrontal– amygdala cou-
pling differs between major depression and
controls. Psychopharmacology, 205(2), 261–
271.
Frijda, N. H. (1986). The emotions. Cambridge,
UK: Cambridge University Press.
Frodl, T., Reinhold, E., Koutsouleris, N., Reiser,
The Neural Basis of Emotion Dysregulation 73
M., & Meisenzahl, E. M. (2010). Interaction
of childhood stress with hippocampus and
prefrontal cortex volume reduction in major
depression. Journal of Psychiatric Research,
44(13), 799 – 807.
Ghashghaei, H. T., Hilgetag, C. C., & Barbas,
H. (2007). Sequence of information process-
ing for emotions based on the anatomic dia-
logue between prefrontal cortex and amyg-
dala. NeuroImage, 34(3), 905–923.
Gilbert, K. E. (2012). The neglected role of posi-
tive emotion in adolescent psychopathology.
Clinical Psychology Review, 32(6), 467–481.
Gillihan, S. J., Rao, H., Wang, J., Detre, J. A.,
Breland, J., Sankoorikal, G. M. V., et al.
(2010). Serotonin transporter genotype modu-
lates amygdala activity during mood regula-
tion. Social Cognitive and Affective Neurosci-
ence, 5(1), 1–10.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross, (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well- being. Journal of Personality and Social
Psychology, 85(2), 348–362.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera,
F., & Weinberger, D. R. (2003). Neocorti-
cal modulation of the amygdala response to
fearful stimuli. Biological Psychiatry, 53(6),
494–501.
Heinz, A., Braus, D. F., Smolka, M. N., Wrase,
J., Puls, I., Hermann, D., et al. (2005).
Amygdala– prefrontal coupling depends on a
genetic variation of the serotonin transporter.
Nature Neuroscience, 8(1), 20–21.
Heller, A. S., Johnstone, T., Light, S. N., Peter-
son, M. J., Kolden, G. G., Kalin, N. H., et al.
(2013). Relationships between changes in sus-
tained fronto- striatal connectivity and posi-
tive affect in major depression resulting from
antidepressant treatment. American Journal
of Psychiatry, 170(2), 197–206.
Heller, A. S., Johnstone, T., Shackman, A. J.,
Light, S. N., Peterson, M. J., Kolden, G. G.,
et al. (2009). Reduced capacity to sustain
positive emotion in major depression reflects
diminished maintenance of fronto- striatal
brain activation. Proceedings of the National
Academy of Sciences, 106(52), 22445–22450.
Holtzheimer, P. E., & Mayberg, H. S. (2011).
Stuck in a rut: Rethinking depression and its
treatment. Trends in Neurosciences, 34(1),
1–9.
Jackson, D. C., Malmstadt, J. R., Larson, C.
L., & Davidson, R. J. (2000). Suppression
and enhancement of emotional responses to
unpleasant pictures. Psychophysiology, 37(4),
515–522.
Johnstone, T., van Reekum, C. M., Urry, H. L.,
Kalin, N. H., & Davidson, R. J. (2007). Failure
to regulate: Counterproductive recruitment of
top-down prefrontal– subcortical circuitry in
major depression. Journal of Neuroscience,
27(33), 8877–8884.
Kalisch, R., Korenfeld, E., Stephan, K. E., Weis-
kopf, N., Seymour, B., & Dolan, R. J. (2006).
Context- dependent human extinction memory
is mediated by a ventromedial prefrontal and
hippocampal network. Journal of Neurosci-
ence, 26(37), 9503–9511.
Kanske, P., Heissler, J., Schönfelder, S., & Wessa,
M. (2012). Neural correlates of emotion reg-
ulation deficits in remitted depression: The
influence of regulation strategy, habitual regu-
lation use, and emotional valence. NeuroIm-
age, 61(3), 686–693.
Karg, K., Burmeister, M., Shedden, K., & Sen,
S. (2011). The serotonin transporter promoter
variant (5-HTTLPR), stress, and depression
meta- analysis revisited: Evidence of genetic
moderation. Archives of General Psychiatry,
68(5), 444–454.
Keedwell, P. A., Andrew, C., Williams, S. C. R.,
Brammer, M. J., & Phillips, M. L. (2005).
The neural correlates of anhedonia in major
depressive disorder. Biological Psychiatry,
58(11), 843–853.
Kim, S. H., & Hamann, S. (2007). Neural cor-
relates of positive and negative emotion regu-
lation. Journal of Cognitive Neuroscience,
19(5), 776–798.
Knutson, B., Bhanji, J. P., Cooney, R. E., Atlas, L.
Y., & Gotlib, I. H. (2008). Neural responses to
monetary incentives in major depression. Bio-
logical Psychiatry, 63(7), 686–692.
Lemogne, C., Gorwood, P., Boni, C., Pessiglione,
M., Lehéricy, S., & Fossati, P. (2011). Cogni-
tive appraisal and life stress moderate the
effects of the 5-HTTLPR polymorphism on
amygdala reactivity. Human Brain Mapping,
32(11), 1856–1867.
74 BIOLOGICAL BASES
Levesque, J., Joanette, Y., Mensour, B., Beaudoin,
G., Leroux, J. M., Bourgouin, P., et al. (2004).
Neural basis of emotional self- regulation in
childhood. Neuroscience, 129(2), 361–369.
Linden, D. E. J., Habes, I., Johnston, S. J., Lin-
den, S., Tatineni, R., Subramanian, L., et al.
(2012). Real- time self- regulation of emotion
networks in patients with depression. PLoS
ONE, 7(6), e38115.
Loo, C. K., Alonzo, A., Martin, D., Mitchell, P.
B., Galvez, V., & Sachdev, P. (2012). Transcra-
nial direct current stimulation for depression:
3-week, randomised, sham- controlled trial.
British Journal of Psychiatry, 200(1), 52–59.
Lozano, A. M., Mayberg, H. S., Giacobbe, P.,
Hamani, C., Craddock, R. C., & Kennedy,
S. H. (2008). Subcallosal cingulate gyrus
deep brain stimulation for treatment- resistant
depression. Biological Psychiatry, 64(6), 461–
467.
MacLeod, C., Mathews, A., & Tata, P. (1986).
Attentional bias in emotional disorders. Jour-
nal of Abnormal Psychology, 95(1), 15–20.
Mathers, C. D., Lopez, A. D., & Murray, C. J.
L. (2006). The burden of disease and mortal-
ity by condition: Data, methods, and results
for 2001. In A. D. Lopez, C. D. Mathers,
M. Ezzati, D. T. Jamison, & C. J. L. Murray
(Eds.), Global burden of disease and risk fac-
tors (pp. 45–241). New York: World Bank/
Oxford University Press.
Mayberg, H. S., Lozano, A. M., Voon, V.,
McNeely, H. E., Seminowicz, D., Hamani,
C., et al. (2005). Deep brain stimulation for
treatment- resistant depression. Neuron, 45(5),
651–660.
Meehl, P. E. (1975). Hedonic capacity: Some
conjectures. Bulletin of the Menninger Clinic,
39(4), 295–307.
Milad, M. R., & Quirk, G. J. (2002). Neurons
in medial prefrontal cortex signal memory for
fear extinction. Nature, 420(6911), 70–74.
Milad, M. R., & Quirk, G. J. (2012). Fear extinc-
tion as a model for translational neuroscience:
Ten years of progress. Annual Review of Psy-
chology, 63(1), 129–151.
Milad, M. R., Wright, C. I., Orr, S. P., Pitman,
R. K., Quirk, G. J., & Rauch, S. L. (2007).
Recall of fear extinction in humans activates
the ventromedial prefrontal cortex and hip-
pocampus in concert. Biological Psychiatry,
62(5), 446–454.
Mitchell, D. G. V. (2011). The nexus between
decision making and emotion regulation: A
review of convergent neurocognitive sub-
strates. Behavioural Brain Research, 217(1),
215–231.
Monk, C. S., McClure, E. B., Nelson, E. E.,
Zarahn, E., Bilder, R. M., Leibenluft, E., et al.
(2003). Adolescent immaturity in attention-
related brain engagement to emotional facial
expressions. NeuroImage, 20(1), 420–428.
Nelson, E. E., McClure, E. B., Monk, C. S.,
Zarahn, E., Leibenluft, E., Pine, D. S., et al.
(2003). Developmental differences in neuro-
nal engagement during implicit encoding of
emotional faces: An event- related fMRI study.
Journal of Child Psychology and Psychiatry
and Allied Disciplines, 44(7), 1015–1024.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 23–42). New York: Guilford
Press.
Padberg, F., & George, M. S. (2009). Repeti-
tive transcranial magnetic stimulation of the
prefrontal cortex in depression. Experimental
Neurology, 219(1), 2–13.
Perlman, G., Simmons, A. N., Wu, J., Hahn,
K. S., Tapert, S. F., Max, J. E., et al. (2012).
Amygdala response and functional connectiv-
ity during emotion regulation: A study of 14
depressed adolescents. Journal of Affective
Disorders, 139(1), 75–84.
Pezawas, L., Meyer- Lindenberg, A., Drab-
ant, E. M., Verchinski, B. A., Munoz, K. E.,
Kolachana, B. S., et al. (2005). 5-HTTLPR
polymorphism impacts human cingulate–
amygdala interactions: A genetic susceptibility
mechanism for depression. Nature Neurosci-
ence, 8(6), 828–834.
Phelps, E. A., Delgado, M. R., Nearing, K. I., &
LeDoux, J. E. (2004). Extinction learning in
humans: Role of the amygdala and vmPFC.
Neuron, 43(6), 897–905.
Posner, M. I., Snyder, C. R., & Davidson, B. J.
(1980). Attention and the detection of signals.
Journal of Experimental Psychology, 109(2),
160–174.
Ridderinkhof, K. R., Ullsperger, M., Crone, E.
A., & Nieuwenhuis, S. (2004). The role of the
medial frontal cortex in cognitive control. Sci-
ence, 306(5695), 443–447.
Rolls, E. T. (2004). The functions of the orbi-
tofrontal cortex. Brain and cognition, 55(1),
11–29.
Savitz, J., & Drevets, W. C. (2009). Bipolar and
major depressive disorder: Neuroimaging the
developmental- degenerative divide. Neuro-
The Neural Basis of Emotion Dysregulation 75
science and Biobehavioral Reviews, 33(5),
699–771.
Schaefer, H. S., Putnam, K. M., Benca, R. M.,
& Davidson, R. J. (2006). Event- related func-
tional magnetic resonance imaging measures
of neural activity to positive social stimuli in
pre- and post- treatment depression. Biological
Psychiatry, 60(9), 974–986.
Schardt, D. M., Erk, S., Nüsser, C., Nöthen, M.
M., Cichon, S., Rietschel, M., et al. (2010).
Volition diminishes genetically mediated
amygdala hyperreactivity. NeuroImage, 53(3),
943–951.
Scherer, K. R., Schorr, A., & Johnstone, T.
(2001). Appraisal processes in emotion: The-
ory, methods, research. New York: Oxford
University Press.
Schönfeldt- Lecuona, C., Lefaucheur, J.-P.,
Cardenas- Morales, L., Wolf, R. C., Kam-
mer, T., & Herwig, U. (2010). The value of
neuronavigated rTMS for the treatment of
depression. Neurophysiologie Clinique, 40(1),
37–43.
Schutter, D. J. L. G. (2009). Antidepressant effi-
cacy of high- frequency transcranial magnetic
stimulation over the left dorsolateral prefron-
tal cortex in double- blind sham- controlled
designs: A meta- analysis. Psychological Medi-
cine, 39(1), 65–75.
Schwartz, C. E., & Rauch, S. L. (2004). Temper-
ament and its implications for neuroimaging
of anxiety disorders. CNS Spectrums, 9(4),
284–291.
Schwartz, C. E., Wright, C. I., Shin, L. M.,
Kagan, J., & Rauch, S. L. (2003). Inhibited
and uninhibited infants “grown up”: Adult
amygdalar response to novelty. Science,
300(5627), 1952–1953.
Shackman, A. J., Sarinopoulos, I., Maxwell,
J. S., Pizzagalli, D. A., Lavric, A., & David-
son, R. J. (2006). Anxiety selectively disrupts
visuospatial working memory. Emotion, 6(1),
40–61.
Shin, L. M., & Liberzon, I. (2010). The neuro-
circuitry of fear, stress, and anxiety disorders.
Neuropsychopharmacology, 35(1), 169–191.
Slotema, C. W., Blom, J. D., Hoek, H. W., &
Sommer, I. E. C. (2010). Should we expand
the toolbox of psychiatric treatment methods
to include repetitive transcranial magnetic
stimulation (rTMS)? Journal of Clinical Psy-
chiatry, 71(7), 873–884.
Somerville, L. H., Wagner, D. D., Wig, G. S.,
Moran, J. M., Whalen, P. J., & Kelley, W. M.
(2012). Interactions between transient and
sustained neural signals support the genera-
tion and regulation of anxious emotion. Cere-
bral Cortex, 23(1), 49– 60.
Speer, A. M., Kimbrell, T. A., Wassermann, E.
M., Repella, J., Willis, M. W., Herscovitch,
P., et al. (2000). Opposite effects of high and
low frequency rTMS on regional brain activity
in depressed patients. Biological Psychiatry,
48(12), 1133–1141.
Staudinger, M. R., Erk, S., & Walter, H. (2011).
Dorsolateral prefrontal cortex modulates
striatal reward encoding during reappraisal of
reward anticipation. Cerebral Cortex, 21(11),
2578–2588.
Tomarken, A. J., & Keener, A. D. (1998). Fron-
tal brain asymmetry and depression: A self-
regulatory perspective. Cognition and Emo-
tion, 12(3), 387–420.
Urry, H. L., van Reekum, C. M., Johnstone, T.,
Kalin, N. H., Thurow, M. E., Schaefer, H.
S., et al. (2006). Amygdala and ventromedial
prefrontal cortex are inversely coupled during
regulation of negative affect and predict the
diurnal pattern of cortisol secretion among
older adults. Journal of Neuroscience, 26(16),
4415–4425.
Van Dillen, L. F., Heslenfeld, D. J., & Koole, S.
L. (2009). Tuning down the emotional brain:
An fMRI study of the effects of cognitive load
on the processing of affective images. Neuro-
Image, 45(4), 1212–1219.
van Reekum, C. M., Schaefer, S. M., Lapate, R.
C., Norris, C. J., Greischar, L. L., & David-
son, R. J. (2011). Aging is associated with
positive responding to neutral information but
reduced recovery from negative information.
Social Cognitive and Affective Neuroscience,
6(2), 177–185.
Wager, T. D., Davidson, M. L., Hughes, B. L.,
Lindquist, M. A., & Ochsner, K. N. (2008).
Prefrontal- subcortical pathways mediating
successful emotion regulation. Neuron, 59(6),
1037–1050.
Walker, D. L., Toufexis, D. J., & Davis, M.
(2003). Role of the bed nucleus of the stria
terminalis versus the amygdala in fear, stress,
and anxiety. European Journal of Pharmacol-
ogy, 463(1–3), 199–216.
Winecoff, A., LaBar, K. S., Madden, D. J.,
Cabeza, R., & Huettel, S. A. (2011). Cognitive
and neural contributors to emotion regulation
in aging. Social Cognitive and Affective Neu-
roscience, 6(2), 165–176.

76
Emotions take center stage in our lives
through the ways they influence how we
think and behave. Yet emotions are not
deterministic; just as much as they influ-
ence us, we have the ability to regulate and
change the way our emotional responses
unfold. Most research on emotion regula-
tion has focused on how people explicitly
(using deliberate, effortful means) accom-
plish the regulation of emotion (e.g., Och-
sner & Gross, 2005). We have previously
argued that it is useful to anchor emotion
regulatory processes along an implicit (non-
conscious, automatic) to explicit (deliberate,
effortful) dimension within a dual- process
framework (Gyurak, Gross, & Etkin, 2011).
In this chapter, we demonstrate the utility of
the implicit– explicit framework to organize
a wealth of neurobiological findings from
basic affective and clinical neuroscience.
We utilize this new expanded framework
to illustrate its benefits for understanding
healthy and disordered emotion regulatory
processes.
We present paradigmatic cases of explicit
and implicit regulation to highlight the par-
allels and differences between implicit and
explicit forms of regulation. We then explore
cases in which implicit and explicit regula-
tory processes blend together, to illustrate
the fluid boundaries between implicit and
explicit regulation in many emotion regula-
tory contexts. By anchoring this conceptual
framework in neurobiological findings, this
model of emotion regulation brings together
basic, clinical, neuroscientific, and trans-
lational research. Our goal in this chapter
is to demonstrate how an understanding
anchored at the implicit– explicit spectrum
informs an understanding of psychopathol-
ogy that cuts across traditional diagnostic
boundaries.
Component Processes
and Definition of Emotion Regulation
The Modal Model of Emotions
Using previous definitions (Gross & Thomp-
son, 2007; Gross, this volume) we define
“emotions” as interpersonal and transac-
tional (Keltner & Haidt, 2001) processes
that play out in the rich fabric of real or
imagined social relationships, for example,
feelings of embarrassment over a social faux
pas, or anger when one is slighted. Emotions
arise when individuals attend to a situation
and see it as relevant to their goals, either
in the short or long term (Levenson, 1999).
As emotions unfold, they engage loosely
coupled changes in the domains of subjec-
tive experience, behavior, and central and
peripheral physiology (Mauss, Levenson,
McCarter, Wilhelm, & Gross, 2005). Many
CHAPTER 5
A Neurobiological Model of Implicit
and Explicit Emotion Regulation
Anett Gyurak
Amit Etkin
Neurobiological Model of Implicit and Explicit Emotion Regulation 77
of the central and peripheral physiologi-
cal reactions have phylogenetic continuity
across species (LeDoux, 2012). Moreover,
these reactions simultaneously include pro-
cesses generally seen as “emotional” (e.g.,
a withdrawal response or a feeling), as well
as “cognitive” (e.g., an attentional focus on
a relevant stimulus; Pessoa, 2010). Finally,
emotions can be viewed as “push” forces
that predispose us to act in certain ways
(Frijda, 1987; Keltner & Gross, 1999; Lev-
enson, 2003).
Together, these features of emotion con-
stitute the modal model of emotion: a goal-
driven person– situation transaction that
structures attention, has particular meaning
to an individual, and gives rise to an evo-
lutionarily rooted coordinated yet flexible
multisystem response (Gross & Thompson,
2007; Gross, this volume). However, just
as emotions have many compulsory fea-
tures that are well- conserved evolutionarily,
emotions have also been understood to be
malleable since the work of William James
(1884). It is this latter aspect of emotion
that is most crucial for an analysis of emo-
tion regulation, because it is this feature that
makes any form of regulation possible.
Emotion Regulation
Following past work, we define “emotion
regulation” as functional processes that
influence the intensity, duration, and type
of emotion experienced (Gross & Thomp-
son, 2007). Emotion regulation permits
flexibility in emotional responding in accord
with one’s momentary as well as longer-
term goals. Emotion regulatory processes
may have their effects at one or more points
in the emotion generative process, and may
dampen, intensify, or simply maintain emo-
tions, depending on an individual’s current
implicit or explicit goals. Emotion regula-
tory processes may also change the degree to
which emotion response components cohere
as the emotion unfolds.
We conceptualize emotion regulation
as occurring along a spectrum from con-
scious, effortful, and controlled regulation
(which we call explicit) to unconscious, and
possibly effortless, or automatic regulation
(implicit). The history of explicit– implicit
process description in psychology has been
marked by arguments over terms and defini-
tions (Bargh, 1994; Moors & De Houwer,
2006). In general, attempts to create a clear
dichotomy capable of completely dividing
implicit and explicit processes have been
unsuccessful. Drawing on this body of lit-
erature, for the purposes of this review, we
define a spectrum, rather than discrete cat-
egories, and characterize explicit emotion
regulation as those processes that require
conscious effort for initiation, demand some
level of monitoring during implementation,
and are associated with some level of insight
and awareness. Implicit regulation, is evoked
automatically, run to completion with-
out monitoring, and can happen without
insight and awareness. As outlined below,
specific examples of types of emotion regu-
lation may vary with respect to the degree
of explicitness or implicitness and, as such,
this definition most clearly describes pro-
cesses at the extreme ends of this spectrum.
Our view is that there is scientific utility in
using the explicit– implicit spectrum: It is
more parsimonious to understand different
emotion regulatory paradigms currently in
the literature within this framework rather
than characterize each paradigm at the level
of constituent processes (level of monitoring,
awareness, etc.).
We view both implicit and explicit
forms of regulation as functional— as hav-
ing arisen to help navigate the rich social
world in which humans exist. The ability
to deploy either form of regulation flexibly
in a context- sensitive manner might be a
marker of mental health. Additionally, the
conscious, effortful operations of explicit
emotion regulatory processes are useful in a
variety of contexts for adjusting emotional
reactions. Because of its cognitively demand-
ing nature, however, explicit regulation is
not something in which one can effectively
engage all of the time. Rather, use of effi-
cient implicit emotion regulation processes,
which may run without awareness, is likely a
critical component to well-being and every-
day emotional functioning.
Most contexts in which emotions are reg-
ulated involve a variable mixture of implicit
and explicit processes. The degree to which
ongoing regulation is implicit or explicit
may also change depending on the individ-
ual, context, or time course of the emotion
or its regulation. For example, a particular
regulatory process may be explicit in healthy

78 BIOLOGICAL BASES
individuals (suppressing a testy response to
a colleague) but implicit in patients (expres-
sive suppression), in whom it may have been
automatized through overrepetition, to the
point that it has become habitual. Addition-
ally, explicit and implicit regulation change
in quick succession within one person over
time. For example, one might go from con-
sciously labeling one’s feelings of frustration
at work to reflexively looking at the positive
outcome that might result from putting in
the extra time.
Taken together, our proposal is that it is
most fruitful to view emotion regulation as
reflecting a spectrum of processes, rang-
ing from the mostly explicit to the mostly
implicit. In applying this framework and
systematically testing its components we
can gain important insights into the neu-
ral circuits that are critical for the dynamic
process of emotion regulation, and into the
utility of implicit and explicit processes and
their relevance for well-being and, by exten-
sion, psychopathology.
Core Neural Structures
of Emotion Regulation
Our focus in this chapter is on neurobiologi-
cally investigated paradigms of emotion reg-
ulation. This section provides a brief over-
view of the core structures in the prefrontal
cortex that are thought to be critical for
the monitoring and regulation of emotion.
Figure 5.1 introduces the lateral– medial
gradient along which explicit and implicit
processes can be usefully lined up along.
We then expand on these as we review each
paradigm below.
The Lateral Prefrontal Cortex
The dorsolateral (dlPFC) and ventrolateral
(vlPFC) prefrontal cortices are commonly
associated with emotion regulation (e.g.,
Kalisch, 2009). Along with their role in
cognitive control and executive function-
ing, these regions are typically described in
the context of deliberate, effortful, and con-
scious regulation of emotion (Gyurak et al.,
2011). However, as described earlier, emo-
tion regulation can occur more reflexively
and outside of our awareness, and this type
of regulation implicates more medial pre-
frontal regions and might be more directly
relevant to fear- and anxiety- related regula-
tion (Gyurak et al., 2011).
The Medial Prefrontal Cortex
Regions of the medial frontal lobes, includ-
ing the ventral portions of the anterior cin-
gulate cortex (vACC) and ventromedial
prefrontal cortex (vmPFC) have long been
implicated in emotional processes, specifi-
cally in regulation of emotions. We review
relevant evidence in turn below. In addi-
tion to emotion regulation, these regions
are important in fear extinction and recall-
ing inhibitory extinction memories a day
or more after training (Milad et al., 2009;
Phelps, Delgado, Nearing, & LeDoux,
2004). Similarly, the vACC/vmPFC is acti-
vated when exposure to a distant versus
close threat occurs, suggesting that it may
be involved in planning adaptive responses,
a regulatory function (Mobbs et al., 2007).
The vACC/vmPFC shows negative func-
tional connectivity with the amygdala in a
range of emotional tasks and has anatomical
connectivity to the amygdala (Myers- Schulz
& Koenigs, 2012). Finally, during explicit
emotion regulation, the vACC may also act
as an intermediary between dlPFC and the
amygdala (Ochsner & Gross, 2005).
Selected Empirical Findings
across the Spectrum
of Emotion Regulation
Below we review a number of neurobiologi-
cally investigated examples of emotion regu-
latory processes along the explicit– implicit
spectrum. Though these examples are
ordered roughly in the degree to which they
involve implicit or explicit processes, we
remind the reader of the fluid and relative
nature of implicit and explicit regulatory
processes. We review the relevance of each
example for increasing our understanding
of psychopathology. We start on the implicit
end.
Regulation of Emotional Conflict
Our first case of implicit emotion regula-
tion is based on the emotional conflict task
(Egner, Etkin, Gale, & Hirsch, 2008; Etkin,
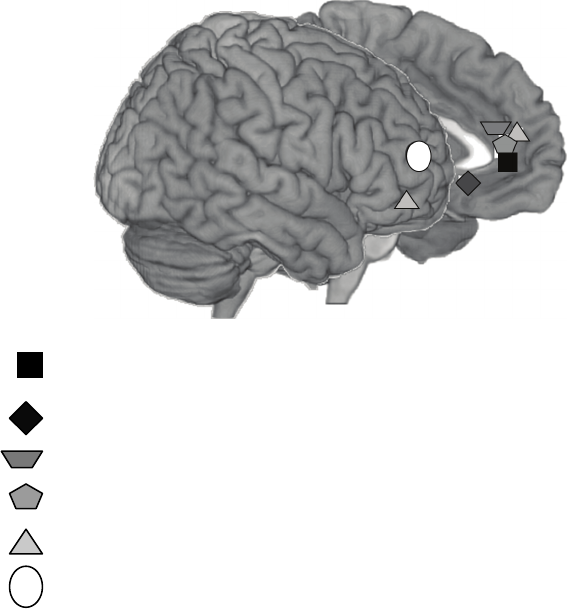
Neurobiological Model of Implicit and Explicit Emotion Regulation 79
Egner, Peraza, Kandel, & Hirsch, 2006).
The task is the emotional analogue of the
classic Stroop paradigm (Stroop, 1935), and
takes advantage of a variant of the congru-
ency sequence effects originally reported by
Gratton, Coles, and Donchin (1992) in non-
emotional conflict tasks (Botvinick, Nys-
trom, Fissell, Carter, & Cohen, 1999). In the
emotional conflict task, participants are pre-
sented with photographs of emotional faces
(fearful or happy) with the words fear or
happy written over them. The task is to indi-
cate whether the facial expression is happy
or fearful by pressing a button and to do this
fast and accurately. The word written on the
photo either matches the facial expression
(no- conflict trials: happy face with the word
happy written over it, or fearful face with
the word fear written over it) or is incongru-
ent with the facial expression (conflict tri-
als: happy face with the word fear written
over it, or fearful face with the word happy
written over it). Because reading is automa-
tized (Stroop, 1935), during conflict tri-
als, participants need to enact control over
reading the word in favor of labeling the
emotional expression. Consistent with this,
it takes longer to respond to conflict trials
than to no- conflict trials; this slow-down in
response time due to emotional conflict has
been termed the congruency effect. Implicit
emotion regulation in this task is indexed
by trial-to-trial changes in the congruency
effect as a function of the congruency effect
on the previous trial (trial n – 1). Specifically,
conflict on trial n – 1 triggers an increase in
regulation, thereby reducing susceptibility
to emotional conflict on trial n. Consistent
with this, response times to incongruent tri-
als are usually faster after incongruent tri-
als (iI = current Incongruent preceded by an
incongruent trial) than after congruent trials
FIGURE 5.1. Putative foci for implicit and explicit forms of emotional regulation on a spectrum. Foci
plotted are approximate; x coordinate = –2 for visualization purposes on the medial slice.
Emotional conflict adaptation, x = –2, y = 44, z = 2; from Egner et al. (2008)
Reappraisal, calculated as the mean of the absolute values of all vLPFC and dlPFC
activation in Study 2a plotted on the right hemisphere for illustrative purposes, x = 42,
y = 27, z = 21; from Kalisch (2009)
Anticipation, x = –2, y = 40, z = 7; from Straube et al. (2009)
Habituation, x = –2, y = 44, z = 2; from Hare et al. (2008)
Extinction, x = –2, y = 23, z = –8; from Milad et al. (2009)
Verbal labeling (triangles in the medial and lateral prefrontal cortices), x = –2, y = 48,
z = 16 and, x = 24, y = –10, z = 44, respectively; from Lieberman et al. (2007)
80 BIOLOGICAL BASES
(cI = current Incongruent preceded by a con-
gruent trial). Implicit emotion regulation is
therefore quantified by contrasting response
times on iI with cI trials. This behavioral
slowdown effect was shown to occur outside
of awareness (Etkin, Prater, Hoeft, Menon,
& Schatzberg, 2010). The task qualifies as
an emotion regulation paradigm, because
the stimulus itself evokes behavioral adjust-
ment in the context of the goal of responding
fast and accurately to emotionally conflict-
ing stimuli. This task fits our definition of
implicit regulation because the process is
uninstructed, likely results from the stimu-
lus properties itself, and is effortless and
proceeds without awareness; despite care-
ful probing, participants do not report any
awareness of the key processes of the task
(Etkin et al., 2010).
Parallel imaging (Egner et al., 2008) and
lesion (Maier & di Pellegrino, 2012) studies
of regulation of nonemotional conflict sug-
gest that key elements of the neural circuitry
involved in implicit regulation of emotional
conflict is specific to the emotional content.
Specifically, neuroimaging studies suggest a
regulatory interplay between anterior cin-
gulate cortex (ACC) and emotion reactivity
regions in the limbic system in the emotional
conflict task: increased activation in the
vACC, and dampened reactivity in the amyg-
dala (Etkin et al., 2006; Etkin et al., 2010;
see Figure 5.1). In the nonemotional ana-
logue of the task, in which subjects judged
the gender of emotional faces, while trying
to ignore congruent or incongruent gender
labels, regulation was achieved through
a dissociable neural pathway, involving
increased activation in the dlPFC, and posi-
tive coupling to target- specific visual corti-
cal areas in the fusiform face area, and as
such, activation of the vACC and dampen-
ing of amygdalar reactivity was specific to
implicit emotion regulation (Egner et al.,
2008).
Applications of this task to clinical disor-
ders revealed a marked deficit both behav-
iorally and in neural network engagement as
a function of anxiety and depression (Etkin
et al., 2010; Etkin & Schatzberg, 2011). Spe-
cifically, nonmedicated patients with either
generalized anxiety disorder (GAD) or
comorbid depression and GAD were unable
to regulate the effect of emotional conflict
as compared to healthy controls. Further-
more, analysis of functional magnetic reso-
nance imaging (fMRI) data acquired during
the emotional conflict task revealed that
patients with GAD or depression failed to
engage the circuitry normally implicated
in this task. Specifically, patients failed to
activate the vACC during regulation of emo-
tional conflict and to dampen emotional
conflict evaluation- related activity in the
amygdala. These data indicate that regula-
tion of emotional conflict, as a prototypi-
cal exemplar of implicit emotion regulation,
captures the critical inability of patients to
manage emotional processing and engage in
task- related behavior.
Extinction and Habituation
Both extinction and habituation are basic
forms of learning that are important for
adaptive behavioral responding to emotional
challenges. Both of these qualify as emotion
regulation because they underlie the ability
to change emotional responding to fit situa-
tional demands. Both of these processes have
aspects that happen outside of conscious
awareness and, as such, share implicit emo-
tion regulatory elements but are likely to be
a blend of implicit and explicit processes in
humans, as detailed below (Delgado, Near-
ing, LeDoux, & Phelps, 2008; Dijksterhuis
& Smith, 2002; Lovibond, 2004).
Extinction involves repeated presenta-
tion of an innocuous stimulus (e.g., a blue
cross) that was previously associated with
an aversive stimulus (e.g., a loud noise) and
had acquired the ability to elicit a defensive
response (e.g., startle eyeblink) in the absence
of the aversive stimulus. Through repeated
presentation without the aversive outcome,
a new inhibitory memory is formed between
the innocuous stimulus and absence of the
aversive outcome, such that the conditioned
defensive reaction is no longer elicited. Even
though extinction can happen without the
individual being aware of the change in
contingency, consciously registering the
contingency changes during extinction has
been shown to facilitate extinction (for a
review, see Lovibond, 2004). This evidence
implicates the presence of both implicit and
explicit processes in extinction.
Importantly, the neural circuitry support-
ing extinction shares common elements with
the circuitry mapped out for the regulation
Neurobiological Model of Implicit and Explicit Emotion Regulation 81
of emotional conflict (i.e., vACC and amyg-
dala). Specifically, the vACC and adjacent
vmPFC reduce fear responses through inter-
connections with the amygdala and hippo-
campus during extinction learning and recall
of extinction memories (Ehrlich et al., 2009;
Milad & Quirk, 2002; Phelps, Delgado,
Nearing, & LeDoux, 2004). More success-
ful extinction is associated with decreased
conditioned stimulus (CS)-driven activa-
tion in the dorsal anterior cingulate cortex
(dACC) in human (Milad et al., 2009) and
animal models (Livneh & Paz, 2012). This
is consistent with a recent review of the role
of the dACC in evaluation and expression of
negative emotion (Etkin, Egner, & Kalisch,
2011) and suggest the presence of a second
pathway between the dACC and the amyg-
dala where activation might be actually det-
rimental to extinction of fear memories.
Clinical models document impaired
extinction behaviorally in posttraumatic
stress disorder (PTSD; Norrholm et al.,
2011; Wessa & Flor, 2007; for a review, see
Lissek et al., 2005). Behavioral symptoms
in GAD and specific anxiety disorders also
indicate an inability to inhibit dysfunctional
fear responses. In addition, the presence of
safety and avoidance behaviors in these dis-
orders might function to prevent the uncou-
pling of fear responses (Graham & Milad,
2011; Milad et al., 2009). Consistent with
this, heightened amygdala activation was
documented in PTSD during fear extinction
following fear conditioning (Milad et al.,
2009). However, there were no differences
in behavioral or neural patterns of extinc-
tion as a function of social anxiety (Pejic,
Hermann, Vaitl, & Stark, 2011), which
might suggest preservations of extinction in
social anxiety.
Habituation is the adaptive reduction of
the initial response to emotional stimuli that
carries no immediate reward or punishment
value to the individual. A typical paradigm
used in studying the neurobiological sub-
strates of habituation in humans is to repeat-
edly present emotionally salient stimuli (e.g.,
emotional faces) over several runs of the scan
and look at the time course of the neuronal
signal change from early to late trials (Hare
et al., 2008) within regions typically asso-
ciated with processing the stimuli. Behav-
ioral manipulations of attention to different
aspects of the stimulus have been shown to
alter the rate of habituation to nonemotional
stimuli (e.g., tones; Gruzelier & Eves, 1987),
suggesting that there are both implicit and
explicit processes at play in habituation.
Studies have shown that the signal from
the amygdala is reliably reduced as partici-
pants repeatedly encounter a salient but emo-
tionally nonrewarding or punishing stimu-
lus (Fisher et al., 2009; Hare et al., 2008).
The extent of habituation, however, appears
to be a function of the engagement of medial
PFC circuitry that overlaps with the circuit
associated with regulation of emotional
conflict (i.e., vACC). For example, during
habituation, engagement of the medial PFC
is related to lower amygdala responses (Hare
et al., 2008). A recent fMRI–PET (positron
emission tomography) study also showed
that higher density of serotonergic neurons
in the vACC—considered an indicator of the
capacity to modulate emotional arousal— is
related to better habituation to negative faces
and lower amygdala responses (Fisher et al.,
2009). Failure of habituation of the amyg-
dala has also been documented in severely
inhibited individuals (Blackford, Allen,
Cowan, & Avery, 2013) and in high trait
anxiety (Hare et al., 2008) as well as PTSD
(Shin et al., 2005). Furthermore, impaired
habituation of auditory startle responses (a
process mediated through the amygdala)
was found to be a marker of later develop-
ment of PTSD in a longitudinal study (Pole
et al., 2009).
Anticipatory Responding
to Emotional Stimuli
Anticipation of negative emotional events
prepares the individual to respond adaptively
and flexibly to actual emotional challenges
as they arise. This process has typically
been studied in experimental paradigms by
presenting cues that with some probability
predict the occurrence of a threatening or
negative event (e.g., electric shock, thermal
pain, negative image) (Drabant et al., 2011;
Nitschke et al., 2009; Straube, Schmidt,
Weiss, Mentzel, & Miltner, 2009). Anticipa-
tory responding fits our definition of emotion
regulation, because it allows the individual
to optimize and prepare future responding
in the face of an emotional challenge. The
conscious anticipatory component gives a
more explicit flavor to these paradigms, but
82 BIOLOGICAL BASES
in the absence of explicit instruction to regu-
late the response, the implicit component is
retained.
More importantly, these paradigms have
proved to be useful in mapping the neu-
ral circuits associated with anticipatory
responses and associated anxiety. These
results show the involvement of the vACC
and the broader ACC with typically higher
activation during anticipation (Butler et
al., 2005; Nitschke et al., 2009; Ploghaus,
Becerra, Borras, & Borsook, 2003;
Ploghaus et al., 1999; Straube et al., 2009),
and often tracking concomitant anxiety.
Sarinopoulos et al. (2010) reported that
anticipatory activation in the vACC follow-
ing uncertain cues was related to systematic
overestimation of the frequency with which
the cue was predictive of aversive images.
In terms of the relationship to anticipa-
tory responding, informative moderation
effects have been found. Specifically, dur-
ing moderate levels of threat (mildly pain-
ful electric shock), higher vACC activation
was related to higher anticipatory prepared-
ness, whereas during high levels of threat
of electric shocks, higher vACC activation
was related to lower anticipatory prepared-
ness (Straube et al., 2009), suggesting that
higher vACC activation indices anticipa-
tory preparedness, whereas deactivation
might track disengagement from the task,
a perhaps more explicit form of regulation.
vACC activation was also documented in
a virtual chasing game while participants
expected painful levels of electric shocks,
and this activation increased as a function
of anticipated pain (Mobbs et al., 2007).
Clinically, abnormalities in activation
to anticipatory cues preceding negative
anxiety- provoking images have been docu-
mented in GAD (Nitschke et al., 2009). Sim-
ilarly, patients with unipolar major depres-
sive disorder (Abler, Erk, Herwig, & Walter,
2007) also showed higher amygdala acti-
vation compared to healthy controls while
anticipating negative pictures.
Incidental Regulation
through Language
Goal- directed engagement of higher order
cognitive processes, such as language, in
emotional contexts can have “acciden-
tal” emotion regulatory consequences. In
experimental paradigms, participants are
instructed to label the emotion displayed
on a face (e.g., indicate whether the face is
angry or afraid), match the affect shown on
the face to another face showing the same
emotion, or match the gender of the face
(Hariri, Bookheimer, & Mazziotta, 2000;
Lieberman et al., 2007; Lieberman, Hariri,
Jarcho, Eisenberger, & Bookheimer, 2005).
There are important similarities between
verbal labeling as done in this task and cre-
ation of a verbal narrative that is supposed
to lessen emotional arousal seen in explicit
regulatory tasks. Because of these parallels,
the task fits our definition of emotion regu-
lation, as it results in the regulation of emo-
tional arousal in a goal- directed fashion.
Semantically labeling emotional stimuli, or
“putting feelings into words,” as these para-
digms are typically described, versus match-
ing the emotion on another face or labeling
the gender has a marked explicit compo-
nent, yet the regulation still happens in the
absence of an explicit goal to do so.
At the neural level, matching emotional
expressions on faces with words that label
affect results in lower amygdala activation,
as compared to matching expressions to
other expressions, matching the gender to
gender labels, or simply viewing the face.
Moreover, labeling the emotion of facial
expressions or focusing on physical rather
than emotional features of an emotionally
evocative scene results in increased activa-
tion in the lateral and medial prefrontal cor-
tices, despite the fact that emotion regulation
in this paradigm is unintentional or implicit
(Hariri et al., 2000; Lieberman et al., 2005).
Importantly, Lieberman and colleagues
(2007) documented that a vmPFC activation
mediated the relationship between lateral
PFC and decreased amygdala activity, sug-
gesting that similar to implicit emotion regu-
lation, it may be the medial PFC–amygdala
pathway that plays a critical role in dampen-
ing emotional reactivity in these tasks (see
Figure 5.1).
Variants of the affect- labeling task have
been used in pediatric anxiety and mood
disorder populations with or at-risk for psy-
chopathology (Beesdo et al., 2009; Monk
et al., 2007; Taylor, Eisenberger, Saxbe,
Lehman, & Lieberman, 2006). These stud-
ies show that among at-risk adolescents,
focusing attention on the physical features
of an emotional face (nose width) or label-
ing one’s subjective emotions while viewing
Neurobiological Model of Implicit and Explicit Emotion Regulation 83
an emotional face, normalized concomitant
neural activation in the PFC and amygdala.
Similarly, McClure and colleagues (2007)
found that the performance of children
with GAD did not differ from that of their
healthy counterparts in rating physical fea-
tures of negative emotional faces, and that
they recruited the same amygdala, dACC,
and vmPFC networks as their healthy ado-
lescent counterparts. However, in another
study, Taylor et al. (2006) found that chil-
dren from high- stress family environments
showed higher amygdala and dlPFC activa-
tion during an affect labeling of faces task
than children from low-risk families, who
showed lower amygdala activation during
affect labeling. Low-risk children did not
differ in dlPFC activation. Taken together,
these result shows that disordered and at-
risk children are capable of labeling as a
form of regulation behaviorally, and they
might do so largely through the normative
circuitry.
Instructed Regulation
of Emotional Responses
A paradigmatic case of explicit regulation
may be found in work by James Gross and
colleagues (e.g., Gross & Levenson, 1993;
McRae et al., 2010; Ochsner, Bunge, Gross,
& Gabrieli, 2002). As reviewed by Ochsner
and Gross (this volume), in a typical test of
explicit emotion regulation, participants are
presented with a task that involves process-
ing stimuli under two different conditions—
one in which participants are instructed to
react naturally (reactivity trial), and another
in which they are instructed to regulate
their emotional responses (regulation trial)
using a strategy specified by the researcher
that participants have had ample opportu-
nity to practice beforehand. Explicit emo-
tion regulation performance is indexed
by contrasting emotional responses in the
reactivity and regulation trials. These tasks
fit our definition of explicit emotion regu-
lation, because the process is effortful and
carried out with considerable awareness
and monitoring. Specifically, individuals are
(1) aware of the cues that elicit emotional
responses (i.e., images, films), (2) aware of
the emotion itself (i.e., they report reduc-
tion in accompanying feelings), and (3)
aware of the effect of the regulation on their
behavior (i.e., if prompted, they can report
having engaged in emotion regulation).
Often- studied forms of explicit regulation
are reappraisal, in which participants are
instructed to attempt to change the way they
think about the emotional event so that they
feel less negative emotions, and suppression,
in which participants are instructed not to
show how they feel.
Neuroimaging studies show that reap-
praisal results in a dynamic interchange
between frontal lobe areas implicated in
cognitive control and executive function,
and emotion reactivity areas (for reviews, see
Ochsner & Gross, 2005; Ochsner & Gross,
this volume; Kalisch, 2009). Specifically,
imaging studies indicate that attempts to
reappraise negative stimuli result in increased
activation in ventrolateral (vlPFC), dlPFC,
dACC—areas traditionally implicated in
non- emotional forms of cognitive control
(Kalisch, 2009). This frontal lobe activa-
tion in turn is related to reduced activation
in emotional reactivity- related areas (amyg-
dala and insula), suggesting that engage-
ment of control- related circuitry dampens
reactivity in these critical emotion reactiv-
ity regions (Banks, Eddy, Angstadt, Nathan,
& Phan, 2007; Goldin, McRae, Ramel, &
Gross, 2008; Ochsner et al., 2002; Wager,
Davidson, Hughes, Lindquist, & Ochsner,
2008). Furthermore, there appears to be a
temporal specificity in the engagement of
frontal lobe regions as a function of imple-
mentation stage (Paret et al., 2011). Specifi-
cally, Kalisch and colleagues (Kalisch, 2009;
Paret et al., 2011) propose that instructed
reappraisal can be partitioned into two
temporarily distinct phases: an early phase
of implementation that comprises strategy
selection and retrieval of reappraisal mate-
rial into working memory, and a later phase
of maintenance of performance- monitoring
processes. Corresponding shifts in the
recruitment of the lateral PFC are observed
as a function of these phases from posterior
to anterior parts of the lateral frontal cortex
(Paret et al., 2011).
Assessment of instructed emotion regula-
tory abilities in clinical populations, how-
ever, has produced mixed results. There
appears to be agreement that behavior-
ally, patients with mood and anxiety dis-
orders are not deficient in using regulation
when instructed to do so in the laboratory
(Ehring, Tuschen- Caffier, Schnülle, Fischer,
& Gross, 2010; Goldin, Manber, Hakimi,

84 BIOLOGICAL BASES
Canli, & Gross, 2009; Goldin, Manber-
Ball, Werner, Heimberg, & Gross, 2009),
but they might spontaneously use certain
beneficial forms of regulation such as reap-
praisal less frequently, or report feeling less
successful in regulating their emotions as
a result (Gruber, Harvey, & Gross, 2012).
By contrast, a variety of neural deficits have
been found in different patient groups. For
example, currently depressed patients acti-
vated bilateral PFC during reappraisal of
negative scenes, while healthy subjects did
so only unilaterally (Johnstone et al., 2007).
in another study, there was a lack of dlPFC
activation in patients with major depressive
disorder altogether, but activated dACC,
amygdala, and insular cortices (Beauregard,
Paquette, & Lévesque, 2006). Patients with
social anxiety disorder showed delayed but
overall enhanced engagement of dlPFC and
dACC when instructed to reappraise nega-
tive self- relevant thoughts (Goldin, Man-
ber-Ball, et al., 2009). By contrast, during
reappraisal of angry/contemptuous faces,
patients with social anxiety disorder failed
to activate dACC and anterior medial fron-
tal cortex (neither dlPFC nor vlPFC were
involved in this task; Goldin, Manber-Ball,
et al., 2009). Patients with GAD and social
phobia both failed to activate the dACC
when reappraising either positive or negative
pictures (no dlPFC or vlPFC was involved in
this task; Blair et al., 2012). Finally, trauma
exposed patients with borderline personality
disorder hypoactivated lateral and medial
PFC more during reappraisal of negative
scenes, relative to trauma- exposed healthy
controls. Taken together, these results
show a rather mixed set of abnormalities in
instructed emotion regulation, with differ-
ences likely due to different tasks, stimuli,
or instructions. Regardless, explicit regula-
tion at the behavioral level does not appear
to be perturbed in psychiatric disorder, even
if patients recruit different neural circuits.
Impairments may exist, however, in the
spontaneous and potentially more implicit
engagement of these effortful forms of regu-
lation.
Implications and Future
Research Directions
Our goal in this review was to provide a
unifying conceptual framework for emotion
regulation, highlighting and expanding our
prior model of an implicit– explicit emotion
regulatory spectrum. We have argued sev-
eral key points.
First, types of emotion regulation vary in
the degree to which they are implicit versus
explicit. We demonstrated that the rela-
tive degree of implicit or explicit features
depends on multiple factors, including the
stimulus, task, context, individual differ-
ences, psychopathology, and time. Com-
pounding this complexity, we showed that
many existing experimental paradigms
involve various admixtures of implicit and
explicit types of emotion regulation. Greater
attention should therefore be paid to the
implications of these design features in light
of a conceptualization of implicit regulation.
Studies need to address the relative degree to
which regulation is at any time implicit or
explicit in both existing and new paradigms.
Probing awareness about regulation is one
way to assess implicitness versus explicit-
ness. Other factors that influence whether
a particular regulatory task is implicit or
explicit are personality features, such as low
trait rumination, which might predispose
individuals to have more automatic tenden-
cies to use reappraisal, and they might do so
in a more implicit, automatic fashion (Ray et
al., 2005). Another example is pathological
worry in GAD (Borkovec, Alcaine, & Behar,
2004), which might parallel explicit forms
of regulation by virtue of engaging effort-
ful resources (e.g., prefrontal areas). This
in turn might influence the ability of people
with GAD engage explicit regulation in a
goal- directed fashion.
Additionally, several researchers have
pointed out the inherent difficulties involved
in differentiating emotional regulation from
emotional reactivity, with some questioning
the utility of emotion regulation as a scien-
tific construct altogether (Campos, Frankel,
& Camras, 2004; Cole, Martin, & Dennis,
2004). Neuroimaging studies get around
this problem and routinely include a con-
trasting control condition when the effects
of emotion regulation are compared to those
of canonical emotional reactivity trials (i.e.,
adaptation to emotional conflict is a contrast
between iI and cI conditions; reappraisal is
a contrast between uninstructed “watch”
trials and instructed regulation). However,
studies that purportedly measure the effects
of spontaneous use of effortful regulation
Neurobiological Model of Implicit and Explicit Emotion Regulation 85
often do not include a contrasting reactiv-
ity condition and do not carefully control
for the effects of reactivity. Future research
has to address emotional reactivity by sys-
tematically including a baseline unregulated
condition, in addition to assessing implicit
and explicit features. Future studies examin-
ing the explicit– implicit dimension system-
atically should also test the separability and
interactions between these circuits.
Second, we showed that there appears
broadly to be a medial– lateral differentiation
between implicit and explicit regulatory pro-
cesses (see Figure 5.1). That is, paradigmatic
forms of implicit emotion regulation (e.g.,
emotional conflict regulation) involve ven-
tromedial prefrontal regions in regulation,
while paradigmatic forms of explicit regula-
tion (e.g., reappraisal) involve mainly dlPFC
and vlPFC for regulation. Other forms of
regulation that contain a mixture of implicit
and explicit processes (e.g., language- based
incidental regulation) involve roles for both
lateral PFC and vmPFC. While we do not
consider this medial– lateral gradient to be
an absolute rule, neither would we be con-
fident in interpreting medial or lateral acti-
vation differences in patients as necessarily
reflecting deficits in implicit or explicit regu-
lation, respectively; this model provides a
useful framework for describing the range of
emotion regulation processes, anchored in
relevant neurocircuitry. Moreover, we would
readily concede that our framework is useful
for anchoring our theoretical understanding
of emotion regulation, but it is unlikely that
implicit and explicit modules per se exist at
the level of the brain. Future research might
parse the operations of implicit and explicit
regulation into circuit- level components
(e.g., working memory, interference resolu-
tion) and our understanding of the implicit–
explicit anchors might transform as our
understanding of the interactions between
participating brain areas develops. Our
goal was to illustrate the fluid boundaries
between implicit and explicit forms of regu-
lation, and move away from characterizing
implicit and explicit regulation in a dichoto-
mous fashion. New paradigms in which the
level of explicitness and implicitness is para-
metrically manipulated (e.g., in the form of
increased explicit regulatory effort in the
context of an otherwise implicit regulatory
process or by repetitively training explicit
regulation until it becomes automatized)
will allow us to ground implicit and explicit
regulation in neurobiology and further chart
the interactive and dynamic processes by
which the brain accomplishes regulation.
Our third point is that in striving for a
neurobiological understanding of emotion
regulation and developing these new para-
digms, it will be important to refine the
circuitry underlying implicit and explicit
regulation. The human brain is likely to
accomplish emotion regulation through an
economical process that relies on a distrib-
uted network of brain areas. Three brain
areas highlighted here as underlying implicit
and explicit forms of regulation (medial
PFC and dlPFC/vlPFC, respectively) have
been documented to be involved in a wide
array of processes besides emotion regula-
tion. In this vein, further research will likely
broaden our understanding of the relevant
circuitry and how these functions relate to
the variety of implicit and explicit regula-
tory mechanisms. Moreover, most studies
focused on corticoamygdalar circuitry, but
less attention has been paid to the role of the
insula and midbrain structures essential for
emotional processing (e.g., periaqueductal
gray). The role of these nodes in the emotion
regulatory circuitry with respect to implicit
and explicit features remains to be explored.
Another important element that has thus
far been unmapped in terms of implicit
and explicit regulation is the modulation
of approach tendencies and positive emo-
tions (e.g., reward). There is evidence that
approach might also be regulated in an
implicit and explicit fashion through some-
what dissociable circuitry (Kable & Glim-
cher, 2007; McClure, Laibson, Loewenstein,
& Cohen, 2004). For example, value-based
decision making about food while focusing
on the health aspect of the stimuli modu-
lates activation in medial prefrontal regions
that overlap with the vACC region mapped
to types of implicit regulation, such as emo-
tional conflict adaptation, and this modula-
tion seems to be orchestrated by the dlPFC,
which is involved in more explicit forms
of regulation (Hare, Malmaud, & Ran-
gel, 2011). Additionally, explicit regulation
of monetary rewards in a group of self-
assessed, successful regulators was related
to lower striatal activation and fewer risky
behavioral choices (Martin & Delgado,
2011), but without medial prefrontal or
dlPFC involvement. These findings in the

86 BIOLOGICAL BASES
realm of positive approach related will need
to be integrated into our explicit– implicit
framework through systematic studies that
manipulate explicitness and implicitness in
the context of positive emotions.
Our fourth point is that better definition
of implicit and explicit tasks at the process
level, and a refined understanding of the
neural circuits involved, will help concret-
ize how we can best improve these functions
using targeted, neurobiologically derived,
customized, and adaptive training meth-
ods (e.g., Vinogradov, Fisher, & de Villers-
Sidani, 2011). One question is whether tar-
geted training aimed at the dlPFC and vlPFC
will impart emotion regulatory benefits, and
whether these benefits will be evident in
implicit or explicit regulatory tasks.
As our fifth point, we presented evidence
that difficulties in emotion regulation in
psychiatric disorders cut across implicit and
explicit types. Specifically, we found behav-
ioral and neural deficits in implicit forms of
regulation: emotional adaptation; habitua-
tion; and documented, more subtle, largely
neural deficits in more explicit forms of reg-
ulation (affect labeling and instructed reap-
praisal). These results support clinical obser-
vations of pervasive emotion regulatory
symptoms in psychopathology and provide
neurobiological anchors for the observed
deficits that might track with treatment.
Conclusions
Strategies that individuals use to alter their
emotions affect not only their current emo-
tional experience but also broader emo-
tional, cognitive, and interpersonal func-
tioning. Thus, emotion regulation is of clear
importance for both basic and applied trans-
lational investigations. Implicit processes
are hidden for measurement reasons but
are now more tractable, thanks to neurosci-
ence methods. We have reached an exciting
point at which neurobiologically anchored
understanding of both implicit and explicit
processes is rapidly advancing. Implicit and
explicit processes arose to help us navigate
our social and emotional worlds, and it is
important that we conduct systematic stud-
ies on this domain. Our hope is that a uni-
fying framework will facilitate this process
and result in research advances for both
basic and translational affective neurosci-
ence fields.
References
Abler, B., Erk, S., Herwig, U., & Walter, H.
(2007). Anticipation of aversive stimuli acti-
vates extended amygdala in unipolar depres-
sion. Journal of Psychiatric Research, 41(6),
511–522.
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan,
P. J., & Phan, K. L. (2007). Amygdala– frontal
connectivity during emotion regulation. Social
Cognitive and Affective Neuroscience, 2(4),
303–312.
Bargh, J. A. (1994). The four horsemen of auto-
maticity: Awareness, intention, efficiency, and
control in social cognition. In R. S. Wyer &
T. K. Srull (Eds.), Handbook of social cogni-
tion (2nd ed., Vol. 1, pp. 1–40). Hillsdale, NJ:
Erlbaum.
Beauregard, M., Paquette, V., & Lévesque, J.
(2006). Dysfunction in the neural circuitry of
emotional self- regulation in major depressive
disorder. NeuroReport, 17(8), 843–846.
Beesdo, K., Lau, J. Y. F., Guyer, A. E., McClure-
Tone, E. B., Monk, C. S., et al. (2009). Common
and distinct amygdala- function perturbations
in depressed vs. anxious adolescents. Archives
of General Psychiatry, 66(3), 275–285.
Blackford, J. U., Allen, A. H., Cowan, R. L.,
& Avery, S. N. (2013). Amygdale and hip-
pocampus fail to habituate to faces in indi-
viduals with an inhibited temperament. Social
Cognitive and Affective Neuroscience, 8(2),
143–150.
Blair, K. S., Geraci, M., Smith, B. W., Hollon, N.,
DeVido, J., Otero, M., et al. (2012). Reduced
dorsal anterior cingulate cortical activity dur-
ing emotional regulation and top-down atten-
tional control in generalized social phobia,
generalized anxiety disorder, and comorbid
generalized social phobia/generalized anxiety
disorder. Biological Psychiatry, 72(6), 476–
482.
Borkovec, T. D., Alcaine, O. M., & Behar, E.
(2004). Avoidance theory of worry and gener-
alized anxiety disorder. In R. G. Heimberg, C.
L. Turk, & D. S. Mennin (Eds.), Generalized
anxiety disorder: Advances in research and
practice (pp. 77–108). New York: Guilford
Press.
Botvinick, M., Nystrom, L. E., Fissell, K., Carter,
C. S., & Cohen, J. D. (1999). Conflict moni-
Neurobiological Model of Implicit and Explicit Emotion Regulation 87
toring versus selection- for- action in anterior
cingulate cortex. Nature, 402(6758), 179–181.
Butler, T., Pan, H., Epstein, J., Protopopescu,
X., Tuescher, O., Goldstein, M., et al. (2005).
Fear- related activity in subgenual anterior cin-
gulate differs between men and women. Neu-
roReport, 16(11), 1233–1236.
Campos, J. J., Frankel, C. B., & Camras, L.
(2004). On the nature of emotion regulation.
Child Development, 75(2), 377–394.
Cole, P. M., Martin, S. E., & Dennis, T. A.
(2004). Emotion regulation as a scientific con-
struct: Methodological challenges and direc-
tions for child development research. Child
Development, 75(2), 317–333.
Delgado, M. R., Nearing, K. I., LeDoux, J.
E., & Phelps, E. A. (2008). Neural circuitry
underlying the regulation of conditioned fear
and its relation to extinction. Neuron, 59(5),
829–838.
Dijksterhuis, A., & Smith, P. K. (2002). Affective
habituation: Subliminal exposure to extreme
stimuli decreases their extremity. Emotion,
2(3), 203–214.
Drabant, E. M., Kuo, J. R., Ramel, W., Blechert,
J., Edge, M. D., Cooper, J. R., et al. (2011).
Experiential, autonomic, and neural responses
during threat anticipation vary as a function
of threat intensity and neuroticism. NeuroIm-
age, 55(1), 401–410.
Egner, T., Etkin, A., Gale, S., & Hirsch, J. (2008).
Dissociable neural systems resolve conflict
from emotional versus nonemotional distract-
ers. Cerebral Cortex, 18(6), 1475–1484.
Ehring, T., Tuschen- Caffier, B., Schnülle, J.,
Fischer, S., & Gross, J. J. (2010). Emotion
regulation and vulnerability to depression:
Spontaneous versus instructed use of emotion
suppression and reappraisal. Emotion, 10(4),
563–572.
Ehrlich, I., Humeau, Y., Grenier, F., Ciocchi,
S., Herry, C., & Lüthi, A. (2009). Amygdala
inhibitory circuits and the control of fear
memory. Neuron, 62(6), 757–771.
Etkin, A., Egner, T., & Kalisch, R. (2011). Emo-
tional processing in anterior cingulate and
medial prefrontal cortex. Trends in Cognitive
Sciences, 15(2), 85–93.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E.
R., & Hirsch, J. (2006). Resolving emotional
conflict: A role for the rostral anterior cingu-
late cortex in modulating activity in the amyg-
dala. Neuron, 51(6), 871–882.
Etkin, A., Prater, K. E., Hoeft, F., Menon, V.,
& Schatzberg, A. F. (2010). Failure of anterior
cingulate activation and connectivity with the
amygdala during implicit regulation of emo-
tional processing in generalized anxiety disor-
der. American Journal of Psychiatry, 167(5),
545–554.
Etkin, A., & Schatzberg, A. F. (2011). Common
abnormalities and disorder- specific compen-
sation during implicit regulation of emotional
processing in generalized anxiety and major
depressive disorders. American Journal of
Psychiatry, 168(9), 968–978.
Fisher, P. M., Meltzer, C. C., Price, J. C., Cole-
man, R. L., Ziolko, S. K., Becker, C., et al.
(2009). Medial prefrontal cortex 5-HT2A
density is correlated with amygdala reactiv-
ity, response habituation, and functional cou-
pling. Cerebral Cortex, 19(11), 2499–2507.
Frijda, N. H. (1987). The emotions. Cambridge,
UK: Cambridge University Press.
Goldin, P. R., Manber, T., Hakimi, S., Canli, T.,
& Gross, J. J. (2009). Neural bases of social
anxiety disorder: Emotional reactivity and
cognitive regulation during social and physical
threat. Archives of General Psychiatry, 66(2),
170–180.
Goldin, P. R., Manber-Ball, T., Werner, K.,
Heimberg, R., & Gross, J. J. (2009). Neural
mechanisms of cognitive reappraisal of nega-
tive self- beliefs in social anxiety disorder. Bio-
logical Psychiatry, 66(12), 1091–1099.
Goldin, P. R., McRae, K., Ramel, W., & Gross,
J. J. (2008). The neural bases of emotion regu-
lation: Reappraisal and suppression of nega-
tive emotion. Biological Psychiatry, 63(6),
577–586.
Graham, B. M., & Milad, M. R. (2011). The
study of fear extinction: Implications for anxi-
ety disorders. American Journal of Psychia-
try, 168(12), 1255–1265.
Gratton, G., Coles, M. G., & Donchin, E. (1992).
Optimizing the use of information: Strategic
control of activation of responses. Journal of
Experimental Psychology. General, 121(4),
480–506.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J, & Levenson, R. W. (1993). Emo-
tional suppression: Physiology, self- report,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64(6), 970–986.
Gross, J. J., & Thompson, R. A. (2007). Emo-
tion regulation: Conceptual foundations. In J.
88 BIOLOGICAL BASES
J. Gross (Ed.), Handbook of emotion regula-
tion (pp. 3–24). New York: Guilford Press.
Gruber, J., Harvey, A. G., & Gross, J. J. (2012).
When trying is not enough: Emotion regula-
tion and the effort- success gap in bipolar dis-
order. Emotion, 12(5), 997–1003.
Gruzelier, J., & Eves, F. (1987). Rate of habitu-
ation of electrodermal orienting responses: A
comparison of instructions to stop respond-
ing, count stimuli, or relax and remain indif-
ferent. International Journal of Psychophysi-
ology, 4(4), 289–291.
Gyurak, A., Gross, J. J., & Etkin, A. (2011).
Explicit and implicit emotion regulation: A
dual- process framework. Cognition and Emo-
tion, 25(3), 400–412.
Hare, T. A., Malmaud, J., & Rangel, A. (2011).
Focusing attention on the health aspects of
foods changes value signals in vmPFC and
improves dietary choice. Journal of Neurosci-
ence, 31(30), 11077–11087.
Hare, T. A., Tottenham, N., Galvan, A., Voss, H.
U., Glover, G. H., & Casey, B. J. (2008). Bio-
logical substrates of emotional reactivity and
regulation in adolescence during an emotional
go–no-go task. Biological Psychiatry, 63(10),
927–934.
Hariri, A. R., Bookheimer, S. Y., & Mazziotta,
J. C. (2000). Modulating emotional responses:
Effects of a neocortical network on the limbic
system. NeuroRreport, 11(1), 43– 48.
James, W. (1884). What is an emotion? Mind, 9,
188–205.
Johnstone, T., Van Reekum, C. M., Urry, H.
L., Kalin, N. H., & Davidson, R. J. (2007).
Failure to regulate: Counterproductive recruit-
ment of top-down prefrontal– subcortical cir-
cuitry in major depression. Journal of Neuro-
science, 27(33), 8877–8884.
Kable, J. W., & Glimcher, P. W. (2007). The
neural correlates of subjective value during
intertemporal choice. Nature Neuroscience,
10(12), 1625–1633.
Kalisch, R. (2009). The functional neuroanat-
omy of reappraisal: Time matters. Neurosci-
ence and Biobehavioral Reviews, 33(8), 1215–
1226.
Keltner, D., & Gross, J. J. (1999). Functional
accounts of emotions. Cognition and Emo-
tion, 13(5), 467–480.
Keltner, D., & Haidt, J. (2001). Social functions
of emotions at four levels of analysis. In W. G.
Parrott (Ed.), Emotions in social psychology:
Essential readings (pp. 175–184). New York:
Psychology Press.
LeDoux, J. (2012). Rethinking the emotional
brain. Neuron, 73(4), 653–676.
Levenson, R. W. (1999). The intrapersonal func-
tions of emotion. Cognition and Emotion,
13(5), 481–504.
Levenson, R. W. (2003). Blood, sweat, and
fears: The autonomic architecture of emotion.
Annals of the New York Academy of Sciences,
1000, 348–366.
Lieberman, M. D., Eisenberger, N. I., Crockett,
M. J., Tom, S. M., Pfeifer, J. H., et al. (2007).
Putting feelings into words: Affect label-
ing disrupts amygdala activity in response to
affective stimuli. Psychological Science, 18(5),
421–428.
Lieberman, M. D., Hariri, A. R., Jarcho, J.
M., Eisenberger, N. I., & Bookheimer, S. Y.
(2005). An fMRI investigation of race- related
amygdala activity in African- American and
Caucasian- American individuals. Nature
Neuroscience, 8(6), 720–722.
Lissek, S., Powers, A. S., McClure, E. B., Phelps,
E. A., Woldehawariat, G., Grillon, C., et al.
(2005). Classical fear conditioning in the
anxiety disorders: A meta- analysis. Behaviour
Research and Therapy, 43(11), 1391–1424.
Livneh, U., & Paz, R. (2012). Amygdala–
prefrontal synchronization underlies resis-
tance to extinction of aversive memories. Neu-
ron, 75(1), 133–142.
Lovibond, P. F. (2004). Cognitive processes in
extinction. Learning and Memory, 11(5),
495–500.
Maier, M. E., & di Pellegrino, G. (2012).
Impaired conflict adaptation in an emotional
task context following rostral anterior cingu-
late cortex lesions in humans. Journal of Cog-
nitive Neuroscience, 24(10), 2070–2079.
Martin, L. N., & Delgado, M. R. (2011). The
influence of emotion regulation on decision-
making under risk. Journal of Cognitive Neu-
roscience, 23(9), 2569–2581.
Mauss, I. B., Levenson, R. W., McCarter, L.,
Wilhelm, F. H., & Gross, J. J. (2005). The tie
that binds? Coherence among emotion expe-
rience, behavior, and physiology. Emotion, 5,
175–190.
McClure, S. M., Laibson, D. I., Loewenstein, G.,
& Cohen, J. D. (2004). Separate neural sys-
tems value immediate and delayed monetary
rewards. Science, 306(5695), 503–507.
McClure, E. B., Monk, C. S., Nelson, E. E.,
Parrish, J. M., Adler, A., Blair, R. J. R., et al.
(2007). Abnormal attention modulation of
fear circuit function in pediatric generalized
Neurobiological Model of Implicit and Explicit Emotion Regulation 89
anxiety disorder. Archives of General Psychia-
try, 64(1), 97–106.
McRae, K., Hughes, B., Chopra, S., Gabrieli, J.
D. E., Gross, J. J., & Ochsner, K. N. (2010).
The neural bases of distraction and reap-
praisal. Journal of Cognitive Neuroscience,
22(2), 248–262.
Milad, M. R., Pitman, R. K., Ellis, C. B., Gold, A.
L., Shin, L. M., Lasko, N. B., et al. (2009). Neu-
robiological basis of failure to recall extinction
memory in posttraumatic stress disorder. Bio-
logical Psychiatry, 66(12), 1075–1082.
Milad, M. R., & Quirk, G. J. (2002). Neurons
in medial prefrontal cortex signal memory for
fear extinction. Nature, 420(6911), 70–74.
Mobbs, D., Petrovic, P., Marchant, J. L., Has-
sabis, D., Weiskopf, N., Seymour, B., et al.
(2007). When fear is near: Threat imminence
elicits prefrontal– periaqueductal gray shifts in
humans. Science, 317(5841), 1079–1083.
Monk, C. S., Klein, R. G., Telzer, E. H., Schroth,
E. A., Mannuzza, S., Moulton, J. L., et al.
(2007). Amygdala and nucleus accumbens
activation to emotional facial expressions
in children and adolescents at risk for major
depression. American Journal of Psychiatry,
165(1), 90 –98.
Moors, A., & De Houwer, J. (2006). Automa-
ticity: A theoretical and conceptual analysis.
Psychological Bulletin, 132(2), 297–326.
Myers- Schulz, B., & Koenigs, M. (2012). Func-
tional anatomy of ventromedial prefrontal
cortex: Implications for mood and anxiety dis-
orders. Molecular Psychiatry, 17(2), 132–141.
Nitschke, J. B., Sarinopoulos, I., Oathes, D. J.,
Johnstone, T., Whalen, P. J., Davidson, R. J.,
et al. (2009). Anticipatory activation in the
amygdala and anterior cingulate in general-
ized anxiety disorder and prediction of treat-
ment response. American Journal of Psychia-
try, 166(3), 302–310.
Norrholm, S. D., Jovanovic, T., Olin, I. W.,
Sands, L. A., Karapanou, I., Bradley, B., et al.
(2011). Fear extinction in traumatized civil-
ians with posttraumatic stress disorder: Rela-
tion to symptom severity. Biological Psychia-
try, 69(6), 556–563.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. E. (2002). Rethinking feelings:
An FMRI study of the cognitive regulation of
emotion. Journal of Cognitive Neuroscience,
14(8), 1215–1229.
Ochsner, K. N., & Gross, J. J. (2005). The cogni-
tive control of emotion. Trends in Cognitive
Sciences, 9(5), 242–249.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 23–42). New York: Guilford
Press.
Paret, C., Brenninkmeyer, J., Meyer, B., Yuen,
K. S. L., Gartmann, N., Mechias, M.-L.,
et al. (2011). A test for the implementation–
maintenance model of reappraisal. Frontiers
in Psychology, 2, 216. [E-publication ahead of
print]
Pejic, T., Hermann, A., Vaitl, D., & Stark, R.
(2011). Social anxiety modulates amygdala
activation during social conditioning. Social
Cognitive and Affective Neuroscience, 8(3),
267–276.
Pessoa, L. (2010). Emergent processes in
cognitive– emotional interactions. Dialogues
in Clinical Neuroscience, 12(4), 433–448.
Phelps, E. A., Delgado, M. R., Nearing, K. I., &
LeDoux, J. E. (2004). Extinction learning in
humans: Role of the amygdala and vmPFC.
Neuron, 43(6), 897–905.
Ploghaus, A., Becerra, L., Borras, C., & Bor-
sook, D. (2003). Neural circuitry underly-
ing pain modulation: Expectation, hypnosis,
placebo. Trends in Cognitive Sciences, 7(5),
197–200.
Ploghaus, A., Tracey, I., Gati, J. S., Clare, S.,
Menon, R. S., Matthews, P. M., et al. (1999).
Dissociating pain from its anticipation in the
human brain. Science, 284(5422), 1979–1981.
Pole, N., Neylan, T. C., Otte, C., Henn-Hasse,
C., Metzler, T. J., & Marmar, C. R. (2009).
Prospective prediction of posttraumatic stress
disorder symptoms using fear potentiated
auditory startle responses. Biological Psychia-
try, 65(3), 235–240.
Ray, R. D., Ochsner, K. N., Cooper, J. C., Rob-
ertson, E. R., Gabrieli, J. D., & Gross, J. J.
(2005). Individual differences in trait rumina-
tion and the neural systems supporting cog-
nitive reappraisal. Cognitive, Affective, and
Behavioral Neuroscience, 5(2), 156–168.
Sarinopoulos, I., Grupe, D. W., Mackiewicz,
K. L., Herrington, J. D., Lor, M., Steege, E.
E., et al. (2010). Uncertainty during anticipa-
tion modulates neural responses to aversion in
human insula and amygdala. Cerebral Cortex,
20(4), 929–940.
Shin, L. M., Wright, C. I., Cannistraro, P. A.,
Wedig, M. M., McMullin, K., Martis, B., et
al. (2005). A functional magnetic resonance
imaging study of amygdala and medial pre-
90 BIOLOGICAL BASES
frontal cortex responses to overtly presented
fearful faces in posttraumatic stress disorder.
Archives of General Psychiatry, 62(3), 273–
281.
Straube, T., Schmidt, S., Weiss, T., Mentzel,
H.-J., & Miltner, W. H. R. (2009). Dynamic
activation of the anterior cingulate cortex dur-
ing anticipatory anxiety. NeuroImage, 44(3),
975–981.
Stroop, J. R. (1935). Studies of interference in
serial verbal reactions. Journal of Experimen-
tal Psychology, 18, 643–662.
Taylor, S. E., Eisenberger, N. I., Saxbe, D.,
Lehman, B. J., & Lieberman, M. D. (2006).
Neural responses to emotional stimuli are
associated with childhood family stress. Bio-
logical Psychiatry, 60(3), 296–301.
Vinogradov, S., Fisher, M., & de Villers- Sidani,
E. (2011). Cognitive training for impaired neu-
ral systems in neuropsychiatric illness. Neuro-
psychopharmacology, 37(1), 43–76.
Wager, T. D., Davidson, M. L., Hughes, B. L.,
Lindquist, M. A., & Ochsner, K. N. (2008).
Prefrontal– subcortical pathways mediating
successful emotion regulation. Neuron, 59(6),
1037–1050.
Wessa, M., & Flor, H. (2007). Failure of extinc-
tion of fear responses in posttraumatic stress
disorder: Evidence from second- order con-
ditioning. American Journal of Psychiatry,
164(11), 1684–1692.
Williams, L. M., Liddell, B. J., Kemp, A. H.,
Bryant, R. A., Meares, R. A., Peduto, A. S., et
al. (2006). Amygdala– prefrontal dissociation
of subliminal and supraliminal fear. Human
Brain Mapping, 27(8), 652–661.

PART III
COGNITIVE APPROACHES

93
Throughout our daily lives, we confront
decisions and situations that require self-
control. Generally, these are of relatively
minor consequence, such as when one resists
the urge to order dessert or spend money on
an expensive electronic gadget. However,
failure to control one’s impulses can also
have serious ramifications, as with eating
disorders, substance abuse, and compulsive
gambling (Dawe & Loxton, 2004). In this
chapter, we discuss the cognition– emotion
interactions that occur with temptations
and self- control as they are understood and
have been studied in psychology and neuro-
science.
Most often this work focuses on a set of
phenomena that together comprise delay
discounting and the class of choices that
require selecting between rewards available
at different points in time, known as inter-
temporal choices. Delay discounting refers
to the simple fact that people tend to prefer
to receive rewards sooner rather than later.
In an intertemporal choice, the subjective
value of each outcome is discounted in pro-
portion to the delay until its receipt, through
the process of delay discounting. One of the
advantages of focusing on delay discounting
is that it is possible to analyze how discount-
ing takes place with the use of discount
functions, which describe mathematically
how subjective value declines as a function
of delay.
There have been tremendous advances in
research on delay discounting over the past
10 years, spurred in part by experiments
using brain imaging as people engage in
intertemporal choices. To a first approxima-
tion, the processes involved in evaluating
outcomes have been distinguished by the
extent to which they rely on “emotion” ver-
sus “reason” (e.g., McClure, Laibson, Loew-
enstein, & Cohen, 2004; Hare, Camerer,
& Rangel, 2009; Figner et al., 2010). This
dichotomy roughly corresponds to the trad-
eoff between satisfying immediate desires
and adhering to abstract, long-term goals.
The terms used to describe these processes
have been diverse: hot and cold; automatic
and controlled; impulsive and deliberative;
passions and reason; visceral and abstract;
and doer and planner, among others (Posner
& Snyder, 1975; Shiffrin & Schneider, 1977;
Thaler & Shefrin, 1981; Chaiken & Trope,
1999; Lieberman, Gaunt, Gilbert, & Trope,
2002; Mischel, Ayduk, & Mendoza- Denton,
2003; Loewenstein, 1996).
The interaction between cognition and
emotion is common to emotion regulation
generally; delay discounting can therefore be
CHAPTER 6
Delay Discounting:
A Two‑Systems Perspective
Eric M. Miller
Christian Rodriguez
Bokyung Kim
Samuel M. McClure

94 COGNITIVE APPROACHES
thought of as one class of behavior in which
emotion regulation strategies are employed.
Indeed, the self- regulation strategies used in
the context of intertemporal choice can be
interpreted through the regulatory process
model proposed by Gross (1998). Specifi-
cally, regulation can occur at several points
in the decision- making process. First, we can
intervene before the decision takes place,
avoiding situations and circumstances that
are likely to require self- control. In inter-
temporal choice, one such strategy referred
to as precommitment involves committing
to one course of behavior ahead of time so
as to avoid unwanted temptations (Strotz,
1956). Second, we can regulate our valua-
tion processes at the time of decision mak-
ing, shifting our attention or reappraising
our perception of an alluring stimulus (e.g.,
Hutcherson, Plassman, Gross, & Rangel,
2012). Third, following the process of valu-
ation, we can attempt to suppress impulsive
choice tendencies by exerting top-down cog-
nitive regulation, a mechanism commonly
related to self- control (Hare et al., 2009).
Choice regulation by self- control implies
that we select an option that is consistent
with long-term goals even while we expe-
rience the temptation to choose a differ-
ent option (cf. Figner et al., 2010). In each
of these regulatory strategies, the interplay
between valuation and cognitive regula-
tion aligns closely with the functions of the
“hot” and “cold” systems described earlier.
As such, studying intertemporal choices as
a combination of these two factors provides
a useful framework for understanding the
mechanisms by which we regulate our deci-
sions.
In the remainder of this chapter we con-
sider the various cognitive and neural pro-
cesses that together compose delay dis-
counting, and emphasize the regulatory
mechanisms involved in controlling our
decision making. We begin with the brain,
and describe the large-scale distinction that
is commonly made between brain networks
linked to the behaviorally defined constructs
of valuation and self- control. We then sum-
marize available evidence suggesting that
these processes account for many aspects of
delay discounting behavior. Recent research
has been expanding on this framework, with
the goal of identifying the diversity of brain
processes involved in intertemporal choice.
Progress along these lines has come in part
from investigations of how different decision
processes interact in guiding behavior, high-
lighting important functions that seem to be
played by regions of the medial prefrontal
cortex. Finally, we return to emotion regu-
lation, and discuss some of the intentional
strategies that we can (and do) employ to
overcome temptations, described from the
perspective of the neural systems involved in
delay discounting.
Neural Systems Involved
in Delay Discounting
Our argument is that multiple systems con-
tribute to delay discounting. Although this
model provides a reasonable account of how
decision making might be thought to occur,
an important question should be addressed
at the outset: Is it necessary to posit the exis-
tence of multiple separate systems? Many
economic theories indicate that decision
making can be wholly explained by the max-
imization of a unitary function of expected
utility. Given that a single utility function
can reasonably account for decision making
in most contexts, why not settle for a more
parsimonious, single- system account? Our
answer is two pronged. First, as will be dis-
cussed, the variability in delay discounting
behavior observed within and between indi-
viduals is difficult to capture with a single-
system account, but it follows naturally from
a multiple systems model (van den Bos &
McClure, 2013). Second, to examine deci-
sion making with techniques such as func-
tional magnetic resonance imaging (fMRI)
provides strong evidence for qualitatively
different neural systems involved in delay
discounting (although some controversy still
remains; McClure et al., 2004; McClure,
Ericson, Laibson, Loewenstein, & Cohen,
2007; Kable & Glimcher, 2007; Peters &
Büchel, 2011). In many aspects of their func-
tion and relationship to behavior, these neu-
ral systems appear to be specialized for the
qualitatively different purposes of valuation
and top-down cognitive control.
Valuation
We face a tremendous number of choices
every day. Occasionally, these choices are
Delay Discounting 95
very significant, such as when we select
which college to attend or decide how to
invest our money. Such decisions warrant
careful deliberation and integration of the
various factors that contribute to the value
of each course of action. However, for the
vast majority of the choices that we face,
careful deliberation would be prohibitive.
If we stopped to think about all of the fac-
tors that determine what we should eat for
breakfast, which clothes to wear, and which
direction to drive to work, it would be diffi-
cult to accomplish anything during the day.
We refer to valuation processes as the
set of relatively automatic processes that
guide our behavior outside of our delibera-
tive control, facilitating the myriad choices
we face every day. In many instances, such
automatic judgments are accompanied by an
affective response (Kahneman, 2003). This
appears to be particularly true for delay
discounting. An immediate reward can be
extremely tempting, producing affectively
charged, high- arousal approach tendencies.
Influential theories of emotions posit that
one of the functions of these processes is to
signal assessments, such as danger, so as to
influence behavior without necessarily mak-
ing us fully aware of the source of the threat
(LeDoux, 2000). Generally, we make these
assessments reflexively, without intention or
deliberate control, and in a manner that is
obscure to our explicit understanding. The
subjective experiences generated by immedi-
ate outcomes share these properties.
At the neural level, a complex network
of brain structures, both cortical and sub-
cortical, underlies these automatic valua-
tion processes (Figure 6.1). A wealth of data
now indicate that positive motivational and
affective signals are encoded in and com-
municated by regions of the brain that are
targeted by midbrain dopamine neurons,
including the nucleus accumbens (NAcc) and
portions of the ventromedial prefrontal cor-
tex (vmPFC; e.g., Knutson, Fong, Bennett,
Adams, & Hommer, 2003). Other regions,
including the amygdala and insular cortex,
are also involved in affective processing,
particularly with regard to signaling salient
or aversive events (LaBar, Gatenby, Gore,
LeDoux, & Phelps, 1998; Wicker et al.,
2003). The NAcc and amygdala, core struc-
tures in these circuits, are evolutionarily
quite old and exist with relatively conserved
function in many animal species. Due partly
to this fact, the motivational processes sup-
ported by this network of brain regions are
thought to form a system that is separable
from deliberative thought and reasoning
that is likely to be specific to humans.
Dopaminergic projections (i.e., neuronal
connections that rely on the neurotransmit-
ter dopamine) into the NAcc are known to
FIGURE 6.1. Two systems in delay discounting. Reward prediction, affect, and automatic behavioral
responses are associated with the ventral striatum (nucleus accumbens), ventromedial PFC, and pos-
terior cingulate cortex. These functions are components of automatic valuation. By contrast, regions
in the dorsolateral and dorsomedial PFC, as well as the posterior parietal cortex, are associated with
cognitive control processes and self- control.
posterior parietal
cortex
lateral PFC
posterior cingulate
cortex
dorsal anterior
cingulate cortex
ventromedial
PFC
ventral striatum
substantia nigra/
VTA
96 COGNITIVE APPROACHES
be an especially crucial motivational sig-
nal. Some of the most compelling evidence
for this comes from studies in which rats
were given the opportunity to perform an
action to self- stimulate different regions of
their brains (Olds, 1977; Gallistel, Shizgal,
& Yeomans, 1981; Ikemoto & Panksepp,
1999). Rats in these studies are especially
motivated to self- stimulate regions of the
midbrain that evoke dopamine release into
the NAcc (Ikemoto & Panksepp, 1999). In
fact, self- stimulation to these brain regions
is so reinforcing that rats will self- stimulate
continuously (i.e., 2,000 times an hour, or
roughly once every 2 seconds), to the exclu-
sion of all other activities, until they are
too physically exhausted to continue (Olds,
1977). Rats also exert significant physical
effort and endure pain (electric shock) to
receive this stimulation (Olds, 1977). They
even prefer brain stimulation to rewards
such as food, when the stimulation is strong
enough (Shizgal & Conover, 1996).
Dopamine release in the NAcc seems to be
the crucial mediator of this self- stimulating
behavior. Pharmacologically inhibiting the
effects of dopamine release in the NAcc
greatly diminishes the rewarding proper-
ties of this stimulation, and direct release of
dopamine into the NAcc can substitute for
electrical stimulation (Wise, 1982). These
reward effects of dopamine also seem to
apply in humans. This is clearly demon-
strated by many drugs of abuse that directly
increase dopamine availability in the brain,
such as cocaine and methamphetamine
(Koob & Bloom, 1988). Together, these data
underscore the importance of dopamine and
the NAcc in motivating behavior (Ikemoto
& Panksepp, 1999; Berridge, Robinson, &
Aldridge, 2009).
Functional neuroimaging experiments in
humans have also demonstrated the involve-
ment of dopamine- associated brain regions
such as the NAcc and vmPFC in track-
ing value and signaling the expectation of
rewarding outcomes (Knutson et al., 2003;
McClure, Berns, & Montague, 2003; Del-
gado, 2007). Neural activity in these regions
can be measured using fMRI, and such mea-
surements have been shown to scale propor-
tionally with the magnitude of expected
reward (Knutson et al., 2003). In most situ-
ations, these dopamine- based value signals
are quite helpful, as they allow us to predict
value and guide our behavior on the basis of
these predictions (Schultz, Dayan, & Mon-
tague, 1997). However, when these pro-
cesses go awry, as is the case in addiction,
exaggerated value signals for things such as
drug- related cues can lead us to overvalue
and seek out maladaptive outcomes (Redish,
2004).
The brain contains a separate network
for encoding aversive signals and detecting
outcomes in our environment that ought
to be avoided. The amygdala has been the
brain structure most clearly implicated with
learning and detecting negative stimuli and
environmental states (although recent evi-
dence suggests that, at the spatial resolu-
tion afforded by fMRI, the amygdala may
respond more generally to salient events,
irrespective of valence; Hamann, Ely, Hoff-
man, & Kilts, 2002; Phelps, 2006). For
example, presenting human or animal sub-
jects with a stimulus that predicts an aver-
sive event, such as a shock, is associated
with increased amygdala activation, even if
the shock does not actually occur (Phillips
& LeDoux, 1992; Phelps et al., 2001). Simi-
larly, amygdala lesions in animals have been
shown to produce a number of odd behav-
ioral effects, including increased approach
behaviors in monkeys toward objects that
they would normally fear (Blanchard &
Blanchard, 1972). As might be expected,
although aversive signals from the amygdala
are generally necessary for adaptive behav-
ior, they can also go awry, as is the case in
social phobias (Phan, Fitzgerald, Nathan, &
Tancer, 2006).
A clear feature of both NAcc and amyg-
dala function is their automaticity. Amyg-
dala responses to fearful or salient stimuli
occur even when participants are unaware
of seeing them, such as when images are
shown so fast that they are only perceived
subliminally (Whalen et al., 1998). Sublimi-
nal responses are also found with respect
to rewards in the NAcc (Pessiglione et al.,
2008). Additionally, the NAcc is a com-
ponent of the basal ganglia, a subcortical
motor circuit that underlies the development
of habits (e.g., Jog, Kubota, Connolly, Hill-
egaart, & Graybiel, 1999). As such, it is well
positioned to facilitate the development of
new automatic behaviors.
Thus, there is strong evidence that both
the amygdala and NAcc underlie the auto-
Delay Discounting 97
matic and stereotyped behaviors that are
often accompanied by affective responses.
It is easy to see why such a system might
have evolved: The automatic generation of
value and behaviors allows quick and effort-
less formulation of responses to rewards or
threats in the environment. These systems
are therefore believed to allow us to get on
with our day, and, perhaps just as impor-
tantly, once helped us in ancestral environ-
ments to respond quickly enough to avoid
predators and obtain fleeting rewards.
Although rapid, effortless, adaptive
responses are a key benefit of this system,
it is at the same time limited by its reliance
on stereotyped behaviors. In the absence of
regulatory influences, our valuation system
seems to overgeneralize responses, leading
to behaviors that are inappropriate in some
circumstances (e.g., Hershberger, 1986).
Moreover, in the domain of delay discount-
ing, automatic valuation processes are inad-
equate for long-term planning. The system is
hypothesized to learn to produce behaviors
that are appropriate given what is occurring
in the immediate environment. For goals
that require planning over extended peri-
ods of time (weeks or years) valuation pro-
cesses are simply not equipped to determine
appropriate behaviors. Instead, in these
cases, decision making requires careful con-
sideration of goals and simulation of future
states. These goal- related functions are com-
monly referred to as cognitive control.
Cognitive Control
The top-down cognitive control system is
defined by deliberative, rule-based consider-
ation of different courses of action (Kahne-
man, 2003). By definition, the computations
involved in cognitive control occur within
our conscious awareness; this is the kind
of reasoning that we think about when we
consider our subjective mental life. These
capacities are particularly important when
we face unfamiliar circumstances for which
we do not have enough experience to know
the appropriate (automatic) response. We
also need to rely on cognitive control when
there is a high chance for error. Consider as
an example the famous Stroop task in which
you have to name the color in which a word
is written, while you suppress reading the
word (think of the word red written in green
ink; Cohen, Dunbar, & McClelland, 1990).
Without explicitly controlling behavior to
arrive at the appropriate response, people
are likely to commit errors by automatically
reading the word.
The brain structures associated with cog-
nitive control are distinct from those that
we previously attributed to valuation. These
structures include dorsolateral and anterior
(frontopolar) regions of the prefrontal cor-
tex, regions within the dorsomedial prefron-
tal cortex, as well as the posterior parietal
cortex (PPC). These brain regions are consis-
tently observed to be involved in a variety of
cognitive processes, such as working mem-
ory (e.g., Cohen et al., 1997), abstract rea-
soning (e.g., Kroger et al., 2002), and general
problem solving (e.g., Duncan et al., 2000).
There is broad consensus that these systems
are central to the brain’s ability to respond
flexibly to rapidly changing task demands,
as well as the pursuit of longer- term, goal-
directed behaviors, especially when faced
with competition from more salient stimuli
or automatic responses (Miller & Cohen,
2001). Although our brain’s cognitive con-
trol system involves an extensive network
of interconnected structures, it is possible
to illustrate some of its mechanistic features
by restricting our focus to two regions: the
dorsolateral prefrontal cortex (dlPFC) and
dorsal anterior cingulate cortex (dACC).
The dlPFC seems to be crucial for success-
ful planning and other executive functions.
Lesions of the dlPFC are associated with dys-
function in organization, planning, working
memory, and attention (Shallice & Burgess,
1991; Stuss & Levine, 2002). Similarly,
brain imaging experiments in healthy adults
have shown that the completion of tasks
that require planning and problem solving
is associated with greater activity in this
region (e.g., Baker et al., 1996). The dlPFC
is also associated with the maintenance and
manipulation of information stored in work-
ing memory, consistent with its hypoth-
esized role in integrating and manipulating
stored mental representations in rational
problem solving (Owen, McMillan, Laird,
& Bullmore, 2005). Finally, the dlPFC is
associated with the control of actions during
tasks that require people to override auto-
matic responses and instead respond based
on a higher- level rule (MacDonald, Cohen,
Stenger, & Carter, 2000).
98 COGNITIVE APPROACHES
The dACC also plays an important role in
high-level cognition. In particular, the dACC
has been shown to be especially important
for the online monitoring of performance, as
well as conflict and error detection (Carter
et al., 1995). According to a dominant the-
ory of dACC function, conflict detection in
the dACC acts as a gating mechanism that
signals when additional control is necessary
(Botvinick, Braver, Barch, Carter, & Cohen,
2001). According to this theory, the dACC
detects when our automatic processes are
leading us awry and modulates activity in
the dlPFC, leading to changes in behavior
(i.e., so that we respond appropriately in cir-
cumstances in which errors are likely). The
dACC’s purported roles in conflict monitor-
ing and dlPFC modulation are evident in
tasks that span a variety of domains, rang-
ing from simple motor responses to moral
and social decision making (MacDonald et
al., 2000; Greene, Nystrom, Engell, Darley,
& Cohen, 2004; Sanfey, Rilling, Aronson,
Nystrom, & Cohen, 2003).
The dlPFC and dACC are two regions in
a network of structures that encode contex-
tual information and facilitate deliberative
processing and planning. These are pre-
cisely the functions ascribed to cognitive
control. A critical limitation of this system
is that information processing is slow and
computationally expensive relative to auto-
matic valuation. Moreover, we can only
explicitly consider one thing at a time, so
processing in this system is serial (Kahne-
man, 2003). These constraints limit the set
of the decisions that are best addressed by
this system.
Intertemporal Choice
To this point, we have considered valuation
and cognitive control in relative isolation.
In many situations, we are presented with
choices for which we must arbitrate between
following our impulses to achieve immediate
gratification and choosing the actions that
we know will be more beneficial in the long
run. Intertemporal choice experiments tar-
get precisely this dilemma, asking people to
decide between smaller rewards (most com-
monly money) that are available sooner in
time and larger rewards that are available
after a longer delay. In such cases, it must be
determined whether the additional amount
to be gained is worth the wait. That is, as in
any value-based decision, the desirability of
the available rewards must be determined,
and a choice must be made on the basis of
these relative values (Rangel, Camerer, &
Montague, 2008). In intertemporal choice,
the desirability of each reward depends on
its magnitude, as well as the delay until its
receipt.
Although everyone would prefer to
receive reward sooner and in greater mag-
nitude when all else is kept equal, people
differ substantially in how they discount the
value of future compensation. For exam-
ple, people with chronic deficits in impulse
control, such as pathological gamblers and
cocaine addicts, tend to discount the value
of delayed reward far more than control
subjects (Bickel, Jarmolowicz, Mueller, Kof-
farnus, & Gatchalian, 2012). People suf-
fering from attention- deficit/hyperactivity
disorder (ADHD), a condition character-
ized by deficits in self- control, also discount
rewards more steeply than healthy controls
(Barkley, Edwards, Lanieri, Fletcher, &
Metevia, 2001). Discounting rates also tend
to decrease as people age from adolescence
into adulthood, consistent with the general
improvements in self- control that occur over
the course of development (Green, Fry, &
Myerson, 1994).
As already stated, from a mechanis-
tic point of view, making intertemporal
choices requires discounting the value of
outcomes based on their delay, and choos-
ing the option with greatest discounted val-
ues. Rationally, preferences generated in this
way should be consistent across time. If you
prefer outcome A over an outcome B that is
to occur some fixed amount of time after A
(say a week), then you should always prefer
A to B, whether A occurs today (and B in a
week) or A occurs in a year (and B in a year
and a week). If this were not the case, then
there would be some critical point in time at
which preferences must switch, so that you
initially prefer B but then reverse to prefer
A some time thereafter. Mathematically,
the only discount function that ensures con-
sistency of preference is one that declines
exponentially with delay (Frederick, Leow-
enstein, & O’Donoghue, 2003). This sounds
reasonable enough, but neither humans nor
animals demonstrate such consistency. In an
often-cited example, when deciding between
Delay Discounting 99
one apple available in a year and two apples
available in a year and a day, people gener-
ally prefer the latter. However, when the
choice is between one apple available today
and two apples available tomorrow, people
generally prefer the former (Thaler, 1981).
Such preference reversals indicate that
humans (and other animals) discount more
steeply over the near term than over the lon-
ger term; that is, people have a tendency to
respond impulsively to tempting rewards
that are immediately available.
Time- dependent preference reversals are
most often explained by positing that delay
discounting follows a hyperbolic discount
function (e.g., Ainslie, 1975; Kirby, 1997;
see Figure 6.2 for more detail). Mathemati-
cally, the hyperbolic function captures the
observation that discount rates decline with
time. However, this framework does little
to explain the broad range of discount rates
that people exhibit for different goods under
different circumstances. In a well-known
example, it was pointed out that it is hard
to imagine people being impulsive for writ-
ing paper or gasoline (Hoch & Loewen-
stein, 1991). Instead, hyperbolic discounting
seems to apply most when we are dealing
with goods that are somehow viscerally
arousing, as is commonly the case with
money and food (Loewenstein, 1996). Simi-
larly, we tend to be impulsive when we are
aroused, either because of some exogenous
emotionally charged event (e.g., Li, 2008) or
because of internal reasons such as hunger
(Giordano et al., 2002; Wang & Dvorak,
2010). While people appear to exhibit hyper-
bolic discounting under all of these condi-
tions, the nature of the discount function
varies substantially by circumstance (van
den Bos & McClure, 2013).
An alternative approach to describ-
ing intertemporal choice views discount-
ing behavior as resulting from the engage-
ment of separate evaluative systems, each
of which uses a different discount function
(e.g., Loewenstein, 1996; van den Bos &
McClure, 2013). The combined effect of
multiple distinct discount functions can pro-
duce hyperbolic- like behavior. The simplest
version of this account suggests that there
are two types of discounting systems: one
that values only goods that are immediately
available, and another system that discounts
more modestly over time (Laibson, 1997).
This corresponds well to the two processing
systems discussed earlier, with the automatic
valuation system exhibiting steep discount-
FIGURE 6.2. Value and preference reversal for smaller, sooner (SS) and larger, later (LL) reward. As
time progresses, and the delay until receipt of SS and LL decreases, delay discounting also decreases
and subjective value grows. (A) With exponential discounting, if LL is preferred to SS initially (solid
line greater than dashed line at Time 0), then this ordinal preference is necessarily maintained. (B)
However, with hyperbolic discounting, preference can reverse as the time to the SS reward approaches.
The gray bar indicates the period of time, just prior to the availability of SS, when preferences favor
the SS outcome.
Time
Smaller, sooner
Larger, later
Time
cilobrepyHlaitnenopxE
Value
Value
BA
100 COGNITIVE APPROACHES
ing and the cognitive control system placing
more equal weight on immediate and future
rewards (McClure et al., 2004, 2007).
A tremendous advantage of the two-
system model of discounting is that it pro-
vides a simple explanation for why people
are impulsive for certain goods and not oth-
ers (e.g., writing paper) for which there is
no associated affective response. Moreover,
it suggests alternative routes to meliorat-
ing impulsive tendencies. One can work to
dampen the emotional impact of a stimu-
lus, exert deliberative control for the sake of
satisfying longer- term goals, or undertake
behaviors to avoid situations in which temp-
tations hold sway. Many of these approaches
have been investigated in recent research,
and they coincide with different strategies
common to instances of emotion regulation.
Neuroscientific studies of intertemporal
choice provide support for the dissociation
between automatic and controlled systems
involved in the decision making. In one of
our early fMRI studies, people were pre-
sented with two types of monetary choices:
a choice between an immediately available
option or a larger, delayed option, and a
choice in which both options were available
only after delays (McClure et al., 2004).
According to the dual- process model of
discounting, one would expect that choices
involving an immediately available reward
would be more affectively arousing in
nature and therefore partially reflect the
function of the impulsive valuation system.
By contrast, choices involving only delayed
rewards would not have this property, and
would instead rely more heavily on delib-
erative processes to form judgments. The
brain activation that was observed while
subjects performed these two types of tri-
als was consistent with this proposed for-
mulation. A number of valuation- associated
brain structures, including the NAcc and
vmPFC, were activated during decisions that
involved choices with an immediately avail-
able option. In contrast, structures involved
in deliberative processing, including the
dACC and dlPFC, were more engaged dur-
ing decisions that involved two delayed out-
comes. Additionally, for choices that pit an
immediate reward against a delayed one,
relative brain activation in the regions that
comprise these two systems was predictive
of the choice that people ultimately made.
In other words, when structures in the auto-
matic valuation system were more active,
people tended to choose more impulsively,
but when structures in the cognitive control
system were more active, people were more
likely to choose the larger, delayed option.
Subsequent fMRI studies have further
elaborated and confirmed the dissociation
of these neural systems. For example, the
results from the experiment just described
were replicated in an experiment in which
thirsty subjects had to make choices between
quantities of juice delivered at different peri-
ods of time, demonstrating that this type of
dual processing is not specific to monetary
rewards (McClure et al., 2007). Moreover,
when deciding between foods, dlPFC activ-
ity tends to support the selection of healthier
options via suppressing responses in vmPFC
(Hare et al., 2009; discussed in greater
detail below). There are important indi-
vidual differences in discounting that are
well explained by this two- system frame-
work as well. Hariri and colleagues (2006)
showed that individual differences in NAcc
responses to winning money predict dis-
count rates. In other words, the more that
the automatic valuation system activates for
the receipt of rewards, the more impulsively
one tends to seek them. By contrast, individ-
ual differences in cognitive control predict
reduced discounting. People who perform
better on a working memory task and show
greater responses in anterior parts of the lat-
eral prefrontal cortex also tend to be more
patient in intertemporal choices (Shamosh et
al., 2008).
One important limitation of the results
that we have discussed up to this point is that
functional brain imaging provides data that
are correlational in nature. In other words,
fMRI provides information regarding which
regions of the brain are associated with a
particular cognitive process but reveals little
about whether these brain regions actually
cause the observed differences in behavior.
To address this limitation, two recent stud-
ies assessed the causal importance of these
structures through more direct manipula-
tions. The first manipulated intertemporal
decision making using repetitive transcra-
nial magnetic stimulation (rTMS; Figner et
al., 2010). In rTMS, electromagnetic pulses
are produced to disrupt activity in particular
regions of an otherwise typically functioning

Delay Discounting 101
brain. In this experiment, rTMS was admin-
istered the left and right dlPFC. After rTMS
administration, subjects completed a set of
intertemporal choice questions to assess the
extent to which the procedure would disrupt
the decision- making process. rTMS to the
left lateral prefrontal cortex caused people to
make more impatient choices, demonstrat-
ing that activation in this region causally
promotes goal- directed decision making. A
second study produced a similar effect by
enhancing dopamine function pharmaco-
logically by giving participants drugs that
increase dopaminergic activity (Pine, Shiner,
Seymour, & Dolan, 2010). Consistent with
the two- system framework, this study found
that discount rates were elevated as well.
Toward Multiple Interacting Systems
The two- system model we discussed earlier
was primarily derived from behavioral stud-
ies. We now know that many of the prop-
erties of this two- system model correspond
well with what is known about brain reward
systems (i.e., dopamine and the NAcc) and
regions linked to executive functions (i.e.,
dlPFC and dACC). However, additional
details of brain function urge a more com-
plex model, with more precise functions
ascribed to individual brain areas, inclusion
of additional systems in the delay discount-
ing processes, as well as a more complete
theory for how brain systems interact dur-
ing choice.
At least three lines of research support
a more refined, multiple- systems model of
delay discounting. First, work by Bechara,
Damasio, Damasio, and Lee (1999) exam-
ining the behavioral deficits associated with
lesions to the vmPFC indicates a role for
this structure in integrating cognitive and
emotional influences on behavior, suggest-
ing that this structure’s function is more
nuanced than allowed for in a two- system
model. Second, although the available evi-
dence suggests that the dACC plays a criti-
cal role in regulating the relative influence
of automatic and controlled processes in
decision making, no studies have clari-
fied exactly how this regulatory function is
implemented in complex scenarios such as
intertemporal choice. Finally, recent studies
suggest an important role for a brain region
that has received very little attention in the
literature on delay discounting— the hippo-
campus— in making intertemporal choices
(Peters & Büchel, 2011). As a whole, these
findings indicate the need to understand
how automatic and controlled processes are
integrated to produce coherent behavior,
and how this integration might be expanded
to include additional brain systems.
Value Integration in the vmPFC
A recent fMRI study dissociated the contri-
butions of the brain systems we have linked
to valuation and self- control in the context
of decision making relative to unhealthy
foods (Hare et al., 2009). In this study, sub-
jects were scanned as they chose between
food options that varied in health and taste
qualities. For example, potato chips are low
on health value but high on taste, and the
opposite is true for broccoli. Subjects were
classified as “self- controllers” or “non-self-
controllers” based on their food preferences
as expressed during the task. Self- controllers
were people who made their decisions based
on both health and taste, whereas non-
self- controllers made their decisions based
solely on taste. Activation in the vmPFC
was shown to be associated with the total
subjective value assigned to individual food
items, such that activity was correlated
with subjects’ rated preference for the item
at the time of choice. Additionally, for self-
controllers, vmPFC activation at the time of
choice was correlated with both the health
and the taste of the items, whereas for non-
self- controllers, vmPFC activation was cor-
related only with taste. Thus, this study indi-
cates that the vmPFC encodes the total value
assigned to individual food items.
Importantly, the separate factors that con-
tribute to total value could also be identified
in this study. The dlPFC showed elevated
activation when participants successfully
engaged self- control, so that dlPFC activity
was greatest when subjects chose to reject
liked but unhealthy foods. Additionally,
comparison of functional activity in the
dlPFC between self- controllers and non-self-
controllers on these trials indicated that self-
controllers displayed greater activation in
this region at the time of choice, providing
confirmatory evidence that activity in this
region facilitates controlled decision making.
102 COGNITIVE APPROACHES
Finally, the investigators found a relation-
ship between activation in the vmPFC and
dlPFC for decisions on liked but unhealthy
foods (i.e., those that should require the most
self- control). In particular, greater activity
in the dlPFC was associated with reduced
activity in the vmPFC on these trials. These
results indicate a means by which valuation
and self- control processes can interact dur-
ing decision making to facilitate successful
self- control. Critically, the vmPFC seems to
function as the site of interaction.
These findings fit into a larger body of
data indicating that the vmPFC is impor-
tant for integrating cognitive and emotional
influences on behavior. Some of the most
compelling evidence for this function comes
from studies of patients with lesions to the
vmPFC. These patients have normal execu-
tive functioning, with normal IQ and over-
all performance on tests of cognitive abili-
ties. They also express normal emotional
responses in tasks such as fear condition-
ing, in which an initially neutral stimulus
(e.g., a visual stimulus) is paired with some-
thing aversive (e.g., an unpleasant sound)
until participants learn to fear the stimulus
(Bechara et al., 1999). Where patients with
vmPFC lesions show striking deficits is in
situations in which cognition and emotion
must be combined to behave appropriately.
Damasio (1994) summarizes this by saying
that reason and emotion “intersect” at the
vmPFC.
This intersection is clearly illustrated by
behavioral deficits expressed by Elliot, a
famous patient with vmPFC impairment
(Eslinger & Damasio, 1985). Elliot had a
tumor surgically removed from his vmPFC,
compromising the function of neighboring
cortical areas. The consequences were disas-
trous. By all accounts, Elliot seemed normal
when in conversations. His IQ was signifi-
cantly above normal, he was appropriately
emotionally expressive, and he passed all
manner of neuropsychological tests. Despite
this, he made terrible financial decisions,
squandering all his money in poor business
decisions. He was unable to hold a job, and
he interacted poorly with others, eventually
leading to his wife divorcing him. Elliot’s
behavior seemed generally normal, but he
had trouble with specific types of choices.
For example, choosing a restaurant for din-
ner could be an onerous chore. He would
study the menu, considering prices and the
different types of food available. He would
even drive to the restaurants to assess their
ambiance. Despite this effort, he would still
be unable to decide. On open-ended neuro-
psychiatric tests, Elliot could provide many
reasonable solutions to complex problems,
only to exclaim at the end “and after all this,
I still wouldn’t know what to do!” (Dama-
sio, 1994, p. 49). Elliot’s problem lay in
linking automatic valuation and regulatory
control when necessary to make a decision.
Specifically, when decisions become too
complex to be handled by the limited capac-
ity of our cognitive control system, we seem
to rely on the combination of affective and
cognitive judgments— something like a gut
feeling— to make the decision. This seems to
be the function of the vmPFC.
Valuation and Control Interactions
Involving the dACC–dlPFC Network
The dlPFC and dACC, key components of
the cognitive control system discussed ear-
lier, have been extensively studied in cogni-
tive neuroscience and are primary regions
involved in deliberated choices. They are
critical nodes in a general- purpose cogni-
tive control network that guides behavior in
a wide variety of tasks, including intertem-
poral decision making (Ridderinkhof et al.,
2004). We understand the most about the
interactions between the dlPFC and dACC
from their function in perceptual decision-
making tasks. Although these perceptual
tasks are mechanistically different from
intertemporal choice, they provide useful
insight regarding the role these regions may
play in more complex decision scenarios.
Two experimental paradigms, the Erik-
sen flanker task and the Stroop task, have
revealed a wealth of mechanistic detail about
dACC and dlPFC function. In both tasks,
successful performance requires participants
to override a prepotent motor response in
order to respond accurately to the stimulus
presented. We discussed the Stroop task ear-
lier, and why deliberation and control are
necessary for appropriate behavior under the
conditions of this experiment. The flanker
task presents similar requirements. In this
task, participants are asked to identify the
central item among a set of distracter stim-
uli (e.g., respond “right” to > > > > > and
Delay Discounting 103
“left” to > > < > >). In incongruent trials, in
which the flanking stimuli support a differ-
ent response than the target stimulus (i.e., >
> < > >), cognitive control becomes essential.
People have a tendency to emit the incorrect
response, as evidenced by an elevated error
rate (Gratton, Coles, & Donchin, 1992).
Furthermore, even when people are able to
suppress the erroneous response, they are
slower to respond than when the flanking
stimuli support the correct answer. Thus, as
in the Stroop task, successful performance
on incongruent trials requires inhibition of
the automatic tendency to respond to the
predominant stimulus attribute.
Imaging and neurophysiological studies
indicate that the type of control required by
these tasks is primarily facilitated by con-
tributions from the dlPFC and the dACC
(Yeung, Botvinick, & Cohen, 2004; Kerns
et al., 2004; Botvinick et al., 2001). As dis-
cussed earlier, these regions play comple-
mentary roles. The dACC is believed to
monitor for the coactivation of incompatible
responses, essentially serving as a detector
for situations in which errors are likely and
control is necessary. The dlPFC is thought
to maintain the appropriate task goals
and to direct action selection accordingly.
When conflict is detected, the dACC shows
increased activity, which then activates
the dlPFC (Kerns et al., 2004). Consistent
with this account, increased activity in this
dACC–dlPFC network is commonly associ-
ated with improved performance during per-
ceptual decision making.
An analogous mechanism seems likely
to be at play in delay discounting. Imag-
ine, for example, that you are asked to
choose between two options that seem
equally appealing (say, $10 today or $20 in
a month). Response conflict is inevitable in
this circumstance, and dACC activity may
be anticipated to increase. Indeed, this has
been found in several studies of intertem-
poral choice (e.g., Marco- Pallarés, Moham-
madi, Samii, & Münte, 2010). In our own
work we find evidence that the dACC sig-
nals conflict as well. For example, Figure
6.3 shows an analysis (McClure et al., 2004)
that identifies the dACC as a region whose
activity scales with reaction time (a surro-
gate for choice difficulty, and hence conflict).
The consequences of increased dlPFC
activity following response conflict in delay
discounting remain somewhat unresolved.
On the one hand, a bulk of evidence suggests
that increased dlPFC activity promotes the
selection of larger, later rewards. In choices
between immediate and delayed rewards,
FIGURE 6.3. dACC activity in intertemporal choice. (A) Reanalysis of the data from McClure et al.
(2004) reveals that dACC responses scale with choice difficulty, as approximated by choice response
time. (B) Trials were split into three groups based on reaction time. Mean hemodynamic responses in
the dACC scale linearly with response time. Time of zero seconds is when participants submitted their
responses. The lag to the peak of the hemodynamic responses (~5–6s) is typical for this measurement
modality.
dACC
Short
Intermediate
Long
-0.1
0.0
0.1
0.2
0.3
0.4
0.5
-4 0 4 8
Time (s)
% Signal Change
BA

104 COGNITIVE APPROACHES
increased dlPFC activity, particularly rela-
tive to NAcc responses, predicts selection of
the latter alternative (McClure et al., 2004,
2007). Furthermore, disruption of the dlPFC
by rTMS increases the proportion of choices
for the immediate rewards, particularly in
conditions where conflict is anticipated to be
high (Figner et al., 2010). These results sup-
port the hypothesis that when conflict arises,
the dACC may modulate the dlPFC, which
in turn implements self- control by directly
biasing choices toward future- oriented goals
or by modulating the integrated valuation
processes occurring in the vmPFC.
On the other hand, recent evidence from
perceptual decision- making experiments
(e.g., Philiastides, Auksztulewicz, Heekeren,
& Blankenburg, 2011), as well as theoreti-
cal proposals (Kable & Glimcher, 2009),
suggests that the dlPFC might instead facili-
tate the selection of the option assigned the
highest discounted value by the valuation
system. This evidence supports an alterna-
tive hypothesis in which the function of
cognitive control is not necessarily linked
to future- oriented goals independent of
automatic valuation processes. Instead, this
hypothesis suggests that automatic valua-
tion and controlled processes may interact
more generally to facilitate decision making.
However, both of these proposals are consis-
tent with the notion that the dACC–dlPFC
network is the basis of deliberative decision
making, guiding choices toward options
that maximize value, either specifically for
the long-term or generally. In either case, the
dACC–dlPFC network appears to influence
behavior through interactions with valua-
tion regions, particularly the vmPFC (Hare
et al., 2009).
Controlled Decisions and Memory
in the Hippocampus
The hippocampus is well known in cognitive
neuroscience primarily because of its role in
remembering specific life events, known as
episodic memories (Burgess, Maguire, &
O’Keefe, 2002). This is best exemplified
by patients such as HM, who are unable
to form new memories after losing hippo-
campal function (Scoville & Milner, 1957).
Episodic memory is also very important for
conceiving of the future (Schacter, Addis,
& Buckner, 2008); patients with hippo-
campal damage are unable to reason about
hypothetical future events, perhaps because
people rely on past experiences in order to
conceive of probable future occurrences.
For delay discounting, this function of the
hippocampus turns out to be very impor-
tant. Intuitively, bringing past experiences to
mind and envisioning future occurrences is
an integral part of controlled decision mak-
ing. Directed memory recall is guided in a
goal- directed manner by dlPFC inputs to
the hippocampus (Levy & Anderson, 2002).
Consequently, rewards promised at future
times linked to specific events (e.g., a $25
payment on December 23, during the holi-
days) elicit increased responses in the dlPFC
and hippocampus (Peters & Büchel, 2010).
Such tangible future episodes have profound
effects on delay discounting; the greater the
recruitment of the hippocampus for future
dates, the less the discounting of future
rewards. Similarly, when people are given
identical intertemporal choices, they are
more patient when future times are linked to
concrete events (e.g., a holiday) than when
they are expressed only with respect to the
delay until receipt (e.g., in 3 months; Peters
& Büchel, 2010). This suggests that con-
trolled processes interact not only with valu-
ation but also with cognitive functions such
as memory, underscoring the need to expand
existing two- system models of intertemporal
choice.
Emotion Regulation
in Intertemporal Choice
We face intertemporal dilemmas every day
and employ a host of strategies to overcome
temptations. In this section we discuss a few
of those strategies and relate them to the
brain systems and cognitive processes on
which they impinge.
The most famous strategy for avoiding
temptations is termed “precommitment,”
in which one commits to a course of action
prior to the actual time of choice to avoid
responding impulsively. One famous exam-
ple of precommitment involves a product
that banks used to offer, known as “Christ-
mas Clubs” (Strotz, 1956). Christmas Clubs
were savings accounts that offered no inter-
est and forbade withdrawals until just before
Christmas. They were popular; people will-
Delay Discounting 105
fully signed up to have access to their money
restricted for months at a time, with no
compensation, so that they could afford to
buy presents at the holidays. Analogous pro-
grams for precommitment continue to exist
today. For example, people pay money to
join weight loss programs that restrict their
calorie intake, and they join the military to
improve self- discipline. Furthermore, there
now exist websites where one can sign up
to be punished financially for not accom-
plishing some goal (e.g., stickk.com and
joesgoals.com).
Precommitment devices are extremely
effective. For example, people ordinarily
perform abysmally at saving for retirement.
However, savings rates increase dramatically
when retirement plans include a precommit-
ment to increase savings progressively by
committing a portion of future raises (Thaler
& Benartzi, 2004). Additionally, when we
understand that we have “weaknesses,” we
often take actions in anticipation of this fact.
Since precommitment requires anticipation
and planning about future situations, we
suspect that precommitment depends on the
involvement of the dlPFC and hippocampus,
because concretely contemplating the future
depends on these structures.
In the absence of precommitment, when
we confront tempting stimuli, a number of
strategies can be applied to curb our emo-
tions and help us respond appropriately.
For example, in a recent study, people were
scanned while they attempted cognitively
to regulate their cravings and to make deci-
sions about whether to eat unhealthy foods
(Hutcherson et al., 2012). As expected,
participants made more healthy decisions
when actively regulating their cravings, and
they showed less controlled decision mak-
ing when they focused on the pleasurable
aspects of the food options. Furthermore,
active regulation of cravings was associated
with decreased activity in regions of the
brain associated with automatic valuation,
whereas value- related activation increased
when these cravings were indulged. Thus,
active regulation of our emotional respond-
ing can facilitate controlled decision making
by suppressing the brain’s automatic value
responses.
Similarly, in one particularly revealing
study by Mischel, Shoda, and Rodriguez
(1989), children chose between receiving one
reward now and receiving two rewards at
some indeterminate time in the future. This
delay is ordinarily very difficult for children
to endure; however, Mischel et al. found that
instructing children to think of the abstract
qualities of the rewards (thereby reducing
their desirability) dramatically improved
their ability to wait for the better outcome.
Furthermore, children who were worse at
delaying gratification expressed greater
activation in reward- related regions when
attempting to suppress responses to an allur-
ing cue (when studied again as adults; Casey
et al., 2011). This provides further evidence
that suppression of reward- related activation
is an important factor in controlled decision
making during intertemporal choice.
A number of other strategies have been
shown to help regulate decision making dur-
ing intertemporal choice. For example, based
on the work by Peters and Büchel (2010)
discussed earlier, thinking of the future in
a more concrete manner can increase the
appeal of obtaining a delayed reward. Such a
change in the way future reward is construed
may compensate for the fact that future ben-
efits tend to be thought of in very general
terms that do not have the same appeal as
the much more precisely considered imme-
diate outcomes (Trope & Liberman, 2003).
Indeed, priming people to think more con-
cretely also makes them more patient (Fujita
& Han, 2009).
Another strategy that has been shown
to regulate decision making in intertempo-
ral choice is focusing one’s attention on the
future consequences of an action. Consider
the manipulation discovered by Magen,
Dweck, and Gross (2008). Usually, inter-
temporal choices are presented as decisions
between smaller, more immediate rewards
(i.e., $10 available right now) and larger,
delayed rewards (i.e., $20 available in a
month). Of course, it is equivalent to reex-
press this choice as either $10 now and noth-
ing in a month or nothing now but $20 in a
month. In this “explicit zero” framing, peo-
ple are significantly more patient. Recently,
we examined the mechanisms underlying
this effect. Based on our studies, we believe
that expressing the future consequences of
immediate reward forces people to think
about the future, thereby reducing a natural
present bias that leads to underconsideration
of future desires. In fact, priming people to

106 COGNITIVE APPROACHES
think about the future is sufficient to reduce
impulsivity in intertemporal choice (Radu,
Yi, Bickel, Gross, & McClure, 2011).
There are undoubtedly many ways that
one can reframe alluring immediate rewards
to reduce impulsivity. Furthermore, there are
numerous known influences on delay dis-
count rates that remain unexplained, such
as the “date-delay effect,” in which people
are more patient when future times are
expressed as exact dates (i.e., July 25 vs. 30
days from now), or the fact that people are
more patient when deciding between larger
rather than smaller rewards (e.g., Thaler,
1981; Read, Frederick, Orsel, & Rahman,
2005). We suspect that these effects work
through a common pathway that ultimately
suppresses reward- related activation in the
NAcc, as meliorating this myopic and impul-
sive signal could facilitate far- sighted behav-
ior. Dampening of NAcc responses can
occur either exogenously (i.e., by presenting
rewards in different ways) or endogenously
(i.e., by deliberatively reconstruing a tempt-
ing outcome). Identifying the precise mecha-
nisms by which these manipulations work
to reduce impulsivity is an exciting future
direction in this research domain.
Conclusions
Delay discounting encompasses a spectrum
of important behaviors in which emotion
regulation strategies have important con-
sequences, particularly for meliorating
impulsivity. Our aim has been to illustrate
cognitive approaches for understanding how
delay discounting occurs, with a particular
emphasis on two- systems models. These
models posit the existence of an automatic,
affect- laden set of processes that interact
with a more flexible, deliberate system to
govern our behavior.
Studying delay discounting from the pur-
view of neuroscience is exciting for a number
of reasons. First, neuroscience offers a com-
plementary empirical approach for establish-
ing the function of constructs such as valu-
ation and self- control in decision making.
Following on this, neuroscience contributes
additional details to a mechanistic under-
standing of delay discounting. For example,
we originally identified vmPFC and dACC
as components of automatic valuation and
controlled decision making, respectively,
based on analyses that sought to identify
qualities of these hypothesized cognitive
processes. Subsequent work has refined our
understanding of these structures. We now
have more detailed understanding of how
self- control works (via dACC–dlPFC inter-
actions) and how valuation and self- control
interact (in part, via the vmPFC). Our sus-
picion is that over the next several years the
nature of these kinds of processes will be
even better understood, and our conceptu-
alization of the overall architecture support-
ing intertemporal decision making will be
expanded to include additional brain sys-
tems and cognitive processes.
Although we have a basic understanding
of how emotion regulation impacts neu-
ral activation during intertemporal choice,
further research is necessary to refine our
understanding of how different regulatory
strategies function with respect to the brain.
This will give us a better understanding of
how known regulatory strategies work,
allow us to understand better individual
differences in impulsivity, and provide a
framework for developing ever more effec-
tive strategies to overcome problems with
self- control. Addiction, obesity, and other
self- control problems are rife in our society.
They can all be conceptualized through the
framework of intertemporal choice, and a
more detailed mechanistic understanding of
delay discounting can only facilitate efforts
to combat these problems.
References
Ainslie, G. (1975). Specious reward: A behavioral
theory of impulsiveness and impulse control.
Psychological Bulletin, 82, 463–496.
Baker, S. C., Rogers, R. D., Owen, A. M., Frith,
C. D., Dolan, R. J., Frackowiak, R. S. J., et al.
(1996). Neural systems engaged by planning:
A PET study of the Tower of London task.
Neuropsychologia, 34, 515–526.
Barkley, R. A., Edwards, G., Laneri, M., Fletcher,
K., & Metevia, L. (2001). Executive function-
ing, temporal discounting, and sense of time in
adolescents with attention deficit hyperactiv-
ity disorder (ADHD) and oppositional defiant
disorder (ODD). Journal of Abnormal Child
Psychology, 29, 541–556.
Bechara, A., Damasio, H., Damasio, A. R., &
Delay Discounting 107
Lee, G. P. (1999). Different contributions of
the human amygdala and ventromedial pre-
frontal cortex to decision- making. Journal of
Neuroscience, 19, 5473–5481.
Bergh, B. V. D., Dewitte, S., & Warlop, L. (2008).
Bikinis instigate generalized impatience in
intertemporal choice. Journal of Consumer
Research, 35, 85–97.
Berridge, K. C., Robinson, T. E., & Aldridge, J.
W. (2009). Dissecting components of reward:
“Liking,” “wanting,” and learning. Current
Opinion in Pharmacology, 9, 65–73.
Bickel, W. K., Jarmolowicz, D. P., Mueller, E.
T., Koffarnus, M. N., & Gatchalian, K. M.
(2012). Excessive discounting of delayed rein-
forcers as a trans- disease process contributing
to addiction and other disease- related vulner-
abilities: Emerging evidence. Pharmacology
and Therapeutics, 134, 287–297.
Blanchard, D. C., & Blanchard, R. J. (1972).
Innate and conditioned reactions to threat in
rats with amygdaloid lesions. Journal of Com-
parative and Physiological Psychology, 81,
281–290.
Botvinick, M. M., Braver, T. S., Barch, D. M.,
Carter, C. S., & Cohen, J. D. (2001). Conflict
monitoring and cognitive control. Psychologi-
cal Review, 108, 624–652.
Burgess, N., Maguire, E. A., & O’Keefe, J.
(2002). The human hippocampus and spatial
and episodic memory. Neuron, 35, 625–641.
Carter, C. S., Braver, T. S., Barch, D. M., Botv-
inick, M. M., Noll, D., & Cohen, J. D. (1995).
Anterior cingulate cortex, error detection, and
the online monitoring of performance. Sci-
ence, 280, 747–749.
Casey, B. J., Somerville, L. H., Gotlib, I. H.,
Ayduk, O., Franklin, N. T., Askren, M. K., et
al. (2011). Behavioral and neural correlates of
delay of gratification 40 years later. Proceed-
ings of the National Academy of Sciences,
108(36), 14998–15003.
Chaiken, S., & Trope, Y. (Eds.). (1999). Dual-
process theories in social psychology. New
York: Guilford Press.
Cohen, J. D., Dunbar, K., & McClelland, J. L.
(1990). On the control of automatic processes:
A parallel distributed processing account of
the Stroop effect. Psychological Review, 97,
332–361.
Cohen, J. D., Perlstein, W. M., Braver, T. S.,
Nystrom, L. E., Noll, D. C., Jonides, J., et al.
(1997). Temporal dynamics of brain activation
during a working memory task. Nature, 386,
604–608.
Damasio, A. (1994). Descartes’ error. New York:
Penguin Books.
Dawe, S., & Loxton, N. J. (2004). The role of
impulsivity in the development of substance
use and eating disorders. Neuroscience and
Biobehavioral Reviews, 28(3), 343–351.
Delgado, M. R. (2007). Reward- related responses
in the human striatum. Annals of the New
York Academy of Sciences, 1104, 70–88.
Duncan, J., Seitz, R. J., Kolodny, J., Bor, D.,
Herzog, H., Ahmed, A., et al. (2000). A neu-
ral basis for general intelligence. Science, 289,
457–460.
Eslinger, P. J., & Damasio, A. R. (1985). Severe
disturbance of higher cognition after bilateral
frontal lobe ablation: Patient EVR. Neurol-
ogy, 35, 1731–1741.
Figner, B., Knoch, D., Johnson, E. J., Krosch,
A. R., Lisanby, S. H., Fehr, E., & Weber, E.
U. (2010). Lateral prefrontal cortex and self-
control in intertemporal choice. Nature Neu-
roscience, 13, 538–539.
Frederick, S., Loewenstein, G., & O’Donoghue,
T. (2003). Time discounting and time prefer-
ence: A critical review. In G. Loewenstein, D.
Read, & R. Baumeister (Eds.), Decision and
time (pp. 13–86). New York: Russell Sage
Foundation.
Fujita, K., & Han, H. (2009). Moving beyond
deliberative control of impulses: The effect of
construal levels on evaluative associations in
self- control conflicts. Psychological Science,
20, 799–804.
Gallistel, C. R., Shizgal, P., & Yeomans, J. S.
(1981). A portrait of the substrate for self-
stimulation. Psychological Review, 88, 228–
273.
Giordano, L. A., Bickel, W. K., Loewenstein,
G., Jacobs, E. A., Marsch, L., & Badger, G. J.
(2002). Opioid deprivation affects how opioid-
dependent outpatients discount the value of
delayed heroin and money. Psychopharmacol-
ogy, 163, 174–182.
Gratton, G., Coles, M. G., & Donchin, E. (1992).
Optimizing the use of information: Strategic
control of activation of responses. Journal
of Experimental Psychology: General, 121,
480–506.
Green, L., Fry, A., & Myerson, J. (1994). Dis-
counting of delayed rewards: A life-span com-
parison. Psychological Science, 5, 33–36.
Greene, J. D., Nystrom, L. E., Engell, A. D., Dar-
ley, J. M., & Cohen, J. D. (2004). The neu-
ral bases of cognitive conflict and control in
moral judgment. Neuron, 44, 389–400.
108 COGNITIVE APPROACHES
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Hamann, S. B., Ely, T. D., Hoffman, J. M., &
Kilts, C. D. (2002). Ecstasy and agony: Acti-
vation of the human amygdala in positive
and negative emotion. Psychological Sci-
ence, 13(2), 135–141.
Hare, T. A., Camerer, C. F., & Rangel, A. (2009).
Self- control in decision- making involves mod-
ulation of the VMPFC valuation system. Sci-
ence, 324, 646–648.
Hariri, A. R., Brown, S. M., Williamson, D.
E., Flory, J. D., de Wit, H., & Manuck, S. B.
(2006). Preference for immediate over delayed
rewards is associated with magnitude of ven-
tral striatal activity. Journal of Neuroscience,
26, 13213–13217.
Hershberger, W. A. (1986). An approach through
the looking glass. Animal Learning and
Behavior, 14, 443–451.
Hoch, S. J., & Loewenstein, G. F. (1991). Time-
inconsistent preferences and consumer self-
control. Journal of Consumer Research, 17,
492–507.
Hutcherson, C. A., Plassmann, H., Gross, J. J.,
& Rangel, A. (2012). Cognitive regulation
during decision- making shifts behavioral con-
trol between ventromedial and dorsolateral
prefrontal value systems. Journal of Neurosci-
ence, 32(39), 13543–13554.
Ikemoto, S., & Panksepp, J. (1999). The role of
nucleus accumbens dopamine in motivated
behavior: A unifying interpretation with
special reference to reward- seeking. Brain
Research Reviews, 31, 6– 41.
Jog, M. S., Kubota, Y., Connolly, C. I., Hill-
egaart, V., & Graybiel, A. M. (1999). Building
neural representations of habits. Science, 286,
1745–1749.
Kable, J. W., & Glimcher, P. W. (2007). The neu-
ral correlates of subjective value during inter-
temporal choice. Nature Neuroscience, 10,
1625–1633.
Kable, J. W., & Glimcher, P. W. (2009). The neu-
robiology of decision: Consensus and contro-
versy. Neuron, 63(6), 733–745.
Kahneman, D. (2003). Maps of bounded ratio-
nality: Psychology for behavioral economics.
American Economic Review, 93, 1449–1475.
Kerns, J. G., Cohen, J. D., MacDonald, A. W.,
Cho, R. Y., Stenger, V. A., & Carter, C. S.
(2004). Anterior cingulate conflict monitor-
ing and adjustments in control. Science, 303,
1023–1026.
Kirby, K. N. (1997). Bidding on the future:
Evidence against normative discounting of
delayed rewards. Journal of Experimental
Psychology: General, 126, 54–70.
Knutson, B., Adams, C. M., Fong, G. W., &
Hommer, D. (2001). Anticipation of increas-
ing monetary reward selectively recruits
nucleus accumbens. Journal of Neuroscience,
21, RC159.
Knutson, B., Fong, G. W., Bennett, S. M.,
Adams, C. M., & Hommer, D. (2003). A
region of mesial prefrontal cortex tracks mon-
etarily rewarding outcomes: Characterization
with rapid event- related fMRI. NeuroImage,
18, 263–272.
Koob, G. F., & Bloom, F. E. (1988). Cellular and
molecular mechanisms of drug dependence.
Science, 242, 715–723.
Kroger, J. K., Sabb, F. W., Fales, C. L.,
Bookheimer, S. Y., Cohen, M. S., & Holyoak,
K. J. (2002). Recruitment of anterior dorso-
lateral prefrontal cortex in human reasoning:
A parametric study of relational complexity.
Cerebral Cortex, 12, 477–485.
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux,
J. E., & Phelps, E. A. (1998). Human amyg-
dala activation during conditioned fear acqui-
sition and extinction: A mixed-trial fMRI
study. Neuron, 20, 937–945.
Laibson, D. I. (1997). Golden eggs and hyper-
bolic discounting. Quarterly Journal of Eco-
nomics, 42, 861–871.
LeDoux, J. E. (2000). Emotion circuits in the
brain. Annual Review of Neuroscience, 23(1),
155–184.
Levy, B. J., & Anderson, M. C. (2002). Inhibitory
processes and the control of memory retrieval.
Trends in Cognitive Sciences, 6, 299–305.
Li, X. (2008). The effects of appetitive stimuli on
out-of-domain consumption impatience. Jour-
nal of Consumer Research, 34, 649–656.
Lieberman, M. D., Gaunt, R., Gilbert, D. T.,
Trope, Y. (2002). Reflection and reflexion:
A social cognitive neuroscience approach to
attributional inference. Advances in Experi-
mental Social Psychology, 34, 199–249.
Loewenstein, G. (1996). Out of control: Vis-
ceral influences on behavior. Organizational
Behavior and Human Decision Processes, 65,
272–293.
Loewenstein, G., & Prelec, D. (1992). Anoma-
Delay Discounting 109
lies in intertemporal choice: Evidence and an
interpretation. Quarterly Journal of Econom-
ics, 107, 573–597.
MacDonald, A. W., Cohen, J. D., Stenger, V.
A., & Carter, C. S. (2000). Dissociating the
role of the dorsolateral prefrontal and anterior
cingulate cortex in cognitive control. Science,
288, 1835–1838.
MacLean, P. D. (1990). The triune brain in evo-
lution: Role in paleocerebral functions. New
York: Plenum Press.
Magen, E., Dweck, C. S., & Gross, J. J. (2008).
The hidden-
z
ero effect: Representing a single
choice as an extended sequence reduces impul-
sive choice. Psychological Science, 19, 648–
649.
Marco-
P
allarés, J., Mohammadi, B., Samii,
A., & Münte, T. F. (2010). Brain activations
reflect individual discount rates in inter
-
t
emporal choice. Brain Research, 1320, 123–
129.
McClure, S. M., Berns, G. S., & Montague, P. R.
(2003). Temporal prediction errors in a passive
learning task activate human striatum. Neu-
ron, 38, 339–346.
McClure, S. M., Ericson, K. M., Laibson, D. I.,
Loewenstein, G., & Cohen, J. D. (2007). Time
discounting for primary rewards. Journal of
Neuroscience, 27, 5796–5804.
McClure, S. M., Laibson, D. I., Loewenstein, G.,
& Cohen, J. D. (2004). Separate neural sys-
tems value immediate and delayed monetary
rewards. Science, 306, 503–507.
Miller, E. K., & Cohen, J. D. (2001). An inte-
grative theory of prefrontal cortex function.
Annual Review of Neuroscience, 24, 167–
202.
Mischel, W., Ayduk, O., & Mendoza-
D
enton,
R. (2003). Sustaining delay of gratification
over time: A hot-cool systems perspective. In
G. Loewenstein, D. Read, & R. Baumeister
(Eds.), Decision and time (pp.
175–200). New
York: Russell Sage Foundation.
Mischel, W., Shoda, Y., & Rodriguez, M. L.
(1989). Delay of gratification in children. Sci-
ence, 244, 933–938.
Olds, J. (1977). Drives and reinforcements:
Behavioral studies of hypothalamic function.
New York: Raven Press.
Owen, A. M., McMillan, K. M., Laird, A. R., &
Bullmore, E. (2005). N-back working memory
paradigm: A meta-
a
nalysis of normative func-
tional neuroimaging studies. Human Brain
Mapping, 25, 46–59.
Pessiglione, M., Petrovic, P., Daunizeau, J.,
Palminteri, S., Dolan, R. J., & Frith, C. D.
(2008). Subliminal instrumental conditioning
demonstrated in the human brain. Neuron,
59, 561–567.
Peters, J., & Büchel, C. (2010). Episodic future
thinking reduces reward delay discount-
ing through an enhancement of prefrontal–
m
ediotemporal interactions. Neuron, 66,
138–148.
Peters, J. & Büchel, C. (2011). The neural mech-
anisms of inter-
t
emporal decision-
m
aking:
Understanding variability. Trends in Cogni-
tive Sciences, 15, 227–239.
Phan, K. L., Fitzgerald, D. A., Nathan, P. J., &
Tancer, M. E. (2006). Association between
amygdala hyperactivity to harsh faces and
severity of social anxiety in generalized social
phobia. Biological Psychiatry, 59(5), 424–
429.
Phelps, E. A. (2006). Emotion and cognition:
Insights from studies of the human amygdala.
Annual Review of Psychology, 57, 27–53.
Phelps, E. A., O’Connor, K. J., Gatenby, J. C.,
Gore, J. C., Grillon, C., & Davis, M. (2001).
Activation of the left amygdala to a cognitive
representation of fear. Nature Neuroscience,
4, 437–441.
Philiastides, M. G., Auksztulewicz, R., Heekeren,
H. R., & Blankenburg, F. (2011). Causal role
of dorsolateral prefrontal cortex in human
perceptual decision making. Current Biology,
21(11), 980–983.
Phillips, R. G., & LeDoux, J. E. (1992). Differen-
tial contribution of amygdala and hippocam-
pus to cued and contextual fear conditioning.
Behavioral Neuroscience, 106, 274–285.
Pine, A., Shiner, T., Seymour, B., & Dolan, R.
J. (2010). Dopamine, time, and impulsivity in
humans. Journal of Neuroscience, 30, 8888–
8896.
Posner, M. I., & Snyder, C. R. R. (1975). Atten-
tion and cognitive control. In R. L. Solso
(Ed.), Information processing and cognition
(pp.
55–85). Hillsdale, NJ: Erlbaum.
Radu, P. T., Yi, R., Bickel, W. K., Gross, J. J.,
& McClure, S. M. (2011). A mechanism for
reducing delay discounting by altering tem-
poral attention. Journal of the Experimental
Analysis of Behavior, 96, 363–385.
Rangel, A., Camerer, C. F., & Montague, P. R.
(2008). A framework for studying the neurobi-
ology of value-based decision-
m
aking. Nature
Reviews Neuroscience, 9, 545–556.
110 COGNITIVE APPROACHES
Read, D., Frederick, S., Orsel, B., & Rahman, J.
(2005). Four Score and Seven Years from Now:
The date/delay effect in temporal discounting.
Management Science, 51(9), 1326–1335.
Redish, A. D. (2004). Addiction as a computa-
tional process gone awry. Science, 306, 1944–
1947.
Ridderinkhof, K. R. (2004). The role of the
medial frontal cortex in cognitive control. Sci-
ence, 306(5695), 443–447.
Sanfey, A. G., Rilling, J. K., Aronson, J. A., Nys-
trom, L. E., & Cohen, J. D. (2003). The neural
basis of economic decision- making in the ulti-
matum game. Science, 300, 1755–1758.
Schacter, D. L., Addis, D. R., & Buckner, R. L.
(2008). Remembering the past to imagine the
future: The prospective brain. Nature Reviews
Neuroscience, 8, 657–661.
Schultz, W., Dayan, P., & Montague, P. R.
(1997). A neural substrate of prediction and
reward. Science, 275, 1593–1599.
Scoville, W. B., & Milner, B. (1957). Loss of
recent memory after bilateral hippocampal
lesions. Journal of Neurology, Neurosurgery,
and Psychiatry, 20, 11–21.
Shallice, T., & Burgess, P. W. (1991). Deficits
in strategy application following frontal lobe
damage in man. Brain, 114, 727–741.
Shamosh, N. A., DeYoung, C. G., Green, A. E.,
Reis, D. L., Johnson, M. R., Conway, A. R. A.,
et al. (2008). Individual differences in delay
discounting: Relation to intelligence, working
memory, and anterior prefrontal cortex. Psy-
chological Science, 19, 904–911.
Shiffrin, R. M., & Snyder, W. (1977). Controlled
and automatic processing: II. Perceptual learn-
ing, automatic attending, and a general theory.
Psychological Review, 84, 127–190.
Shizgal, P., & Conover, K. (1996). On the neural
computation of utility. Current Directions in
Psychological Science, 5, 37–43.
Simon, H. A. (1959). Theories of decision-
making in economics and behavioral science.
American Economic Review, 49, 253–283.
Strotz, R. (1956). Myopia and inconsistency in
dynamic utility maximization. Review of Eco-
nomic Studies, 23, 165–180.
Stuss, D. T., & Levine, B. (2002). Adult clinical
neuropsychology: Lessons from studies of the
frontal lobes. Annual Review in Psychology,
53, 401–433.
Thaler, R. H. (1981). Some empirical evidence on
time inconsistency. Review of Economic Stud-
ies, 23, 165–180.
Thaler, R. H., & Benartzi, S. (2004). Save More
Tomorrow
TM
: Using behavioral economics to
increase employee saving. Journal of Political
Economy, 112, S164–S187.
Thaler, R. H., & Shefrin, H. (1981). An eco-
nomic theory of self- control. Journal of Politi-
cal Economy, 89, 392–406.
Trope, Y., & Liberman, N. (2003). Temporal con-
strual. Psychological Review, 110, 403–421.
van den Bos, W., & McClure, S. M. (2013).
Towards a general model of delay discount-
ing. Journal of the Experimental Analysis of
Behavior, 99, 58–73.
Wang, X. T., & Dvorak, R. D. (2010). Sweet
future: Fluctuating blood glucose levels affect
future discounting. Psychological Science, 21,
183–188.
Whalen, P. J., Rauch, S. L., Etcoff, N. L., McIn-
erney, S. C., Lee, M. B., & Jenike, M. A.
(1998). Masked presentations of emotional
facial expressions modulate amygdala activity
without explicit knowledge. Journal of Neuro-
science, 18, 411– 418.
Wicker, B., Keysers, C., Plailly, J., Royet, J. P.,
Gallese, V., & Rizzolatti, G. (2003). Both of
us disgusted in my insula: The common neu-
ral basis of seeing and feeling disgust. Neuron,
40, 655–664.
Wise, R. A. (1982). Neuroleptics and operant
behavior: The anhedonia hypothesis. Behav-
ioral and Brain Sciences, 5, 39 – 87.
Yeung, N., Botvinick, M. M., & Cohen, J. D.
(2004). The neural basis of error detection:
Conflict monitoring and the error- related
negativity. Psychological Review, 111(4), 931–
959.

111
You would be hard pressed to find a psy-
chologist, professional or budding, who is
not familiar with research on delay of grati-
fication (DG)—the ability to put off a small,
but immediate, reward in favor of receiving
a larger reward later (e.g., Mischel & Ayduk,
2011). In fact, more and more, this work is
being written about in the popular media
(e.g., Brooks, 2006; Lehrer, 2009), and thus
likely discussed in nonacademic circles, such
as parents, educators, and clinicians. What
makes DG, as a topic, so interesting? This
fascination is likely due to the longitudinal
studies linking performance on the classic
DG paradigm, during which a child tries
to wait for two treats instead of having one
right away, to a variety of critical outcomes
in later life, including drug use, social com-
petence, and even Scholastic Aptitude Test
(SAT) scores (e.g., Mischel, Shoda, & Rodri-
guez, 1989).
Although this early longitudinal research
popularized DG for the general public,
researchers have certainly expanded beyond
it. For example, research has linked DG to
other forms of cognitive control (e.g., Casey
et al., 2011; Eigsti et al., 2006), identified
mechanisms that underlie DG ability (e.g.,
Rodriguez, Mischel, & Shoda, 1989), and
examined how these processes play out in
adults, with whom treats are no longer entic-
ing rewards (e.g., Green, Fry, & Myerson,
1994). The bulk of this work, however, has
most often described and discussed DG as a
form of behavioral self- control and focused
on its cognitive and attentional underpin-
nings.
More recently, there has been a growing
body of research linking DG to affective
processes— emotional experience and emo-
tion regulation. In this chapter, we examine
these findings. To do so, we first provide
a brief overview of DG, including its mea-
surement across different age groups and a
description of the most studied mechanism
thought to underlie performance. Subse-
quently, we review four bodies of research
on DG and emotion that in many ways over-
lap. First, we discuss research on the devel-
opmental precursors of DG ability, focus-
ing on how caregivers’ emotion expression
and responsivity to child distress impact the
development of this competency in young
children (e.g., Rodriguez et al., 2005). Sec-
ond, we describe how an individual’s own
affective state impacts DG—more particu-
larly, the damaging effect of negative emo-
tion on one’s ability to put off long-term
rewards (e.g., Tice, Baumeister, & Zhang,
2004). Third, we draw on literature sug-
gesting that DG ability may serve an impor-
tant role in the regulation of emotion— as
CHAPTER 7
The Role of Emotion
and Emotion Regulation
in the Ability to Delay Gratification
Anna Luerssen
Ozlem Ayduk

112 COGNITIVE APPROACHES
a resource for individuals to utilize in emo-
tionally taxing situations (e.g., Ayduk et al.,
2000). Finally, we describe neuroimaging
research that has provided clues about the
links between affective processes and DG
(e.g., McClure, Laibson, Loewenstein, &
Cohen, 2004).
Review of DG
Early research on DG was conducted pri-
marily with preschool- age children using
what has become the classic DG paradigm,
referred to fondly as the “marshmallow
task” (see Mischel & Ayduk, 2011, for
review). First, the experimenter asks the
child whether they would prefer a smaller
or larger reward (e.g., one marshmallow or
two marshmallows). Most children prefer
the larger option, and once they say so, the
experimenter goes on to explain the task con-
tingencies. If, indeed, the child would like to
have the larger reward, he or she must wait
until the experimenter returns to the room,
ostensibly after setting up another task. The
child can have the smaller reward anytime,
however, by ringing a bell and bringing the
experimenter back. The experimenter leaves
the child alone with their options sitting on
a plate in front of them (e.g., one marshmal-
low on one side of the plate, two marshmal-
lows on the other side) and the amount of
time the child can wait is recorded— the pri-
mary measure of DG ability.
Longer wait-time scores on this task have
been linked to varied long-term outcomes
such as higher SAT scores, lower drug use,
and better social- cognitive competence
(e.g., Mischel et al., 1989; Shoda, Mischel,
& Peake, 1990). As such, researchers have
sought to understand what mechanisms
underlie the ability of children to wait (e.g.,
Rodriguez et al., 1989). Experimental and
individual- differences research has found
that attention deployment during the wait-
ing period is an important predictor of wait
time. The more time a child spends looking
at the rewards and the bell (i.e., hot- focused
attention), the harder it is for him or her to
wait. Alternatively, the more time a child
spends looking away from the rewards and
bell and instead focuses on other things in
the room (i.e., cool- focused attention), the
easier it is to wait. In this way, contempo-
rary researchers using the classic paradigm
often measure both wait time and attention
deployment in their evaluation of DG per-
formance.
Although the classic paradigm is the most
studied DG measure, it was designed for pre-
school children and expanded for children
up to 11 years of age (Rodriguez et al., 1989).
Prior to preschool (before approximately age
4), children typically have neither the cogni-
tive capacity to understand the contingencies
of the task nor the ability to wait extended
periods of time alone. Alternatively, for ado-
lescents and adults, cookies and marshmal-
lows may not be tempting in the way they
are for children. Moreover, the experimental
situation with a capped wait time may not
tax adults’ self- regulatory competencies suf-
ficiently. For these reasons the classic task is
unlikely to be diagnostic of individual differ-
ences in DG ability after childhood. Given
that researchers have wanted to study DG in
other age groups, they developed a variety of
tasks better suited to these populations.
Researchers evaluating DG ability in very
young children often employ variations of
either the gift delay or snack delay tasks,
both developed by Kochanska and Knaack
(2003). These tasks require the child to
exert impulse control before obtaining a
desired reward. In a standard gift delay
task, for example, the experimenter pres-
ents a wrapped gift to the child, then has to
leave the room and asks the child to wait to
open it until he or she returns. The child’s
behavior is scored with codes ranging from
not touching or peeking inside the bag to
opening the gift completely. In a typical
snack delay task, children have to wait for
the experimenter to ring a bell before tak-
ing M&M’s “hidden” under a clear cup.
There are a series of trials that vary in the
time delay before the experimenter rings the
bell (e.g., 10–30 seconds). Halfway through
the delay, the experimenter lifts the cup but
does not ring the bell. The child’s behavior
is observed and coded as waiting, touching,
or eating the M&M’s before the bell rings,
with the assumption that these behaviors
reflect decreasing levels of DG ability.
Choice delay tasks are used with adoles-
cents and adults, though many studies also
employ these tasks with children. In a stan-
dard choice delay task, the participant is
asked to make a series of choices between

Delay and Emotion 113
a smaller, immediate reward and a larger,
delayed reward (e.g., $5 today vs. $10 in 1
week) (e.g., Seeman & Schwarz, 1974; Wert-
heim & Schwarz, 1983). The sizes of the
small and large options, as well as the delay
intervals, are varied between trials. In some
studies, researchers calculate participants’
temporal or delay discounting rate—how
quickly larger rewards become unappeal-
ing when a delay is required to obtain them.
For more on choice delay tasks, see Miller,
Rodriguez, Kim, and McClure (this volume).
While we do not claim that these tasks
perfectly parallel the classic paradigm, in
this review we include research using these
measures, with the assumption that there is
at least a moderate degree of overlap in the
psychological conditions between them. In
summary, researchers have developed a vari-
ety of tasks to evaluate DG ability. In child-
hood, the classic paradigm or “marshmal-
low task” is the predominant measure, with
both wait time and attention deployment
scored. Other measures have been developed
for young children and for use with adoles-
cents and adults. In the subsequent review,
we link performance on these measures to
emotion and emotion regulation.
Caregiver Emotion and Responsivity
Given the connection between DG perfor-
mance and such varied and critical long-
term outcomes, the question of how this
ability develops becomes important. Of par-
ticular relevance for our discussion of DG
and emotion is whether there is an associa-
tion between affective environments in early
childhood and the successful development of
DG ability later on.
Researchers have found that caregiver
variables, including caregiver emotion and
caregiver responsivity to children’s emotion,
are important correlates of subsequent DG
development. These variables are often inter-
related or measured interchangeably— with
the nature of caregivers’ expressed emotion
serving as a common signal of whether the
caregiver is responsive to the child’s emo-
tion. In one such study, Eiden, Edwards, and
Leonard (2007) coded parental affect from
videos of the parent and child interacting
at home when the child was age 2. DG was
measured 1 year later with a composite of
performance on age- appropriate DG tasks,
including the snack and gift delay tasks.
Children of parents who displayed lower lev-
els of positive affect were worse at DG 1 year
later. In a similar study, maternal responsive-
ness to a child’s verbal communications was
coded during home visits when the child was
age 24 months. The more responsive his or
her mother was, the more patient and task-
oriented the child was on a gift delay task
1 year later (Olson, Bates, & Bayles, 1990).
Houck and Lecuyer- Maus (2004) had
similar results when evaluating the associa-
tion between maternal responsivity and DG
behavior in a narrower context— during
child limit setting. During laboratory ses-
sions at child ages 12, 24, and 36 months,
parents were asked to prevent their child
from touching and playing with an attractive
toy. The researchers coded four limit- setting
approaches, which varied in the degree of
parent responsivity to the child’s emotion.
Children of parents who predominantly
used a power-based limit- setting approach,
characterized by little responsivity and high
negative affect, waited the least amount of
time during the DG task, significantly less
than children whose parents’ approaches
were more responsive to the child’s feelings.
Rodriguez and colleagues (2005) also
evaluated the role of maternal responsiv-
ity in their study linking behaviors during
the Strange Situation paradigm (used to
measure caregiver– child attachment) when
the child was 18 months old to DG ability
at age 4 (Rodriguez et al., 2005). Mater-
nal responsiveness during the free-play and
reunion episodes was coded using facial and
vocal expression, position and body contact,
and expression of affection, among other
variables. In addition, during the reunion
episode, the researchers identified instances
when a mother disengaged from her child
specifically following the child’s expression
of negative affect. Children of unresponsive
and disengaged mothers waited marginally
less time for a larger reward, and spent more
time directing their attention toward the
rewards and bell during the waiting period,
which, again, is an important mechanism
underlying DG ability (Rodriguez et al.,
1989).
In fact, children’s attachment style with
their caregivers, a more general reflection of
caregiver emotion and responsivity (Shaver
114 COGNITIVE APPROACHES
& Mikulincer, 2011), has also been cor-
related with DG performance. For exam-
ple, in one study, children who were more
securely attached to their caregivers waited
the longest on the classic DG task (Jacob-
sen, Huss, Fendrich, Kruesi, & Ziegenhain,
1997). The greatest difference in wait time
was found between children who displayed
a secure attachment pattern and those who
displayed a disorganized attachment pat-
tern (characterized by fearful and disorga-
nized responses during the reunion phase).
For more on attachment and emotion, see
Shaver and Mikulincer (this volume).
A variety of studies have corroborated
the finding that a caregiver’s hostile, over-
controlling behavior is associated with poor
DG ability. Jacobsen (1998) coded expressed
emotion during a 5-minute speech in which
mothers were asked to describe what their
child was like. Children of mothers who
expressed criticism (resentment of the child’s
behavior or characteristics) waited less time
on the classic DG paradigm. In another
study, researchers coded caregivers’ behav-
ior for directives (commands) during a free-
play time, along with take-overs (completing
the game themselves) and contingent posi-
tive affect (responding to the child’s correct
response with affect signaling approval) dur-
ing a teaching– learning task (Silverman &
Ippolito, 1995). Children of mothers who
used directives and engaged in take-overs
performed worse on age- appropriate ver-
sions of the DG task, including the snack
and gift delay tasks, 6 months later. Chil-
dren whose mothers engaged in contingent
positive affect were better at DG.
Other studies have provided evidence that
a caregiver’s approach to emotion more gen-
erally relates to DG development. Brophy-
Herb, Stansbury, Bocknek, and Horodyn-
ski (2011) evaluated the relation between
children’s DG and a variety of parental
emotion- related socialization behaviors
(ERSBs)—parenting behaviors thought to
be critical for a child’s socioemotional devel-
opment. ERSBs were scored during parent–
child interactions and with questionnaires.
These included positive emotional respon-
sivity and general expressivity in the home,
among other measures. Higher scores on
the ERSB composite factor (more emotion-
related socialization) positively predicted
performance on age- appropriate versions of
the DG task, including the snack and gift
delay tasks.
Collectively, these studies suggest that
caregiver emotion and responsivity are asso-
ciated with the development of DG ability
in childhood. But why exactly is a home life
characterized by caregiver positive affect,
limited hostility, and clear awareness and
sensitivity to a child’s emotion related to
superior DG? How do these affective vari-
ables relate to the development of the ability
to put off immediate gratification in order
to achieve long-term goals? Though there
have been little to no studies exploring this
question of mechanism, researchers have put
forth a few hypotheses, as described below.
Children’s negative affect, emerging
in the context of stress or frustration, is
likely short- circuited by responsive caregiv-
ers. These caregivers attend to their child’s
emotional needs and may provide support,
for example, through soothing or distract-
ing behavior (e.g., Garner, 2006; Jahromi
& Stifter, 2007). This external source of
emotion regulation may serve dual func-
tions. In the immediate, it helps to mitigate
children’s negative affective states. As fur-
ther described in the next section, negative
emotion can destabilize DG (e.g., Mischel,
Ebbesen, & Zeiss, 1972).
At a more global level, the external forms
of regulation provided by responsive care-
givers serve an important modeling func-
tion. Children of responsive caregivers may
have more opportunities to observe and
learn successful emotion regulation strate-
gies that they themselves can employ at an
appropriate age (e.g., Eisenberg, Fabes, &
Murphy, 1996; Garner, 2006). These skills
may be useful for successful DG, which is
in itself a frustrating task. In contrast, chil-
dren who lack such regulatory models may
not develop these skills, therefore becoming
more vulnerable to DG failure.
Caregivers’ own affective profiles may
serve to intensify these processes. Whereas
warm caregivers who score high in posi-
tive affect likewise engender positive affect
in their children, caregivers who score high
in negative affect regularly respond to their
children’s emotions and behaviors in nega-
tive ways, thereby maintaining or even
escalating their children’s negative affect
(Malatesta, Culver, Tesman, & Shepard,
1989). Over time, a caregiver’s character-

Delay and Emotion 115
istic affective tone serves as a model for
the development of a child’s own affective
profile— teaching the child which emotional
responses are appropriate across a range of
situations (e.g., Eisenberg et al., 1999, 2003;
Garner, 1995). Children with more chronic
negative affect may be regularly diverting
resources from DG goals, toward the regula-
tion of their negative affect.
In summary, warm and responsive care-
givers may raise children with high posi-
tive affect and low negative affect, who are
armed with a host of regulation strategies,
whereas cold and distant caregivers may
raise children with low positive affect and
high negative affect, who have fewer regu-
latory strategies. Future research should
evaluate these hypotheses more directly, for
example, by linking parental emotion and
regulatory profiles to the types of affective
reactions and regulatory strategies employed
by children during the DG task. For more on
the role of family on emotion, see Thompson
(this volume).
Affective State and DG
Does an individual’s prior affective state
impact his or her DG ability? For example,
how might children perform if they started
the classic DG task right after learning that
they received a failing grade on a test? What
if they had earned the top score in their
class? As briefly mentioned in the previous
section, researchers have found that negative
affect, in particular, is associated with poor
performance on both laboratory measures
of DG and during real-world DG scenarios,
such as dietary restraint. The evidence on
positive affect is more mixed: Some studies
indicate that positive affect helps DG; others
find that it hurts DG; and still others find
that it does not exert an impact all that dif-
ferent from neutral emotional states.
Negative Affect
Analyses conducted with various laboratory
measures have collectively supported the
prediction that negative emotion is associ-
ated with poor DG performance. In an early
study, researchers hypothesized that behav-
iors, or even thoughts, that successfully dis-
tract the child from the frustrating nature of
the classic DG task will help the child wait
for the larger reward (Mischel et al., 1972).
Indeed, children directed to think “fun”
thoughts during the task (e.g., playing with
toys) did wait longer. However, the con-
tent of the distraction was found to matter.
Directing children to think “sad” thoughts
(e.g., crying with no one to help you) actu-
ally hurt wait time. Children performed at
the same poor level when directed to think
sad thoughts, as when they were directed to
focus their attention on the rewards them-
selves, that is, hot attentional focus.
Similar results have been found with
choice delay tasks (e.g., Schwarz & Pollack,
1977). For example, in one study, 9-year-old
participants drew a picture, and then went
through either a positive affect induction—
receiving feedback that the drawing was
good and would be featured in an art
exhibit, or a negative affect induction—
receiving feedback that the drawing was
not good and would not be featured (See-
man & Schwarz, 1974). Subsequently, they
were asked a series of choice delay questions.
Participants in the negative affect condition
chose large, delayed rewards significantly
less often than participants in the positive
affect condition. In a similar study, partici-
pants, ages 3 to 5 years, directed to think
sad thoughts were more likely to choose an
immediate, mediocre reward over a desir-
able, delayed reward than were children in
either neutral or positive affect conditions
(Moore, Clyburn, & Underwood, 1976).
Similar results have been found with adults
(Gray, 1999). In one study, participants in
a negative affective state (exposed to aver-
sive pictures; in a state of heightened stress
about upcoming midterms) showed a prefer-
ence for short-term gains at the expense of
long-term gains, thus performing worse on
the task overall.
Individual- difference studies have cor-
roborated this body of experimental work.
For example, developmental researchers
have argued that temperament (biologically
based personality differences; see Rothbart,
Sheese, & Posner, 2007, for review) is com-
prised of three factors: arousal, emotion, and
self- regulation. These researchers found that
temperamental levels of negative affectivity
are inversely related to temperamental levels
of self- regulation (Derryberry & Rothbart,
1988). Relatedly, in studies with toddlers,
116 COGNITIVE APPROACHES
more frequent negative affect during the
separation (Rodriguez et al., 2005; Sethi,
Mischel, Aber, Shoda, & Rodriguez, 2000)
and reunion (Rodriguez et al., 2005) phases
of the Strange Situation procedure is associ-
ated with more hot attentional focus (look-
ing toward the rewards and bell) during the
classic DG task at ages 4 and 5. Moreover,
in a choice delay task, mild and moderately
depressed adults were more likely to choose
immediate, less desirable rewards over large,
delayed rewards than individuals who were
not depressed (Wertheim & Schwarz, 1983).
Similar effects have been found in more
real-world DG scenarios. For example,
dietary restraint, like laboratory measures of
DG, requires an individual to put off short-
term gratification (e.g., the pleasure derived
from eating delicious foods), for the sake of
a long-term goal (e.g., losing weight, being
healthy). Because restrained eaters are con-
stantly trying to delay gratification to obtain
a thinner and healthier body, how their eating
changes in response to their current affective
state can inform our understanding of how
affective states impact DG performance more
generally. According to the restraint theory
of Herman and Polivy (1975), negative affect
increases eating in restrained eaters (the rea-
sons for which we evaluate below).
In an experimental evaluation of this
prediction, Frost, Goolkasian, Ely, and
Blanchard (1982) had participants, who var-
ied on degree of dietary restraint, go through
either a negative, positive, or neutral affect
induction. During this affect induction, a
bowl of M&M’s was placed in front of par-
ticipants, and they were told to eat freely
from the bowl. Restrained eaters in the nega-
tive affect condition ate more M&M’s than
nonrestrained eaters in the same condition,
and ate more than restrained eaters in both
the positive and neutral affect conditions.
Though there was not a significant differ-
ence in the eating behavior of nonrestrained
eaters across conditions, they appeared to eat
the least when in a negative affective state.
Baucom and Aiken (1981) had similar
results. Their participants, obese and non-
obese, restrained and nonrestrained eaters,
were asked to solve a task and received either
success feedback (a positive affect induction)
or failure feedback (a negative affect induc-
tion). Participants were then asked to com-
plete an ostensible taste test study in which
they tried different crackers and rated their
flavor preferences. Among restrained eat-
ers, participants who received failure feed-
back ate significantly more crackers than
those who received success feedback. In
contrast, nonrestrained eaters ate margin-
ally fewer crackers after failure feedback
than after success feedback. This pattern
of results held for both obese and nonobese
participants. The link between negative
affect and weakened dietary restraint was
also found among participants experiencing
clinical depression (characterized by strong
negative affect; Polivy & Herman, 1976).
In this study, restrained eaters reported a
considerable weight gain since the onset of
their depression (~6 lbs on average), while
nonrestrained eaters reported a considerable
weight loss (~5 lbs on average), with the dif-
ference between them highly significant.
Collectively, both experimental and
individual- difference studies, involving
classic laboratory measures and more eco-
logically valid study designs, suggest that
negative affect is associated with worse DG
performance. A variety of theories have been
posited to explain this association. The most
common account argues that negative affec-
tive states are incredibly distressing, and as
such, relief from them may take precedence
over long-term goals (e.g., Heatherton &
Baumeister, 1991). Energy or resources ini-
tially directed toward putting off immedi-
ate gratification may be diverted and used
toward emotion regulation instead. With
fewer resources aimed specifically at the DG
attempt, the ability to wait thus suffers (see
Baumeister, Vohs, & Tice, 2007, for review).
For example, restrained eaters, when put into
a negative affective state, may need to divert
the self- regulatory resources they typically
use to restrict their caloric intake, to regu-
late their emotions, resulting in more eating.
Note, however, that some researchers have
argued that individuals who report dieting
may in fact be worse at self- regulation more
generally (see Pratt & Wardle, 2012, for
review).
Moreover, in some DG scenarios a proxi-
mal source of affect relief is available— the
smaller, but immediately available, option.
For example, a child might experience a
reduction in his or her sad feelings as a
Delay and Emotion 117
result of thinking sad thoughts by enjoy-
ing an immediately available, albeit small,
treat. At a more global level, this suggests
that negative affect may change the subjec-
tive reinforcement values of immediate and
delayed rewards (e.g., Baucom & Aiken,
1981; Schwarz & Pollack, 1977; Seeman &
Schwarz, 1974). For individuals in a negative
affective state, the value of the more immedi-
ate option, though objectively smaller, may
increase, because it has the potential to ame-
liorate negative affect. On the other hand,
the value of the long-term goal, or delayed
option, is likely to decrease, because it serves
less of a purpose in removing the negative
affect and may even exacerbate it by adding
additional frustration.
Positive Affect
Although the link between DG and nega-
tive affect seems well established, the find-
ings on positive affect are more mixed. Some
researchers find that, like negative affect,
positive affect is associated with poor DG
performance. For example, Cools, Schotte,
and McNally (1992) exposed participants to
a neutral film, a comedy (i.e., positive affect
induction), or a horror film (i.e., negative
affect induction). Participants were given a
bag of popcorn during the movie and told
to eat as much as they liked. In the neutral
condition, participants higher in dietary
restraint ate less popcorn than those lower
in dietary restraint. In both the comedy
and horror film conditions, however, par-
ticipants higher in dietary restraint ate more
popcorn.
In many other studies, however, partici-
pants in a positive affective state performed
better at DG tasks than those in a negative
affective state. As previously described, chil-
dren who received positive feedback regard-
ing the quality of their drawings chose
larger, delayed rewards more often than
those who received negative feedback (See-
man & Schwarz, 1974). Similarly, children
completing the classic DG task performed
better when asked to think fun, distract-
ing thoughts than when they were directed
to think about the rewards (Mischel et al.,
1972). On a choice delay task, children
in the positive affect condition tended to
choose larger, delayed rewards even more
often than those in the neutral affect condi-
tion, though these results were not statisti-
cally significant (Moore et al., 1976). Stud-
ies have also shown that putting restrained
eaters in a positive or neutral affective state,
in comparison to a negative affective state, is
associated with greater DG ability (Baucom
& Aiken, 1981; Frost et al., 1982). Positive
affective states did not consistently improve
DG performance more than neutral states,
however (Frost et al., 1982).
In summary, the majority of evidence sug-
gests that being in a positive affective state
does not hurt DG the way being in a nega-
tive affective state does, and in some cases it
may even help DG. Although some research-
ers’ findings implied that there are specific
conditions under which positive affect may
thwart DG, we suspect that positive affect
is likely to serve a facilitative function under
most conditions. This assumption is con-
sistent with studies linking positive affect
to improved self- regulation more generally
(e.g., Shmueli & Prochaska, 2012; Tice,
Baumeister, Shmueli, & Muraven, 2007;
Tice et al., 2004).
Although there is need for more research
to firmly establish the facilitative impact of
positive affect on DG, what mechanisms
might explain such an effect? As previously
described, positive affect has been found
to restore self- regulatory resources (Tice
et al., 2007), suggesting that individuals
in a positive affective state may have addi-
tional resources available for DG. More-
over, research in support of Fredrickson’s
broaden- and-build theory of positive emo-
tion, has found that positive affect increases
one’s attentional focus, which may help
transcend the here and now, and thus DG
(see Fredrickson, 2001, for review; cf. Gable
& Harmon-Jones, 2011). Relatedly, positive
affect appears to mitigate the adverse effects
of negative affect (e.g., Fredrickson, 2001;
Fredrickson & Levenson, 1998), and per-
haps by extension the damaging effect nega-
tive affect has on DG performance. Finally,
it is possible that positive affect has informa-
tional value—affirming that things are safe
or certain (see Schwarz, 2011, for review),
which may lead individuals to be more will-
ing to wait for the delayed rewards during a
DG task. Future work should examine these
hypotheses directly.

118 COGNITIVE APPROACHES
Emotion Regulation and DG
Since the initial studies, researchers have
readily assumed that DG tasks induce emo-
tion, and thus by extension, require emotion
regulation— the process of changing the way
we think, feel, and express emotions (e.g.,
Gross & Thompson, 2007). Does the way
an individual deals with these task- induced
emotions impact his or her ability to wait for
the larger reward? What about the reverse?
Is there an association between an individu-
al’s DG ability and how adept he or she is at
regulating emotions? Indeed, a large body of
research demonstrates that one’s DG ability
is predictive of emotion regulation behaviors
in a variety of emotionally taxing situations.
Whether, and how, children regulate their
emotions during DG tasks likely impacts per-
formance. Here we refer to emotions stimu-
lated by the task itself, above and beyond
affective states that precede task onset (as
described in the previous section). For exam-
ple, if a child feels frustration and anger that
he or she cannot have the two marshmallows
immediately, does down- regulating negative
affect help or hurt efforts to wait? On the
flip side, if a child’s predominant affective
reaction to the DG situation is eagerness and
excitement, does down- regulating such posi-
tive emotions facilitate or undermine DG?
Unfortunately, there has been little to no
research that provides clear-cut answers
to these questions. This shortage may be
due, in part, to the difficulty of disentan-
gling emotional reactivity during DG tasks
(e.g., how frustrated– excited the individual
feels) and emotion regulation (e.g., how
well an individual can regulate frustration–
excitement if it indeed emerges). One of the
few studies evaluating emotion during DG
demonstrates this conundrum. Researchers
found that children, ages 4–7, who expressed
more anger and sadness during three age-
appropriate DG tasks, including the gift
delay task and a modified version of the clas-
sic task, also focused their attention more
on the rewards (an important mechanism
underlying poor DG performance; Santucci
et al., 2008). If more expressed negative
emotion reflects less emotion regulation, the
results would suggest that regulating nega-
tive emotion during the DG task should help
performance. It is also possible, however,
that children who performed better were
not regulating their negative emotion; rather
they were less emotionally reactive— and
this is why they displayed fewer expressions
of anger and sadness. These possibilities
need to be disentangled in future research.
Although research that examines emotion
regulation during the DG task is scarce, a
growing body of work shows that individu-
als strong in DG ability are better able to
regulate their emotions across a variety of
situations. In fact, this literature suggests
that individuals at risk for emotion regu-
latory difficulties may be protected from
negative life outcomes if they are also good
at DG. To illustrate, in one set of studies,
researchers evaluated the link between DG
and emotion regulation outcomes in people
with high rejection sensitivity (RS; Ayduk et
al., 2000). These are individuals who anx-
iously expect, readily perceive, and overreact
to social rejection (e.g., Downey & Feldman,
1996). Prior research indicates that individu-
als with high RS respond to perceived social
rejection with dysregulated emotion, includ-
ing jealousy (Downey & Feldman, 1996),
depressed affect (Ayduk, Downey, & Kim,
2001), and hostility directed toward the
source of rejection (Ayduk, Downey, Testa,
Yen, & Shoda, 1999).
Ayduk and colleagues (2000) hypoth-
esized that individuals with high RS and
strong DG ability would be better able to
focus on their longer- term goals, such as
relationship maintenance, during perceived
rejection, and thus be more likely to regu-
late their reactive emotional responses. In
support of this prediction, inner-city middle
school students with high RS were rated by
their teachers as more aggressive and less
socially accepted than those with low RS,
but only if they had poor DG ability, as
measured with the classic paradigm 2 years
earlier. In fact, participants with high RS
and good DG were rated as marginally less
aggressive and better liked than their peers
with low RS. Correspondingly, in a sample
of middle- aged participants, those with high
RS were rated by their parents as worse at
emotion regulation than were those with low
RS (e.g., more likely to go to pieces under
stress), but again, only if they performed
poorly on the classic DG task as preschool-
ers (Ayduk et al., 2000).
A similar pattern of results was found
with respect to borderline personality disor-

Delay and Emotion 119
der (BPD) symptoms (Ayduk et al., 2008). A
core feature of this disorder is extreme affec-
tive lability that bears a resemblance to that
of high RS, including intense hostility and
depression. Ayduk and colleagues hypoth-
esized, and found, that although there was
a positive association between RS and BPD
symptoms, this association was weaker in
individuals with good DG ability, as mea-
sured with the classic DG task in childhood.
A host of other studies corroborate these
findings— that individuals with good DG
ability seem better able to regulate their
emotions in a wide range of situations. For
example, researchers have found that good
DG ability is related to lower verbal and
physical aggression (Mischel et al., 1989),
along with less cheating behavior (Mischel
& Gilligan, 1964). Another study found that
adolescents rated by observers as less likely
to wait for a larger, but delayed, payment for
study participation were also rated higher in
hostility (Funder & Block, 1989). Kochan-
ska, Murray, and Harlan (2000) found that
among toddlers (i.e., 22–33 months olds),
effortful control (of which DG is a com-
ponent) was related to better regulation of
anger, both concurrently and prospectively.
Relatedly, inhibitory control ability (similar
to effortful control) among preschoolers,
in part measured by a gift delay task, posi-
tively correlated with emotion regulation in
controlling both positive and negative affect
(Carlson & Wang, 2007). For more infor-
mation on emotion regulation and effortful–
inhibitory control, see Eisenberg, Hofer,
Sulik, and Spinrad (this volume).
Studies with clinical populations yield
similar results. In one study, early adoles-
cents with externalizing disorder (character-
ized by hostility and aggression) performed
worse on a modified DG task that involved
a series of choices between pressing a button
immediately, which provided a 40% chance
of winning a nickel, or waiting to press a
button, which provided an 80% chance of
winning a nickel (Krueger, Caspi, Moffitt,
White, & Stouthamer- Loeber, 1996). Math-
ias and colleagues (2011) found that adoles-
cent girls’ preference for small, immediate
rewards on a choice delay task was associ-
ated with higher self- reported depression,
aggression, impulsivity, and suicide intent.
In another study, male parolees were pre-
sented with a choice between a small mon-
etary reward available after a short, fixed
delay and a large monetary reward avail-
able after a longer delay (Cherek, Moeller,
Dougherty, & Rhoades, 1997). The number
of times parolees chose the immediate, small
reward was correlated with the number of
aggressive responses they made in another
paradigm. In a follow- up study, parolees
who were imprisoned for violent crimes (e.g.,
aggravated assault, manslaughter) displayed
a preference for small, immediate rewards
significantly more than parolees imprisoned
for nonviolent crimes (e.g., theft, forgery)
(Cherek & Lane, 1999).
Collectively these studies show that there
is a strong association between DG and emo-
tion regulation, and they may suggest that
both forms of regulation are drawing on a
domain- general regulatory competence or
resource that can be utilized in a wide vari-
ety of situations. In the next section we pres-
ent evidence in support of this thesis with
work evaluating the neural underpinnings of
both emotion regulation and DG processes.
Emotion Regulation and DG
in the Brain
Why does an individual’s DG ability relate to
how well they can regulate their emotions?
One theory suggests that DG and emotion
regulation are related because both reflect
an individual’s more general self- regulation
ability (e.g., Cohen & Lieberman, 2010;
Heatherton & Wagner, 2011)—how well
the individual is able to overcome prepotent,
automatic, or stimulus- driven responses
(e.g., Metcalfe & Mischel, 1999). Accord-
ing to this theory, the ability to overcome an
automatic response, be it to grab a marsh-
mallow (in the case of DG) or to become
angry and lash out (in the case of emotion
regulation), is served by a regulatory “con-
trol center” that varies among individuals
in its efficiency or strength. Performance
on tasks diagnostic of self- regulatory ability
in one domain (e.g., DG) are therefore pre-
dictive of self- regulatory ability in another
domain (e.g., emotion regulation), in that
both utilize and reflect the power of this
more general regulatory ability.
This control center may emerge at a
neural level, with common brain regions
or networks serving self- regulation of all
120 COGNITIVE APPROACHES
types, including, but not limited to, DG
and emotion regulation. From this perspec-
tive, individual differences in self- regulation
reflect the relative or effective recruitment of
these regions. Neuroimaging work support-
ing this thesis has found that dorsolateral
(dlPFC) and ventrolateral (vlPFC) regions of
the lateral prefrontal cortex (lPFC) are acti-
vated during both DG and emotion regula-
tion tasks (e.g., Cohen & Lieberman, 2010;
Kober et al., 2010; Ochsner, Bunge, Gross,
& Gabrieli, 2002). This lPFC activation
is associated with the down- regulation of
activity in subcortical regions, such as those
implicated in emotional reactivity in the case
of emotion regulation (e.g., amygdala; Kober
et al., 2010), and reward- related reactivity in
the case of DG (e.g., ventral striatum; Och-
sner, Bunge, Gross, & Gabrieli, 2002).
For example, in one study participants pre-
sented with aversive pictures were directed
to regulate their negative emotion by either
increasing or decreasing it (Ochsner et al.,
2004). When engaging in both forms of
emotion regulation, the dlPFC and vlPFC
became active. Moreover, up- regulation
of negative emotion was associated with
increases in amygdala activation, while
down- regulation of negative emotion was
associated with decreases in amygdala acti-
vation. In another study, participants viewed
negative pictures and were instructed to cog-
nitively reappraise (i.e., emotion regulation),
or just attend to the pictures (Ochsner et al.,
2002). During reappraisal, the lPFC became
more active and the amygdala became less
active. In fact, there was an inverse corre-
lation between vlPFC and amygdala activa-
tion. These results suggest that, during reap-
praisal, vlPFC may down- regulate activity
in the amygdala, a region, again, known for
its role in emotion processing. For further
review on the neural bases of emotion regu-
lation, see Ochsner and Gross (this volume).
DG studies have similarly found the
lPFC is important for self- regulation. For
example, in choice delay tasks, both dlPFC
(Christakou, Brammer, & Rubia, 2011;
McClure et al., 2004) and vlPFC (McClure
et al., 2004) activation is associated with
delayed, rather than immediate, choices.
Additionally, participants who showed low
levels of dlPFC activation more quickly dis-
counted delayed rewards (i.e., worse DG
performance; Hariri et al., 2006). In a more
naturalistic study, cigarette smokers viewed
pictures of cigarettes known to induce crav-
ings (Kober et al., 2010). During the NOW
condition, participants were directed to
focus on the immediate consequences of
consuming the substance (how great that
cigarette would taste; i.e., immediate gratifi-
cation), while during the LATER condition,
participants were directed to focus on the
long-term consequences (risk for heart dis-
ease; i.e., delayed gratification). There was
greater activation of the dlPFC and vlPFC
during LATER trials than during NOW tri-
als. Moreover, dlPFC activation was associ-
ated with decreased activation in the ventral
striatum. In fact, ventral striatal activation
mediated the relationship between dlPFC
activation and self- reported craving. This
suggests that the dlPFC may down- regulate
reward- related activity in the ventral stria-
tum, which then may decrease the subjective
craving experience.
Together these studies show that the lPFC,
including both dorsolateral and ventrolat-
eral subregions, serve an important role
in both DG and emotion regulation tasks.
These results suggest that the lPFC may
be a critical component of a general self-
regulation control center, or network, in the
brain. A recent review conducted by Cohen
and Lieberman (2010) supports this theory.
They found that vlPFC activity, in particu-
lar, was associated with self- regulation in a
variety of domains, including emotion regu-
lation and DG, as well as motor response
inhibition, suppressing risky behavior, mem-
ory inhibition, and thought suppression.
Imaging studies also have the poten-
tial to clarify the relative contributions of
both emotional reactivity and regulation
to behavioral indices of DG. For example,
Casey and colleagues (2011) found that
participants with good DG ability showed
greater right lPFC activation when inhibit-
ing responses to appetitive stimuli (happy
faces). During these same trials, those with
good DG also showed less ventral striatum
activation. Importantly, there was no rela-
tionship between the lPFC and the ventral
striatum, suggesting that activation in each
region independently related to DG. These
results imply that DG ability may as much
reflect diminished emotional reactivity to
rewards, here social rewards in the form of
smiling faces, as regulatory control. Future

Delay and Emotion 121
work is needed, however, to more fully dis-
entangle how and when these regions work
independently versus inversely in predicting
DG performance.
Before concluding this section, it is neces-
sary that we mention a few caveats. First, it is
important to note that the lPFC, a relatively
large region of the brain, has been related
to diverse functions above and beyond self-
regulation. Additionally, though we have
focused on the lPFC in this review, we by
no means suggest that this is the only region
involved in self- regulation. For example, acti-
vation in the medial prefrontal (e.g., Kober
et al., 2010; Ochsner et al., 2002, 2004),
anterior cingulate (e.g., Hariri, Mattay, Tes-
sitore, Fera, & Weinberger, 2003; Ochsner
et al., 2004) posterior cingulate (e.g., Witt-
man, Leland, & Paulus, 2007), and parietal
(e.g., McClure et al., 2004) cortices is also
related to self- regulation in many studies,
including some of those mentioned earlier.
These regions are activated somewhat less
consistently, however, and may thus reflect
more specific aspects of regulation, forms
of regulation, or even the particulars of the
tasks used.
Concluding Remarks
In this chapter, we have reviewed research
showing that emotion and emotion regu-
lation are strongly connected to DG. Our
analysis focused on four subtopics: (1)
the link between early caregiver emotion/
responsivity and DG; (2) the role of affec-
tive state in DG; (3) the association between
emotion regulation and DG abilities; and (4)
the common neural foundations of emotion
regulation and DG. We found that children
best able to DG typically have been raised by
caregivers who have high positive affect and
low negative affect (especially hostility), and
are responsive and attentive to their chil-
dren’s emotional needs. A negative affective
state has been found to hinder successful
DG attempts, both in laboratory contexts
and in a real-world DG scenario— dietary
restraint. DG ability appears to support
individuals’ attempts to navigate emotion-
ally evocative situations. Finally, the litera-
ture indicates that both DG and emotion
regulation activate or rely on common brain
regions, including the lPFC. These findings
suggest that performance on both emotion
regulation and DG tasks may reflect an indi-
vidual’s domain- general regulatory ability,
with regulatory control largely influenced
by the lPFC.
Though there has been important research
on DG and emotion, many questions remain
unanswered. Throughout our review we have
highlighted a variety of important directions
for future research. For example, given that
prior results have been mixed, more studies
must be conducted to clarify the role of posi-
tive emotion in DG performance. Relatedly,
how emotion regulation functions within
DG tasks needs to be better understood. In
one study, reconstruing temptations as a
test of willpower was found to improve self-
regulation (Magen & Gross, 2007; see Leroy,
Grégoire, Magen, Gross, & Mikolajczak,
2012, for similar results). Therefore, reap-
praising one’s frustration during DG tasks
may similarly help performance. However,
it is also possible that letting task- induced
emotions flow freely to reserve regulatory
resources would actually enhance DG per-
formance. Therefore, whether emotion regu-
lation attempts help or hurt DG is an open
question that awaits further research.
In addition to emotional reactivity and
regulation, other factors, such as environ-
mental conditions, may also relate to DG.
For example, Kidd, Palmeri, and Aslin
(2013) evaluated performance on a classic
DG task in children previously exposed to
a reliable experimenter (one who promised
new art supplies and delivered them), and
those exposed to an unreliable experimenter
(one who failed to deliver said art supplies).
Children previously exposed to the unreli-
able environment were less likely to wait
for the larger rewards— here, a practical
and rational decision. These findings sug-
gest that DG ability may as much reflect the
reliability of the environments in which chil-
dren live as their self- regulatory abilities.
Most of the neuroimaging studies evalu-
ating DG reported here have utilized choice
delay tasks in adult populations. Future
studies should focus analysis on child pop-
ulations given that prefrontal regions are
among the last to mature (e.g., Casey, Gal-
van, & Hare, 2005) and may not be reliably
utilized by children in their efforts to exert
self- regulation. Finally, much of the research
outlined here is correlational. More experi-

122 COGNITIVE APPROACHES
mental research is needed to answer ques-
tions about the causal connection between
emotion, emotion regulation, and DG.
References
Ayduk, O., Downey, G., & Kim, M. (2001).
Rejection sensitivity and depressive symptoms
in women. Personality and Social Psychology
Bulletin, 27(7), 868– 877.
Ayduk, O., Downey, G., Testa, A., Yen, Y., &
Shoda, Y. (1999). Does rejection elicit hostil-
ity in rejection sensitive women? Social Cogni-
tion, 17(2), 245–271.
Ayduk, O., Mendoza- Denton, R., Mischel, W.,
Downey, G., Peake, P. K., & Rodriguez, M.
(2000). Regulating the interpersonal self: Stra-
tegic self- regulation for coping with rejection
sensitivity. Journal of Personality and Social
Psychology, 79(5), 776–792.
Ayduk, O., Zayas, V., Downey, G., Cole, A. B.,
Shoda, Y., & Mischel, W. (2008). Rejection
sensitivity and executive control: Joint predic-
tors of borderline personality features. Journal
of Research in Personality, 42(1), 151–168.
Baucom, D. H., & Aiken, P. A. (1981). Effect of
depressed mood on eating among obese and
nonobese dieting and nondieting persons.
Journal of Personality and Social Psychology,
41(3), 577–585.
Baumeister, R. F., Vohs, K. D., & Tice, D. M.
(2007). The strength model of self- control.
Current Directions in Psychological Science,
16(6), 351–355.
Brooks, D. (2006, May 7). Marshmallows and
public policy. Retrieved from www.nytimes.
com/2006/05/07/opinion/07brooks.html?n
=top%2fopinion%2feditorials%20and%20
op%2ded%2fop%2ded%2fcolumnists%2fda
vid%20brooks.
Brophy-Herb, H. E., Stansbury, K., Bocknek,
E., & Horodynski, M. A. (2011). Modeling
maternal emotion- related socialization behav-
iors in a low- income sample: Relations with
toddlers’ self- regulation. Early Childhood
Research Quarterly, 27, 352–364.
Carlson, S. M., & Wang, T. S. (2007). Inhibitory
control and emotion regulation in preschool
children. Cognitive Development, 22(4), 489–
510.
Casey, B. J., Galvan, A., & Hare, T. A. (2005).
Changes in cerebral functional organization
during cognitive development. Current Opin-
ion in Neurobiology, 15(2), 239–244.
Casey, B. J., Somerville, L. H., Gotlib, I. H.,
Ayduk, O., Franklin, N. T., Askren, M. K., et
al. (2011). Behavioral and neural correlates of
delay of gratification 40 years later. Proceed-
ings of the National Academy of Sciences
USA, 108(36), 14998–15003.
Cherek, D. R., & Lane, S. D. (1999). Laboratory
and psychometric measurements of impul-
sivity among violent and nonviolent female
parolees. Biological Psychiatry, 46(2), 273–
280.
Cherek, D. R., Moeller, F. G., Dougherty, D.
M., & Rhoades, H. (1997). Studies of violent
and nonviolent male parolees: II. Laboratory
and psychometric measurements of impulsiv-
ity. Biological Psychiatry, 41(5), 523–529.
Christakou, A., Brammer, M., & Rubia, K.
(2011). Maturation of limbic corticostriatal
activation and connectivity associated with
developmental changes in temporal discount-
ing. NeuroImage, 54(2), 1344–1354.
Cohen, J. R., & Lieberman, M. D. (2010). The
common neural basis of exerting self- control
in multiple domains. In R. R. Hassin, K. N.
Ochsner, Y. Trope, R. R. Hassin, K. N. Och-
sner, & Y. Trope (Eds.), Self control in society,
mind, and brain (pp. 141–160). New York:
Oxford University Press.
Cools, J., Schotte, D. E., & McNally, R. J.
(1992). Emotional arousal and overeating in
restrained eaters. Journal of Abnormal Psy-
chology, 101(2), 348–351.
Derryberry, D., & Rothbart, M. K. (1988).
Arousal, affect, and attention as components
of temperament. Journal of Personality and
Social Psychology, 55(6), 958–966.
Downey, G., & Feldman, S. I. (1996). Implica-
tions of rejection sensitivity for intimate rela-
tionships. Journal of Personality and Social
Psychology, 70(6), 1327–1343.
Eiden, R. D., Edwards, E. P., & Leonard, K.
E. (2007). A conceptual model for the devel-
opment of externalizing behavior problems
among kindergarten children of alcoholic
families: Role of parenting and children’s self-
regulation. Developmental Psychology, 43(5),
1187–1201.
Eigsti, I., Zayas, V., Mischel, W., Shoda, Y.,
Ayduk, O., Dadlani, M. B., et al. (2006). Pre-
dicting cognitive control from preschool to
late adolescence and young adulthood. Psy-
chological Science, 17(6), 478–484.
Eisenberg, N., Fabes, R. A., & Murphy, B. C.
(1996). Parents’ reactions to children’s nega-
tive emotions: Relations to children’s social
Delay and Emotion 123
competence and comforting behavior. Child
Development, 67(5), 2227–2247.
Eisenberg, N., Fabes, R. A., Shepard, S. A., Guth-
rie, I. K., Murphy, B. C., & Reiser, M. (1999).
Parental reactions to children’s negative emo-
tions: Longitudinal relations to quality of chil-
dren’s social functioning. Child Development,
70(2), 513–534.
Eisenberg, N., Hofer, C., Sulik, J. & Spinrad, T.
L. (this volume, 2014). Self- regulation, effort-
ful control, and their socioemotional corre-
lates. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 157–172). New
York: Guilford Press.
Eisenberg, N., Valiente, C., Morris, A. S., Fabes,
R. A., Cumberland, A., Reiser, M., et al.
(2003). Longitudinal relations among parental
emotional expressivity, children’s regulation,
and quality of socioemotional functioning.
Developmental Psychology, 39(1), 3–19.
Fredrickson, B. L. (2001). The role of positive
emotions in positive psychology: The broaden-
and-build theory of positive emotions. Ameri-
can Psychologist, 56(3), 218–226.
Fredrickson, B. L., & Levenson, R. W. (1998).
Positive emotions speed recovery from the
cardiovascular sequelae of negative emo-
tions. Cognition and Emotion, 12(2), 191–
220.
Frost, R. O., Goolkasian, G. A., Ely, R. J., &
Blanchard, F. A. (1982). Depression, restraint,
and eating behavior. Behaviour Research and
Therapy, 20(2), 113–121.
Funder, D. C., & Block, J. (1989). The role of
ego- control, ego- resiliency, and IQ in delay of
gratification in adolescence. Journal of Per-
sonality and Social Psychology, 57(6), 1041–
1050.
Gable, P. A., & Harmon-Jones, E. (2011). Atten-
tional consequences of pregoal and postgoal
positive affects. Emotion, 11(6), 1358–1367.
Garner, P. W. (1995). Toddlers’ emotion regula-
tion behaviors: The roles of social context and
family expressiveness. Journal of Genetic Psy-
chology, 156(4), 417– 430.
Garner, P. W. (2006). Prediction of prosocial and
emotional competence from maternal behav-
ior in African American preschoolers. Cul-
tural Diversity and Ethnic Minority Psychol-
ogy, 12(2), 179–198.
Gray, J. R. (1999). A bias toward short-term
thinking in threat- related negative emotional
states. Personality and Social Psychology Bul-
letin, 25(1), 65–75.
Green, L., Fry, A. F., & Myerson, J. (1994). Dis-
counting of delayed rewards: A life-span com-
parison. Psychological Science, 5(1), 33–36.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 3–24). New York: Guilford Press.
Hariri, A. R., Brown, S. M., Williamson, D.
E., Flory, J. D., de Wit, H., & Manuck, S. B.
(2006). Preference for immediate over delayed
rewards is associated with magnitude of ven-
tral striatal activity. Journal of Neuroscience,
26(51), 13213–13217.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera,
F., & Weinberger, D. R. (2003). Neocortical
modulation of the amygdala response to fear-
ful stimuli. Biological Psychiatry, 53, 494–
501.
Heatherton, T. F., & Baumeister, R. F. (1991).
Binge eating as escape from self- awareness.
Psychological Bulletin, 110(1), 86–108.
Heatherton, T. F., & Wagner, D. D. (2011). Cog-
nitive neuroscience of self- regulation failure.
Trends in Cognitive Sciences, 15(3), 132–139.
Herman, C. P., & Polivy, J. (1975). Anxiety,
restraint, and eating behavior. Journal of
Abnormal Psychology, 84(6), 666–672.
Houck, G. M., & Lecuyer- Maus, E. A. (2004).
Maternal limit setting during toddlerhood,
delay of gratification, and behavior problems
at age five. Infant Mental Health Journal,
25(1), 28– 46.
Jacobsen, T. (1998). Delay behavior at age six:
Links to maternal expressed emotion. Journal
of Genetic Psychology, 159(1), 117–120.
Jacobsen, T., Huss, M., Fendrich, M., Kruesi, M.
J. P., & Ziegenhain, U. (1997). Children’s abil-
ity to delay gratification: Longitudinal rela-
tions to mother– child attachment. Journal of
Genetic Psychology, 158(4), 411–426.
Jahromi, L. B., & Stifter, C. A. (2007). Individ-
ual differences in the contribution of maternal
soothing to infant distress reduction. Infancy,
11(3), 255–269.
Kidd, C., Palmeri, H., & Aslin, R. N. (2013).
Rational snacking: Young children’s decision-
making on the marshmallow task is moder-
ated by beliefs about environmental reliability.
Cognition, 126, 109–114.
Kober, H., Mende- Siedlecki, P., Kross, E. F.,
Weber, J., Mischel, W., Hart, C. L., et al.
(2010). Prefrontal– striatal pathway underlies
cognitive regulation of craving. Proceedings
of the National Academy of Sciences USA,
107(33), 14811–14816.
Kochanska, G., & Knaack, A. (2003). Effort-
124 COGNITIVE APPROACHES
ful control as a personality characteristic of
young children: Antecedents, correlates, and
consequences. Journal of Personality, 71(6),
1087–1112.
Kochanska, G., Murray, K. T., & Harlan, E. T.
(2000). Effortful control in early childhood:
Continuity and change, antecedents, and
implications for social development. Develop-
mental Psychology, 36(2), 220–232.
Krueger, R. F., Caspi, A., Moffitt, T. E., White,
J., & Stouthamer- Loeber, M. (1996). Delay of
gratification, psychopathology, and personal-
ity: Is low self- control specific to externaliz-
ing problems? Journal of Personality, 64(1),
107–129.
Lehrer, J. (2009, May 18). Don’t!: The
secret of self- control. The New Yorker.
Retrieved from www.newyorker.com/
reporting/2009/05/18/090518fa_fact_lehrer.
Leroy, V., Grégoire, J., Magen, E., Gross, J. J.,
& Mikolajczak, M. (2012). Resisting the
sirens of temptation while studying: Using
reappraisal to increase focus, enthusiasm, and
performance. Learning and Individual Differ-
ences, 22(2), 263–268.
Magen, E., & Gross, J. J. (2007). Harnessing
the need for immediate gratification: Cogni-
tive reconstrual modulates the reward value of
temptations. Emotion, 7(2), 415–428.
Malatesta, C. Z., Culver, C., Tesman, J. R., &
Shepard, B. (1989). The development of emo-
tion expression during the first two years of
life. Monographs of the Society for Research
in Child Development, 54(1–2), 1–104.
Mathias, C. W., Dougherty, D. M., James, L.
M., Richard, D. M., Dawes, M. A., Ache-
son, A., et al. (2011). Intolerance to delayed
reward in girls with multiple suicide attempts.
Suicide and Life- Threatening Behavior, 41(3),
277–286.
McClure, S. M., Laibson, D. I., Loewenstein, G.,
& Cohen, J. D. (2004). Separate neural sys-
tems value immediate and delayed monetary
rewards. Science, 306(5695), 503–507.
Metcalfe, J., & Mischel, W. (1999). A hot/
cool- system analysis of delay of gratifica-
tion: Dynamics of willpower. Psychological
Review, 106(1), 3–19.
Miller, E. M., Rodriguez, C., Kim, B., &
McClure, S. M. (this volume, 2014). Delay
discounting: A two- systems perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 93–110). New York: Guil-
ford Press.
Mischel, W., & Ayduk, O. (2011). Willpower in
a cognitive- affective processing system: The
dynamics of delay of gratification. In K. D.
Vohs & R. F. Baumeister (Eds.), Handbook of
self- regulation: Research, theory, and applica-
tions (2nd ed., pp. 83–105). New York: Guil-
ford Press.
Mischel, W., Ebbesen, E., & Zeiss, A. (1972).
Cognitive and attentional mechanisms in
delay of gratification. Journal of Personality
and Social Psychology, 21(2), 204–218.
Mischel, W., & Gilligan, C. (1964). Delay of grat-
ification, motivation for the prohibited gratifi-
cation, and responses to temptation. Journal
of Abnormal and Social Psychology, 69(4),
411–417.
Mischel, W., Shoda, Y., & Rodriguez, M. L.
(1989). Delay of gratification in children. Sci-
ence, 244(4907), 933–938.
Moore, B. S., Clyburn, A., & Underwood, B.
(1976). The role of affect in delay of gratifica-
tion. Child Development, 47(1), 273–276.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. (2002). Rethinking feelings:
An fMRI study of the cognitive regulation of
emotion. Journal of Cognitive Neuroscience,
14(8), 1215–1229.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 23–42). New York: Guilford
Press.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D., et al.
(2004). For better or for worse: Neural sys-
tems supporting the cognitive down-and up-
regulation of negative emotion. NeuroImage,
23, 483– 499.
Olson, S. L., Bates, J. E., & Bayles, K. (1990).
Early antecedents of childhood impulsivity:
The role of parent– child interaction, cogni-
tive competence, and temperament. Journal of
Abnormal Child Psychology, 18(3), 317–334.
Polivy, J., & Herman, C. P. (1976). Clinical
depression and weight change: A complex rela-
tion. Journal of Abnormal Psychology, 85(3),
338–340.
Pratt, F. J., & Wardle, J. (2012). Dietary restraint
and self- regulation in eating behavior. Inter-
national Journal of Obesity, 36, 665– 674.
Rodriguez, M., Ayduk, O., Aber, J. L., Mischel,
W., Sethi, A., & Shoda, Y. (2005). A con-
textual approach to the development of self-
regulatory competencies: The role of maternal
unresponsivity and toddlers’ negative affect
Delay and Emotion 125
in stressful situations. Social Development,
14(1), 136 –157.
Rodriguez, M. L., Mischel, W., & Shoda, Y.
(1989). Cognitive person variables in the delay
of gratification of older children at risk. Jour-
nal of Personality and Social Psychology,
57(2), 358–367.
Rothbart, M. K., Sheese, B. E., & Posner, M. I.
(2007). Executive attention and effortful con-
trol: Linking temperament, brain networks,
and genes. Child Development Perspectives,
1(1), 2 –7.
Santucci, A. K., Silk, J. S., Shaw, D. S., Gentzler,
A., Fox, N. A., & Kovacs, M. (2008). Vagal
tone and temperament as predictors of emo-
tion regulation strategies in young children.
Developmental Psychobiology, 50(3), 205–
216.
Schwarz, J. C., & Pollack, P. R. (1977). Affect
and delay of gratification. Journal of Research
in Personality, 11(2), 147–164.
Schwarz, N. (2011). Feelings-
a
s-
i
nformation the-
ory. In P. A. M. Van Lange, A. W. Kruglanski,
& E. T. Higgins (Eds.), Handbook of theories
of social psychology (pp.
289–308). Thousand
Oaks, CA: Sage.
Seeman, G., & Schwarz, J. C. (1974). Affective
state and preference for immediate versus
delayed reward. Journal of Research in Per-
sonality, 7(4), 384–394.
Sethi, A., Mischel, W., Aber, J. L., Shoda, Y., &
Rodriguez, M. L. (2000). The role of strate-
gic attention deployment in development of
self-
re
gulation: Predicting preschoolers’ delay
of gratification from mother–
t
oddler inter-
actions. Developmental Psychology, 36(6),
767–777.
Shaver, P. R., & Mikulincer, M. (this volume,
2014). Adult attachment and emotion regula-
tion. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp.
237–250). New
York: Guilford Press.
Shaver, P. R., & Mikulincer, M. (2011). Attach-
ment theory. In P. A. M. Van Lange, A. W.
Kruglanski, & E. T. Higgins (Eds.), Handbook
of theories of social psychology (pp.
160–179).
Thousand Oaks, CA: Sage.
Shmueli, D., & Prochaska, J. J. (2012). A test
of positive affect induction for countering
self- control depletion in cigarette smokers.
Psychology of Addictive Behaviors, 26(1),
157–161.
Shoda, Y., Mischel, W., & Peake, P. K. (1990). Pre-
dicting adolescent cognitive and self-
r
egulatory
competencies from preschool delay of gratifica-
tion: Identifying diagnostic conditions. Devel-
opmental Psychology 26(6), 978–986.
Silverman, I. W., & Ippolito, M. F. (1995).
Maternal antecedents of delay ability in young
children. Journal of Applied Developmental
Psychology, 16(4), 569–591.
Thompson, R. A. (this volume, 2014). Socializa-
tion of emotion and emotion regulation in the
family. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp.
173–186). New
York: Guilford Press.
Tice, D. M., Baumeister, R. F., Shmueli, D., &
Muraven, M. (2007). Restoring the self: Posi-
tive affect helps improve self-
r
egulation fol-
lowing ego depletion. Journal of Experimen-
tal Social Psychology, 43(3), 379–384.
Tice, D. M., Baumeister, R. F., & Zhang, L.
(2004). The role of emotion in self-
r
egulation:
Differing role of positive and negative emo-
tions. In P. Philippot & R. S. Feldman (Eds.),
The regulation of emotion (pp.
213–226).
Mahwah, NJ: Erlbaum.
Wertheim, E. H., & Schwarz, J. C. (1983).
Depression, guilt, and self-
m
anagement of
pleasant and unpleasant events. Journal of
Personality and Social Psychology, 45(4),
884–889.
Wittman, M., Leland, D. S., & Paulus, M. P.
(2007). Time and decision making: Differ-
ential contribution of the posterior insular
cortex and the striatum during a delay dis-
counting task. Experimental Brain Research,
179(4), 643–653.

126
A few months ago, I had to take a long,
12-hour international flight in order to get
home. Having more than enough time to
spend, I started looking around me. While
some people were sleeping (in extremely cre-
ative body postures), or watching movies
(with delight that they finally had enough
time to watch Tolkien’s full trilogy), others
appeared quite alert and fearful, especially
when the captain announced that the plane
had started its final descent. While appear-
ing fearful, luckily these individuals were
able to choose between different regulatory
options to control their fear. For example,
they could choose whether they want to deal
with their flight phobia by disengagement,
closing their window shades while immers-
ing themselves in unrelated yet demand-
ing conversations. Alternatively, they could
choose to look out the window while
explaining some facts, including a decline in
crash statistics since autopilots took charge.
Emotion regulatory choices are an inte-
gral part of our daily lives in a dynamically
changing affective world. By emotion regu-
lation choice I mean the choices individuals
make as to how they should regulate their
emotions in a particular context when regu-
lation is warranted and more than one regu-
latory option is active. Furthermore, given
the abundance of knowledge on explicit
emotion regulation strategies and the rela-
tive lack of evidence on implicit emotion reg-
ulation, I limit this chapter to deliberate and
explicit forms of emotion regulation choice.
Can we consistently predict the regulatory
choices individuals make to deal with their
emotions? Are there specific emotional,
cognitive, and motivational influences that
systematically affect these regulatory prefer-
ences? What are the major factors underly-
ing the regulatory choice process? And what
are the broad implications of regulatory
choices? In this chapter I try to answer these
important questions. Specifically, I begin by
introducing the importance of emotion reg-
ulation choice and its relative lack of empiri-
cal investigation. I then introduce a recent
conceptual model to explain dominant emo-
tional, cognitive, and motivational determi-
nants, and underlying mechanisms of emo-
tion regulation choice between dominant
cognitive emotion regulation categories. I
close by describing broad implications and
future directions.
The Importance of Emotion
Regulation Choice
The different ways individuals can go about
controlling their emotions has attracted
scholars for centuries. Nevertheless, emo-
tion regulation has become an independent
CHAPTER 8
Emotion Regulation Choice:
Theory and Findings
Gal Sheppes
Emotion Regulation Choice 127
field only recently (Gross, 1998, 2007, 2010;
Koole, 2009; Tamir, 2011). From its early
days a central question in this field has been
whether different forms of emotion regula-
tion have different consequences. However,
in a first generation of studies, different
forms of emotion regulation have generally
been considered to be either adaptive or mal-
adaptive.
Of two well-known examples, consider
first Nolen- Hoeksema’s influential response
styles theory contrasting maladaptive rumi-
nation with distraction (Nolen- Hoeksema,
1991; see Nolen- Hoeksema, Wisco, & Lyu-
bomirsky, 2008, for reviews). Multiple stud-
ies have widely established that ruminating
on negative attributes of one’s self rather
than distracting attention away from them
is strongly related to the development, main-
tenance, and recurrence of depressive epi-
sodes. As a second example, consider Gross’s
canonical process model and supporting evi-
dence from multiple studies showing the rel-
ative superiority of reappraising the mean-
ing of negative events over suppression of
affective expressions, with respect to a wide
range of affective, cognitive, and social indi-
cators of adaptive functioning (Gross, 2002;
see Gross & Thompson, 2007, for reviews).
The centrality of the alleged dichotomy
between “adaptive” and “maladaptive”
forms of emotion regulation is captured by
a recent meta- analysis that summarizes a
decade of work on the relationship between
some regulation strategies (rumination, sup-
pression) and psychopathology, and other
strategies (reappraisal, problem solving) and
resilience (Aldao, Nolen- Hoeksema, & Sch-
weizer, 2010).
There is no doubt that the first generation
of studies has enormously advanced the field
of emotion regulation. However, with its
rapid growth a second generation of studies
to emerge has begun to find inconsistencies
in the formerly unconditional maladaptive–
adaptive label given to different strategies.
For example, the ostensibly maladaptive
strategy of rumination was found to have
both adaptive and maladaptive forms (Wat-
kins, 2008). For example, rumination was
found to be advantageous in situations in
which a single goal needs to be maintained
in the face of distractors (Altamirano,
Miyake, & Whitmer, 2010). Similarly, the
ostensibly maladaptive strategy of suppres-
sion was shown to be beneficial in extremely
adverse situations (e.g., Bonanno & Kelt-
ner, 1997). At the same time, the ostensibly
adaptive strategy of distraction was found to
be maladaptive when long-term adjustment
is required (Kross & Ayduk, 2008), and the
ostensibly adaptive strategy of reappraisal
was found to be less effective when dealing
with particularly high- intensity emotional
situations (Sheppes, Catran, & Meiran,
2009; Sheppes & Meiran, 2007, 2008).
The main conclusion derived from the
second generation of studies is that emotion
regulation strategies have different conse-
quences in different contexts. This means
that healthy adaptation is the result of flex-
ibly choosing between regulation strategies
to adapt to differing situational demands
(e.g., Bonanno, 2005; Kashdan & Rotten-
berg, 2010; see Troy & Mauss, 2011, for
reviews). To illustrate, Kashdan and Rot-
tenberg (2010) argue that different forms
of psychopathology can be characterized by
different ways in which flexible regulation
choice breaks down, and Troy and Mauss’s
(2011) and Bonanno’s (2005) influential
accounts highlight how flexible regulation
choice promotes resilience in the face of
stress and trauma.
Although emotion regulation choice is now
viewed as a crucial element in healthy adap-
tation, until recently it has only been studied
indirectly. Two forms of important yet indi-
rect evidence come from correlational stud-
ies involving self- report questionnaires that
assess individual differences in people’s fre-
quency of use of different regulatory strate-
gies across situations (e.g., Aldao & Nolen-
Hoeksema, 2012a; Garnefski et al., 2001;
Gross & John, 2003; John & Gross, 2007)
and from laboratory experiments that evalu-
ate spontaneous use of emotion regulation
strategies in emotion- inducing situations
(e.g., Aldao & Nolen- Hoeksema, 2012b;
Ehring, Tuschen- Caffier, Schnülle, Fischer,
& Gross, 2010; Gruber, Harvey, & Gross,
2012). While showing important links to
well-being and various forms of psychopa-
thology (see Aldao et al., 2010, for a review),
these studies do not assess the factors that
influence individuals predominantly to pre-
fer using one particular regulatory strategy
over another. Even more conventional stud-
ies of emotion regulation have not exam-
ined which regulation strategies are chosen

128 COGNITIVE APPROACHES
in different emotional contexts, because
the experimental design involved directly
instruct participants to use regulation strat-
egies in different situations. Consider, for
example, the most direct and convincing evi-
dence of the importance of flexible regula-
tion patterns, which has shown that the abil-
ity to alternate flexibly between enhancing
and suppressing emotion strongly predicts
healthy adaptation (Bonanno, Papa, Lal-
ande, Westphal, & Coifman, 2004) over an
extended time period (Westphal, Seivert, &
Bonanno, 2010), and that flexible regulation
can protect people from complicated grief
patterns in bereavement (Gupta & Bonanno,
2011). In these and other studies, the regu-
lation strategies employed by participants
were determined by the experimenter, leav-
ing the important topic of determinants and
consequences of emotion regulation choice
unexplored.
Conceptualizing Emotion
Regulation Choice
Recently my colleagues and I developed a
conceptual framework to explain (1) major
determinants and (2) underlying mecha-
nisms of emotion regulation choice (Sheppes,
Scheibe, Suri, Radu, Blechert, & Gross,
2012). A central working hypothesis in this
framework is that healthy individuals would
be sensitive to the central costs and benefits
associated with the implementation of each
regulatory option under different contexts.
With this working hypothesis in mind, cer-
tain emotional, cognitive, and motivational
contextual factors are likely to bias regula-
tory choices in ways that are congruent with
the differential consequences of implement-
ing these strategies under these conditions.
In illuminating the underlying mechanisms
of emotion regulation choice a further argu-
ment suggests that healthy regulation choice
should require in some contexts an ability to
recruit deliberate executive control processes
that can override contrasting associative
emotional processes. This interplay of higher
control processes that can override opposing
associative processes is at the heart of many
models of self- regulation (e.g., Muraven &
Baumeister, 2000). Moreover, the regula-
tion choice process gives a central weight to
differences between strategies’ underlying
engagement– disengagement dimension rela-
tive to other potent factors, such as cogni-
tive effort. To help us better appreciate the
broader context of this emotion regulation
choice conceptual framework I elaborate it
below.
The starting point of this conceptual
framework is that individuals’ limited cog-
nitive capacity poses permanent processing
constraints. These constraints result in a
constant competition between emotion gen-
eration and emotion regulation processes
(Gross, Sheppes, & Urry, 2011a, 2011b)
for dominance over behavior. Our concep-
tual account draws on major information-
processing theories (e.g., Hübner, Stein-
hauser, & Lehle, 2010; Pashler, 1998) and
the process model of emotion regulation
(Gross & Thompson, 2007) to suggest that
regulating one’s emotions involves recruit-
ing deliberate executive control mechanisms
that try to modify the nature of emotional
information processing at two major cogni-
tive stages (Sheppes & Gross, 2011, 2012),
which include an early attentional selection
stage and a late semantic meaning stage.
Specifically, regulation can be achieved via
early disengagement from emotional pro-
cessing at the attention selection stage, or via
an engagement with emotional processing
that is modulated at a late semantic meaning
stage (e.g., Johnston & Heinz, 1978; Lehle
& Hübner, 2008). This conceptual frame-
work focuses on two particular regulatory
strategies that are frequently used in every-
day life and have their major influence in
each of these two cognitive stages of infor-
mation processing.
Incoming emotional information can
be regulated at an early attentional selec-
tion processing stage by disengaging from
emotional information processing before
it undergoes elaborated processing. A clas-
sic early selection strategy is distraction,
which involves disengaging attention from
emotional processing before it is represented
in working memory by producing neutral
thoughts that are independent from and not
in conflict with emotional information (e.g.,
van Dillen & Koole, 2007).
Engagement with incoming emotional
information that passes the early atten-
tional selection stage can still be regulated
at a late semantic meaning processing stage
before it affects behavior. A classic late
Emotion Regulation Choice 129
selection regulation strategy is reappraisal,
which involves engaging with and elaborat-
ing emotional information prior to changing
its meaning in a late processing stage (e.g.,
Gross, 2002). In reappraisal, the neutral
reinterpretation is semantically dependent
and in direct conflict with the original emo-
tional information. According to our con-
ceptual framework, these underlying char-
acteristics of disengagement distraction and
engagement reappraisal result in a differen-
tial cost– benefit tradeoff (Sheppes & Gross,
2012). Specifically, the benefits of block-
ing emotional information early, before it
gathers force via distraction, are that emo-
tionally high- intensity information can be
successfully modulated. Cognitively, this
successful modulation involves relatively
simple processes, because the generation of
regulatory neutral thoughts in distraction
is independent from and does not conflict
with the original emotional information.
Nevertheless, the major cost of distraction
is that because it does not allow processing,
evaluating, and remembering of emotional
information, motivationally it is less benefi-
cial for one’s long-term goals and adaptation
(see Wilson & Gilbert, 2008, for a review).
Specifically, distraction does not allow one
to attend to emotional events that are being
repeatedly encountered and be provided
with adequate explanation, a requirement
that is at the heart of many long-term goals
in which an individual has to face difficul-
ties in order to adapt.
The underlying characteristics of engage-
ment reappraisal result in a different set of
costs and benefits. Specifically, the elabo-
rated semantic processing that occurs prior
to late modulation should be emotionally
costly, because it can less successfully block
high- intensity emotional information. Cog-
nitively, reappraisal engages relatively com-
plex processes, because the generation of
alternative construals is dependent on and
in conflict with the original emotional infor-
mation. Nevertheless, the major benefit of
engaging with emotional information is that
motivationally it allows one to process, eval-
uate, and remember emotional information,
which are crucial for long-term goals and for
adaptation.
Direct empirical support for the costs and
benefits of employing (rather than freely
choosing) distraction and reappraisal comes
from behavioral and electrophysiological
studies. Specifically, several behavioral stud-
ies showed that employing early disengage-
ment distraction in high- sadness emotion-
ally intense situations resulted in successful
regulation (Sheppes & Meiran, 2007), and
did not result in an increased expenditure
of cognitive resources (Sheppes et al., 2009;
Sheppes & Meiran, 2008). At the same time,
distraction’s lack of emotional processing
was demonstrated in an impaired memory
for emotional information (Sheppes & Mei-
ran, 2007, 2008), and distraction’s motiva-
tional costs were evident in a lack of long-
term attenuation of the intensity or quality
of important autobiographical, negative
emotional events (Kross & Ayduk, 2008).
By contrast, these studies showed that while
employing late engagement reappraisal in
low- sadness emotionally intense situations
was successful, high- sadness emotionally
intensity situations resulted in less success-
ful modulation and in an increased expendi-
ture of cognitive resources. The elaborated
emotional processing was demonstrated in
intact memory for emotional information
(see also Dillon, Ritchey, Johnson, & LaBar,
2007; Richards & Gross, 1999, 2000), and
reappraisal’s motivational benefits evinced
in adaptation to distressing events that are
important for one’s long-term goals and
functioning.
In two recent electrophysiological stud-
ies, my colleagues and I took advantage of
the excellent temporal resolution of electro-
encephalography (EEG) and event- related
potentials (ERPs) to provide further sup-
port for the differential underlying cognitive
mechanisms and consequences of employ-
ing distraction and reappraisal (Blechert,
Sheppes, Di Tella, Williams, & Gross, 2012;
Thiruchselvam, Blechert, Sheppes, Ryd-
strom, & Gross, 2011). Recent ERP stud-
ies in emotion regulation showed that dis-
traction (e.g., Dunning & Hajcak, 2009;
Hajcak, Dunning, & Foti, 2009) and reap-
praisal (e.g., Foti & Hajcak, 2008; Hajcak
& Nieuwenhuis, 2006) modulate the late
positive potential (LPP)—an electrocortical
component that is enhanced during emo-
tionally arousing viewing and that reflects
enhanced attentional and semantic meaning
processing of emotionally salient informa-
tion (Hajcak, MacNamara, & Olvet, 2010).
Consistent with the framework, we found

130 COGNITIVE APPROACHES
that implementing distraction resulted in
a strong modulation of an initial phase of
the LPP that represents early disengage-
ment of emotional information before it is
represented in working memory, and reap-
praisal only modulated the late phase of the
LPP representing engagement and elabo-
rated meaning prior to late modulation
(Thiruchselvam et al., 2011). In that same
study, we also tested our prediction that,
motivationally, distraction relative to reap-
praisal cannot be in accord with major long-
term goals. Specifically, distraction does not
allow attending to and explaining emotional
information, which result in subsequent
attenuation of emotional responses relative
to novel emotional situations (Wilson &
Gilbert, 2008). To that end, our participants
were reexposed to emotional materials they
had previously distracted or reappraised.
Consistent with our prediction, we found
that emotional materials with a distraction
but not reappraisal history demonstrated
an enhanced LPP during reexposure, which
represents an extended influence of negative
emotional processing beyond the regulatory
episode. Prolonged influence of emotional
events is considered maladaptive, with many
long-term goals that require dealing with
emotional events that are repeatedly encoun-
tered (see also MacNamara, Ochsner, &
Hajcak, 2011). In a similar vein, we recently
showed that repeated reappraisal efforts
with biologically significant emotional stim-
uli (i.e., angry facial expressions) resulted
in a gradual change to the basic evaluation
and thus representation of these emotional
stimuli (Blechert et al., 2012).
Emotional, Cognitive,
and Motivational Determinants
of Emotion Regulation Choice
In a recent set of studies my colleagues and I
have evaluated central determinants of emo-
tion regulation choice (Sheppes, Scheibe,
Suri, & Gross, 2011; Sheppes et al., 2012).
Prior to discussing these studies I wish
briefly to describe the novel paradigm we
developed to enable the evaluation of emo-
tion regulation choice. In short, partici-
pants that come to the laboratory initially
undergo a learning phase, followed by a
training phase in which they are taught the
differences between distraction and reap-
praisal, as well as how to implement each. In
the actual task, participants are exposed to
series of trials that involve a brief presenta-
tion of an emotional stimulus followed by a
choice screen, where they decide their pre-
ferred regulatory strategy. Following a short
preparation period, the emotional stimulus
reappears and participants are instructed
to implement their chosen strategy and to
rate how they feel. In general, participants
are instructed to choose freely between dis-
traction and reappraisal, and to base their
choice on the strategy they think will make
them feel less negative. The main dependent
measure is the proportion of choice of each
regulatory option. To evaluate adherence of
participants’ reports of their chosen regula-
tory strategies (achieved via which button
they pressed during the choice screen), we
either asked participants to talk out loud
about their chosen strategies during strat-
egy implementation or we administered a
surprise memory test for emotional materi-
als that were presented (see Sheppes et al.,
2011). These two methods have proven to
be satisfactory. Specifically, adherence levels
were evident in high agreement between par-
ticipants’ button responses and their talk-
out-loud protocols, and in the finding that
in trials on which participants indicated that
they chose to distract, memory was worse
(and not significantly different from chance)
than that in trials of participants who indi-
cated that they chose to reappraise (Sheppes
et al., 2011).
The first determinant of regulation choice
examined is emotional intensity, which is a
key dimension of variation across emotional
contexts (Sheppes et al., 2011). Based on the
emotion regulation choice conceptual frame-
work, we predicted that under low- negative
emotionally intense situations, individuals
would prefer to choose late selection engage-
ment reappraisal over early selection disen-
gagement distraction, because reappraisal
can both successfully modulate emotional
responding and provide long-term affec-
tive adaptation. However, we predicted that
under high- negative intensity situations,
participants would switch, to prefer choos-
ing early disengagement distraction over
reappraisal, because only distraction can
successfully block emotional information
early before it gathers force.

Emotion Regulation Choice 131
To test our predictions, we manipulated
emotional intensity with emotional images
or unpredictable electric stimulation and
had participants choose between distraction
and reappraisal (Sheppes et al., 2011). The
results strongly supported our predictions in
both emotional contexts. Specifically, par-
ticipants preferred to reappraise their emo-
tional reactions to low- negative- intensity
pictures and to a threat of low- intensity
electric shocks, but they preferred to dis-
tract from their emotional reactions to high-
negative- intensity pictures and to a threat of
high- intensity electric shocks.
In a follow- up study we wanted to exam-
ine the robustness of the effect of emotional
intensity on regulation choice (Sheppes
et al., 2012). To that end, we examined
whether individuals would keep their regu-
latory preferences under different emotional
intensities even when offered a potent rein-
forcement (monetary incentive) to engage
in a counter preference regulatory option.
Although we found that monetary incentives
influenced regulatory choices in the expected
direction, the basic preference to reappraise
low- intensity emotional situations and to
distract high- intensity emotional situations
remained evident, even when participants
were paid high monetary amounts to choose
the contrasting strategy. These results sug-
gest that emotional intensity strongly influ-
ences regulatory preferences.
The second determinant we examined was
the cognitive complexity of generating an
emotion regulation strategy (Sheppes et al.,
2012). Emotion regulation can be viewed as
involving several sequential cognitive pro-
cesses that involve generation, implementa-
tion, and maintenance (Kalisch, 2009; Och-
sner & Gross, 2008). Generation involves
finding an adequate regulatory option that
can replace emotional information pro-
cessing. Implementation involves activat-
ing a regulatory strategy, and maintenance
involves holding it in an active state as long
as regulation is required. Because generation
operates early in the sequence of an emo-
tion regulation episode, it is likely to affect
emotion regulation choice. In distraction,
the generation process is simple, because the
neutral thoughts that are produced can be
of any content as long as they are absorbing.
However, in reappraisal, the generation pro-
cess is complex, because the formation of an
alternative neutral reinterpretation depends
on the original emotional information. To
examine this disparity we showed that when
the generation process was simplified, by
providing participants concrete regulatory
options for distraction and reappraisal,
reappraisal was more frequently chosen.
The third determinant of emotion regula-
tion choice involved investigating the influ-
ence of motivational goals (Sheppes et al.,
2012). An important motivational goal
for choosing a regulatory strategy involves
evaluating whether an emotional stimulus
will be encountered once or multiple times.
While an emotional stimulus that is encoun-
tered once can be regulated with strategies
such as distraction, which provide short term
relief, emotional stimuli that are encoun-
tered multiple times can be better regulated
with strategies such as reappraisal, which
involves engaging with emotional process-
ing that results in gradual adaptation (e.g.,
Wilson & Gilbert, 2008; Blechert et al.,
2012). As we therefore expected and found,
when participants were told that they would
encounter emotional stimuli more than
once, they preferred to reappraise more than
they did when they expected to encounter an
emotional stimulus once.
Underlying Mechanisms of Emotion
Regulation Choice
The previous section established that cen-
tral emotional, cognitive, and motivational
factors strongly influence individuals’ pref-
erences between two regulation strategies
that modulate emotional responding at an
early attentional stage (distraction) or a late
semantic meaning stage (reappraisal). In this
section I turn to the issue of the mechanisms
at the core of emotion regulation choice.
According to our conceptual framework,
emotion regulation choice should involve a
general ability of deliberate executive con-
trol processes to override competing asso-
ciative emotional processes. In line with cen-
tral models of self control (e.g., Muraven &
Baumeister, 2000), an ability to recruit cen-
tral control processes that can moderate the
influence of drives and emotions is crucial
for daily functioning. In addition, the actual
regulatory choice process is heavily influ-
enced by the engagement– disengagement
132 COGNITIVE APPROACHES
dimension where people are weighting their
preference to employ early attentional disen-
gagement from emotional processing (dis-
traction) versus engagement with emotional
processing prior to late modulation at the
semantic meaning processing stage (reap-
praisal).
Provision of supporting evidence for the
involvement of deliberate executive con-
trol processes that override associative
emotional processes in emotion regulation
choice is important given a potential alterna-
tive, more parsimonious account. According
to this more parsimonious account, emotion
regulation choice can be fully explained by
a direct influence from simple associative
emotional processes (e.g., Bradley, Codis-
poti, Cuthbet, & Lang, 2001). Specifically,
according to an associative emotional pro-
cess account, as negative emotional intensity
increases, it directly activates a basic defen-
sive system to shift from an engagement (or
sensory intake) preference, which leads to
preferring reappraisal, to a disengagement
(or sensory rejection) preference, which may
result in an increased preference to distract.
Alternatively, according to the emotion reg-
ulation choice account, individuals can use
deliberate executive processes in preferring
to reappraise in low- negative intensity situa-
tions and to distract in high- negative inten-
sity situations. Because both accounts lead
to the same regulatory choice prediction in
negative emotional situations, in order to
determine between them we investigated
down- regulation of positive emotional situa-
tions— an emotional context where the two
accounts diverge. Specifically, the associative
emotional process account would argue that
as positive emotional intensity increases, it
directly activates a basic appetitive system
that would lead to an increased preference
to engage. By contrast, the emotion regula-
tion choice account would predict that the
operation of deliberate control processes,
whose goal is to provide down- regulation of
positive emotional situations, would involve
overriding the associative tendency to
engage, resulting in an increased preference
to disengage as positive emotional intensity
increases. The results, which showed par-
ticipants’ clear tendency to disengage via
distraction as positive emotional intensity
increased, clearly support the involvement
of deliberate executive control processes
that override opposing associative emotional
processes originating from the appetitive
system (Sheppes et al., 2012).
While these results support the involve-
ment of deliberate executive control pro-
cesses in emotion regulation choice, an
important question remains: What are the
dimensions that receive central weight in the
choice between distraction and reappraisal?
One central dimension in my conceptual
account is engagement– disengagement, in
which people who prefer to reappraise may
wish to engage in emotional processing,
and when they want to distract may want
to disengage from emotional processing.
However, in a second potential key dimen-
sion, the amount of cognitive effort people
wish to exert (e.g., Chajut & Algom, 2003;
Kool, McGuire, Rosen, & Botvinick, 2010;
Muraven & Baumeister, 2000), when people
prefer to reappraise they are willing to exert
considerable effort, and when they want
to distract they wish to preserve cognitive
effort. Accordingly, when people prefer to
distract in high- negative emotionally intense
situations, do they prefer to disengage from
emotional processing or do they prefer to
reserve their cognitive resources and go with
the easy regulatory option?
To determine between these two options,
we had participants choose between two
types of distractions that involve making
mathematical computations (e.g., see Erber
& Tesser, 1992; van Dillen & Koole, 2007
for similar operationalization of atten-
tional distractions): One regulatory option
was cognitively simple and involved minor
disengagement from emotional processing
(subtract 2’s) and a second regulatory option
was cognitively effortful yet highly disen-
gaged from emotional processing (subtract
7’s). The logic was that if the cognitive effort
dimension is central in regulatory choice,
then one should expect that the preference to
use the more simple subtract 2’s distraction
would increase as negative emotional inten-
sity increases. However, if the engagement–
disengagement dimension is central in
regulatory choice, we should expect that
the preference to use the more disengaging
(despite it being also more effortful) subtract
7’s distraction would increase as negative
emotional intensity increases.
Results strongly supported the centrality
of the engagement– disengagement dimen-
sion, where participants preferred to disen-
gage from emotional processing in the high-

Emotion Regulation Choice 133
intensity condition despite the fact that this
regulatory option was clearly more effort-
ful (Sheppes et al., 2012). In addition, when
participants were performing the subtract
7’s option, their actual mathematical perfor-
mance was interfered with less by the inten-
sity of emotional stimuli relative to perform-
ing subtract 2’s. This result indicates that
the more effortful subtract 7’s option indeed
resulted in a stronger disengagement from
emotional processing relative to subtracting
2’s.
In a complementary fashion we wished to
show that the engagement– disengagement
dimension is central within the reappraisal
category. Although, reappraisal is gener-
ally considered to be an engagement strat-
egy (Gross & Thompson, 2007; Sheppes &
Gross, 2011), there is also a considerable
variation in this broad regulation category
(Ochsner et al., 2004; McRae, Ciesielski,
& Gross, 2012). Specifically, in a classic
situation- focused reappraisal, engagement
with emotional processing is central in
order to reinterpret the meaning of ongo-
ing events. However, other forms of reap-
praisal, such as reality challenge, involve a
disengagement element in which emotional
consequences are simply not considered and
the basic authenticity of the event is being
questioned. In a regulation choice study we
showed that whereas participants preferred
to engage via situation- focused reappraisal
under a low- intensity condition, they pre-
ferred to disengage via reality challenge
reappraisal under a high- intensity condition
(Sheppes et al., 2012).
Broader Implications
In the two previous sections, I tried to estab-
lish key emotional, cognitive, and motiva-
tional determinants, and underlying mech-
anisms of emotion regulation choice. In
this section I zoom out in order to provide
broader implications of emotion regulation
choice. In doing so I concentrate on impli-
cations for emotion regulation science, deci-
sion making, and clinical science.
Implications for Emotion Regulation
Emotion regulation choice provides an
important extension to the general field
of emotion regulation. Previous studies of
emotion regulation have almost exclusively
focused on the consequences of implement-
ing different emotion regulation strategies
(Gross, 2007; see Koole, 2009, for reviews).
Multiple studies have instructed participants
to engage with different emotion regulation
strategies and have examined the costs and
benefits associated with successful imple-
mentation. Understanding the consequences
of employing different regulation strategies
is a crucial and important step toward an
understanding of the basic elements of emo-
tion regulation strategies. Nevertheless, the
emotion regulation choice findings extend
this work by illuminating a step that pre-
cedes the implementation of emotion regu-
lation. Specifically, when an emotion regu-
lation goal has been activated, individuals
need to select a regulation strategy out of
all the available options. Our conceptual
account has highlighted some emotional,
cognitive, and motivational factors that can
strongly influence the regulatory choices
individuals are inclined to make in any par-
ticular context.
Combining the recent knowledge on emo-
tion regulation choice with existing knowl-
edge on the consequences of implement-
ing emotion regulation strategies leads to
important conceptual extensions. For exam-
ple, individuals’ regulatory preferences as
indexed by their regulatory choices should
be considered when evaluating individuals’
ability to implement different strategies.
Specifically, initiating a particular strategy
may require not only generation, implemen-
tation, and maintenance but also overrid-
ing a default regulatory preference. To give
laboratory and real-life analogues, it may be
that when participants in the laboratory or
patients in the clinic are asked to engage via
reappraisal when dealing with high- intensity
situations, in addition to generating, imple-
menting, and maintaining reinterpretation
of an emotional situation, they also need to
override their strong default preference to
disengage via distraction.
Implications for Decision Making
Emotion regulation choice is an instance
of general choice behavior, and as such
it is important to place it under the broad
umbrella of general decision making. Classic
examples of choice behavior involve decid-
ing between different external outcomes in
134 COGNITIVE APPROACHES
one’s environment. For example, in intertem-
poral choice paradigms, such as temporal
discounting (Reynolds, 2006), individuals
choose between receiving smaller monetary
rewards sooner (e.g., $5 today) and receiv-
ing bigger monetary rewards later (e.g.,
$8 tomorrow). Other examples of choice
behavior involve deciding between differ-
ent internal processes to deal with external
demands, such as the mathematical strate-
gies children choose in order to solve math
problems (Siegler, 2005) or the strategies
adults choose to solve chess problems (de
Groot, 1978). Emotion regulation choice is
a special case of decision making, because it
involves choosing between internal cognitive
processes to control one’s internal emotional
environment.
Although emotion regulation choice
appears to be unique in some ways, it shares
with other theories basic assumptions about
strategy choice. Like classic theories in deci-
sion sciences (e.g., Payne, Bettman, & John-
son, 1988, 1993), the emotion regulation
choice account argues that individuals are
sensitive to central factors (emotional, cog-
nitive, and motivational factors in the case
of emotion regulation choice) when mak-
ing their regulatory selections. In addition,
just as other models highlight the role of
learning (e.g., Rieskmap, 2006; Rieskmap
& Otto, 2006), in the emotion regulation
choice account regulatory decisions are to
some extent based on prior knowledge, with
the consequences of implementing different
strategies in different contexts.
Implications for Clinical Psychology
Emotion regulation choice also has impor-
tant clinical implications. Central concep-
tual accounts argue that psychological well-
being requires flexibly adapting emotion
regulation strategies to fit with differing situ-
ational demands (Gross, 2007; Kashdan &
Rottenberg, 2010; Watkins, 2011). The flip
side of flexible regulatory choice is a rigid
and maladaptive regulation choice that may
be related to various forms of psychopathol-
ogy.
The recent empirical evidence maps emo-
tional, cognitive, and motivational determi-
nants of regulation choice in healthy adults;
thus, deviations from healthy regulatory
choice can be used to understand different
forms of psychopathology.
Consider first the emotional intensity one
is facing. As previously described, healthy
individuals prefer to use reappraisal in low-
intensity emotional situations and distrac-
tion in high- intensity situations. Regulatory
preferences that deviate from the flexible
regulation choice observed in healthy indi-
viduals might be related to different psy-
chopathologies. Specifically, deviation
from a preference to choose to disengage
from very high- intensity stimuli is expected
from individuals who are prone to develop
major depression. According to the response
style theory, rumination involves engag-
ing with strong emotional experiences, and
repeatedly thinking about their causes and
consequences in an abstract and repeti-
tive way (e.g., Nolen- Hoeksema, 1991; see
Nolen- Hoeksema et al., 2008, for reviews).
Although rumination has been proven to be
related to onset maintenance and relapse of
depression, depressed individuals who pre-
fer to use positive distractions when dealing
with strong emotional experiences show a
better prognosis (e.g., Nolen- Hoeksema &
Morrow, 1991, 1993). Emotion regulation
choice is likely to be an important target in
the context of depression, because empirical
studies have shown that although depressed
individuals are able to implement distrac-
tion effectively when instructed to do so
(e.g., Joormann & Siemer, 2004; Joormann,
Siemer, & Gotlib, 2007), they hold a favor-
able view of rumination, believing that it
helps them understand better the reasons
for depressed mood. Nevertheless, an over-
generalized preference to engage with emo-
tional contents may also depend on impaired
ability to shift attention away from negative
contents (e.g., Joormann & Gotlib, 2008).
Another type of deviation from the healthy
regulatory choice patterns involves diverg-
ing from engagement with low- intensity
emotional stimuli. Common to several anxi-
ety disorders is a tendency to overgeneral-
ize a disengagement or avoidance regulatory
response (for reviews, see Campbell- Sills &
Barlow, 2007; Foa & Kozak, 1986). Avoid-
ance usually starts in response to high-
intensity emotional stimuli, but over time,
it ends up spilling over to seemingly low-
intensity stimuli. As pointed in our concep-
tual model, while disengagement strategies
are helpful in providing short-term relief,
they are maladaptive in the long run and can
perpetuate anxiety and fears.

Emotion Regulation Choice 135
The cognitive factor highlighted is the
ease with which a regulation strategy is gen-
erated. Specifically, aiding the generation
process resulted in increased reappraisal
choice. These results are relevant for many
cognitive- behavioral therapies in which
patients are encouraged to reappraise strong
emotional events (e.g., Campbell- Sills &
Barlow, 2007). While the final objective is
to have patients generate their own regula-
tion strategies, therapists should be mindful
that their patients might need aid in gener-
ating alternative ways to think about upset-
ting events, until they can gradually build
their skill. Complementarily, one can expect
that with continued practice in reappraisal,
patients would improve its generation and
choose to use it more frequently when it’s
adaptive.
The motivational factor highlighted indi-
viduals’ goals when choosing to regulate
their emotions. It was shown that when
participants expect to encounter emotional
events repeatedly, they increase reappraisal
choice, which offers long-term benefits. The
ability to override regulatory preferences
that offer short-term relief (i.e., distraction)
in favor of regulatory preferences that offer
long-term benefits (i.e., reappraisal) is likely
to require self- control, and impairments
in self- control ability have been linked to
various psychopathologies, including addic-
tions and eating disorders such as bulimia
(for comprehensive reviews, see Heatherton
& Baumeister, 1991; Vohs & Baumeister,
2011).
Future Directions
Despite promising preliminary results and
clear, broad implications, research in emo-
tion regulation choice has only begun to
develop. In closing I wish to point out sev-
eral potential future research directions.
First, to date, researchers have examined
the influence of only one emotional (emo-
tional intensity), one cognitive (generation
of a strategy), and one motivational (short-
vs. long-term goals) determinant of emotion
regulation choice. While these factors are
important, future studies should evaluate
the influence of the many additional factors
that likely influence regulatory preference.
To give just one example, the availability
of cognitive resources is likely to influence
individuals’ regulatory choices. Specifically,
a temporary state of self- control resource
depletion (e.g., Baumeister, Vohs, & Tice,
2007; Muraven & Baumeister, 2000) is
likely to lead individuals to prefer strategies
such distraction, which provide short-term
relief. Therefore, studying how various novel
factors affect regulatory choice is an impor-
tant future research direction.
Second, while distraction and reappraisal
are considered classic disengagement and
engagement strategies (Parkinson & Tot-
terdell, 1999), and they are widely used in
everyday life, studies of choices between
other emotion regulation strategies are
urgently needed. In everyday life, individu-
als choose from many more regulatory
options that should be studied in the future.
Several promising avenues involve allowing
people to choose from regulatory options
that are considered less adaptive in certain
contexts (e.g., suppression and rumination).
Another option involves investigating how
individuals switch their regulatory choices
when dealing with dynamic and prolonged
emotional events that constitute many of
our daily experiences (see Aldao & Nolen-
Hoeksema, 2012c, for a related discussion).
Relatedly, a crucial test of the applicability of
the emotion regulation choice paradigm and
its supporting results should involve study-
ing regulatory choices in daily emotional
experiences, such as when patients wait to
receive potentially stressful news in medi-
cal settings, or when clients face a stimulus
about which they feel anxious. Although
distraction and reappraisal are widely used
in real-life situations, it remains unclear
whether we would observe somewhat simi-
lar preferences when individuals spontane-
ously choose between regulatory options in
daily emotional situations.
Third, the goals of this study were gener-
ally to characterize the influence of differ-
ent factors on emotion regulatory choice.
Nevertheless, it is quite clear that studying
individual differences in emotion regula-
tion choice is crucial. Recent relevant studies
have shown that individual differences in the
ability to modify emotions are tightly linked
to long-term adaptation (e.g., Bonanno et
al., 2004; Westphal et al., 2010). Therefore,
future studies should evaluate how mul-
tiple individual differences can moderate
the influence of central factors on emotion
regulation choice. One promising venue,

136 COGNITIVE APPROACHES
which I mentioned in discussing the clinical
implications, involves studying impairments
in regulatory preferences of individuals with
mood, anxiety, and personality disorders,
whose psychopathology revolves around
emotion dysregulation.
Fourth, according to the emotion regula-
tion choice account, healthy individuals are
sensitive to central costs and benefits of dif-
ferent regulation strategies when trying to
choose between regulatory options, and in
general these individuals showed adaptive
regulatory choice profiles. Nevertheless,
multiple demonstrations in general decision-
making studies have shown important limi-
tations in human reasoning (e.g., Tversky &
Kahneman, 1974); therefore, future studies
should investigate situations in which indi-
viduals’ regulatory choices would not nec-
essarily lead them to the best outcome. In
a related vein, while conscious regulation
strategies have been a major focus in the
field of emotion regulation, and an integral
part of many cognitive- behavioral thera-
pies targeting emotion dysregulation (e.g.,
Linehan, 1993), many emotion regulation
choices are likely to be determined implicitly
and without deliberate control.
Finally, until now, emotion regulation
choice has been investigated separately from
studies on the consequences of implement-
ing regulation strategies. Future studies
should make stronger connections between
the developing studies on emotion regula-
tion choice and the well- established studies
on the consequences of regulation imple-
mentation. For example, studies should
evaluate whether the effectiveness of imple-
menting a given strategy is moderated by an
ability to override default regulatory choice
preferences. At the same time, future studies
in emotion regulation choice should utilize
multiple levels of analysis that combine the
concurrent assessment of the effectiveness of
a chosen regulatory strategy.
Acknowledgments
This chapter draws on and updates previous
reviews by Sheppes and Gross (2011, 2012) and
two recent empirical manuscripts (Sheppes et al.,
2011, 2012). The writing of this chapter was sup-
ported by the Israel Science Foundation (Grant
No. 1393/12).
References
Aldao, A., & Nolen- Hoeksema, S. (2012a).
When are adaptive strategies most predictive
of psychopathology? Journal of Abnormal
Psychology, 121, 276–281.
Aldao, A., & Nolen- Hoeksema, S. (2012b). The
influence of context on the implementation of
adaptive emotion regulation strategies. Behav-
iour Research and Therapy, 50, 493–501.
Aldao, A., & Nolen- Hoeksema, S. (2012c). One
versus many: Capturing the use of multiple
emotion regulation strategies in response to
an emotion- eliciting stimulus. Cognition and
Emotion. [E-publication ahead of print]
Aldao, A., Nolen- Hoeksema, S., & Schweizer,
S. (2010). Emotion regulation strategies
across psychopathology: A meta- analytic
review. Clinical Psychology Review, 30, 217–
237.
Altamirano, L. J., Miyake, A., & Whitmer, A.
J. (2010). When mental flexibility facilitates
cognitive control: Beneficial side effects of
ruminative tendencies on goal maintenance.
Psychological Science, 21, 1377–1382.
Baumeister, R. F., Vohs, K. D., & Tice, D.
M. (2007). The strength model of self-
control. Current Directions in Psychological
Science, 16, 396–403.
Blechert, J., Sheppes, G., Di Tella, C., Williams,
H., & Gross, J. J. (2012). See what you think:
Reappraisal modulates behavioral and neural
responses to social stimuli. Psychological Sci-
ence, 4, 356–363.
Bonanno, G. A. (2005). Resilience in the face of
potential trauma. Current Directions in Psy-
chological Science, 14, 135–138.
Bonanno, G. A., & Keltner, D. (1997). Facial
expressions of emotion and the course of con-
jugal bereavement. Journal of Abnormal Psy-
chology, 106, 126 –137.
Bonanno, G. A., Papa, A., Lalande, K., West-
phal, M., & Coifman, K. (2004). The impor-
tance of being flexible: The ability to both
enhance and suppress emotional expression
predicts long term adjustment. Psychological
Science, 15, 482–487.
Bradley, M. M., Codispoti, M., Cuthbert, B. N.,
& Lang, P. J. (2001). Emotion and motivation
I: Defensive and appetitive reactions in picture
processing. Emotion, 1, 276–298.
Bradley, M. M., Codispoti, M., Sabatinelli, D.,
& Lang, P. J. (2001). Emotion and motivation
II: Sex differences in picture processing. Emo-
tion, 1, 300–319.
Emotion Regulation Choice 137
Campbell- Sills, L., & Barlow, D. H. (2007).
Incorporating emotion regulation into con-
ceptualizations and treatments of anxiety and
mood disorders. In J. J. Gross (Ed.), Hand-
book of emotion regulation (pp.
542–559).
New York: Guilford Press.
Chajut, E., & Algom, D. (2003). Selective atten-
tion improves under stress: Implications for
theories of social cognition. Journal of Per-
sonality and Social Psychology, 85, 231–248.
de Groot, A. D. (1978). Thought and choice in
chess (2nd ed.). The Hague: Mouton.
Dillon, D. G., Ritchey, M., Johnson, B. D., &
LaBar, K. S. (2007). Dissociable effects of con-
scious emotion regulation strategies on explicit
and implicit memory. Emotion, 7, 354–365.
Dunning, J. P., & Hajcak, G. (2009). See no
evil: Directed visual attention modulates the
electrocortical response to unpleasant images.
Psychophysiology, 46, 28–33.
Ehring, T., Tuschen-
C
affier, B., Schnülle, J.,
Fischer, S., & Gross, J. J. (2010). Emotion
regulation and vulnerability to depression:
Spontaneous versus instructed use of emotion
suppression and reappraisal. Emotion, 10,
563–572.
Erber, R., & Tesser, A. (1992). Task effort
and the regulation of mood: The absorption
hypothesis. Journal of Experimental Social
Psychology, 28, 339–359.
Foa, E. B., & Kozak, M. J. (1986). Emotional pro-
cessing of fear: Exposure to corrective infor-
mation. Psychological Bulletin, 99, 20–35.
Foti, D., & Hajcak, G. (2008). Deconstructing
reappraisal: Descriptions preceding arous-
ing pictures modulate the subsequent neural
response. Journal of Cognitive Neuroscience,
20, 977–988.
Garnefski, N., Kraaij, V., & Spinhoven, P.
(2001). Negative life events, cognitive emotion
regulation and emotional problems. Personal-
ity and Individual differences, 30, 1311–1327.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J. (2001). Emotion regulation in adult-
hood: Timing is everything. Current Direc-
tions in Psychological Science, 10, 214–219.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39, 281–291.
Gross, J. J. (Ed.). (2007). Handbook of emotion
regulation. New York: Guilford Press.
Gross, J. J. (2010). The future’s so bright, I gotta
wear shades. Emotion Review, 2, 212–216.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., Sheppes, G., & Urry, H. L. (2011a).
Emotion generation and emotion regulation: A
distinction we should make (carefully). Cogni-
tion and Emotion, 25, 765–781.
Gross, J. J., Sheppes, G., & Urry, H. L. (2011b).
Taking one’s lumps while doing the splits: A
big tent perspective on emotion generation and
emotion regulation. Cognition and Emotion,
25, 789–793.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp.
3–24). New York: Guilford Press.
Gruber, J., Harvey, A. G., & Gross, J. J. (2012).
When trying is not enough: Emotion regula-
tion and the effort-
s
uccess gap in bipolar dis-
order. Emotion, 12(5), 997–1003.
Gupta, S., & Bonanno, G. A. (2011). Compli-
cated grief and deficits in emotional expressive
flexibility. Journal of Abnormal Psychology,
120, 635–643.
Hajcak, G., Dunning, J. P., & Foti, D. (2009).
Motivated and controlled attention to emo-
tion: Time-
course of the late positive poten-
tial. Clinical Neurophysiology, 120, 505–510.
Hajcak, G., MacNamara, A., & Olvet, D. M.
(2010). Event-
r
elated potentials, emotion, and
emotion regulation: An integrative review.
Developmental Neuropsychology, 35, 129–
155.
Hajcak, G., & Nieuwenhuis, S. (2006). Reap-
praisal modulates the electrocortical response
to negative pictures. Cognitive, Affective, and
Behavioral Neuroscience, 6, 291–297.
Heatherton, T. F., & Baumeister, R. F. (1991).
Binge eating as escape from self-
a
wareness.
Psychological Bulletin, 110, 86–108.
Hübner, R., Steinhauser, M., & Lehle, C. (2010).
A dual-stage two-phase model of selective
attention. Psychological Review, 117, 759–
784.
John, O. P., & Gross, J. J. (2007). Individual
differences in emotion regulation. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp.
351–372). New York: Guilford Press.
Johnston, W. A., & Heinz, S. P. (1978). Flexibil-
ity and capacity demands of attention. Journal
of Experimental Psychology: General, 107,
420–435.
Joormann, J., & Gotlib, I. H. (2008). Updating
138 COGNITIVE APPROACHES
the contents of working memory in depres-
sion: Interference from irrelevant negative
material. Journal of Abnormal Psychology,
117, 182–192.
Joormann, J., & Siemer, M. (2004). Memory
accessibility, mood regulation and dysphoria:
Difficulties in repairing sad mood with happy
memories? Journal of Abnormal Psychology,
113, 179–188.
Joormann, J., Siemer, M., & Gotlib, I. H. (2007).
Mood regulation in depression: Differential
effects of distraction and recall of happy mem-
ories on sad mood. Journal of Abnormal Psy-
chology, 116, 484–490.
Kalisch, R. (2009).The functional neuroanatomy
of reappraisal: Time matters. Neuroscience
Biobehavioral Reviews, 33, 1215–1226.
Kashdan, T. B., & Rottenberg, J. (2010). Psy-
chological flexibility as a fundamental aspect
of health. Clinical Psychology Review, 30,
865–878.
Kool, W., McGuire, J. T., Rosen, Z., & Botv-
inick, M. M. (2010). Decision making and
the avoidance of cognitive demand. Journal
of Experimental Psychology: General, 139,
665–682.
Koole, S. L. (2009). The psychology of emotion
regulation: An integrative review. Cognition
and Emotion, 23, 4– 41.
Kross, E., & Ayduk, O. (2008). Facilitating
adaptive emotional analysis: Distinguishing
distanced- analysis of depressive experiences
from immersed- analysis and distraction. Per-
sonality and Social Psychology Bulletin, 34,
924–938.
Lehle, C., & Hübner, R. (2008). On-the-fly
adaptation of selectivity in the flanker task.
Psychonomic Bulletin and Review, 15, 814–
818.
Linehan, M. M. (1993). Skills training manual
for treating borderline personality disorder.
New York: Guilford Press.
MacNamara, A., Ochsner, K. N., & Hajcak, G.
(2011). Previously reappraised: The lasting
effect of description type on picture- elicited
electrocortical activity. Social, Cognitive, and
Affective Neuroscience, 6, 348–358.
Mancebo, M. C., Eisen, J. L., Grant, J. E., & Ras-
mussen, S. A. (2005). Obsessive– compulsive
personality disorder and obsessive– compulsive
disorder: Clinical characteristics, diagnostic
difficulties, and treatment. Annals of Clinical
Psychiatry, 17, 197–204.
McRae, K., Ciesielski, B. R. G., & Gross, J. J.
(2012). Unpacking cognitive reappraisal:
Goals, tactics and outcomes. Emotion, 12,
250–255.
Muraven, M., & Baumeister, R. F. (2000). Self-
regulation and depletion of limited resources:
Does self- control resemble a muscle? Psycho-
logical Bulletin, 126, 247–259.
Nolen- Hoeksema, S. (1991). Responses to
depression and their effects on the duration of
depressive episodes. Journal of Abnormal Psy-
chology, 100, 569–582.
Nolen- Hoeksema, S., & Morrow, J. (1991). A
prospective study of depression and post-
traumatic stress symptoms following a natural
disaster: The 1989 Loma Prieta Earthquake.
Journal of Personality and Social Psychology,
61, 115–121.
Nolen- Hoeksema, S., & Morrow, J. (1993).
Effects of rumination and distraction on
naturally- occurring depressed mood. Cogni-
tion and Emotion, 7, 561–570.
Nolen- Hoeksema, S., Wisco, B. E., & Lyubomir-
sky, S. (2008). Rethinking rumination. Per-
spectives on Psychological Science, 3, 400–
424.
Ochsner, K. N., & Gross, J. J. (2008). Cogni-
tive emotion regulation: Insights from social
cognitive and affective neuroscience. Current
Directions in Psychological Science, 17, 153–
158.
Ochsner, K. N., Ray, R. D., Robertson, E. R.,
Cooper, J. C., Chopra, S., Gabrieli, J. D. E.,
et al. (2004). For better or for worse: Neural
systems supporting the cognitive down- and
up- regulation of negative emotion. NeuroIm-
age, 23(2), 483– 499.
Parkinson, B., & Totterdell, P. (1999). Classify-
ing affect regulation strategies. Cognition and
Emotion, 13, 277–303.
Pashler, H. (1998). The psychology of attention.
Cambridge, MA: MIT Press.
Payne, J. W., Bettman, J. R., & Johnson, E. J.
(1988). Adaptive strategy selection in decision
making. Journal of Experimental Psychol-
ogy: Learning, Memory, and Cognition, 14,
534–552.
Payne, J. W., Bettman, J. R., & Johnson, E. J.
(1993). The adaptive decision maker. New
York: Cambridge University Press.
Reynolds, B. (2006). A review of delay-
discounting research with humans: Relations
to drug use and gambling. Behavioural Phar-
macology, 17, 651– 667.
Richards, J. M., & Gross, J. J. (1999). Compo-
Emotion Regulation Choice 139
sure at any cost?: The cognitive consequences
of emotion suppression. Personality and Social
Psychology Bulletin, 25, 1033–1044
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79, 410–424.
Rieskamp, J. (2006). Perspectives on probabi-
listic inferences: Reinforcement learning and
an adaptive network compared. Journal of
Experimental Psychology: Learning, Mem-
ory, and Cognition, 32, 1355–1370.
Rieskamp, J., & Otto, P. E. (2006). SSL: A the-
ory of how people learn to select strategies.
Journal of Experimental Psychology: Gen-
eral, 135, 207–236.
Sheppes, G., Catran, E., & Meiran, N. (2009).
Reappraisal (but not distraction) is going to
make you seat: Physiological evidence for self
control effort. International Journal of Psy-
chophysiology, 71, 91–96.
Sheppes, G., & Gross, J. J. (2011). Is timing
everything?: Temporal considerations in emo-
tion regulation. Personality and Social Psy-
chology Review, 15, 319–331.
Sheppes, G., & Gross, J. J. (2012). Emotion regu-
lation effectiveness: What works when. In H.
A. Tennen & J. M. Suls (Eds.), Handbook of
psychology (2nd ed., pp. 391–406). Indianap-
olis, IN: Wiley- Blackwell.
Sheppes, G., & Meiran, N. (2007). Better late
than never?: On the dynamics of on-line regu-
lation of sadness using distraction and cogni-
tive reappraisal. Personality and Social Psy-
chology Bulletin, 33, 1518–1532.
Sheppes, G., & Meiran, N. (2008). Divergent
cognitive costs for online forms of reappraisal
and distraction. Emotion, 8, 870–874.
Sheppes, G., Scheibe, S., Suri, G., & Gross, J. J.
(2011). Emotion- regulation choice. Psycholog-
ical Science, 22, 1391–1396.
Sheppes, G., Scheibe, S., Suri, G., Radu, P.,
Blechert, J., & Gross, J. J. (2012). Emotion
regulation choice: A conceptual framework
and supporting evidence. Journal of Experi-
mental Psychology: General. [E-publication
ahead of print]
Siegler, R. S. (2005). Children’s learning. Ameri-
can Psychologist, 60, 769–778.
Tamir, M. (2011). The maturing field of emotion
regulation. Emotion Review, 3, 3 –7.
Thiruchselvam, R., Blechert, J., Sheppes, G.,
Rydstrom, A., & Gross, J. J. (2011). The tem-
poral dynamics of emotion regulation: An
EEG study of distraction and reappraisal. Bio-
logical Psychology, 87, 84–92.
Troy, A. S., & Mauss, I. B. (2011). Resilience in
the face of stress: Emotion regulation ability
as a protective factor. In S. Southwick, B. Litz,
D. Charney, & M. Friedman (Eds.), Resiliency
and mental health (pp. 30–44). Cambridge,
UK: Cambridge University Press.
Tversky, A., & Kahneman, D. (1974). Judgment
under uncertainty: Heuristics and biases. Sci-
ence, 185, 1124–1131.
van Dillen, L. F., & Koole, S. L. (2007). Clear-
ing the mind: A working memory model of
distraction from negative mood. Emotion, 7,
715–723.
Vohs, K. D., & Baumeister, R. (Eds.). (2011).
Handbook of self- regulation: Research, the-
ory, and applications (2nd ed.). New York:
Guilford Press.
Vohs, K. D., Baumeister, R. F., Schmeichel, B. J.,
Twenge, J. M., Nelson, N. M., & Tice, D. M.
(2008). Making choices impairs subsequent
self- control: A limited resource account of
decision making, self- regulation, and active
initiative. Journal of Personality and Social
Psychology, 94, 883–898.
Watkins, E. R. (2008). Constructive and uncon-
structive repetitive thought. Psychological
Bulletin, 134, 163–206.
Watkins, E. (2011). Dysregulation in level of goal
and action identification across psychological
disorders. Clinical Psychology Review, 31,
260–278.
Westphal, M., Seivert, N. H., & Bonanno, G.
A. (2010). Expressive flexibility. Emotion, 10,
92–100.
Wilson, T. D., & Gilbert, D. T. (2008). Explain-
ing away: A model of affective adaptation.
Perspectives on Psychological Science, 3,
370–386.

140
Emotion and Decision Making
Definition of the Field
Recent years have seen a notable increase
in interest in examining the processes that
underlie human decision making, with one
of the more energetic new directions being
that of how emotions can impact our deci-
sions and choices (Sanfey, 2007). Though a
relatively recent development, this research
area has already uncovered some compelling
findings with regard to the emotional and
social factors involved in how we make deci-
sions. Indeed, it is now commonly argued
that the principal way in which actual
decision- making behavior deviates from the
choices prescribed by economic models of
decision making may be in large part due
to emotional factors that weigh heavily on
our actual decision but are seldom taken
into account by the standard models. Hence,
greater knowledge of the exact role that
emotions play in decision making is invalu-
able in building complete, accurate, models
of choice.
The Role of Emotion
in Decision Making
Historically, the study of how we make
decisions has been tremendously influenced
by economic theories grounded in math-
ematical models of behavior. These models
typically strive to outline what the correct
decision might be when selecting from a
specified choice set, with these models gen-
erally assuming that decisions are based on a
“rational,” cognitive evaluation of their con-
sequences (Sanfey, 2007). While the explicit
and analytically tractable solutions have
certainly been of use in examining how we
make decisions, and in particular perhaps
how we should make decisions, factors such
as emotions, mood, and social cues have
typically been excluded from these models.
However, in recent decades there has been a
welcome increased focus on the roles these
factors play in decision making, and experi-
mental evidence has begun to clarify greatly
how emotions impact our decision behavior.
Emotions can affect individual’s goals,
attitudes, and social decision making (For-
gas, 2003; Zajonc, 2000). For example,
unpleasant emotions are associated with
lower confidence and a more risk- averse
and vigilant processing style (Clark & Isen,
1982; Isen & Daubman, 1984).
On the other hand, pleasant emotions
have been associated with higher confi-
dence, more optimistic framing, and greater
cooperation (Forgas, Bower, & Moylan,
1990). More related to economic decisions
is the work done by researchers in the field
of neuroeconomics. One of the first well-
CHAPTER 9
Emotion Regulation and Decision Making
Alessandro Grecucci
Alan G. Sanfey
Emotion Regulation and Decision Making 141
characterized demonstrations of the rela-
tionship between emotions and economic
choices famously showed that damage to
particular neural areas can radically bias
decision making, with the lesioned areas
of interest thought to contribute important
emotional information to choice (Damasio,
1994; Sanfey, Rilling, Aronson, Nystrom,
& Cohen, 2003). Continuing in this vein,
many other basic decision- making studies
have now shown that physiological indi-
ces, such as skin conductance, correlate
with other decision- relevant information,
such as imminent losses in a gambling task
(Bechara, Damasio, Tranel, & Damasio,
1997) and anger during negative interper-
sonal interactions (Van’t Wout, Kahn, San-
fey, & Aleman, 2006).
Other studies have looked at how emo-
tional responses to the stimuli themselves
can directly impact decision making. For
example, in a functional magnetic resonance
imaging (fMRI) experiment, Sanfey et al.
(2003) showed that activation of the anterior
insula, known to be associated with disgust
and anger (Phillips et al., 1997), correlated
with the rejection of inequitable financial
offers made by an opponent in an economic
task known as the Ultimatum Game. The
interpretation given was that insula activa-
tion reflected negative emotional reactiv-
ity in response to the unfair offers (Sanfey
et al., 2003). This hypothesis was tested
in a follow- up study (Van’t Wout et al.,
2006), which showed that skin conductance
responses, a measure for emotional arousal,
were higher for unfair offers, and this mea-
sure discriminated between acceptances and
rejections of these offers.
Researchers have also carefully manipu-
lated emotions either before or during choices
to demonstrate the causality of the relation-
ship (Lerner, Small, & Loewenstein, 2004;
Winkielman, Berridge, & Willbarger, 2005;
Harlé & Sanfey, 2007; Andrade & Ariely,
2009; Sokol- Hessner, Camerer, & Phelps,
2013). Recent research in this vein suggests
that task- irrelevant mood states may bias
decision making (Harlé, Chang, Van’t Wout,
& Sanfey, 2012). For example, Harlé and
Sanfey (2007) showed that negative emotion
is associated more with lower acceptance
rates of unfair monetary. What rational mod-
els consider suboptimal (i.e., turning down
monetary gain) therefore appears likely to
reflect the activation of emotional variables
that can be additionally increased by subtle
incidental mood states (Harlé & Sanfey,
2007), and, given that the role of emotions in
human social and individual decision mak-
ing has been widely demonstrated (Slovic,
Finucane, Peters, & MacGregor, 2004),
suggests that the existing models of behav-
ior need to incorporate affective factors to
arrive at a more comprehensive accounting
of human decision- making behavior.
As a result of this understanding,
researchers have begun to incorporate emo-
tional influences into decision process mod-
els by assuming the reciprocal modulation
of affective and more deliberative modes of
thought (Harlé & Sanfey, 2007). One useful
framework to interpret the role of emotions
in decision making suggests that our choices
may be best understood as the operation of
multiple underlying systems that interact to
form our decisions (Sanfey & Chang, 2008).
There is a long tradition in psychology for
a dual- systems approach in the understand-
ing of cognitive processes, and one aspect of
dual systems that is relevant for this chap-
ter is the distinction between emotion- based
and cognitive- based judgments. Emotions
are rapid and automatic responses to spe-
cific stimuli (Sanfey & Chang, 2008); how-
ever, in some circumstances we are required
to adopt more flexible and context- specific
solutions. At a neural level, emotional pro-
cesses activate brainstem reward processing
regions, such as the basal ganglia; ventral
parts of the brain, such as the ventromedial
frontal, orbitofrontal, and anterior cingulate
cortex; and amygdala and insular cortex
(Dalgleish, 2004; Sanfey & Chang, 2008).
Deliberative processing engages anterior
and dorsolateral and posterior regions of
the brain (Duncan, Emslie, Williams, John-
son, & Freer, 1996; Smith & Jonides, 1999;
Miller & Cohen, 2001; Stuss & Knight,
2002), whereas automatic processes acti-
vate more parietal and subcortical systems.
It seems evident that emotions play a role
in decision making in general; however,
it is still largely unknown how these pro-
cesses interact with deliberative systems and
whether they can be controlled in a “top-
down” fashion (Grecucci, Giorgetta, Bonini,
& Sanfey, 2013a, 2013b). One proposal on
how the two systems may interact comes
from Evans (2009) who conceptualized the

142 COGNITIVE APPROACHES
conflict between these two styles of thinking
as a “cognitive control problem,” referring
to the fundamental question of the mecha-
nism by which control over the answer is
ultimately allocated, and suggested how this
conflict could be resolved. Evans pointed out
that when confronted with a decision, auto-
matic processing produces default intuitive
responses, unless slower and more reflective
deliberative processing intervenes. Impor-
tantly, such an intervention may or may not
lead to a different decision that in turn may
or may not be the correct one. However,
the linkage between emotions and decision
making may be more complex and multi-
faceted, as suggested by the “fourfold clas-
sification of emotions and decision- making”
model of Pfister and Böhm (2008), accord-
ing to which emotions provide information
about pleasure and pain during preference
construction, enable rapid choices under
time pressure, lead attention on relevant
aspects of a decision problem, and may gen-
erate commitment concerning morally and
socially significant decisions.
Emotion Regulation
and Decision Making
Overview
One useful approach to investigate how
emotion and cognition interact is to exam-
ine whether regulatory strategies applied to
decision- making tasks have the ability to
alter subjects’ decisions by modulating the
ongoing emotions (Van’t Wout, Chang, &
Sanfey, 2010). Emotion regulation refers to a
set of different strategies by which individu-
als influence which emotions they have, when
they have them, and how they experience
and express them. Emotions can intervene
in different ways in our decisions, starting
from the assessment of potential alternative
options, the evaluation of a choice, the vari-
ous possible outcomes, and so on. Although
there is surprisingly little empirical work
on the link between top-down control of
emotions and the act of decision making,
the experimental use of emotion regulation
strategies has great potential to elucidate
this relationship (Van’t Wout et al., 2010),
that is, to clarify how emotions and decision
making interact. Broadly speaking, emo-
tion regulation strategies applied to deci-
sion making have two notable advantages as
compared to basic emotion regulation stud-
ies: (1) It provides the opportunity to see
how these regulatory strategies can actually
affect behavioral responses themselves and
not only emotional perception per se, and (2)
it allows for the study of complex emotions
that are often difficult to elicit via standard
sets of simple perceptual stimuli.
Though there has been much important
recent progress on understanding emotion
regulation, most studies to date have mainly
measured the ability to regulate negative
emotions induced by the viewing of images
of valenced and arousing scenes (see Och-
sner & Gross, 2008, for a review). How-
ever, in daily life we are confronted with an
often bewildering range of decisions, each
containing an evaluation of gains, losses,
and associated risks, and usually laden with
emotional value. Therefore, investigating
whether emotion regulation strategies can
have an effect on actual decisions provides
an exciting opportunity to extend emotion
regulation research beyond the modulation
of emotional responses per se.
The effectiveness of emotion regulation
strategies is dependent on both the specific
type of stimulus and the emotion to be regu-
lated (Grecucci, 2012; Ochsner & Gross,
2008), and the emotions elicited by decision-
making situations are substantially differ-
ent than those experienced while passively
viewing emotional images (Van’t Wout et al.,
2010). In addition to the benefits of better
understanding the processes of emotional
regulation by examining these mechanisms
in concert with decision making, the field of
decision making itself also stands to benefit
from this research program. Decision mak-
ing and emotion regulation are linked by the
role that emotion plays in both (Mitchell,
2011), and emotions appear to play a fun-
damental role in choices associated with
reward or punishment. Moreover, psychiat-
ric disorders are often associated with emo-
tion regulation deficits and abnormal deci-
sion making (Mitchell, 2011). For example,
neuropsychological studies have shown that
damage in brain areas implicated in emo-
tional regulation (i.e., ventromedial cortex)
often result in suboptimal decision making
(Koenigs & Tranel, 2007; Moretti, Drag-
one, & di Pelegrino, 2009). A recent line of
research has studied how emotion regulation
Emotion Regulation and Decision Making 143
strategies can affect decision making directly
and bias our decision- making behavior as
presented in the following sections.
Individual Decision Making
One useful organizational distinction when
talking about decision making is to separate
the field into two main branches: individual
decision making and social decision mak-
ing. This categorization is not trivial, and
it has particular implications for emotion
regulation, because the affective experience
associated with these two kinds of decision
can be quite different. Individual decision
making refers to situations in which the
decision makers assess the available options,
then choose the best course of action for
themselves based on their own value and
beliefs, for example, when choosing to play
a risky bet. Factors typically considered here
include risk evaluation, reward, loss aver-
sion, regret, and quantity evaluation. Social
decision scenarios also require that these
factors be taken into account, but what dis-
tinguishes these situations is the presence of
another (involved) agent. A good example of
this is making decisions in a business nego-
tiation context. These contexts additionally
involve evaluations of motivations such as
fairness, equity, cooperation, and in general
more socially driven emotions. Here, we
first examine the experimental evidence of
emotion regulation on individual decision
making, before turning our attention to the
social contexts.
The Modulation of Risk Evaluation
and Risk‑Seeking Behavior
One method to study the interaction between
emotion and decision making is examin-
ing risky choices, for example, when there
is uncertainty about the exact outcome of
the decision and, typically, a safe, relatively
low payout or low-cost option is weighed
against an option with both higher costs
and benefits. A canonical example would
be a choice between a sure gain of $10 and
a risky choice, with a 50% chance of pay-
ing out $25. The importance of studying
the effect of emotion regulation strategies
in modulating risky behaviors becomes par-
ticularly salient when considering that many
pathological behaviors stem from seemingly
irrational evaluation of the risk associated
with a given choice (pathological gambling,
substance abuse, etc.).
Two recent studies have manipulated
people’s emotion regulation strategies when
making risky decisions. One study from
Heilman, Crişan, Houser, Miclea, and
Miu (2010) used cognitive reappraisal (an
antecedent- focused strategy that changes the
meaning of the situation), expressive sup-
pression (a response- focused strategy that
involves inhibiting behavioral expressions
of emotions), and a control baseline condi-
tion in a between- subjects design (Study 1).
Subjects were required to apply these strat-
egies while playing the Balloon Analogue
Risk Task (BART; Lejuez et al., 2002),
as well as the Iowa Gambling Task (IGT;
Bechara, Damasio, Damasio, & Anderson,
1994). The BART provides a vivid measure
of risk taking by giving players the opportu-
nity to progressively earn money by pump-
ing up computerized balloons (Lejuez et al.,
2002). While each additional pump provides
an extra monetary payoff, the balloons can
explode without warning, in which case the
participant loses everything. Therefore, the
task assesses the degree to which players are
prepared to risk an extra pump (for an extra
reward) at the risk of a total loss (Lejuez et
al., 2002). The well-known IGT is a comput-
erized card- searching game that measures
the degree to which individuals shift from
choices with large immediate gains (which,
however, are also associated with even larger
losses in the long term) toward the more pru-
dent and eventually more rewarding choices
with small, immediate gains (Bechara et al.,
1994).
Heilman et al. (2010) found that when
compared with the control group, the group
of reappraisers reported fewer negative emo-
tions and demonstrated reduced risk aver-
sion; that is, they made more risky deci-
sions in the BART task, operationalized as
a greater number of pumps on average per
unexploded balloon. In the IGT, reapprais-
ers demonstrated improved performance in
the prehunch/hunch period of the task, in
which players are endeavoring to discover
the exact traits of the various cards; there-
fore, they yielded significantly higher earn-
ings at the end of the game. Importantly,
suppressors and controls showed no sig-
nificant difference in both tasks in terms
144 COGNITIVE APPROACHES
of emotional ratings and decision- making
behavior. Overall, then, this study showed
that cognitive reappraisal appears to lead to
increased risk taking by reducing the expe-
rience of negative emotions elicited by the
decision scenario.
Another study (Martin & Delgado, 2011)
explored how emotion regulation strategies
influence participants’ preferences when
choosing between two monetary options:
a gamble (risky option) and a guaranteed
amount (safe option). Here, participants
engaged in imagery- focused regulation strat-
egies during the presentation of a cue that
preceded an economic decision. The emotion
regulation strategies placed subjects in the
following experimental conditions: “Look,”
“Relax,” or “Excite.” When instructed to
“Relax,” participants were asked to imagine
a calming scene; when in the “Excite” condi-
tion, participants were to imagine an excit-
ing scene, in order to increase their reactiv-
ity. After the imagery phase, participants
made a choice between a risky and a safe
monetary lottery while undergoing fMRI.
Participants who successfully used the emo-
tion regulation strategy “Relax” made fewer
risky choices in comparison to the “Look”
condition. The “Excite” condition was not
effective in producing any change in deci-
sion behavior. To better understand the
effect of the “Relax” strategy on decision
making, the authors divided participants
into two groups on the basis of their success
at implementing the strategy. Regulators
were then compared with nonregulators,
and the former showed a significant pro-
portion of fewer risky choices as compared
with “Look” trials, suggesting that emotion
regulation strategies are able to modulate
risky decisions (Martin & Delgado, 2011).
Moreover, at the neural level, responses in
the striatum, a key region associated with
risky choice, were attenuated during deci-
sion making when participants adopted the
“Relax” emotion regulation strategy (Mar-
tin & Delgado, 2011). In contrast, partici-
pants who did not effectively use emotion
regulation strategies (i.e., the nonregulator
group) failed to show neural differences
during decision making. In conclusion, the
authors argued that imagery- focused strate-
gies “can foster more goal- directed behavior
and promote safer, compared with riskier,
decision- making.”
Anticipation and Processing of Losses:
The Regulation of Loss Aversion
Kahneman and Tversky (1979) famously
codified the notion that losses loom larger
than their equivalent gains, terming this
phenomenon “loss aversion.” Experimental
studies over the past 30 years indicate that
people demonstrate loss aversion for objects
(Kahneman, Knetsch, & Thaler, 1990) and
money (Tversky & Kahneman, 1992). Two
studies from Sokol- Hessner and his col-
leagues (2009, 2013) showed that applying
emotion regulation strategies to decision
making can affect the experience of loss
aversion. In the first study (Sokol- Hessner et
al., 2009), participants were asked to reap-
praise, by taking a different perspective,
about a choice between a binary gamble
and a guaranteed amount. Outcomes were
displayed following the decision phase (e.g.,
“You won” or “You lost”). For half of the
trials, participants applied an “Attend”
strategy, which considered each choice in
isolation, and for the other half, a “Regu-
late” strategy, which emphasized choices in
their broader context, such as “thinking like
a trader.” In other words, they were asked to
imagine themselves to be experienced pro-
fessional stock traders. Risk aversion and
loss aversion indices were then computed,
based on participants’ actual choice behav-
ior. The authors were able to show that the
“Regulate” strategy had a strong effect in
decreasing individuals’ initial levels of loss
aversion but not risk aversion. Importantly,
to identify the degree of change in loss aver-
sion according to differences in regulation
success, subjects were divided into “regu-
lators” and “nonregulators.” Reduced loss
aversion was also associated with reduced
physiological arousal responses to loss out-
comes, further showing that the reappraisal
strategy was also associated with decreased
emotional reaction to bad outcomes.
In the second study, using a similar para-
digm, Sokol- Hessner et al. (2013) found
that success in regulating loss aversion by
using emotion regulation strategies (reap-
praisal), was associated with a reduction
in amygdala responses to losses but not to
gains. Moreover, the application of the reap-
praisal strategy increased the baseline activ-
ity in prefrontal cortices and the striatum, in
line with previous studies (Ochsner, Bunge,
Emotion Regulation and Decision Making 145
Gross, & Gabrieli, 2002; Banks, Eddy, Ang-
stadt, Nathan, & Phan, 2007; Eippert et al.,
2007; Delgado, Gillis, & Phelps, 2008; Del-
gado, Nearing, LeDoux, & Phelps, 2008).
These results support the idea that different
modes of thinking can alter both choices and
arousal responses related to loss aversion.
Regulation of Rewards, Expected Values,
and Prediction Error
Another line of research has involved the
study of rewards in an economic decision-
making context. A reward can be defined as
“an object or event that generates approach
and consummatory behavior, represents
positive outcomes of decisions and may
engage positive emotions” (cf. Schultz, 2009,
p. 323). In other words, rewards are positive
outcomes that occur after specific events.
The importance of studying emotion reg-
ulation strategies of reward stems from the
fact that dysregulation of reward seems to
be associated with pathological addiction
syndromes (Heinz, 2002; Robinson & Ber-
ridge, 2003; Volkow & Wise, 2005; Wrase
et al., 2007). At the neural level, when the
reward is greater than expected, there is a
positive prediction error (greater ventral
striatal activity), and when the reward is
less than expected (or missing altogether),
there is a negative prediction error (less ven-
tral striatal activity) (Hollerman & Schultz,
1998; McClure, Berns, & Montague, 2003;
Staudinger, Erk, Abler, & Walter, 2009).
Prediction errors have been interpreted as
“teaching” signals, helping participants
to adapt their behavior so as to maximize
future rewards acquisition (Sutton, 1988;
Montague, Hyman, & Cohen, 2004;
Staudinger et al., 2009).
Two studies from Staudinger and col-
leagues (2009; Staudinger, Erk, & Walter,
2011) addressed the question of whether
emotion regulation might also impact reward
processing. In the first study, Staudinger
et al. (2009), the authors used a monetary
incentive delay (MID) task (Knutson, Tay-
lor, Kaufman, Peterson, & Glover, 2005)
to investigate whether regulation strategies
affect the neural processing of reward and
its associated motivated behavior. Subjects
were scanned with fMRI while playing the
MID task, in which subjects observed an
abstract cue indicating the amount of money
to be won (€0.50, €0.10), followed by a wait-
ing period. A target (square or triangle) was
then presented, after which subjects became
eligible to win the announced amount of
money. Immediately afterwards, a feed-
back display informed the subjects whether
they had actually won (€0.50, €0.10) or
not (€0.00). Subjects in one condition were
asked to rethink an emotion- eliciting situ-
ation in nonemotional terms by distancing
themselves from feelings and behaving as
neutral observers (distancing; Gross, 2002).
In another condition (“Permit”) they were
allowed to experience their feelings. Dis-
tancing significantly lowered subjects’ per-
ceived feelings. More importantly, a modu-
lation of expected value and its associated
prediction error was observed in the right
ventral striatum of subjects while applying
the distancing strategy. The comparison of
the “Distance” and “Permit” conditions
showed a significant activation in the right-
hemispheric frontoparietal network (includ-
ing the rostral cingulate zone, lateral orbi-
tofrontal cortex [OFC] and inferior parietal
lobe/temporal parietal junction). Interest-
ingly, reappraisal success was positively cor-
related with activation in the rostral cingu-
late zone and the lateral OFC (Staudinger et
al., 2009). The authors were therefore able
to show that emotion regulation strategies
generally impact reward coding, and also
modulate expected value and prediction
error encoding in the ventral striatum.
In the second study, Staudinger and col-
leagues (2011) used a similar task but
explored whether emotion regulation would
also be effective in decreasing the moti-
vation to obtain a reward. The authors
found reduced positive evaluative feelings
for larger gains (€1) during the “Distance”
instruction (from 4.08 to 2.50 points), as
well as a slowdown in reaction times, which
they interpreted as a lowered motivation to
obtain the reward. At a neural level, using
psychophysiological interaction analyses,
they found that the dorsolateral prefrontal
cortex played a fundamental role in exerting
direct modulatory control over the striatum
by promoting low- reward responses. In the
same fashion, another experiment showed
that imagery- based strategies successfully
modulate reward expectation in a monetary
reward– conditioning procedure (Delgado,
Nearing, et al., 2008).
146 COGNITIVE APPROACHES
Overall, these experiments showed that
emotion regulation strategies applied to indi-
vidual decisions have the power to alter our
choices. Emotions such as risk perception,
reward, and loss evaluation are modulated
by the usage of different kind of strategies
(cognitive or relaxation- based imagery), and
this alters our decision behavior toward safer
behavior (Martin & Delgado, 2011), toward
less sensitivity to outcomes (Sokol- Hessner
et al., 2013), and toward lower motivation
to obtain an economic reward (Staudinger et
al., 2011).
Social Decision Making
Despite the extensive literature on emotional
“self- regulation” (see Ochsner & Gross,
2008), evidence of emotion regulation in
social interactive situations is still relatively
scant. Indeed, interest in such “social regu-
lation” has been explored only recently in
a study examining the emotion regulation
of subjects looking at pictures depicting
social versus nonsocial scenes. Studying the
mechanisms involved in the regulation of
real social situations is particularly impor-
tant when considering the failures to regu-
late interpersonal interactions exhibited in
psychiatric disorders such as borderline per-
sonality disorder or social anxiety disorder
(Grecucci, 2012, p. 135); Ochsner, 2008;
Ochsner & Gross, 2008). Recent experi-
ments on emotion regulation and social deci-
sion making hold the promise to elucidate
the mechanisms subserving interpersonal
emotion regulation (Grecucci, Giorgetta,
Bonini, & Sanfey, 2013). Moreover, emo-
tions elicited in social interactions may well
be of a qualitatively different nature than
those experienced while making individual
decisions about monetary gains, rewards, or
risky choices.
Anger
A potentially useful approach to measuring
socially driven emotions is to adopt tasks
derived from “game theory.” Game theory is
the study of mathematical models of conflict
and cooperation between decision makers
(Myerson, 1991), and offers useful models
for the psychological investigation of social
exchange (Sanfey & Dorris, 2009). One of
the more well-known tasks in this field is
the Ultimatum Game (UG; Guth, Schmitt-
berger, & Schwarz, 1982), in which two
players must agree on the division of a sum of
money provided by the experimenter (Van’t
Wout et al., 2010). One player, the proposer,
makes an offer as to how this money should
be split. The responder must then make a
decision to either accept or reject this offer.
If the offer is accepted, then the money is
split as proposed, but if the responder rejects
the offer, then neither player receives any-
thing. A central focus of the literature has
been the well- replicated result that low offers
(i.e., less than 30% of the total amount) are
rejected approximately 50% of the time
(Camerer, 2003). That is, UG choices are
not purely driven by financial self- interest,
but also incorporate affective information
about the social interaction, with evidence
that negative emotions play an important
role in punishment behavior (Pillutla &
Murnighan, 1996; Xiao & Houser, 2005).
Put simply, the UG gives us the opportunity
to elicit anger reactions in subjects via real
social interactions, then to measure their
willingness to punish the perpetrator (and,
of course, themselves) by examining rejec-
tion rates of these unequal offers.
One study from Van’t Wout and col-
leagues (2010) showed that participants
who were asked to reappraise their emotions
accepted a greater number of unfair offers
(i.e., $1 or $2 offered from a pot of $10)
than participants who did not use any regu-
lation strategy (Experiment 1). Importantly,
in another condition, subjects were asked
to use a response- focused strategy, namely,
suppression of any negative emotions toward
the proposer; however, this strategy was not
effective in altering the decision- making of
subjects. Moreover, when acting later in the
experimental session as proposers them-
selves (Experiment 2), participants who had
used reappraisal strategies made more gen-
erous offers to their previously unfair part-
ners than those who had use the strategy of
suppression, demonstrating an interesting
“follow- on” effect of reappraisal.
Following this line of research, Grecucci,
Giorgetta, Van’t Wout, Bonini, and Sanfey
(2013) applied a similar paradigm to that of
Van’t Wout et al. (2010) but also employed
fMRI to explore the neural bases of regulated

Emotion Regulation and Decision Making 147
interpersonal decisions. In addition they
studied the effects of both up- and down-
regulation in participants. This allowed
them to explore whether the negative emo-
tions induced by an inequitable proposal can
be both reduced and increased depending
on the strategy, and, importantly, whether
this regulation in turn affects the decision to
punish the other player for his or her unfair
treatment. When using a down- regulation
strategy (rethink the situation as less nega-
tive), a greater number of unfair offers were
accepted (i.e., fewer punishment behaviors).
Up- regulation, on the other hand, yielded a
greater number of rejections of unfair offers
(i.e., more punishment behaviors), with both
strategy conditions compared with a base-
line “Look” condition. Subjective ratings
analyses clarified that subjects’ anger about
the unfair proposed division was the main
emotion regulated by the reappraisal strat-
egies. At the neural level, significant acti-
vations during reappraisal were observed
in the dorsolateral prefrontal cortex and
cingulate gyrus, but only for unfair offers.
“Regulated” decisions activated the left infe-
rior frontal gyrus for up- regulation and the
middle frontal gyrus for down- regulation
strategies, respectively. The insula, one key
region that was active when participants
were treated unfairly (Sanfey et al., 2003),
showed less activation for down- regulation
and more activation for up- regulation. In
conclusion, these studies provide strong
evidence that interpersonal emotions (e.g.,
anger when treated unfairly) can be success-
fully modulated by emotion regulation strat-
egies. And, as an important consequence,
these studies also clearly show that emotion
regulation can alter our social behavior (in
this case, punishment of unfair behavior),
with these effects observed in within- subject
interactions. This has important implica-
tions for the regulation of social behavior in
interactive contexts.
Selfishness
Another recent study by the same group
(Grecucci, Giorgetta, Bonini, et al., 2013)
used a similar socioeconomic game, namely,
the Dictator Game, to measure emotional
responses to both the altruistic and selfish
behavior of others. The Dictator Game is
similar to the UG, with the important excep-
tion that responders cannot reject the divi-
sion of money made by the proposer; that
is, they must accept whatever the proposer
decides to give them from the experimenter-
provided pot. Here, subjects were trained to
reappraise the other player (a form of “men-
talizing”) in one condition, and to distance
from the situation (“distancing”) in another.
This kind of reappraisal comprised refram-
ing the intention and behavior of the other
player in either a more or less negative way.
Following both altruistic and selfish divi-
sions of money, emotional ratings were col-
lected separately for arousal and valence.
Reappraisal strategies increased the valence
ratings (less negative perception) for self-
ish offers ($1, $2, $3, from a total of $10),
and subjective ratings also indicated that the
main emotion involved when receiving such
offers was “disappointment” (but also to
some extent “anger” and “disgust”) in rela-
tion to the selfish proposers, while distanc-
ing did not produce any change compared
with the “Look” condition. This study
provisionally demonstrates that disappoint-
ment regarding selfish behavior can be suc-
cessfully modulated by mentalizing strate-
gies (reappraisal of the intention of others),
whereas distancing from others (an interper-
sonal strategy that is typical of social and
generalized anxiety disorders) is not effec-
tive in modulating these reactions.
Overall, these experiments have begun
to show that emotions in reaction to com-
plex social motivations such as inequity and
selfishness can be successfully modulated by
emotion regulation strategies, and that these
strategies can lead to changes in observed
decision behaviors.
Conclusion
Our aim in this chapter has been to review
the extant studies that have used regula-
tory strategies to moderate affective inputs
to the decision- making process. Though
relatively few in number to date, there has
been encouraging success with these type of
manipulations, and overall these studies pro-
vide compelling evidence that emotion regu-
lation strategies can have a strong impact
on not only perceptions of stimuli, but also
148 COGNITIVE APPROACHES
real, consequential, decisions in both social
and nonsocial contexts. This turn can lead
to interesting hypotheses about how our
daily decisions can be modulated by emo-
tion regulation strategies. Though it seems
evident that emotional factors play a role in
decision- making situations, the mechanisms
integrating affective and more deliberative
information in decision making are still
relatively understudied. Recent experiments
have demonstrated changes in decision
making following both incidental emotion
induction (Harlé & Sanfey 2007; Bonini et
al., 2011) and neurophysiological disruption
via transcranial magnetic stimulation (Van’t
Wout, Kahn, Sanfey, & Aleman, 2005;
Knoch, Pascual- Leone, Meyer, Treyer, &
Fehr, 2006), but emotion regulation studies
of decision making have already extended
these findings in important ways by dem-
onstrating that emotions directly related to
the choice situation itself can be controlled
through top-down effort, leading to changes
in how the choice sets are represented and
the associated decisions themselves. More-
over, these studies are important because
they extend the range of emotions that can
be affected by emotion regulation strategies.
Neuroanatomical Bases of Emotion
Regulation of Decision Making
Overall, the brain regions implicated in the
aforementioned studies can be grouped into
two main functional clusters: the regions
that implement the regulation strategies,
and the regions regulated by the strategies.
For the former, regions acting as “regula-
tors” are primarily the dorsolateral (dlPFC)
and ventrolateral (vlPFC) portions of the
prefrontal cortex, and in some cases also
the ventromedial regions. The dlPFC and
vlPFC have been implicated in active cogni-
tive control and inhibition (Miller & Cohen,
2001; Smith & Jonides, 1999), and may
underlie the generation and maintenance
of reappraisal strategies (Ochsner et al.,
2002, 2004). Importantly, the ventral part
of the prefrontal cortex is well- connected
with brain structures such as the insula, the
amygdala, and the striatum. One hypothesis
derived from these activations is that the pre-
frontal cortex actively modulates the activity
in more emotional regions under conditions
of emotional regulation (Grecucci, Gior-
getta, Bonini, et al., 2013a, 2013b). Interest-
ingly, dlPFC and vlPFC have been previously
reported as active when reappraising emo-
tional pictures (Ochsner et al., 2004). One
interpretation might therefore be that dlPFC
is involved in modulating both behavioral
and emotional outputs in order to satisfy
contextual demands (Mitchell, 2011).
Interestingly, there is an overlap between
“decision- making” and “regulatory” brain
areas. Indeed, the role of the lateral prefron-
tal cortex in decision making has been con-
ceptualized along four main lines: (1) nega-
tive feedback encoding; (2) motor response
inhibition; (3) stimulus– response reconfigu-
ration; and (4) attention to behaviorally sig-
nificant stimuli (Mitchell, 2011). The same
considerations apply to the role of the lat-
eral PFC in emotion regulation. For exam-
ple, Mitchell hypothesized that the lateral
prefrontal cortex is involved in modulating
behavioral and emotional output given par-
ticular contextual demands. Another key
region connected to both emotion regulation
and decision making is the cingulate cortex,
which may best be interpreted in terms of
performance monitoring. One of these mon-
itoring functions is the detection of response
conflict between top-down “deliberative”
signals and bottom- up “emotional” signals.
Other relevant regions for these processes
are the temporoparietal junction (TPJ) and
the inferior parietal lobe (IPL). Activation
of the right TPJ/IPL is in line with previous
reappraisal studies (Ochsner et al., 2002;
Delgado, Nearing, et al., 2008; Staudinger
et al., 2009) and might indicate allocation
and dislocation of attention (Corbetta &
Shulman, 2002).
The regions modulated by the strategies are
generally connected with the emotions elic-
ited in the tasks. For example, tasks involving
risk assessment found reduced activity in the
ventral striatum (Martin & Delgado, 2011);
tasks involving reward found alterations in
the activity of OFC, cingulate cortex, and
the ventral striatum (Staudinger et al., 2009,
2011); experiments involving the experience
of monetary loss found reduced amygdala
activity (Sokol- Hessner et al., 2013); and
social decision- making tasks such as the UG
yielded reduced activity in the insula, known
to be associated with anger and feelings of
disgust (Grecucci, Giorgetta, Bonini, et al.,
2013a). See Table 9.1 for a summary.
149
TABLE 9.1. Emotion Regulation and Decision Making Studies and Their Main Findings
Emotion or emotion-
related behavior
Decision-making
task
Emotion
regulation strategy Behavioral effect Main regulated regions
Regulating
regions Reference
Risky behavior Balloon Analogue
Risk Task; Iowa
Gambling Task
Suppression,
reappraisal
Reduced risk aversion;
increased monetary
performance
— — Heilman et al.
(2010)
Risk perception Gambling Imagery-focused
regulation
Reduced risky choices Ventral striatum Martin and Delgado
(2011)
Loss aversion Gambling Reappraisal Reduced loss aversion Amygdala dlPFC, vmPFC Sokol-Hessner et al.
(2009, 2013)
Reward Monetary Incentive
Delay Task
Distancing Attenuation of expected value;
modulation of prediction
error; attenuation of pleasant
anticipation of gains
Ventral striatum, rostral
cingulate, orbitofrontal
cortex, putamen
dlPFC, CC,
IPL/TPJ
Staudinger et al.
(2009, 2011)
Anger Ultimatum Game Reapprasial,
suppression
Reduced rejection rates Insula dlPFC, CC Van’t Wout et al.
(2010); Grecucci,
Giorgetta, Van’t
Wout, et al. (2013)
Responses to selfish
behavior of others
Dictator Game Reapprasial,
distancing
Less negative emotions Insula, Striatum,
posterior cingulate
dIPFC, TPJ Grecucci, Giorgetta,
Bonini, and Sanfey
(2013a, 2013b)
Note. dlPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial PFC; CC, cingulate cortex; IPL, inferior parietal lobe; TPJ, temporoparietal junction.

150 COGNITIVE APPROACHES
Concluding Remarks
and Future Directions
In this chapter, we have reviewed the cur-
rent experimental evidence concerning emo-
tion regulation as applied to decision mak-
ing. While relatively few experiments to
date have explicitly used regulation strate-
gies in the context of decision and choice,
the results thus far are encouraging and
point to the usefulness of regulation in
potentially modifying decision making. The
experimental findings are consistent with a
key role of emotional regulatory process in
decision making. For example, expressing
emotions when bargaining in the UG has
effects on the subsequent punishment behav-
ior (Xiao & Houser, 2005). Individual and
social decision- making experiments have
shown that emotions connected to relevant
decision factors such as loss, risk, reward,
interpersonal anger, and moral disgust can
be successfully regulated by various strate-
gies. Notably, not all the strategies result
in equally successful changes in subjects’
decision- making behavior. While rein-
terpretative strategies such as reappraisal
appear effective at altering decision mak-
ing and associated emotions, the same does
not necessarily apply to expressive strate-
gies such as suppression (Heilman et al.,
2010; Van’t Wout et al., 2010) or distancing
(Grecucci, Giorgetta, Bonini, et al., 2013;
but see Staudinger et al., 2009, for a posi-
tive result). Although researchers are now
beginning to investigate the role of regula-
tion in decision making, several factors still
need to be understood and explored. For
example, there are many different types of
decision making that have been hitherto
unexamined in terms of how regulation may
impact choice, such as impulsivity, temporal
discounting, charitable donations, assess-
ments of uncertainty and so on. Moreover,
some (e.g., Sokol- Hessner et al., 2009)
have argued that ability to regulate may be
related to more efficient decision- making
behavior. One interesting question is there-
fore whether individual differences in the
way we treat and regulate our emotions are
predictive of more optimal choices and the
better avoidance of detrimental behaviors.
To date, researchers have focused their
attention primarily on the effect of nega-
tive emotions on decision making. However,
positive emotions may also have a large
impact on decision making, and future stud-
ies could productively examine these affec-
tive influences. Another intriguing finding
is that, in some studies, augmented activity
in the more “decision- related” brain areas
has been reported. At least two studies have
reported a baseline shift in dlPFC (Grecucci
et al., 2013; Sokol- Hessner et al., 2013). The
exact interpretation of this shift is a matter
for future research studies, but one specula-
tive account is that this may be involved in
increased intentional control (Sokol- Hessner
et al., 2013) or more flexibility in satisfying
contextual demands (Grecucci, 2012; Gre-
cucci, Giorgetta, Van’t Wont, et al., 2013).
One important point that remains unsolved
is how emotion regulation strategies affect
decisions. Do regulatory strategies alter deci-
sions themselves, without impacting emo-
tions? Or does emotion regulation influence
decision- making processes by altering the
emotion, which in turn alters decision mak-
ing? Even if the latter seems to be the more
plausible explanation, the two hypotheses
have rarely been tested in the same experi-
ment. Finally, recent studies of experimental
psychopathology are providing important
insights into how emotional dysregulation
(excessive anxiety or depression, etc.) affects
our decisions. These studies may provide
further proof of the importance of regula-
tory mechanisms (and their failure) in deci-
sion making.
Overall, studying the processes and mech-
anisms involved in how emotions are regu-
lated in concert with actual decision- making
behavior offers an appealing template for
future research. Demonstrating how regula-
tion can actually impact both behavior and
subsequent choice outcomes extends the cur-
rent research topic in interesting directions
and also has the potential to outline practi-
cal strategies that can be usefully employed
in real-life situations.
References
Andrade, E. B., & Ariely, D. (2009). The endur-
ing impact of transient emotions on decision-
making. Organizational Behavior and Human
Decision Processes, 109(1), 1–8.
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan,
P. J., & Phan, K. L. (2007). Amygdala– frontal
Emotion Regulation and Decision Making 151
connectivity during emotion regulation. Social
Cognitive and Affective Neuroscience, 2(4),
303–312.
Bechara, A., Damasio, A. R., Damasio, H., &
Anderson, S. W. (1994). Insensitivity to future
consequences following damage to human
prefrontal cortex. Cognition, 50, 7–15.
Bechara, A., Damasio, H., Tranel, D., & Dama-
sio, A. R. (1997). Deciding advantageously
before knowing the advantageous strategy.
Science, 275(5304), 1293–1295.
Bonini, N., Hadjichristidis, C., Mazzocco, K.,
Demattè, M. L., Zampini, M., Sbarbati, A.,
et al. (2011). Pecunia olet: The role of inciden-
tal disgust in the Ultimatum Game. Emotion,
11(4), 965–969.
Camerer, C. F. (2003). Behavioral game theory.
New York: Russell Sage Foundation.
Clark, M. S., & Isen, A. M. (1982). Towards
understanding the relationship between feel-
ing states and social behavior. In A. H. Has-
torf & A. M. Isen (Eds.), Cognitive social psy-
chology (pp.
73–108). New York: Elsevier.
Corbetta, M., & Shulman, G. L. (2002). Control
of goal-
d
irected and stimulus driven attention
in the brain. Nature Review Neuroscience, 3,
201–215.
Dalgleish, T. (2004). The emotional brain.
Nature Review Neuroscience, 5, 583–589.
Damasio, A. (1994). Descartes’ error: Emotion,
reason, and the human brain. New York: Put-
nam.
Delgado, M. R., Gillis, M., & Phelps, E. A.
(2008). Regulating the expectation of reward
via cognitive strategies. Nature Neuroscience,
11(8), 880–881.
Delgado, M. R., Nearing, K. I., LeDoux, J. E., &
Phelps, E. A. (2008). Neural circuitry underly-
ing the regulation of conditioned fear and its
relation to extinction. Neuron, 59, 829–838.
Duncan, J., Emslie, H., Williams, P., Johnson,
R., & Freer, C. (1996). Intelligence and the
frontal lobe: The organization of goal-
d
irected
behavior. Cognitive Psychology, 30, 257–303.
Eippert, F., Veit, R., Weiskopf, N., Erb, M.,
Birbaumer, N., & Anders, S. (2007). Regula-
tion of emotional responses elicited by threat-
r
elated stimuli. Human Brain Mapping, 28(5),
409–423.
Evans, J. St. B. T. (2009). How many dual-
p
rocess
theories do we need: One, two or many? In J. St.
B. T. Evans & K. Frankish (Eds.), In two minds:
Dual processes and beyond (pp.
31–54). New
York: Oxford University Press.
Forgas, J. P. (1995). Mood and judgment: The
affect infusion model (AIM). Psychological
Bulletin, 73(117), 39–66.
Forgas, J. P. (2003). Affective influences on atti-
tudes and judgments. In R. J. Davidson, K. J.
Scherer, & H. H. Goldsmith (Eds.), Hand-
book of affective sciences (pp.
596–618). New
York: Oxford University Press.
Forgas, J. P., Bower, G. H., & Moylan, S. J.
(1990). Praise or blame?: Affective influences
on attributions for achievement. Journal of
Personality and Social Psychology, 59, 809–
818.
Grecucci, A. (2012). Il Conflitto Epistemologico.
Psicoanalisi e Neuroscienze dei Processi anti-
conoscitivi. Francavilla, Italy: Edizioni Psi-
conline.
Grecucci, A., Giorgetta, C., Bonini, N., & San-
fey, A. (2013a). Living emotions, avoiding
emotions: behavioral investigation of the regu-
lation of socially driven emotions. Frontiers in
Psychology, 3(616).
Grecucci, A., Giorgetta, C., Bonini, N., &
S
anfey, A. (2013b). Reappraising social emo-
tions: The role of inferior frontal gyrus,
temporo-
p
arietal junction and insula in inter-
personal regulation. Frontiers in Human
Neuroscience.
Grecucci, A., Giorgetta, C., Van’t Wout, M.,
Bonini, N., & Sanfey, A. G. (2013). Reapprais-
ing the ultimatum: An fMRI study of emotion
regulation and decision-
m
aking. Cerebral
Cortex, 23(2), 399–410.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39, 281–291.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp.
3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85(2), 348–362.
Guth, W., Schmittberger, R., & Schwarz, B.
(1982). An experimental analysis of ultima-
tum bargaining. Journal of Economic and
Behavioral Organization, 3, 376–388.
Harlé, K., & Sanfey, A. G. (2007). Incidental
sadness biases social economic decisions in the
ultimatum game. Emotion, 7(4), 876–881.
Harlé, K. M., Chang, L. J., Van’t Wout, M., &
Sanfey, A. G. (2012). The neural mechanisms
of affect infusion in social economic decision-
152 COGNITIVE APPROACHES
making: A mediating role of the anterior
insula. NeuroImage, 61(1), 32–40.
Heilman, R. M., Crişan, L. G., Houser, D.,
Miclea, M., & Miu, A. C. (2010). Emotion
regulation and decision- making under risk
and uncertainty. Emotion, 10(2), 257–265.
Heinz, A. (2002). Dopaminergic dysfunc-
tion in alcoholism and schizophrenia—
Psychopathological and behavioral correlates.
European Psychiatry, 17, 9–16.
Hollerman, J. R., & Schultz, W. (1998). Dopa-
mine neurons report an error in the temporal
prediction of reward during learning. Nature
Neuroscience, 1, 304–309.
Isen, A. M., & Daubman, K. A. (1984). The
influence of affect on categorization. Jour-
nal of Personality and Social Psychology, 47,
1206–1217.
Kahneman, D., Knetsch, J. L., & Thaler, R. H.
(1990). Experimental tests of the endowment
effect and the Coase theorem. Journal of Polit-
ical Economy, 98, 1325–1348.
Kahneman, D., & Tversky, A. (1979). Prospect
theory: An analysis of decision under risk.
Econometrica, 47, 263–291.
Knoch, D., Pascual- Leone, A., Meyer, K., Treyer,
V., & Fehr, E. (2006). Diminishing reciprocal
fairness by disrupting the right prefrontal cor-
tex. Science, 314, 829–832.
Knutson, B., Taylor, J., Kaufman, M., Peterson,
R., & Glover, G. (2005). Distributed neural
representation of expected value. Journal of
Neuroscience, 25, 4806–4812.
Koenigs, M., & Tranel, D. (2007). Irrational
economic decision- making after ventromedial
prefrontal damage: Evidence from the Ulti-
matum Game. Journal of Neuroscience, 27,
951–956.
Lejuez, C. W., Read, J. P., Kahler, C. W., Rich-
ards, J. B., Ramsey, S. E., Stuart, G. L., et al.
(2002). Evaluation of a behavioral measure of
risk taking: The Balloon Analogue Risk Task
(BART). Journal of Experimental Psychology:
Applied, 8, 75–84.
Lerner, J. S., Small, D. A., & Loewenstein, G. F.
(2004). Heart strings and purse strings: Car-
ryover effects of emotions on economic deci-
sions. Psychological Science, 15(5), 337–341.
Martin, L. N., & Delgado, M. R. (2011). The
influence of emotion regulation on decision-
making under risk. Journal of Cognitive Neu-
roscience, 23(9), 2569–2581.
McClure, S. M., Berns, G. S., & Montague, P. R.
(2003). Temporal prediction errors in a passive
learning task activate human striatum. Neu-
ron, 38, 339–346.
Miller, E. K., & Cohen, J. D. (2001). An inte-
grative theory of prefrontal cortex function.
Annual Review of Neuroscience, 24, 167–
202.
Mitchell, D. G. V. (2011). The nexus between
decision- making and emotion regulation:
A review of convergent neurocognitive sub-
strates. Behavioral Brain Research, 217, 215–
231.
Montague, P. R., Hyman, S. E., & Cohen, J. D.
(2004). Computational roles for dopamine in
behavioural control. Nature, 431, 760–767.
Moretti, L., Dragone, D., & di Pellegrino, G.
(2009). Reward and social valuation deficits
following ventromedial prefrontal damage.
Journal of Cognitive Neuroscience, 21(1),
128–140.
Myerson, R. B. (1991). Game theory: Analysis of
conflict. Cambridge, MA: Harvard University
Press.
Ochsner, K. (2008). The social- emotional pro-
cessing stream: Five core constructs and their
translational potential for schizophrenia and
beyond. Biological Psychiatry, 64(1), 48– 61.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. (2002). Rethinking feelings: An
fMRI study of the cognitive regulation of emo-
tion. Journal of Cognitive Neuroscience, 14,
1215–1229.
Ochsner, K. N., & Gross, J. J. (2008). Cogni-
tive emotion regulation: Insights from social
cognitive and affective neuroscience. Current
Directions in Psychological Science, 17, 153–
158.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D., et al.
(2004). For better or for worse: Neural sys-
tems supporting the cognitive down- and up-
regulation of negative emotion. NeuroImage,
23(2), 483–499.
Pfister, H.-R., & Böhm, G. (2008). The multi-
plicity of emotions: A framework of emotional
functions in decision making. Judgment and
Decision Making, 3(1), 5–17.
Phillips, M. L., Drevets, W. C., Rauch, S. L., &
Lane, R. (2003). Neurobiology of emotion
perception: II. Implications for major psychi-
atric disorders. Biological Psychiatry, 54(5),
515–528.
Phillips, M. L., Young, A. W., Senior, C., Bram-
mer, M., Andrew, C., Calder, A. J., et al.
(1997). A specific neural substrate for perceiv-
ing facial expressions of disgust. Nature, 389,
495–498.
Pillutla, M. M., & Murnighan, J. K. (1996).
Unfairness, anger, and spite: Emotional rejec-
Emotion Regulation and Decision Making 153
tions of ultimatum offers. Organization
Behavior and Human Decision Process, 68,
208–224.
Robinson, T. E., & Berridge, K. C. (2003).
Addiction. Annual Review of Psychology, 54,
25–53.
Sanfey, A. G. (2007). Social decision- making:
Insights from game theory and neuroscience.
Science, 318(5850), 598–602.
Sanfey, A. G., & Chang, L. J. (2008). Multiple
systems in decision making. Annals of the
New York Academy of Sciences, 1128, 53–62.
Sanfey, A. G., & Dorris, M. (2009). Games in
humans and non-human primates: Scanners
to single units. In P. W. Glimcher, C. F. Cam-
erer, E. Fehr, & R. A. Poldrack (Eds.), Neu-
roeconomics (pp. 63–80). London: Academic
Press.
Sanfey, A. G., Rilling, J. K., Aronson, J. A., Nys-
trom, L. E., & Cohen, J. D. (2003). The neural
basis of economic decision- making in the ulti-
matum game. Science, 300, 1755–1758.
Schultz, W. (2009). Midbrain dopamine neurons:
A retina of the reward system? In P. W. Glim-
cher, C. F. Camerer, E. Fehr, & R. A. Poldrack
(Eds.), Neuroeconomics (p. 323). London:
Academic Press.
Slovic, P., Finucane, M. L., Peters, E., & Mac-
Gregor, D. G. (2004). Risk as analysis and risk
as feelings: Some thoughts about affect, rea-
son, risk, and rationality. Risk Analysis, 24(2),
311–322.
Smith, E. E., & Jonides, J. (1999). Storage and
executive processes in the frontal lobes. Sci-
ence, 283(5408), 1657–1661.
Sokol- Hessner, P., Camerer, C. F., & Phelps, E.
A. (2013). Emotion regulation reduces loss
aversion and decreases amygdala responses to
losses. Social Cognitive and Affective Neuro-
science, 8(3), 341–350.
Sokol- Hessner, P., Hsu, M., Curley, N. G., Del-
gado, M. R., Camerer, C. F., & Phelps, E.
A. (2009). Thinking like a trader selectively
reduces individuals’ loss aversion. Proceeding
of the National Academy of Sciences, 106(13),
5035–5040.
Staudinger, M. R., Erk, S., Abler, B., & Walter,
H. (2009). Cognitive reappraisal modulates
expected value and prediction error encod-
ing in the ventral striatum. NeuroImage, 47,
713–721.
Staudinger, M. R., Erk, S., & Walter, H. (2011).
Dorsolateral prefrontal cortex modulates
striatal reward encoding during reappraisal of
reward anticipation. Cerebral Cortex, 21(11),
2578–2588.
Stuss, D. T., & Knight, R. T. (2002). Principles
of frontal lobe function. New York: Oxford
University Press.
Sutton, R. (1988). Learning to predict by the
methods of temporal differences. Machine
Learning, 3, 9–44.
Tversky, A., & Kahneman, D. (1992). Advances
in prospect theory: Cumulative representation
of uncertainty. Journal of Risk and Uncer-
tainty, 5, 297–323.
Van’t Wout, M., Chang, L. J., & Sanfey, A. G.
(2010). The influence of emotion regulation on
social interactive decision- making. Emotion,
10(6), 815–821.
Van’t Wout, M., Kahn, R. S., Sanfey, A. G., &
Aleman, A. (2005). Repetitive transcranial
magnetic stimulation over the right dorsolat-
eral prefrontal cortex affects strategic decision-
making. NeuroReport, 16, 1849–1852.
Van’t Wout, M., Kahn, R. S., Sanfey, A. G.,
& Aleman, A. (2006). Affective state and
decision- making in the ultimatum game.
Experimental Brain Research, 169(4), 564–
568.
Volkow, N. D., & Wise, R. A. (2005). How can
drug addiction help us understand obesity?
Nature Neuroscience, 8, 555–560.
Winkielman, P., Berridge, K. C., & Wilbarger,
J. L. (2005). Unconscious affective reactions
to masked happy versus angry faces influ-
ence consumption behavior and judgments of
value. Personality and Social Psychology Bul-
letin, 31(1), 121–135.
Wrase, J., Schlagenhauf, F., Kienast, T., Wusten-
berg, T., Bermpohl, F., Kahnt, T., et al. (2007).
Dysfunction of reward processing correlates
with alcohol craving in detoxified alcoholics.
NeuroImage, 35, 787–794.
Xiao, E., & Houser, D. (2005). Emotion expres-
sion in human punishment behavior. Proceed-
ings of the National Academy of Sciences,
102, 7398–7401.
Zajonc, R. B. (2000). Feelings and thinking:
Closing the debate over the independence of
affect. In J. P. Forgas (Ed.), Feeling and think-
ing: The role of affect in social cognition
(pp. 31–58). New York: Cambridge University
Press.

PART IV
DEVELOPMENTAL
CONSIDERATIONS

157
In this chapter, we focus on the nature and
development of temperamentally based
effortful control (EC) processes and the
role of effortful self- regulation in children’s
socioemotional development. First we con-
sider definitional and conceptual issues.
Then we briefly summarize the normative
development of self- regulation (including
EC), followed by review of research illustrat-
ing the importance of individual differences
in children’s self- regulatory skills (including
effortful control) for their socioemotional
development. We conclude by discussing a
few current conceptual and methodological
issues and gaps in the research.
Conceptual Issues
Our discussion of EC must be considered
in the broader context of conceptions of
children’s self- regulation. In the work with
children, there has been a strong focus
on self- regulatory processes involved in
the modulation of emotion— an aspect of
regulation labeled as emotion regulation.
Definitions of this construct vary consider-
ably (Gross, this volume). Our definition
of emotion- related self- regulation includes
many of the elements of others’ definitions
but is broader in some respects and narrower
in others. For us, it refers to processes used
to manage and change whether, when, and
how (e.g., how intensely) one experiences
emotions and emotion- related motivational
and physiological states, as well as how
emotions are expressed behaviorally. Thus,
it includes processes used to change one’s
own emotional state, to prevent or initiate
emotional responding (e.g., by selecting or
changing situations), to modify the signifi-
cance of an event for the self, and to modu-
late the behavioral expression of emotion
(e.g., through verbal or nonverbal cues). Our
definition is consistent with Gross’s (this
volume) model, in which emotion regulation
includes situation selection, situation modi-
fication, attentional deployment, cognitive
change, and response modulation.
We have used the term emotion- related
self- regulation because many of the pro-
cesses frequently involved in emotional self-
regulation (see below) are also used for the
self- regulation in the absence of emotion.
Although we fully acknowledge that emo-
tional control/regulation often is externally
imposed, especially early in life, we believe
it is useful to differentiate between inter-
CHAPTER 10
Self‑Regulation, Effortful Control,
and Their Socioemotional Correlates
Nancy Eisenberg
Claire Hofer
Michael J. Sulik
Tracy L. Spinrad
158 DEVELOPMENTAL CONSIDERATIONS
nally generated self- regulatory processes
and those processes that the child does not
execute (e.g., parental soothing of an infant;
Eisenberg et al., 2004; Eisenberg, Spinrad,
& Eggum, 2010). Thus, we tend to use the
term self- regulation only for internally gen-
erated regulatory processes (although in the
past we used the term regulation), similar
to Gross’s (this volume) distinction between
intrinsic and extrinsic regulatory processes.
There are other variations in how the term
regulation or even self- regulation is used.
Some people use it to refer to a host of pro-
cesses that have a controlling effect on cog-
nition, attention, or behavior. As we discuss
later, we believe that it is useful to make a
distinction between more and less volitional
processes involved in regulation/control.
Although it is clear that many nonvolitional
processes have important modulating (even,
in a sense, regulating) effects on attention,
cognition, behavior, and physiological
responding, it would be useful to differenti-
ate the two types of processes semantically.
A term such as potentially volitional self-
regulatory processes would be more precise
than self- regulation in referring to volitional
processes; however, such terminology is
quite awkward. We ask readers to focus less
on the use of the word self- regulation than
on the distinctions we address.
Because it is extremely difficult to differen-
tiate emotion from its regulation, we believe
it is useful to focus on the processes used
to manage attention, emotion, and associ-
ated behavior rather than on the amount of
emotion experienced or expressed. In this
respect, we find EC to be a useful construct.
Effortful Control
EC, the regulatory component of tempera-
ment, is defined as “the efficiency of execu-
tive attention, including the ability to inhibit
a dominant response and/or to activate a
subdominant response, to plan, and to detect
errors” (Rothbart & Bates, 2006, p. 129). It
involves the ability to deploy attention will-
fully (often called attention focusing and
shifting, and including cognitive distraction)
and to inhibit or activate behavior willfully
(inhibitory control and activational control,
respectively). Some executive functioning
(EF) skills— especially effortful deployment
of attention, integration of information
to which one attends, and planning— are
involved in EC; thus, EF and EC are par-
tially overlapping constructs. In our view,
EC and, as a result, aspects of EF, include
an array of processes or capabilities that can
be used to manage emotion and behavior;
however, EC is not completely analogous to
emotional self- regulation because EC can
be used for other purposes (e.g., to focus
on schoolwork). Moreover, self- regulation
capacities likely include more than just EC;
for example, self- regulation may include the
motivation to utilize EC/EF in the service of
goals (Eisenberg & Zhou, in press). We view
the skills inherent in EC as building blocks
for the development of self- regulation, emo-
tional and otherwise, across the lifespan,
and thus, as the core of internally based,
volitional self- regulation. Therefore, despite
some distinctions between EC and self-
regulation, the constructs are overlapping
and we tend to use the terms interchange-
ably in this chapter.
The fact that EC is effortful or willful
does not mean that the individual is always
aware that he or she is modulating emotion
or behavior. Some aspects of EC, especially
after rehearsal and practice, may often be
automatic and executed without much con-
scious awareness in contexts with relevant
trigger cues (Mischel & Ayduk, 2011);
however, if needed, the individual is able to
shift into a more volitional mode of func-
tioning. Effortful regulatory processes are
distinguished from less voluntary reactive
processes (see below) by the ease with which
they can be volitionally controlled.
Any particular form of self- regulation (or
EC) is not necessarily good or bad in terms
of its consequences. Nonetheless, we believe
that effortful self- regulatory processes are
more likely than some less volitional aspects
of control (see below) to result in adaptive
outcomes in normative contexts, or at least
in desired goals, be they actually adaptive or
not. This is because effortful capacities can
be applied at will and adapted flexibly to
the demands of specific contexts. Of course,
individuals may also use EC in a manner
that is not adaptive. For example, EC can be
used to achieve socially inappropriate goals,
such as a youth planning and carrying out a
series of well- regulated actions to humiliate
a peer or to steal items to impress friends.
Moreover, an effortful mode of regulation
Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 159
could be adaptive in the short run but not
in the long run, or vice versa. In part, the
adaptiveness of EC depends on the goals
that an individual is striving to achieve and
the social context.
Control and Regulation: Is Control
Always Regulation?
Like a number of other investigators (e.g.,
Block & Block, 1980), we have argued that
well- regulated people are neither overly con-
trolled nor undercontrolled; rather, they can
respond flexibly to varying demands with
a range of responses, depending on the cir-
cumstances. Optimally self- regulated chil-
dren can effortfully initiate or inhibit behav-
iors when appropriate or when required to
achieve goals, but they can also be sponta-
neous (uncontrolled) when control is not
needed. Thus, their regulation is flexible and
generally (although not always) adaptive.
Because of the importance of both will-
fulness and flexibility in EC, it is useful to
try to differentiate EC from less voluntary
over- or undercontrolled processes. Some
control is nearly always automatic and
nonvolitional, and often it is emotionally
based; such control is likely less flexible and
often is less adaptive than volitional self-
regulation. An example is children who have
been labeled “behaviorally inhibited.” They
tend to be wary and overly constrained in
novel or stressful situations and have dif-
ficulty modulating (e.g., relaxing) their
inhibition (Kagan & Fox, 2006). Although
they may appear to be self- regulated, their
inhibition or constraint is relatively involun-
tary or so automatic that it is difficult for
them to modulate effortfully (Eisenberg &
Morris, 2002). In addition, the impulse to
approach people or inanimate objects in the
environment (sometimes called surgency;
Rothbart & Bates, 2006) often may be rela-
tively involuntary. For example, individuals
may be “pulled” toward rewarding or posi-
tive situations or stimuli, and this pull may
be nonvolitional; such people generally are
viewed as “impulsive.” We have labeled such
overly inhibited and impulsive behavior as
two aspects of reactive control—reactive
overcontrol and undercontrol— based on the
distinction by Rothbart and Bates (2006)
between effortful and reactive tempera-
mental processes. The constructs of reactive
undercontrol and overcontrol are somewhat
similar to what the Block and Block (1980)
labeled as the extremes of ego control (i.e.,
ego undercontrol and overcontrol), which is
defined as the “threshold or operating char-
acteristic of an individual with regard to
the expression or containment of impulses,
feelings, and desires” (p. 43). In the Blocks’
model, ego resiliency provides control and
modulation of impulses (i.e., ego control).
We consider EC, but not reactive control,
to be part of volitional self- regulation. As
noted previously, we certainly acknowledge
that reactive control processes influence and
even have controlling or “regulatory” effects
on individuals’ functioning, but we prefer
not to equate these effects with volitional
self- regulation (see Compas, Connor-Smith,
Saltzman, Thomsen, & Wadsworth, 2001,
for a similar distinction when defining cop-
ing; also see Carver, 2005, for a review
of similar perspectives, including dual-
processing models).
Rothbart and colleagues (e.g., Derryberry
& Rothbart, 1997) linked what we have
labeled reactive overcontrol and undercon-
trol processes to reactive emotional pro-
cesses—fear versus desire, hope, and relief,
respectively—and their associated moti-
vational systems, defensive and appetitive,
respectively. Although we agree that these
associations between behavioral inhibi-
tion or impulsivity and emotion/motivation
exist, we wish to differentiate between emo-
tional reactivity and the aspects of behavior
relevant to control that typically are associ-
ated with emotion. It is quite possible that
children who display behavioral inhibition
(reactive overcontrol) do not experience fear
or anxiety every time they display overly
inhibited behavior. Such behavior, although
probably originally based on fear or a reac-
tion to novelty (Kagan & Fox, 2006), seems
to become a habitual style of response to
novel or potentially stressful contexts. More-
over, highly inhibited children often look
controlled (restrained) in their behavior but
tend to be prone to fear and anxiety; thus,
control of overt behavior is not the same
as control in regard to emotional reactivity
(although both are elements of temperamen-
tal reactivity). Similarly, impulsive behavior
that may be linked with both desire/posi-
tive affect (e.g., Rothbart, Ahadi, Hershey,
& Fisher, 2001) and anger in some contexts

160 DEVELOPMENTAL CONSIDERATIONS
might, in other contexts, not be linked to any
clear emotion. Thus, emotional reactivity of
a particular sort is not strictly paired with
impulsivity, and it is worthwhile to differ-
entiate between impulsivity and emotional
reactivity, and among associated motiva-
tions in specific interactions. In support of
this argument, in factor analyses, disposi-
tional negative emotionality tends to load
on one factor, whereas reactive processes
that involve avoidance or approach behavior
(e.g., shyness, impulsivity) tend to load on
a separate surgency factor (Rothbart et al.,
2001).
A disadvantage to invoking the distinction
between effortful self- regulation and reac-
tive control is that it is difficult to catego-
rize some aspects of regulation/control. For
example, it is difficult to know whether the
child who is constrained in a new context is
self- regulated (i.e., voluntarily constrained)
or overly controlled (behaviorally inhibited).
In our view, a major challenge for the field is
differentiating between these two contribu-
tors to constrained behavior.
Although impulsivity and EC are fairly
consistently negatively related (Aksan &
Kochanska, 2004; Valiente et al., 2003), the
two constructs have been found to load on
different latent constructs (Eisenberg et al.,
2004, 2013; Valiente et al., 2003). The rela-
tion between EC and reactive overcontrol
is less consistent (e.g., Aksan & Kochan-
ska, 2004; Spinrad et al., 2007), and there
is a need for research testing the relation
between reactive over- and undercontrol.
A comparison with Ursache, Blair, and
Raver’s (2012) definition helps to highlight
some of the subtle differences in definitions
(some other definitions differ from ours
in numerous, more obvious ways, includ-
ing some of those mentioned earlier). They
defined self- regulation as
the primarily volitional management of
arousal or activity in attention, emotion, or
stress response systems in ways that facilitate
the use of EF abilities in the service of goal-
directed actions. . . . EF is the top-down or voli-
tional component of self- regulation. . . .This
volitional component, however, is reciprocally
related to and dependent on bottom- up, less
volitional, and more automatic regulation of
responses to the environment through atten-
tion, emotion, and stress response systems.
(p. 123)
We would argue that some responses that
are relatively automatic usually can be voli-
tional if necessary, so the critical distinction
is whether a capacity can easily become voli-
tional if needed for adaptation, not whether
it can be automatic. In addition, Ursache et
al. seem to include what we have labeled as
reactive control in the construct of regulation
(“the bottom- up, less volitional” aspects).
Although we prefer to confine the term self-
regulation to the aspects that can be voli-
tional when needed, as a semantic way to
differentiate between volitional and reactive
control processes, we agree that both pro-
cesses occur. We also agree that the relation
between more effortful and less volitional
processes is bidirectional; moreover, reactive
over- and undercontrol systems likely affect
one another and are affected by emotions
(Derryberry & Rothbart, 1997). Not com-
pletely in accord with Ursache et al. (2012),
we (Eisenberg & Zhou, in press) suggested
that aspects of EF related to inhibition (e.g.,
in regard to thoughts or behavior and the
ability to shift attention) overlap with the
construct of self- regulation (as well as the
construct of EC), whereas other parts of
EF, such as working memory, facilitate self-
regulation but are separate capacities from
both self- regulation and EC.
In summary, numerous conceptual issues
are unresolved regarding the definition of
self- regulation and the relations among vari-
ous processes that have controlling/regulat-
ing effects. We believe that more rather than
less conceptual differentiation is useful, even
if in reality the various processes are inter-
connected in complex and intricate ways.
The Normative Development
of Self-Regulation/EC
Because EC is viewed as providing the build-
ing blocks for self- regulation, its develop-
ment is of importance to many aspects of
children’s functioning. Children begin to
regulate their own emotions and emotion-
related behavior in the first few years of
life, and there are improvements in regula-
tion skills across childhood and adolescence
(see Eisenberg, Spinrad, et al., 2010). Even
young children have been found to modulate
their distress using a variety of methods. For
example, by 6 months of age, infants some-

Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 161
times reduce their own distress in response
to novelty by looking away from the novel
object and by using self- soothing strate-
gies (Crockenberg & Leerkes, 2004). Such
behaviors appear to be effective in reduc-
ing arousal in the first year of life because
they are associated with lower negativity in
frustrating situations (Stifter & Braungart,
1995). Stifter and Braungart found that
self- soothing was the most preferred regu-
latory strategy at both 5 and 20 months of
age. In contrast, Mangelsdorf, Shapiro, and
Marzolf (1995) found that (1) 6-month-old
infants tended to use gaze aversion as their
primary regulatory strategy, (2) 12-month-
olds engaged in more self- soothing (e.g.,
thumb sucking and hair twirling) than did
18-month-olds, and (3) 12- and 18-month-
old toddlers used more behavioral avoid-
ance and self- distracting strategies than did
6-month-olds.
There appears to be a decline in the use
of self- soothing between 24 and 48 months,
coupled with the emergence of new and more
complex use of objects and interactions to
regulate emotional state (see Diener & Man-
gelsdorf, 1999, for a review). By 24 months
of age, self- distraction may be the most
common and successful regulatory strategy
in fearful and frustrative situations (Grol-
nick, Bridges, & Connell, 1996). Advances
in cognitive, sociocognitive, motor, and
language development that occur between
2 and 5 years of age contribute to the emer-
gence of more sophisticated, diverse, and
successful models of self- regulation and EF
skills (Garon, Bryson, & Smith, 2008), such
that many children are relatively skilled at
managing impulses by age 4 or 5 (Mischel
& Ayduk, 2011; Posner & Rothbart, 2000;
Rothbart, 2011).
Researchers who have focused on the
development of EC processes such as atten-
tion shifting and attention focusing or the
ability to inhibit behavior voluntarily (rather
than as specific reactions to distress or
frustration) have also noted developmental
changes in their use. Eight- to 10-month-
olds demonstrate some capacity to focus
their attention (Kochanska, Coy, Tjebkes,
& Husarek, 1998), and voluntary control
of attention increases somewhat between
9 and 18 months of age (Ruff & Rothbart,
1996). Around 12 months of age, infants
develop the ability to inhibit predominant
responses. For example, Diamond (1991)
found that infants were able to inhibit pre-
dominant response tendencies when reach-
ing for objects (e.g., move around an object),
an ability believed to involve the execution
of intentional behavior, planning, and the
resistance of more automatic or reactive
action tendencies.
According to Posner and Rothbart (2000),
a transition in the development of execu-
tive attention and the effortful inhibition of
behavior can be seen around 30 months of
age. Much of the relevant work involved the
use of a Stroop-like task that required tod-
dlers to switch attention and inhibit behav-
ior. Children show significant improvement
in performance on such a task between 24
and 30 months of age and often perform
with high accuracy by 36 to 38 months
of age, and this ability has been positively
related to adults’ ratings of EC (Rothbart
& Bates, 2006). In addition, with the matu-
ration of attentional mechanisms, the abil-
ity to inhibit motor behavior effortfully
greatly improves between 22 and 44 months
(Kochanska, Murray, & Harlan, 2000;
Rothbart & Bates, 2006) and is fairly good
by age 4 (Posner & Rothbart, 2000). Wil-
loughby, Wirth, Blair, and Family Life Proj-
ect Investigators (2012) found substantial
improvements in a latent factor of EF abili-
ties in children between ages 3 and 5 years,
with the average child improving more than
one standard deviation in EF skills over any
12-month period. Moreover, there appears
to be a further increase in the use of inter-
nal mental or cognitive regulatory strategies
in the school years. Nonetheless, the capac-
ity for EC continues to improve in adoles-
cence and may even continue to develop at a
slower pace into adulthood (Murphy, Eisen-
berg, Fabes, Shepard, & Guthrie, 1999; Wil-
liams, Ponesse, Schachar, Logan, & Tan-
nock, 1999; also see Eisenberg, Spinrad, et
al., 2010).
The Relation of EC
to Socioemotional Development
Children who differ in their self- regulatory
capacities are expected to differ in aspects
of socioemotional functioning involving
self- regulation. Based on the distinction
between reactive control and EC, Eisen-
162 DEVELOPMENTAL CONSIDERATIONS
berg and Morris (2002) constructed a heu-
ristic model including descriptions of three
styles of control— overcontrolled, undercon-
trolled, and optimally controlled— to gen-
erate predictions regarding the relations of
various aspects of EC and reactive control
to children’s socioemotional adjustment and
development. In this heuristic model, the
authors described different constellations of
self- regulation/reactive control expected to
cluster together for some children and the
expected socioemotional correlates of each
constellation.
In the model, overcontrolled individuals
are those high in involuntary reactive con-
trol (e.g., high in behavioral inhibition) and
low in reactive undercontrol (impulsivity),
average in the ability to inhibit behavior
effortfully as needed (inhibitory control),
low in effortful attentional regulation (e.g.,
the abilities to shift and focus attention
effortfully), and low in the ability to activate
behavior effortfully as needed (i.e., effort-
ful activational control). Volitional shifting
or focusing attention is expected to be use-
ful in reducing the negative emotions, such
as fear, associated with highly inhibited
behavior. Individuals with this constellation
are expected to be prone to internalizing
problems (e.g., depression, anxiety, social
withdrawal) and relatively low in social
competence, especially if they are also pre-
disposed to experience negative emotions.
These children might also lack the ability
to be relaxed, socially interactive, and spon-
taneous in all but very familiar settings,
which also is likely to undermine their social
attractiveness and peer status.
In contrast, undercontrolled individuals
are those high in reactive approach tenden-
cies (i.e., impulsive); low in reactive overcon-
trol; and low in all types of EC, including
attentional, inhibitory, and activation con-
trol. People with this style of control are
predicted to be relatively low in social com-
petence and prone to externalizing behav-
ior problems such as aggression, defiance,
and antisocial behaviors (e.g., delinquency).
These children would also be expected to
be low in empathy, prosocial behavior, and
moral development, because of deficits in
social cognition and regulated behavior.
Finally, optimally regulated individuals—
those who are most flexible and appropriate
in their regulation— are those fairly high in
all modes of EC and, in regard to reactive
control, are neither overcontrolled (inhib-
ited) nor undercontrolled (impulsive). These
individuals are expected to be well adjusted,
socially competent (including moral and
prosocial), and resilient when faced with
stress because they typically regulate their
behavior in a goal- directed manner but can
also be spontaneous and unconstrained.
Consistent with the heuristic model, EC
and self- regulation more generally have been
related in predictable ways to a variety of
children’s developmental outcomes. Next
we provide a selective summary of studies
on the relation of EC and/or other measures
of self- regulation to some positive and nega-
tive outcomes expected to be affected by
EC and/or reactive control, including social
competence, social cognition, (mal)adjust-
ment, school- related outcomes, internaliza-
tion of parental rules/demands and guilt,
empathy/sympathy, and prosocial behavior.
Social Competence
In a number of studies with preschoolers,
children, and adolescents, EC has been asso-
ciated with relatively high levels of social
competence (see Eisenberg, Smith, & Spin-
rad, 2011, for a review). Children’s abilities
to modulate their attentional resources likely
lead to optimal levels of emotional arousal in
stressful contexts and, thus, accurate infor-
mation processing linked to understanding
the causes of emotion, effective planning,
and action suited to the situation. Moreover,
the abilities to inhibit and activate behavior
effortfully allow children to implement or
inhibit behaviors as is adaptive in a given
context, hence facilitating socially appropri-
ate responses. Consistent with these argu-
ments, EC (assessed with EF and/or delay
of gratification tasks) in the preschool years
has been found to predict parent- reported
social competence 6 months later (Lengua,
Honorado, & Bush, 2007), as well as socio-
emotional competencies in adolescence and
adulthood (Mischel & Ayduk, 2011). Eisen-
berg and colleagues (2011) have found sup-
port for the positive relation between EC
and social competence in multiple longi-
tudinal samples, and this relation has also
been observed in other cultures (e.g., Hofer,
Eisenberg, & Reiser, 2010; Zhou, Eisenberg,
Wang, & Reiser, 2004).
Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 163
EC (or aspects thereof) frequently has
been examined as a mediator of the rela-
tion of parenting to social competence.
For example, Mintz, Hamre, and Hat-
field (2011) found that mothers’ reports of
54-month-olds’ inhibitory control, but not
attentional focusing, partially mediated the
relation between early maternal sensitiv-
ity (prompt and appropriate responses to
the child, nonintrusive, positive regard for
child) and observed and teacher- rated social
and relational competence, as well as social
and relational competence in first grade.
Similarly, EC has been found to mediate
the relation between maternal support and
children’s social competence concurrently
(at 18 and 30 months), albeit not over time
(Spinrad et al., 2007). In addition, other
competencies appear to mediate the rela-
tion of self- regulation to social competence.
For example, the positive relation between
EC and peer popularity appears to be medi-
ated by ego- resiliency (personality resil-
iency; the ability to cope with and rebound
from stress), both in longitudinal work with
American 6- to 8-year-olds (Spinrad et al.,
2006) and concurrently with French ado-
lescents (Hofer et al., 2010). Children with
high EC are expected to be able to bounce
back easily in new or stressful situations,
and this resiliency is expected to improve
their social competence.
Social Cognition
Children who can avoid emotional over-
arousal and focus their attention are more
likely than less regulated children to focus
on relevant information about emotions
and the environment in social interactions
and, hence, develop a better understand-
ing of emotions. In support of these ideas,
preschoolers’ self- regulation has predicted
their understanding of emotion 2 years later
(Schultz, Izard, Ackerman, & Youngstrom,
2001), and emotion understanding mediates
the relation of self- regulation to adaptive
social behavior (Izard, Schultz, Fine, Young-
strom, & Ackerman, 1999). It is also likely
that emotion understanding fosters, as well
as stems from, emotion- related regulation.
Denham and Burton (2003) suggested that
emotion understanding gives children labels
for their internal feelings, which can then be
made conscious. Such conscious emotional
awareness allows children to immediately
attach feelings to events, which can then
facilitate successful and appropriate regula-
tion. Researchers have found that children
who are able to understand and communi-
cate about emotions, and who know how to
manage emotions, are better able to regulate
themselves (Denham & Burton, 2003).
With the development of EF, children also
improve on theory of mind (ToM) tasks,
which involve an understanding of others’
internal mental representations. Researchers
have identified inhibitory control as espe-
cially relevant to the development of ToM
because it enables children to suppress irrele-
vant perspectives (Carlson, Moses, & Clax-
ton, 2004). In a number of studies using a
variety of observed and reported measures,
inhibitory control has been positively related
to ToM skills in preschool and kindergar-
ten children (Blair & Razza, 2007; Carlson
et al., 2004). Moreover, inhibitory control
appears to precede the development of ToM
in the preschool years (e.g., Flynn, 2007). In
brief, self- regulated children appear to have
an advantage in regard to emotional under-
standing and ToM skills.
Maladjustment
Children with adjustment problems tend to
have difficulties managing and controlling
their attention, behavior, and emotions in a
willful and flexible manner. These difficul-
ties can result in processing information and
modulating emotions and behaviors in ways
that hinder adaptive functioning (Eisenberg,
Spinrad, et al., 2010). In addition, because
children with high EC are probably better
able to avoid negative interactions and mod-
ulate negative emotions such as sadness and
anger, it is not surprising that low EC has
been related to maladjustment (for reviews,
see Eisenberg et al., 2011; Eisenberg, Spin-
rad, et al., 2010).
Consistent with our heuristic model,
many investigators have found negative rela-
tions between EC and externalizing prob-
lems in preschoolers, elementary schoolchil-
dren, and adolescents (Eisenberg, Spinrad,
et al., 2010). For example, Kochanska and
Knaack (2003) reported negative associa-
tions between EC (assessed with a battery
of behavioral measures) and externalizing
problems, concurrently and across time,
164 DEVELOPMENTAL CONSIDERATIONS
in young children (Kochanska & Knaack,
2003). In multiple longitudinal samples,
Eisenberg and colleagues have found nega-
tive relations of teacher- reported, parent-
reported, and observed measures of EC
with children’s externalizing behaviors
(Eisenberg et al., 2011; Eisenberg, Spinrad,
et al., 2010). For example, Eisenberg et al.
(2009) found that change in at-risk elemen-
tary schoolchildren’s externalizing over the
4 years— whether pure externalizing prob-
lems or co- occurring with internalizing
problems— was related to change in EC over
the same period. Such associations appear to
continue throughout adolescence, including
in European samples (Hofer et al., 2010). For
example, Bakker, Ormel, Verhulst, and Old-
ehinkel (2011) found that parent- reported
EC at age 13½ appeared to buffer Dutch
adolescents against externalizing problems
at age 16 in families with high adversity.
EC also appears to act as a mediator or
moderator when predicting maladjustment
(e.g., Hofer et al., 2010; Spinrad et al., 2007).
For example, Hardaway, Wilson, Shaw, and
Dishion (2012) found that inhibitory control
at age 4 mediated the positive and negative
relations, respectively, between chaos in
the home and positive parental behavior at
age 3 to externalizing behaviors at age 5½.
Moreover, EC appears to reduce the positive
relation of inconsistent discipline and physi-
cal punishment to 8- to 12-year-olds’ exter-
nalizing problems (Lengua, 2008) and the
relation of adolescents’ proneness to frustra-
tion to externalizing problems (Oldehinkel,
Hartman, Ferdinand, Verhulst, & Ormel,
2007).
Theoretically, as delineated in the afore-
mentioned model, reactive control and low
EC are related to internalizing problems:
Children with internalizing problems are
often overcontrolled and struggle with the
flexible control of their emotions, cognition,
attention, and behaviors. Although the lit-
erature is not highly consistent (Eisenberg,
Spinrad, et al., 2010), a number of studies
support a negative relation between EC and
internalizing problems. For example, moth-
ers’ reports of preschoolers’ attention focus-
ing and inhibitory control have been mod-
estly, negatively related with internalizing
problems at 5½ years old, even when items
that overlapped between the constructs of
self- regulation and internalizing problems
were removed from the scales (Lemery,
Essex, & Smider, 2002). Similarly, negative
relations between EC and depressive symp-
toms have been reported for 11- to 18-year-
olds (Verstraeten, Vasey, Raes, & Bijttebier,
2008). Moreover, EC seems to moderate the
relation between affectivity and internaliz-
ing problems. In one study, adolescents’ low
positive and high negative affectivity pre-
dicted more depressive symptoms for youth
with low EC (Verstraeten et al., 2008); in
another study, EC buffered the relation of
fearfulness to adolescents’ internalizing
problems (Oldehinkel et al., 2007).
School‑Related Outcomes
The role of EC in school- related outcomes
has aroused increasing interest (Eisenberg,
Valiente, & Eggum, 2010; Ursache et al.,
2012), and both direct and indirect rela-
tions between EC and school- related out-
comes have been predicted. Consistent with
our heuristic model, because children high
in EC are expected to manage their atten-
tion, behavior, and emotion flexibly, they
are predicted to have an advantage when
learning. Moreover, the effects of EC on
academic outcomes are hypothesized to be
mediated by children’s adjustment, quality
relationships with peers and teachers, and
school engagement (Eisenberg, Valiente, et
al., 2010).
In fact, EC predicts a variety of school-
related outcomes, both academic and social,
concurrently and longitudinally (Allan &
Lonigan, 2011; Eisenberg, Valiente, et al.,
2010; Ursache et al., 2012). In recent work,
mediators of these relations have been exam-
ined. For example, in a 1-year longitudinal
study with 3- to five-year-olds, Silva et al.
(2011) found that a high- quality teacher–
child relationship midyear (i.e., low conflict
and high closeness) mediated the positive
relation between high EC measured early in
the year and positive school attitudes mea-
sured at the end of the year with reports
of school liking and avoidance. In another
study with multiple reporters, high social
competence and low externalizing prob-
lems at ages 6–10 fully mediated the positive
relation between high EC at ages 4–8 and
high academic achievement at ages 10–14
(Valiente et al., 2011). In brief, optimally
regulated children tend to have better school
Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 165
outcomes, and these relations appear to be
both direct and indirect.
Internalization of Parental Rules/
Demands and Guilt
EC and related measures of emotion- related
regulation or control have also been linked
to a variety of measures of moral develop-
ment, including compliance, internalization
of rules, and conscience. Committed com-
pliance—when children appear to comply
willingly and even enthusiastically with
adults’ expectations/requests—is thought to
reflect children’s rudimentary internaliza-
tion of their mothers’ commands. In addi-
tion, when toddlers comply with their moth-
ers’ rules when left alone, they are believed
to have internalized the rules (Kochanska,
Coy, & Murray, 2001).
Toddlers who exhibit more committed
compliance tend to have higher EC than
their less compliant peers (e.g., Kochanska
et al., 2001). In addition, EC at 30 and 42
months predicts higher committed compli-
ance 1 year later, even after researchers con-
trol for earlier levels of committed compli-
ance (although these findings did not hold
in models accounting for time- invariant
covariates; Spinrad et al., 2012). Infants’
attention regulation (components of EC)
predicts later committed compliance (Hill &
Braungart- Rieker, 2002; Kochanska et al.,
1998). In addition, Kochanksa and Knaack
(2003) have found a positive relation, often
across time, between EC and the develop-
ment of conscience (i.e., moral self, refrains
from cheating on a task, and moral reason-
ing) during the toddler, preschool, and early
school years (Kochanska & Knaack, 2003).
There are few data on the relation between
conscience and EC in schoolchildren. How-
ever, parent reports of children’s EC have
been related to reports of their 7-year-olds’
guilt (Rothbart, Ahadi, & Hershey, 1994).
More research is needed to clarify the rela-
tion of EC to conscience and other aspects
of moral development in older elementary
school- age children and adolescents.
Empathy/Sympathy
and Prosocial Behavior
Eisenberg and Fabes (1992) hypothesized
that individuals high in effortful emotion-
related self- regulation tend to experience
sympathy (an other- oriented response to
another’s emotion or condition) rather than
personal distress (i.e., a self- focused, aver-
sive response to another’s emotional state or
condition). Personal distress is believed to be
associated with empathic overarousal (pos-
sibly due to underregulation), which in turn
can lead to a self-focus and self- concerned
behavioral response (rather than concern for
others; see Eisenberg, Wentzel, & Harris,
1998). Consistent with this premise, Guthrie
et al. (1997) found that children rated high
on EC exhibited greater facial sadness (but
not distress)—presumed to reflect sympa-
thy/empathy— during an empathy- inducing
film than did less regulated peers. Children’s
postfilm reports of sadness and sympathy
during the film were also positively related
to parents’ ratings of EC. Conversely, chil-
dren low in parent- rated EC were prone to
experience personal distress (e.g., anxiety
and tension) during the film.
Children’s EC (or aspects thereof) also has
been positively related to self- or other- report
measures of dispositional empathy/sympathy
in children (Eisenberg et al., 1998; Panfile
& Laible, 2012; Rothbart et al., 1994; also
see Eisenberg, Fabes, & Spinrad, 2006). In
an 8-year longitudinal study, relatively high
levels of EC and growth in EC were related
to high sympathy, although this pattern was
somewhat stronger for boys than for girls
(Eisenberg et al., 2007). Similar concurrent
relations have been found in studies in which
adults reported on their own sympathy and
regulation, although sometimes the associa-
tion was not significant until the effects of
individual differences in negative emotion-
ality were controlled (see Eisenberg et al.,
1998). In addition, EC has been negatively
related to children’s and adults’ reports of
personal distress (Bandstra, Chambers,
McGrath, & Moore, 2011; see Eisenberg et
al., 1998, 2006).
Consistent with the empirical relation
between EC and sympathy, adults’ ratings of
elementary school children’s effortful atten-
tional control and/or a behavioral measure
of persistence have been correlated with
peers’ ratings (e.g., Eisenberg et al., 1997)
or teachers’ ratings (Diener & Kim, 2004)
of prosocial behavior. Thus, people who
are skilled at regulating their emotion and
behavior are not only more likely to feel con-

166 DEVELOPMENTAL CONSIDERATIONS
cern for others but also they are relatively
likely to help them.
Conclusions and Future Directions
The study of emotion regulation and related
processes has progressed tremendously in
the last decade. Yet definitional and meth-
odological issues need to be addressed, and
there are gaps in the literature, as well as
new directions to exploit.
Terminology
Currently, there is considerable variation in
what behavioral scientists mean when they
use words such as regulation, self- regulation,
and emotion regulation. The term regulation
is used so broadly by different investigators
that it is often hard to understand what is
meant. Even though there may be somewhat
of a continuum, we suggest that researchers
clearly distinguish volitional self- regulatory
processes (e.g., EC) from other, less voli-
tional processes that also “regulate” behav-
ior (e.g., reactive control). It is also impor-
tant for researchers to distinguish between
self- regulation and external regulation.
Distinguishing between EC and EF
Despite the fact that EF and EC typically
have been examined in two separate litera-
tures, researchers have begun to recognize
that there is considerable overlap between
these constructs (Eisenberg & Zhou, in
press). The integration of these two bodies
of knowledge has great potential to be mutu-
ally informative. For example, considerable
attention has been given to the factor struc-
ture of EF (Garon et al., 2008), whereas sim-
ilar work on EC is only now beginning (e.g.,
Allan & Lonigan, 2011; Sulik et al., 2010).
The conceptualization of EF as compris-
ing interrelated but independent abilities—
working memory, response inhibition, and
set shifting— has led to the understanding
that task performance may be differentially
related to these abilities and consequently to
a more fine- grained analysis of what indi-
vidual tasks are measuring (Best & Miller,
2010). For example, working memory, which
is considered an important aspect of EF but
not EC, has been examined as a contribu-
tor to performance on EF tasks. In contrast,
working memory has generally not been
considered a contributor to performance on
measures of EC, even though it likely plays an
important role for very young children and
in tasks with more complex working mem-
ory demands. Finally, researchers studying
EF have made the distinction between hot
(i.e., emotional, for example, in delay tasks)
and cold (i.e., cognitive, for example, in typi-
cal EF measures such as flanker or Stroop
tasks) aspects of EF, which may have impor-
tant implications for emotion- related self-
regulation (e.g., Willoughby, Kupersmidt,
Voegler- Lee, & Bryant, 2011). However, we
would argue that it is not EF that is hot or
cold, but that EF may function somewhat
differently in hot and cold contexts (Eisen-
berg & Zhou, in press; see also Kim, Nor-
dling, Yoon, Boldt, & Kochanska, 2013).
Researchers studying EF can also benefit
from theoretical distinctions made in the
EC literature. For example, temperament
researchers have begun to study the devel-
opment and correlates of activational con-
trol, which is the ability to activate behavior
willfully. This is an aspect of EC that has
no corresponding construct in the field of EF
but merits further study due to the impor-
tance of up- regulating emotional reactions
in certain situations— for example, show-
ing gratitude when receiving a disappointing
gift—as well as its likely role in persistence
on unpleasant tasks and the achievement of
long-term goals.
Design of Research Studies
There is growing recognition of the bidi-
rectional nature of the relations between
temperament and environmental influences.
Researchers have come to understand that
although temperament has a biological
basis, it is also susceptible to environmental
influences. This may be especially true for
EC, which develops more slowly than more
emotionally reactive aspects of tempera-
ment. Conversely, there is also evidence that
children’s characteristics may affect parent-
ing behavior (e.g., Eisenberg, Vidmar, et al.,
2010). Longitudinal designs in which tem-
poral hypotheses can be tested are becoming
more common and can help to clarify the
degree to which the early environment influ-
ences children’s temperament and vice versa.
Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 167
Although such research designs have clear
strengths, even well- designed correlational
studies cannot definitively test causal rela-
tions. There is still a need for experimental
studies, such as intervention and prevention
trials that promote self- regulatory skills in
children, to determine how these skills are
causally related to self- regulation processes
and other child outcomes. School-based
prevention trials have demonstrated effects
on self- regulation in low- income preschool-
ers (Diamond, Barnett, Thomas, & Munro,
2007), but more research is needed to deter-
mine whether gains are maintained over long
periods of time, the extent to which increases
in EC are causally related to a number of dif-
ferent child outcomes, whether such effects
can be achieved in other age groups, and
whether the intervention effects generalize
to other populations (e.g., middle- income
children or children in other cultures). Simi-
larly, experimental studies that modify par-
enting behavior are needed to understand
the causal effects of parenting on the devel-
opment of EC.
Genetically informed research designs
could also contribute to an understanding
of the influences on, and effects of, chil-
dren’s self- regulation. Not only can twin,
adoption, or sibling studies be used to assess
the role of genetics and environment in self-
regulation (see Rothbart & Bates, 2006), but
they also can be used to assess environmen-
tal influences, especially when genetic influ-
ences are held constant. For example, one
could examine factors related to differences
between identical twins (who are genetically
the same) in EC. A relatively new and prom-
ising area of research is the use of measured
genes in studies of EC (Kochanska, Kim,
Barry, & Philibert, 2011; Voelker, Sheese,
Rothbart, & Posner, 2009). More work is
needed in this area to determine whether
such effects are replicable, but such work
may help investigators target interventions
and understand the biological mechanisms
underlying the development of emotion and
self- regulation.
The Measurement of Emotion‑Related
Self‑Regulation
It is now common for studies of self-
regulation to include a number of behavioral
measures, allowing researchers to study
the relations among tasks and to measure
more accurately the underlying construct.
However, a limitation of the majority of
these measures is that performance quickly
reaches a ceiling in early school- age chil-
dren. One challenge for EC researchers is to
establish measures that can be used across
a relatively wide age range to establish con-
tinuity in measurement. In particular, mea-
sures appropriate for adolescents and adults
are needed, and it would be advantageous if
these measures also could be used with chil-
dren. There is now evidence that it is pos-
sible to develop tasks appropriate for a wide
age range, including adolescents and adults,
making it possible to measure more accu-
rately the development of EC beyond early
childhood (Lagattuta, Sayfan, & Monsour,
2011; Tottenham, Hare, & Casey, 2011).
Although there has been recent progress
in this area, further work is still needed
because a single laboratory measure of EC
is relatively unreliable, and a battery of
tasks is recommended in order to assess the
construct more accurately (Kochanska &
Knaack, 2003).
Psychophysiological methods have been
underutilized in the existing research, even
though it is possible to assess many psycho-
physiological variables inexpensively and
noninvasively. Of particular interest for
the assessment of EC is respiratory sinus
arrhythmia (RSA), a cardiac measure of
parasympathetic nervous system function.
There is now a consistent body of evidence
linking resting RSA to EC (e.g., Marcovitch
et al., 2010). RSA reactivity— a change in
RSA in response to task demands— has also
been found in some studies to correlate with
EC and EF, but the exact nature of this rela-
tion is still not clear. Although RSA reactiv-
ity may also be a promising as a means of
assessing emotion- related regulation, fur-
ther research is needed to determine how
task demands relate to the interpretation of
this measure (Obradović, Bush, & Boyce,
2011).
Process Models of Emotion‑Related
Self‑Regulation
Finally, there is a need to understand how EC
supports emotion- related self- regulation. EC
likely plays a supporting role in a wide vari-
ety of emotion regulation strategies, includ-

168 DEVELOPMENTAL CONSIDERATIONS
ing reappraisal, attentional strategies such as
distraction or avoidance, choosing or modi-
fying situations, and inhibiting emotionally
motivated behavior. Although there is ample
evidence that EC is related to successful
emotion regulation, we know relatively little
about the extent to which the effectiveness
of specific emotion regulation strategies is
dependent on individual differences in EC,
or whether specific aspects of EC such as
activational control, inhibitory control, and
attention focusing are differentially related
to emotion- related self- regulation.
Conclusion
In summary, although the study of children’s
self- regulation is flourishing, there is much
yet to be explored and understood. A multi-
method approach, influenced by conceptual
work in developmental, clinical, personality,
social, and neurophysiological psychology,
including greater integration of research and
theory in the adult and child literatures, is
likely to be productive in future attempts to
expand our knowledge.
Acknowledgments
Work on this chapter was supported by grants
from the National Institute of Child Health and
Human Development to Nancy Eisenberg, Tracy
L. Spinrad, and Carlos Valiente.
References
Aksan, N., & Kochanska, G. (2004). Links
between systems of inhibition from infancy
to preschool years. Child Development, 75,
1477–1490.
Allan, N. P., & Lonigan, C. J. (2011). Examin-
ing the dimensionality of effortful control in
preschool children and its relation to academic
and socioemotional indicators. Developmen-
tal Psychology, 47, 905–915.
Bakker, M. P., Ormel, J., Verhulst, F. C., &
Oldehinkel, A. J. (2011). Adolescent family
adversity and mental health problems: The
role of adaptive self- regulation capacities: The
TRAILS study. Journal of Abnormal Child
Psychology, 39, 341–350.
Bandstra, N. F., Chambers, C. T., McGrath,
P. J., & Moore, C. (2011). The behavioural
expression of empathy to others’ pain versus
others’ sadness in young children. PAIN, 152,
1074–1082.
Best, J. R., & Miller, P. H. (2010). A develop-
mental perspective on executive function.
Child Development, 81, 1641–1660.
Blair, C., & Razza, R. P. (2007). Relating effort-
ful control, executive function, and false belief
understanding to emerging math and literacy
ability in kindergarten. Child Development,
78, 647–663.
Block, J. H., & Block, J. (1980). The role of ego-
control and ego- resiliency in the organization
of behavior. In W. A. Collins (Ed.), Minne-
sota Symposia on Child Psychology (Vol. 13,
pp. 39–101). Hillsdale, NJ: Erlbaum.
Carlson, S. M., Moses, L. J., & Claxton, L. J.
(2004). Individual differences in executive
functioning and theory of mind: An investiga-
tion of inhibitory control and planning ability.
Journal of Experimental Child Psychology,
87, 299–319.
Carver, C. S. (2005). Impulse and constraint:
Perspectives from personality psychology,
convergence with theory in other areas, and
potential for integration. Personality and
Social Psychology Review, 9, 312–333.
Compas, B. E., Connor-Smith, J. K., Saltzman,
H., Thomsen, A. H., & Wadsworth, M. E.
(2001). Coping with stress during childhood
and adolescence: Problems, progress, and
potential in theory and research. Psychologi-
cal Bulletin, 127, 87–127.
Crockenberg, S. C., & Leerkes, E. M. (2004).
Infant and maternal behaviors regulate infant
reactivity to novelty at 6 months. Develop-
mental Psychology, 40, 1123–1132.
Denham, S. A., & Burton, R. (2003). Social and
emotional prevention and intervention pro-
gramming for preschoolers. New York: Klu-
wer Academic/Plenum Press.
Derryberry, D., & Rothbart, M. K. (1997). Reac-
tive and effortful processes in the organization
of temperament. Development and Psychopa-
thology, 9, 633–652.
Diamond, A. (1991). Neuropsychological insights
into the meaning of object concept develop-
ment. In S. Carey & R. Gelman (Eds.), The
epigenesis of mind: Essays on biology and cog-
nition (pp. 67–110). Hillsdale, NJ: Erlbaum.
Diamond, A., Barnett, W. S., Thomas, J., &
Munro, S. (2007). The early years: Preschool
program improves cognitive control. Science,
318, 1387–1388.
Self-Regulation, Effortful Control, and Their Socioemotional Correlates 169
Diener, M. L., & Kim, D. (2004). Maternal and
child predictors of preschool children’s social
competence. Journal of Applied Developmen-
tal Psychology, 25, 3–24.
Diener, M. L., & Mangelsdorf, S. C. (1999).
Behavioral strategies for emotion regulation in
toddlers: Associations with maternal involve-
ment and emotional expressions. Infant
Behavior and Development, 22, 569–583.
Eisenberg, N., Edwards, A., Spinrad, T. L., Sal-
lquist, J., Eggum, N. D., & Reiser, M. (2013,
February 11). Are effortful and reactive con-
trol unique constructs in young children?
Developmental Psychology.
Eisenberg, N., & Fabes, R. A. (1992). Emotion,
regulation, and the development of social
competence. In M. S. Clark (Ed.), Review of
personality and social psychology. Vol 14.
Emotion and Social Behavior (pp.
119–150).
Newbury Park, CA: Sage Publications.
Eisenberg, N., Fabes, R. A., & Spinrad, T. L.
(2006). Prosocial development. In W. Damon
& R. M. Lerner (Series Eds.), & N. Eisenberg
(Vol. Ed.), Handbook of child psychology, Vol
3. Social, emotional, and personality develop-
ment (pp.
646–718). New York: Wiley.
Eisenberg, N., Guthrie, I. K., Fabes, R. A., Reiser,
M., Murphy, B. C., Holgreen, R., et al. (1997).
The relations of regulation and emotionality
to resilience and competent social functioning
in elementary school children. Child Develop-
ment, 68, 295–311.
Eisenberg, N., Michalik, N., Spinrad, T. L.,
Hofer, C., Kupfer, A., Valiente, C., et al.
(2007). Relations of effortful control and
impulsivity to children’s sympathy: A longi-
tudinal study. Cognitive Development, 22,
54 4 –567.
Eisenberg, N., & Morris, A. S. (2002). Children’s
emotion-
r
elated regulation. In R. Kail (Ed.),
Advances in child development and behavior
(pp.
190–229). Amsterdam: Academic Press.
Eisenberg, N., Smith, C. L., & Spinrad, T. L.
(2011). Effortful control: Relations with emo-
tion regulation, adjustment, and socializa-
tion in childhood. In R. F. Baumeister & K.
D. Vohs (Eds.), Handbook of self-
r
egulation:
Research, theory, and applications (pp.
263–
283). New York: Guilford Press.
Eisenberg, N., Spinrad, T. L., & Eggum, N. D.
(2010). Emotion-
related self- regulation and its
relation to children’s maladjustment. Annual
Review of Clinical Psychology, 6, 495–525.
Eisenberg, N., Spinrad, T. L., Fabes, R. A., Rei-
ser, M., Cumberland, A., Shepard, S. A., et al.
(2004). The relations of effortful control and
impulsivity to children’s resiliency and adjust-
ment. Child Development, 75, 25–46.
Eisenberg, N., Valiente, C., & Eggum, N. D.
(2010). Self-
r
egulation and school readiness.
Early Education and Development, 21, 681–
698.
Eisenberg, N., Valiente, C., Spinrad, T. L., Cum-
berland, A., Liew, J., Reiser, M., et al. (2009).
Longitudinal relations of children’s effortful
control, impulsivity, and negative emotional-
ity to their externalizing, internalizing, and
co-
o
ccurring behavior problems. Develop-
mental Psychology, 45, 988–1008.
Eisenberg, N., Vidmar, M., Spinrad, T. L.,
Eggum, N. D., Edwards, A., Gaertner, B., et
al. (2010). Mothers’ teaching strategies and
children’s effortful control: A longitudinal
study. Developmental Psychology, 46, 1294–
1308.
Eisenberg, N., Wentzel, M., & Harris, J. D.
(1998). The role of emotionality and regula-
tion in empathy-
r
elated responding. School
Psychology Review, 27, 506–521.
Eisenberg, N., & Zhou, Q. (in press). Concep-
tions of executive functioning and regulation:
When and to what degree do they overlap? In J.
A. Griffin, L. S. Freund, & P. McCardle (Eds.),
Executive function in preschool age children:
Integrating measurement, neurodevelopment
and translational research. Washington, DC:
American Psychological Association.
Flynn, E. (2007). The role of inhibitory control
in false belief understanding. Infant and Child
Development, 16, 53–69.
Garon, N., Bryson, S. E., & Smith, I. M. (2008).
Executive function in preschoolers: A review
using an integrative framework. Psychological
Bulletin, 134, 31–60.
Grolnick, W. S., Bridges, L. J., & Connell, J. P.
(1996). Emotion regulation in two-year-olds:
Strategies and emotional expression in four
contexts. Child Development, 67, 928.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp.
3–20). New York:
Guilford Press.
Guthrie, I. K., Eisenberg, N., Fabes, R. A., Mur-
phy, B. C., Holmgren, R., Mazsk, P., et al.
(1997). The relations of regulation and emo-
tionality to children’s situational empathy-
r
elated responding. Motivation and Emotion,
21, 87–108.
Hardaway, C. R., Wilson, M. N., Shaw, D. S., &
170 DEVELOPMENTAL CONSIDERATIONS
Dishion, T. J. (2012). Family functioning and
externalizing behaviour among low- income
children: Self- regulation as a mediator. Infant
and Child Development, 21, 67–84.
Hill, A. L., & Braungart- Rieker, J. M. (2002).
Four-month attentional regulation and its pre-
diction of three-year compliance. Infancy, 3,
261–273.
Hofer, C., Eisenberg, N., & Reiser, M. (2010).
The role of socialization, effortful control,
and ego resiliency in French adolescents’ social
functioning. Journal of Research on Adoles-
cence, 20, 555–582.
Izard, C. E., Schultz, D., Fine, S. E., Youngstrom,
E., & Ackerman, B. P. (1999). Temperament,
cognitive ability, emotion knowledge, and
adaptive social behavior. Imagination, Cogni-
tion and Personality, 19(4), 305–330.
Kagan, J., & Fox, N. A. (2006). Biology, culture,
and temperamental biases. In W. Damon & R.
M. Lerner (Series Eds.) & N. Eisenberg (Vol.
Ed.), Handbook of child psychology: Vol. 3.
Social, emotional, and personality develop-
ment (6th ed., pp. 167–225). Hoboken, NJ:
Wiley.
Kim, S., Nordling, J. K., Yoon, J. E., Boldt, L. J.,
& Kochanska, G. (2013). Effortful control in
“hot” and “cool” tasks differentially predicts
children’s behavior problems and academic
performance. Journal of Abnormal Child Psy-
chology, 41(1), 43–56.
Kochanska, G., Coy, K. C., & Murray, K. T.
(2001). The development of self- regulation in
the first four years of life. Child Development,
72, 1091–1111.
Kochanska, G., Coy, K. C., Tjebkes, T. L., &
Husarek, S. J. (1998). Individual differences in
emotionality in infancy. Child Development,
69, 375–390.
Kochanska, G., Kim, S., Barry, R. A., & Philib-
ert, R. A. (2011). Children’s genotypes inter-
act with maternal responsive care in predict-
ing children’s competence: Diathesis– stress or
differential susceptibility? Development and
Psychopathology, 23, 605–616.
Kochanska, G., & Knaack, A. (2003). Effort-
ful control as a personality characteristic of
young children: Antecedents, correlates, and
consequences. Journal of Personality, 71,
1087–1112.
Kochanska, G., Murray, K. T., & Harlan, E. T.
(2000). Effortful control in early childhood:
Continuity and change, antecedents, and
implications for social development. Develop-
mental Psychology, 36, 220–232.
Lagattuta, K. H., Sayfan, L., & Monsour, M.
(2011). A new measure for assessing execu-
tive function across a wide age range: Chil-
dren and adults find happy–sad more difficult
than day–night. Developmental Science, 14,
481–489.
Lemery, K. S., Essex, M. J., & Smider, N. A.
(2002). Revealing the relation between tem-
perament and behavior problem symptoms
by eliminating measurement confounding:
Expert ratings and factor analyses. Child
Development, 73, 867–882.
Lengua, L. J. (2008). Anxiousness, frustration,
and effortful control as moderators of the
relation between parenting and adjustment in
middle- childhood. Social Development, 17,
554 –577.
Lengua, L. J., Honorado, E., & Bush, N. R.
(2007). Contextual risk and parenting as pre-
dictors of effortful control and social compe-
tence in preschool children. Journal of Applied
Developmental Psychology, 28, 40–55.
Liew, J., Eisenberg, N., Spinrad, T. L., Eggum,
N. D., Haugen, R. G., Kupfer, A., et al. (2011).
Physiological regulation and fearfulness as
predictors of young children’s empathy- related
reactions. Social Development, 20, 111–134.
Mangelsdorf, S. C., Shapiro, J. R., & Marzolf,
D. (1995). Developmental and temperamental
differences in emotion regulation in infancy.
Child Development, 66, 1817–1828.
Marcovitch, S., Leigh, J., Calkins, S. D., Leerks,
E. M., O’Brien, M., & Blankson, A. N. (2010).
Moderate vagal withdrawal in 3.5-year-old
children is associated with optimal perfor-
mance on executive function tasks. Develop-
mental Psychobiology, 52, 603–608.
Mintz, T. M., Hamre, B. K., & Hatfield, B. E.
(2011). The role of effortful control in medi-
ating the association between maternal sen-
sitivity and children’s social and relational
competence and problems in first grade. Early
Education and Development, 22, 360–387.
Mischel, W., & Ayduk, O. (2011). Willpower in
a cognitive- affective processing system: The
dynamics of delay of gratification. In K. D.
Vohs & R. F. Baumeister (Eds.), Handbook of
self- regulation: Research, theory, and applica-
tions (2nd ed., pp. 83–105). New York: Guil-
ford Press.
Murphy, B. C., Eisenberg, N., Fabes, R. A.,
Shepard, S., & Guthrie, I. K. (1999). Consis-
tency and change in children’s emotionality
and regulation: A longitudinal study. Merill–
Palmer Quarterly, 46, 413–444.
Self‑Regulation, Effortful Control, and Their Socioemotional Correlates 171
Obradović, J., Bush, N. R., & Boyce, W. T.
(2011). The interactive effect of marital con-
flict and stress reactivity on externalizing and
internalizing symptoms: The role of labora-
tory stressors. Development and Psychopa-
thology, 23, 101–114.
Oldehinkel, A. J., Hartman, C. A., Ferdinand,
R. F., Verhulst, F. C., & Ormel, J. (2007).
Effortful control as modifier of the association
between negative emotionality and adoles-
cents’ mental health problems. Development
and Psychopathology, 19, 523–539.
Panfile, T. M., & Laible, D. J. (2012). Attach-
ment security and child’s empathy: The medi-
ating role of emotion regulation. Merrill–
Palmer Quarterly, 58, 1–21.
Posner, M. I., & Rothbart, M. K. (2000). Devel-
oping mechanisms of self- regulation. Devel-
opment and Psychopathology, 12, 427–441.
Rothbart, M. K. (2011). Becoming who we are:
Temperament and personality in develop-
ment. New York: Guilford Press.
Rothbart, M. K., Ahadi, S. A., & Hershey, K.
L. (1994). Temperament and social behavior
in childhood. Merill–Palmer Quarterly, 40,
21–39.
Rothbart, M. K., Ahadi, S. A., Hershey, K., &
Fisher, P. (2001). Investigations of tempera-
ment at three to seven years: The children’s
behavior questionnaire. Child Development,
72, 1394–1408.
Rothbart, M. K., & Bates, J. E. (2006). Tempera-
ment. In W. Damon & R. M. Lerner (Series
Eds.) & N. Eisenberg (Vol. Ed.), Handbook
of child psychology: Vol. 3. Social, emo-
tional, and personality development (6th ed.,
pp. 99–166). Hoboken, NJ: Wiley.
Ruff, H. A., & Rothbart, M. K. (1996). Atten-
tion in early development: Themes and varia-
tions. London: Oxford University Press.
Schultz, D., Izard, C. E., Ackerman, B. P., &
Youngstrom, E. A. (2001). Emotion knowl-
edge in economically disadvantaged children:
Self- regulatory antecedents and relations to
social difficulties and withdrawal. Develop-
ment and Psychopathology, 13, 53 – 67.
Silva, K. M., Spinrad, T. L., Eisenberg, N., Sulik,
M. J., Valiente, C., Huerta, S., et al. (2011).
Relations of children’s effortful control and
teacher– child relationship quality to school
attitudes in a low- income sample. Early Edu-
cation and Development, 22, 434–460.
Spinrad, T. L., Eisenberg, N., Cumberland, A.,
Fabes, R. A., Valiente, C., Shepard, S., et al.
(2006). Relation of emotion- related regulation
to children’s social competence: A longitudinal
study. Emotion, 6, 498–510.
Spinrad, T. L., Eisenberg, N., Gaertner, B., Popp,
T., Smith, C. L., Kupfer, A., et al. (2007). Rela-
tions of maternal socialization and toddlers’
effortful control to children’s adjustment and
social competence. Developmental Psychol-
ogy, 43, 1170–1186.
Spinrad, T. L., Eisenberg, N., Silva, K. M.,
Eggum, N. D., Reiser, M., Edwards, A., et al.
(2012). Longitudinal relations among mater-
nal behaviors, effortful control and young
children’s committed compliance. Develop-
mental Psychology, 48, 552–566.
Stifter, C. A., & Braungart, J. M. (1995). The
regulation of negative reactivity in infancy:
Function and development. Developmental
Psychology, 31, 448–455.
Sulik, M. J., Huerta, S., Zerr, A. A., Eisenberg,
N., Spinrad, T. L., Valiente, C., et al. (2010).
The factor structure of effortful control and
measurement invariance across ethnicity and
sex in a high-risk sample. Journal of Psychopa-
thology and Behavioral Assessment, 32, 8–22.
Tottenham, N., Hare, T. A., & Casey, B. J.
(2011). Behavioral assessment of emotion
discrimination, emotion regulation, and cog-
nitive control in childhood, adolescence, and
adulthood. Frontiers in Developmental Psy-
chology, 2, 1–9.
Ursache, A., Blair, C., & Raver, C. C. (2012).
The promotion of self- regulation as a means of
enhancing school readiness and early achieve-
ment in children at risk for school failure.
Child Development Perspectives, 6, 122–128.
Valiente, C., Eisenberg, N., Haugen, R., Spinrad,
T. L., Hofer, C., Liew, J., et al. (2011). Chil-
dren’s effortful control and academic achieve-
ment: Mediation through social functioning.
Early Education and Development, 22, 411–
433.
Valiente, C., Eisenberg, N., Smith, C. L., Reiser,
M., Fabes, R. A., Losoya, S., et al. (2003).
The relations of effortful control and reactive
control to children’s externalizing problems: A
longitudinal assessment. Journal of Personal-
ity, 71, 1172–1196.
Verstraeten, K., Vasey, M. W., Raes, F., & Bijt-
tebier, P. (2008). Temperament and risk for
depressive symptoms in adolescence: Media-
tion by rumination and moderation by effort-
ful control. Journal of Abnormal Child Psy-
chology, 37, 349–361.
Voelker, P., Sheese, B. E., Rothbart, M. K., &
Posner, M. I. (2009). Variations in catechol-
172 DEVELOPMENTAL CONSIDERATIONS
O-methyltransferase gene interact with par-
enting to influence attention in early develop-
ment. Neuroscience, 164, 121–130.
Williams, B. R., Ponesse, J. S., Schachar, R. J.,
Logan, G. D., & Tannock, R. (1999). Develop-
ment of inhibitory control across the life span.
Developmental Psychology, 35, 205–213.
Willoughby, M. T., Kupersmidt, J., Voegler- Lee,
M., & Bryant, D. (2011). Contributions of hot
and cool self- regulation to preschool disruptive
behavior and academic achievement. Develop-
mental Neuropsychology, 36, 162–180.
Willoughby, M. T., Wirth, R. J., Blair, C. B.,
& Family Life Project Investigators. (2012).
Executive function in early childhood: Longi-
tudinal measurement invariance and develop-
mental change. Psychological Assessment, 24,
418–431.
Zhou, Q., Eisenberg, N., Wang, Y., & Reiser,
M. (2004). Chinese children’s effortful con-
trol and dispositional anger/frustration: Rela-
tions to parenting styles and children’s social
functioning. Developmental Psychology, 40,
352–366.

173
The development of emotion regulation is
one of the central goals of early socialization
because of its importance to social compe-
tence, academic achievement, and psycho-
logical well-being (Saarni, 1999). It is also
important to parent– child relationships (Cas-
sidy, 1994). Parents typically overestimate
how much young children can manage their
feelings, especially when their judgments are
compared with what research has shown
about the development of self- regulatory
ability (Newton & Thompson, 2010). In a
recent national survey, for example, many
parents reported that children under the age
of 3 could control their emotions when frus-
trated, and most thought that children of this
age could competently share and take turns
with other children (Newton & Thompson,
2010). This may help to explain why paren-
tal efforts to foster emotional self- control in
offspring are so concerted and multifaceted.
Their efforts include proactive strategies to
manage the everyday emotional demands
that children face, direct interventions to
regulate children’s emotions, parents’ evalu-
ations of children’s emotional displays, sanc-
tions on unregulated emotional displays,
and conversation in which emotional control
strategies are discussed and the benefits of
self- management are described. The social-
ization of emotion regulation in the family is
particularly interesting, therefore, as a pro-
cess of considerable importance to parents
but also as one in which parents’ efforts are
leading, sometimes significantly, children’s
developing behavioral and neurobiological
capacities.
As the study of the development of emo-
tion regulation has expanded, research on
the socialization of emotion regulation has
changed. Emotion regulation was conven-
tionally viewed in terms of the top-down
imposition of cognitive or neurobiological
control on basic emotional processes, and
socialization was perceived primarily as
helping children acquire the understand-
ing, self- control strategies, motivation, and
competence needed to enact these control
processes. In recent years, however, devel-
opmental researchers have recognized that
emotion regulation also involves bottom-
up processes, such as how basic emotion
appraisals originating in limbic structures
influence higher control processes (Thomp-
son, 2011). From this view, therefore, the
socialization of emotion regulation involves
family processes that influence the growth
of emotional reactivity and the demands
this imposes on emotional self- control. This
chapter addresses both top-down and bot-
tom- up facets of the socialization of emo-
tion regulation, because each is important
to how family experiences affect children’s
emotional self- control.
CHAPTER 11
Socialization of Emotion
and Emotion Regulation in the Family
Ross A. Thompson

174 DEVELOPMENTAL CONSIDERATIONS
The chapter begins with a definition of
emotion regulation that highlights its rel-
evance to understanding these socialization
processes. Next, the discussion turns to
family processes that influence bottom- up
aspects of emotion regulation, with partic-
ular attention to experiences of stress that
can shape the neurobiology of emotion. In
the third section, the socialization of emo-
tion regulation as a top-down process is con-
sidered with respect to the diverse parental
influences that affect how children inter-
pret and appraise their feelings, learn about
strategies of emotion management, achieve
competence in controlling their feelings, and
acquire cultural and gender expectations
for emotion regulation (for a more extended
discussion of these influences, see Morris,
Silk, Steinberg, Myers, & Robinson, 2007;
Thompson & Meyer, 2007). In the fourth
section I integrate these perspectives with
respect to the broader security of parent–
child attachment, then conclude the chapter
with final reflections.
Defining Emotion Regulation
Definitions of emotion regulation are built
on broader conceptualizations of emotion
(see Gross, this volume). A developmental
approach to emotion regulation highlights
the changes that occur over time in multiple
components of emotion and their interac-
tion, and how these emotion components
unfold through biological maturation, social
influences, the growth of self- referential
processes, and many other developmental
influences (Thompson, 1990, 1994, 2011).
The following definition of emotion regula-
tion reflects this developmental approach:
Emotion regulation consists of the extrinsic
and intrinsic processes responsible for moni-
toring, evaluating, and modifying emotional
reactions, especially their intensive and tem-
poral features, to accomplish one’s goals
(Thompson, 1994, pp. 27–28). Incorporated
within this definition are several assump-
tions about emotion and emotion regula-
tion.
First, emotion regulation processes target
positive as well as negative emotions and can
entail diminishing, heightening, or simply
maintaining one’s current level of emotional
arousal in particular circumstances. Emo-
tion regulation usually alters the dynamics
of emotion rather than changing its quality.
In other words, individuals alter the inten-
sity, escalation (i.e., latency to onset and rise
time), or duration of an emotional response,
speed its recovery, or reduce or enhance the
lability or range of emotional responding in
particular situations depending on the indi-
vidual’s goals for that situation (Thompson,
1990). We usually think of emotionally well-
regulated people as those who are capable
of altering how long, how intensely, or how
quickly they feel as they do, rather than
transforming the valence of emotion (e.g.,
changing anger into happiness).
Second, consistent with broader under-
standing of emotional development, emotion
regulation can occur through the influence
of other people or through the individual’s
own efforts. Both extrinsic and intrinsic
processes of emotion regulation are impor-
tant. From birth, children’s emotional lives
are managed by others, and this remains so
throughout life. Moreover, extrinsic influ-
ences on emotion regulation can be either
facilitative (i.e., those that assist the child
in emotional self- control) or hindering (i.e.,
those that impair the child’s emotional self-
control), with the latter occurring often in
contexts of family disruption. In addition,
extrinsic influences on emotion regulation
can be either deliberate (i.e., intentionally
managing the child’s emotions) or unin-
tended (i.e., not meant to have the effects on
children’s emotions that they do).
Third, consistent with a functionalist
approach to emotion, strategies of emotion
regulation are rarely inherently optimal or
maladaptive. Rather, emotion regulation
strategies must be evaluated in relation to
the individual’s goals for the situation. This
functionalist orientation is especially impor-
tant for developmental analysis. A toddler’s
petulant crying or an adolescent’s sullenness
may be intuitively interpreted by others as
revealing deficient skills in emotion regu-
lation until one realizes that the toddler’s
crying causes parents to accede and the ado-
lescent’s sullenness causes peers to provide
support, each of which may be the child’s
goal (even if these emotional reactions have
other, negative consequences). It is impor-
tant, however, to recognize that a person
can have multiple goals in a situation requir-
ing emotion regulation (e.g., managing fear
Socialization of Emotion and Emotion Regulation in the Family 175
and defending oneself when in the presence
of a bully). These alternative goals may con-
flict, which further complicates evaluating
whether specific emotion regulation strate-
gies are adaptive.
This functionalist orientation is also
important for understanding emotion regu-
latory processes relevant to developmental
psychopathology. Children with anxiety
disorders are typically viewed as deficient
in emotion regulation, for example, but
their hypervigilance to threatening events,
fear- oriented cognitions, and sensitivity to
internal visceral cues of anxiety are part of
a constellation of self- regulatory strategies
for anticipating and avoiding encounters
with fear- provoking events. In light of their
genetic vulnerability and family processes
that heighten risk for anxious pathology,
these emotion regulatory strategies may be
the most adaptive options available to the
child (Thompson, 2000). Characterizing
such children simply as emotionally dysreg-
ulated seems oversimplified. To be sure, the
same emotion regulatory strategies that pro-
vide immediate relief can exact long-term
costs that make anxious children vulnerable
to continued pathology. This double- edged
sword is typical of emotional regulatory
processes for many forms of developmental
psychopathology (Thompson & Goodman,
2010). Understanding emotion regulation
for children at risk requires comprehend-
ing the emotional goals the child is seeking
to achieve and interpreting his or her self-
regulatory efforts in light of these goals.
Fourth, consistent with a developmental
analysis, emotion regulation includes under-
standing how people monitor and evaluate,
as well as modify, their emotions. Children’s
developing capacities for emotional self-
awareness and for appraising their feelings
in light of personal and cultural expectations
are core features of developing emotion reg-
ulation (Saarni, 1999). Emotion monitoring
and evaluation function nonconsciously as
well as consciously to affect self- regulation.
A preschooler arriving at child care after
witnessing her parents’ morning argument
may not be consciously monitoring how
upset she is, for example, but the argument
might affect her emotional self- control when
she is later provoked by a peer. In this situa-
tion, antecedent emotional arousal creates a
lowered emotional threshold for subsequent
negative arousal that impairs the child’s
emotion management.
Emotion regulation can be studied at
multiple levels of analysis, from genetic and
neurobiological processes to developing cog-
nition and personality, to family processes
and overarching cultural values. A devel-
opmental analysis highlights that although
psychologists tend to regard “emotion regu-
lation” as if it were a single, coherent per-
sonality construct or developmental process,
the growth of emotion regulation is actually
based on a multidimensional network of
loosely allied processes arising from within
and outside the child. Developmental study
of emotion regulation requires that theo-
rists keep the broader view in mind even as
they are studying specific components of the
growth of emotion regulation, such as its
socialization in the family.
The developmental study of emotion
regulation requires distinct methodologi-
cal strategies. Studies of emotion regulation
in adults or adolescents typically rely on
respondent self- report, commonly through
questionnaires, to index individual differ-
ences in emotion self- regulation. Infants
and young children are not very informative
reporters. Thus, developmental researchers
must use other procedures. These include
detailed observations of emotional reac-
tions in carefully structured experimental
situations, often with convergent behavioral
and psychophysiological measures, some-
times accompanied by the reports of moth-
ers and other secondary sources concern-
ing the child’s emotional qualities. These
methods are informative, but behavioral
measures (whether of infants or adults) are
also complicated by the following interpre-
tive difficulties: (1) behavior that reflects the
influence of emotion regulatory processes
is multidetermined; (2) emotional reactions
and emotional regulatory influences are not
easily distinguished behaviorally; and (3)
the situational context can have a profound
effect on children’s emotional reactions
(Cole, Martin, & Dennis, 2004). Studying
emotion regulation in vivo in this manner
is thus conceptually and methodologically
more difficult than enlisting self- report.
Adding further complexity to the study
of emotion regulation is the functional-
ist requirement of understanding the goals
motivating self- regulatory efforts. Although

176 DEVELOPMENTAL CONSIDERATIONS
these goals are usually assumed in studies
of adults (e.g., diminishing negative affect
and enhancing positive emotion), behavioral
studies of emotion regulation with chil-
dren require carefully designed assessments
in which the goals for managing emotion
are either explicit or incorporated into the
design (e.g., observing children as they cope
with a disappointing gift). In short, because
of the special methodological challenges it
presents, developmental research into emo-
tion regulation is not for the fainthearted.
Family Influences on Emotional
Reactivity and Self-Regulation
Two conclusions from recent research have
changed how developmental researchers
understand the growth of emotion regula-
tion (Thompson, 2011; Thompson, Lewis,
& Calkins, 2008). First, as noted earlier,
neurobiological systems that are higher and
lower on the neuroaxis exert mutual regu-
latory influences related to emotion. “Top-
down” regulatory control (e.g., projections
from the prefrontal cortex to the amygdala)
is important, but so is the “bottom- up”
regulatory influence (e.g., from the limbic
system to higher cortical regions). Second,
these higher and lower neurobiological sys-
tems are shaped by the quality of early expe-
rience, particularly family interactions.
The view that emotion regulation involves
bidirectional influences between higher and
lower systems is consistent with current work
in the neuroscience of emotion. Neuroimag-
ing studies show that responses to emotion
tasks are widely distributed throughout the
brain, including areas commonly regarded as
relevant to affective activation (including the
amygdala, hypothalamus, brainstem, and
striatum) and areas often viewed as impor-
tant to emotion regulation (including medial
and lateral prefrontal cortex and anterior
cingulate). Neuroimaging studies show that
cortical and limbic areas are coactive in
responses to emotion probes (Kober et al.,
2008; Ochsner et al., 2009), and that lim-
bic systems exert influence over cortical
systems in affective arousal, as well as the
reverse. Amygdala activation is associated,
for example, with enhanced perceptual sen-
sitivity to cues of danger, consistent with its
role in affective learning and appraisals that
influence higher- order emotion processing
(Barrett & Bar, 2009; Ochsner et al., 2009;
Woltering & Lewis, 2009).
Consistent with the view that early expe-
riences shape brain architecture, research
has shown that experience is important to
the functioning of higher and lower brain
regions relevant to emotion. The best evi-
dence concerns the development of stress
responding, which involves neural systems
shared with emotion. Developmental stud-
ies with humans and animals indicate that
children growing up in adverse conditions
develop altered autonomic nervous system
functioning that causes them to become
more sensitive to environmental demands,
more likely to become biologically and
emotionally reactive (or underresponsive)
to challenge, and less capable of adaptive
self- regulation (Loman & Gunnar, 2010;
Lupien, McEwen, Gunnar, & Heim, 2009).
These consequences have been documented
in the limbic– hypothalamic– pituitary–
adrenocortical (LHPA) axis (which is rel-
evant to affect and stress responding),
the parasympathetic system, and multiple
areas of the prefrontal cortex, indicating
that early stress can have diverse and per-
vasive effects on emotion and emotion reg-
ulation (Blair, 2010; El- Sheikh & Erath,
2011). Importantly, early family experiences
have been the model for early adversity in
this research literature, related both to the
stresses directly imposed by parental behav-
ior (e.g., experiences of abuse or chronic
neglect) and the failure of caregivers to buf-
fer the effects of other stresses on children.
Children with a history of abuse, for exam-
ple, exhibit multiple indications of difficulty
in emotion processing relevant to their mal-
treatment: They are hypersensitive to adult
expressions of anger, have a lower threshold
for detecting anger in maternal vocal expres-
sions, and have more difficulty attentionally
disengaging from perceived angry cues when
these responses are measured both behavior-
ally and neurobiologically (see Pollak, 2008,
for a review). Hypersensitivity to anger and
threat is probably an adaptive emotional
regulatory strategy in contexts in which chil-
dren cannot avert an adult attack but may be
capable of anticipating it and flee, avoid, or
otherwise prepare for it. But it comes with
a cost: Children who are hyperreactive to
cues of threat are less likely to be capable
Socialization of Emotion and Emotion Regulation in the Family 177
of managing their arousal, as this research
indicates. Moreover, outside the home,
hyperreactivity to potential threat is likely
to be a liability in dealing with other adults
and peers, whose social cues are more likely
to be misinterpreted and imbued with hos-
tile intent (Thompson & Goodman, 2010).
It is important to note that these deficien-
cies in emotion regulation are not pervasive,
and abused children are not hyperreactive
to cues of adult sadness or happiness (Pol-
lak, 2008). Rather, children’s self- regulatory
problems are related to the circumstances
of their family adversity, consistent with a
functionalist view of emotion regulation.
The research does not permit an evalu-
ation of the nature, severity, or chronicity
of early stresses in the family that prompt
these neurobiological and behavioral adap-
tations. There is some evidence that the
association between family stress and its
effects on behavioral functioning increment
in a dose– response fashion (Repetti, Robles,
& Reynolds, 2011). Nor is there clear evi-
dence concerning the extent to which early
experiences have privileged influence on the
growth of neurobiological and behavioral
systems related to emotion and its regula-
tion, or whether later experiences of adver-
sity have comparable effects. There is reason
to believe, however, that early experiences
may be especially important because of the
plasticity of immature brain systems related
to emotion and stress (Goldsmith, Pollak, &
Davidson, 2008). Taken together, however,
this research indicates that one way emo-
tion regulation is socialized in the family
is through children’s exposure to the emo-
tional demands and stresses of family life,
especially in the context of the (lack of)
support that exists for children’s competent
management of the emotional demands on
them. These experiences shape the func-
tioning of lower and higher neurobiological
systems associated with emotional arousal
and its regulation, and equip children with
response propensities that are likely to influ-
ence their emotional responding outside of
the home.
Such a conclusion is consistent with con-
siderable behavioral research conducted in
the context of less extreme family contexts.
In the following sections, I discuss develop-
mental studies of emotion regulation that
are consistent with the foregoing account of
neurobiological growth in two areas: par-
ents’ direct interventions to manage chil-
dren’s emotions, and effects of the broader
emotional climate of the family.
Parents’ Interventions
to Manage Emotion
From birth, parents exert considerable effort
to manage the emotions of their infants and
young children by soothing distress, provok-
ing positive emotion, and allaying fear. They
proactively seek to make the everyday emo-
tional demands on children manageable and
predictable by organizing the child’s daily
routine, scheduling meals and naps, choos-
ing appropriate child care arrangements,
and in other ways. Parents also seek to alter
children’s direct appraisals of emotionally
evocative situations, such as distracting chil-
dren away from potentially frightening or
distressing events, assisting in solving prob-
lems that children find frustrating, striving
to alter the children’s interpretations of neg-
atively arousing experiences (e.g., “It’s just a
game”), and through social referencing. By
directly intervening in these ways, parents
act as external regulators of young children’s
emotions.
Beyond their immediate consequences,
these parental interventions have broader
consequences for early emotion and emo-
tion regulation. With respect to parental
soothing of infant distress, for example,
Lamb (1981) has argued that such distress
relief sequences are easily learned by infants
and contribute to the emergence of rudi-
mentary expectations that the parent’s
arrival will relieve distress. By 6 months of
age, for example, distressed infants begin
quieting in anticipation of the arrival of
their mother when they hear her approach-
ing footsteps; infants also protest loudly if
mother approaches but does not pick them
up to soothe them (Gekoski, Rovee- Collier,
& Carulli- Rabinowitz, 1983; Lamb & Mal-
kin, 1986). The parent’s sensitive respond-
ing thus contributes to anticipatory soothing
(an early self- regulatory response) before the
parent’s arrival. Similarly, the mother’s sen-
sitivity during episodes of animated, face-
to-face play with the baby contributes to the
growth of early capacities for self- regulation
as the infant learns how to maintain man-
ageable arousal in the context of social play
178 DEVELOPMENTAL CONSIDERATIONS
with the caregiver (Feldman, Greenbaum, &
Yirmiya, 1999; Gianino & Tronick, 1988).
Parents who provide support to very
young children facing emotional challenges
have children who are more emotionally
competent. Calkins and Johnson (1998)
found that 18-month-olds who became
more distressed during frustration tasks had
mothers who were independently observed
to be more interfering when interacting with
their offspring, while children who could
use problem solving and distraction during
frustration had mothers who had earlier
offered greater support, suggestions, and
encouragement. In another study, mothers
who insisted that their toddlers approach
and confront potentially fearful objects in
the laboratory had children who exhibited
greater stress, as indexed by postsession cor-
tisol levels (Nachmias, Gunnar, Mangels-
dorf, Parritz, & Buss, 1996). These findings
suggest that parental assistance is important
not only for managing immediate emotional
behavior but also for the development of
children’s emotional self- control through
the child’s expectation that distress is man-
ageable and that adults can assist in manag-
ing emotionally challenging situations.
These studies focus primarily on paren-
tal interventions that buffer children’s emo-
tional arousal and support self- regulatory
capacity. As the research on stress indicates,
however, parental behavior may not always
be so supportive, and when this occurs there
is greater risk for problems with emotional
reactivity and self- regulation. Another
research literature supporting this view con-
cerns parental expressed emotion, which is
an index of parental attitudes of criticism
or emotional overinvolvement in the child’s
problems that can undermine competent
emotional functioning (e.g., Hooley & Rich-
ters, 1995). Although expressed emotion has
been examined most extensively in clinical
studies of schizophrenia, depression, and
bipolar disorder, because of its relevance to
the maintenance or relapse of clinical symp-
tomatology, developmental studies have
found that expressed emotion is associated
with the onset of psychological problems
in children (Caspi et al., 2004). For exam-
ple, one study found expressed emotion to
be particularly prevalent in homes with a
depressed parent (Rogosch, Cicchetti, &
Toth, 2004). In the context of expressed
emotion, therefore, parental responses that
are critical of a child’s emotional behavior
can contribute to the child’s risk of devel-
oping psychopathology involving emotion
dysregulation. Risk is enhanced because
the parent’s critical demeanor is stressful,
undermines opportunities for the child to
learn more adaptive forms of emotional cop-
ing, and creates a more difficult family emo-
tional climate for troubled children.
Direct parental interventions to manage
children’s emotions decline in frequency in
early childhood as young children acquire
their own self- regulatory strategies. How-
ever, direct interventions remain an impor-
tant source of extrinsic influence on emo-
tion regulation throughout life as they are
supplemented by other socialization influ-
ences. Adults commonly rely on the support
of family and friends when facing emotional
stresses. Social support is thus a significant
buffer of psychosocial stress, and social iso-
lation is a risk factor for stress- related prob-
lems (Thompson, Flood, & Goodvin, 2006).
Emotional Climate of Family Life
The emotional climate of family life can
make emotion management easier or more
difficult because of the emotional demands
that children encounter in the home. As sug-
gested by the research on expressed emotion,
when children must cope with frequent,
intensive negative emotion from other fam-
ily members, particularly when it is directed
at them, it can contribute to their hypersen-
sitivity to threat and undermine emotion
regulation capacities. The family emotional
climate is also relevant to emotion regula-
tion because of the models of emotion reg-
ulation to which children are exposed and
how the family environment shapes chil-
dren’s developing schemas for emotion and
its management (e.g., Are emotions threat-
ening? Empowering? Uncontrollable?).
Another illustration of the importance
of the family emotional climate for devel-
oping child emotional reactivity and self-
regulation is research on marital conflict
(Davies & Woitach, 2008). According to
the Cummings and Davies (2010) emo-
tional security hypothesis, children seek to
reestablish the emotional security they have
lost in families with marital conflict, even
when parental conflict is not directed at
Socialization of Emotion and Emotion Regulation in the Family 179
them. They do so by intervening in parental
arguments to end them, monitoring parental
moods to anticipate the outbreak of argu-
ments, and otherwise striving to manage
their emotions in a conflicted home environ-
ment. As a consequence, they show height-
ened sensitivity to parental distress and
anger, tend to become overinvolved in their
parents’ emotional conflicts, have difficulty
managing the strong emotions that conflict
arouses in them (in a manner resembling the
“emotional flooding” described by emotion
theorists), and exhibit signs of early devel-
opment of internalizing problems. Research
derived from this view has found that grade
school children who witness the most
intense marital conflict not only exhibit the
greatest enmeshment in family conflict but
also go to greater efforts to avoid conflict,
while also showing more signs of internal-
izing symptomatology (Davies & Forman,
2002; see also Davies, Harold, Goeke-
Morey, & Cummings, 2002). An impor-
tant facet of the family emotional life is, of
course, parents’ emotional expressiveness
(Halberstadt, Crisp, & Eaton, 1999; Hal-
berstadt & Eaton, 2003). A series of stud-
ies by Eisenberg and her colleagues showed
that children’s social competence is affected
by how mothers convey positive or nega-
tive feelings in the home—and this associa-
tion is mediated by differences in children’s
self- regulatory behavior (Eisenberg et al.,
2001, 2003; Valiente, Fabes, Eisenberg, &
Spinrad, 2004). These findings suggest that
a family climate characterized by moderate
to high amounts of positive emotion among
family members contributes to the growth of
emotion regulation, perhaps because emo-
tional demands are manageable within the
home and children are exposed to models of
skillful emotion self- regulation.
With respect to the influence of negative
emotional expressiveness in the family, sev-
eral studies report that negative maternal
expressions of emotion are associated with
children’s lack of self- regulation and cop-
ing, but others have found a positive asso-
ciation (Eisenberg, Cumberland, & Spinrad,
1998; Eisenberg et al., 2001, 2003; Valiente
et al., 2004). Viewed in the context of the
research on expressed emotion and mari-
tal conflict, the effects of negative parental
emotional expressiveness may depend on
whether emotions are directed toward the
child or not, the frequency and intensity of
these emotions, and whether negative emo-
tions are negative dominant (e.g., anger and
hostility) or negative submissive (e.g., sad-
ness and distress), with the former more
likely to elicit the child’s fear or defensive-
ness. One can see how emotion regulatory
skills might be enhanced (rather than under-
mined) by a child’s exposure to nonhostile
negative emotions of moderate intensity in
contexts in which negative feelings can be
safely expressed and managed, in contrast
with exposure to a more hostile, threatening
family emotional environment.
An important influence on the emotional
climate of the family— which also affects
how parents evaluate and respond to the
emotions of offspring— is parental beliefs
about emotion and its expression. These
beliefs can be considered part of a parent’s
“meta- emotion philosophy,” which includes
the adult’s awareness of her or his own emo-
tions, an understanding and acceptance of
the child’s emotions, and the parent’s own
approach to managing the child’s feelings
(Gottman, Katz, & Hooven, 1997; Hooven,
Gottman, & Katz, 1995). Based on paren-
tal interviews, Gottman and his colleagues
distinguish between “emotion coaching”
and “emotion dismissing” parenting styles.
Emotion- coaching parents are attentive
to their own emotions and the child’s feel-
ings, and do not believe that feelings should
be stifled. They consider the child’s emo-
tional expressions an occasion to validate
the child’s feelings, and an opportunity to
teach the child about emotions, expression,
and coping. Thus, emotion- coaching par-
ents foster the growth of emotion regula-
tion in offspring by offering warm support
and specific guidance for managing feel-
ings. Dismissive parents tend to ignore their
own emotions or belittle the importance of
emotions, and they may not constructively
attend to their children’s feelings. They view
emotions (especially negative ones) as poten-
tially harmful and believe that parents are
responsible for promptly subduing negative
outbursts in offspring and teaching their
children that negative emotions are fleeting
and unimportant.
Several studies testing this provocative
formulation have yielded supportive but
mixed results (Katz, Maliken, & Stettler,
2012). One study indicated that emotion

180 DEVELOPMENTAL CONSIDERATIONS
coaching and emotion dismissing interact
complexly as influences on children’s emo-
tion regulation (Lunkenheimer, Shields,
& Cortina, 2007; see also Gottman, Katz,
& Hooven, 1996; Ramsden & Hubbard,
2002). Research in my laboratory confirmed
that the association between maternal rep-
resentations of emotion and children’s emo-
tion regulation was mediated by mothers’
constructive emotion- related socialization
practices (Thompson, Virmani, Waters,
Meyer, & Raikes, 2013). Mothers’ emotion-
ally supportive representations (i.e., atten-
tion to her feelings; her focus on construc-
tive emotion self- regulation) were associated
with children’s constructive emotion regu-
lation (e.g., problem- focused and emotion-
focused strategies) primarily through their
association with positive, supportive emo-
tion regulation socialization behaviors (i.e.,
maternal problem- solving encouragement,
emotion- focused comfort, validation of chil-
dren’s feelings, and positive family expres-
sivity).
Taken together, the emotional climate of
family life influences the development of
emotion regulation through both the emo-
tional demands and supports it provides
that affect children’s developing emotional
reactivity. In studies of the development of
stress reactivity, the effects of marital con-
flict, and the consequences of parental posi-
tive and negative emotional expressivity, it
is clear that skills in emotion management
develop, in part, in reaction to the emo-
tional demands with which children must
daily cope. The research on parental meta-
emotion philosophy highlights one source of
variability in the family emotional climate,
and it also identifies the basis for parental
coaching of emotion regulatory strategies
in offspring. In the next section, I consider
these and other kinds of top-down regula-
tory influences.
Family Influences on Emotion
Self-Regulatory Strategies
As prefrontal regions of the brain progres-
sively mature, these higher brain systems
enable children to become more capable
of deliberately managing their feelings,
impulses, and behavior. Growth in children’s
capacities to understand and enact cogni-
tive and attentional strategies to manage
emotional reactions accompanies the devel-
opment of these neurobiological control
processes. These top-down influences on
emotion regulation highlight another avenue
for socialization influences in the family. In
direct and indirect ways, parents guide how
children learn to appraise their feelings,
confront the demands of emotion regulation
at home or in other social settings, acquire
specific skills for managing their feelings,
understand cultural expectations for emo-
tional self- control, and represent emotion
processes relevant to self- regulation. In con-
trast to the family influences (e.g., marital
conflict) that shape developing emotional
reactivity and usually have unintended
effects on children’s emotion regulation,
these socialization influences are usually
deliberate. In coaching, conversation, and
other modes of family interaction, parents
encourage the development of well- regulated
emotion and children’s understanding, moti-
vation, and competence to accomplish this.
Because these socialization processes
extend throughout life and mediate cultural
and gender differences in emotion manage-
ment, individuals reach adulthood with
emotion self- regulation skills that have been,
to a large extent, socially constructed. In the
following sections, two general socialization
influences on developing emotion regulation
are considered: parental evaluations of chil-
dren’s emotions, and parent– child conversa-
tion about emotion and its regulation.
Parental Evaluations
of Children’s Emotions
Emotion regulation can be facilitated or
impaired by how others evaluate one’s feel-
ings. Sympathetic, constructive responses
affirm that one’s feelings are justified and
provide social support in understanding and
advice that help people cope with difficult
situations. However, denigrating, critical, or
dismissive responses contain implicit mes-
sages that demean the appropriateness of the
feelings or their expression, or the compe-
tence of the person feeling this way. Indeed,
others’ dismissiveness can exacerbate the
negative emotions that one is trying to man-
age (in part by arousing further emotion), as
well as diminish opportunities for acquiring
more adaptive modes of emotion regulation.
Socialization of Emotion and Emotion Regulation in the Family 181
For children, these parental evaluations also
become internalized guides for how children
judge their own emotional reactions.
Developmental studies indicate that
children cope more adaptively with their
emotions in immediate circumstances and
acquire more constructive emotion regula-
tory capacities when parents respond accept-
ingly and supportively to their negative emo-
tional displays. By contrast, outcomes are
more negative when parents are denigrating,
punitive, or dismissive, or when the child’s
negative emotions elicit parents’ personal
distress (for reviews, see Denham, 1998;
Denham, Bassett, & Wyatt, 2007; Eisen-
berg et al., 1998). In a socioeconomically
disadvantaged sample, for example, mothers
who reported exerting more positive control
(using warmth and approval) over their sons
at age 1½ had children who were observed to
manage their negative emotions more con-
structively (e.g., by using self- distraction) at
age 3½ (Gilliom, Shaw, Beck, Schonberg, &
Lukon, 2002). Eisenberg, Fabes, and Mur-
phy (1996) found that mothers’ self- reported
problem- solving responses to their grade
school children’s negative emotions were
associated with independent reports of their
children’s constructive coping with prob-
lems (e.g., seeking support, problem solving,
and positive thinking), while mothers’ puni-
tive and minimizing reactions to children’s
emotions were negatively associated with
children’s constructive coping and positively
associated with avoidant coping. Research
in my laboratory indicates that parents who
accepted and validated young children’s feel-
ings during conversations about recent dif-
ficult events had children who were more
willing to talk about these experiences and
as a result more likely to learn about their
emotions and how to manage them (Waters,
Virmani, Thompson, Meyer, Raikes, &
Jochem, 2010). These studies indicate that
how parents respond to children’s emotions,
and the behaviors that result, predict chil-
dren’s emotion- related coping in later assess-
ments.
Parent–Child Conversation and
Developing Emotion Representations
From an early age, parents actively coach
children on strategies of emotion regula-
tion when they encourage offspring to “use
words to say how you feel,” take a deep
breath, or think of something nice when
emotionally aroused. Research in my labo-
ratory indicates that in conversation with
their preschoolers about recent emotional
events, mothers directly suggest strategies of
emotional self- control and explicitly endorse
certain strategies over others (Thompson,
Waters, Meyer, Raikes, Jochem, & Virmani,
2009). They do so in light of their expecta-
tions for age- appropriate emotional behav-
ior in social settings (Cassano & Zeman,
2010).
Parent–child conversations have, however,
broader significance for the growth of emo-
tion regulation. Consistent with the work
of Gottman and his colleagues (1997) on
parental coaching, conversation shapes chil-
dren’s broader representations of emotion.
Understanding the causes and consequences
of their feelings, comprehending gender
and cultural expectations for emotional
expression, learning the social functions
of emotional behavior, and other aspects
of emotion understanding are advanced
by parent– child conversation (Thompson,
2006a, 2006b). These broader aspects of
emotion understanding are important to
developing an informed comprehension of
emotion regulation. As their conceptual
skills mature, children begin to appreciate,
especially through conversations with par-
ents, that they can enlist internal constitu-
ents of emotion to manage external emotion
(e.g., redirecting attention; cognitive reap-
praisal; mental distraction), that one need
not always show what one feels, and that
mental processes (e.g., expectations, memo-
ries, thoughts) affect how one feels.
Parent–child conversation has further sig-
nificance for emotion regulation, moreover,
as a means of guiding children’s immediate
appraisals of events. By managing infor-
mation the child receives about potentially
stressful events (e.g., describing an antici-
pated dental visit as “teeth tickling”) or
introducing alternative interpretations of an
event (e.g., eliciting sympathy for a physi-
cally challenged person the child fears or
finds amusing), parents influence emotion-
related appraisals that facilitate emotion
regulation. In an experimental probe, Mor-
ris et al. (2011) found that mothers’ use of
attention refocusing and cognitive reframing
was associated with less sadness and anger

182 DEVELOPMENTAL CONSIDERATIONS
in children who received a disappointing
gift in her presence (see Kliewer, Fearnow,
& Miller, 1996, for similar results with
mothers and fathers). Comparable to how
parent– child conversations influence mem-
ory through attentional redirection, there-
fore, these conversations can influence emo-
tion through reorienting appraisal processes
(Thompson, 2006b).
It is important to note that conversations
with peers and siblings are also important
catalysts to the growth of emotion regula-
tion in childhood. Young children talk about
their feelings more frequently with friends
and siblings than they do with their moth-
ers, and these conversations also contrib-
ute to developing emotional understanding
(Brown, Donelan- McCall, & Dunn, 1996).
Peer conversations are important; there is
evidence that adaptive strategies of emotion
regulation with peers are not the same as
those with family members and adult inter-
actions (Thompson & Waters, 2010). Vent-
ing distress does not typically elicit as much
support from peers as from parents, for
example. Thus, as children travel between
different social settings, they must develop
competence in applying different norms for
emotional behavior and accepted strategies
for emotion regulation.
An Integrative Perspective:
Parent–Child Attachment Security
The preceding discussion focused on imme-
diate and longer- term socialization processes
by which children become competent at
managing their emotions. But what happens
and who does it are both important in emo-
tion socialization. Most of the socialization
influences on emotion regulation discussed
in this chapter occur in a relational context,
and the quality of the parent– child relation-
ship colors the influence of parents’ direct
interventions, appraisals of children’s emo-
tions, coaching, and conversations about
emotion and its regulation. In this section,
I discuss the significance of the security of
the parent– child relationship, and in doing
so reintegrate the bottom- up and top-down
processes discussed earlier.
According to attachment researchers,
differences in the security of child– parent
attachment may be especially significant for
the growth of emotion regulation (Cassidy,
1994; Thompson, 1994; see also Shaver &
Mikulincer, this volume). Young children
in secure relationships have more sensitive
mothers who are accepting of children’s
positive and negative feelings, and open to
talking about intense, disturbing, or con-
fusing feelings with them. Consequently,
like the offspring of emotion- coaching par-
ents, securely attached children are likely
to become more emotionally self-aware,
acquire deeper emotion understanding, and
develop a more flexible capacity to manage
their emotions appropriate to circumstances.
Moreover, the security of the parent– child
relationship provides a continuing resource
of support on which the child can rely. By
contrast, young children in insecure rela-
tionships have mothers who are less sensitive
and more inconsistently responsive to their
feelings, and less likely to be comfortable
talking with them about difficult emotional
experiences. These children are likely to
have a more limited understanding of emo-
tion and to become more easily emotionally
dysregulated, especially in stressful circum-
stances, because of the lack of support in the
parent– child relationship.
There is research evidence in support of
this view. In a longitudinal study of chil-
dren’s first 3 years, Kochanska (2001)
reported that over time, insecurely attached
children, compared with secure children,
exhibited progressively greater fear and/or
anger, and diminished joy, in standardized
assessments. Even by age 1, the mothers of
secure infants commented on both positive
and negative emotions when interacting
with their children, while the mothers of
insecurely attached infants rarely discussed
their feelings or commented primarily about
negative emotions (Goldberg, MacKay-
Soroka, & Rochester, 1994). By early child-
hood, securely attached preschoolers talked
more about emotions in everyday conversa-
tions with their mothers, and their mothers
were more richly elaborative than mothers
of insecurely attached preschoolers in their
discussions of emotion with them. This may
help to explain why secure children are more
advanced in emotion understanding (Laible
& Thompson, 1998; Raikes & Thompson,
2006). There is also evidence that children
in secure relationships are better at manag-
ing negative emotions. Gilliom and his col-

Socialization of Emotion and Emotion Regulation in the Family 183
leagues (2002) reported that boys who were
securely attached at age 1½ were observed
to use more constructive anger manage-
ment strategies at age 3½. In a study of the
responses of 18-month-olds to moderate
stressors, Nachmias and colleagues (1996)
reported that postsession cortisol elevations,
indicating stressful responding, were found
only in temperamentally inhibited toddlers
in insecure relationships with their mothers.
For inhibited toddlers in secure relationships,
the mother’s presence helped to buffer the
physiological effects of challenging events.
In another study, Contreras, Kerns, Weimer,
Gentzler, and Tomich (2000) reported that
by middle childhood, attachment security
was significantly associated with children’s
constructive coping with stress, and the
extent of coping mediated the association
between attachment and children’s peer
competence. Research in our laboratory has
also shown that mothers in secure relation-
ships with their children are more accurate
in judging what their children are feeling
and potentially are thus more capable of
providing helpful guidance about managing
those feelings (Waters et al., 2010).
These findings indicate that the rela-
tional context in which emotion regulation
develops is important not only for the spe-
cific ways that parents respond to children’s
feelings but also for the relational support
that shapes the growth of emotion self-
regulation.
Conclusions
In the broadest sense, the research surveyed
in this chapter confirms how significantly
social influences shape the growth of emo-
tional experience and emotion regulation.
This literature underscores the multifaceted
ways that socialization processes in the fam-
ily affect children’s emotional reactivity and
self- regulation.
Viewed in terms of bottom- up influences,
family processes shape children’s emotional
reactivity through the everyday emotional
demands to which children must adapt,
anticipate, and cope. These influences are
most apparent in studies of at-risk children,
for whom the challenges of family dysfunc-
tion, marital conflict, and even maltreatment
underscore how emotional development and
self- regulation conform to the functional
requirements of managing difficult every-
day emotional demands at home. Viewed in
terms of top-down influences, family pro-
cesses also shape children’s understanding
of emotion, and their motivation and com-
petence to manage their feelings. These pro-
cesses contribute to significant individual
differences in children’s emotional apprais-
als, goals for emotion regulation, and strate-
gies for self- control.
Although study of the socialization of
emotion regulation has long been a topic of
vigorous research interest, a considerable
research agenda remains. Continuing work
in this field is justified not only for theoreti-
cal reasons, but also for its importance to
understanding the challenges faced by chil-
dren at risk for problems of emotion- related
psychopathology.
References
Barrett, L. F., & Bar, M. (2009). See it with
feeling: Affective predictions during object
perception. Philosophical Transactions of the
Royal Society B: Biological Sciences, 364,
1325–1334.
Blair, C. (2010). Stress and the development of
self- regulation in context. Child Development
Perspectives, 4, 181–188.
Brown, J. R., Donelan- McCall, N., & Dunn, J.
(1996). Why talk about mental states?: The
significance of children’s conversations with
friends, siblings, and mothers. Child Develop-
ment, 67, 836–849.
Calkins, S. D., & Johnson, M. C. (1998). Tod-
dler regulation of distress to frustrating
events: Temperamental and maternal corre-
lates. Infant Behavior and Development, 21,
379–395.
Caspi, A., Moffitt, T. E., Morgan, J., Rutter,
M., Taylor, A., Arseneault, L., et al. (2004).
Maternal expressed emotion predicts chil-
dren’s antisocial behavior problems: Using
monozygotic- twin differences to identify envi-
ronmental effects on behavioral development.
Developmental Psychology, 40, 149–161.
Cassano, M. C., & Zeman, J. L. (2010). Parental
socialization of sadness regulation in middle
childhood: The role of expectations and gen-
der. Developmental Psychology, 46, 1214–
1226.
Cassidy, J. (1994). Emotion regulation: Influ-
184 DEVELOPMENTAL CONSIDERATIONS
ences of attachment relationships [Special
issue]. Monographs of the Society for Research
in Child Development, 59(2–3, Serial No.
240), 228–249.
Cole, P., Martin, S., & Dennis, T. (2004). Emo-
tion regulation as a scientific construct: Meth-
odological challenges and directions for child
development research. Child Development,
75, 317–333.
Contreras, J. M., Kerns, K. A., Weimer, B. L.,
Gentzler, A. L., & Tomich, P. L. (2000). Emo-
tion regulation as a mediator of associations
between mother– child attachment and peer
relationships in middle childhood. Journal of
Family Psychology, 14, 111–124.
Cummings, E. M., & Davis, P. T. (2010). Chil-
dren and marital conflict: An emotional secu-
rity perspective. New York: Guilford Press.
Davies, P. T., & Forman, E. M. (2002). Chil-
dren’s patterns of preserving emotional secu-
rity in the interparental subsystem. Child
Development, 73, 1880 –1903.
Davies, P. T., Harold, G. T., Goeke-Morey, M.
C., & Cummings, E. M. (2002). Child emo-
tional security and interparental conflict.
Monographs of the Society for Research in
Child Development, 67(Serial No. 270),
1–127.
Davies, P. T., & Woitach, M. J. (2008). Chil-
dren’s emotional security in the interparental
relationship. Current Directions in Psycho-
logical Science, 17, 269–274.
Denham, S. (1998). Emotional development in
young children. New York: Guilford Press.
Denham, S., Bassett, H. H., & Wyatt, T. (2007).
The socialization of emotional competence. In
J. Grusec & P. Hastings (Eds.), Handbook of
socialization (pp. 614–637). New York: Guil-
ford Press.
Eisenberg, N., Cumberland, A., & Spinrad, T. L.
(1998). Parental socialization of emotion. Psy-
chological Inquiry, 9, 241–273.
Eisenberg, N., Fabes, R. A., & Murphy, B. C.
(1996). Parents’ reactions to children’s nega-
tive emotions: Relations to children’s social
competence and comforting behavior. Child
Development, 67, 2227–2247.
Eisenberg, N., Gershoff, E. T., Fabes, R. A.,
Shepard, S. A., Cumberland, A. J., Losoya, S.
H., et al. (2001). Mothers’ emotional expres-
sivity and children’s behavior problems and
social competence: Mediation through chil-
dren’s regulation. Developmental Psychology,
37, 475–490.
Eisenberg, N., Valiente, C., Morris, A. S., Fabes,
R. A., Cumberland, A., Reiser, M., et al.
(2003). Longitudinal relations among parental
emotional expressivity, children’s regulation,
and quality of socioemotional functioning.
Developmental Psychology, 39, 3–19.
El- Sheikh, M., & Erath, S. A. (2011). Family
conflict, autonomic nervous system function-
ing, and child adaptation: State of the science
and future directions. Development and Psy-
chopathology, 23, 703–721.
Feldman, R., Greenbaum, C. W., & Yirmiya, N.
(1999). Mother– infant affect synchrony as an
antecedent of the emergence of self- control.
Developmental Psychology, 35, 223–231.
Gekoski, M., Rovee- Collier, C., & Carulli-
Rabinowitz, V. (1983). A longitudinal analy-
sis of inhibition of infant distress: The origins
of social expectations? Infant Behavior and
Development, 6, 339–351.
Gianino, A., & Tronick, E. (1988). The mutual
regulation model: The infant’s self and inter-
active regulation and coping and defensive
capacities. In T. Field, P. McCabe, & N.
Schneiderman (Eds.), Stress and coping (Vol.
2, pp. 47–68). Hillsdale, NJ: Erlbaum.
Gilliom, M., Shaw, D. S., Beck, J. E., Schonberg,
M. A., & Lukon, J. L. (2002). Anger regula-
tion in disadvantaged preschool boys: Strat-
egies, antecedents, and the development of
self- control. Developmental Psychology, 38,
222–235.
Goldberg, S., MacKay- Soroka, S., & Rochester,
M. (1994). Affect, attachment, and maternal
responsiveness. Infant Behavior and Develop-
ment, 17, 335–339.
Goldsmith, H. H., Pollak, S. D., & Davidson,
R. J. (2008). Developmental neuroscience per-
spectives on emotion regulation. Child Devel-
opment Perspectives, 2, 132–140.
Gottman, J. M., Katz, L. F., & Hooven, C.
(1996). Parental meta- emotion philosophy
and the emotional life of families: Theoretical
models and preliminary data. Journal of Fam-
ily Psychology, 10, 243–268.
Gottman, J. M., Katz, L. F., & Hooven, C.
(1997). Meta- emotion: How families commu-
nicate emotionally. Mahwah, NJ: Erlbaum.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Halberstadt, A. G., Crisp, V. W., & Eaton, K. L.
Socialization of Emotion and Emotion Regulation in the Family 185
(1999). Family expressiveness: A retrospective
and new directions for research. In P. Philip-
pot & R. S. Feldman (Eds.), The social context
of nonverbal behavior (pp. 109–155). New
York: Cambridge University Press.
Halberstadt, A. G., & Eaton, K. L. (2003). A
meta- analysis of family expressiveness and
children’s emotion expressiveness and under-
standing. Marriage and Family Review, 34,
35–62.
Hooley, J. M., & Richters, J. E. (1995). Expressed
emotion: A developmental perspective. In D.
Cicchetti & S. Toth (Eds.), Emotion, cogni-
tion, and representation (pp. 133–166). Roch-
ester, NY: University of Rochester Press.
Hooven, C., Gottman, J. M., & Katz, L. F.
(1995). Parental meta- emotion structure pre-
dicts family and child outcomes. Cognition
and Emotion, 9, 229–264.
Katz, L. F., Maliken, A. C., & Stettler, N. M.
(2012). Parental meta- emotion philosophy: A
review of research and theoretical framework.
Child Development Perspectives, 6, 417– 422.
Kliewer, W., Fearnow, M. D., & Miller, P. A.
(1996). Coping socialization in middle child-
hood: Tests of maternal and paternal influ-
ences. Child Development, 67, 2339–2357.
Kober, H., Barrett, L. F., Joseph, J., Bliss-
Moreau, E., Lindquist, K., & Wager, T. D.
(2008). Functional grouping and cortical–
subcortical interactions in emotion: A meta-
analysis of neuroimaging studies. NeuroIm-
age, 42, 998–1031.
Kochanska, G. (2001). Emotional development
in children with different attachment histories:
The first three years. Child Development, 72,
474–490.
Laible, D. J., & Thompson, R. A. (1998). Attach-
ment and emotional understanding in pre-
school children. Developmental Psychology,
34(5), 1038–1045.
Lamb, M. (1981). Developing trust and per-
ceived effectance in infancy. In L. Lipsitt (Ed.),
Advances in infancy research (Vol. 1, pp. 101–
127). New York: Ablex.
Lamb, M., & Malkin, C. (1986). The develop-
ment of social expectations in distress– relief
sequences: A longitudinal study. International
Journal of Behavioral Development, 9, 235–
249.
Loman, M. M., & Gunnar, M. R. (2010). Early
experience and the development of stress reac-
tivity and regulation in children. Neuroscience
and Biobehavioral Reviews, 34, 867–876.
Lunkenheimer, E. S., Shields, A. M., & Cortina,
K. S. (2007). Parental emotion coaching and
dismissing in family interaction. Social Devel-
opment, 16, 232–248.
Lupien, S. J., McEwen, B. S., Gunnar, M. R., &
Heim, C. (2009). Effects of stress through-
out the lifespan on the brain, behaviour and
cognition. Nature Reviews Neuroscience, 10,
434–445.
Morris, A. S., Silk, J. S., Morris, M. D. S., Stein-
berg, L., Aucoin, K. J., & Keyes, A. W. (2011).
The influence of mother– child emotion regu-
lation strategies on children’s expression of
anger and sadness. Developmental Psychol-
ogy, 47, 213–225.
Morris, A. S., Silk, J. S., Steinberg, L., Myers, S.
S., & Robinson, L. R. (2007). The role of the
family context in the development of emotion
regulation. Social Development, 16, 361–388.
Nachmias, M., Gunnar, M., Mangelsdorf, S.,
Parritz, R. H., & Buss, K. (1996). Behavioral
inhibition and stress reactivity: The moderat-
ing role of attachment security. Child Devel-
opment, 67, 508–522.
Newton, E. K., & Thompson, R. A. (2010).
Parents’ views of early social and emotional
development: More and less than meets the
eye. Zero to Three, 31, 10–15.
Ochsner, K. N., Ray, R. R., Hughes, B., McRae,
K., Cooper, J. C., Weber, J., et al. (2009).
Bottom-up and top-down processes in emo-
tion generation: Common and distinct neu-
ral mechanisms. Psychological Science, 20,
1322–1331.
Pollak, S. D. (2008). Mechanisms linking early
experience and the emergence of emotions:
Illustrations from the study of maltreated chil-
dren. Current Directions in Psychological Sci-
ence, 17, 370–375.
Raikes, H. A., & Thompson, R. A. (2006). Fam-
ily emotional climate, attachment security,
and young children’s emotion understand-
ing in a high-risk sample. British Journal of
Developmental Psychology, 24, 989–1004.
Ramsden, S. R., & Hubbard, J. A. (2002). Fam-
ily expressiveness and parental emotion coach-
ing: Their role in children’s emotion regulation
and aggression. Journal of Abnormal Child
Psychology, 30, 657–667.
Repetti, R. L., Robles, T. F., & Reynolds, B.
(2011). Allostatic processes in the family.
Development and Psychopathology, 23, 921–
938.
Rogosch, F., Cicchetti, D., & Toth, S. (2004).
186 DEVELOPMENTAL CONSIDERATIONS
Expressed emotion in multiple subsystems of
the families of toddlers with depressed moth-
ers. Development and Psychopathology, 16,
689–709.
Saarni, C. (1999). The development of emo-
tional competence. New York: Guilford Press.
Shaver, P. R., & Mikulincer, M. (this volume,
2014). Adult attachment and emotion regula-
tion. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 237–250). New
York: Guilford Press.
Thompson, R. A. (1990). Emotion and self-
regulation. In R. A. Thompson (Ed.), Socio-
emotional development: Nebraska Sympo-
sium on Motivation (Vol. 36, pp. 383–483).
Lincoln: University of Nebraska Press.
Thompson, R. A. (1994). Emotion regulation:
A theme in search of definition. Monographs
of the Society for Research in Child Develop-
ment, 59(2–3, Serial No. 240), 25–52.
Thompson, R. A. (2000). Childhood anxiety dis-
orders from the perspective of emotion regula-
tion and attachment. In M. W. Vasey & M.
R. Dadds (Eds.), The developmental psycho-
pathology of anxiety (pp. 160–182). Oxford,
UK: Oxford University Press.
Thompson, R. A. (2006a). The development of
the person: Social understanding, relation-
ships, self, conscience. In W. Damon & R. M.
Lerner (Eds.), N. Eisenberg (Vol. Ed.), Hand-
book of child psychology: Vol. 3. Social, emo-
tional, and personality development (6th ed.,
pp. 24–98). New York: Wiley.
Thompson, R. A. (2006b). Conversation and
developing understanding: Introduction to
the special issue. Merrill– Palmer Quarterly,
52(1), 1–16.
Thompson, R. A. (2011). Emotion and emotion
regulation: Two sides of the developing coin.
Emotion Review, 3(1), 53–61.
Thompson, R. A., Flood, M. F., & Goodvin,
R. (2006). Social support and developmental
psychopathology. In D. Cicchetti & D. Cohen
(Eds.), Developmental psychopathology: Vol.
III. Risk, disorder, and adaptation (2nd ed.,
pp. 1–37). New York: Wiley.
Thompson, R. A., & Goodman, M. (2010).
Development of emotion regulation: More
than meets the eye. In A. Kring & D. Sloan
(Eds.), Emotion regulation and psychopathol-
ogy (pp. 38–58). New York: Guilford Press.
Thompson, R. A., Lewis, M., & Calkins, S. D.
(2008). Reassessing emotion regulation. Child
Development Perspectives, 2(3), 124–131.
Thompson, R. A., & Meyer, S. (2007). The
socialization of emotion regulation in the fam-
ily. In J. J. Gross (Ed.), Handbook of emotion
regulation (pp. 249–268). New York: Guilford
Press.
Thompson, R. A., Virmani, E., Waters, S. F.,
Meyer, S., & Raikes, A. (2013). The develop-
ment of emotion self- regulation: The whole and
the sum of the parts. In K. Barrett, N. A. Fox,
G. A. Morgan, D. J. Fidler, & L. A. Daunhauer
(Eds.), Handbook of self- regulatory processes
in development (pp. 5–26). New York: Taylor
& Francis.
Thompson, R. A., & Waters, S. F. (2010). The
development of emotion regulation: Parent and
peer influences. In R. Sanchez- Aragon (Ed.),
Emotion regulation: A crossing from culture
to the development of personal relationships
(pp. 125–157). Mexico City: National Auton-
omous University of Mexico.
Thompson, R. A., Waters, S. F., Meyer, S. C.,
Raikes, A., Jochem, R. A., & Virmani, E.
A. (2009, April). Conversational catalysts to
developing understanding of emotion regula-
tion. In L. J. Levine (Chair), How kids keep
their cool: Pathways to effective emotion reg-
ulation. Symposium presented at the biennial
meeting of the Society for Research in Child
Development, Denver, CO.
Valiente, C., Fabes, R. A., Eisenberg, N., & Spin-
rad, T. L. (2004). The relations of parental
expressivity and support to children’s coping
with daily stress. Journal of Family Psychol-
ogy, 18, 97–106.
Waters, S., Virmani, E., Thompson, R. A.,
Meyer, S., Raikes, A., & Jochem, R. (2010).
Emotion regulation and attachment: Unpack-
ing two constructs and their association.
Journal of Psychopathology and Behavioral
Assessment, 32(1), 37– 47.
Woltering, S., & Lewis, M. D. (2009). Devel-
opmental pathways of emotion regulation in
childhood: A neuropsychological perspective.
Mind, Brain, and Education, 3, 160–169.

187
Adolescents’ emotional lives are distinct
from those of children or adults: They react
more strongly to emotion- eliciting situa-
tions (e.g., Miller & Shields, 1980; Stroud
et al., 2009), experience negative and mixed
emotions more frequently (e.g., Larson &
Asmussen, 1991; Larson & Lampman-
Petraitis, 1989; Riediger, Schmiedek, Wag-
ner, & Lindenberger, 2009; Riediger, Wrzus,
& Wagner, 2012), and fluctuate more rap-
idly in their emotional states (e.g., Larson,
Moneta, Richards, & Wilson, 2002). Emo-
tion regulation is assumed to play a key
role in these characteristics of adolescents’
emotional experiences (e.g., Opitz, Gross,
& Urry, 2012). It has also been proposed to
be a central component of adolescents’ more
general socioemotional adaptation, that is,
their ability to adjust to the socioemotional
challenges they face (e.g., Silk, Steinberg,
& Morris, 2003; Yap, Allen, & Sheeber,
2007). Studying emotion regulation is there-
fore essential for understanding the develop-
mental phase of adolescence.
In line with the widely accepted definition
by Gross (e.g., 1999), we conceive of emo-
tion regulation as comprising those delib-
erate and automatic processes that allow
individuals to influence which emotional
experiences they have, when they have
them, and how they experience and express
them. This chapter provides an overview
of the current status of research on adoles-
cent emotion regulation in Western societ-
ies. To set the stage, we briefly characterize
the developmental period of adolescence and
the relevance of emotion regulation for ado-
lescents’ socioemotional adjustment. The
main part of the chapter integrates evidence
on three questions that are important for
the understanding of emotion regulation in
adolescence: Which factors contribute to the
development of emotion regulation skills in
that life phase? Does adolescents’ motiva-
tions to regulate their feelings differ from
those of other age groups? Which strategies
do adolescents use to regulate their emo-
tions, and how adaptive and effective are
these strategies? We conclude with an out-
look on important future research direc-
tions.
Setting the Stage
The life phase “adolescence” refers to the
transition from being a child to being an
independent adult. It can roughly be defined
as beginning with the physical changes of
puberty and ending with the assumption of
adult social roles. Thus, the particular age
range of adolescence varies widely between
individuals, depending on the onset of
puberty and life circumstances. Empirical
CHAPTER 12
Emotion Regulation in Adolescence
Michaela Riediger
Kathrin Klipker
188 DEVELOPMENTAL CONSIDERATIONS
investigations often approximate adoles-
cence as a period within the time window
from about 10 to about 25 years of age.
In Western industrialized societies, the life
phase of adolescence has undergone a major
expansion in recent history. Throughout the
past century, the average onset of pubertal
processes has occurred progressively earlier,
particularly in girls. Nowadays, hormonal
changes of female puberty begin between 9
and 12 years of age, and most of the physical
changes of puberty are typically completed
by the midteens. The acceleration of puberty
onset has coincided with a progressive delay
of assuming adult roles in Western societ-
ies. This is often postponed until around the
early 20s (for an overview, see Dahl, 2004).
Adolescence is characterized by vast
changes in multiple domains of functioning
(for an overview, see, e.g., Eccles, Temple-
ton, Barber, & Stone, 2003). Puberty- related
hormonal changes, for example, lead to
sexual maturation, the development of sec-
ondary gender characteristics, and dramatic
changes in body size and composition. Ado-
lescence is also a phase of profound psycho-
logical change, such as marked growth in
cognitive functioning or moral reasoning.
Furthermore, many aspects of social experi-
ence change as adolescents move away from
their family and toward their peers. Impor-
tantly, there is large variability in the timing
of these various changes, both between ado-
lescents (e.g., regarding the onset of puberty)
and within adolescents (i.e., development in
one domain of functioning does not neces-
sarily imply parallel development in another;
e.g., Dahl, 2004).
During adolescence, emotionally chal-
lenging situations typically become more
frequent and intense. In Western industri-
alized societies, for example, this derives
from an increased potential for conflict with
parents, from adolescents’ greater sensitivity
to peer- related social interactions, or from
early romantic and sexual experiences. In
addition, the physical changes of puberty
make social demands regarding adolescents’
behavior more challenging. Social environ-
ments expect adolescents who look like
adults to behave like adults (Dahl, 2004).
Furthermore, cognitive growth during
adolescence provides the basis for greater
engagement with emotion- relevant aspects
of one’s own and others’ existence and future
(e.g., Thorne, 2004). These challenges can
stipulate the development of skills that are
necessary for identity formation, autonomy
increase, and other developmental tasks of
adolescence (Erikson, 1968). This, however,
is only true as long as the challenges do not
overtax the individual (Yap et al., 2007).
Temporary increases in affective reactivity
(which we address in greater detail when
discussing neurophysiological aspects of
emotion regulation in adolescence) may
amplify adolescents’ vulnerability to being
overwhelmed by the emotional challenges
they face (e.g., Gunnar, Wewerka, Frenn,
Long, & Griggs, 2009; Miller & Shields,
1980; Stroud et al., 2009; Sumter, Bokhorst,
Miers, Van Pelt, & Westenberg, 2010). In
fact, increases during adolescence in inter-
nalizing (e.g., depressiveness) and external-
izing (e.g., aggressiveness) problems, as well
as in various forms of psychopathology (e.g.,
Allen & Sheeber, 2009; Shortt, Stoolmiller,
Smith-Shine, Mark Eddy, & Sheeber, 2010;
Silk et al., 2003) demonstrate that develop-
mental demands of adolescence may over-
stretch the adaptational capacity of vulner-
able individuals.
Emotion regulation has been proposed
to play a core role in adolescents’ ability to
weather the developmental challenges they
face (e.g., McLaughlin, Hatzenbuehler,
Mennin, & Nolen- Hoeksema, 2011; Silk et
al., 2007; Yap et al., 2007). Both over- and
underregulation of emotion are assumed
to imply risks for adolescents’ socioemo-
tional adaptation, that is, for their ability to
adjust to the socioemotional challenges they
encounter. Available evidence indeed links
emotion regulation skills to higher social
competence, more prosocial behavior, bet-
ter academic achievements, and fewer inter-
nalizing and externalizing problems (e.g.,
Bell & Calkins, 2000; Buckley & Saarni,
2009; Silk et al., 2003; Zeman, Cassano,
Perry- Parrish, & Stegall, 2006). Emotion
regulation has also been related to more
peer acceptance, higher peer status, and a
lower likelihood of experiencing chronic
victimization and bullying by peers (for an
overview, see Buckley & Saarni, 2009). In
terms of adolescent psychopathology, emo-
tion regulation has been implicated in the
development of diverse problems, including
anxiety, depressive or conduct disorders,
as well as eating disorders in females (e.g.,

Emotion Regulation in Adolescence 189
McLaughlin et al., 2011; Silk et al., 2007;
Sim & Zeman, 2006; Yap et al., 2007).
To date, the vast majority of studies are
cross- sectional (but see, e.g., McLaughlin et
al., 2011). Thus, whether emotion dysregu-
lation is a risk factor for, or a consequence
of adaptational problems in adolescence
cannot yet be conclusively anwered. There
also is substantial variability across studies
in the particular aspects of emotion regula-
tion investigated. Taken together, however,
ample evidence links emotion regulation
skills to socioemotional adjustment in ado-
lescence. Insight into the development of
emotion regulation skills during adolescence
can thus have important practical implica-
tions. This chapter reviews three fundamen-
tal research topics in this respect that refer to
influences on emotion regulation in adoles-
cence, emotion regulation motivations, and
the ways and effectiveness of adolescents’
attempts to regulate emotions.
Influences on Emotion Regulation
The development of emotion regulation
skills in adolescence is shaped by the interac-
tion of multiple influences. We focus on two
examples in this chapter, namely, individu-
als’ neurophysiological development and
their familial context. A discussion of other
factors— such as genetic disposition, gen-
der, personality, temperament, attachment
style, or influences from developments dur-
ing infancy and childhood— is beyond the
scope of this chapter (but see, e.g., Shaver &
Mikulincer, and Mesquita, De Leersnyder,
& Albert, this volume; Bariola, Gullone, &
Hughes, 2011).
Neurophysiology
and Emotion Regulation
Advances in neuroimaging technologies have
led to a recent upsurge of interest in the role
of neurophysiological development in emo-
tion regulation during adolescence. Research
has shown that similar brain regions are acti-
vated when participants regulate emotions
and when they control cognitive operations
(Mauss, Bunge, & Gross, 2007; Mohanty et
al., 2007; Ochsner & Gross, 2005). Thus,
neurophysiologically oriented research typi-
cally ascribes the exertion of cognitive con-
trol an essential role for effective emotion
regulation (Gray & Braver, 2007; Ochsner
& Gross, 2005, this volume). Cognitive con-
trol encompasses the various processes that
are necessary for successful goal pursuit,
which include maintaining a current goal
in working memory or shielding it from dis-
traction (Best & Miller, 2010). Intentional
emotion regulation can be conceived of as an
instance of such goal- directed behavior (e.g.,
to not express one’s anger). Indeed, several
studies with participants from various age
groups indicate associations between mea-
sures of cognitive control and the effective-
ness of emotion regulation (e.g., Compton et
al., 2008; Hoeksma, Oosterlaan, & Schip-
per, 2004; Robinson, 2007). For example,
Schmeichel and Demaree (2010; Schmeichel,
Volokhov, & Demaree, 2008) demonstrated
that undergraduates with higher working
memory capacity (a facet of cognitive con-
trol) were better able to suppress negative
emotional expressions and to reappraise
stimuli in an unemotional manner.
Cognitive control capacities become
increasingly complex and efficient through-
out childhood and adolescence and into
young adulthood (for review, see Yurgelun-
Todd, 2007). Maturation of the prefrontal
cortex has been associated with the devel-
opment of these skills (e.g., Casey, Getz,
& Galvan, 2008; Casey et al., 2010; Dahl,
2001, 2004; Steinberg, 2008). Structural
maturation includes a decrease in gray mat-
ter. This is reflective of synaptic pruning,
that is, the elimination of unused neural
connections, which possibly enables more
focal activation of these brain regions. These
structural developments are also associated
with functional changes. Studies examining
performance on tasks requiring cognitive
control (e.g., Stroop or flanker tasks), for
example, suggest an age- related increase in
the activation of the dorsolateral prefrontal
cortex during childhood and adolescence,
and a further age- related increase from ado-
lescence to young adulthood in the focaliza-
tion and efficiency of selective recruitment
of cognitive control areas (for reviews, see
Casey et al., 2008; Steinberg, 2008). Behav-
ioral studies show that these structural and
functional changes are associated with per-
formance improvements in a variety of cog-
nitive tasks from childhood to late adoles-
cence (e.g., Best & Miller, 2010; Forman,
190 DEVELOPMENTAL CONSIDERATIONS
Mäntylä, & Carelli, 2011; Ordaz, Davis, &
Luna, 2010).
The pattern of improving cognitive control
associated with maturation of the prefrontal
cortex suggests that self- regulation compe-
tence in general, and emotion regulation
competence in particular, should improve
from childhood to adulthood (Casey et al.,
2010). However, the well- documented peak
in impulsivity and risky/reckless behavior
in adolescents and emerging adults demon-
strates limitations in the ability or willing-
ness to resist temptations and peer influence
(see also Luerssen & Ayduk, Grecucci &
Salfey, and Cole, this volume) and suggests
a nonlinear development of self- regulation
competence (Steinberg, 2008). The tempo-
rary increase in negative emotionality (e.g.,
Ciarrochi, Heaven, & Supavadeeprasit,
2008) and affective instability (e.g., Larson
et al., 2002) during adolescence gives rise
to the assumption that a similar nonlinear
development is characteristic of emotion
regulation skills as well.
Several contemporary positions on ado-
lescent brain development conclude that an
exclusive focus on prefrontal cognitive con-
trol areas is insufficient for understanding
the neurophysiological correlates of ado-
lescent emotion regulation. These models
propose that it is also necessary to take into
consideration subcortical regions involved in
the processing of emotional information, as
well as the coordination between these and
prefrontal regions (e.g., Casey et al., 2010;
Dahl, 2001; Steinberg, 2008). Central to
these models is the assumption that struc-
tural and functional maturation of these
various brain systems do not occur in par-
allel. For example, subcortical regions criti-
cal to affective processing are assumed to
mature earlier in adolescence than cortical
regions subserving cognitive control. The
differential timing of maturation in differ-
ent brain regions is expected to result in a
temporarily increased disjunction between
younger adolescents’ affective experiences
and their emotion regulation abilities. This
disjunction is smaller during childhood,
when both systems are still developing, and
becomes smaller again during adulthood,
when both systems have fully matured.
The assumption of differential develop-
mental timing in prefrontal and subcortical
regions is consistent with nonhuman pri-
mate and human postmortem studies but
needs further empirical confirmation (e.g.,
Steinberg, 2008). Furthermore, Pfeifer and
Allen (2012) recently criticized this assump-
tion as being based on an overly simplifying
conception of structure– function mappings
in the brain. Development of the prefrontal
cortex, for example, may also be associated
with more emotional understanding and
complexity, which in turn could be related
to emotional experiences. In addition, while
neurophysiological maturation undeniably
plays a central role in emotion regulation in
adolescence, external influences, to which
we turn next, are also important for those
learning processes.
Familial Contexts
and Emotion Regulation
Emotion regulation abilities are funda-
mentally shaped by the continual interac-
tions between individuals and their social
environments. On the one hand, demands
on emotion regulation skills are often par-
ticularly high in social situations (Bell &
Calkins, 2000). Individuals are required
to comply with sociocultural norms and
expectations regarding appropriate emo-
tional experiences and expressions in social
situations. Furthermore, attainment of situ-
ational goals would often not be possible if
individuals were not able to regulate their
emotions effectively (e.g., to control their
anxiety or anger appropriately; Thompson,
1994).
On the other hand, social contexts also
present important influences that facilitate
or hinder emotion regulation skills (Bariola
et al., 2011). Most of the available research
focuses on the respective role of familial con-
texts. Morris, Silk, Steinberg, Myers, and
Robinson (2007), for example, propose that
family contexts influence the development
of emotion regulation during childhood
and adolescence in three important ways:
through observation learning (e.g., when
parents’ own emotion regulation serves
as a social role model for their offspring),
through parenting practices and explicit
instruction (e.g., when parents coach emo-
tion regulation strategies), and through the
emotional climate in the family (e.g., when
parental behaviors and attitudes toward
the offspring’s emotional expressions and
Emotion Regulation in Adolescence 191
experiences reinforce or discourage emotion
regulation).
Empirical research on the role of paren-
tal influences on the emotion regulation of
offspring has typically focused on families
with infants or children (see Thompson, this
volume). Fewer investigations are available
on the role of familial contexts for emotion
regulation in adolescence. This work sug-
gests that parents’ emotion– socialization
behaviors continue to be important when
their children become adolescents. This is
the case even though adolescents’ orienta-
tion toward more autonomy increases the
relative importance of extrafamilial influ-
ences, such as peers, media, neighborhood,
or culture, as socialization agents of emotion
regulation (Bariola et al., 2011). Shortt and
colleagues (2010), for example, found that
maternal emotion coaching (e.g., approach-
ing the adolescent when he or she is upset
to talk about the situation and experienced
emotions) was associated with fewer diffi-
culties of their 10- to 13-year-old children
in regulating anger. Better anger control, in
turn, was associated with fewer external-
izing problems of the adolescent. Maternal
emotion coaching in this study also pre-
dicted fewer externalizing problems of the
adolescent 3 years later (i.e., at ages 13–16).
Sheeber, Allen, Davis, and Sorensen (2000)
found that maternal approval or affirma-
tion of adolescent depressive behaviors was
predictive of longer episodes of negative
affect on the part of the adolescent (ages
12–19 years). The authors assumed that this
reflects a poorer ability to regulate negative
affect. Yap, Allen, and Ladouceur (2008)
found a relationship between self- reported
maternal tendencies to be restrictive and
unaccepting of positive affective displays
and adolescents’ use of maladaptive emotion
regulation strategies in conflict situations.
More restrictive maternal tendencies were
associated with a higher tendency among
early adolescents to use maladaptive strate-
gies, such as venting or dysregulated expres-
sion of negative affect, which in turn were
associated with more depressive symptoms.
Evidence also suggests that emotion–
socialization styles in the family need to be
adjusted to the age and developmental status
of the offspring. Direct intervention in the
form of soothing or directive instructions
is effective in children at younger ages. For
adolescents, however, indirect influences,
such as talking about possible emotion
regulation strategies, fit better with their
cognitive and self- regulation competen-
cies, and their increased need for autonomy
(Bell & Calkins, 2000; Morris et al., 2007).
Thus, parental balancing of the adolescent’s
opposing needs for autonomy on the one
hand, and for guidance and structure on the
other is particularly important for emotion–
socialization in adolescence. Imbalance in
either direction can have potentially disad-
vantageous effects on adolescents’ socio-
emotional adaptation. Adolescents who
remain overly dependent on their parents
appear to be at a higher risk of internaliz-
ing problems (e.g., depression; for review,
see Morris et al., 2007). Lack of emotion
regulation skills has been proposed to play a
mediating role in this regard (for review, see
Bell & Calkins, 2000). In contrast, adoles-
cents who lack or refuse emotional guidance
from their parents seem to be at a higher risk
for externalizing problems (e.g., rule break-
ing and delinquency, substance use, aggres-
sion). These behaviors, again, have been
associated with a lack of skills to regulate
emotions, particularly anger (for review, see
Morris et al., 2007).
Interaction between Internal
and External Factors
Evidence suggests that a complex set of
internal and external factors contributes to
the development of emotion regulation skills
during adolescence. We illustrated this using
the examples of individuals’ neurophysi-
ological development, which represents an
essential internal factor, and their famil-
ial context, which can provide significant
external influences on emotion regulation.
Importantly, the effects of these internal and
external factors are not unidirectional. They
reciprocally shape each other. Familial influ-
ences on emotion regulation competence,
for example, are modulated by the propen-
sity of adolescents to experience and express
intense affect. The latter, as discussed ear-
lier, can in turn be related to a temporary
dissociation of neurophysiological changes
yielding increased emotional reactivity at
a time when control functions have not
yet fully developed. In a parent– adolescent
interaction study, Schulz, Waldinger,

192 DEVELOPMENTAL CONSIDERATIONS
Hauser, and Allen (2005) indeed showed
that parents were less positively engaged and
showed more hostility when their adolescent
children fully expressed their emotions than
when they controlled their emotion expres-
sions. Other studies also indicate that par-
ents may have difficulties in coping with
adolescents’ uninhibited displays of intense
emotions, and that these parental difficulties
can shape emotion coaching and the emo-
tional climate in the family, and thus feed
back to the adolescent (e.g., Dishion, Nel-
son, & Bullock, 2004).
Taken together, the research reviewed so
far suggests that interactions among a com-
plex set of internal and external factors are
associated with the development of emotion
regulation skills during adolescence. Struc-
tural and functional changes of the pre-
frontal cortex subserving cognitive control
abilities might facilitate cognitive emotion
regulation skills throughout adolescence.
Simultaneous increases in affective reac-
tivity associated with the development of
subcortical regions, however, might over-
tax these evolving regulatory abilities. Such
neurobiological factors could interact with
aspects of the individual’s social context in
promoting or hindering the development of
emotion regulation skills.
Emotion Regulation Motivation
To date, only little attention has been paid to
the fact that emotion regulatory behaviors
are preceded and fundamentally shaped by
motivational processes (see John & Eng, this
volume). One reason for this is that most
investigators seem to assume that emotion
regulation is inevitably “prohedonic,” that
is, always directed at optimizing one’s well-
being. Only recently has awareness arisen
that occasional exceptions are possible, that
is, that emotion regulation can sometimes be
“contrahedonic.” People may occasionally
be inclined to dwell on or intensify negative
emotional experiences, such as anger or sad-
ness, or to lessen positive ones, such as pride
or amusement (e.g., Erber & Wang Erber,
2000; Riediger et al., 2009; Tamir, 2009).
Various experience- sampling studies
reported by Riediger and colleagues demon-
strated adolescent peaks in the prevalence of
contra- hedonic motivation in samples rang-
ing in age from 12 years to late adulthood
(Riediger et al., 2009; Riediger, Wrzus, &
Wagner, 2013). Participants reported their
momentary emotional experiences, on aver-
age 54 times over 3 weeks, and whether they
momentarily wanted to influence their feel-
ings. Contrahedonic motivation (wanting
to maintain or enhance negative affect, or
to dampen positive affect) was considerably
less prevalent than prohedonic motivation
(wanting to maintain or enhance positive
affect, or to dampen negative affect) in all
investigated age groups. There were, how-
ever, pronounced age differences. Adoles-
cents reported contrahedonic motivation
most frequently, namely, in about 25% of
the measurement occasions. There were
steep decreases in the prevalence of contra-
hedonic motivation between the adolescent
and the young adult subsamples, and a fur-
ther decline throughout the adult subsam-
ples into old age. Prohedonic motivation,
in contrast, showed an opposite prevalence
pattern. It was least prevalent among adoles-
cent and young adult participants, and most
prevalent in later adulthood (Riediger et al.,
2009, 2013).
Furthermore, analyses of associations
with within- person fluctuations in work-
ing memory capacity indicated that contra-
hedonic motivation was more cognitively
demanding than prohedonic motivation. The
more contrahedonic motivation participants
reported, the lower their momentary work-
ing memory capacity. Prohedonic motiva-
tion, in contrast, was only weakly associated
with fluctuations in working memory. This
was the case irrespective of participants’
ages. Therefore, despite the pronounced
age- related differences in the prevalence
of different affect regulation motivations,
their cognitive requirements appeared to be
independent of the individual’s age (Riedi-
ger, Wrzus, Schmiedek, Wagner, & Linden-
berger, 2011).
Two complementary theoretical perspec-
tives on possible reasons for contrahedonic
motivation are currently being discussed in
the literature. From an instrumental perspec-
tive, it has been argued that there may be sit-
uations in which negative affect is useful, or
when positive affect is disadvantageous for
the individual (e.g., Tamir, 2009). Anger, for
example, can help to assert one’s interests in
an argument, whereas expressions of joy can
be inappropriate when attending a funeral.
Contrahedonic orientation may therefore

Emotion Regulation in Adolescence 193
derive from people being (consciously or
unconsciously) strategic in seeking affec-
tive states that are instrumental in a given
context (Ford & Tamir, 2012). Supporting
this position, Riediger and colleagues (2009)
found contrahedonic motivation to be less
strongly related to people’s current emo-
tional experiences than prohedonic orienta-
tions. That is, while participants tended to
report that they wanted to maintain positive
affect when it was high, to enhance positive
affect when it was low, and to dampen nega-
tive affect when it was high, contrahedonic
orientations were less strongly associated
with participants’ momentary affect. This
is consistent with the idea that contrahe-
donic motivation may serve instrumental
functions that are not necessarily related to
the individual’s current affect. The authors
speculated that the relatively higher preva-
lence of contrahedonic motivation in adoles-
cence might reflect an instrumental value in
tackling the developmental tasks of that life
phase. Repudiating prevailing hedonic con-
ventions, for example, might put adolescents
in situations where they have to deal with
negative emotional experiences. This, in
turn, might help them to establish emotional
autonomy from their parents, affirm a sense
of maturity, develop their sense of identity,
or refine their self- regulatory competencies.
To date, however, these assumptions have
not yet been empirically verified.
A complementary account of possible
reasons for contrahedonic motivation is the
mixed- affect perspective. It proposes that
contrahedonic motivation may also arise
when apparently negative emotional states
are accompanied or followed by positive
experiences, that is, when the emotional
episode is mixed (Andrade & Cohen, 2007;
Riediger et al., 2009). Such mixed affective
experiences might motivate individuals, for
example, to seek or maintain a given nega-
tive affective state because of the positive
aspects they associate with it. In fact, the
experience sampling studies by Riediger
and colleagues demonstrated that mixed
affective experiences in everyday life were
related to participants’ being more likely to
report contrahedonic orientations. In addi-
tion, these studies also showed pronounced
age- related differences in the prevalence of
mixed- affective experiences that followed
the same pattern as those of contrahedonic
motivation: Both mixed affective experi-
ences and contrahedonic motivation were
most prevalent among adolescent partici-
pants and least prevalent among older adults
(Riediger et al., 2009, 2013).
1
Importantly,
this pattern of age- related differences was
not restricted to participants’ self- report; it
was also reflected in implicit representations
of affect valence, as assessed with implicit
association tests. Compared to adults from
various age groups, adolescents associated
positive affect least distinctively with pleas-
antness (vs. unpleasantness) and unhappi-
ness least distinctively with unpleasantness
(vs. pleasantness). The older the participants,
however, the more differentiated their repre-
sentations of the valence of affective states.
Furthermore, the less differentiated people’s
mental representations of affect valence, the
more likely they were to report mixed affect
and contrahedonic motivation in their every-
day lives (Riediger et al., 2013). Although
causal conclusions are not possible given the
correlational nature of these studies, these
findings are in line with the mixed affective
perspective. Thus, the comparatively higher
prevalence of mixed affective experiences in
adolescence could be among the factors that
contribute to a comparatively higher preva-
lence of self- reported contrahedonic moti-
vation in that age group. This seems to be
associated with relatively more undifferenti-
ated mental representations of the valence of
affective states in adolescence.
Emotion Regulation Strategies
The research reviewed so far demonstrates
that internal and external influences on
emotion regulation abilities undergo pro-
found changes during childhood, adoles-
cence, and into young adulthood, and that
adolescents’ emotion regulation motivation
differs from those of adults. But what are
the consequences? Are there age- related dif-
ferences in the strategies individuals use to
regulate their emotions, or in their emotion
regulation effectiveness? An integrated and
interpretable body of research addressing
this question has yet to emerge. Only few
studies have investigated cross- sectional age
differences within or across developmen-
tal periods, and longitudinal evidence on
change within persons over time is scarce.
These studies differ widely in their concep-
tualization of regulatory strategies. This is
194 DEVELOPMENTAL CONSIDERATIONS
partly due to the fact that these studies were
conducted either in the research tradition
of emotion regulation or (more frequently)
in that of coping, with the latter including
emotion regulation as one of many ways of
dealing with stress (Compas, 2009; Gross,
1999). Despite considerable overlap in
research interests, there has been little cross-
talk between these two traditions.
Research within the emotion regula-
tion tradition has most typically proceeded
from the process model of emotion regula-
tion (e.g., Gross, 1999), which distinguishes
antecedent- focused strategies (directed at
changing the emotional input prior to the
actual emotional experience) from response-
focused strategies (directed at modifying
emotional responses after they have been
elicited). Most research has centered around
two prototypical examples, namely, “cog-
nitive reappraisal” (i.e., changing one’s
thinking about an emotion- eliciting situa-
tion or about the capacity to manage it) as
an instance of antecedent- focused emotion
regulation, and “expressive suppression”
(i.e., inhibiting the outward expression of
an emotional experience) as an instance of
response- focused emotion regulation.
More research on regulatory strategies
in adolescence has been conducted within
the coping research tradition, which views
emotion regulation as one of various ways
of dealing with stress. These studies, how-
ever, often referred to different classifica-
tion systems of regulatory strategies, mak-
ing an integration of findings difficult. Two
widely used classification systems are the
primary– secondary control model (e.g.,
Rothbaum, Weisz, & Snyder, 1982) and
the ways-of- coping model (e.g., Lazarus &
Folkman, 1984). The primary– secondary
control model distinguishes regulatory strat-
egies according to their underlying goals.
The aim of primary coping is to influence
characteristics of the stress- eliciting condi-
tions, whereas the aim in secondary coping
is to adapt oneself to the conditions as they
are. The ways-of- coping model, in contrast,
distinguishes regulatory strategies according
to their respective target. Problem- focused
coping refers to modifying the source of
stress, whereas emotion- focused coping
refers to modifying the elicited emotional
response. Primary and secondary coping, as
well as problem- focused coping, partly over-
lap with Gross’s (1999) conceptualization
of antecedent- focused emotion regulation.
Central to these strategies is that they are
directed at factors influencing the process
of emotion generation. Emotion- focused
coping, in contrast, overlaps with response-
focused emotion regulation in Gross’s model,
as both conceptualizations refer to strategies
directed at modifying an emotional response
after it has been activated.
Use of Emotion Regulation Strategies
Zimmer- Gebeck and Skinner (2011) inte-
grated findings from 58 studies compar-
ing coping strategies within the age range
from childhood to adolescence. Overall, the
authors concluded that age differences in
regulatory behaviors are characterized by
two trends.
First, there appears to be an age- graded
increase in regulatory capacities. This is
reflected in not only an increased under-
standing of emotional situations (Labouvie-
Vief, DeVoe, & Bulka, 1989) but also a
broader and more sophisticated repertoire
of regulatory strategies. For example, from
childhood to adolescence, instrumental
action in response to stressors appears to
become gradually supplemented by planful
problem solving. Furthermore, distraction
tactics seem to become more diverse and
increasingly include cognitive distraction,
in addition to behavioral distraction. Also,
compared to children, adolescents appear
to be better able to attend to and reflect on
their own emotional states. They also seem
to use progressively more sophisticated cog-
nitive strategies to deal with emotions, such
as positive self-talk and reappraisal.
The second developmental trend from
childhood to adolescence, according to the
analysis by Zimmer- Gebeck and Skinner
(2011), reflects age- related improvement in
tailoring regulatory attempts to the situa-
tion. That is, adolescents seem to become
increasingly able to engage in those regu-
latory strategies that are most effective in
dealing with particular kinds of stressors.
For example, evidence suggests increasing
use of both problem solving to deal with
modifiable difficulties (e.g., in school or
sports) and distraction to deal with uncon-
trollable stressors (e.g., parental illness)
from childhood to adolescence. Another
example derives from a longitudinal study
on anger regulation among friends. Salisch
Emotion Regulation in Adolescence 195
and Vogelgesang (2005) observed a decline
from older childhood to adolescence in indi-
viduals’ self- reported use of confrontation
and harming, redirection of attention, and
ignoring and self-blame when dealing with
situations involving anger toward a friend.
The authors also found an increase in par-
ticipants’ use of explanation and reconcilia-
tion in such situations.
Zimmer- Gembeck and Skinner (2011)
also emphasized that the apparent improve-
ment in emotion regulatory competence
does not necessarily need to be linear. Some
of the reviewed evidence points to tempo-
rarily increased struggles with regulation in
response to stressors during the transition
from late childhood to early adolescence.
Compared to older adolescents, for exam-
ple, younger adolescents have occasionally
shown lower levels of help- seeking behaviors
and effort expenditure in domains in which
this would be helpful (e.g., regarding school-
related stressors). The authors also reviewed
evidence of a temporary rise in the use of
potentially more maladaptive regulation
strategies during early adolescence, such as
cognitive escape, rumination, verbal aggres-
sion, or venting.
Most of the extant research investigated
the age range from childhood to adolescence.
Only a few available studies compare the use
of regulatory strategies in adolescence and
adulthood. Overall, this research suggests
continued change into young adulthood
(e.g., Blanchard- Fields & Coats, 2008; Gar-
nefski, Legerstee, Kraaij, van den Kommer,
& Teerds, 2002). Garnefski and colleagues,
for example, found that adolescents around
13 years of age reported using various cog-
nitive change strategies (e.g., positive reap-
praisal or refocus on planning) significantly
less frequently than did adults of various age
groups.
Effects of Emotion Regulation
Strategies: Adaptiveness
and Effectiveness
Most studies on the effects of emotion regu-
lation strategies proceed from the idea that it
is meaningful to conceive of an adolescent’s
regulatory style as a trait. These studies
characterize adolescents according to their
overarching tendency to use particular types
of regulatory strategies more than others.
The focus is on the adaptiveness of different
regulatory styles, as characterized by their
association with self- or other- reports of
various indicators of psychological adjust-
ment, such as emotional or behavioral prob-
lems, psychosomatic health, subjective well-
being, and social or academic competence.
Within the research tradition on emotion
regulation, investigations of the adaptive-
ness of specific regulatory strategies have
most typically focused on reappraisal and
expressive suppression. Investigations with
young adults suggest that habitual use of
reappraisal is associated with a healthier
profile of socioemotional adjustment than is
habitual use of expressive suppression, and
that the latter is also associated with greater
cognitive and physiological costs (for an
overview, see Gross, 2002). Investigations in
adolescent samples are rare, but they have
yielded similar patterns of findings (Betts,
Gullone, & Allen, 2009; Hughes, Gullone,
Dudley, & Tonge, 2010).
More research on the adaptiveness of
regulatory styles in adolescence has been
conducted in the research tradition on cop-
ing. Compas and colleagues (2001) reviewed
63 of these studies and concluded that the
majority of the studies demonstrate that
problem- focused and engagement coping
(which overlap with antecedent- focused
emotion regulation) were associated with
better psychological adjustment during
childhood and adolescence. The specific
subtypes most consistently associated with
better adjustment included the generation
of positive and hopeful thoughts (i.e., cog-
nitive reappraisal), careful analysis of the
stressful situation, and selective attention
to positive aspects of the situation. In con-
trast, disengagement and emotion- focused
coping (which overlap largely with response-
focused emotion regulation) were associated
with poorer psychological adjustment in
most studies. However, it was not focusing
on one’s emotions in general that was related
to lower psychological adjustment. Rather,
these effects were due to disengagement
from the stressor or from one’s emotions
(e.g., expressive suppression, avoidance,
or withdrawal), negative cognitions about
the self and the situation, and unregulated
release or ventilation of emotions.
Compas and colleagues (2001) also
emphasized that notable differences in
results across studies may be associated with
the nature of the investigated stressor, par-
196 DEVELOPMENTAL CONSIDERATIONS
ticularly its controllability. This is consistent
with the view that an adaptive regulatory
style may not be characterized primarily by
more frequent use of particular regulation
strategies relative to others, but by a high
level of flexibility (e.g., Gross, 1999). This
flexibility should be evident in positive asso-
ciations between socioemotional adaptation
and adolescents’ ability to tailor specific
regulation attempts to the nature (e.g., the
controllability) of a given situation. While
this claim seems intuitively appealing, future
research needs to strengthen the empirical
foundation supporting it.
The research on the adaptiveness of affect
regulation styles takes a person- centered
(i.e., trait- oriented) approach. Another
approach is process- centered (i.e., state-
oriented) and focuses on how effective indi-
viduals actually are in influencing their emo-
tional experiences in intended ways. Hardly
any evidence is available on the effectiveness
of emotion regulation in adolescence. This
is probably due to the methodological chal-
lenges involved in operationalizing emo-
tion regulation effectiveness. In fact, there
is an ongoing debate whether and to what
degree it is possible to disentangle emotion
regulation from the respective emotional
experience. While some researchers regard
emotion regulation as being experientially
and structurally indistinguishable from the
emotional experience, others claim that
it is possible and meaningful to differenti-
ate between emotion and emotion regula-
tion (for an overview of positions, see, e.g.,
Matarazzo, 2008).
In line with the latter position, emotion
regulation effectiveness in adolescence has
recently been addressed from two different
perspectives. One focuses on the effective-
ness of particular emotion regulation strat-
egies in adolescence. Here, adolescents’ use
of regulatory strategies is not conceived of
as a trait but as a process that can fluctuate
within persons over time. The covariation
of this process with fluctuations in affec-
tive experiences is of particular interest.
Reijntjes, Stegge, Terwogt, Kamphuis, and
Telch (2006), for example, experimentally
manipulated experiences of social rejec-
tion during an ostensible computer game in
10- to 13-year-olds. Contrary to what one
would expect from research on the adaptive-
ness of regulatory styles, problem- focused
engagement in this study was unrelated to
mood improvement after social rejection.
In contrast, better mood improvement was
associated with more behavioral distrac-
tion and less passive behaviors. Silk and col-
leagues (2003) used experience sampling in
12- to 17-year-old participants to investigate
the role of regulatory strategies for mood
dynamics after participants had encoun-
tered negative events in their daily lives.
When adolescents used disengagement-
related strategies in response to subjectively
severe negative events, or when they reacted
involuntarily (e.g., by ruminating or act-
ing impulsively), they maintained higher
levels of negative affect over time, suggest-
ing limited effectiveness of these strategies.
Engagement in primary and secondary con-
trol attempts, however, was unrelated to
the dynamics of negative affect. The latter,
again, is not consistent with what one would
expect based on the majority of studies on
associations between regulatory styles and
more general outcomes of socioemotional
adaptation. Differences in findings across
studies on the effectiveness versus the adap-
tiveness of regulatory strategies might be
attributable to differences in assessment
approaches (process- vs. person- centered)
and/or in the investigated emotion- eliciting
situations. Future research is necessary to
better integrate the findings from these two
lines of research.
A second perspective on emotion regula-
tion effectiveness in adolescence focuses on
respective age- related differences. In two
recent investigations (McRae et al., 2012;
Silvers et al., 2012), participants between 10
and 23 years of age were presented neutral
and aversive picture stimuli from the Inter-
national Affective Picture System (IAPS).
Viewing instructions varied across studies
but generally included a reappraisal condi-
tion (e.g., thinking about the picture in a
way that makes one feel less negative) and
a comparison condition (e.g., reacting natu-
rally to the picture, but not reappraising).
After viewing each picture, participants
rated their current negative affect. Regula-
tion effectiveness was determined as the rel-
ative decrease in negative affect after view-
ing aversive stimuli in the reappraisal versus
the comparison condition. Silvers and col-
leagues observed an age- related increase in
regulation effectiveness from late childhood

Emotion Regulation in Adolescence 197
to late adolescence that tapered off in young
adulthood. McRae and colleagues (2012),
in contrast, found relative age invariance in
regulation effectiveness from ages 10 to 17
years, but an increase in effectiveness from
late adolescence to young adulthood. These
inconsistencies in findings might be due to
methodological differences between the
studies. Procedures in both studies varied,
for example, in the selection of stimuli for
the analyses, and in whether or not partici-
pants were trained in the reappraisal task.
More empirical investigations are necessary
to clarify and expand the empirical picture
on emotion regulation effectiveness through-
out adolescence.
Wrapping Up and Looking Forward
Adolescence is characterized by an upsurge
in the frequency and intensity of emotional
challenges, in a time of life when individuals
have to manage such challenging situations
more and more independently. This provides
developmental stimulation, but it can also
increase vulnerability to adaptational dif-
ficulties. Emotion regulation has been pro-
posed to play an important role in how ado-
lescents adapt to these challenges. Empirical
investigations of this claim vary considerably
regarding their operationalizations of what
“better” emotion regulation entails. System-
atic comparisons of implications of different
facets of emotion regulation for adolescents’
socioemotional adaptation are still lacking.
In addition, causal relations between emo-
tion regulation and developmental adapta-
tion need more systematic investigation in
the future. Another open task for the future
is a more rigorous investigation of possible
interindividual differences in adaptational
outcomes of emotion regulation. There are,
for example, indications that outcomes may
differ between male and female adolescents
(Bowie, 2010; Perry- Parrish & Zeman,
2011).
In addition to assuming implications of
emotion regulation for adolescents’ socio-
emotional adjustment in general, it has also
been proposed that emotion regulation is
involved in the unique emotional lives of
adolescents in particular. Compared to chil-
dren and adults, adolescents report more
negative, more mixed, and more rapidly
varying affective states. Empirical evidence
that directly links emotion regulation to the
characteristic emotional experiences of ado-
lescents is rare. Providing such evidence rep-
resents an important undertaking for future
studies. Knowledge about emotion regula-
tion in adolescence can thus have important
practical implications. We believe that an
integration of findings from three research
areas— influences on the development of
emotion regulation, emotion regulation
motivation, and emotion regulation strate-
gies— is crucial for a better understanding of
adolescents’ emotion regulation. This chap-
ter has reviewed contemporary positions
within each of these areas of inquiry.
A complex set of internal and external
factors contribute to the development of
emotion regulation skills during adoles-
cence. A temporal dissociation in the matu-
ration of prefrontal and subcortical brain
regions, for example, is assumed to result in
a temporary decrease in emotion regulation
capacity when cognitive control capacities
have not yet sufficiently improved to provide
adequate control of the progressively more
active emotion system. It seems plausible
that this may be one of the factors contribut-
ing to the unique quality of emotional expe-
riences observed during adolescence. How-
ever, predictions about relations between
neurophysiological changes during adoles-
cence and adolescents’ development of emo-
tion regulation abilities are still in need of
systematic empirical verification. So far, lit-
tle is known about the possibility that differ-
ent emotion regulation strategies might vary
in terms of how much cognitive control they
require, and that people can recruit external
resources when internal resources are insuf-
ficient for effective emotion regulation (e.g.,
by asking somebody else to distract one-
self from emotion- eliciting thoughts when
attempts of self- initiated distraction are not
successful, Opitz et al., 2012). Furthermore,
emotion regulation skills in adolescence are
also influenced (i.e., facilitated or impaired)
by many other factors, such as varying
degrees of parental structure and supervi-
sion. Future research needs to provide more
empirical evidence on the interplay of these
various internal and external factors to
arrive at a more integrated understanding of
influences on emotion regulation in adoles-
cence.

198 DEVELOPMENTAL CONSIDERATIONS
In comparison to adults from various
age groups, adolescents have been found to
report more contrahedonic and less prohe-
donic motivation. This suggests that part
of the negative emotionality that is charac-
teristic of adolescence may be intentionally
sought and maintained by the individual.
Many questions, however, still remain open.
For example, future research will need to
employ well- controlled experiments to dis-
entangle the causal mechanisms involved in
these associations. This will also contribute
to a better understanding of the reasons for
adolescent patterns of pro- and contrahe-
donic orientations. Another open question
pertains to potential functions of contrahe-
donic motivation, for example, related to the
development of autonomy, self- regulatory
skills, or other aspects of socioemotional
development of adolescents. Finally, rela-
tions between adolescents’ emotion regula-
tion motivation and their actual engagement
in emotion regulatory behaviors, as well as
the effectiveness of these emotion regula-
tion attempts, need to be explored in future
investigations.
Available research on age- related differ-
ences in the use, adaptiveness, and effective-
ness of adolescents’ strategies for regulating
their emotions differs widely in the con-
ceptual frameworks employed. This leads
to difficulty in deriving a cohesive picture
about possible developmental changes dur-
ing adolescence and adjacent developmental
periods. Tentatively, however, this research
suggests an age- related increase in regulation
competence from childhood to adolescence,
as reflected in an increasingly sophisticated
understanding of emotional situations and a
broadening repertoire of regulatory strate-
gies. This appears to coincide with an age-
related increase in the ability to tailor regu-
latory strategies to the specific requirements
of the emotion- eliciting situation. Future
research should further clarify the complex
empirical picture on adolescent development
in the use and adaptiveness of regulatory
strategies. Meta- analyses of the available
empirical evidence would be helpful in this
respect. Furthermore, more empirical evi-
dence is needed on differences in the actual
effectiveness of regulatory attempts between
children, adolescents, and adults.
The three themes on emotion regulation
development in adolescence reviewed in this
chapter currently represent relatively inde-
pendent bodies of research. A more explicit
integration of these various research per-
spectives will help us to arrive at a more
integrated and interpretable picture of the
development of emotion regulation during
adolescence. In addition, future research is
necessary to address various limitations of
the currently available research. For exam-
ple, more longitudinal evidence is necessary
to portray developmental changes within
persons as they move from childhood to
adolescence and young adulthood. Such por-
trayals should also take into account more
explicitly the possibility of nonlinear trends
in development, such as temporary pertur-
bations or deviations from growth trajecto-
ries. Furthermore, in addition to adolescents’
chronological ages, the role of their pubertal
status (which can vary substantially by age)
should be taken into consideration more
comprehensively, because affective pro-
cesses during adolescence are strongly asso-
ciated with puberty- related developments
(Steiner, Dunn, & Born, 2003). Finally,
multimethodological enrichment of research
approaches would be desirable to overcome
the methodological limitations associated
with the currently prevailing dominance of
self- report approaches in most research on
emotion regulation in adolescence.
Note
1. Note that research on age differences in the
frequency of mixed affect during adulthood
has yielded inconclusive findings. Results
from these studies range from age- related
decrease, to age invariance, to age- related
increase in the propensity to experience mixed
affect. Heterogeneity between studies regard-
ing the operationalization of mixed affect may
be among the reasons for the inconclusiveness
of findings with this measure (for a review, see
Riediger & Rauers, in press).
References
Allen, N., & Sheeber, L. (Eds.). (2009). Adoles-
cent emotional development and the emer-
gence of depressive disorders. New York:
Cambridge University Press.
Andrade, E. B., & Cohen, J. B. (2007). On the
Emotion Regulation in Adolescence 199
consumption of negative feelings. Journal of
Consumer Research, 34, 283–300.
Bariola, E., Gullone, E., & Hughes, E. K. (2011).
Child and adolescent emotion regulation:
The role of parental emotion regulation and
expression. Clinical Child and Family Psy-
chology Review, 14, 198–212.
Bell, K. L., & Calkins, S. D. (2000). Relation-
ships as inputs and outputs of emotion regula-
tion. Psychological Inquiry, 11, 160–163.
Best, J. R., & Miller, P. H. (2010). A develop-
mental perspective on executive function.
Child Development, 81, 1641–1660.
Betts, J., Gullone, E., & Allen, J. S. (2009). An
examination of emotion regulation, tempera-
ment, and parenting style as potential predic-
tors of adolescent depression risk status: A
correlational study. British Journal of Devel-
opmental Psychology, 27, 473–485.
Blanchard- Fields, F., & Coats, A. H. (2008). The
experience of anger and sadness in everyday
problems impacts age differences in emotion
regulation. Developmental Psychology, 44,
1547–1556.
Bowie, B. H. (2010). Understanding the gender
differences in pathways to social deviancy:
Relational aggression and emotion regulation.
Archives of Psychiatric Nursing, 24, 27–37.
Buckley, M., & Saarni, C. (2009). Emotion regu-
lation: Implications for positive youth devel-
opment. In R. Gilman, E. S. Huebner, & M.
J. Furlong (Eds.), Handbook of positive psy-
chology in schools (pp. 107–118). New York:
Routledge.
Casey, B., Getz, S., & Galvan, A. (2008). The
adolescent brain. Developmental Review, 28,
62 –77.
Casey, B., Jones, R. M., Levita, L., Libby, V.,
Pattwell, S. S., Ruberry, E. J., et al. (2010).
The storm and stress of adolescence: Insights
from human imaging and mouse genetics.
Developmental Psychobiology, 52, 225–235.
Ciarrochi, J., Heaven, P. C., & Supavadeeprasit,
S. (2008). The link between emotion identifi-
cation skills and socio- emotional functioning
in early adolescence: A 1-year longitudinal
study. Journal of Adolescence, 31, 565–582.
Cole, S. W. (this volume, 2014). Emotion regula-
tion and gene expression. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 571–585). New York: Guilford Press.
Compas, B. E. (2009). Coping, regulation, and
development during childhood and adoles-
cence. New Directions for Child and Adoles-
cent Development, 2009, 87–99.
Compas, B. E., Connor-Smith, J. K., Saltzman,
H., Thomsen, A. H., & Wadsworth, M. E.
(2001). Coping with stress during childhood
and adolescence: Problems, progress, and
potential in theory and research. Psychologi-
cal Bulletin, 127, 87–127.
Compton, R. J., Robinson, M. D., Ode, S.,
Quandt, L. C., Fineman, S. L., & Carp, J.
(2008). Error- monitoring ability predicts daily
stress regulation: Research article. Psychologi-
cal Science, 19, 702–708.
Dahl, R. E. (2001). Affect regulation, brain
development, and behavioral/emotional health
in adolescence. CNS Spectrums, 6, 60–72.
Dahl, R. E. (2004). Adolescent brain develop-
ment: A period of vulnerabilities and oppor-
tunities. Annals of the New York Academy of
Sciences, 1021, 1–22.
Dishion, T. J., Nelson, S. N., & Bullock, B. M.
(2004). Premature adolescent autonomy: Par-
ent disengagement and deviant peer process in
the amplification of problem behavior. Jour-
nal of Adolescence, 27, 515–530.
Eccles, J. S., Templeton, J., Barber, B., & Stone,
M. (2003). Adolescence and emerging adult-
hood: The critical passage ways to adulthood.
In M. H. Bornstein, L. Davidson, C. L. M.
Keyess, & K. A. Moore (Eds.), Well-being:
Positive development across the life course
(pp. 383–406). Mahwah, NJ: Erlbaum.
Erber, R., & Wang Erber, M. (2000). The self-
regulation of moods: Second thoughts on the
importance of happiness in everyday life. Psy-
chological Inquiry, 11, 142–148.
Erikson, E. E. (1968). Identity: Youth and crisis.
New York: Norton.
Ford, B. Q., & Tamir, M. (2012). When getting
angry is smart: Emotional preferences and
emotional intelligence. Emotion, 12, 685–
689.
Forman, H., Mäntylä, T., & Carelli, M. G.
(2011). Time keeping and working memory
development in early adolescence: A 4-year
follow- up. Journal of Experimental Child
Psychology, 108, 170 –179.
Garnefski, N., Legerstee, J., Kraaij, V., van den
Kommer, T., & Teerds, J. (2002). Cognitive
coping strategies and symptoms of depression
and anxiety: A comparison between adoles-
cents and adults. Journal of Adolescence, 25,
603–611.
Gray, J. R., & Braver, T. S. (2007). Integration of
emotion and cognitive control: A neurocom-
putational hypothesis of dynamic goal regula-
tion. In S. C. Moore & M. Oaksford (Eds.),
200 DEVELOPMENTAL CONSIDERATIONS
Emotional cognition: From brain to behavior
(pp. 289–316). Amsterdam: Benjamins.
Grecucci, A., & Sanfey, A. G. (this volume,
2014). Emotion regulation and decision mak-
ing. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed.). New York: Guilford
Press.
Gross, J. J. (1999). Emotion regulation: Past,
present, and future. Cognition and Emotion,
13, 551–573.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39, 281–291.
Gunnar, M. R., Wewerka, S., Frenn, K., Long,
J. D., & Griggs, C. (2009). Developmental
changes in hypothalamus– pituitary– adrenal
activity over the transition to adolescence:
Normative changes and associations with
puberty. Development and Psychopathology,
21, 69–85.
Hoeksma, J. B., Oosterlaan, J., & Schipper,
E. M. (2004). Emotion regulation and the
dynamics of feelings: A conceptual and meth-
odological framework. Child Development,
75, 354–360.
Hughes, E. K., Gullone, E., Dudley, A., & Tonge,
B. (2010). A case control study of emotion
regulation and school refusal in children and
adolescents. Journal of Early Adolescence, 30,
691–706.
Labouvie- Vief, G., DeVoe, M., & Bulka, D.
(1989). Speaking about feelings: Conceptions
of emotion across the life span. Psychology
and Aging, 4, 425– 437.
Larson, R., & Asmussen, L. (1991). Anger,
worry, and hurt in early adolescence: An
enlarging world of negative emotions. In M.
E. Colton & S. Gore (Eds.), Adolescent stress:
Causes and consequences (pp. 21–41). New
York: de Gruyter.
Larson, R., & Lampman- Petraitis, C. (1989).
Daily emotional states as reported by children
and adolescents. Child Development and Psy-
chopathology, 60, 1250–1260.
Larson, R., Moneta, G., Richards, M. H., &
Wilson, S. (2002). Continuity, stability, and
change in daily emotional experience across
adolescence. Child Development, 73, 1151–
1165.
Lazarus, R. S., & Folkman, S. (1984). Coping
and adaption. In W. D. Gentry (Ed.), Hand-
book of behavioral medicine (pp. 282–325).
New York: Guilford Press.
Luerssen, A., & Ayduk, O. (this volume, 2014).
The role of emotion and emotion regulation in
the ability to delay gratification. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp. 111–125). New York: Guilford Press.
Matarazzo, O. (2008). Use and effectiveness of
three modalities of emotion regulation after
negative life events: Rumination, distraction
and social sharing. In A. B. Turley & G. C.
Hofmann (Eds.), Life style and health research
progress (pp. 87–138). Hauppauge, New York:
Nova Biomedical Books.
Mauss, I. B., Bunge, S. A., & Gross, J. J. (2007).
Automatic emotion regulation. Social and Per-
sonality Psychology Compass, 1, 146 –167.
McLaughlin, K. A., Hatzenbuehler, M. L., Men-
nin, D. S., & Nolen- Hoeksema, S. (2011).
Emotion dysregulation and adolescent psy-
chopathology: A prospective study. Behaviour
Research and Therapy, 49, 544–554.
McRae, K., Gross, J. J., Weber, J., Robertson,
E. R., Sokol- Hessner, P., Ray, R. D., et al.
(2012). The development of emotion regula-
tion: An fMRI study of cognitive reappraisal
in children, adolescents and young adults.
Social Cognitive and Affective Neuroscience,
7, 11–22.
Mesquita, B., De Leersnyder, J., & Albert, D.
(this volume, 2014). The cultural regulation
of emotions. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 284–302).
New York: Guilford Press.
Miller, E. M., & Shields, S. A. (1980). Skin con-
ductance response as a measure of adolescents’
emotional reactivity. Psychological Reports,
46, 587–590.
Mohanty, A., Engels, A. S., Herrington, J. D.,
Heller, W., Ho, M. H., Banich, M. T., et al.
(2007). Differential engagement of anterior
cingulate cortex subdivisions for cognitive and
emotional function. Psychophysiology, 44,
343–351.
Morris, A. S., Silk, J. S., Steinberg, L., Myers, S.
S., & Robinson, L. R. (2007). The role of the
family context in the development of emotion
regulation. Social Development, 16, 361–388.
Ochsner, K. N., & Gross, J. J. (2005). The cogni-
tive control of emotion. Trends in Cognitive
Sciences, 9, 242–249.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 23–42). New York: Guilford
Press.
Emotion Regulation in Adolescence 201
Opitz, P. C., Gross, J. J., & Urry, H. L. (2012).
Selection, optimization, and compensation
in the domain of emotion regulation: Appli-
cations to adolescence, older age, and major
depressive disorder. Social and Personality
Psychology Compass, 6, 142–155.
Ordaz, S., Davis, S., & Luna, B. (2010). Effects
of response preparation on developmental
improvements in inhibitory control. Acta Psy-
chologica, 134, 253–263.
Perry-
P
arrish, C., & Zeman, J. (2011). Relations
among sadness regulation, peer acceptance,
and social functioning in early adolescence:
The role of gender. Social Development, 20,
135–153.
Pfeifer, J. H., & Allen, N. B. (2012). Arrested
development? Reconsidering dual-
s
ystems
models of brain function in adolescence and
disorders. Trends in Cognitive Sciences, 16,
322–329.
Reijntjes, A., Stegge, H., Terwogt, M. M., Kam-
phuis, J. H., & Telch, M. J. (2006). Emotion
regulation and its effects on mood improve-
ment in response to an in vivo peer rejection
challenge. Emotion, 6, 543–552.
Riediger, M., & Rauers, A. (in press). Do everyday
affective experiences differ throughout adult-
hood? A review of ambulatory-
a
ssessment evi-
dence. In P. Verhaeghen & C. Hertzog (Eds.),
The Oxford handbook of emotion, social
cognition, and everyday problem solving dur-
ing adulthood. New York: Oxford University
Press.
Riediger, M., Schmiedek, F., Wagner, G., & Lin-
denberger, U. (2009). Seeking pleasure and
seeking pain: Differences in pro- and contra-
h
edonic motivation from adolescence to old
age. Psychological Science, 20, 1529–1535.
Riediger, M., Wrzus, C., Schmiedek, F., Wagner,
G. G., & Lindenberger, U. (2011). Is seeking
bad mood cognitively demanding? Contra-
hedonic orientation and working- memory
capacity in everyday life. Emotion, 11, 656–
665.
Riediger, M., Wrzus, C., & Wagner, G. G.
(2013). Happiness is pleasant, or is it? Rep-
resentations of affect valence are associated
with contra-
h
edonic motivation and mixed
affect in daily life. Manuscript submitted for
publication.
Robinson, M. D. (2007). Gassing, braking, and
self-
r
egulating: Error self-
r
egulation, well-
being, and goal-
r
elated processes. Journal of
Experimental Social Psychology, 43, 1–16.
Rothbaum, F., Weisz, J. R., & Snyder, S. S.
(1982). Changing the world and changing the
self: A two-
p
rocess model of perceived control.
Journal of Personality and Social Psychology,
42, 5–37.
Salisch, M. V., & Vogelgesang, J. (2005). Anger
regulation among friends: Assessment and
development from childhood to adolescence.
Journal of Social and Personal Relationships,
22, 837–855.
Schmeichel, B. J., & Demaree, H. A. (2010).
Working memory capacity and spontaneous
emotion regulation: High capacity predicts
self-
e
nhancement in response to negative feed-
back. Emotion, 10, 739–744.
Schmeichel, B. J., Volokhov, R. N., & Demaree,
H. A. (2008). Working memory capacity and
the self-
re
gulation of emotional expression
and experience. Journal of Personality and
Social Psychology, 95, 1526–1540.
Schulz, M. S., Waldinger, R. J., Hauser, S. T., &
Allen, J. P. (2005). Adolescents’ behavior in
the presence of interparental hostility: Devel-
opmental and emotion regulatory influences.
Development and Psychopathology, 17, 489–
507.
Shaver, P. R., & Mikulincer, M. (this volume,
2014). Adult attachment and emotion regula-
tion. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp.
237–250). New
York: Guilford Press.
Sheeber, L., Allen, N., Davis, B., & Sorensen, E.
(2000). Regulation of negative affect during
mother–
child problem-
solving interactions:
Adolescent depressive status and family pro-
cesses. Journal of Abnormal Child Psychol-
ogy, 28, 467–479.
Shortt, J. W., Stoolmiller, M., Smith-Shine, J. N.,
Mark Eddy, J., & Sheeber, L. (2010). Mater-
nal emotion coaching, adolescent anger regu-
lation, and siblings externalizing symptoms.
Journal of Child Psychology and Psychiatry,
51, 799–808.
Silk, J. S., Steinberg, L., & Morris, A. S. (2003).
Adolescents’ emotion regulation in daily life:
Links to depressive symptoms and problem
behavior. Child Development, 74, 1869–1880.
Silk, J. S., Vanderbilt-
A
driance, E., Shaw, D. S.,
Forbes, E. E., Whalen, D. J., Ryan, N. D., et
al. (2007). Resilience among children and ado-
lescents at risk for depression: Mediation and
moderation across social and neurobiological
contexts. Development and Psychopathology,
19, 841–865.
202 DEVELOPMENTAL CONSIDERATIONS
Silvers, J. A., McRae, K., Gabrieli, J. D., Gross,
J. J., Remy, K. A., & Ochsner, K. N. (2012).
Age- related differences in emotional reactiv-
ity, regulation, and rejection sensitivity in ado-
lescence. Emotion, 12(6), 1235–1247.
Sim, L., & Zeman, J. (2006). The contribution
of emotion regulation to body dissatisfaction
and disordered eating in early adolescent girls.
Journal of Youth and Adolescence, 35, 219–
228.
Steinberg, L. (2008). A social neuroscience per-
spective on adolescent risk- taking. Develop-
mental Review, 28, 78–106.
Steiner, M., Dunn, E., & Born, L. (2003). Hor-
mones and mood: From menarche to meno-
pause and beyond. Journal of Affective Disor-
ders, 74, 67–83.
Stroud, L. R., Foster, E., Papandonatos, G. D.,
Handwerger, K., Granger, D. A., Kivlighan,
K. T., et al. (2009). Stress response and the
adolescent transition: Performance versus peer
rejection stressors. Development and Psycho-
pathology, 21, 47–68.
Sumter, S., Bokhorst, C., Miers, A., Van Pelt,
J., & Westenberg, P. (2010). Age and puberty
differences in stress responses during a public
speaking task: Do adolescents grow more sen-
sitive to social evaluation? Psychoneuroendo-
crinology, 35, 1510–1516.
Tamir, M. (2009). What do people want to feel
and why? Pleasure and utility in emotion regu-
lation. Current Directions in Psychological
Science, 18, 101–105.
Thompson, R. A. (1994). Emotion regulation:
A theme in search of definition. Monographs
of the Society for Research in Child Develop-
ment, 59, 25–52.
Thompson, R. A. (this volume, 2014). Socializa-
tion of emotion and emotion regulation in the
family. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 173–186). New
York: Guilford Press.
Thorne, A. (2004). Putting the person into social
identity. Human Development, 47, 361–365.
Yap, M. B., Allen, N. B., & Ladouceur, C. D.
(2008). Maternal socialization of positive
affect: The impact of invalidation on adolescent
emotion regulation and depressive symptom-
atology. Child Development, 79, 1415–1431.
Yap, M. B., Allen, N. B., & Sheeber, L. (2007).
Using an emotion regulation framework to
understand the role of temperament and fam-
ily processes in risk for adolescent depressive
disorders. Clinical Child and Family Psychol-
ogy Review, 10, 180–196.
Yurgelun- Todd, D. (2007). Emotional and cog-
nitive changes during adolescence. Current
Opinion in Neurobiology, 17, 251–257.
Zeman, J., Cassano, M., Perry- Parrish, C., &
Stegall, S. (2006). Emotion regulation in chil-
dren and adolescents. Journal of Developmen-
tal and Behavioral Pediatrics, 27, 155–168.
Zimmer- Gembeck, M. J., & Skinner, E. A.
(2011). Review: The development of coping
across childhood and adolescence: An integra-
tive review and critique of research. Interna-
tional Journal of Behavioral Development,
35, 1–17.

203
Developmental psychologists have docu-
mented age- related changes in the regulation
of emotions across childhood that paral-
lel biological maturation. As children and
adolescents acquire and develop abilities
to control impulses and gain awareness of
themselves and others, they begin to achieve
mastery over their environments and become
increasingly effective at describing and regu-
lating their emotions. Brain maturation and
patterns of neurological functioning related
to emotional processes continue to develop
throughout childhood and adolescence.
Greater inhibitory control, for example, is
reflected in increased activity in the prefron-
tal cortex from childhood into the early 20s
(e.g., McRae et al., 2012). In the latter half
of the lifespan, however, the role of biologi-
cal development in emotional experience and
regulation is relatively understudied. Physi-
ological functioning is gradually degraded
with age, and it has long been assumed that
such changes affect emotional functioning.
A growing literature, however, suggests that
aging is associated with relatively positive
emotional experience in everyday life. As the
understanding of emotional aging improves,
it is becoming increasingly clear that older
people typically engage in strategies that
maintain positive emotional experiences
and limit negative ones. It is also becoming
clear that circumstances that restrict strat-
egy usage may have especially dire outcomes
for older adults.
For our purposes herein, we define emo-
tion regulation as efforts to experience rela-
tively low levels of negative affect and elicit
relatively high levels of positive affect (see
Gross, this volume, for a more complete
description of emotion regulation). The
study of age differences in emotion regula-
tion has grown, although direct comparisons
across the lifespan remain limited. Similarly,
studies that focus on thoughts and behav-
iors that enable people to avoid or mitigate
exposure to distressing events rarely link
such strategies to affective outcomes. Only
a few studies, which we describe below,
examine age differences in regulation when
people are intentionally increasing (i.e., up-
regulating or amplifying) or decreasing (i.e.,
down- regulating) their positive and negative
emotional states.
We focus our review on normal aging as
opposed to disease- related aging, and on the
general experience of positive and negative
affect as opposed to discrete emotions. We
begin with a brief overview of age differ-
ences in physiological processes involved in
emotional experience. We then describe the
scope of the existing literature examining
age and affective well-being. Next, we pres-
CHAPTER 13
Emotion Regulation and Aging
Susan Turk Charles
Laura L. Carstensen

204 DEVELOPMENTAL CONSIDERATIONS
ent socioemotional selectivity theory (SST)
and strength and vulnerability integration
(SAVI), both of which guide our understand-
ing of emotional development in adulthood.
SST has received considerable empirical
support since it was proposed in the early
1990s (see Charles & Carstensen, 2010). Its
core tenets concern the ways that time hori-
zons influence goals. SST initially addressed
emotion somewhat indirectly, based on the
premise that as people become increasingly
focused (selectivity) on emotionally mean-
ingful goals, well-being likely benefits. As
evidence for social selection accrued along
with evidence that emotional well-being is
relatively high in later life, we began to con-
sider selection as a highly effective form of
antecedent emotion regulation (Carstensen,
Fung, & Charles, 2003). SAVI, in contrast,
was proposed relatively recently. It aims to
integrate the relatively positive profile of
emotional well-being with concurrent evi-
dence of repercussions for older adults who
experience sustained and inescapable expo-
sure to toxic situations. We review empirical
findings that are consistent with the premise
that motivational shifts lead to differences
in attention, memory, social partner prefer-
ences, and problem- solving strategies that
serve to maintain or optimize emotional
experience. Next, we discuss situations pos-
ited by SAVI, in which benefits of age may
be less pronounced and may even reverse in
direction. We then review areas that we pre-
viously highlighted as especially important
and how they have been addressed since the
previous edition of this volume. We conclude
that the emerging literature suggests that age
differences in emotional experience, cogni-
tive processes, and physiological functioning
operate in concert to yield relatively well-
preserved if not enhanced emotional well-
being in later life.
Emotion and the Aging
Physiological System
Emotional experience, like all psychological
phenomena, is inextricably linked to physi-
ological functioning. Aging in three bio-
logical systems— the central nervous system
(specifically the brain), the cardiovascular
system, and the neuroendocrine system— is
relevant for emotional aging.
Age Changes in Brain Structure
and Functioning
Researchers have documented normative
age- related changes in both brain structure
and functioning. Brain volume decreases
with age, with reductions in the synaptic
density of white matter and a consequent
slowing of neural processing that accom-
panies demyelination (e.g., Westlye et al.,
2010). These age- related decreases are
observed after the fourth decade of life, and
exhibit an accelerating decline beginning in
the early to mid-60s (Westyle et al., 2010).
Brain activation in response to emotional
stimuli shifts throughout childhood and into
early adulthood toward less activation in the
amygdala and greater activation of the pre-
frontal cortex, findings that researchers use
to explain developmental increases in emo-
tion regulation abilities (McRae et al., 2012).
The same difference in relative activity of
these brain regions is found in comparisons
of younger and older adults, a phenomenon
referred to as the posterior– anterior shift in
aging (PASA; Davis, Dennis, Dasellar, Fleck,
& Cabeza, 2008). In memory and percep-
tual studies, with and without emotional
stimuli, older adults show greater activity in
the prefrontal regions and reduced activity
in medial and temporal regions compared to
younger adults (Davis et al., 2008).
Age Changes in Cardiovascular
and Neuroendocrine Functioning
Cardiovascular and neuroendocrine
responses also change with age. Although
the overall pattern of cardiovascular reactiv-
ity to stressors is the same, aging is related
to relative differences in the degree of this
reactivity. Specifically, older age is related to
a smaller increase in heart rate in response
to stressors but greater increases in blood
pressure reactivity (see review by Uchino,
Birmingham, & Berg, 2010). The reduced
heart rate reactivity most likely represents
age- related reductions in heart rate variabil-
ity (HRV) that are normative and begin in
adolescence, and that are a risk factor for
mortality (Zulfigar, Jurivich, Gao, & Singer,
2010).
The neuroendocrine response also varies
with age (Björntorp, 2002). The perception
of a real or imagined threat mobilizes the

Emotion Regulation and Aging 205
body for action through a series of reac-
tions along the hypothalamic– pituitary–
adrenal (HPA) axis that results in the release
of cortisol by the adrenal glands. In both
human and animal models, cortisol reactiv-
ity in response to psychological and physi-
cal stressors is prolonged with age, such that
more time is necessary to return to baseline
levels (see review by Björntorp, 2002).
Age Differences
in Emotional Experience
Early theories positing that emotional well-
being and regulation would parallel age-
related biological decline were so pervasive
that researchers did not bother to test them.
In the last few decades, however, researchers
have dispelled many myths about inevitable
depression and despair in late life. Older age
is not associated with high levels of emo-
tional distress in examinations of people
ranging from ages 18 to 84 years (Kobau,
Safran, Zack, Moriarty, & Chapman,
2004). Rates of anxiety and major depres-
sive disorder are lower among adults age 65
years and older than in younger cohorts (see
review by Piazza & Charles, 2006; Villa-
mil, Huppert, & Melzer, 2006). Reports of
subdromal depression— feeling sad, blue, or
depressed within the past 30 days— decrease
linearly across increasingly older age groups
(Kobau et al., 2004).
This age- related decrease in self- reported
negative affect is observed longitudinally in
normal populations as well (e.g., Carstensen,
Pasupathi, Mayr, & Nesselroade, 2000;
Carstensen et al., 2011), although patterns
vary across studies when examining peo-
ple over age 60. Findings from a number
of cross- sectional studies suggest that the
experience of negative emotion continues
to decline after age 60 (e.g., Grühn, Kotter-
Grühn, & Röcke, 2010; Stone, Schwartz,
Broderick, & Deaton, 2010). This decrease
has also been found over time among
cohorts of younger, middle- aged, and older
adults followed across 20 years (Charles,
Reynolds, & Gatz, 2001). In one experi-
ence sampling study, the decrease in nega-
tive emotional experience peaked and then
stabilized somewhere between ages 60 and
70 (Carstensen et al., 2011). Other studies
have found slight age- related increases in
depressive symptoms in advanced old age
(e.g., after age 80 in one study; Teachman,
2006), although levels were still lower than
those observed in young adults. In another
study, adjusting for functional limitations
and chronic illness eliminated the upturn
in negative affect among the octogenarians
(e.g., Kunzmann, Little, & Smith, 2000).
Findings from studies that examine posi-
tive affect are mixed but overall point to
considerable stability. Although age- related
linear decreases (about half a point on a
5-point Positive Affect Scale) were observed
among people from a large, multinational
sample of people ranging in age from their
20s to 80s (Diener & Suh, 1998), this pattern
has not been found in other studies. Using
the same scale, a large longitudinal study
found age- related stability across 20 years
among younger and middle- aged adults, and
a small decline only among people age 65
and older who began the study (Charles et
al., 2001). Another large U.S. national sur-
vey observed slightly higher levels of positive
affect with age in a sample of people rang-
ing in age from 25 to 74 years (Mroczek
& Kolarz, 1997). Two other large surveys
found that reports of daily happiness and
enjoyment (in a U.S. sample) and reports of
joviality and serenity (in a German sample)
exhibited an age- related U-shaped pattern,
decreasing among successively older adults
from ages 18 to the mid-50s, but increasing
thereafter, such that people in their 70s and
80s had levels of these emotions similar to
those of people in their late teens and early
20s (Grühn et al., 2010; Stone et al., 2010);
people in their 50s reported the lowest levels
of these positive emotions in both the U.S.
and German samples.
Taking the findings on negative and posi-
tive affect together, overall well-being does
not follow the physiological trajectories
of linear decline across adulthood. One
potential explanation for these findings is
that age- related reductions in physiologi-
cal ability may paradoxically lead to flat-
tened, or diminished, emotional experiences
that are easier to regulate. Studies provide
little evidence, however, for this hypoth-
esis. Older adults report less negative emo-
tion in daily life, but the reported intensity
of both positive and negative emotions is
similar across age groups (Carstensen et al.,
2000, 2011). In other words, when older

206 DEVELOPMENTAL CONSIDERATIONS
people do experience emotions, the inten-
sity is similar to that of younger people.
Older and middle- aged adults report similar
levels of emotional experience when asked
to relive emotionally charged events or to
engage in a confrontation with their spouses
(Levenson, Carstensen, & Gottman, 1994;
Magai, Consedine, Krivoshekova, Kudadjie-
Gyamfi, & McPherson, 2006; Story et al.,
2007). Some studies in which people react
to film clips have revealed higher subjective
ratings of intensity in older adults than in
younger adults in response to scenes elicit-
ing sadness, especially when scenes were
particularly salient for older people, for
example, in a depiction of a person realiz-
ing that she has Alzheimer’s disease (Kun-
zmann & Grühn, 2005; Kunzmann & Rich-
ter, 2009; Kliegel, Jäger, & Phillips, 2007;
Seider, Shiota, Whalen, & Levenson, 2011).
Older adults also reported greater reports
of intensity to film clips depicting injustice
(Charles, 2005; Phillips, Henry, Hosie, &
Milne, 2008). Other film-based studies have
not observed age differences (Tsai, Leven-
son, & Carstensen, 2000).
Socioemotional Selectivity Theory
SST, a lifespan theory of motivation that
addresses how and why emotional well-
being changes across the lifespan, posits that
at conscious and subconscious levels, human
monitor time, continuously adjusting their
time horizons as they move through life (see
Carstensen, 1992, 2006; Carstensen, Isaa-
cowitz, & Charles, 1999). This accounting
of time influences the relative importance
of two constellations of goals that dominate
human thought and behavior. One set of
goals includes knowledge and information-
based goals, and is prioritized when time is
perceived as expansive. The other goal con-
stellation includes a focus on emotional life,
encompassing motivations to derive mean-
ing in actions and to invest in activities for
their emotional significance.
Because time perspective is strongly related
to place in the life cycle, growing older
comes with an increasing awareness of the
ephemeral nature of existence: As a result,
emotional goals increase in relative impor-
tance to knowledge- related goals as people
age. People become increasingly selective in
their goals as they age, so that experiences
are more emotionally meaningful and satis-
fying. This selection process falls under the
rubric of antecedent- focused emotion regu-
lation, whereby people engage in actions to
avoid the experience of distress and to main-
tain or enhance the experience of positive
emotions. Accordingly, people allocate more
effort to maintaining emotional balance,
attempting to improve important relation-
ships, and setting aside relatively trivial mat-
ters. Of course, experience grows with age
as well, so older people are well- positioned
to manage social relationships and have the
skills to solve important conflicts. Accord-
ing to SST, motivational shifts that also
operate at a subconscious level influence
cognitive processing in which people favor
positive material and disattend to negative
information. Goals always direct attention
and memory, and with age, cognition comes
to operate in the service of emotion regula-
tion.
Strength and Vulnerability
Integration
SAVI endorses SST and its tenet that aware-
ness of time left to live directs priorities,
such that a focus on emotional experience,
and specifically positive emotional experi-
ences above negative ones, gradually comes
to influence thought and action (Charles,
2010; Charles & Piazza, 2009). People act
on these motivations based on their accrued
knowledge about themselves and their social
environment, and their experience from
time lived. As a result, aging is associated
with motivational shifts— both conscious
and subconscious— that direct attention,
influence memorial processing, and change
preferences in ways that allow people to
circumvent negative experiences. If they
cannot avoid these experiences altogether,
cognitive- behavioral emotion regulation
strategies allow them to decrease their expo-
sure and mitigate relatively minor stressors
in their day-to-day lives.
According to SAVI, older adults more
often engage in emotion regulation strategies
(i.e., thoughts and behaviors whose aim is to
reduce negative affect and maintain or even
enhance positive affect) than do younger
adults who fail to shy away from negative

Emotion Regulation and Aging 207
situations, presumably because there is
informational value in negative experiences
that prepares people for the future. When
confronted with minor events, older adults
appraise them as less stressful (e.g., Charles
& Carstensen, 2008). When older adults
cannot employ strengths of aging to avoid or
mitigate their exposure to a negative event,
however, they experience distress. During
these demanding and highly arousing situ-
ations, SAVI posits that the age- related ben-
efits disappear or even reverse in direction.
Because age is related to biological changes
that present greater threats to a less robust
physiological system, SAVI posits that high
levels of physiological reactivity and conse-
quent recovery in response to sustained neg-
ative arousal are more costly to older adults.
Age, Cognitive Functioning,
and Antecedent-Focused
Emotion Regulation
Emotional Salience
and Emotion Regulation
Brains do not operate like computers, treat-
ing all information equally. Rather, cogni-
tive resources are directed to goal- relevant
information. SST posits that older adults
place more importance and prominence on
emotional than on nonemotional aspects
of stimuli they encounter in the labora-
tory or in their daily lives, and they do so
without explicit awareness of their actions.
Laboratory studies using incidental memory
paradigms reveal that the proportion of
emotional versus nonemotional informa-
tion increases linearly as a function of age
(Carstensen & Turk- Charles, 1994), and
emotional details are recalled better than
perceptual details of stories (e.g., Mather,
Johnson, & De Leonardis, 1999). In addi-
tion, the salience of emotion is greater with
age when people recall autobiographical
information (Alea, Bluck, & Semegon,
2004). Overall, this pattern of findings sug-
gests that older adults pay attention to emo-
tionally relevant information that in and of
itself may aid emotion regulation.
Rather than “positivity bias” we coined
the term positivity effect to describe the
age- related pattern of change (Carstensen &
Mikels, 2005; Mather & Carstensen, 2005).
Age differences are as frequently driven by a
preference for negative material in the young
as a preference for positive material in the
old. The positivity effect refers to the age-
related pattern of change.
Brain Activity and the Positivity Effect
Studies of brain activity during the process-
ing of emotional information support the
positivity effect. Mather et al. (2004) specu-
late that subconscious changes in motivation
influence activation of the amygdala, con-
sistent with their finding that older adults
show greater amygdalar activity when view-
ing positive rather than negative images.
Other brain regions also show age differ-
ences in activation depending on the emo-
tional valence of the information (Samanez-
Larkin et al., 2007). In a study examining
brain activity in response to decision mak-
ing, older adults exhibited less activation in
insula and caudate regions than did younger
adults when they anticipated loss, but
similar activation when anticipating gains
(Samanez- Larkin et al., 2007).
The general PASA in processing infor-
mation discussed previously (St. Jacques,
Bessette- Symons, & Cabeza, 2009) may
serve emotion regulation goals. For exam-
ple, one study revealed that older adults
display less amygdalar activity and greater
activation of areas associated with emotion
regulation (prefrontal cortex) when viewing
negative images compared to positive images
relative to younger adults (Leclerc & Kens-
inger, 2011). Another study revealed age
differences in connectivity of the amygdala
to other brain regions, consistent with age-
related increases in emotion regulation pro-
cesses (St. Jacques, Dolcos, & Cabeza, 2009,
2010). Specifically, older adults showed more
activity in a brain region associated with
emotion regulation (ventral anterior cingu-
late cortex), and less activity in areas asso-
ciated with perceptual processing (visual
cortex) than did the younger adults (St.
Jacques et al., 2010). Age- related reductions
in activities related to perceptual processing
are consistent with the well- established age-
related reductions in facial emotion recogni-
tion in the literature (e.g., Lambrecht, Krei-
felts, & Wildgruber, 2012). Similar patterns
emerge with brain activation and consequent
memory for these pictures (St. Jacques et al.,
2009). Older adults exhibit greater activity
208 DEVELOPMENTAL CONSIDERATIONS
in frontal regions associated with emotional
control (bilateral dorsolateral prefrontal cor-
tex) and less activity in regions associated
with later memory encoding (hippocampus
and bilateral ventrolateral prefrontal corti-
ces) for negative stimuli relative to neutral
stimuli than do younger adults. Behavioral
data again reflected these findings: Older
adults were less likely than younger adults
to remember the negative stimuli relative to
the neutral stimuli (St. Jacques et al., 2009).
Age‑Related Changes in Attention
and Working Memory
The positivity effect explains how chronic
activation of emotion- related goals directs
cognitive resources systematically toward
positive and away from negative stimuli—
often without conscious awareness. Older
adults focus their attention on more posi-
tive and less negative stimuli than do
younger adults in studies of simple atten-
tion, as assessed by reaction times (Mather
& Carstensen, 2003) and eye gaze (e.g.,
Isaacowitz, Toner, Goren, & Wilson, 2008;
Nikitin & Freund, 2011).
SST predicts that top-down motivational
changes with age lead to changes in atten-
tion and memory that serve emotion regu-
lation goals, such that performance may be
better for emotional information than for
nonemotional information. One such exam-
ple lies in working memory, which is vital
for emotional functioning and planning,
because working memory allows an individ-
ual to focus on the potential for rewards and
enhances goal- seeking behavior (Davidson,
Jackson, & Kalin, 2000). In a test of SST,
the working memory performance of older
and younger adults was examined on tasks
in which participants compared images
based on either their brightness intensity
or emotional intensity (Mikels, Larkin,
Reuter- Lorenz, & Carstensen, 2005). Find-
ings reveal the usual age- related decrement
in working memory for visual brightness but
no age difference in working memory for
affective arousal. Moreover, the lack of age
differences was driven by older adults’ bet-
ter performance for positive stimuli than for
negative stimuli compared to younger adults
(Mikels et al., 2005). Other studies suggest
that emotion regulation is less cognitively
taxing for older adults than for younger
adults (e.g., Scheibe & Blanchard- Fields,
2009). In one study, Scheibe and Blanchard-
Fields asked younger and older adults to
perform a working memory task after hav-
ing been induced into a negative mood and
asked to down- regulate or maintain current
mood, or were provided no specific instruc-
tions. Although the performance of younger
adults deteriorated when they were asked to
down- regulate their emotions, the perfor-
mance of older adults did not vary from that
in the other conditions. Similarly, another
study found that emotional suppression was
related to worse memory for younger adults
but not for older adults (Emery & Hess,
2011).
Evidence That Memory Supports
Emotion Regulation
Antecedent- focused emotion regulation
strategies prevent or reduce exposure to
events and thoughts that threaten well-
being. To the extent that memories influence
current mood states, thoughts, and behav-
ior (see review by Levine & Pizarro, 2004),
memory can be a powerful regulation strat-
egy. This shift in valence toward positive
information with age has been documented
in studies examining memory for both life
events and laboratory stimuli. Memory
about past events is more positive for older
compared to younger adults (Kennedy,
Mather, & Carstensen, 2004). Even when
asked about prior negative events, older
adults report them as having been more
positive than do younger adults (Schryer &
Ross, 2012).
Laboratory studies underscore the shift
toward older people remembering events
more positively. In a study in which younger,
middle- aged, and older adults viewed posi-
tive, negative, and neutral images, and were
later asked to recall and to recognize these
previously viewed images from a larger set
of images, younger adults recalled similar
levels of positive and negative stimuli, and
they recognized a greater proportion of neg-
ative images than positive images (Charles,
Mather, & Carstensen, 2003). Older adults
displayed no such negative bias in their recog-
nition, and they recalled significantly fewer
negative than positive images (Charles et
al., 2003). This age- associated shift toward
positive emotion and away from negative
Emotion Regulation and Aging 209
information has been replicated in a num-
ber of studies from many laboratories (e.g.,
Petrican, Moscovitch, & Schimmack, 2008;
Piguet, Connally, Krendl, Huot, & Corkin,
2008; see reviews by Reed & Carstensen,
2012; Scheibe & Carstensen, 2010).
Cognitive Appraisal of Negative Events
Older adults not only focus their attention
on less negative aspects of the environment
but they also appraise ambiguous or unpleas-
ant events more benignly than do younger
adults. Older adults perceive daily stressors
as less severe and less threatening than do
younger adults in a sample ranging from
25 to 74 years (Charles & Almeida, 2007).
They appraise aversive stimuli less nega-
tively than do younger adults, and are more
likely to focus their thoughts away from the
negative content (Charles & Carstensen,
2008). Even when asked about their diag-
nosis and treatment of cancer, older adults
report lower levels of threat appraisals (e.g.,
the amount of tension people experience in
response to their illness) and are more likely
than younger adults to frame the event as
a challenge rather than a threat (Hart &
Charles, 2013).
The Positivity Effect Is Malleable
SST maintains that—all things being equal—
chronically activated goals differ for older
and younger people. However, because posi-
tivity reflects top-down motivational shifts
as opposed to bottom- up brain- driven shifts,
the effect is theoretically malleable. Younger
people sometimes pursue emotional goals,
and older adults sometimes pursue informa-
tional goals. In circumstances where stakes
are high, younger and older people may
pursue goals that direct attention toward or
away from emotional material accordingly.
Indeed, Löckenhoff and Carstensen (2007)
examined whether experimental instruc-
tions provided to participants would elimi-
nate age differences. In an initial phase of
the study, participants were simply asked
to review information about a variety of
alternative health care plans. In this condi-
tion, older people focused more on positive
than on negative characteristics. In a second
condition, researchers explicitly asked older
adults to focus on informational aspects of
the plans; that is, they gave the participants
a specific goal, namely, to pursue informa-
tion. And in this condition, age differences
were eliminated. Reed and Carstensen
(2013) recently reviewed the positivity effect
literature and concluded that positivity pref-
erences are strongest during controlled pro-
cessing when motivations are hypothesized
to play a strong role (for discussion, see
Kensinger, 2004).
Whether or not positivity in cognitive pro-
cessing is adaptive or maladaptive depends
on the task. Certainly there are situations
where selective attention to positivity may
disadvantage performance. Decision mak-
ing likely benefits from evenhanded process-
ing of advantages and disadvantages associ-
ated with options. Focusing on the positive
interpersonal qualities of a charming con-
man could have disastrous financial conse-
quences. Ignoring negative information or
appraising a situation as less negative than
it is may lead to short-term benefits to well-
being but longer term problems that lead
to distress (Scheibe & Carstensen, 2010).
Focusing on positive aspects and avoiding
negative realities of fiscal concerns may lead
to “optimistic, but not necessarily realistic,
financial planning” (Weierich et al., 2011,
p. 197). Ignoring negative information may
contribute to misremembering negative
information as more positive than it actually
was (Shamaskin, Mikels, & Reed, 2010).
Finally, there is some evidence that not
only emotional valence but also arousal con-
tribute to age differences in positivity. Grühn
and Scheibe (2008) found that older people
perceived positive pictures in the Interna-
tional Affective Picture System (IAPS) as
more positive and less arousing than did
younger adults. Keil and Freund (2009) later
observed that older adults prefer less arous-
ing positive and negative images and words
than do younger adults, and Streubel and
Kunzmann (2011) reported that the subjec-
tive experience of positivity in older adults
is reduced under high arousal. Using experi-
ence sampling data, Scheibe, English, Tsai,
and Carstensen (2013) recently examined
the effect of age differences in the value
placed on high- versus low- arousal states
and concluded that older people placed more
value on low- arousal positive states and were
more successful in achieving them than their
younger counterparts. Taken together, these

210 DEVELOPMENTAL CONSIDERATIONS
findings suggest that older adults may orient
to positive material that is more calming and
peaceful than to positive material associated
with excitement and surgency.
Social Partners
and Emotion Regulation
This review has focused largely on age-
related changes in cognitive processes that
serve emotion regulation goals. SST also
makes specific predictions about how age-
related behaviors, and explicitly changes in
social partner preferences and interaction
patterns, serve emotion regulatory goals.
Older adults have smaller and more care-
fully pruned social networks that contain
larger percentages of emotionally close
social partners than do those of younger
adults (Lang & Carstensen, 1994). Younger
peoples’ networks, by way of contrast, often
include many social partners who were not
chosen freely but have been incorporated
because of relationships to work or offspring
(Lang & Carstensen, 1994; Lansford, Sher-
man, & Antonucci, 1998). Thus, the social
networks of older adults may be easier to
navigate emotionally. Because most strong
emotions occur in social contexts, careful
selection of social partners is arguably a
highly effective strategy. When faced with
interpersonal conflict in their networks,
older adults engage in more conflict avoid-
ance, with the goal of preserving harmony in
their relationships, than do middle- aged and
younger adults (Blanchard- Fields, Mienal-
towski, & Seay, 2007).
Responding to Negative Events
The previously discussed literature points
to older adults using— either knowingly or
not— thoughts and behaviors that fall under
the category of antecedent- focused emo-
tion regulation strategies. Some researchers
argue, however, that older adults’ greater
well-being reflects not actions by the older
adults but instead age- related differences
in environmental demands (see review by
Lawton, 2001). According to this view, the
end of child- rearing responsibilities and the
onset of retirement decreases the frequency
of negative events more in the lives of older
than younger adults (see review by Lawton,
2001; but see Villamil et al., 2006). On the
other hand, older people face many chal-
lenges that younger people are less likely to
face, such as chronic health problems, loss of
loved ones, and age discrimination.
Although questions about environmental
demands are important and there is some
evidence that changes in life circumstances
may contribute to the decreased number of
stressors older adults report in their daily
lives (e.g., Charles & Almeida, 2007), there
is evidence that older adults are also active
agents in influencing their well-being. Com-
pared to younger people, older adults report
better control over their emotions (Gross
et al., 1997), enhanced abilities to regulate
emotions in general (Kessler & Staudinger,
2009), as well as more success at calming
strategies and other thoughts to control their
feelings of anger (Phillips, Henry, Hosie, &
Milne, 2008). Although the veracity of sub-
jective reports alone may be questioned, they
are consistent with low levels of psychiatric
disease and reported experience, and behav-
ioral evidence in the literature. For exam-
ple, when people ranging in age from 18 to
94 years reported the emotions they were
experiencing at five random times each day
throughout the course of a week, the proba-
bility of continuing to feel a negative emotion
from one reporting interval to the next was
lower with advancing age (Carstensen et al.,
2000). Researchers have discussed how older
adults select and are adept at using emotion
regulation strategies given their decrements
in others area of their lives, such as cognitive
functioning (Urry & Gross, 2010).
Questions remain, however, as to how
well older adults regulate their emotions
when they cannot avoid or mitigate expo-
sure to a negative event. SAVI posits that
when older adults cannot avoid experienc-
ing high and sustained levels of emotional
distress, this arousal reduces any age- related
benefits previously observed for regulation
abilities. One study explicitly compared age
differences in affective outcomes in two situ-
ations: one in which people disengaged from
a negative event and another in which they
did not (Charles, Piazza, Luong, & Almeida,
2009). When older adults encountered an
unpleasant situation in which they chose to
avoid further conflict, they reported lower
levels of affective reactivity than did younger
adults. When they engaged in a conflict, no
Emotion Regulation and Aging 211
age differences in reactivity were apparent
(Charles et al., 2009).
Other studies also suggest that high lev-
els of arousal reduce age- related benefits
in emotional outcomes and may even show
age- related decrements. When older and
younger adults report stressors of equally
high severity, older adults display greater
affective reactivity than do younger adults
(Mroczek & Almeida, 2004). Longitudinal
data confirm this age- related pattern, in that
when people report experiencing a stressor,
affective reactivity increases with age (Sli-
winski, Almeida, Smyth, & Stawski, 2009).
The studies described earlier use informa-
tion gathered from the daily lives of adults.
Researchers attempting to capture emo-
tion regulation in the laboratory face the
challenge of devising paradigms that elicit
responses sufficiently strong to demand
active and sustained regulatory efforts.
Although use of weak stimuli, such as pic-
tures of facial expressions, has become
commonly accepted, we strongly question
the validity of this approach when research-
ers are interested in participants’ efforts to
regulate emotional responses actively. Stron-
ger inductions are possible with film clips,
personal memories, music, and interactions
with confederates or spouses. Of course, a
tradeoff exists between carefully controlled
elicitors and responsivity. Only a handful of
such studies that focus on age differences in
regulation effectiveness have been published
to date.
In one study, subjective reports of distress
were compared across groups of younger (18
to 40 years old) and older adults (60 to 88
years old) who watched emotional film clips
with instructions to react naturally, suppress
all facial expressions, or positively refocus
on a pleasant memory if they felt any nega-
tive emotions (Phillips et al., 2008). Results
indicate that older adults reported less
intense negative affect in the positive reap-
praisal condition compared to the other con-
ditions. When comparing across age groups,
older and younger adults had similar ratings
in the positive reappraisal condition, but
younger adults reported less negative affect
than older adults in the conditions where
they were instructed to react naturally or to
suppress their facial expressions.
Another study examined the baseline,
reactivity, and recovery ratings of nega-
tive affect in older (60 to 75 years old)
and younger (20 to 30 years old) adults in
response to a film clip that elicited disgust
(Scheibe & Blachard- Fields, 2009). After
having viewed the film clips, participants
were given instructions to attempt to change
the negative feelings into positive ones or to
continue feeling their negative emotions and
not try to change them, or they were given no
instructions. Results indicated no significant
age differences in the reported affect during
the recovery period. However, older adults
showed less deterioration in performance on
one of the three trials of a n-back task fol-
lowing the induction, suggesting that fewer
resources were required for regulation.
A third study examined three types of
regulation strategies in older (60–69 years),
middle- aged (40–49 years) and younger
adults (20–29 years) in response to emo-
tional film clips (Shiota & Levenson, 2009).
The three strategies were detached appraisal,
in which participants were told to watch
objectively in order to feel less negative emo-
tion; positive reappraisal, in which they
were told to think about positive aspects of
what they were viewing so that they would
feel less negative emotion; or emotional sup-
pression, in which they were asked to hide
negative facial expressions. When compar-
ing subjective reports across different con-
ditions within each age group, researchers
found that detached appraisal resulted in
significantly lower levels of distress among
the younger adults, marginally signifi-
cant lower levels of distress in middle- aged
adults, but the same level of distress in older
adults when compared to the “just watch”
condition. Only older adults showed sig-
nificantly lower levels of subjective distress
in the positive reappraisal condition com-
pared to the “just watch” condition. When
examining mean levels of the target emo-
tions (sadness or disgust) across age groups,
subgroups reporting the lowest mean levels
were the youngest and middle- aged adults
in the detached appraisal group (3.25 and
4.08, respectively), and the two groups with
the highest reported target emotions were
the middle- aged and oldest adults in the
positive reappraisal groups (5.42 and 5.20,
respectively).
One recent study compared younger (18-
to 30-year-olds) and older (60- to 80-year-
olds) participants’ responses as they viewed
212 DEVELOPMENTAL CONSIDERATIONS
negative and neutral images in three condi-
tions: a “just watch” condition, in which
they were told simply to look at the image; a
condition in which each image was preceded
by a string of digits they need to remember
immediately after their 10-second exposure
to the image; and a condition in which they
were instructed to reinterpret the picture in
such a way that they would feel less negative
affect (Tucker, Feuerstein, Mende- Siedlecki,
Ochsner, & Stern, 2012). After each image,
participants rated how much negative emo-
tion they felt. Findings indicate that younger
adults reported less negative reactivity in
the reappraisal condition than did the older
adults, but age groups were similar in levels
of reactivity in the distraction condition.
In summary, few studies have examined
emotion regulation strategies that target
decreasing subjective distress when people
are exposed to unpleasant stimuli under con-
trolled laboratory conditions. Findings from
the handful of existing studies do not point to
consistent evidence for age- related improve-
ments or impairment in emotion regulation.
As we note below, however, we believe that
more of this type of research is needed.
Physical Costs of Emotion Regulation
SAVI further posits that circumstances
marked by higher levels of sustained arousal
will have greater physiological consequences
for older adults. Support for this tenet comes
from literature examining both cardiovas-
cular and HPA reactivity responses to emo-
tional events in younger and older adults.
For example, blood pressure reactivity is
more pronounced in older adults relative
to younger adults in response to stressors
of daily life and laboratory- based stressors
such as social evaluative threat (Ong, Roth-
stein, & Uchino, 2012; Uchino et al., 2010)
and when dealing with cognitively com-
plex stressors of daily life (Wrusz, Müller,
Wagner, Lindenberger, & Riediger, 2013).
In addition, the effect of a more prolonged
negative experience— loneliness— is more
strongly related to blood pressure recovery
in older than in younger adults (Ong et al.,
2012).
Age- related changes in HPA reactivity
have been studied less frequently. Although
researchers document prolonged HPA reac-
tivity in animal studies, they have conducted
fewer studies using human samples (Björn-
torp, 2002). Still, findings suggest that nega-
tive affect is related more strongly to cortisol
output for older than for younger adults.
For example, one study found that high lev-
els of negative affect were related to higher
level of daily cortisol output for relatively
older participants (54 to 74 years old), but
unrelated to this biomarker for people 53
and younger (Piazza, Charles, Stawski, &
Almeida, 2013).
When Avoiding Negative
Emotions Does Not Occur:
Aging with Negative Affect
SAVI posits that affective well-being remains
relatively stable and may increase with age,
with one important caveat. When older
people find themselves in unavoidable cir-
cumstances that elicit sustained physiologi-
cal arousal, the consequences for well-being
will be exacerbated. Researchers have long
discussed the powerful role of the environ-
ment in shaping emotion- related outcomes
and how age is related to different life cir-
cumstances. These circumstances are pos-
ited to explain why not all groups of older
adults show continuing, positive trajecto-
ries of emotional experience. Loss of social
belonging, prolonged caregiving, and neuro-
logical dysregulation may result in an upturn
in depressive symptoms and negative affect
in the last decades of life (Charles, 2010).
For example, in the last years of life—often
characterized by neurological and physi-
ological dysregulation— there are reliable
declines in emotional well-being. Indeed,
Gerstorf and colleagues (2010) found that
terminal decline is better predicted by close-
ness to death than by chronological age. On
the one hand, age and closeness to death
are strongly coupled in the population, but
Gerstorf and his colleagues found that years
from birth may be less important than the
circumstances that define the years to death
in predicting emotional well-being.
In addition, experiencing chronically high
levels of trait- negative affect also makes peo-
ple more vulnerable to emotional distress
with age. For example, two studies have
found that declines in subjective reports of
negative affect do not continue into old age
for those high in neuroticism (Charles et al.,
2001; Ready, Akertedt, & Mroczek, 2012).

Emotion Regulation and Aging 213
Future Directions and Conclusions
Empirical findings amassed over the past 30
years have dramatically changed the land-
scape of research on emotion and aging.
Among healthy adults, emotional experi-
ences and emotion regulation do not decline
across adulthood but are instead well-
maintained and, in some ways, improve. We
review below the aims for future studies we
recommended 5 years ago.
We stated that future studies will need to
link hypothesized emotion regulation strat-
egies (e.g., attention allocation) directly to
emotion regulation. This area has begun to
receive some attention (e.g., Isaacowitz &
Blanchard- Fields, 2012), but more work and
conceptual clarity are needed. SST, for exam-
ple, focuses on selection as the key mecha-
nism involved in greater well-being with age,
and views selective attention and memory
as consequences of changes in goals. In the
theoretical context of SST, the positivity
effect, rather than being a coping strategy,
reflects goal- directed attention. Whether or
not older people also deplore positive atten-
tion in response to distress remains an open
question. SAVI posits that when selection
is not possible, age advantages disappear
or even reverse. Further study is needed to
examine how emotion regulation strategies
and effectiveness change with age, relative
to the way age reflects both birth cohort
and age- associated experience. In addi-
tion, we mentioned the need to study mul-
tiple response- focused regulation strategies
and include conditions specifying main-
tenance, exacerbation, and attenuation of
emotional experience in the investigations.
As we reviewed earlier, several studies have
begun to address different types of strategies
among people of different ages (e.g., Shiota
& Levenson, 2009). We hope that research-
ers will continue to investigate age differ-
ences in online emotion regulation tasks.
We mentioned the need to examine the
importance of the arousal of emotional stim-
uli when studying age differences (Wurm,
Labouvie- Vief, Aycock, Rebucal, & Koch,
2004), as well as the complexity of emo-
tional stimuli (Charles, 2005). Researchers
who take into account the role of arousal
in processing and regulating emotions find
that more arousing conditions may be more
challenging for older adults (e.g., Ong et al.,
2012). It will be important to consider both
valence and arousal. We mentioned the need
to examine discrete emotions in the previ-
ous edition of this volume, and provided
the example that no large study to date has
reported increases in levels of anger with age,
but one study did observe age differences in
reports of depressive symptoms (e.g., Teach-
man, 2006) although others have not. A
growing number of studies has examined
discrete emotions (Grühn et al., 2010) and
especially emotion recognition (e.g., Slessor,
Miles, Bull, & Phillips, 2010), yet theories
still focus mostly on positive and negative
valence and arousal when examining emo-
tion regulation processes (see Charles &
Carstensen, 2010).
One methodological issue that frequently
arises in lifespan developmental research is
the issue of cohort effects. Cross- sectional
research points to potentially interesting
developmental phenomena, but only longi-
tudinal research can confirm developmen-
tal processes postulated by socioemotional
selectivity theory. Researchers have made
great strides in longitudinal investigation of
more complex emotional stimuli in the past
5 years (e.g., Carstensen et al., 2011; Ger-
storf et al., 2010; Sliwinski et al., 2009) and
the maintenance of large longitudinal data-
sets promises to illuminate the field in this
important area.
Methodological innovations in the past
5 years have advanced the field in several
ways. For example, functional imaging of
brain activity has illuminated associations
between behavior and brain activity, and
promises to provide insights into the neuro-
logical correlates of age differences. As this
area grows, the study of emotion and aging
will continue to benefit. A second innova-
tion that will shape the future of psycho-
logical science is the growing area of health
psychology and new techniques allowing
for affordable assessments of cardiovascu-
lar, neuroendocrine, and immunological
responses in the laboratory and in daily life.
With these studies of physiological stabil-
ity and reactivity, researchers will be able
to examine how thoughts and behaviors are
related to physiological arousal and reactiv-
ity accompanying emotional experience. In
addition, smart phones provide the ability to
assess cognitive functioning rapidly, along
with momentary sampling of emotion across

214 DEVELOPMENTAL CONSIDERATIONS
the course of the days, which provides more
online collection of cognitive and emotional
experiences (see Riediger, Schmiedek, Wag-
ner, & Lindenberger, 2009).
Finally, psychologists have long recog-
nized the importance of the environment
in shaping behavior (see review by Hess,
2005), but we need to continue to recognize
the importance of context when interpret-
ing age differences. Although notable excep-
tions exist, such as studies examining age
differences in social cognition (e.g., Hess,
2005) and in health- related contexts (e.g.,
Consedine & Magai, 2006), further stud-
ies are needed to understand how similar
behaviors or cognitions may have different
outcomes according to age- specific contexts.
Perceptions are guided by experiences, and
these experiences may alter the meaning and
interpretation of emotional stimuli. A pic-
ture of a surgical procedure, for example,
may elicit more sadness in older adults but
more disgust in younger adults (e.g., Kun-
zmann & Grühn, 2005). The death of a par-
ent is often an imagined event for a college
student and a long past reality for an older
adult. To this end, the study of emotions and
aging is an examination of how cognition
and behavior shape, and are shaped by, the
interplay between physiological processes
and the environment across the lifespan.
In conclusion, emotions arouse physi-
ological systems and direct attention, and
they motivate people to action. Emotions
are critical for successful adaptation across
the lifespan, but developmental processes
alter multiple facets of emotional experi-
ence, including cognitive appraisals, behav-
ioral responses, physiological reactivity, and
environmental context. Research on emo-
tion and aging has revealed that shifts in
cognitive processing can offset physiologi-
cal decline, such that even though biologi-
cal functioning peaks relatively early in life,
emotional experience remains vital and well
maintained into late adulthood.
Acknowledgments
This work was supported by National Institute
on Aging Grant No. R37-AGO8816 to Laura L.
Carstensen.
References
Alea, N., Bluck, S., & Semegon, A. B. (2004).
Young and older adults’ expression of emo-
tional experience: Do autobiographical nar-
ratives tell a different story? Journal of Adult
Development, 11, 235–250.
Björntorp, P. (2002). Alterations in the ageing
corticotropic stress- response axis. In Novartis
Foundation Symposium (Ed.), Novartis Foun-
dation Symposium 242—Endocrine facets of
ageing (pp. 46–58). London: Wiley.
Blanchard- Fields, F., Mienaltowski, A., & Seay,
R. B. (2007). Age differences in everyday
problem- solving effectiveness: Older adults
select more effective strategies for interper-
sonal problems. Journals of Gerontology: Psy-
chological Science, 62, P61–P64.
Carstensen, L. L. (1992). Social and emotional
patterns in adulthood: Support for socio-
emotional selectivity theory. Psychology and
Aging, 7, 331–338.
Carstensen, L. L. (2006). The influence of a
sense of time on human development. Science,
312, 1913–1915.
Carstensen, L. L., Isaacowitz, D. M., & Charles,
S. T. (1999). Taking time seriously: A theory
of socioemotional selectivity. American Psy-
chologist, 54, 165–181.
Carstensen, L. L., Fung, H., & Charles, S.
(2003). Socioemotional selectivity theory and
the regulation of emotion in the second half
of life. Motivation and Emotion, 27, 103–123.
Carstensen, L. L., & Mikels, J. A. (2005). At the
intersection of emotion and cognition: Aging
and the positivity effect. Current Directions in
Psychological Science, 14, 117–121.
Carstensen, L. L., Pasupathi, M., Mayr, U., &
Nesselroade, J. R. (2000). Emotional experi-
ence in everyday life across the adult lifespan.
Journal of Personality and Social Psychology,
79, 644–655.
Carstensen, L. L., Turan, B., Scheibe, S., Ram,
N., Ersner- Hershfield, H., Samanez- Larkin,
G. R., et al. (2011). Emotional experience
improves with age: Evidence based on over 10
years of experience sampling. Psychology and
Aging, 26, 21–33.
Carstensen, L. L., & Turk- Charles, S. (1994).
The salience of emotion across the adult lifes-
pan. Psychology and Aging, 9, 259–264.
Charles, S. T. (2005). Viewing injustice: Age dif-
ferences in emotional experience. Psychology
and Aging, 20, 159–164.
Emotion Regulation and Aging 215
Charles, S. T. (2010). Strength and Vulnerabil-
ity Integration (SAVI): A model of emotional
well-being in later adulthood. Psychological
Bulletin, 136, 1068–1091.
Charles, S. T., & Almeida, D. M. (2007).
Genetic and environmental effects on daily
life stressors: More evidence for greater varia-
tion in later life. Psychology and Aging, 22,
231–240.
Charles, S. T., & Carstensen, L. L. (2008).
Unpleasant situations elicit different emo-
tional responses in younger and older adults.
Psychology and Aging, 23, 495–504.
Charles, S. T., & Carstensen, L. L. (2010).
Social and emotional aspects of aging. Annual
Review of Psychology, 61, 383– 409.
Charles, S. T., Mather, M., & Carstensen, L. L.
(2003). Aging and emotional memory: The
forgettable nature of negative images for older
adults. Journal of Experimental Psychology:
General, 132, 310–324.
Charles, S. T., & Piazza J. R. (2009). Age differ-
ences in affective well-being: Context matters.
Social and Personality Psychology Compass,
3, 1–14.
Charles, S. T., Piazza, J., Luong, G., & Almeida,
D. M. (2009). Now you see it, now you don’t:
Age differences in affective reactivity to social
tensions. Psychology and Aging, 24, 645–653.
Charles, S. T., Reynolds, C. A., & Gatz, M.
(2001). Age- related differences and change
in positive and negative affect over 23 years.
Journal of Personality and Social Psychology,
80, 136–151.
Consedine, N. S., & Magai, C. (2006). Emotion
development in adulthood: A developmental
functionalist review and critique. In C. Hoare
(Ed.), The Oxford handbook of adult develop-
ment and learning (pp. 209–244). New York:
Oxford University Press.
Davidson, R. J., Jackson, D. C., & Kalin, N.
H. (2000). Emotion, plasticity, context, and
regulation: Perspectives from affective neuro-
science [Special issue]. Psychological Bulletin,
126, 890–909.
Davis, S. W., Dennis, N. A., Daselaar, S. M.,
Fleck, M. S., & Cabeza, R. (2008). Que
PASA?: The posterior– anterior shift in aging.
Cerebral Cortex, 18, 1201–1209.
Diener, E., & Suh, E. M. (1998). Subjective
well-being and age: An international analysis.
Annual Review of Gerontology and Geriat-
rics, 17, 304–324.
Emery, L., & Hess, T. M. (2011). Cognitive con-
sequences of expressive regulation in older
adults. Psychology and Aging, 26, 388–396.
Gerstorf, D., Ram, N., Mayraz, G., Hidajat,
M., Lindenberger, U., Wagner, G. G., et al.
(2010). Late-life decline in well-being across
adulthood in Germany, the United Kingdom,
and the United States: Something is seriously
wrong at the end of life. Psychology and
Aging, 25, 577–485.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., Carstensen, L. L., Pasupathi, M.,
Tsai, J., Götestam Skorpen, C., & Hsu, A.
Y. C. (1997). Emotion and aging: Experi-
ence, expression, and control. Psychology and
Aging, 12, 590–599.
Grühn, D., Kotter-Grühn, D., & Röcke, C.
(2010). Discrete affects across the lifespan:
Evidence for multidimensionality and multidi-
rectionality of affective experiences in young,
middle- aged and older adults. Journal of
Research in Personality, 44, 492–500.
Grühn, D., & Scheibe, S. (2008). Age- related
differences in valence and arousal ratings of
pictures from the International Affective Pic-
ture System (IAPS): Do ratings become more
extreme with age? Behavior Research Meth-
ods, 40, 512–521.
Hart, S. L., & Charles, S. T. (2013). Age- related
patterns in negative affect and appraisals
about colorectal cancer over time. Health Psy-
chology, 32, 302–310.
Hess, T. M. (2005). Memory and aging in con-
text. Psychological Bulletin, 131, 383–406.
Isaacowitz, D. M., & Blanchard- Fields, F.
(2012). Linking process and outcome in the
study of emotion and aging. Perspectives on
Psychological Science, 7, 3 –17.
Isaacowitz, D. M., Toner, K., Goren, D., & Wil-
son, H. R. (2008). Looking while unhappy:
Mood congruent gaze in young adults, posi-
tive gaze in older adults. Psychological Sci-
ence, 22, 147–159.
Keil, A., & Freund, A. M. (2009). Changes in the
sensitivity to appetitive and aversive arousal
across adulthood. Psychology and Aging, 24,
668–680.
Kensinger, E. A. (2004). Remembering emotional
experiences: The contribution of valence and
arousal. Reviews in the Neurosciences, 15(4),
231–307.
216 DEVELOPMENTAL CONSIDERATIONS
Kennedy, Q., Mather, M., & Carstensen, L. L.
(2004). The role of motivation in the age-
related positivity effect in autobiographical
memory. Psychological Science, 15, 208–214.
Kessler, E., & Staudinger, U. M. (2009). Affec-
tive experience in adulthood and old age: The
role of affective arousal and perceived affect
regulation. Psychology and Aging, 24, 349–
362.
Kliegel, M., Jäger, T., & Phillips, L. H. (2007).
Emotional development across adulthood:
Differential age- related emotional reactivity
and emotion regulation in a negative mood
induction procedure. International Journal
of Aging and Human Development, 64, 217–
244.
Kobau, R., Safran, M. A., Zack, M. M., Mori-
arty, D. G., & Chapman, D. (2004). Sad,
blue, or depressed days, health behaviors
and health- related quality of life, Behavioral
Risk Factor Surveillance System, 1995–2000.
Health and Quality of Life Outcomes, 2, 40.
Kunzmann, U., & Grühn, D. (2005). Age differ-
ences in emotional reactivity: The sample case
of sadness. Psychology and Aging, 20, 47–59.
Kunzmann, U., Little, T. D., & Smith, J. (2000).
Is age- related stability of subjective well-being
a paradox?: Cross- sectional and longitudinal
evidence from the Berlin aging study. Psychol-
ogy and Aging, 15, 511–526.
Kunzmann, U., & Richter, D. (2009). Emotional
reactivity across the adult life span: The cogni-
tive pragmatics make a difference. Psychology
and Aging, 24, 879–889.
Lambrecht, L., Kreifelts, B., & Wildgruber, D.
(2012). Age- related decrease in recognition
of emotional facial and prosodic expressions.
Emotion, 12, 529–539.
Lang, F. R., & Carstensen, L. L. (1994). Close
emotional relationships in late life: Further
support for proactive aging in the social
domain. Psychology and Aging, 9, 315–324.
Lansford, J. E., Sherman, A. M., & Antonucci, T.
C. (1998). Satisfaction with social networks:
An examination of socioemotional selectivity
theory across cohorts. Psychology and Aging,
13, 544–552.
Lawton, M. P. (2001). Emotion in later life. Cur-
rent Directions in Psychological Science, 10,
120–123.
Leclerc, C. M., & Kensinger, E. A. (2011). Neural
processing of emotional pictures and words: A
comparison of young and older adults. Devel-
opmental Neuropsychology, 26, 519–538.
Levenson, R. W., Carstensen, L. L., & Gottman,
J. M. (1994). The influence of age and gender
on affect, physiology, and their interrelations:
A study of long-term marriages. Journal of
Personality and Social Psychology, 67, 56–68.
Levine, L. J., & Pizarro, D. A. (2004). Emotion
and memory research: A grumpy overview.
Social Cognition, 22, 530–554.
Löckenhoff, C. E., & Carstensen, L. L. (2007).
Aging, emotion, and health- related decision
strategies: Motivational manipulations can
reduce age differences. Psychology and Aging,
22, 134–146.
Magai, C., Consedine, N. S., Krivoshekova, Y.
S., Kudadjie- Gyamfi, E., & McPherson, R.
(2006). Emotion experience and expression
across the adult life span: Insights from a mul-
timodal assessment study. Psychology and
Aging, 21, 303–317.
Mather, M., Canli, T., English, T., Whitfield, S.,
Wais, P., Ochsner, K., et al. (2004). Amygdala
responses to emotionally valenced stimuli in
older and younger adults. Psychological Sci-
ence, 15, 259–263.
Mather, M., & Carstensen, L. L. (2003). Aging
and attentional biases for emotional faces.
Psychological Science, 14, 409–415.
Mather, M., & Carstensen, L. L. (2005). Aging
and motivated cognition: The positivity effect
in attention and memory. Trends in Cognitive
Sciences, 9, 496–502.
Mather, M., Johnson, M. K., & De Leonardis,
D. M. (1999). Stereotype reliance in source
monitoring: Age differences and neuropsycho-
logical test correlates. Cognitive Neuropsy-
chology, 16, 437–458.
McRae, K., Gross, J. J., Weber, J., Robertson,
E. R., Solkol- Hessner, P., Ray, R. D., et al.
(2012). The development of emotion regula-
tion: An fMRI study of cognitive reappraisal
in children, adolescents and young adults.
Scan, 7, 11–22.
Mikels, J. A., Larkin, G. R., Reuter- Lorenz, P.,
& Carstensen, L. L. (2005). Divergent trajec-
tories in the aging mind: Changes in working
memory for affective versus visual information
with age. Psychology and Aging, 20, 542–553.
Mroczek, D. K., & Almeida, D. M. (2004). The
effect of daily stress, personality, and age on
daily negative affect. Journal of Personality,
72, 355–378.
Mroczek, D. K., & Kolarz, C. M. (1998). The
effect of age on positive and negative affect: A
developmental perspective on happiness. Jour-
nal of Personality and Social Psychology, 75,
1333–1349.
Emotion Regulation and Aging 217
Nikitin, J., & Freund, A. M. (2011). Age and
motivation predict gaze behavior for facial
expressions. Psychology and Aging, 26, 695–
700.
Ong, A. D., Rothstein, J. D., & Uchino, B. N.
(2012). Loneliness accentuates age differences
in cardiovascular responses to social evalua-
tive threat. Psychology and Aging, 27, 190–
198.
Petrican, R., Moscovitch, M., & Schimmack,
U. (2008). Cognitive resources, valence, and
memory retrieval of emotional events in older
adults. Psychology and Aging, 23, 585–594.
Phillips, L. H., Henry, J. D., Hosie, J. A., &
Milne, A. B. (2008). Effective regulation of
the experience and expression of negative
affect in old age. Journals of Gerontology B:
Psychological Sciences and Social Sciences,
63, P138–P145.
Piazza, J., & Charles, S. T. (2006). Mental health
and the baby boomers. In S. K. Whitbourne
& S. L. Willis (Eds.), The baby boomers at
midlife: Contemporary perspectives on mid-
dle age (pp. 111–148). Mahwah, NJ: Erlbaum.
Piazza, J. R., Charles, S. T., Stawski, R. S., &
Almeida, D. M. (2013). Age and the asso-
ciation between negative affective states and
diurnal cortisol. Psychology and Aging, 28,
47–56.
Piguet, O., Connally, E., Krendl, A. C., Huot,
J. R., & Corkin, S. (2008). False memory in
aging: Effects of emotional valence on word
recognition accuracy. Psychology and Aging,
23, 85–92.
Ready, R. E., Akerstedt, A. M., & Mroczek, D.
K. (2012). Emotional complexity and emo-
tional well-being in older adults: Risks of neu-
roticism. Aging and Mental Health, 16, 17–26.
Reed, A., & Carstensen, L. L. (2012) The theory
behind the age- related positivity effect. Fron-
tiers in Cognitive Science, 3, 1–9.
Riediger, M., Schmiedek, F., Wagner, G., & Lin-
denberger, U. (2009). Seeking pleasure and
seeking pain: Differences in prohedonic and
contra- hedonic motivation from adolescence
to old age. Psychological Science, 20, 1529–
1535.
Samanez- Larkin, G. R., Gibbs, S. E. B., Khanna,
K., Nielsen, L., Carstensen, L. L., & Knutson,
B. (2007). Anticipation of monetary gain but
not loss in healthy older adults. Nature Neuro-
science, 10, 787–791.
Scheibe, S., & Blanchard- Fields, F. (2009).
Effects of regulating emotions on cognitive
performance: What is costly for young adults
is not so costly for older adults. Psychology
and Aging, 24, 217–223.
Scheibe, S., & Carstensen, L. L. (2010). Emo-
tional aging: Recent findings and future
trends. Journals of Gerontology B: Psycho-
logical and Social Sciences, 65, 135–144.
Scheibe, S., English, T., Tsai, J. L., & Carstensen,
L. L. (2013). Striving to feel good: Ideal affect,
actual affect, and their correspondence across
adulthood. Psychology and Aging, 28, 160–
171.
Schryer, E., & Ross, M. (2012). Evaluating the
valence of remembered events: The impor-
tance of age and self- relevance. Psychology
and Aging, 27, 237–242.
Seider, B. H., Shiota, M. N., Whalen, P., & Lev-
enson, R. W. (2011). Greater sadness reactiv-
ity in late life. Social Cognitive and Affective
Neuroscience, 6, 186–194.
Shamaskin, A. M., Mikels, J. A., & Reed, A. E.
(2010). Getting the message across: Age differ-
ences in the positive and negative framing of
health care messages. Psychology and Aging,
25, 746–751.
Shiota, M. N., & Levenson, R. W. (2009).
Effects of aging on experimentally instructed
detached reappraisal, positive reappraisal, and
emotional behavioral suppression. Psychology
and Aging, 24, 890–900.
Slessor, G., Miles, L. K., Bull, R., & Phillips, L.
H. (2010). Age- related changes in detecting
happiness: Discriminating between enjoyment
and nonenjoyment smiles. Psychology and
Aging, 25, 246–250.
Sliwinski, M. J., Almeida, D. M., Smyth, J., &
Stawski, R. S. (2009). Intraindividual change
and variability in daily stress processes: Find-
ings from two measurement- burst diary stud-
ies. Psychology and Aging, 24, 828–840.
St. Jacques, P., Dolcos, F., & Cabeza, R. (2009).
Effects of aging on functional connectivity of
the amygdala during subsequent memory for
negative pictures: A network analysis of fMRI
data. Psychological Science, 20, 74–84.
St. Jacques, P., Dolcos, F., & Cabeza, R. (2010).
Effects of aging on functional connectivity of
the amygdala during negative evaluation: A
network analysis of fMRI data. Neurobiology
of Aging, 31, 315–331.
St. Jacques, P. L., Bessette- Symons, B., & Cabeza,
R. (2009). Functional neuroimaging studies of
aging and emotion: Fronto- amygdalar differ-
ences during emotional perception and epi-
sodic memory. Journal of the International
Neuropsychological Society, 15, 819–825.
218 DEVELOPMENTAL CONSIDERATIONS
Stone, A. A., Schwartz, J. E., Broderick, J. E.,
& Deaton, A. (2010). A snapshot of the age
distribution of psychological well-being in the
United States. Proceedings of the National
Academy of Sciences USA, 107(22), 9985–
9990.
Story, T. N., Berg, C. A., Smith, T. W., Beveridge,
R., Henry, N. J. M., & Pearce, G. (2007). Age,
marital satisfaction, and optimism as predic-
tors of positive sentiment override in middle-
aged and older married couples. Psychology
and Aging, 22, 719 –727.
Streubel, B., & Kunzmann, U. (2011). Age dif-
ferences in emotional reactions: Arousal and
age- relevance count. Psychology and Aging,
26, 966–978.
Teachman, B. A. (2006). Aging and negative
affect: The rise and fall and rise of anxiety and
depressive symptoms. Psychology and Aging,
21, 201–207.
Tsai, J. L., Levenson, R. W., & Carstensen, L.
L. (2000). Autonomic, expressive and subjec-
tive responses to emotional films in younger
and older adults of European American and
Chinese descent. Psychology and Aging, 15,
684–693.
Tucker, A. M., Feuerstein, R., Mende- Siedlecki,
P., Ochsner, K. N., & Stern, Y. (2012). Dou-
ble dissociation: Circadian off-peak times
increase emotional reactivity; Aging impairs
emotion regulation via reappraisal. Emotion,
12, 869–874.
Uchino, B. N., Birmingham, W., & Berg, C. A.
(2010). Are older adults less or more physi-
ologically reactive?: A meta- analysis of age-
related differences in cardiovascular reactivity
to laboratory tasks. Journals of Gerontology
B: Psychological Sciences and Social Sciences,
65, P154–P162.
Urry, H. L., & Gross, J. J. (2010). Emotion regu-
lation in older age. Current Directions in Psy-
chological Science, 19, 352–357.
Villamil, E., Huppert, F. A., & Melzer, D. (2006).
Low prevalence of depression and anxiety is
linked to statutory retirement ages rather than
personal work exit: A national survey. Psycho-
logical Medicine, 36, 999–1009.
Weierich, M. R., Kensinger, E. A., Munnell, A.
H., Sass, S. T., Dickerson, B. D., Wright, C.
I., et al. (2011). Older and wiser?: An affec-
tive science perspective on age- related chal-
lenges in financial decision making. Scan, 6,
195–206.
Westlye, L. T., Walhovd, K., B., Dale, A. M.,
Bjørnerud, A., Due-Tønnessen, P., Engvig, A.,
et al. (2010). Life-span changes of the human
brain white matter: Diffusion tensor imaging
(DTI) and volumetry. Cerebral Cortex, 20,
2055–2068.
Wrzus, C., Müller, V., Wagner, G. G., Linden-
berger, U., & Riediger, M. (2013). Affective
and cardiovascular responding to unpleasant
events from adolescence to old age: Complex-
ity of events matters. Developmental Psychol-
ogy, 49, 384 –397.
Wurm, L. H., Labouvie- Vief, G., Aycock, J.,
Rebucal, K. A., & Koch, H. E. (2004). Perfor-
mance in auditory and visual emotional Stroop
tasks: A comparison of older and younger
adults. Psychology and Aging, 19, 523–535.
Zulfiqar, U., Jurivich, D. A., Gao, W., & Singer,
D. H. (2010). Relation of high heart rate vari-
ability to healthy longevity. American Journal
of Cardiology, 105, 1181–1185.

PART V
SOCIAL ASPECTS

221
To survive and reproduce, organisms must
take in more energy than they expend,
a principle of behavioral ecology called
economy of action (Krebs & Davies, 1993).
Social baseline theory (SBT), a framework
based on this principle, organizes decades
of observed links between social relation-
ships, health, and well-being, in order to
understand how humans utilize each other
as resources to optimize individual energy
expenditures. This general strategy helps us
manage the costs of our very long period of
ontogenetic development and, we argue, the
many behavioral and psychological capa-
bilities of our uniquely powerful and costly
brain (Smith, 2003). Below, we review evi-
dence that social relationships serve the
energy- saving functions that we claim and
describe the reasons SBT refers to social
proximity as a “baseline” condition. For
this chapter we place special emphasis on the
role of social proximity in the regulation of
emotion. In our view, the social regulation
of emotion serves, in the aggregate and on
average, to decrease the cost of coping with
many of life’s difficulties (Cohen & Hober-
man, 1983; Cohen & McKay, 1984)—a
function that involves the brain’s ability
to use both internal and external informa-
tion to make “bets” about how to deploy its
own resources in light of expected returns.
This perspective (1) highlights the impor-
tance of emotion as a source of information
about current and predicted resources, and
(2) entails a view of psychological measure-
ment that extends beyond the individual to
systemic processes within dyads and groups
(Schilbach et al., in press).
Social Proximity and the Economy
of Action
The economy of action suggests that adap-
tations to prevailing environmental con-
ditions must, at the very least, ensure that
more energy is acquired than lost (Krebs
& Davies, 1993). The alternative— more
energy out than in—leads ultimately to
death. But because environments are rife
with danger and competition, the economy
of action tends toward optimization, where
resources are both acquired and conserved
whenever possible. For example, foraging
animals optimize energy intake per unit
of time by abandoning even plentiful food
sources if the amount of energy (e.g., calo-
ries) acquired is not optimal (MacArthur
& Pianka, 1966; Schmidhempel, Kacelnik,
& Houston, 1985). Optimization strategies
CHAPTER 14
Social Baseline Theory
and the Social Regulation of Emotion
James A. Coan
Erin L. Maresh

222 SOCIAL ASPECTS
are likely mediated through changes in sen-
sory perception. So simply wearing a heavy
backpack can make distances appear far-
ther away and hills seem steeper (Stefanucci,
Proffitt, Banton, & Epstein, 2005). Indeed,
it is increasingly apparent that perception is
influenced by not only the properties of sen-
sory stimuli but also emotions— emotions
that are themselves embodied instantia-
tions of prevailing circumstances, goals,
and physiological states (Stefanucci, Proffitt,
Clore, & Parekh, 2008).
SBT is rooted in the proposition that, for
humans, social proximity and interaction
powerfully optimize energy expenditures
(e.g., actual and perceived effort) devoted to
navigating potentially dangerous environ-
ments. Moreover, the best evidence suggests
these effects are mediated through rela-
tively automatic, bottom- up, unconditioned
perceptual mechanisms that modulate—
and regulate— emotional responding, as
opposed to mechanisms often associated
with the self-regulation of emotion (e.g., the
dorsolateral [dlPFC] and ventromedial pre-
frontal cortex [vmPFC]). SBT invokes the
economy of action here, too, by suggesting
that perceptual mechanisms of the central
nervous system are “inexpensive”
1
when
compared to more effortful, self- regulatory,
vigilant, “future- thinking” processes associ-
ated with the operation of the PFC—a dif-
ference that leads to both phylogenetic and
ontogenetic pressures to achieve regulatory
ends by perceptual and hence social means.
We suggest that the ability to depend on oth-
ers for as many costly processes as possible
leads to (1) decreased overall cost in deal-
ing with an uncertain and potentially deadly
environment, and (2) pressure— throughout
the lifespan and over the course of evolu-
tion— to form and maintain close social
relationships.
Before going any further, we would like to
emphasize three important points. First, our
view of social emotion regulation does not
imply that any and all effects an individual’s
behavior may have on the emotional behav-
ior of another constitute regulatory effects.
If one individual insults or compliments
another, either behavior could cause the
receiver to activate an emotional response.
This would not, however, constitute an
instance of emotion regulation. An indi-
vidual regulates another’s emotions when
his or her behavior (which might encompass
social history with the other person, an offer
of aid, or simply close physical proximity)
influences another’s emotional response to
some additional current or potential stimu-
lus or situation. Second, we acknowledge
that people vary in the extent to which they
both seek out and derive benefit from social
contact (leading to individual differences in
the broad processes described here). But on
average and in the aggregate, it is probably
the case that for humans and other social
mammals, proximity to social resources
reduces the net cost of survival. Third,
although most of the empirical evidence we
review entails the regulatory impact of social
proximity and interaction on the regulation
of negative affect, we do not intend to argue
that negative affect is the only broad form of
emotional responding that can be regulated.
Indeed, below we address some instances of
the social regulation of positive emotion.
Our Social Baseline
There is little doubt that supportive social
behaviors can modify or quell our subjec-
tive, behavioral, and physiological responses
to stress (Holt- Lunstad, Smith, & Lay-
ton, 2010). Such effects are likely rooted
in human phylogeny. Indeed, Beckes and
Coan (2011) and others (Berscheid, 2003;
Brewer & Caporeal, 1990) have suggested
that the dominant ecology to which humans
are adapted is not any one terrain, diet, or
climate, but rather each other. One of the
defining features of human beings— our
adaptability— is yoked to our ability to
cooperate with others, an ability that was
likely shaped by periods of great environ-
mental instability (Richerson, Bettinger,
& Boyd, 2005). Cooperative behavior was
likely selected as a means of managing the
energy costs of our exceedingly expensive
bodies, brains, and activities (Hill et al.,
2011; McNally, Brown, & Jackson, 2012;
Moll & Tomasello, 2007). So the first sense
in which SBT refers to a social baseline is
that social relatedness and its psychological
correlates constitute the normal, baseline
ecology of the functional human brain. That
is, the human brain is designed to operate
within a relatively predictable network of
social relationships characterized by famil-

Social Baseline Theory 223
iarity, shared intentionality, and interdepen-
dence (Tomasello, Carpenter, Call, Behne,
& Moll, 2005).
The other sense in which SBT refers to
the social “baseline” results from work
using functional magnetic resonance imag-
ing (fMRI; Coan, Schaefer, & Davidson,
2006), which suggests that social proximity
and interaction regulate many aspects of the
brain’s response to potential threats. Spe-
cifically, the authors subjected 16 married
women to the threat of mild electric shock
under three conditions, counterbalanced
across subjects: while alone in the scanner,
while holding a stranger’s hand, and while
holding her husband’s hand. The brain was
maximally active while facing the threat
alone, exhibiting a set of responses repli-
cating a large body of work on the brain’s
response to threat, fear, stress, and pain
(Bishop, Duncan, Brett, & Lawrence, 2004;
Brooks, Nurmikko, Bimson, Singh, & Rob-
erts, 2002; Dedovic et al., 2005; Stark et al.,
2003). As levels of contact with perceived
social resources increased, however, many
of these activations either decreased in inten-
sity or vanished altogether.
For example, although both spouse and
stranger handholding caused decreased
threat responding in regions associated
with the regulation of bodily arousal and
the mobilization of behavioral action
plans—such as the ventral anterior cingu-
late cortex, supramarginal and postcentral
gyri, and posterior cingulate— only spouse
hand- holding was specifically associated
with additional attenuation in circuits asso-
ciated with effortful emotion regulation
and threat- related homeostatic functions,
such as the right dlPFC and superior col-
liculus. Most strikingly, women inhabiting
the highest quality relationships realized
dramatic attenuations in all the aforemen-
tioned threat- responsive regions in addition
to those supporting subjective suffering and
the release of stress hormones, such as the
right anterior insula and hypothalamus.
Putting it all together, there appeared to be
a relatively linear, monotonic decrease in
the degree of threat- related neural process-
ing as one progressed from being alone to
being with anyone (stranger or spouse), to
being with a spouse, to being with a spouse
in a very high- quality relationship. This sug-
gests that the perception of threat decreases
as a function of not only proximity to social
resources but also the perceived quality of
those resources.
From the perspective of SBT, the alone
condition used in the hand- holding para-
digm described earlier is not a “baseline”
condition against which the experimental
hand- holding conditions are compared. On
the contrary, the reverse is true—the hand-
holding conditions are closer to the environ-
mental conditions to which the human brain
is adapted, and the alone condition is far-
ther away. This conclusion forms part of the
basis of SBT—that social resources decrease
the perceived cost of managing a threaten-
ing environment, providing a primary and
largely unconditional opportunity for econ-
omizing both neural and behavioral activity.
Importantly, these effects are not depen-
dent upon physical touch per se. For
example, there is less hypothalamic activ-
ity during social rejection among individu-
als asked to imagine an attachment figure
(Karremans, Heslenfeld, van Dillen, & Van
Lange, 2011). Moreover, imagining a strong
attachment figure seems to have resulted in
not more but less activity in frontal regions
supporting self- regulatory effort, suggesting
that even imagining the presence of a secure
attachment figure may regulate one’s emo-
tions in such a way as to conserve perceived
resources.
Social Systems Regulate
the Emotions of Individuals
Critically, what we experience and catego-
rize as emotion powerfully impacts the way
we perceive and engage with the world (Ste-
fanucci, Gagnon, & Lessard, 2011). Emo-
tional processes carry vital, often implicit,
and embodied information about the good-
ness and badness of things (Clore & Tamir,
2002)—information that modifies percep-
tion and guides action (Barrett & Bar, 2009;
Schwarz & Clore, 1983). It follows that
emotions are indicators of, among other
things, the tension between perceived per-
sonal resources and perceived environmen-
tal demands (Moore, Vine, Wilson, & Free-
man, 2012; Tomaka, Blascovich, Kelsey,
& Leitten, 1993). In this way, emotion is
probably at or near the center of decisions
that economize activity (Bechara, 2011;
224 SOCIAL ASPECTS
Stefanucci et al., 2008). Accordingly, SBT
suggests that the brain uses affective, expe-
riential, conceptual, and contextual knowl-
edge— in effect, emotion (Barrett, 2006;
Lindquist & Barrett, 2008)—to bias percep-
tion in ways that guide actions toward more
favorable outcomes (Beckes & Coan, 2011;
Coan, 2008).
Emotion has been identified by others
as a key link between social relationships
and health. For example, the social buffer-
ing hypothesis suggests that social relation-
ships can provide support that is tangible,
such as material support when facing loss
of income, appraisal- based support, such as
when familiar others reduce the incidence
or intensity of threat- related appraisals, or
emotional support, such as when famil-
iar others repair threats to self- esteem or
increase a sense of felt belonging (Cohen &
McKay, 1984). It has been suggested further
that social relationships grow into part of
the brain’s understanding of itself and its
available resources (Beckes, Coan, & Has-
selmo, 2012). Moreover, as it makes predic-
tions about the potential cost of coping with
a given situation, the brain follows Bayes-
ian rules of inference (Friston, 2010; Knill
& Pouget, 2004), in which judgments about
the level of personal resources to deploy are
based on (1) the current situation (particu-
larly constraints, risks, and opportunities),
(2) predicted possible future situations, (3)
situational goals, (4) current energy states,
and (5) expected future energy states (Sali-
nas, 2011).
For example, hills appear to be less steep
when standing next to a friend (Schnall,
Harber, Stefanucci, & Proffitt, 2008), and
this effect is correlated with friendship dura-
tion: the longer the friendship, the less steep
the hill appears to be. The perception that
the hill is less steep serves as a marker that
one has, in effect, more bioenergetic capi-
tal in one’s budget— capital that can either
be spent more freely walking up the hill, or
conserved over time. Diverse studies hint
at similar conclusions regarding the brain’s
Bayesian properties. For example, Kraus,
Huang, and Keltner (2010) have observed
that more frequent physical touching among
professional basketball players corresponds
with increased late- season performance,
even after accounting for player status, pre-
season expectations, and early season per-
formance. Others have observed that one of
the best predictors of collective IQ is not the
IQs of individual group members but rather
the degree to which each member is sensitive
to social cues expressed on the face (Wool-
ley, Chabris, Pentland, Hashmi, & Malone,
2010). From the perspective of SBT, each
of these findings is attributable to the way
social resources help to conserve costly vigi-
lance and self- regulation efforts, either by
imposing less interference with motor and
perceptual activity (in the case of players in
the NBA), or by devoting those resources to
solving other kinds of problems (in the case
of collective IQ). But the question of why
social resources economize human neural
and behavioral activity remains. We pro-
pose at least two broad, distal mechanisms
by which this is accomplished: risk distribu-
tion and load sharing.
Risk Distribution
In group settings, many species adjust the
level of their energy expenditure in accor-
dance with the size of the group they inhabit
(Krause & Ruxton, 2002). This benefit
of group living is called risk distribution
(Coan, 2008), in that environmental risk is
probabilistically distributed across the group
rather than being concentrated on one indi-
vidual. A clear example of this can be seen
in optimal foraging theory, which proposes
that animals forage in a way that maximizes
energy intake per unit of time (MacArthur
& Pianka, 1966). For example, animals
that forage must spend a certain amount
of energy maintaining vigilance for preda-
tors. Foraging in a larger group decreases
the burden of vigilance on any one member,
and indeed, in many species, the number of
individuals that are vigilant for predators
at any given time decreases as group size
increases (Elgar, 1989; Roberts, 1996). With
regard to the social regulation of emotion,
we can construe threat vigilance as a form
of negative affect— or at least as the result
of a negative affect process that reflects the
demand for vigilance. If true, then social
proximity can be viewed as decreasing vigi-
lance behavior via the regulation of negative
affect. Evidence for this can even be seen
in the modulation by group size of physi-
Social Baseline Theory 225
ological indicators of stress in nonhuman
animals. For example, salivary cortisol con-
centration in sheep is higher when the flock
size is smaller (Michelena et al., 2012). Risk
distribution suggests further that the num-
ber of proximal conspecifics decreases nega-
tive affect, because doing so conserves effort
that would otherwise be spent either on vigi-
lance, self- regulation, or both. This is exem-
plified in humans in the hand- holding study
discussed earlier, by the finding that holding
any hand—even that of a stranger— reduces
neural activity related to threat (Coan et
al., 2006), especially in regions supporting
responses to acute threats (Mobbs et al.,
2007).
Interestingly, the individual benefits of
group living described earlier often benefit
the group as well, such that as each individ-
ual’s vigilance effort decreases, total group
vigilance actually increases (Bertram, 1978;
Pulliam, Pyke, & Caraco, 1982) and time
to detect predators decreases (Siegfried &
Underhill, 1975). It is important to note,
however, that group living introduces certain
risks and stressors as well, such as increased
competition for resources, increased likeli-
hood of disease transmission, and increased
conspicuousness (Alexander, 1974). Groups
therefore iteratively move toward an optimal
size given the availability of food and other
resources (Higashi & Yamamura, 1993).
Note that risk distribution concerns only the
number of members in a group and makes
no reference to the nature and quality of the
relationships between the group members.
Coan (2008) has speculated that the brain
is sensitive to risk distribution as a general
purpose strategy that employs Bayesian- like
calculations to assess the cost- effectiveness
of affective behaviors at any given time.
Load Sharing
Load sharing builds on the benefits of risk
distribution by adding information that
increases certainty about the availability of
a given social resource— namely, familiar-
ity, reliability, and interdependence. The
presence of an individual with whom one
has established a close relationship not only
probabilistically reduces one’s risk of envi-
ronmental threat but also signals access to a
person who will use his or her own resources
on one’s behalf, thus further reducing the
need to expend one’s own energy, and com-
mensurably decreasing the need, for exam-
ple, for negative affect. In other words, a
close companion can be trusted to share an
interest in one’s well-being. He or she may
provide additional vigilance not only in some
general sense but also specifically, with one’s
own welfare in mind (Davis, 1984). A close
companion may share resources (e.g., Rog-
ers & DeBoer, 2001), help care for young
(e.g., Ehrenberg, Gearing- Small, Hunter, &
Small, 2001), and help when one is sick or
injured (e.g., Townsend & Franks, 1995).
These additional benefits provide a much
greater opportunity for economizing neural
and behavioral activity, as can be seen in
the enhanced effect of holding hands with
a close friend or a spouse (Coan, Beckes, &
Allen, 2013; Coan et al., 2006).
SBT maintains that load sharing impacts
decisions about personal resource budget-
ing in part by altering the way the brain
encodes what constitutes the “self,” with
implications for the level of resources the
self is able to access. This is consistent with
the observation that the neural representa-
tions of threats directed at the self are highly
correlated with those directed at friends,
implying a kind of “involuntary breach of
individual separateness” (Langer, 1974,
p. 129) that does not seem to generalize to
strangers (Beckes et al., 2012). In this way,
an individual’s social support system can be
construed as an extension of the self and,
in turn, of how one perceives and interacts
with the world.
Capitalization
Importantly, social proximity and interac-
tion serve not only to down- regulate negative
emotions, but also to maintain and increase
positive, approach- related emotions. Admit-
tedly, our research has focused on the
social regulation of response to threat, but
we believe the basic ideas behind SBT are
generalizable to positive emotions as well.
People frequently expend energy to induce,
maintain, or even dampen experiences of
positive emotion, and this is reflected in
neural activity in prefrontal areas similar to
those seen in the self- regulation of negative
emotion (Kim & Hamann, 2007). Social
226 SOCIAL ASPECTS
relationships may act to conserve effortful
positive self- regulation, possibly by reducing
reliance on prefrontal activity and increas-
ing activity in reward circuits. Interestingly,
neural activity in the medial PFC decreases
in mothers observing pictures of their chil-
dren, and in adults observing pictures of
their romantic partner, as compared to
when they are observing pictures of a friend
(Bartels & Zeki, 2000, 2004). Moreover,
positive responses by a relational partner to
one’s own positive news, a process known
as capitalization, may be a mechanism by
which social relationships enhance positive
emotions in ways that are less individually
effortful. Capitalization increases daily posi-
tive affect over and above the impact of the
positive event itself (Gable, Reis, Impett, &
Asher, 2004; Langston, 1994). Furthermore,
capitalization may strengthen trust and pro-
social behavior, even when one is interacting
with a stranger (Reis et al., 2010). Following
the SBT model, then, receiving supportive,
positive feedback from a partner may sig-
nal an increase in both personal and social
resources. It is interesting to note that the
perceived responsiveness of one’s partner
during positive events may be more strongly
linked with the relationship’s well-being
and longevity than perceived responsiveness
during negative events (Gable, Gonzaga, &
Strachman, 2006).
Importantly, SBT views all forms of
social emotion regulation as systemic
and dynamic, challenging the widely held
assumption that the basic unit of analysis in
human psychology is the single individual
(Beckes & Coan, 2011). By extending the
unit of analysis to the dyad or group, social
interaction and proximity emerge as adap-
tive strategies rooted in the sharing of efforts
that, left only to each individual member of
a dyad or group, would be redundant, costly,
and inefficient. Ultimately, it may be that
dyads or groups benefit from social emo-
tion regulation at least as much as the indi-
viduals who inhabit them (McComb, Moss,
Durant, Baker, & Sayialel, 2001). In consid-
ering mechanisms for these dyadic or group
effects, social emotion regulation can be
mediational, reflecting a direct intervention
on an emotional process by social resources,
or moderational, modifying the perception
of potentially emotional situations. In the
following sections, we briefly describe and
propose potential mechanisms for the dis-
tinction.
Mediating Mechanisms of Social
Emotion Regulation
As mediators of emotion regulation (Baron
& Kenny, 1986), social resources serve as a
proximal mechanism through which emo-
tion regulation effects are achieved. Attach-
ment theory provides the quintessential
example of socially regulated emotion in
its description of mother– child attachment
interactions, in which infants seek attach-
ment figures during periods of distress and
are soothed by their caregiver’s presence
(Ainsworth, Blehar, Waters, & Wall, 1978;
Bowlby, 1969/1982). A common example
might be a child’s vaccination procedure,
where the child— distressed at the prospect
of receiving a shot—calls for the mother’s
support. In response, the mother may engage
in soothing behaviors like hand- holding and
offering reassuring words in a calm tone of
voice. Here, the mother’s behavior provides
an obvious mechanism through which the
child’s distress is attenuated. Specifically,
the child identifies a potential threat (the
needle), and this threat causes two reactions,
distress and the seeking of support from the
mother. In turn, the mother’s support behav-
ior exerts a down- regulatory influence on
the distress response.
The mother– child dynamic is a par-
ticularly powerful example of how social
resources can mediate emotion regulation
in part because the child in the example is
developmentally limited in his or her self-
regulatory abilities, a limitation that is itself
rooted in the slow development of neural
systems that will eventually serve the child’s
self- regulatory needs (Coan, 2008). That
the mother’s soothing works is obvious and
well documented (Cassidy & Shaver, 2008).
Less obvious is that the child is relatively
incapable of emotion regulation without
the mother. Thus, we can view the mother
and child as an interactive system that is
intimately linked in an emotion activation–
regulation dynamic. Importantly, however,
the mother– child behavioral and experiential
dynamics described by attachment theorists
play out similarly within adult relationships
(Mikulincer & Shaver, 2007), where stress-
ful situations motivate social proximity, and
Social Baseline Theory 227
social proximity lowers autonomic arousal,
attenuates hypothalamic– pituitary– adrenal
(HPA) axis activity, and improves immune
function (Baron, Cutrona, Hicklin, Russell,
& Lubaroff, 1990; Heinrichs, Baumgart-
ner, Kirschbaum, & Ehlert, 2003; Uchino,
Cacioppo, & Kiecolt- Glaser, 1996). Of
interest with regard to adult relationships
is that, unlike mother– infant dyads, two or
more adults each possess fully developed and
functioning brains. Thus, although children
require maternal support for emotion regu-
lation, adults appear to utilize each other for
similar purposes more strategically.
A major focus of our work has been the
identification of proximal neural mechanisms
linking social relationships to decreased
threat responding and, in turn, increased
health and well-being. Although many can-
didate mechanisms have been postulated,
few or none have been definitively identified.
Recently, Eisenberger and colleagues (2011)
provided what they characterized as media-
tional evidence for the social regulation of
emotion by portions of the vmPFC. In this
work, participants were shown pictures of
a romantic partner, pictures of strangers, or
pictures of objects, while receiving painful
stimulation. Putative pain- related activa-
tions in regions such as the dorsal anterior
cingulate cortex (dACC) and anterior insula
were less active while participants viewed
images of a romantic partner. Moreover, the
vmPFC was more active while participants
viewed romantic partners, even during pain
stimuli, and vmPFC activation was positively
associated with relationship duration and
perceived partner support, while negatively
associated with both subjective pain ratings
and pain- related neural activity. In a simi-
lar study, Younger, Aron, Parke, Chatterjee,
and Mackey (2010) collected brain images
from 15 participants as they experienced
moderate levels of thermal pain while they
viewed pictures of their romantic partner,
pictures of attractive strangers, or engaged
in a distraction task, hypothesizing that pic-
tures of romantic partners would attenuate
pain processing via putative “reward sys-
tems.” Indeed, viewing pictures of roman-
tic partners both reduced subjective pain
reports and attenuated circuits associated
with pain processing. Moreover, subjective
pain reports were inversely correlated dur-
ing romantic partner picture viewing with
the activation of neural circuits associated
with reward processing (e.g., the caudate
nucleus, the nucleus accumbens) and effort-
ful self- regulation (e.g., the dlPFC).
Thus, emerging evidence for proximal
mechanisms supporting socially medi-
ated emotion regulation appears to include
regions associated with both the automatic
and effortful self-regulation of emotion
(vmPFC and dlPFC, respectively), as well
as putative reward circuits (e.g., the nucleus
accumbens). However, our laboratory has
not been able to produce similar results, in
our original study of hand- holding by mar-
ried couples (Coan et al., 2006), in a more
recent study of hand- holding by platonic
friends (Coan et al., 2013), or in a study of
the regulation of threat responses in chil-
dren by the presence of adult caregivers
(Conner et al., 2012). Indeed, in the latter
study, we observed decreased activation spe-
cifically in the vmPFC and ventrolateral PFC
(vlPFC), where we used a region of interest
(ROI) approach for the purpose of address-
ing the question of prefrontal mediation of
social support. Ultimately, we have thus far
failed to reliably identify any regions of the
brain that are normatively more active under
threat conditions during supportive presence
or hand- holding than while alone. More-
over, none of the regions identified by Eisen-
berger et al. (2011) and Younger et al. (2010)
are negatively correlated with any of the
threat- responsive circuits apparently down-
regulated in our work by hand- holding.
What may account for these apparently
inconsistent results? The answer, we think,
has to do with the difference between touch
and picture viewing. Specifically, there is
ample evidence that touch acts as an uncon-
ditioned or primary stimulus in social ani-
mals, not least humans (Francis et al., 1999),
who may also use touch to exchange impor-
tant social information (Gazzola et al.,
2012; Morrison, Löken, & Olausson, 2010).
By contrast, picture viewing likely involves
associative learning, particularly involv-
ing the expected value of the photographic
image—a task often mediated through
vmPFC activity (Kable & Glimcher, 2007).
Thus, vmPFC activity may be necessary
when utilizing photographs of loved ones to
regulate emotion. But a careful reading of
Younger et al. (2010) reveals that, in addi-
tion to showing their participants pictures
228 SOCIAL ASPECTS
of loved ones, they instructed participants to
“focus on the picture and think about the
displayed person” (p. 2), effectively encour-
aging self-mediated rather than socially
mediated emotion regulation. Ultimately,
we would argue that both the Eisenberger et
al. (2011) and Younger et al. (2010) experi-
ments manipulated self- regulatory systems
and are not best understood as examples of
socially mediated emotion regulation at all.
Moderating Mechanisms of Social
Emotion Regulation
Strictly speaking, the hand- holding study
discussed earlier may also provide an
example of socially moderated emotion
regulation, in part because the provision
of social support preceded the presentation
of threat cues, thus potentially altering the
perception of those threat cues and obviat-
ing the activation of portions of the threat
response. This illustrates one of the many
ways in which social proximity and interac-
tion can be viewed as moderating emotional
responses and self- regulation needs, acting
as third variables that modify the conditions
under which emotional responses and self-
regulation strategies are either called upon
or maximally effective. Taking the example
of the vaccination procedure discussed ear-
lier, it may be the case that, as in our hand-
holding experiments, the mother holds the
child’s hand not in response to the child’s
obvious distress but in anticipation of the
child’s potential distress. In this case, mater-
nal hand- holding may cause relaxation of
the child’s vigilance processing, rendering
the appearance of the needle less threaten-
ing when it does arrive.
Past social experiences also impact an
individual’s self- regulation requirements
and capabilities (Allen, Moore, Kuperminc,
& Bell, 1998; Prinstein & La Greca, 2004;
Weaver et al., 2004). For example, ongo-
ing current daily social support may mod-
erate the perception of threats encountered
while alone, which may in turn reduce the
perceived need for engaging self- regulation
capabilities (Eisenberger, Taylor, Gable,
Hilmert, & Lieberman, 2007). Similarly,
mental representations of loved ones may be
used as methods by which one can exert self-
regulatory strategies related to distraction
or reappraisal. Any or all of these possibili-
ties can be modeled by SBT, such that past
social experiences or even prevailing social
conditions alter an individual’s perception of
potential threats and rewards in his or her
immediate environment even when he or she
is alone at the occasion of measurement.
A number of researchers have argued
that early childhood experiences critically
influence the ways in which individuals
view social relationships later in life. For
humans and many other species, filial bond-
ing—bonding with siblings and caregivers—
occurs rapidly, unconditionally, and during a
period of neural development that may hold
powerful consequences for subsequent func-
tioning (Bowlby, 1969/1982; Coan, 2008).
In infants, the PFC is particularly under-
developed in comparison to that of adults,
and its development takes approximately
two decades to complete. The human brain
in general grows exponentially during just
the first 2 years of life (Franceschini et al.,
2007)—growth reflected in the brain’s con-
sumption of glucose, which continues to be
approximately double that of adults until 10
years of age (Chugani, 1998). Throughout
this early development, the brain is in effect
adapting itself to environmental contingen-
cies through a process of axonal, dendritic,
and synaptic “pruning”—a process that is
itself dependent upon neural activity associ-
ated with environmental stimulation (Can-
cedda et al., 2004; Reichardt, 2006). Put
another way, the brain in early development
adheres roughly to the colloquialism “use it
or lose it”; environmental demands shape a
developing brain prepared for a wide range
of behavioral possibilities. By the process of
pruning, the brain’s responses to prevail-
ing contextual demands become more fixed
and hence more rapid—it becomes adapted
ontogenetically to its expected environment
(Hebb, 1949; Posner & Rothbart, 2007).
During this development, interactions
between social experiences, self- regulatory
activity and socially bound activations in
medial prefrontal, temporoparietal, and
posterior temporal cortices, as well as in
the amygdala, ventral tegmentum, nucleus
accumbens, periaqueductal gray and else-
where, may shape expectations about the
availability of social resources, opportuni-
ties for affiliation, expectations about the
reliability of close relational partners, and so
on (Lieberman, 2007). This in turn is likely

Social Baseline Theory 229
to shape individual differences in the way
social resources are approached and main-
tained, perhaps especially in situations that
blend social proximity with potential stress-
ors. Indeed, it is just this sort of experience-
based developmental divergence that early
attachment researchers described as mani-
festing in “working models” of attachment
figures, a theoretical perspective expressed
in more recent terms as attachment styles
(Ainsworth et al., 1978; Main, 1996; Miku-
lincer & Shaver, 2007).
A large and growing body of research has
documented effects consistent with early psy-
chosocial brain development. For example,
primates are highly sensitive to early proxim-
ity to and interaction with caregivers (Har-
low, 1958). In rats, maternal grooming may
influence the methylation of glucocorticoid
receptor genes throughout the hippocampus
and possibly elsewhere, influencing in turn
the stress reactivity of the pups throughout
their lives and even into subsequent genera-
tions (Weaver et al., 2004). Evidence sug-
gests a similar methylation process may
occur in humans (McGowan et al., 2009;
van IJzendoorn, Bakermans- Kranenburg, &
Ebstein, 2011). Hofer (2006) has proposed
that early interactions with caregivers slowly
evolve from the indirect regulation of sen-
sorimotor, thermal, and nutrient functions
via responses to expressed emotion. At first
the infant is totally dependent upon caregiv-
ers for access to primary reinforcers (food,
water, warmth, touch). The only way for the
infant to access these resources is to get the
caregiver’s attention with expressed emo-
tion. A predictable pattern emerges where
the infant cries out when a basic resource is
needed, followed by the caregiver’s response,
after which the infant stops crying. As the
infant develops, his or her emotional reper-
toire expands to include preferences beyond
simple metabolic or thermal needs, but the
process of caregiver responsiveness contin-
ues in much the same way, until it can be
said that the emotional feelings and expres-
sions are themselves the targets of the care-
giver’s responses.
Attachment theorists have had perhaps
the most to say about how social experiences
contribute to individual differences in the
social regulation of emotion (Mikulincer &
Shaver, 2005; Mikulincer, Shaver, & Pereg,
2003). Specifically, attachment theory posits
the existence of trait-like expectations— the
aforementioned attachment styles—regard-
ing the availability of social resources.
Dominant views of attachment style can
be expressed as two independent axes rep-
resenting tendencies toward social anxi-
ety and social avoidance. Individuals low
in both are said to be secure, in that their
appraisals of potential threats and views of
emotional disclosure tend to be less negative
(Mikulincer & Florian, 1998; Mikulincer &
Orbach, 1995). Critically, such individuals
are likelier to seek out, utilize, and benefit
from emotional support from others (Fraley
& Shaver, 2000; Larose, Bernier, Soucy, &
Duchesne, 1999; Rholes, Simpson, & Grich
Stevens, 1998). Secure individuals may even
benefit more from capitalization attempts:
Shallcross, Howland, Bemis, Simpson, and
Frazier (2011) found that insecurely attached
individuals more often underestimated
the responsiveness of their partners when
sharing a positive event than did securely
attached individuals. Although neuroscien-
tific investigations of attachment style are
rare, Gillath, Bunge, Shaver, Wendelken,
and Mikulincer (2005) have observed that
greater trait attachment anxiety is associ-
ated with more activity in the dACC and
temporal pole, areas associated with alarm
and negative affect— and decreased activity
in the orbitofrontal cortex— when partici-
pants are asked to think about a threatening
relationship scenario.
Future Directions
We feel these perspectives on social relation-
ships and social emotion regulation have
implications that extend both to basic and
applied domains of psychological science,
and that the ecological framework of SBT
may be useful for understanding a number
of important new findings. Clinically, we
have recently noted that SBT holds implica-
tions for how we understand the frontolim-
bic dysfunction and emotional dysregula-
tion that characterize borderline personality
disorder (Hughes, Crowell, Uyeji, & Coan,
2012), and we believe that SBT may be rel-
evant to the development and maintenance
of other psychopathologies as well. For
example, although speculative, it is possible
that the disruptions in social relationships

230 SOCIAL ASPECTS
that characterize autism spectrum disorders
result in an overreliance on self- regulation,
leading to chronic depletion and the use of
self- regulatory strategies associated with
small children. We suggest further that SBT
may be useful for understanding emerging
approaches to relationship therapy that are
proving to be efficacious for a number of
problems traditionally treated by individu-
ally oriented psychotherapies alone (Mon-
son et al., 2012; Naaman, Radwan, & John-
son, 2009).
Ultimately, we emphasize that human life
is in many ways defined by social relation-
ships. According to SBT, a key function of
social relationships is the social regulation
of emotion. Emotions provide rapid, embod-
ied information about current states and
contextual demands (Clore & Tamir, 2002),
guiding decision making and modifying per-
ception (Bechara, 2011; Stefanucci et al.,
2008). The modification of perception via
emotional responding is itself yoked to the
management of bioenergetic resources for the
purpose of economizing action (Stefanucci
et al., 2011). Because emotion is powerfully
regulated by social proximity (Coan et al.,
2013; Coan et al., 2006), all of the above is
controlled by proximity to social resources
(Schnall et al., 2008). Thus, the impact of
social relationships ripples through virtu-
ally everything humans do, because social
relationships are tightly linked to emotional
processes, and emotional processes inform,
bias, and direct many, if not most, of the
brain’s activities. This may not always work
in ways that are broadly socially desirable.
For example, the social regulatory pro-
cesses described throughout this chapter
may contribute to phenomena such as social
loafing (Karau & Williams, 1993), and the
“outsourcing” of health behaviors (Fitzsi-
mons & Finkel, 2011). The impact of social
relationships— manifest as perceived prox-
imity, interaction, and history— is thus likely
to be felt within any specific subdomain of
psychological science, suggesting that any
such subdomain that does not account for
social processes will be limited in impor-
tant ways. On the other hand, the extent to
which social processes impact psychological
phenomena opens wide the possibilities for
new testable hypotheses and exciting pro-
grams of research.
Note
1. Accumulating evidence from a diverse col-
lection of laboratories suggests the psycho-
logical functions supported by the cortex—
including the PFC—are computationally, and
hence bioenergetically, expensive, especially
relative to more computationally basic per-
ceptual processes (Dietrich, 2009; Halford,
Wilson, & Phillips, 1997, 1998). At the very
least, psychological processes supported by
the PFC are often subjectively experienced
as effortful (Muraven & Baumeister, 2000;
Sheppes, Catran, & Meiran, 2009). Sus-
tained prefrontally mediated behaviors (e.g.,
attention, self- control, vigilance, behavioral
inhibition) cause subjective exhaustion and a
steady decrease in the ability to perform those
very behaviors (Baumeister, Vohs, & Tice,
2007; Vohs & Heatherton, 2000). Because
the brain maintains a fairly constant meta-
bolic rate regardless of task (Sokoloff, Man-
gold, Wechsler, Kennedy, & Kety, 1955), an
extended reliance on prefrontally mediated
processes may reduce resources (e.g., blood)
available to other regions. Indeed, there is
evidence of intrabrain competition for blood,
even within the cortex (Dietrich, 2009).
Although open to serious criticism (Kurzban,
2010), it may yet be possible that the PFC
even places a higher metabolic demand on
the brain’s resources. For example, the ratio
of glia to neurons— a ratio that covaries with
metabolic demand— is higher in the PFC of
humans than in other primates (Sherwood
et al., 2006). The PFC is among the last neu-
ral systems to develop fully (Fuster, 2002)
and among the first to show functional defi-
cits in the case of extreme emotional stress,
malnourishment, intoxication, addiction,
old age, and even exercise (Arnsten, 2009;
Del Giorno, Hall, O’Leary, Bixby, & Miller,
2010; Goldstein & Volkow, 2011; Lipina &
Colombo, 2009; Paxton, Barch, Racine, &
Braver, 2008). All of this is despite a general
consensus that the human PFC is one of the
defining characteristics of human phylogeny
(Ehrlich & Ehrlich, 2008)—a resource shaped
over evolutionary development that allows
humans a level of abstraction, creativity, vigi-
lance, self- control, and anticipatory judgment
that is unprecedented in the animal kingdom
and largely responsible for the globally domi-
nant status that humans enjoy.

Social Baseline Theory 231
References
Ainsworth, M. D. S., Blehar, M. C., Waters, E.,
& Wall, S. (1978). Patterns of attachment: A
psychological study of the strange situation.
Hillsdale, NJ: Erlbaum.
Alexander, R. D. (1974). The evolution of social
behavior. Annual Review of Ecology and Sys-
tematics, 5, 325–383.
Allen, J. P., Moore, C., Kuperminc, G., & Bell,
K. (1998). Attachment and adolescent psycho-
social functioning. Child Development, 69(5),
1406–1419.
Arnsten, A. F. T. (2009). Stress signalling path-
ways that impair prefrontal cortex structure
and function. Nature Reviews Neuroscience,
10(6), 410– 422.
Baron, R. M., & Kenny, D. A. (1986). The
moderator– mediator variable distinction in
social psychological research: Conceptual,
strategic, and statistical considerations. Jour-
nal of Personality and Social Psychology,
51(6), 1173–1182.
Baron, R. S., Cutrona, C. E., Hicklin, D., Rus-
sell, D. W., & Lubaroff, D. M. (1990). Social
support and immune function among spouses
of cancer patients. Journal of Personality and
Social Psychology, 59(2), 344–352.
Barrett, L. F. (2006). Solving the emotion para-
dox: Categorization and the experience of
emotion. Personality and Social Psychology
Review, 10, 20–46.
Barrett, L. F., & Bar, M. (2009). See it with
feeling: Affective predictions during object
perception. Philosophical Transactions of the
Royal Society B: Biological Sciences, 364,
1325–1334.
Bartels, A., & Zeki, S. (2000). The neural basis
of romantic love. NeuroReport, 11(17), 3829–
3834.
Bartels, A., & Zeki, S. (2004). The neural corre-
lates of maternal and romantic love. NeuroIm-
age, 21(3), 1155–1166.
Baumeister, R. F., Vohs, K. D., & Tice, D. M.
(2007). The strength model of self- control.
Current Directions in Psychological Science,
16(6), 351–355.
Bechara, A. (2011). The somatic marker hypoth-
esis and its neural basis: Using past experiences
to forecast the future in decision making. In
M. Bar (Ed.), Predictions in the brain: Using
our past to generate a future (pp. 122–133).
New York: Oxford University Press.
Beckes, L., & Coan, J. A. (2011). Social baseline
theory: The role of social proximity in emo-
tion and economy of action. Social and Per-
sonality Psychology Compass, 5, 976–988.
Beckes, L., Coan, J. A., & Hasselmo, K. (2012).
Familiarity promotes the blurring of self and
other in the neural representation of threat.
Social Cognitive and Affective Neuroscience.
[E-publication ahead of print]
Berscheid, E. (2003). The human’s greatest
strength: Other humans. In U. M. Staudinger
(Ed.), A psychology of human strengths: Fun-
damental questions and future directions for a
positive psychology (pp. 37–47). Washington,
DC: American Psychological Association.
Bertram, B. C. R. (1978). Living in groups: Pred-
ators and prey. In J. R. Krebs & N. B. Davies
(Eds.), Behavioural ecology: An evolutionary
approach (pp. 64–96). Oxford, UK: Black-
well.
Bishop, S., Duncan, J., Brett, M., & Lawrence,
A. D. (2004). Prefrontal cortical function and
anxiety: Controlling attention to threat- related
stimuli. Nature Neuroscience, 7, 184–188.
Bowlby, J. (1982). Attachment and loss: Vol. 1.
Attachment (2nd ed.). New York: Basic Books.
(Original work published 1969)
Brewer, M. B., & Caporeal, L. R. (1990). Selfish
genes vs. selfish people: Sociobiology as origin
myth. Motivation and Emotion, 14, 237–243.
Brooks, J. C. W., Nurmikko, T. J., Bimson, W.
E., Singh, K. D., & Roberts, N. (2002). fMRI
of thermal pain: Effects of stimulus laterality
and attention. NeuroImage, 15, 293–301.
Cancedda, L., Putignano, E., Sale, A., Viegi, A.,
Berardi, N., & Maffei, L. (2004). Acceleration
of visual system development by environmen-
tal enrichment. Journal of Neuroscience, 24,
4840–4848.
Cassidy, J., & Shaver, P. R. (2008). Handbook
of attachment: Theory, research, and clinical
applications (2nd ed.). New York: Guilford
Press.
Chugani, H. T. (1998). A critical period of brain
development: Studies of cerebral glucose uti-
lization with PET. Preventive Medicine, 27,
184–188.
Clore, G. L., & Tamir, M. (2002). Affect as
embodied information. Psychological Inquiry,
13, 37–45.
Coan, J. A. (2008). Toward a neuroscience
of attachment. In J. Cassidy & P. R. Shaver
(Eds.), Handbook of attachment: Theory,
research, and clinical applications (2nd ed.,
pp. 241–265). New York: Guilford Press.
232 SOCIAL ASPECTS
Coan, J. A., Beckes, L., & Allen, J. P. (2013).
Childhood maternal support and social capital
moderate the regulatory impact of social rela-
tionships in adulthood. International Journal
of Psychophysiology, 88(3), 224–231.
Coan, J. A., Schaefer, H. S., & Davidson, R. J.
(2006). Lending a hand: Social regulation of
the neural response to threat. Psychological
Science, 17, 1032–1039.
Cohen, S., & Hoberman, H. M. (1983). Positive
events and social supports as buffers of life
change stress. Journal of Applied Social Psy-
chology, 13(2), 99–125.
Cohen, S., & McKay, G. (1984). Social support,
stress, and the buffering hypothesis: A theo-
retical analysis. Handbook of Psychology and
Health, 4, 253 –267.
Conner, O. L., Siegle, G. J., McFarland, A. M.,
Silk, J. S., Ladouceur, C. D., Dahl, R. E., et
al. (2012). Mom—It helps when you’re right
here!: Attenuation of neural stress markers in
anxious youths whose caregivers are present
during fMRI. PloS ONE, 7(12), e50680.
Davis, L. S. (1984). Alarm calling in Richard-
son’s ground squirrels (Spermophilus richard-
sonii). Zeitschrift für Tierpsychologie, 66(2),
152–164.
Dedovic, K., Renwick, R., Mahani, N. K.,
Engert, V., Lupien, S. J., & Pruessner, J. C.
(2005). The Montreal Imaging Stress Task:
Using functional imaging to investigate the
effects of perceiving and processing psychoso-
cial stress in the human brain. Journal of Psy-
chiatry and Neuroscience, 30, 319–325.
Del Giorno, J. M., Hall, E. E., O’Leary, K. C.,
Bixby, W. R., & Miller, P. C. (2010). Cogni-
tive function during acute exercise: A test of
the transient hypofrontality theory. Journal of
Sport and Exercise Psychology, 32(3), 312–
323.
Dietrich, A. (2009). The transient hypofrontal-
ity theory and its implications for emotion
and cognition. In T. McMorris, P. D. Tompo-
rowski, & M. Audiffren (Eds.), Exercise and
cognitive function (pp. 69–90). Hoboken, NJ:
Wiley- Blackwell.
Ehrenberg, M. F., Gearing- Small, M., Hunter,
M. A., & Small, B. J. (2001). Childcare task
division and shared parenting attitudes in
dual- earner families with young children.
Family Relations, 50(2), 143–153.
Ehrlich, P. R., & Ehrlich, A. H. (2008). The
dominant animal: Human evolution and the
environment. Washington, DC: Island Press.
Eisenberger, N. I., Master, S. L., Inagaki, T. K.,
Taylor, S. E., Shirinyan, D., Lieberman, M.
D., et al. (2011). Attachment figures activate a
safety signal- related neural region and reduce
pain experience. Proceedings of the National
Academy of Sciences, 108, 11721–11726.
Eisenberger, N. I., Taylor, S. E., Gable, S. L.,
Hilmert, C. J., & Lieberman, M. D. (2007).
Neural pathways link social support to attenu-
ated neuroendocrine stress responses. Neuro-
Image, 35(4), 1601–1612.
Elgar, M. A. (1989). Predator vigilance and
group-size in mammals and birds—A critical
review of the empirical evidence. Biological
Reviews of the Cambridge Philosophical Soci-
ety, 64(1), 13–33.
Fitzsimons, G. M., & Finkel, E. J. (2011). Out-
sourcing self- regulation. Psychological Sci-
ence, 22(3), 369–375.
Fraley, R. C., & Shaver, P. R. (2000). Adult
romantic attachment: Theoretical develop-
ments, emerging controversies, and unan-
swered questions. Review of General Psychol-
ogy, 4, 132–154.
Franceschini, M. A., Thaker, S., Themelis, G.,
Krishnamoorthy, K. K., Bortfeld, H., Dia-
mond, S. G., et al. (2007). Assessment of
infant brain development with frequency-
domain near- infrared spectroscopy. Pediatric
Research, 61, 546–551.
Francis, S., Rolls, E. T., Bowtell, R., McGlone,
F., O’Doherty, J., Browning, A., et al. (1999).
The representation of pleasant touch in the
brain and its relationship with taste and olfac-
tory areas. NeuroReport, 10, 453–459.
Friston, K. (2010). The free- energy principle: A
unified brain theory? Nature Reviews Neuro-
science, 11(2), 127–138.
Fuster, J. M. (2002). Frontal lobe and cogni-
tive development. Journal of Neurocytology,
31(3), 373–385.
Gable, S. L., Gonzaga, G. C., & Strachman, A.
(2006). Will you be there for me when things
go right?: Supportive responses to positive
event disclosures. Journal of Personality and
Social Psychology, 91(5), 904 –917.
Gable, S. L., Reis, H. T., Impett, E. A., & Asher,
E. R. (2004). What do you do when things go
right?: The intrapersonal and interpersonal
benefits of sharing positive events. Journal
of Personality and Social Psychology, 87(2),
228–245.
Gazzola, V., Spezio, M. L., Etzel, J. A., Castelli,
F., Adolphs, R., & Keysers, C. (2012). Primary
Social Baseline Theory 233
somatosensory cortex discriminates affective
significance in social touch. Proceedings of the
National Academy of Sciences, 109, E1657–
E1666.
Gillath, O., Bunge, S. A., Shaver, P. R., Wen-
delken, C., & Mikulincer, M. (2005).
Attachment- style differences in the ability to
suppress negative thoughts: Exploring the neu-
ral correlates. NeuroImage, 28, 835–847.
Goldstein, R. Z., & Volkow, N. D. (2011). Dys-
function of the prefrontal cortex in addiction:
Neuroimaging findings and clinical implica-
tions. Nature Reviews Neuroscience, 12(11),
652– 669.
Halford, G. S., Wilson, W. H., & Phillips, S.
(1997). Abstraction: Nature, costs, and ben-
efits. International Journal of Educational
Research, 27(1), 21–35.
Halford, G. S., Wilson, W. H., & Phillips, S.
(1998). Processing capacity defined by rela-
tional complexity: Implications for compara-
tive, developmental, and cognitive psychol-
ogy. Behavioral and Brain Sciences, 21(6),
803–831.
Harlow, H. F. (1958). The nature of love. Ameri-
can Psychologist, 13, 673–685.
Hebb, D. O. (1949). The organization of behav-
ior. New York: Wiley.
Heinrichs, M., Baumgartner, T., Kirschbaum,
C., & Ehlert, U. (2003). Social support and
oxytocin interact to suppress cortisol and sub-
jective responses to psychosocial stress. Bio-
logical Psychiatry, 54(12), 1389–1398.
Higashi, M., & Yamamura, N. (1993). What
determines animal group size?: Insider–
outsider conflict and its resolution. American
Naturalist, 142(3), 553–563.
Hill, K. R., Walker, R. S., Bozicevic, M., Eder, J.,
Headland, T., Hewlett, B., et al. (2011). Co-
residence patterns in hunter– gatherer societies
show unique human social structure. Science,
331(6022), 1286–1289.
Hofer, M. A. (2006). Psychobiological roots of
early attachment. Current Directions in Psy-
chological Science, 15, 84–88.
Holt- Lunstad, J., Smith, T. B., & Layton, J. B.
(2010). Social relationships and mortality risk:
A meta- analytic review. PLoS Medicine, 7,
e1000316.
Hughes, A. E., Crowell, S. E., Uyeji, L., & Coan,
J. A. (2012). A developmental neuroscience of
borderline pathology: Emotion dysregulation
and social baseline theory. Journal of Abnor-
mal Child Psychology, 40, 21–33.
Kable, J. W., & Glimcher, P. W. (2007). The neu-
ral correlates of subjective value during inter-
temporal choice. Nature Neuroscience, 10,
1625–1633.
Karau, S. J., & Williams, K. D. (1993). Social
loafing: A meta- analytic review and theoreti-
cal integration. Journal of Personality and
Social Psychology, 65(4), 681–706.
Karremans, J. C., Heslenfeld, D. J., van Dillen, L.
F., & Van Lange, P. A. (2011). Secure attach-
ment partners attenuate neural responses
to social exclusion: An fMRI investigation.
International Journal of Psychophysiology,
81(1), 44–50.
Kim, S. H., & Hamann, S. (2007). Neural cor-
relates of positive and negative emotion regu-
lation. Journal of Cognitive Neuroscience,
19(5), 776–798.
Knill, D. C., & Pouget, A. (2004). The Bayesian
brain: The role of uncertainty in neural coding
and computation. Trends in Neurosciences,
27(12), 712–719.
Kraus, M. W., Huang, C., & Keltner, D. (2010).
Tactile communication, cooperation, and per-
formance: An ethological study of the NBA.
Emotion, 10, 745–749.
Krause, J., & Ruxton, G. (2002). Living in
groups. New York: Oxford University Press.
Krebs, J. R., & Davies, N. B. (1993). An intro-
duction to behavioural ecology (3rd ed.). Mal-
den, MA: Blackwell.
Kurzban, R. (2010). Does the brain consume
additional glucose during self- control tasks?
Evolutionary Psychology, 8(2), 244–259.
Langer, S. K. (1974). Mind: An essay on human
feeling (Vol. 2). Baltimore: Johns Hopkins
University Press.
Langston, C. A. (1994). Capitalizing on and cop-
ing with daily-life events: Expressive responses
to positive events. Journal of Personality and
Social Psychology, 67(6), 1112–1125.
Larose, S., Bernier, A., Soucy, N., & Duchesne,
S. (1999). Attachment style dimensions, net-
work orientation and the process of seeking
help from college teachers. Journal of Social
and Personal Relationships, 16, 225–247.
Lieberman, M. D. (2007). Social cognitive neu-
roscience: A review of core processes. Annual
Review of Psychology, 58, 259–289.
Lindquist, K. A., & Barrett, L. F. (2008). Con-
structing emotion. Psychological Science, 19,
898–903.
Lipina, S. J., & Colombo, J. A. (2009). Poverty
and brain development during childhood: An
234 SOCIAL ASPECTS
approach from cognitive psychology and neu-
roscience. Washington, DC: American Psy-
chological Association.
MacArthur, R. H., & Pianka, E. R. (1966). On
optimal use of a patchy environment. Ameri-
can Naturalist, 100, 603–609.
Main, M. (1996). Introduction to the special sec-
tion on attachment and psychopathology: 2.
Overview of the field of attachment. Journal
of Consulting and Clinical Psychology, 64,
237–243.
McComb, K., Moss, C., Durant, S. M., Baker, L.,
& Sayialel, S. (2001). Matriarchs as reposito-
ries of social knowledge in African elephants.
Science, 292, 491–494.
McGowan, P. O., Sasaki, A., D’Alessio, A. C.,
Dymov, S., Labonté, B., Szyf, M., et al. (2009).
Epigenetic regulation of the glucocorticoid
receptor in human brain associates with child-
hood abuse. Nature Neuroscience, 12, 342–
348.
McNally, L., Brown, S. P., & Jackson, A. L.
(2012). Cooperation and the evolution of intel-
ligence. Proceedings of the Royal Academy B:
Biological Sciences, 279(1740), 3027–3034.
Michelena, P., Pillot, M. H., Henrion, C., Toulet,
S., Boissy, A., & Bon, R. (2012). Group size
elicits specific physiological response in herbi-
vores. Biology Letters, 8(4), 537–539.
Mikulincer, M., & Florian, V. (1998). The rela-
tionship between adult attachment styles and
emotional and cognitive reactions to stressful
events. In J. A. Simpson & W. S. Rholes (Eds.),
Attachment theory and close relationships
(pp. 143–165). New York: Guilford Press.
Mikulincer, M., & Orbach, I. (1995). Attach-
ment styles and repressive defensiveness: The
accessibility and architecture of affective
memories. Journal of Personality and Social
Psychology, 68, 917–925.
Mikulincer, M., & Shaver, P. R. (2005). Attach-
ment theory and emotions in close relation-
ships: Exploring the attachment- related
dynamics of emotional reactions to relational
events. Personal Relationships, 12, 149–168.
Mikulincer, M., & Shaver, P. R. (2007). Attach-
ment in adulthood: Structure, dynamics, and
change. New York: Guilford Press.
Mikulincer, M., Shaver, P. R., & Pereg, D.
(2003). Attachment theory and affect regula-
tion: The dynamics, development, and cogni-
tive consequences of attachment- related strat-
egies. Motivation and Emotion, 27, 77–102.
Mobbs, D., Petrovic, P., Marchant, J. L., Has-
sabis, D., Weiskopf, N., Seymour, B., et al.
(2007). When fear is near: Threat imminence
elicits prefrontal– periaqueductal gray shifts in
humans. Science, 317(5841), 1079–1083.
Moll, H., & Tomasello, M. (2007). Cooperation
and human cognition: The Vygotskian intelli-
gence hypothesis. Philosophical Transactions
of the Royal Society B: Biological Sciences,
362(1480), 639–648.
Monson, C. M., Fredman, S. J., Macdonald, A.,
Pukay- Martin, N. D., Resick, P. A., & Schnurr,
P. P. (2012). Effect of cognitive- behavioral
couple therapy for PTSD: A randomized con-
trolled trial. Journal of the American Medical
Association, 308, 700–709.
Moore, L. J., Vine, S. J., Wilson, M. R., & Free-
man, P. (2012). The effect of challenge and
threat states on performance: An examination
of potential mechanisms. Psychophysiology,
49(10), 1417–1425.
Morrison, I., Löken, L. S., & Olausson, H.
(2010). The skin as a social organ. Experimen-
tal Brain Research, 204, 305–314.
Muraven, M., & Baumeister, R. F. (2000). Self-
regulation and depletion of limited resources:
Does self- control resemble a muscle? Psycho-
logical Bulletin, 126(2), 247–259.
Naaman, S., Radwan, K., & Johnson, S. (2009).
Coping with early breast cancer: Couple
adjustment processes and couple- based inter-
vention. Psychiatry: Interpersonal and Bio-
logical Processes, 72(4), 321–345.
Paxton, J. L., Barch, D. M., Racine, C. A., &
Braver, T. S. (2008). Cognitive control, goal
maintenance, and prefrontal function in
healthy aging. Cerebral Cortex, 18(5), 1010–
1028.
Posner, M. I., & Rothbart, M. K. (2007).
Research on attention networks as a model
for the integration of psychological science.
Annual Review of Psychology, 58, 1–23.
Prinstein, M. J., & La Greca, A. M. (2004).
Childhood peer rejection and aggression as
predictors of adolescent girls’ externalizing
and health risk behaviors: A 6-year longitudi-
nal Study. Journal of Consulting and Clinical
Psychology, 72, 103–112.
Pulliam, H. R., Pyke, G. H., & Caraco, T. (1982).
The scanning behavior of juncos: A game–
theoretical approach. Journal of Theoretical
Biology, 95(1), 89–103.
Reichardt, L. F. (2006). Neurotrophin- regulated
signalling pathways. Philosophical Transac-
tions of the Royal Society B: Biological Sci-
ences, 361, 1545–1564.
Reis, H. T., Smith, S. M., Carmichael, C. L.,
Social Baseline Theory 235
Caprariello, P. A., Tsai, F. F., Rodrigues, A.,
et al. (2010). Are you happy for me?: How
sharing positive events with others provides
personal and interpersonal benefits. Journal
of Personality and Social Psychology, 99(2),
311–329.
Rholes, W. S., Simpson, J. A., & Grich Stevens,
J. (1998). Attachment orientations, social sup-
port, and conflict resolution in close relation-
ships. In J. A. Simpson & W. S. Rholes (Eds.),
Attachment theory and close relationships
(pp. 166–188). New York: Guilford Press.
Richerson, P. J., Bettinger, R. L., & Boyd, R.
(2005). Evolution on a restless planet: Were
environmental variability and environmental
change major drivers of human evolution? In
F. M. Wuketits & C. Antweiler (Eds.), Hand-
book of evolution: The evolution of living sys-
tems (Vol. 2, pp. 223–242). New York: Wiley.
Roberts, G. (1996). Why individual vigilance
declines as group size increases. Animal
Behaviour, 51, 1077–1086.
Rogers, S. J., & DeBoer, D. D. (2001). Changes
in wives’ income: Effects on marital happi-
ness, psychological well-being, and the risk
of divorce. Journal of Marriage and Family,
63(2), 458–472.
Salinas, E. (2011). Prior and prejudice. Nature
Neuroscience, 14, 943–945.
Schilbach, L., Timmermans, B., Reddy, V., Cos-
tall, A., Bente, G., Schlicht, T., et al. (in press).
Toward a second- person neuroscience. Behav-
ioral and Brain Sciences.
Schmidhempel, P., Kacelnik, A., & Houston, A.
I. (1985). Honeybees maximize efficiency by
not filling their crop. Behavioral Ecology and
Sociobiology, 17(1), 61– 66.
Schnall, S., Harber, K. D., Stefanucci, J. K., &
Proffitt, D. R. (2008). Social support and
the perception of geographical slant. Journal
of Experimental Social Psychology, 44(5),
1246–1255.
Schwarz, N., & Clore, G. L. (1983). Mood,
misattribution, and judgments of well-being:
Informative and directive functions of affec-
tive states. Journal of Personality and Social
Psychology, 45, 513–523.
Shallcross, S. L., Howland, M., Bemis, J., Simp-
son, J. A., & Frazier, P. (2011). Not “capitaliz-
ing” on social capitalization interactions: The
role of attachment insecurity. Journal of Fam-
ily Psychology, 25(1), 77–85.
Sheppes, G., Catran, E., & Meiran, N. (2009).
Reappraisal (but not distraction) is going to
make you sweat: Physiological evidence for
self- control effort. International Journal of
Psychophysiology, 71(2), 91–96.
Sherwood, C. C., Stimpson, C. D., Raghanti, M.
A., Wildman, D. E., Uddin, M., Grossman, L.
I., et al. (2006). Evolution of increased glia–
neuron ratios in the human frontal cortex.
Proceedings of the National Academy of Sci-
ences, 103(37), 13606–13611.
Siegfried, W. R., & Underhill, L. G. (1975).
Flocking as an anti- predator strategy in doves.
Animal Behaviour, 23, 504–508.
Smith, E. A. (2003). Human cooperation: Per-
spectives from behavioral ecology. In P. Ham-
merstein (Ed.), Genetic and cultural evolution
of cooperation (pp. 401–427). Cambridge,
MA: MIT Press.
Sokoloff, L., Mangold, R., Wechsler, R. L., Ken-
nedy, C., & Kety, S. S. (1955). The effect of
mental arithmetic on cerebral circulation and
metabolism. Journal of Clinical Investigation,
34(7, Pt. 1), 1101–1108.
Stark, R., Schienle, A., Walter, B., Kirsch, P.,
Sammer, G., Ott, U., et al. (2003). Hemody-
namic responses to fear and disgust- inducing
pictures: An fMRI study. International Jour-
nal of Psychophysiology, 50, 225–234.
Stefanucci, J. K., Gagnon, K. T., & Lessard, D.
A. (2011). Follow your heart: Emotion adap-
tively influences perception. Social and Per-
sonality Psychology Compass, 5, 296–308.
Stefanucci, J. K., Proffitt, D. R., Banton, T., &
Epstein, W. (2005). Distances appear different
on hills. Attention, Perception, and Psycho-
physics, 67, 1052–1060.
Stefanucci, J. K., Proffitt, D. R., Clore, G. L.,
& Parekh, N. (2008). Skating down a steeper
slope: Fear influences the perception of geo-
graphical slant. Perception, 37, 321–323.
Tomaka, J., Blascovich, J., Kelsey, R. M., & Leit-
ten, C. L. (1993). Subjective, physiological,
and behavioral effects of threat and challenge
appraisal. Journal of Personality and Social
Psychology, 65, 248–260.
Tomasello, M., Carpenter, M., Call, J., Behne,
T., & Moll, H. (2005). Understanding and
sharing intentions: The origins of cultural
cognition. Behavioral and Brain Sciences, 28,
675– 690.
Townsend, A. L., & Franks, M. M. (1995). Bind-
ing ties: Closeness and conflict in adult chil-
dren’s caregiving relationships. Psychology
and Aging, 10(3), 343–351.
Uchino, B. N., Cacioppo, J. T., & Kiecolt- Glaser,
J. K. (1996). The relationship between social
support and physiological processes: A review
236 SOCIAL ASPECTS
with emphasis on underlying mechanisms and
implications for health. Psychological Bulle-
tin, 119, 488–531.
van IJzendoorn, M. H., Bakermans- Kranenburg,
M. J., & Ebstein, R. P. (2011). Methylation
matters in child development: Toward devel-
opmental behavioral epigenetics. Child Devel-
opment Perspectives, 5, 305–310.
Vohs, K. D., & Heatherton, T. F. (2000). Self-
regulatory failure: A resource- depletion
approach. Psychological Science, 11(3), 249–
254.
Weaver, I. C. G., Cervoni, N., Champagne, F. A.,
D’Alessio, A. C., Sharma, S., Seckl, J. R., et al.
(2004). Epigenetic programming by maternal
behavior. Nature Neuroscience, 7, 847–854.
Woolley, A. W., Chabris, C. F., Pentland, A.,
Hashmi, N., & Malone, T. W. (2010). Evi-
dence for a collective intelligence factor in the
performance of human groups. Science, 330,
686–688.
Younger, J., Aron, A., Parke, S., Chatterjee, N.,
& Mackey, S. (2010). Viewing pictures of a
romantic partner reduces experimental pain:
Involvement of neural reward systems. PloS
ONE, 5, e13309.

237
In the past 30 years, attachment theory
(Bowlby, 1973, 1980, 1969/1982) has
become one of the most influential concep-
tual frameworks for understanding emotion
regulation. Although Bowlby did not devote
much attention to abstract theorizing about
emotion itself (he included only a single brief
chapter about it in Volume 1 of his Attach-
ment and Loss trilogy), his writings were
motivated by clinical and ethological obser-
vations of humans and other primates who
were experiencing, expressing, and regu-
lating emotions such as affection, anxiety,
anger, grief, and despair. He was especially
interested in the anxiety- buffering func-
tion of close relationships and the capacity
for dysfunctional relationships to generate
negative emotions, and, in the extreme, to
precipitate debilitating forms of psychopa-
thology. Bowlby (1973, 1980) described and
conceptualized the relatively stable individ-
ual differences in emotion regulation that
emerge from prolonged reliance on particu-
lar attachment figures, people who provide
either adequate or inadequate protection,
safety, support, and guidance concerning
emotions and emotion regulation.
With the accumulation of empirical
knowledge about what Bowlby called the
“attachment behavioral system” and about
individual differences in attachment orienta-
tions in childhood and adulthood, Bowlby’s
ideas (as elaborated and tested initially by
Ainsworth, Blehar, Waters, & Wall, 1978)
have been expanded, tested, and organized
by social– personality psychologists into a
theoretical model of the attachment system
in adulthood (e.g., Mikulincer & Shaver,
2007a; Shaver & Mikulincer, 2002). The
model focuses on the emotion- regulatory
function of the attachment system and
explains many of the emotional corre-
lates and consequences of normative and
individual- differences aspects of attach-
ment system functioning. In this chapter, we
elaborate on these normative and individual-
differences aspects, and review studies that
have examined the links between attachment
system activation and emotion regulation,
and the ways in which individual differences
in attachment are reflected in patterns of
coping with attachment- irrelevant stressful
events and attachment- related stressors (e.g.,
a partner’s hurtful behavior, the breakup of
a relationship, the death of a relationship
partner).
Attachment Theory: Basic Concepts
Bowlby (1969/1982) claimed that human
beings are born with an innate psychobio-
logical system (the attachment behavioral
system) that motivates them to seek proxim-
CHAPTER 15
Adult Attachment and Emotion Regulation
Phillip R. Shaver
Mario Mikulincer
238 SOCIAL ASPECTS
ity to significant others (attachment figures)
in times of need. This system accomplishes
basic regulatory functions (protection from
threats and alleviation of distress) in human
beings of all ages, but it is most directly
observable during infancy and early child-
hood (Bowlby, 1988). According to Bowlby
(1969/1982), attachment figures can pro-
vide a physical and emotional safe haven
that facilitates the down- regulation of nega-
tive affective states, and a secure base that
encourages positive affective states condu-
cive to exploration and learning, thus sup-
porting the development of knowledge and
skills.
Bowlby (1973) also described important
individual differences in attachment system
functioning shaped by the responsiveness
and supportiveness of attachment figures.
Interactions with attachment figures who
are responsive in times of need facilitate
the optimal functioning of the attachment
system and promote a sense of attachment
security. This deep and pervasive sense of
security is based on implicit beliefs that
the world is generally safe, that attachment
figures are helpful when called upon, and
that it is possible to explore the environ-
ment curiously and to engage enjoyably with
other people without undue fear. This sense
of security is rooted in positive mental repre-
sentations of self and others, which Bowlby
called internal working models.
Unfortunately, when attachment figures
are not reliably available and supportive,
and when they fail to provide adequate
relief from distress, children who are depen-
dent on them may form negative work-
ing models of self and others and develop
defensive secondary attachment strategies
that involve hyperactivation or deactiva-
tion of the attachment system (e.g., Cassidy
& Kobak, 1988). Hyperactivation (which
Bowlby, 1982, called “protest”) is charac-
terized by intense efforts to attain proximity
to attachment figures and insistent attempts
to induce a relationship partner, viewed as
insufficiently available or responsive, to
provide more satisfying and reassuring care
and support. Hyperactivating strategies
include clinging, controlling, and coercive
behaviors; cognitive and behavioral efforts
to establish physical contact and a sense
of “oneness”; and up- regulation of nega-
tive affective states (Shaver & Mikulincer,
2002). In contrast, deactivation involves the
inhibition of proximity- seeking inclinations
and actions, suppression or discounting of
threats that might activate the attachment
system, down- regulation of both negative
and positive affective states, and determi-
nation to handle stressors alone (a defen-
sive stance that Bowlby [1969/1982] called
“compulsive self- reliance”). People who rely
on these strategies tend to maximize auton-
omy and distance from relationship partners
and experience discomfort with closeness
and intimacy.
When testing this theory in studies of
adults, most researchers have focused on the
organized pattern of relational expectations,
emotions, and behavior that results from a
history of interactions with attachment
figures— often called “attachment style” by
social psychologists (e.g., Fraley & Shaver,
2000). Psychometric research (e.g., Bren-
nan, Clark, & Shaver, 1998) has shown that
attachment styles can be measured in terms
of two independent dimensions: attachment
anxiety and attachment- related avoidance.
A person’s position on the attachment anxi-
ety dimension indicates the degree to which
he or she worries that a partner will not be
responsive in times of need. A person’s posi-
tion on the avoidance dimension indicates
the extent to which he or she distrusts rela-
tionship partners’ goodwill and their will-
ingness and capacity to help without causing
further distress. People who score low on
both dimensions are said to be secure with
respect to attachment. A person’s location
in the continuous two- dimensional anxiety-
by- avoidance space can be measured with
reliable and valid self- report scales (e.g.,
Brennan et al., 1998) and is associated in
theoretically predictable ways with many
aspects of psychological adjustment (see
Mikulincer & Shaver, 2007a, for a review).
Attachment styles begin to develop in
interactions with primary caregivers during
early childhood, as a large body of research
demonstrates (see Cassidy & Shaver, 2008,
for an anthology of reviews), but Bowlby
(1988) claimed that relational experiences
throughout life can move a person from
one region to another in the anxiety- by-
avoidance conceptual space. Moreover,
although attachment style is often mea-
sured as a single global orientation toward
close relationships, a person’s orientation to

Attachment and Emotion Regulation 239
attachment is rooted in a complex cognitive
and affective associative neural network that
includes both secure and insecure episodic
and semantic memories and mental repre-
sentations (Mikulincer & Shaver, 2007a).
Indeed, a growing body of research shows
that attachment style can change, subtly
or dramatically, depending on current con-
text and recent relational experiences (see
Mikulincer & Shaver, 2007b, for a review).
For example, we have found that priming
thoughts of an available and supportive
attachment figure leads people who score
relatively high on scales tapping attach-
ment insecurities momentarily to behave
like more secure people (e.g., Mikulincer &
Shaver, 2001).
In the next two sections, we present both
theoretical ideas and empirical evidence
concerning the link between attachment
and emotion regulation. In the first section,
we focus on normative aspects of this link
and the emotion regulation functions of
the attachment behavioral system. That is,
we discuss and review empirical evidence
concerning the normative activation of the
attachment system in response to threats
and the down- regulation of distress caused
by optimal functioning of the system. In the
second section, we focus on individual dif-
ferences and review empirical evidence con-
cerning the up- regulation of negative affect
caused by anxious attachment, and the
ways in which avoidant attachment leads to
down- regulation of both negative and posi-
tive affective states.
Normative Aspects of the Link
between Attachment
and Emotion Regulation
According to attachment theory (Bowlby,
1969/1982), the attachment system evolved
because it increased the likelihood that off-
spring would survive until they were old
enough to reproduce and also taught chil-
dren how to regulate emotions effectively.
This system is automatically activated by
external threats or internal sources of dis-
tress and, when it functions appropriately,
leads to emotional security. Its optimal
functioning is associated with a relational
if–then script, which Waters and Waters
(2006) called a secure- base script, para-
phrased by us as follows: “If I encounter
an obstacle and/or become distressed, then
I can approach a significant other for help.
He or she is likely to be available and sup-
portive. I will experience relief and comfort
as a result of proximity to this person, and
I can then return to other activities.” Once
activated, this script can, by itself, mitigate
distress, promote optimism and hope, and
help a person cope effectively with life’s
inevitable difficulties (Mikulincer, Shaver,
Sapir-Lavid, & Avihou-Kanza, 2009).
Adult attachment researchers have
designed experimental procedures to exam-
ine these normative regulatory properties
of the attachment system. For example, in
a series of experiments, Mikulincer, Gil-
lath, and Shaver (2002) showed that men-
tal representations of attachment figures
(e.g., names of security- enhancing attach-
ment figures) are automatically activated in
a person’s mind when he or she is exposed
to threatening stimuli, even if that exposure
is unconscious (i.e., primed subliminally).
Specifically, when a threat- related word
(e.g., death) was presented subliminally on
a computer screen, participants were faster
to detect the name of one of their attachment
figures when it appeared on the screen and
slower to name the color in which such names
were printed on the screen— an indication
that the names had been automatically and
unconsciously activated in memory (Miku-
lincer et al., 2002). In other words, threats,
even when arising unconsciously, can auto-
matically activate mental representations
of security providers. Similar findings have
been obtained in experiments examining
automatic activation of representations of
symbolic sources of security, such as a per-
son’s pet or God, following a threat (Gran-
qvist, Mikulincer, Gurwitz, & Shaver, 2012;
Zilcha-Mano, Mikulincer, & Shaver, 2011).
In another set of studies of what we call
security priming, Mikulincer, Hirschberger,
Nachmias, and Gillath (2001) showed that
activation of representations of security-
enhancing attachment figures can automati-
cally infuse a previously neutral stimulus
with positive affect. For example, subliminal
presentation of the names of people who were
nominated by study participants as attach-
ment figures, compared with others who
were not nominated as attachment figures,
led to greater liking of previously unfamil-

240 SOCIAL ASPECTS
iar stimuli. Moreover, subliminal exposure
to names of attachment figures eliminated
the detrimental effects that threats other-
wise had on liking for previously neutral
stimuli. These effects of security priming on
positive affect have been replicated in sub-
sequent studies (see Mikulincer & Shaver,
2007b, for a review). In addition, Eisen-
berger and colleagues (2011; Master et al.,
2009) found that viewing a photograph of
one’s romantic partner (vs. a stranger or an
object) reduced one’s subjective experience
of pain while receiving thermal stimulation
at levels slightly higher than the pain thresh-
old. Moreover, Selcuk, Zayas, Günaydin,
Hazan, and Kross (2012) found that both
explicit and implicit priming of attachment
figure representations speeded up emotional
recovery and reduced negative thoughts
after recalling an upsetting experience.
Considering all such findings, we con-
clude that people automatically search
for internal representations of security-
enhancing attachment figures during times
of stress, and mental activation of these
representations produces positive emotions
(e.g., relief, satisfaction, gratitude, love) that
facilitate effective coping and restore emo-
tional equanimity. That is, actual or sym-
bolic interactions with available and sup-
portive attachment figures, and the resulting
sense of safety, can be viewed as psycho-
logical resources for dealing with problems
and adversities, and sustaining well-being
and mental health (Mikulincer & Shaver,
2007a).
Attachment-Related Individual
Differences in Emotion Regulation
Theoretical Account
According to Bowlby (1973), disruptions in
the sense of attachment security are viewed
as risk factors for emotional problems and
psychopathology. Although secondary
attachment strategies (anxious hyperacti-
vation and avoidant deactivation) are ini-
tially adaptive, in the sense that they adjust
a child’s behavior to the requirements of
an inconsistently available or consistently
distant or unavailable attachment figure,
they are maladaptive when used in later
relationships in which support seeking and
relational interdependence could be reward-
ing and help a person maintain a sense of
well-being even in stressful conditions or
difficult periods of life (Shaver & Miku-
lincer, 2002). Moreover, these attachment
strategies encourage repeated activation
and suppression of negative emotions and
continued reliance on negative or distorted
mental representations of self and others,
which can erode and destroy mental health
(Mikulincer & Shaver, 2007a).
The early attachment experiences of inse-
cure people (whether anxious, avoidant,
or both) often involve unstable and inad-
equate distress regulation (Bowlby, 1973),
which interferes with the development of
inner resources needed for coping success-
fully with stressors. This impairment is
particularly likely during prolonged, highly
demanding stressful experiences that require
active support seeking and actual confronta-
tion with a problem (Berant, Mikulincer, &
Shaver, 2008). In such cases, anxious hyper-
activating strategies can become extreme,
damaging not only a person’s own mental
health but that of key relationship partners.
Moreover, avoidant defenses can collapse,
resulting in a marked decline in psychologi-
cal well-being.
When regulating their emotions, avoid-
ant people attempt to block or inhibit any
emotional state that is incongruent with
the goal of keeping their attachment system
deactivated (Mikulincer & Shaver, 2007a).
These inhibitory efforts are directed mainly
at fear, anxiety, anger, sadness, shame, guilt,
and distress, because these emotions are
associated with threats and feelings of vul-
nerability. In addition, anger often implies
emotional involvement or investment in a
relationship, and such involvement is incon-
gruent with avoidant people’s preference
for independence and self- reliance (Cassidy,
1994). Avoidant individuals also attempt
to block or inhibit emotional reactions to
potential or actual threats to attachment
figure availability (rejection, betrayal, sepa-
ration, loss), because such threats are direct
triggers of attachment system activation.
Like secure people, avoidant ones attempt to
down- regulate threat- related emotions. But
whereas secure people’s regulatory attempts
usually promote communication, compro-
mise, and relationship maintenance, avoid-
ant people’s efforts are aimed mainly at
keeping the attachment system deactivated,
Attachment and Emotion Regulation 241
regardless of the deleterious effects this can
have on a relationship.
Deactivating strategies cause people to
avoid noticing their own emotional reac-
tions. Avoidant individuals often deny or
suppress emotion- related thoughts and
memories, divert attention from emotion-
related material, suppress emotion- related
action tendencies, or inhibit or mask ver-
bal and nonverbal expressions of emotion
(Mikulincer & Shaver, 2007a). By averting
the conscious experience and expression of
unpleasant emotions, avoidant individuals
make it less likely that emotional experi-
ences will be integrated into their cognitive
structures and that such feelings and mental
structures will be used effectively in informa-
tion processing and social behavior. Bowlby
(1980) described this strategy as “defensive
exclusion” and the creation of “segregated
mental systems.”
Unlike secure and avoidant people, who
tend to view negative emotions as goal-
incongruent states that should either be
managed effectively or suppressed, anx-
iously attached individuals tend to perceive
these emotions as congruent with attach-
ment goals, and they may seek to sustain and
even exaggerate them. Attachment- anxious
people are guided by an unfulfilled wish
to cause attachment figures to pay more
attention and provide more reliable protec-
tion and support (Cassidy & Kobak, 1988;
Mikulincer & Shaver, 2007a). Therefore,
they tend to exaggerate the presence and
seriousness of threats and to overemphasize
their sense of helplessness and vulnerability,
because signs of weakness and neediness can
sometimes elicit attachment figures’ atten-
tion and care (Cassidy & Berlin, 1994).
How is anxious hyperactivation sustained?
One method is to exaggerate the appraisal
process, perceptually heightening the threat-
ening aspects of even fairly benign events,
to hold pessimistic beliefs about one’s ability
to manage distress, and to attribute threat-
related events to uncontrollable causes or
global personal inadequacies (Mikulincer &
Shaver, 2007a). Another method is to attend
to internal indicators of distress (Cassidy
& Kobak, 1988). This includes hypervigi-
lant attention to the physiological aspects
of emotional states, heightened recall of
threat- related experiences, and rumination
on real and potential threats (Mikulincer
& Shaver, 2007a). Another hyperactivating
strategy is to intensify negative emotions by
favoring an approach, counterphobic ori-
entation toward threatening situations or
making self- defeating decisions and taking
ineffective actions that are likely to end in
failure. All of these strategies create a self-
amplifying cycle of distress even after a
threat objectively recedes.
Empirical Evidence
There is now a large body of evidence sup-
porting the hypothesized links between
attachment- related avoidance and emotional
inhibition, and between attachment anxiety
and distress intensification. In particular,
theory- congruent findings have been found
in studies of responses to both attachment-
irrelevant stressful events and attachment-
related stressors (e.g., a partner’s hurtful
behavior, a relationship breakup, or the
death of a relationship partner).
Attachment Orientations and Responses
to Stressful Events
Several studies have examined attachment
style differences in the ways people appraise,
cope with, and emotionally and physiologi-
cally react to attachment- irrelevant stress-
ful events (i.e., events that have no direct
implications for one’s close relationships or
attachment figures). These attachment style
differences have been examined in response
to a wide variety of stressful experiences,
such as combat training (e.g., Mikulincer
& Florian, 1995), transition to college (e.g.,
Lopez & Gormley, 2002), abortion (e.g.,
Cozzarelli, Sumer, & Major, 1998), infertil-
ity (e.g., Amir, Horesh, & Lin-Stein, 1999),
pregnancy (e.g., Mikulincer & Florian,
1999), workload (e.g., Raskin, Kummel, &
Bannister, 1998), financial problems (e.g.,
Bartley, Head, & Stansfeld, 2007), and job
loss (e.g., Hobdy et al., 2007). They have
also been examined in studies that did not
focus on a specific stressful event but asked
participants to report on any major stress-
ors recently experienced (e.g., Holmberg,
Lomore, Takacs, & Price, 2011). In addi-
tion, the link between attachment orienta-
tions and stress- related reactions has been
examined in response to several laboratory-
induced stressors, such as aversive noise (e.g.,
242 SOCIAL ASPECTS
Quirin, Pruessner, & Kuhl, 2008), the Trier
Social Stress Test (e.g., Smeets, 2010), and
difficult cognitive tasks (e.g., Kidd, Hamer,
& Steptoe, 2011).
With regard to the cognitive appraisal
of stressful events, attachment anxiety has
been associated with distress- intensifying
appraisals— in which stressful events are
appraised as threats rather than challenges
and one’s coping resources are viewed
as deficient (e.g., Cozzarelli et al., 1998;
Mikulincer & Florian, 1998). For avoidant
attachment, however, the findings are more
complex. Whereas avoidant attachment is
associated with appraising stressful events
in more threatening terms, it is not associ-
ated with appraisals of coping resources
(e.g., Williams & Riskind, 2004). This dis-
sociation might reflect avoidant individuals’
reluctance to recognize that they are vulner-
able and weak, and therefore need to rely
on others for assistance and support (Miku-
lincer & Shaver, 2007a).
There is also theoretically consistent evi-
dence concerning attachment- related dif-
ferences in self- reports of ways of coping
with stressful events. Whereas attachment
anxiety is associated with emotion- focused
coping, such as wishful thinking, self-blame,
and distress- related rumination, avoidant
attachment is associated with reliance on
distancing strategies, such as stress denial,
diversion of attention, and behavioral or
cognitive disengagement (e.g., Holmberg et
al., 2011; Zhang & Labouvie- Vief, 2004).
Several adult attachment studies have
involved asking participants to report on
their psychological distress or well-being
during and following stressful events.
Overall, findings indicate that attach-
ment insecurities— anxiety, avoidance, or
both—are associated with self- reports of
heightened distress and deteriorated well-
being (see Mikulincer & Shaver, 2007a, for
a review). Moreover, some of the studies
compared the emotional reactions of secure
and insecure people undergoing stressful
experiences with those of controls, reveal-
ing that stressful events arouse distress
mainly among insecurely attached people.
For secure people, there is often no notable
difference in emotion between neutral and
stressful situations (e.g., Amir et al., 1999).
That is, secure people seem to be relatively
calm under stressful conditions.
Recent studies have found that attachment-
related differences in coping are reflected in
physiological responses to stressful events.
For example, two experiments (Diamond,
Hicks, & Otter- Henderson, 2006; Maunder,
Lancee, Nolan, Hunter, & Tannenbaum,
2006) exposed participants to various labo-
ratory stressors (e.g., recalling a stressful sit-
uation, performing demanding math tasks)
and found that self- reports of avoidant
attachment were associated with heightened
physiological reactivity: decreased heart rate
variability, increased skin conductance, and
heightened blood pressure. With regard to
attachment anxiety, Maunder et al. found
that attachment- anxious people reported
higher levels of distress but did not exhibit
heightened physiological reactivity, perhaps
suggesting, as mentioned earlier, that anx-
ious people exaggerate their own distress.
In addition, four studies have assessed
attachment style differences in the activ-
ity of the hypothalamic– pituitary– adrenal
(HPA) axis, which can be measured by
salivary cortisol levels during and following
a laboratory- induced stressor. One study
found that avoidant attachment was associ-
ated with increased levels of salivary corti-
sol (Kidd et al., 2011); another study (Qui-
rin et al., 2008) found a positive association
between attachment anxiety and heightened
cortisol reactivity; and two studies found no
significant association between attachment
orientations and cortisol levels (Ditzen et
al., 2008; Smeets, 2010). These inconsisten-
cies might have been due, in part, to varia-
tions in the laboratory- induced stressors
(e.g., aversive noise, the Trier Social Stress
Test) and participants’ age (young adults,
midlife adults). More systematic research is
needed to determine the presence or absence
of links between attachment insecurities
and HPA dysregulation following stressful
events.
There is also preliminary evidence that
an association between attachment inse-
curities and psychological distress follow-
ing stressful events can be observed in
brain responses. Using event- related func-
tional magnetic resonance imaging (fMRI),
Lemche et al. (2006) found that self- reports
of attachment anxiety or avoidance were
associated with heightened activation in
bilateral amygdalae to a stressful stimulus.
That is, less secure people tended to react to
Attachment and Emotion Regulation 243
stress with increased amygdala activity— a
neural indication of distress- related arousal.
Considering longer- term neural effects
of attachment insecurities, Quirin, Gil-
lath, Pruessner, and Eggert (2010) found
that self- reports of attachment anxiety and
avoidance were associated with reduced
hippocampal cell density, which was asso-
ciated with poorer emotion regulation. In
particular, attachment- related avoidance
was associated with bilateral hippocampal
cell reduction, and attachment anxiety was
significantly related to reduced cell concen-
tration in the left hippocampus. These find-
ings are compatible with a neurotoxic model
of stress- induced cell reduction in the hip-
pocampus, contributing to poorer emotion
regulation abilities in individuals with inse-
cure attachment orientations.
Attachment Orientations and Responses
to a Partner’s Hurtful Behaviors
Explicit or implicit signs of a partner’s disap-
proval, criticism, rejection, or betrayal can
evoke hurt feelings, arouse hostility toward
a partner, and even destroy a relationship.
However, the ways people react to these
relational stressors seem to depend on their
attachment orientation. There is accumu-
lating evidence that insecure people tend to
react more intensely than secure people—
cognitively, emotionally, and behavior-
ally— to actual or imagined partner disap-
proval, criticism, rejection, or betrayal (e.g.,
Besser & Priel, 2009, 2010; Carnelley, Israel,
& Brennan, 2007). For example, Dewitte,
De Houwer, Goubert, and Buysse (2010)
found that whereas attachment anxiety was
related to greater physiological arousal and
stronger negative affect following relational
stress, attachment- related avoidance pre-
dicted withdrawal responses.
Using Rusbult, Verette, Whitney, Slovik,
and Lipkus’s (1991) typology of accom-
modation responses to a partner’s negative
behavior, several studies (e.g., Gaines et al.,
1997; Scharfe & Bartholomew, 1995) found
that, compared with insecure people, secure
ones were more likely to rely on “voice”
(an active, relational approach to solving a
problem) and “loyalty” (understanding the
temporary nature of a partner’s behavior
and working with him or her to improve)—
the two more accommodative responses to a
partner’s transgressions. Moreover, whereas
secure individuals tended to respond to rejec-
tion threats by adopting approach- oriented
goals that improved relationship quality,
less secure people tended to respond to such
threats by decreasing approach motivation
and emphasizing avoidance goals (Park,
2010). In a test of responses to a hurtful
interaction, Perunovic and Holmes (2008)
found that more secure individuals reported
inhibition of negative impulses and the
adoption of relationship- enhancing behav-
ior even under time pressure, implying that
for them accommodation was automatic.
Dewitte and De Houwer (2008; Dewitte,
Koster, De Houwer, & Buysse, 2007) used
a classic dot-probe task and found that
both attachment anxiety and avoidance
were associated with attentional avoid-
ance of signs of a partner’s hurtful behavior
(attachment- threat words and angry faces).
In addition, using the same dot-probe task,
Dewitte, De Houwer, Koster, and Buysse
(2007) found that attachment anxiety was
associated with hypervigilance regarding
attachment figures’ names in both threaten-
ing and positive relational situations. This
finding is conceptually similar to Miku-
lincer et al.’s (2002) finding of faster cogni-
tive processing of attachment figure’s names
regardless of context.
Attachment insecurities also tend to
inhibit forgiveness— one of the most effec-
tive accommodation responses for improv-
ing relationship quality and reestablishing
relational harmony following a partner’s
negative behavior (e.g., Burnette, Taylor,
Worthington, & Forsyth, 2007; Yárnoz-
Yaben, 2009). Mikulincer, Shaver, and
Slav (2006) found that less secure people
were more inclined to report intense feel-
ings of vulnerability or humiliation and a
strong sense of relationship deterioration
when “forgiving” a partner. In other words,
attachment insecurities were associated
with a more negative conception and experi-
ence of forgiveness. Burnette, Davis, Green,
Worthington, and Bradfield (2009) provided
evidence concerning potential mediators
of such effects: Whereas the link between
attachment anxiety and forgiveness was
mediated by excessive rumination on rela-
tional injuries, the link between attachment-
related avoidance and forgiveness was medi-
ated by lack of empathy.
244 SOCIAL ASPECTS
Beyond these associations between dispo-
sitional measures of attachment insecurities
and deficient forms of forgiveness, there is
increasing evidence that state-like senses of
security or insecurity can alter the tendency
to forgive a hurtful partner. For example,
Finkel, Burnette, and Scissors (2007) exper-
imentally enhanced attachment anxiety or
measured weekly fluctuations in attachment
anxiety for 6 months and found that height-
ened attachment anxiety reduced forgiveness
of a partner’s offenses. In addition, Han-
non, Rusbult, Finkel, and Kumashiro (2010)
found that, following an act of betrayal, the
offending partner’s provision of a sense of
security to the injured partner (by genu-
inely expressing interest in being responsive
to the victim’s needs) promoted forgiveness
and restoration of relational harmony. Kar-
remans and Aarts (2007) found that sub-
liminal security priming (with the name
of a loving other), compared with neutral
priming, elicited more automatic forgiveness
responses to interpersonal transgressions.
Following this line of reasoning, Cassidy,
Shaver, Mikulincer, and Lavy (2009) showed
that experimentally heightening the sense of
attachment security can reduce detrimental
effects of dispositional attachment insecu-
rities on cognitive and emotional reactions
to hurtful experiences. Participants wrote a
description of an incident in which a close
relationship partner criticized, disapproved,
rejected, or ostracized them. They then com-
pleted a computerized task in which they
were repeatedly exposed subliminally (for
22 milliseconds on each occasion) to either
a security- enhancing prime word (love,
secure, affection) or a neutral prime word
(lamp, staple, building). Immediately after
the priming trials, participants were asked
to think again about the hurtful event they
had described and to rate how they would
react to such an event if it happened in the
future— for example, how rejected they
would feel, and how they would feel about
themselves.
In the neutral priming condition, the find-
ings confirmed the usual deactivating and
hyperactivating strategies of avoidant and
anxious people. Whereas avoidance scores
were associated with more defensive/hostile
reactions, attachment anxiety was associ-
ated with more intense feelings of rejec-
tion, more crying, and more negative emo-
tions. These typical correlational findings
were dramatically reduced in size (most
approached zero) in the security- priming
condition. In other words, security priming
reduced the tendency of avoidant people to
rely on cool hostility or denial, and the ten-
dency of anxious people to react histrioni-
cally to a partner’s hurtful behaviors. Over-
all, research indicates that (1) people who
are dispositionally secure can deal construc-
tively with hurtful relational events, and (2)
contextual activation of the sense of security
can soften the typical maladaptive responses
of dispositionally insecure people.
Responses to Separations
and Relationship Breakups
Several studies have shown that attach-
ment orientations predict the intensity and
duration of distress following a romantic
relationship breakup (e.g., Sbarra, 2006;
Sbarra & Emery, 2005), divorce (Birn-
baum, Orr, Mikulincer, & Florian, 1997),
wartime separation from a marital partner
(e.g., Medway, Davis, Cafferty, Chappell, &
O’Hearn, 1995), or temporary separations
from a romantic partner (e.g., Diamond,
Hicks, & Otter- Henderson, 2008). In these
studies, distress intensification was a com-
mon response of anxiously attached people,
whereas attachment security was associated
with faster emotional recovery and adjust-
ment. For example, Sbarra (2006) collected
daily emotion data for 4 weeks from a sam-
ple of young adults who had recently experi-
enced a romantic relationship breakup and
found that attachment anxiety was associ-
ated with slower recovery from sadness and
anger. In a 21-day diary study, Diamond et
al. (2008) found that attachment anxiety was
associated with sleeping problems, physical
symptoms, and higher levels of salivary cor-
tisol during and following days of physical
separation from a romantic partner brought
about by work- related travel. Using fMRI
to observe brain responses to the recall of a
painful separation, Gillath, Bunge, Shaver,
Wendelken, and Mikulincer (2005) found
that attachment anxiety was associated with
higher activation of the left anterior tempo-
ral pole and left hippocampus (areas associ-
ated with recall of sad thoughts) and lower
activation of the orbitofrontal cortex (an
area associated with emotional control).
Attachment and Emotion Regulation 245
For avoidant individuals, the findings
depended on the nature of the separation
(Mikulincer & Shaver, 2007a). Avoidance
was associated with higher levels of distress
following divorce and wartime separations
but lower levels of distress and greater relief
following temporary separations from, or
permanent breakups with, dating part-
ners. It seems that avoidant people who
can handle the distress of brief separations
or the dissolution of casual bonds are less
successful in dealing with major separations
requiring reorganization of relational rou-
tines, goals, and plans. This fits with other
evidence, including that from experiments
(e.g., Mikulincer, Dolev, & Shaver, 2004),
indicating that avoidant defenses collapse
under pressure.
There is also evidence that people with
different attachment orientations differ in
the ways they cope with separation. For
example, following divorce (Birnbaum et
al., 1997) and temporary separations from a
dating partner (Feeney, 1998), attachment-
anxious individuals were more likely to rely
on emotion- focused coping strategies, and
avoidant individuals, on distancing strate-
gies. Similar coping strategies were noted by
Davis, Shaver, and Vernon (2003) in a sur-
vey of more than 5,000 Internet respondents
who described romantic relationship break-
ups. Avoidant respondents were less likely
to seek support and more likely to cope
with the breakup alone, while avoiding new
romantic involvements. Anxious respon-
dents reacted with angry protests, height-
ened sexual attraction to the former partner,
intense preoccupation with the lost partner,
a damaged sense of identity, and interference
with school and work activities. In addition,
as a means of coping with separation, both
anxious and avoidant individuals used alco-
hol and drugs, which is not generally an
effective coping strategy.
Studies that induced thoughts about hypo-
thetical or actual separations also provide
important information about attachment-
related differences in regulating the distress
resulting from these thoughts. For example,
using a thought suppression task, Fraley
and Shaver (1997) showed that avoidant
people were highly effective in suppressing
separation- related thoughts, as indicated
by less frequent thoughts of loss following
the suppression task and lower skin con-
ductance during the task. However, Miku-
lincer et al. (2004) found that avoidant peo-
ple’s ability to suppress separation- related
thoughts was disrupted when a cognitive
load— remembering a 7-digit number— was
added to the experimental task. Under a high
cognitive load, avoidant individuals sud-
denly evinced high availability of thoughts
of separation and negative self- traits. That
is, the suppressed material resurfaced in
experience and behavior when a high cogni-
tive demand was imposed. We suspect that
a similar resurfacing occurs when a high
emotional demand is imposed, as in the
studies reviewed earlier that dealt with life-
threatening traumatic events (e.g., Berant et
al., 2008). Recently, Ehrenthal, Friederich,
and Schauenburg (2011) found that avoidant
attachment was associated with impaired
blood pressure recovery following recall of a
painful separation, further emphasizing the
fragility of avoidant defenses.
Reactions to the Death of a Close
Relationship Partner
Few studies have directly examined associa-
tions between attachment orientations and
grief reactions following the death of a close
relationship partner. However, these stud-
ies have consistently found that attachment
anxiety is associated with complicated grief
reactions (e.g., Fraley & Bonanno, 2004;
Jerga, Shaver, & Wilkinson, 2011). For
example, Field and Sundin (2001) found that
anxious attachment, assessed 10 months
after the death of a spouse, predicted higher
levels of psychological distress 14, 25, and
60 months after the loss, and Fraley and
Bonanno (2004) found that attachment
anxiety assessed 4 months after the loss of
a spouse predicted higher levels of anxiety,
depression, grief, trauma- related symptoms,
and alcohol consumption 18 months follow-
ing the loss.
With regard to avoidant attachment,
researchers have generally found no signifi-
cant association between this attachment
pattern and depression, grief, or distress
following the death of a relationship part-
ner (e.g., Field & Sundin, 2001; Fraley &
Bonanno, 2004). However, Wayment and
Vierthaler (2002) found that avoidance was
associated with increased somatic symp-
tom levels following the death of a spouse.

246 SOCIAL ASPECTS
Recently, Jerga et al. (2011) found that
avoidant attachment was positively associ-
ated with prolonged grief symptoms but not
with typical or normative grief symptoms.
That is, people who are generally avoidant
in close relationships experience long-term
difficulties adjusting to the death of a rela-
tionship partner, even though they do not
necessarily experience more intense typical
grief symptoms.
Jerga et al. (2011) also found that
relationship- specific avoidance was nega-
tively associated with both typical and
prolonged grief symptoms. However, other
findings indicated that this association dis-
appeared when measures of relationship
closeness and strength were statistically con-
trolled, suggesting that avoidant individuals
may maintain relatively weak and emotion-
ally distant relationships with the deceased,
which in turn leaves them with less to grieve
about. In other words, it may not be avoid-
ant attachment per se that protects avoidant
individuals from grief symptoms; it may be
the weakness of the emotional bonds they
have to contend with when a relationship
partner dies.
Conclusions
In summarizing recent research on adult
attachment patterns and its implications
for emotion regulation, we have shown
that attachment security is associated with
appraisals and regulation efforts that are
compatible with a balanced, open mind,
generally low levels of stress and distress,
and constructive approaches to relationship
maintenance. The two major dimensions
of insecure attachment are associated with
characteristic strategies for dealing with
painful experiences in previous attachment
relationships.
Avoidance and attachment system deac-
tivation are reactions to important relation-
ships in which attachment figures, often
beginning with one or both parents, reacted
negatively to expressions of need, vulner-
ability, and negative emotions. To cope
with that powerfully painful relationship
influence, avoidant people have learned to
downplay threats (i.e., trying not to appraise
events as threatening), suppress or deny feel-
ings of vulnerability and negative emotions,
and view themselves as superior, autono-
mous, and properly unemotional. This does
not keep them from reacting to relationship
partners with frustration, hostility, and
denigration or from boosting their own self-
esteem in the face of relationship losses by
focusing disproportionately on their own
strengths and other people’s weaknesses.
Attachment anxiety and attachment
system hyperactivation are reactions to
important relationships in which attach-
ment figures, often beginning with one or
both parents, reacted inconsistently to a
person’s expressions of need, vulnerability,
and negative emotions, sometimes providing
support but at other times being frustrating
or inattentive (often because of their own
self- focused anxiety). This caregiver regi-
men causes a person to believe that constant
vigilance, worry, and expressions of need,
vulnerability, and retaliatory anger pay off,
because they sometimes do capture a rela-
tionship partner’s attention. Unfortunately,
they can also alienate a person from his or
her initially favorable and loving relation-
ship partners and evoke exactly what the
anxious person fears: rejection or abandon-
ment. Thus, what began as a response to a
partial reinforcement schedule of attention
and support, and what became a pattern of
noisy negativity, seems to the anxious per-
son to lead to outcomes that confirm his or
her expectations and worst fears.
These different patterns of emotional
reaction and defense have been documented
in a remarkable variety of studies using
experimental, interview, and observational
research methods. They are now being illu-
minated further by neuroscientific stud-
ies. They provide strong specific support
for Bowlby and Ainsworth’s attachment
theory and its extension into the realm of
adult relationships (Mikulincer & Shaver,
2007a). However, more research is needed
if we are to understand better (1) the spe-
cific hyperactivating or deactivating strate-
gies a particular insecure person will use in
particular situations, (2) the link between
attachment orientations and the relative
balance of implicit and explicit emotion-
regulation attempts, and (3) the ways people
scoring high on both attachment anxiety
and avoidance regulate their emotions. In
addition, future studies should attempt to
integrate Bowlby’s (1988) notion that effec-

Attachment and Emotion Regulation 247
tive therapeutic interventions amount to
providing a secure base and help with emo-
tion regulation— precisely what was missing
in a client’s previous attachment relation-
ships—with the findings we have reviewed
here concerning normative and individual-
differences aspects of links between attach-
ment orientations and emotion regulation
strategies. Further development and evalua-
tion of attachment- related therapeutic inter-
ventions will foster greatly improved lives
and close relationships.
References
Ainsworth, M. D. S., Blehar, M. C., Waters, E.,
& Wall, S. (1978). Patterns of attachment:
Assessed in the Strange Situation and at home.
Hillsdale, NJ: Erlbaum.
Amir, M., Horesh, N., & Lin-Stein, T. (1999).
Infertility and adjustment in women: The
effects of attachment style and social support.
Journal of Clinical Psychology in Medical Set-
tings, 6, 463– 479.
Bartley, M., Head, J., & Stansfeld, S. (2007). Is
attachment style a source of resilience against
health inequalities at work? Social Science and
Medicine, 64, 765–775.
Berant, E., Mikulincer, M., & Shaver, P. R.
(2008). Mothers’ attachment style, their men-
tal health, and their children’s emotional vul-
nerabilities: A seven-year study of children
with congenital heart disease. Journal of Per-
sonality, 76, 31–66.
Besser, A., & Priel, B. (2009). Emotional
responses to a romantic partner’s imaginary
rejection: The roles of attachment anxiety,
covert narcissism, and self-
e
valuation. Journal
of Personality, 77, 287–325.
Besser, A., & Priel, B. (2010). Grandiose narcis-
sism versus vulnerable narcissism in threaten-
ing situations: Emotional reactions to achieve-
ment failure and interpersonal rejection.
Journal of Social and Clinical Psychology, 29,
874–902.
Birnbaum, G. E., Orr, I., Mikulincer, M., &
Florian, V. (1997). When marriage breaks up:
Does attachment style contribute to coping
and mental health? Journal of Social and Per-
sonal Relationships, 14, 643–654.
Bowlby, J. (1973). Attachment and loss: Vol. 2.
Separation: Anxiety and anger. New York:
Basic Books.
Bowlby, J. (1980). Attachment and loss: Vol.
3. Sadness and depression. New York: Basic
Books.
Bowlby, J. (1982). Attachment and loss: Vol. 1.
Attachment (2nd ed.). New York: Basic Books.
(Original work published 1969)
Bowlby, J. (1988). A secure base: Clinical appli-
cations of attachment theory. London: Rout-
ledge.
Brennan, K. A., Clark, C. L., & Shaver, P. R.
(1998). Self-
r
eport measurement of adult
attachment: An integrative overview. In J. A.
Simpson & W. S. Rholes (Eds.), Attachment
theory and close relationships (pp. 46–76).
New York: Guilford Press.
Burnette, J. L., Davis, D. E., Green, J. D.,
Worthington, E. L., & Bradfield, E., Jr.
(2009). Insecure attachment and depressive
symptoms: The mediating role of rumination,
empathy, and forgiveness. Personality and
Individual Differences, 46, 276–280.
Burnette, J. L., Taylor, K., Worthington, E. L., Jr.,
& Forsyth, D. R. (2007). Attachment working
models and trait forgivingness: The mediat-
ing role of angry rumination. Personality and
Individual Differences, 42, 1585–1596.
Carnelley, K. B., Israel, S., & Brennan, K. (2007).
The role of attachment in influencing reac-
tions to manipulated feedback from romantic
partners. European Journal of Social Psychol-
ogy, 37, 968–986.
Cassidy, J. (1994). Emotion regulation: Influence
of attachment relationships [Special issue].
Monographs of the Society for Research in
Child Development, 59, 228–249.
Cassidy, J., & Berlin, L. J. (1994). The insecure/
ambivalent pattern of attachment: Theory and
research. Child Development, 65, 971–981.
Cassidy, J., & Kobak, R. R. (1988). Avoidance
and its relationship with other defensive pro-
cesses. In J. Belsky & T. Nezworski (Eds.),
Clinical implications of attachment (pp.
300–
323). Hillsdale, NJ: Erlbaum.
Cassidy, J., & Shaver, P. R. (Eds.). (2008). Hand-
book of attachment: Theory, research, and
clinical applications (2nd ed.). New York:
Guilford Press.
Cassidy, J., Shaver, P. R., Mikulincer, M., &
Lavy, S. (2009). Experimentally induced secu-
rity influences responses to psychological
pain. Journal of Social and Clinical Psychol-
ogy, 28, 463–478.
Cozzarelli, C., Sumer, N., & Major, B. (1998).
Mental models of attachment and coping with
abortion. Journal of Personality and Social
Psychology, 74, 453 – 467.
248 SOCIAL ASPECTS
Davis, D., Shaver, P. R., & Vernon, M. L. (2003).
Physical, emotional, and behavioral reactions
to breaking up: The roles of gender, age, emo-
tional involvement, and attachment style. Per-
sonality and Social Psychology Bulletin, 29,
871–884.
Dewitte, M., & De Houwer, J. (2008). Adult
attachment and attention to positive and nega-
tive emotional face expressions. Journal of
Research in Personality, 42, 498–505.
Dewitte, M., De Houwer, J., Goubert, L., &
Buysse, A. (2010). A multi-modal approach to
the study of attachment- related distress. Bio-
logical Psychology, 85, 149–162.
Dewitte, M., De Houwer, J., Koster, E. H. W.,
& Buysse, A. (2007). What’s in a name?:
Attachment- related attentional bias. Emotion,
7, 535–545.
Dewitte, M., Koster, E. H. W., De Houwer, J.,
& Buysse, A. (2007). Attentive processing
of threat and adult attachment: A dot-probe
study. Behaviour Research and Therapy, 45,
1307–1317.
Diamond, L. M., Hicks, A. M., & Otter-
Henderson, K. A. (2006). Physiological evi-
dence for repressive coping among avoidantly
attached adults. Journal of Social and Per-
sonal Relationships, 23, 205–229.
Diamond, L. M., Hicks, A. M., & Otter-
Henderson, K. A. (2008). Every time you go
away: Changes in affect, behavior, and physi-
ology associated with travel- related separa-
tions from romantic partners. Journal of Per-
sonality and Social Psychology, 95, 385–403.
Ditzen, B., Schmidt, S., Strauss, B., Nater, U.
M., Ehlert, U., & Heinrichs, M. (2008).
Adult attachment and social support inter-
act to reduce psychological but not cortisol
responses to stress. Journal of Psychosomatic
Research, 64, 479–486.
Ehrenthal, J. C., Friederich, H. C., & Schauen-
burg, H. (2011). Separation recall: Psy-
chophysiological response- patterns in an
attachment- related short-term stressor. Stress
and Health, 27, 251–255.
Eisenberger, N. I., Master, S. L., Inagaki, T. K.,
Taylor, S. E., Shirinyan, D., Lieberman, M.
D., et al. (2011). Attachment figures activate a
safety signal- related neural region and reduce
pain experience. Proceedings of the National
Academy of Sciences USA, 108, 11721–11726.
Feeney, J. A. (1998). Adult attachment and
relationship- centered anxiety: Responses to
physical and emotional distancing. In J. A.
Simpson & W. S. Rholes (Eds.), Attachment
theory and close relationships (pp. 189–219).
New York: Guilford Press.
Field, N. P., & Sundin, E. C. (2001). Attachment
style in adjustment to conjugal bereavement.
Journal of Social and Personal Relationships,
18, 347–361.
Finkel, E. J., Burnette, J. L., & Scissors, L. E.
(2007). Vengefully ever after: Destiny beliefs,
state attachment anxiety, and forgiveness.
Journal of Personality and Social Psychology,
92, 871–886.
Fraley, R., & Bonanno, G. A. (2004). Attachment
and loss: A test of three competing models on
the association between attachment- related
avoidance and adaptation to bereavement.
Personality and Social Psychology Bulletin,
30, 878–890.
Fraley, R. C., & Shaver, P. R. (1997). Adult
attachment and the suppression of unwanted
thoughts. Journal of Personality and Social
Psychology, 73, 1080–1091.
Fraley, R. C., & Shaver, P. R. (2000). Adult
romantic attachment: Theoretical develop-
ments, emerging controversies, and unan-
swered questions. Review of General Psychol-
ogy, 4, 132–154.
Gaines, S. O. Jr., Reis, H. T., Summers, S., Rus-
bult, C. E., Cox, C. L., Wexler, M. O., et al.
(1997). Impact of attachment style on reactions
to accommodative dilemmas in close relation-
ships. Personal Relationships, 4, 93–113.
Gillath, O., Bunge, S. A., Shaver, P. R., Wen-
delken, C., & Mikulincer, M. (2005).
Attachment- style differences in the ability to
suppress negative thoughts: Exploring the neu-
ral correlates. NeuroImage, 28, 835–847.
Granqvist, P., Mikulincer, M., Gurwitz, V., &
Shaver, P. R. (2012). Experimental findings
on God as an attachment figure: Normative
processes and moderating effects of internal
working models. Journal of Personality and
Social Psychology, 103, 804–818.
Hannon, P. A., Rusbult, C. E., Finkel, E. J.,
& Kumashiro, M. A. (2010). In the wake of
betrayal: Perpetrator amends, victim forgive-
ness, and the resolution of betrayal incidents.
Personal Relationships, 17, 253–278.
Hobdy, J., Hayslip, B., Kaminski, P. L., Crowley,
B. J., Riggs, S., & York, C. (2007). The role of
attachment style in coping with job loss and
the empty nest in adulthood. International
Journal of Aging and Human Development,
65, 335–371.
Holmberg, D., Lomore, C. D., Takacs, T. A., &
Price, E. L. (2011). Adult attachment styles
Attachment and Emotion Regulation 249
and stressor severity as moderators of the
coping sequence. Personal Relationships, 18,
502–517.
Jerga, C., Shaver, P. R., & Wilkinson, R. B.
(2011). Attachment insecurities and identifica-
tion of at-risk individuals following the death
of a loved one. Journal of Social and Personal
Relationships, 28, 891–914.
Karremans, J. C., & Aarts, H. (2007). The role
of automaticity in the inclination to forgive
close others. Journal of Experimental Social
Psychology, 43, 902–917.
Kidd, T., Hamer, M., & Steptoe, A. (2011).
Examining the association between adult
attachment style and cortisol responses to
acute stress. Psychoneuroendocrinology, 36,
771–779.
Lemche, E., Giampietro, V. P., Surguladze, S.
A., Amaro, E. J., Andrew, C. M., Williams, S.
C., et al. (2006). Human attachment security
is mediated by the amygdala: Evidence from
combined fMRI and psychophysiological mea-
sures. Human Brain Mapping, 27, 623–635.
Lopez, F. G., & Gormley, B. (2002). Stability
and change in adult attachment style over
the first-year college transition: Relations to
self- confidence, coping, and distress patterns.
Journal of Counseling Psychology, 49, 355–
364.
Master, S. L., Eisenberger, N. I., Taylor, S. E.,
Naliboff, B. D., Shirinyan, D., & Lieberman,
M. D. (2009). A picture’s worth: Partner pho-
tographs reduce experimentally induced pain.
Psychological Science, 20, 1316–1318.
Maunder, R. G., Lancee, W. J., Nolan, R. P.,
Hunter, J. J., & Tannenbaum, D. (2006). The
relationship of attachment insecurity to subjec-
tive stress and autonomic function during stan-
dardized acute stress in healthy adults. Journal
of Psychosomatic Research, 60, 283–290.
Medway, F. J., Davis, K. E., Cafferty, T. P., Chap-
pell, K. D., & O’Hearn, R. E. (1995). Family
disruption and adult attachment correlates
of spouse and child reactions to separation
and reunion due to Operation Desert Storm.
Journal of Social and Clinical Psychology, 14,
97–118.
Mikulincer, M., Dolev, T., & Shaver, P. R.
(2004). Attachment- related strategies during
thought- suppression: Ironic rebounds and vul-
nerable self- representations. Journal of Per-
sonality and Social Psychology, 87, 940–956.
Mikulincer, M., & Florian, V. (1995). Appraisal
of and coping with a real-life stressful situa-
tion: The contribution of attachment styles.
Personality and Social Psychology Bulletin,
21, 406–414.
Mikulincer, M., & Florian, V. (1998). The rela-
tionship between adult attachment styles and
emotional and cognitive reactions to stressful
events. In J. A. Simpson & W. S. Rholes (Eds.),
Attachment theory and close relationships
(pp. 143–165). New York: Guilford Press.
Mikulincer, M., & Florian, V. (1999). Maternal–
fetal bonding, coping strategies, and mental
health during pregnancy: The contribution of
attachment style. Journal of Social and Clini-
cal Psychology, 18, 255–276.
Mikulincer, M., Gillath, O., & Shaver, P. R.
(2002). Activation of the attachment system
in adulthood: Threat- related primes increase
the accessibility of mental representations of
attachment figures. Journal of Personality and
Social Psychology, 83, 881–895.
Mikulincer, M., Hirschberger, G., Nachmias, O.,
& Gillath, O. (2001). The affective component
of the secure base schema: Affective priming
with representations of attachment security.
Journal of Personality and Social Psychology,
81, 305–321.
Mikulincer, M., & Shaver, P. R. (2001). Attach-
ment theory and intergroup bias: Evidence
that priming the secure base schema attenu-
ates negative reactions to out- groups. Jour-
nal of Personality and Social Psychology, 81,
97–115.
Mikulincer, M., & Shaver, P. R. (2007a). Attach-
ment in adulthood: Structure, dynamics, and
change. New York: Guilford Press.
Mikulincer, M., & Shaver, P. R. (2007b). Boost-
ing attachment security to promote mental
health, prosocial values, and inter-group toler-
ance. Psychological Inquiry, 18, 139–156.
Mikulincer, M., Shaver, P. R., Sapir-Lavid, Y.,
& Avihou-Kanza, N. (2009). What’s inside
the minds of securely and insecurely attached
people?: The secure- base script and its associa-
tions with attachment- style dimensions. Jour-
nal of Personality and Social Psychology, 97,
615–633.
Mikulincer, M., Shaver, P. R., & Slav, K. (2006).
Attachment, mental representations of others,
and gratitude and forgiveness in romantic rela-
tionships. In M. Mikulincer & G. S. Goodman
(Eds.), Dynamics of romantic love: Attach-
ment, caregiving, and sex (pp. 190–215). New
York: Guilford Press.
Park, L. E. (2010). Responses to self- threat:
Linking self and relational constructs with
approach and avoidance motivation. Social
250 SOCIAL ASPECTS
and Personality Psychology Compass, 4, 201–
221.
Perunovic, M., & Holmes, J. G. (2008). Auto-
matic accommodation: The role of personal-
ity. Personal Relationships, 15, 57–70.
Quirin, M., Gillath, O., Pruessner, J. C., & Egg-
ert, L. D. (2010). Adult attachment insecurity
and hippocampal cell density. Social Cogni-
tive and Affective Neuroscience, 5, 39– 47.
Quirin, M., Pruessner, J. C., & Kuhl, J. (2008).
HPA system regulation and adult attachment
anxiety: Individual differences in reactive and
awakening cortisol. Psychoneuroendocrinol-
ogy, 33, 581–590.
Raskin, P. M., Kummel, P., & Bannister, T.
(1998). The relationship between coping
styles, attachment, and career salience in part-
nered working women with children. Journal
of Career Assessment, 6, 403–416.
Rusbult, C. E., Verette, J., Whitney, G. A.,
Slovik, L. F., & Lipkus, I. (1991). Accommo-
dation processes in close relationships: Theory
and preliminary empirical evidence. Journal of
Personality and Social Psychology, 60, 53–78.
Sbarra, D. A. (2006). Predicting the onset of emo-
tional recovery following nonmarital relation-
ship dissolution: Survival analyses of sadness
and anger. Personality and Social Psychology
Bulletin, 32, 298–312.
Sbarra, D. A., & Emery, R. E. (2005). The emo-
tional sequelae of nonmarital relationship
dissolution: Analysis of change and intrain-
dividual variability over time. Personal Rela-
tionships, 12, 213–232.
Scharfe, E., & Bartholomew, K. (1995). Accom-
modation and attachment representations in
couples. Journal of Social and Personal Rela-
tionships, 12, 389–401.
Selcuk, E., Zayas, V., Günaydin, G., Hazan, C.,
& Kross, E. (2012). Mental representations of
attachment figures facilitate recovery follow-
ing upsetting autobiographical memory recall.
Journal of Personality and Social Psychology,
103, 362–378.
Shaver, P. R., & Mikulincer, M. (2002).
Attachment- related psychodynamics. Attach-
ment and Human Development, 4, 133–161.
Smeets, T. (2010). Autonomic and hypothalamic–
pituitary– adrenal stress resilience: Impact of
cardiac vagal tone. Biological Psychology, 84,
290–295.
Waters, H. S., & Waters, E. (2006). The attach-
ment working models concept: Among other
things, we build scriptlike representations
of secure base experiences. Attachment and
Human Development, 8, 185–198.
Wayment, H. A., & Vierthaler, J. (2002). Attach-
ment style and bereavement reactions. Journal
of Loss and Trauma, 7, 129–149.
Williams, N. L., & Riskind, J. H. (2004). Adult
romantic attachment and cognitive vulnerabil-
ities to anxiety and depression: Examining the
interpersonal basis of vulnerability models.
Journal of Cognitive Psychotherapy, 18, 7–24.
Yárnoz-Yaben, S. (2009). Forgiveness, attach-
ment, and divorce. Journal of Divorce and
Remarriage, 50, 282–294.
Zhang, F., & Labouvie- Vief, G. (2004). Stabil-
ity and fluctuation in adult attachment style
over a 6-year period. Attachment and Human
Development, 6, 419– 437.
Zilcha-Mano, S., Mikulincer, M., & Shaver, P.
R. (2011). Pets as safe havens and secure bases:
The moderating role of pet attachment orien-
tations. Journal of Research in Personality,
45, 345–357.

251
Research and theory in the domain of atti-
tudes and evaluation are relevant to emotion
due to their many overlaps and parallels.
Most fundamentally, they share at their core
the concept of valence. Attitudes encode the
valence of an object: that is, goodness or
badness, desirability or undesirability, plea-
sure or pain. Valence is similarly integral to
the concept of emotion; some theories argue
that all emotions, at their core, can be dis-
tilled to whether they are positive or nega-
tive (e.g., Russell, 2003; Watson & Tellegen,
1985). Valence’s function is to guide appro-
priate action. In the case of attitudes, that
functionality lies in the ability of valence
to provide a rapid, default assessment of an
object’s value; we need not constantly judge
the world anew.
Yet this tool that may even be lifesaving in
times of peril can also be a liability. Reflex-
ively acting in accordance with one’s feelings
is not always appropriate; indeed, the feeling
itself may be deemed problematic. For exam-
ple, prejudiced group attitudes and evalua-
tions may conflict with social norms or one’s
values, leading to reconciliatory processes of
justification or suppression (Crandall, Eshle-
man, & O’Brien, 2002). We also argue that
evaluation entails a process of affect regula-
tion in and of itself. Even in the absence of
a particular desire to change one’s evalua-
tion, regulation is occurring. Increasingly,
contemporary perspectives on attitudes view
evaluations as the product of dynamic pro-
cesses that are shaped by the demands of the
immediate situation. Relatively stable men-
tal representations underlie the very earli-
est evaluative processing; however, in a way
that can be viewed as fundamentally self-
regulatory (e.g., Carver & Scheier, 1982),
evaluations are sensitive to various goals and
standards for thoughts, feelings, and behav-
ior.
Regarding the volitional self- regulation
of evaluation, it will be uncontroversial
to note that people are not merely pas-
sive receivers of their psychological experi-
ences; valence may be not only experienced
but also assessed and modified. Though
evaluation has some characteristics of cued
responding grounded by preexisting struc-
ture, people do not respond to the same cue
in an invariable fashion. One may generally
dislike something (say, golf) but temper that
affective response, or even reverse it, when
the situation demands. For example, when
offered the opportunity to golf with a work
supervisor, one might be motivated to deem-
phasize the object’s disliked features and
emphasize its liked ones (e.g., defining golf
more by its aesthetic value than by, say, its
early- morning tee times), or reevaluate its
features (e.g., considering how golf’s diffi-
culty might constitute a virtue rather than
CHAPTER 16
Attitudes, Evaluation,
and Emotion Regulation
Christopher R. Jones
Tabitha Kirkland
William A. Cunningham
252 SOCIAL ASPECTS
an annoyance). Evaluations are often shaped
to conform to appropriate action. As such,
when considering the use of attitudes to
guide behavior, the regulation of affect is
important. One type of evaluative regulation
entails the inhibition and reshaping of “gut
reactions” of liking in accordance with their
perceived appropriateness. Beyond this, we
emphasize processes within even quite rapid
and spontaneous evaluations (those very gut
reactions) that evidence self- regulatory flex-
ibility in experienced object- directed affect.
Relatedly, Gross and Barrett (2011) sug-
gest the possibility that emotion generation
and regulation can be considered the same
process: Information may be simultane-
ously processed as affectively relevant and
informed by top-down processes that take
contextual cues (e.g., situational appropri-
ateness) into account. Similarly, Todd, Cun-
ningham, Anderson, and Thompson (2012)
have suggested that emotion can be simulta-
neously shaped and regulated through early
attentional biases. In making this argument,
we hope to highlight the potential for greater
integration between the literatures on affect
regulation and attitudes, which as yet have
stood largely separate.
At the outset, some clarification of our
usage of the terms attitude and evaluation
will be useful. Some prominent definitions
have defined attitudes generally: predisposi-
tions to respond positively or negatively to
an object (e.g., Allport, 1935; Eagly & Chai-
ken, 1993), or as a constellation of affec-
tive, cognitive, and behavioral responses
associated with an object (Zanna & Rem-
pel, 1988). In contrast, in a more specific
and limited definition, Fazio (2007) defines
attitudes as associations in memory between
objects and summated valenced information
about those objects. This approach is the
one we take here, defining attitudes as repre-
sentational, which means that attitudes sig-
nify valenced information stored in memory
that has the potential to be activated. We do,
however, distinguish representational atti-
tudes from evaluation— a process by which
valence is constructed in both bottom- up
and top-down fashion. In this way, we inte-
grate a representational definition, with the
approach to attitudes typically considered
most antithetical to it: the constructivist
approach (e.g., Schwarz & Bohner, 2001).
This approach tends not to deny the exis-
tence of mental representations but chooses
to deemphasize them, focusing instead on
contextual factors that shape experiences of
liking and disliking. In summary, we use the
term attitude to refer to a structural entity
in memory, and the term evaluation to refer
to an appraisal process informed by an atti-
tude that unfolds over time in a particular
context. Thus, evaluation can be considered
a process of affect regulation in the moment,
fostering context appropriateness. Given our
terminology, attitudes may also be subject to
self- regulation, such as when one attempts to
change how much one generally likes some-
thing. We primarily focus on the former.
It is the interplay of attitudes and evalua-
tion that provides true functionality. A rigid
retrieval system would be insensitive to con-
text and beholden to even limited past expe-
rience. A fully constructed system would be
slow, inefficient, and too unresponsive to
lessons learned. Instead, we have both capa-
bilities. The transformation of attitudes into
evaluations is a form of emotion regulation
in which situational factors can play a role
even in immediate responses. We argue that
an attitude is a triggered affective response
shaped into a contextually appropriate eval-
uation via a process of iterative reprocessing
(Cunningham & Zelazo, 2007). This updat-
ing occurs as the situation unfolds; thus,
affect regulation is critical to all stages of
the attitude- to- evaluation transformation—
from the processes typically considered
automatic to the processes that are typically
considered controlled. Thus, although the
effortful inhibition of evoked attitudes is a
major topic, our conceptualization of regu-
lation goes beyond this to include a variety
of processes that shape adaptive responding.
This broader view of emotion regulation
is consistent with evolving views on self-
regulation generally, in which inhibition is
not a sine qua non (e.g., Fujita, 2011).
In this chapter, we review contemporary
attitude theories that entail an emphasis
on regulation. Following a discussion of
two foundational models to most recent
approaches, we focus in detail on contem-
porary treatments of attitude and evaluation
that illustrate the self- regulatory shaping
of rapid response. We highlight the itera-
tive reprocessing (IR) model (Cunningham
& Zelazo, 2007), which not only addresses
such processes descriptively but also provides
a perspective from neuroscience regarding
their underlying brain substrates. We use the

Attitudes and Emotion Regulation 253
IR model as a framework to address differ-
ences in theory and empirical controversy
that characterize this literature.
Two Foundational Attitude Models
Models of attitudes commonly empha-
size some distinction between implicit and
explicit attitudes or evaluative processes.
Undoubtedly, this is in large part due to
the introduction of reaction- time-based
implicit attitude measures, such as evalu-
ative priming (Fazio, Jackson, Dunton,
& Williams, 1995) and the Implicit Asso-
ciation Test (IAT; Greenwald, McGhee, &
Schwarz, 1998). Researchers had long hoped
to find a solution to the problem of dishon-
est responding to attitude measures out of
social desirability concerns. These implicit
measures offered the best solution to date by
engaging participants in a task that might
reveal attitudes without actually calling on
participants to express them.
Interestingly, the new implicit attitude
measures appear to diverge from explicit
measures (self- report) particularly when
participants’ self- presentational concerns
are high (e.g., Fazio et al., 1995; Olson,
Fazio, & Hermann, 2007), yet the mea-
sures also diverge even when participants’
incentives to misrepresent themselves are
minimal. For example, Karpinski and Hil-
ton (2001) found null relations between
IAT and self- report measures of preference
for insects versus flowers and apples versus
candy bars, seemingly noncontroversial atti-
tude objects. Furthermore, it appeared that
social influence (not necessarily intentional
misrepresentation) was especially evident on
the implicit measure rather than the explicit
measure. That is, the IAT suggested a greater
affinity for the healthy (i.e., socially val-
ued) alternative than was evident in explicit
behavior. This example highlights the find-
ing that dissociations between implicit and
explicit measures are common and complex
(for a meta- analytic review, see Hofmann,
Gawronski, Gschwendner, Le, & Schmitt,
2005), and that these dissociations cannot
be attributed exclusively to misrepresenta-
tion on self- report measures or measurement
error and lack of structural fit (Payne, Burk-
ley, & Stokes, 2008).
Models need to account for these disso-
ciations, and incorporating some distinction
between implicit and explicit attitudes or
evaluations is a common way of doing so.
However, something like this distinction has
been long recognized. For example, Hov-
land, Janis, and Kelley (1953, cited in Petty
& Briñol, 2006, p. 740) distinguished atti-
tudes (“implicit responses” that are “some-
times unconscious”) from opinions (“verbal
answers that one covertly expresses”). The
recent accumulated evidence from implicit
measures can be understood as validation
of the long- appreciated observation that
rapid, unintentional responses (what one
might call “gut reactions”) can differ a great
deal from carefully considered evaluations.
Accounting fully for the processes that dis-
tinguish them is a central issue in contem-
porary attitude theory and obviously impor-
tant for self- regulation. We first discuss two
influential models that have been recently
built upon to explain differences between
immediate and subsequent responses.
The MODE Model
The acronym MODE refers to Motiva-
tion and Opportunity as DEterminants of
whether the attitude- to- behavior process
is relatively spontaneous or deliberative in
nature. The MODE model (Fazio, 1990;
Olson & Fazio, 2009) posits a single atti-
tude representation, and defines an attitude
as the association in memory between an
object and a summary evaluation thereof (see
Fazio, 2007). The model is largely agnostic
about the nature of attitude representation.
It is readily understood in terms of a “schema
plus tag” model, in which summary evalu-
ations are a special form of tag associated
with an attitude object. However, it is also
consistent with more recent dynamic and
connectionist models if the term summary
is understood to refer to distributed infor-
mation in memory (Eiser, Fazio, Stafford,
& Prescott, 2003). Regardless, the MODE
model is primarily concerned with how and
when attitudes determine behavior.
The MODE model predicts whether the
attitude- to- behavior process is relatively
spontaneous or deliberative in nature. The
model notes that the primary function of
attitudes is to provide a rapid assessment
of objects in the environment to facilitate
appropriate behavior in a timely fashion.
Attitudes can be activated automatically,
and the likelihood of automatic attitude
254 SOCIAL ASPECTS
activation is a function of the associative
strength between the attitude object and the
associated evaluation (see Fazio, 2001). If
there is little motivation for further consid-
eration of the object, for whatever reason, or
low opportunity (e.g., time pressure or cog-
nitive load) to do so, attitudes should guide
behavior in a spontaneous, largely auto-
matic fashion, in which they promote con-
gruent behaviors afforded by the situation.
Controlled deliberation about the attitude
and behavior is likely only when the motiva-
tion and opportunity to do so are present.
This deliberation might lead to the alteration
or rejection of an attitude and the behavior
it facilitates, but it could also affirm it. In
fact, the model suggests that affirmation
is most typical, because the activated atti-
tude fosters biased processing. Alteration or
rejection of the attitude depends on various
factors, including contextual influences on
the object’s utility, social influence, object
reappraisal, and so on.
In summary, the MODE model posits a
single attitude associated with each discrete
representation of an attitude object that may
be retrieved from memory, often automati-
cally. That attitude is likely to shape sub-
sequent judgment and behavior; however,
the attitude’s influence is especially likely
to be direct for spontaneous behaviors.
When the motivation and opportunity to
deliberate are present, the attitude might
be disregarded, adjusted for reasons includ-
ing context or apparent appropriateness, or
validated by deliberation. The MODE mod-
el’s implications for emotion regulation are
straightforward. The model applies directly
to circumstances in which one wishes to
control automatic emotional responses to a
particular object. It points to motivation and
opportunity as broad categories encompass-
ing many specific variables and emphasizes
that any major impediment to motivation or
ability drastically reduces the likelihood of
deliberative regulation.
To account for discrepancies between
implicit and explicit attitudes, the MODE
perspective suggests that implicit measures
largely reflect automatically activated atti-
tudes, while explicit measures capture delib-
erative evaluations, which may or may not
reflect attitudes for the reasons described.
Another way of explaining these differences
is to evoke two different attitude representa-
tions to which the measures are differentially
sensitive. The notion of an implicit attitude
is often attributed to Greenwald and Banaji
(1995), who wrote: “Implicit attitudes are
introspectively unidentified (or inaccurately
identified) traces of past experience that
mediate favorable or unfavorable feeling,
thought, or action toward social objects”
(p. 8). It was not until Wilson, Lindsey, and
Schooler’s (2000) dual- attitudes model,
however, that a major process model of dual
attitudes was articulated.
The Dual‑Attitudes Model
In the original conception of “implicit atti-
tudes,” such attitudes, by definition, were
considered to be unknown to the evaluator
(Greenwald & Banaji, 1995). The absence
of awareness is most directly the facet of
automaticity (see Bargh, 1994) referred to
by the term implicit. However, the term has
been used in a wide variety of ways, lead-
ing some to argue that because implicit de
facto has become a catchall descriptor of
(relatively) automatic processes, it should be
understood as such (De Houwer & Moors,
2007). Wilson and colleagues (2000), for
example, do not define implicit attitudes as
being characterized by unawareness. They
suggested that “people are often aware,
at least fleetingly, of [implicit attitudes]”
(p. 105). What, then, is an implicit attitude
in this model?
According to the dual- attitudes model
(Wilson et al., 2000), implicit attitudes are,
in a word, old (or prior). That is, when an
attitude representation changes, it is not
fully supplanted by a new, altered attitude.
Rather, both representations can coexist in
memory. Thus, an implicit attitude is a prior,
often deeply ingrained attitude. By virtue of
its precedence and having been rehearsed,
the implicit attitude is strongly associated
with the attitude object. Thus, it will likely
be automatically activated upon encounter-
ing an attitude object. Similar to the MODE
model, the dual- attitudes model posits that
“capacity and motivation” are required to
retrieve the newer, explicit attitude effort-
fully. Retrieval of the explicit attitude is
expected to lead to its application, especially
for controlled behaviors. The simultaneous
activation of implicit and explicit attitudes
may also demonstrate unintended influ-
ence of the implicit attitude, especially on
unmonitored or uncontrollable nonverbal

Attitudes and Emotion Regulation 255
responses (e.g., Dovidio, Kawakami, John-
son, Johnson, & Howard, 1997).
Wilson et al. (2000) further distinguish
a typology of four kinds of implicit atti-
tudes, which have different characteristics
due to their differing origins. Briefly, an
implicit attitude might arise from repres-
sion, in which an anxiety- provoking implicit
attitude is expelled from consciousness
and is thus implicit in the sense of being
unknown. Another implicit attitude arises
from motivated overriding, when an attitude
is unwanted and effortfully suppressed. A
third implicit attitude arises from automatic
overriding, when the process of overrid-
ing such an implicit attitude itself becomes
automatized through repetition. In this case,
capacity and motivation are not actually
required for the explicit rather than implicit
attitude to predominate. Finally, the dual-
attitudes model suggests that implicit atti-
tudes may differ from explicit attitudes sim-
ply by virtue of having an entirely separate
neurocognitive substrate (i.e., independent
systems; see Lieberman, 2007, for such a
view) but does not elaborate much on this
possibility, which distinguishes some more
recent models.
In some ways, the implications of the
dual- attitudes model for emotion regulation
are similar to those of the MODE model.
Certainly, the necessity of motivation and
ability/capacity for any sort of controlled
emotion regulation is again highlighted.
The different types of implicit attitudes
described, however, are uniquely relevant.
The case of repression, though its very exis-
tence is controversial, poses an interesting
problem. In this case, one’s own affective
reactions to an object are anxiety provok-
ing, instigating defensive reactions that sup-
press and exclude them from consciousness.
Motivated overriding essentially describes a
phenomenon of successful affective regula-
tion, but it does not elaborate much about
how this occurs, especially under difficult
circumstances, with the exception that
automatizing a regulatory response may
occur through repetition. This is an interest-
ing possibility in the realm of emotion regu-
lation.
Summary of Historical Perspectives
To summarize, these models in their early
incarnations posited implicit or activated
attitudes that were largely unregulated.
Schema-plus-tag or implicit- attitude- as-
habitual- response conceptualizations of
attitude share the consequence of rapid
responses that are quite inflexible and
context- free. Affective regulation is then
possible if and only if one is motivated and
able to suppress, adjust, enhance, or replace
(i.e., effortfully retrieve an explicit atti-
tude) an evaluation. However, the strong
form of this view is inconsistent with find-
ings that are indicative of self- regulatory
responding even in rapid responses evident
on implicit measures. For example, Lowery,
Hardin, and Sinclair (2001) demonstrated
that racial attitudes as measured by an IAT
were sensitive to social tuning— that is, they
were influenced by the presumed attitudes
of other individuals present. Increasingly,
recent models address adaptive flexibility in
processes occurring during the construction
of an evaluation. Notably, a similar devel-
opment has occurred in theorizing about
stereotyping. Automatic stereotype activa-
tion was often viewed as inevitable (Bargh,
1999; Devine, 1989) and could only be over-
ridden by controlled processes. Subsequent
research in this domain suggested a much
more optimistic view of flexibility and self-
regulation at the earliest stages of process-
ing (e.g., Jones & Fazio, 2010; Moskowitz,
Gollwitzer, Wasel, & Schaal, 1999; Sassen-
berg & Moskowitz, 2005). In contemporary
attitude models, we see a greater emphasis
on malleability in rapid responses. This mal-
leability can be viewed as demonstrating the
fundamentally regulatory nature of evalua-
tion. We turn now to recent models includ-
ing several variations on this theme.
Contemporary Attitude Models
The Associative–Propositional
Evaluation Model
Probably the most influential contemporary
approach to attitudes and evaluation is the
associative– propositional evaluation (APE)
model, which originally was introduced
to explain the complex literature on the
impact of interventions that form or change
attitudes on implicit and explicit measures
(Gawronski & Bodenhausen, 2006). Since
then, the APE model has been broadened to
address attitudes and evaluation more gener-
ally (see Gawronski & Bodenhausen, 2011).
256 SOCIAL ASPECTS
The APE model describes two interactive
but distinct types of processes that under-
lie evaluation: associative and propositional
processes.
1
Associative processes activate
associated representations from memory.
This activation is presumed to follow the
principles of contiguity and similarity; con-
tiguity refers to the spatiotemporal proxim-
ity of stimuli that determines the structure
of memory, whereas similarity refers to the
fit between encountered objects and men-
tal representations. Propositional processes
involve the validation of information acti-
vated by associative processes. Unlike asso-
ciative processes, propositional processes
entail subjective assessments of truth values
(i.e., veridicality of information). They are
posited to operate according to principles of
logical consistency, though in the sense of
perceived coherence rather than formal logic
per se. Associative processes are argued to
underlie implicit evaluations, and propo-
sitional processes are argued to underlie
explicit evaluations. Thus, if implicit evalua-
tions are perceived as valid, they will be used
for explicit evaluations; if they are not, they
are modified.
The APE model suggests that, following
connectionist models of associative memory,
information associated with a given atti-
tude object is distributed across a network
of weighted connections. The activation
of a given piece of information associated
with an object is typically probabilistic, in
a manner that maximizes the likelihood of
the most relevant information being acti-
vated, which can be viewed as regulatory.
The particularities of the input stimulus, as
well as incidentally active information due
to recency of use, determine which subset of
associations is activated. This allows for dif-
ferent implicit evaluations as a function of
context, consistent with malleability often
observed on implicit attitude measures (e.g.,
Barden, Maddux, Petty, & Brewer, 2004).
These implicit evaluations provide the grist
for propositional processing. If the propo-
sitional implications of an implicit evalu-
ation are inconsistent with other salient
propositions, an aversive state of cognitive
dissonance is evoked. Cognitive dissonance,
induced by the simultaneous activation of
conflicting information, is an aversive state
of arousal that motivates efforts to allevi-
ate it (Festinger, 1957). Various methods of
restoring balance and consistency have long
been recognized, such as rejecting one of the
propositions or introducing another propo-
sition that reconciles or trivializes the appar-
ent conflict.
Importantly, associative and proposi-
tional processes are argued to be interactive.
In addition to the bottom- up processes in
which activated associations determine the
kinds of propositions that are evoked, there
are also top-down processes that create
or activate further associations (see Gaw-
ronski & Bodenhausen, 2011, for details).
The APE model is useful for explaining the
highly varied patterns of results that have
been observed when implicit and explicit
measures follow manipulations meant to
form or change attitudes. Earlier models,
particularly those that emphasize the sta-
bility of implicit evaluations (e.g., Wilson
et al., 2000), have trouble explaining cir-
cumstances in which implicit measures are
more sensitive to manipulation than explicit
measures (e.g., Gawronski & LeBel, 2008;
Karpinski & Hilton, 2001). The APE model,
in contrast, can explain when information
will recruit associative versus propositional
processes.
Some types of interventions are likely to
have direct effects on associative processes
(Gawronski & Bodenhausen, 2011). For
example, evaluative conditioning, a phenom-
enon in which an attitude toward an object
forms or changes due to its co- occurrence
with one or more valenced objects, is
likely to have these types of direct associa-
tive effects (for reviews, see De Houwer,
Thomas, & Baeyens, 2001; Jones, Olson, &
Fazio, 2010). The repeated pairing of objects
during conditioning procedures facilitates
association formation and strengthening
for obvious reasons. Other types of inter-
ventions are likely to have direct effects
on propositional processes. Inductions of
cognitive inconsistency (e.g., Gawronski
& Strack, 2004) impact propositional pro-
cesses directly but may leave relevant asso-
ciations intact. Events might also directly
influence both associative and propositional
processes. For example, a persuasive mes-
sage could change the propositions that are
considered and also create new associations
in memory.
Finally, events might directly influence
one process and indirectly influence the
Attitudes and Emotion Regulation 257
other. Any change in associative structure
can potentially influence the propositions
that are generated and assessed, the path
taken to reduce cognitive dissonance, and so
forth. The propositions an individual con-
siders can create or change the associative
strength of associations, even in the absence
of external stimulation. A fuller account of
the details of the APE model (see Gawronski
& Bodenhausen, 2006, 2011) is beyond the
scope of this chapter, but it should be noted
that the APE model offers many specific
hypotheses about the eliciting circumstances
and processes underlying patterns of atti-
tude change evident in implicit and explicit
measures.
One issue addressed by the APE model
that has interesting implications for affect
regulation concerns negation. The act of
negating a proposition may be insufficient
to change the relevant associative struc-
ture in memory. Indeed, though negation
may influence propositional processes in
a straightforward manner (i.e., processing
consistent with the negated proposition), the
act of negation may have ironic effects at
the associative level due to the coactivation
of elements (e.g., Deutsch, Gawronski, &
Strack, 2006). For example, telling oneself,
“I am not afraid of giving a speech,” may
have some immediate positive consequences
due to its consciously processed implica-
tion. However, this also risks increasing the
(automatic) association in memory between
fear and public speaking merely because of
their simultaneous activation. Gawronski
and Bodenhausen (2011) suggest that the
overall success of regulating future affec-
tive reactions depends on whether unwanted
propositions are negated (“I’m not afraid of
public speaking”) or preferable propositions
are affirmed (“Public speaking is fun”),
implying that affirmation works better than
negation for emotion regulation.
2
For exam-
ple, a study in which stereotypical associ-
ates were negated led to increased automatic
stereotype activation and, significantly for
the question of affect regulation, increased
bias on an evaluative priming task (Gaw-
ronski, Deutsch, Mbirkou, Seibt, & Strack,
2008). Gawronski and Bodenhausen (2011)
suggested that such ironic effects may bear
some responsibility for the general superi-
ority in emotion regulation (Gross, 1998)
of an affirmative strategy of reappraisal as
opposed to a negative strategy of suppres-
sion. However, other work suggests that
generating or encountering negations may
have positive influences on affect regulation.
For example, Herbert, Deutsch, Sütterlin,
Kübler, and Pauli (2011; see also Mauss,
Evers, Wilhelm, & Gross, 2006) examined
startle eyeblink responses to pleasant and
unpleasant nouns that had or had not been
negated. They found (with ample processing
time) online effects of negation consistent
with the logical meaning: reduced startle
response to negated unpleasant words and
increased response to negated pleasant
words. Thus, the role of negation in affect
regulation remains controversial.
The APE model suggests that the inter-
play of associative and propositional pro-
cesses underlies evaluation. It is sometimes
misunderstood to be a dual- system or
dual- representation model but it explicitly
is not (Gawronski & Bodenhausen, 2011,
pp. 104–105). The APE model argues that
all information is stored in the form of asso-
ciations, and that there is not a separate
store for propositions, thus rejecting the
possibility of dual representations. The APE
model does not explicitly endorse or reject
a dual- system approach, which emphasizes
separate mental systems (i.e., neurocognitive
substrates).
3
Some researchers believe that
the sometimes stark dissociations between
observed implicit and explicit evaluations
are best explained by their origination from
separate systems. We turn now to a recent
model of dual systems to explore this idea in
greater detail.
The Systems of Evaluation Model
The systems of evaluation model (SEM)
describes an associative system that pro-
duces implicit evaluations and a rule-based
system that produces explicit evaluations
(McConnell & Rydell, in press; Rydell &
McConnell, 2006). It builds on prior dual-
system models of cognition, particularly
Sloman’s (1996) model of fast and slow
learning systems of reasoning. These par-
tially independent systems operate in par-
allel and differ in the type of knowledge
they use and the operations conducted on
that knowledge. The associative system
reflects associations governed by principles
of similarity and contiguity, whereas the
258 SOCIAL ASPECTS
rule-based system operates on symbolic rep-
resentations (e.g., language) that are sub-
jected to operations of logic and deductive
reasoning. Because implicit measures tend
to reflect construct activation and explicit
measures tend to reflect symbolic reason-
ing, the measures differ in their sensitivity
to the output of the two systems. This sug-
gests that the primary distinction between
these systems is similar to the APE model.
However, a major difference is that the SEM
emphasizes distinctive characteristics of the
two systems that lead to differential rates of
change. The SEM suggests that the explicit
system is faster, while the implicit system
is slower, both to form and to change atti-
tudes. Explicit evaluations “can be formed
and modified relatively quickly because
logic and syllogism are responsive to one’s
deliberate goals and deductive reasoning
processes,” whereas implicit evaluations
“typically are slower to form and change
because they are based on accumulated atti-
tude object- evaluation pairings in memory”
(McConnell & Rydell, in press). In this way,
the SEM bears some resemblance to the
earlier dual- attitudes model of Wilson and
colleagues (2000), insofar as an implicit atti-
tude tends to be distinguished by stability.
However, the SEM does not posit separate
stores for “old” and “new” attitudes, nor
does it consider prior attitude change neces-
sarily responsible for divergence on implicit
and explicit measures. Though implicit mea-
sures are sometimes quite labile, this can be
reconciled with the SEM by noting that this
often appears to be due to activation of dif-
ferent subset of associations from memory
that influence an implicit measure, among
other reasons, rather than a rapid change in
particular associative representations.
The SEM is supported by research dem-
onstrating strong dissociations between
implicit and explicit measures, consistent
with a dual- systems view. For example,
Rydell and McConnell (2006, Experiment
2) presented participants with a series of
behaviors performed by a target individual.
Presenting a large number of positive or neg-
ative behaviors established positive or nega-
tive attitudes, respectively, that were evident
on both implicit and explicit measures. This
was followed by a series of counterattitudinal
behaviors. When 100 initial behaviors were
followed by 20 conflicting behaviors (i.e.,
positive followed by negative, or vice versa),
explicit attitude measures showed consider-
able sensitivity to new information, while
implicit measures were largely unaffected by
it. When 100 counterattitudinal behaviors
followed the initial 100, both types of mea-
sures reflected the new information. This is
interpreted as being consistent with the pres-
ence of a slow- learning associative system
and fast rule-based system. In other work
supportive of the SEM, a series of sublimi-
nal primes was paired with target individu-
als (Rydell, McConnell, Mackie, & Strain,
2006). Because repeated subliminal priming
might produce the gradual association of
affect with an attitude object, it would be
expected to influence the associative sys-
tem. Moreover, by virtue of being sublimi-
nal, such a manipulation would not provide
grist for the rule-based system. However,
the rule-based system should be especially
sensitive to the consciously accessible behav-
ioral information that was presented, as evi-
denced by influences on explicit measures.
Results in line with these hypotheses were
obtained: Across several studies, implicit
attitudes formed and changed in response
to subliminal primes, and explicit attitudes
formed and changed in response to con-
sciously accessible information.
An interesting aspect of the SEM with
wide implications for affect regulation is the
study of the consequences of discrepancy
between implicit and explicit evaluations.
One consequence of implicit– explicit dis-
crepancy involves the extent of information
processing. It has been suggested that these
discrepancies lead to increased information
processing (e.g., Petty, Tormala, Briñol, &
Jarvis, 2006). Moreover, recent work has
suggested that this may be due to the arousal
of cognitive dissonance caused by the con-
flicting outputs of the two systems (Rydell,
McConnell, & Mackie, 2008). Seeking out
information to resolve the discrepancy is a
major route to dissonance reduction. How-
ever, the source of these aversive feelings
can be mysterious to those experiencing
them, and consequently, the feelings might
be misattributed to inappropriate sources.
Specifically, negative arousal caused by atti-
tude formation with valence- inconsistent
information led participants to report lower
subjective well-being (Rydell & Durso,
2012). Thus, the SEM suggests that when
Attitudes and Emotion Regulation 259
the systems of evaluation produce discrep-
ant evaluations, negative affect and affective
regulation tend to follow. Another relevant
consequence of implicit– explicit discrepancy
involves affective forecasting. It has also been
suggested that thoughtful affective forecast-
ing relies on explicit evaluations of objects,
ignoring implicit evaluations (McConnell,
Dunn, Austin, & Rawn, 2011). Consistent
with this idea, implicit, but not explicit, atti-
tudes predicted error in affective forecasting
when considering future enjoyment of foods.
The Meta‑Cognitive Model
Petty and colleagues’ meta- cognitive model
(MCM; Petty, 2006; Petty et al., 2006; Petty,
Briñol, & DeMarree, 2007) has elements of
most of the other models we have discussed.
It focuses on how attitudes might be stored
in memory, with particular emphasis on
meta- cognitive perceptions of the validity of
responses. Moreover, the MCM draws on
research arguing that the neural substrates
of positive and negative valence are dis-
tinct (e.g., Cacioppo, Gardner, & Berntson,
1997), suggesting that separate positive and
negative evaluations of an object might be
represented in memory.
According to the MCM, though many
attitudes are predominantly univalent, it is
not uncommon for an object to be associ-
ated with both positive and negative sum-
mary evaluations. Whether the positive or
negative attitude associated with an object
is activated depends on associative strength,
recency of prior positive and negative expe-
riences, and whether the current context
is a better fit with the positive or negative
association. Reflecting on these attitudes
can lead them to become associated with a
tag, marking them as either valid or invalid.
The associated confidence indicator serves
as a signal to utilize or disregard the atti-
tude with minimal reflection required.
However, the MCM notes that validity tags
also vary in their associative strength to the
evaluation and in accessibility, meaning that
sometimes the tag may not be retrieved from
memory when the attitude object is. Often,
attitudes marked with an invalidity tag are
prior assessments that have been changed
but still may exert an influence (Petty et al.,
2006). The MCM concurs with the APE in
the assumption that the default response to
association activation is acceptance, the pre-
sumption of validity (see also Gilbert, 1991).
Therefore, it tends to matter more when an
invalidity tag is not retrieved, in which case a
rejected evaluation is likely to influence judg-
ment and behavior. The MCM distinguishes
between explicit ambivalence, in which both
positive and negative evaluations are associ-
ated with validity tags, and implicit ambiva-
lence, in which one association is validated
and the other is not. Explicit ambivalence is
perceived as subjectively conflicting when it
is experienced, but implicit ambivalence is
not, due to the conscious rejection of either
the positive or negative evaluation.
In summary, the MCM suggests that
the creation and retrieval of validity tags
serve a self- regulatory function by mark-
ing which associations are (in)appropriate
for guiding action. Unlike the other models
discussed, the MCM particularly may be
seen as clearly concerning the regulation of
attitudes per se. Validity tags concern the
associative network of an attitude and the
adaptive regulation of relatively general-
ized responses that can guide later action.
Though all models concern the regulation of
attitudes insofar as the process of evaluation
not only recruits but also changes attitudes
in memory, the MCM is particularly use-
ful for considering how attitude structure
changes over time.
The Iterative Reprocessing Model
and Dynamism
Our final contemporary perspective empha-
sizes dynamism in evaluation and regula-
tion. Dynamism refers to the notion that
mental representations are constantly evolv-
ing states in which partial representations
are cascading, competing, and interactive.
The dynamical approach to processing (e.g.,
Spivey, 2007) has been applied to multiple
social psychological concepts, including
categorization (Freeman, Ambady, Rule, &
Johnson, 2008), stereotyping (Freeman &
Ambady, 2009), and evaluation (Wojnowicz,
Ferguson, Dale, & Spivey, 2009). Wojnow-
icz and colleagues argue that explicit evalu-
ations are “merely the end result of a com-
plex, non- linear, time- dependent process of
multiple less- explicit attitudes competing
with one another over hundreds of millisec-
onds” (p. 1428). In early processing, mul-
260 SOCIAL ASPECTS
tiple patterns of evoked activity are partially
consistent with various evaluations. Over
time, “a continuous accrual of information
causes the distributed pattern to dynami-
cally ‘sharpen’ into a confident (selected)
interpretation, forcing other, partially acti-
vated, competing alternative[s] . . . to gradu-
ally die out” (p. 1429), an idea consistent
with the connectionist modeling notion of
an “attractor state” (e.g., Conrey & Smith,
2007). Selection is spurred by a cyclical pro-
cessing loop between higher- order integra-
tive brain regions and lower-level informa-
tional sources, in which the former enact
representation competition that promotes
or inhibits alternatives. Wojnowicz and col-
leagues (2009) drew on research on racial
attitudes (e.g., Fazio et al., 1995) suggesting
that negative partial evaluations of black tar-
gets would be common early in participants’
processing streams but would be subsumed
by more positive evaluations due to the moti-
vation to control prejudice. They anticipated
that the dynamic evolution of an evaluation
would be evident in subtle behavioral traces
as participants moved a cursor to associ-
ate “black” with “like” or “dislike.” Their
theory specifically predicted a curvature in
trajectory toward the eventually unchosen
alternative (“dislike”) when expressing lik-
ing for the target “black” that would not be
evident when the target was “white.” This is
what they observed.
The IR model (Cunningham & Zelazo,
2007) considers such dynamism central in
regulating evaluative responses. As noted,
the terms attitude and evaluation are often
used interchangeably, but we believe that
it is useful to refer to them as qualitatively
different. Whereas an attitude is a relatively
stable set of representations of a stimulus, an
evaluation reflects one’s current appraisal of
the stimulus. Evaluative processes transform
attitudes into evaluations. In other words, to
generate an evaluation, one uses preexisting
attitudes to retrieve useful affective informa-
tion about a stimulus, but the evaluation also
takes into consideration information about
the environment and context, as well as cur-
rent goals (see Figure 16.1). Furthermore,
because information is distributed, not all
aspects of the attitude are activated; as such,
only the currently active aspects of attitude
can shape an evaluation. Critically, because
active representations shape perception and
construal of a situation, and because cate-
gories and construals shape which attitude
aspects are foregrounded and made more
active, stimuli initiate an iterative sequence
of evaluative processes (the evaluative cycle)
through which the stimuli are interpreted
and reinterpreted in light of an increasingly
rich set of contextually meaningful repre-
sentations. Such a view blurs the traditional
distinctions found in dual- systems or dual-
process models of attitudes and attitude
regulation. Indeed, although evaluations
that are based on few iterations of the evalu-
ative cycle may be thought to be relatively
automatic or implicit, they can be shaped
by higher- order processes before they are
ever encountered (Cunningham, Van Bavel,
Arbuckle, Packer, & Waggoner, 2012; Todd
et al., 2012). Furthermore, although evalua-
tions that are based on additional iterations
may become more integrated, they do not
necessarily require or use new representa-
tions to generate ongoing evaluations.
Given the iterative nature of the model,
evaluations are proposed to be the dynamic
result of an integrated set of distributed pro-
cesses, each of which responds to and resolves
specific computational problems (see Cun-
ningham & Johnson, 2007). Evaluation is
an emergent property of multiple processes
that unfold over time. Critically, the model
proposes that a common set of processes is
consistently involved in generating current
evaluations that are shaped by both bottom-
up and top-down influences. Thus, evalua-
tive processes are part of an iterative cycle:
With every iteration, the current evaluation
of a stimulus is adjusted in light of addi-
tional contextual and motivational informa-
tion in order to create an updated evalua-
tion. Information is continually passed back
from relatively higher- order to relatively
lower-order processes, and the evaluation is
recalculated. This “reseeding” of informa-
tion allows for the foregrounding of relevant
(and backgrounding of irrelevant) attitude
representations and contextual information
in order to develop incorporate current goals
and standards, and allows for the regulation
of an evaluation response to come into line
with situational or motivational constraints.
At each iteration, the current evaluation
serves as input for ongoing evaluative pro-
cessing; as such, earlier evaluations are likely
to bias subsequent evaluative processing by
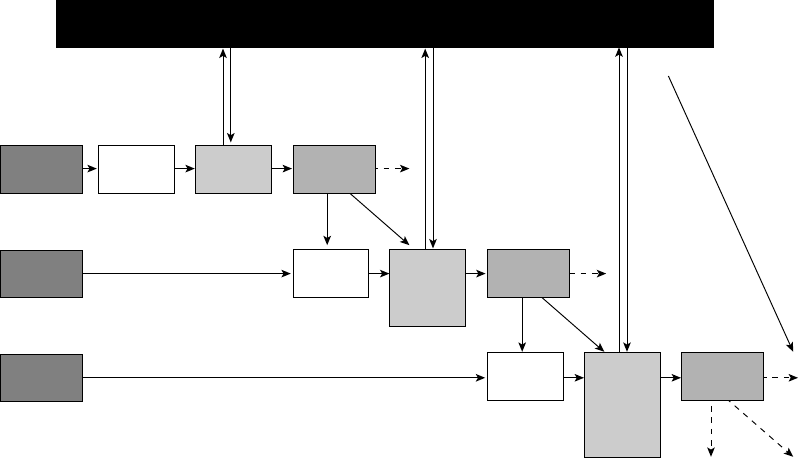
Attitudes and Emotion Regulation 261
influencing attention, information seeking,
stimulus construal, and so forth. Impor-
tantly, while conscious deliberation exerts
an influence on evaluative processing, infor-
mation about the valence and arousal value
of a stimulus continues to be represented in
subcortical structures.
To understand when people are more
likely to generate complex evaluations, the IR
model proposes that two competing motiva-
tional drives influence the extent of evalua-
tive processing. First, a drive to minimize the
discrepancy between one’s evaluation and
the hedonic environment (i.e., to minimize
error) increases reflective processing dur-
ing evaluation. Second, a drive to minimize
processing demands decreases in reflective
processing during evaluation. These oppos-
ing drives create a dynamic tension that can
help individuals to strike a delicate balance
between an initial “gut” response and evalu-
ations that are more nuanced but not com-
putationally overwhelming. The influence of
these competing motivations likely varies as
a function of situational demands, current
goals, and individual differences in process-
ing style.
Thus, unlike models that propose a stark
difference between automatic and controlled
processes, the IR model suggests a contin-
uum from relatively automatic to controlled
evaluative processes that can operate on a set
of representations. At the early stages of pro-
cessing, the strongest weights associated with
an attitude give rise to a specific pattern of
activation, and result in quick and automatic
evaluations. However, with more iterations
and the potential for reflective processing
in areas of the brain responsible for higher
processing (e.g., prefrontal cortex), evalua-
tions are shaped by a dynamic interaction of
several bottom- up and top-down processes.
This interaction allows for the foreground-
ing and backgrounding of particular pat-
terns of activation in accordance with cur-
rent contexts and goals. Evaluations based
on additional iterations are generally more
reflective. As individuals engage in reflec-
tive reprocessing, they are able to formulate
more complex, nuanced representations of
a stimulus (e.g., allowing a stimulus to be
understood in terms of multiple conflicting
dimensions of evaluation). This hierarchical
approach, which views reflection as a matter
FIGURE 16.1. The iterative reprocessing model. From Cunningham, Zelazo, Packer, and Van Bavel
(2007). Copyright 2007 by The Guilford Press. Reprinted by permission.
Evaluative
Processes
Evaluative
Processes
Evaluative
Processes
Stimulus
Construal
1
Stimulus
Construal
2
Stimulus
Construal
3
Evaluation
1
Evaluation
2
Evaluation
3
Stimulus
Stimulus
Stimulus
Attitude Representations
Iteration
T
Iteration
T+~200ms
Iteration
T+~400ms
Iteration
n
Time

262 SOCIAL ASPECTS
of degree, is consistent with contemporary
characterizations of prefrontal cortical func-
tion (Bunge & Zelazo, 2006).
But although it is possible to draw parallels
between standard dual- process models and
the IR model, the nature of the relationship
between what can be considered automatic
and controlled differs in important ways.
The IR model proposes that what is typically
considered more controlled or reflective pro-
cessing in dual- process models merely biases
which representations remain active and the
complexity of the evaluation that is possible
given this set of active representations. Thus,
the biasing of representations can come prior
to processing any given stimulus, allowing
for automatic regulation— the initial rep-
resentation, categorization, and evaluation
of an object can be modified at the earliest
levels of processing. Indeed, attention to one
category (race) or another (age) for stimuli
that can be multiply categorized leads to
greater affective priming to the focal cat-
egory (Gawronski, Cunningham, LeBel, &
Deutsch, 2010). That is, foregrounding one
category or the other changed the initial
evaluation as measured by affective priming.
Linking the Literatures on Attitudes,
Evaluation, and Emotion Regulation
In this chapter, we suggest that the pro-
cesses by which attitudes are transformed
into evaluations may be more dynamic that
previously considered. As such, the classical
distinctions between automatic/implicit and
controlled/explicit processes may need to
be modified to articulate the dynamic and
iterative nature of evaluative processes. In
doing so, many parallels with the literature
on affect and its regulation are noted. For
example, to the extent that evaluations are
constantly updated, and regulation (defined
as biasing representations) exists through-
out the process, this suggests that the ways
that we shape affect will be important for
the types of representations that are active,
and how we use them to generate appropri-
ate evaluations. For example, the processes
of appraisal and reappraisal will be essential
for determining the meaning of an object in
a situation, and determining its value. To
the extent that one is a successful dieter, by
reappraising a donut as an efficient calorie
delivery system rather than a tasty snack,
the nature of active representations will be
more negative, and a more negative evalu-
ation will be constructed. This process
is central to emotion regulation, because
changing the value of the goal state or the
experienced outcome is the most effective
means to feel better (e.g., “I didn’t actually
want that donut, because it is bad for me”).
Furthermore, attitudes and their transfor-
mation into information are essential for all
stages of emotion regulation, from situation
selection (one can use attitudes to determine
whether a situation is likely to be good or
bad) to attention deployment (attitudes
guide attention; Roskos- Ewoldsen & Fazio,
1992). Attitudes provide the expectations
for a stimulus, and this information is criti-
cal to guiding responses that lead to good
outcomes and more positive emotions.
Conclusion
We hope the reader will find conceptual
parallels within the models reviewed and
emotion regulation. Many of the automatic
influences of goals and standards on auto-
matic evaluation, and the means and eliciting
circumstances of effortful self- regulation of
attitudes and evaluation, are likely relevant
to the regulation of emotion. Unfortunately,
these literatures have as yet seen little inte-
gration despite their potential to inform one
another. For example, attitude theory has
often neglected the role of arousal, which
has been integral to the study of emotion.
We end with a final note on the applicability
of models of evaluation for emotion regula-
tion. Although typically studied in isolation
from one another, the processes involved in
attitudes greatly overlap with those involved
in emotion regulation. Indeed, as suggested
in this chapter, the critical question con-
cerning how one converts attitudes (stored
representations about the world) into cur-
rent evaluations (temporary affective experi-
ences that can be used to drive thoughts and
behavior) can be viewed fundamentally as a
regulatory process in which an evoked affec-
tive response is shaped to reflect the exigen-
cies of the moment.
This view goes beyond more traditional
notions of regulation, which typically involve

Attitudes and Emotion Regulation 263
the inhibition or alteration of an automatic
response, dependent upon conscious will
and ability. We argue that the translation of
representation (attitude) to experience (eval-
uation) involves regulation, as active repre-
sentations are shaped and reshaped across
multiple levels of organization. At lower lev-
els, the perceptual system helps to regulate
what stimuli to attend to and what stimuli to
ignore by biasing attention, while at higher
levels, the more reflective regulatory systems
make use of similar foregrounding and back-
grounding processes. As such, the ability to
regulate one’s emotions effectively has been
linked to the ways in which these lower- and
higher- order processes interact (Lee, Heller,
van Reekum, Nelson, & Davidson, 2012).
Critically, the ability to reinterpret or recon-
strue incoming information flexibly— or
cognitive flexibility (Scott, 1962)—is an
essential feature of these models and may
be vital for emotion regulation. Although
dynamic perspectives on attitudes are only
beginning to receive attention, it is our hope
that these perspectives will be influential in
guiding future research beyond the scope of
attitudes alone. After all, these models, at
their core, are models of cognitive process-
ing, and as such are relevant to and overlap
with many domains, emotion regulation
included.
Notes
1. This basic distinction between associative and
propositional processes is shared with Strack
and Deutsch’s (2004) reflexive– impulsive
model (RIM), which in turn drew from mul-
tiple earlier dual- process theories (see Smith
& DeCoster, 2000, for a review). However,
the APE model focuses on evaluation per
se, whereas the RIM is explicitly a model of
behavior.
2. It should be noted that some negations
are familiar (“no way”) and/or very easily
reversed (“not good”), such that their appro-
priate meaning may be encoded even when
cognitive resources are strained (e.g., Mayo,
Schul, & Burnstein, 2004), rendering ironic
effects unlikely in these cases.
3. However, its authors express skepticism that
a strong dual- systems approach is consistent
with emerging evidence from neuroscience.
References
Allport, G. W. (1935). Attitudes. In C. Murchi-
son (Ed.), Handbook of social psychology.
Worcester, MA: Clark University Press.
Barden, J., Maddux, W. W., Petty, R. E., &
Brewer, M. B. (2004). Contextual modera-
tion of racial bias: The impact of social roles
on controlled and automatically activated atti-
tudes. Journal of Personality and Social Psy-
chology, 87, 5–22.
Bargh, J. A. (1994). The four horsemen of auto-
maticity: Awareness, intention, efficiency, and
control in social cognition. In R. S. Wyer, Jr.
& T. K. Srull (Eds.), Handbook of social cog-
nition (Vol. 1, 2nd ed., pp. 1–40). Hillsdale,
NJ: Erlbaum.
Bargh, J. A. (1999). The cognitive monster: The
case against controllability of automatic ste-
reotyping effects. In S. Chaiken & Y. Trope
(Eds.), Dual process theories in social psy-
chology (pp. 361–382). New York: Guilford
Press.
Bunge, S. A., & Zelazo, P. D. (2006). A brain-
based account of the development of rule use
in childhood. Current Directions in Psycho-
logical Science, 15, 118–121.
Cacioppo, J. T., Gardner, W. L., & Berntson, G.
G. (1997). Beyond bipolar conceptualizations
and measures: The case of attitudes and evalu-
ative space. Personality and Social Psychology
Review, 1, 3–25.
Carver, C. S., & Scheier, M. F. (1982). Control
theory: A useful conceptual framework for
personality— Social, clinical, and health psy-
chology. Psychological Bulletin, 92, 111–135.
Conrey, F. R., & Smith, E. R. (2007). Attitude
representation: Attitudes as patterns in a dis-
tributed, connectionist representational sys-
tem. Social Cognition, 25, 718–735.
Crandall, C. S., Eshleman, A., & O’Brien, L.
(2002). Social norms and the expression and
suppression of prejudice: The struggle for
internalization. Journal of Personality and
Social Psychology, 82, 359–378.
Crone, E. A., & Van der Molen, M. W. (2004).
Developmental changes in real-life decision-
making: Performance on a gambling task pre-
viously shown to depend on the ventromedial
prefrontal cortex. Developmental Neuropsy-
chology, 25, 251–279.
Cunningham, W. A., & Johnson, M. K. (2007).
Attitudes and evaluation: Toward a component
process framework. In E. Harmon-Jones & P.
264 SOCIAL ASPECTS
Winkielman (Eds.), Social neuroscience: Inte-
grating biological and psychological explana-
tions of social behavior (pp. 227–245). New
York: Guilford Press.
Cunningham, W. A., Johnson, M. K., Raye, C.
L., Gatenby, J. C., Gore, J. C., & Banaji, M.
R. (2004). Separable neural components in the
processing of Black and White faces. Psycho-
logical Science, 15, 806–813.
Cunningham, W. A., Raye, C. L., & Johnson,
M. K. (2004). Implicit and explicit evalua-
tion: fMRI correlates of valence, emotional
intensity, and control in the processing of atti-
tudes. Journal of Cognitive Neuroscience, 16,
1717–1729.
Cunningham, W. A., Van Bavel, J. J., Arbuckle,
N. L., Packer, D. J., & Waggoner, A. S. (2012).
Rapid social perception is flexible: Approach
and avoidance motivational states shape P100
responses to other-race faces. Frontiers in
Human Neuroscience, 6, 140.
Cunningham, W. A., & Zelazo, P. D. (2007).
Attitudes and evaluations: A social cognitive
neuroscience perspective. Trends in Cognitive
Sciences, 11, 97–104.
Cunningham, W. A., Zelazo, P. D., Packer, D.
J., & Van Bavel, J. J. (2007). The Iterative
Reprocessing Model: A multilevel framework
for attitudes and evaluation. Social Cognition,
25, 736–760.
De Houwer, J., & Moors, A. (2007). How to
define and examine the implicitness of implicit
measures. In B. Wittenbrink & N. Schwarz
(Eds.), Implicit measures of attitudes: Proce-
dures and controversies (pp. 179–194). New
York: Guilford Press.
De Houwer, J., Thomas, S., & Baeyens, F. (2001).
Associative learning of likes and dislikes: A
review of 25 years of research on human eval-
uative conditioning. Psychological Bulletin,
127, 853–869.
Deutsch, R., Gawronski, B., & Strack, F. (2006).
At the boundaries of automaticity: Negation
as reflective operation. Journal of Personality
and Social Psychology, 91, 385–405.
Devine, P. G. (1989). Stereotypes and prejudice:
Their automatic and controlled components.
Journal of Personality and Social Psychology,
56, 5–18.
Dovidio, J. F., Kawakami, K., Johnson, C., John-
son, B., & Howard, A. (1997). On the nature
of prejudice: Automatic and controlled pro-
cesses. Journal of Experimental Social Psy-
chology, 33, 510–540.
Eagly, A. H., & Chaiken, S. (1993). The psychol-
ogy of attitudes. Fort Worth, TX: Harcourt
Brace Jovanovich.
Eiser, J. R., Fazio, R. H., Stafford, T., & Prescott,
T. J. (2003). Connectionist simulation of atti-
tude learning: Asymmetries in the acquisition
of positive and negative evaluations. Personal-
ity and Social Psychology Bulletin, 29, 1221–
1235.
Fazio, R. H. (1990). Multiple processes by which
attitudes guide behavior: The MODE model
as an integrative framework. In M. P. Zanna
(Ed.), Advances in experimental social psy-
chology (Vol. 23, pp. 75–109). New York:
Academic Press.
Fazio, R. H. (2001). On the automatic activation
of associated evaluations: An overview. Cog-
nition and Emotion, 15, 115–141.
Fazio, R. H. (2007). Attitudes as object-
evaluation associations of varying strength.
Social Cognition, 25, 603– 637.
Fazio, R. H., Jackson, J. R., Dunton, B. C., &
Williams, C. J. (1995). Variability in auto-
matic activation as an unobtrusive measure
of racial attitudes: A bona fide pipeline? Jour-
nal of Personality and Social Psychology, 69,
1013–1027.
Festinger, L. (1957). A theory of cognitive dis-
sonance. Evanston, IL: Row Peterson.
Freeman, J. B., & Ambady, N. (2009). Motions
of the hand expose the partial and parallel
activation of stereotypes. Psychological Sci-
ence, 20, 1183–1188.
Freeman, J. B., Ambady, N., Rule, N. O., &
Johnson, K. L. (2008). Will a category cue
attract you?: Motor output reveals dynamic
competition across person construal. Journal
of Experimental Psychology: General, 137,
673–690.
Fujita, K. (2011). On conceptualizing self-
control as more than the effortful inhibition of
impulses. Personality and Social Psychology
Review, 15, 352–366.
Gawronski, B., & Bodenhausen, G. V. (2006).
Associative and propositional processes in
evaluation: An integrative review of implicit
and explicit attitude change. Psychological
Bulletin, 132, 692–731.
Gawronski, B., & Bodenhausen, G. V. (2011).
The associative– propositional evaluation
model: Theory, evidence, and open ques-
tions. Advances in Experimental Social Psy-
chology, 44, 59–127.
Gawronski, B., Cunningham, W. A., Lebel, E.
P., & Deutsch, R. (2010). Attentional influ-
ences on affective priming: Does categoriza-
Attitudes and Emotion Regulation 265
tion influence spontaneous evaluations of
multiply categorizable objects? Cognition, 24,
1008–1026.
Gawronski, B., Deutsch, R., Mbirkou, S., Seibt,
B., & Strack, F. (2008). When “just say no”
is not enough: Affirmation versus negation
training and the reduction of automatic ste-
reotype activation. Journal of Experimental
Social Psychology, 44, 370–377.
Gawronski, B., & LeBel, E. P. (2008). Under-
standing patterns of attitude change: When
implicit measures show change, but explicit
measures do not. Journal of Experimental
Social Psychology, 44, 1355–1361.
Gawronski, B., & Strack, F. (2004). On the
propositional nature of cognitive consistency:
Dissonance changes explicit, but not implicit
attitudes. Journal of Experimental Social Psy-
chology, 40, 535–542.
Gilbert, D. T. (1991). How mental systems
believe. American Psychologist, 46, 107–119.
Greenwald, A. G., & Banaji, M. R. (1995).
Implicit social cognition: Attitudes, self-
esteem, and stereotypes. Psychological
Review, 102, 4 –27.
Greenwald, A. G., McGhee, D. E., & Schwartz,
J. L. (1998). Measuring individual differences
in implicit cognition: The Implicit Association
Test. Journal of Personality and Social Psy-
chology, 74, 1464–1480.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J., & Barrett, L. F. (2011). Emotion
generation and emotion regulation: One or
two depends on your point of view. Emotion
Review, 3, 8–16.
Herbert, C., Deutsch, R., Sütterlin, S., Kübler,
A., & Pauli, P. (2011). Negation as a means
for emotion regulation?: Startle reflex modu-
lation during processing of negated emotional
words. Cognitive, Affective, and Behavioral
Neuroscience, 11, 199–206.
Hofmann, W., Gawronski, B., Gschwendner, T.,
Le, H., & Schmitt, M. (2005). A meta- analysis
on the correlation between the Implicit Asso-
ciation Test and explicit self- report measures.
Personality and Social Psychology Bulletin,
31, 1369–1385.
Hovland, C. I., Janis, I. L., & Kelley, H. H.
(1953). Communication and persuasion: Psy-
chological studies of opinion change. New
Haven, CT: Yale University Press.
Jones, C. R., & Fazio, R. H. (2010). Person cat-
egorization and automatic racial stereotyping
effects on weapon identification. Personality
and Social Psychology Bulletin, 36, 1073–
1085.
Jones, C. R., Olson, M. A., & Fazio, R. H.
(2010). Evaluative conditioning: The “how”
question. In M. P. Zanna & J. M. Olson
(Eds.), Advances in experimental social psy-
chology (Vol. 43, pp. 205–255). New York:
Academic Press.
Karpinski, A., & Hilton, J. L. (2001). Attitudes
and the Implicit Association Test. Journal of
Personality and Social Psychology, 81, 774–
788.
Lee, H., Heller, A. S., van Reekum, C. M., Nel-
son, B., & Davidson, R. J. (2012). Amygdala–
prefrontal coupling underlies individual differ-
ences in emotion regulation. NeuroImage, 62,
1575–1581.
Lieberman, M. D. (2007). Social cognitive neu-
roscience: A review of core processes. Annual
Review of Psychology, 58, 259–289.
Lowery, B. S., Hardin, C. D., & Sinclair, S.
(2001). Social influence effects on automatic
racial prejudice. Journal of Personality and
Social Psychology, 81, 842–855.
Mauss, I. B., Evers, C., Wilhelm, F. H., & Gross,
J. J. (2006). How to bite your tongue without
blowing your top: Implicit evaluation of emo-
tion regulation predicts affective responding
to anger provocation. Personality and Social
Psychology Bulletin, 32, 589–602.
Mayo, R., Schul, Y., & Burnstein, E. (2004). “I
am not guilty” vs. “I am innocent”: Successful
negation may depend on the schema used for
its encoding. Journal of Experimental Social
Psychology, 40, 433–449.
McConnell, A. R., Dunn, E. W., Austin, S. N., &
Rawn, C. D. (2011). Blind spots in the search
for happiness: Implicit attitudes and nonverbal
leakage predict affective forecasting errors.
Journal of Experimental Social Psychology,
47, 628–634.
McConnell, A. R., & Rydell, R. J. (in press). The
systems of evaluation model: A dual- systems
approach to attitudes. In J. Sherman, B. Gaw-
ronski, & Y. Trope (Eds.), Dual process theories
of the social mind. New York: Guilford Press.
Moskowitz, G. B., Gollwitzer, P. M., Wasel, W.,
& Schaal, B. (1999). Preconscious control of
stereotype activation through chronic egali-
tarian goals. Journal of Personality and Social
Psychology, 77, 167–184.
Olson, M. A., & Fazio, R. H. (2009). Implicit
and explicit measures of attitudes: The per-
spective of the MODE model. In R. E. Petty,
266 SOCIAL ASPECTS
R. H. Fazio, & P. Briñol (Eds.), Insights from
the new implicit measures (pp. 19–63). New
York: Psychology Press.
Olson, M. A., Fazio, R. H., & Hermann, A. D.
(2007). Reporting tendencies underlie discrep-
ancies between implicit and explicit measures
of self- esteem. Psychological Science, 18, 287–
291.
Payne, B. K., Burkley, M. A., & Stokes, M. B.
(2008). Why do implicit and explicit attitude
tests diverge?: The role of structural fit. Jour-
nal of Personality and Social Psychology, 94,
16–31.
Petty, R. E. (2006). A metacognitive model of
attitudes. Journal of Consumer Research, 33,
22–24.
Petty, R. E., & Briñol, P. (2006). A metacognitive
approach to “implicit” and “explicit” evalua-
tions: Comment on Gawronski and Boden-
hausen (2006). Psychological Bulletin, 132,
740–744.
Petty, R. E., Briñol, P., & DeMarree, K. G.
(2007). The Meta- Cognitive Model (MCM) of
attitudes: Implications for attitude measure-
ment, change, and strength. Social Cognition,
25, 657–686.
Petty, R. E., Tormala, Z. L., Briñol, P., & Jarvis,
W. B. G. (2006). Implicit ambivalence from
attitude change: An exploration of the PAST
model. Journal of Personality and Social Psy-
chology, 90, 21– 41.
Roskos- Ewoldsen, D. R., & Fazio, R. H. (1992).
On the orienting value of attitudes: Attitude
accessibility as a determinant of an object’s
attraction of visual attention. Journal of Per-
sonality and Social Psychology, 63, 198–211.
Russell, J. A. (2003). Core affect and the psycho-
logical construction of emotion. Psychological
Review, 110, 145–172.
Rydell, R. J., & Durso, G. R. O. (2012). Can I
borrow a feeling?: Spillover of negative arousal
from inconsistent information during attitude
formation diminishes perceptions of well-
being. Journal of Experimental Social Psy-
chology, 48, 575–578.
Rydell, R. J., & McConnell, A. R. (2006). Under-
standing implicit and explicit attitude change:
A systems of reasoning analysis. Journal of
Personality and Social Psychology, 91, 995–
1008.
Rydell, R. J., McConnell, A. R., & Mackie, D.
(2008). Consequences of discrepant explicit
and implicit attitudes: Cognitive dissonance
and increased information processing. Jour-
nal of Experimental Social Psychology, 44,
1526–1532.
Rydell, R. J., McConnell, A. R., Mackie, D. M.,
& Strain, L. M. (2006). Of two minds: Form-
ing and changing valence- inconsistent implicit
and explicit attitudes. Psychological Science,
17, 954–959.
Sassenberg, K., & Moskowitz, G. B. (2005).
Don’t stereotype, think different!: Overcom-
ing automatic stereotype activation by mind-
set priming. Journal of Experimental Social
Psychology, 41, 506–514.
Scott, W. A. (1962). Cognitive complexity and
cognitive flexibility. Sociometry, 25, 405–414.
Schwarz, N., & Bohner, G. (2001). The construc-
tion of attitudes. In A. Tesser & N. Schwarz
(Eds.), Blackwell handbook of social psychol-
ogy: Intraindividual processes (pp. 436–457).
Oxford, UK: Blackwell.
Sloman, S. A. (1996). The empirical case for two
systems of reasoning. Psychological Bulletin,
119, 3–22.
Smith, E. R., & DeCoster, J. (2000). Dual pro-
cess models in social and cognitive psychol-
ogy: Conceptual integration and links to
underlying memory systems. Personality and
Social Psychology Review, 4, 108–131.
Spivey, M. J. (2007). The continuity of mind.
Oxford, UK: Oxford University Press.
Strack, F., & Deutsch, R. (2004). Reflective and
impulsive determinants of social behavior.
Personality and Social Psychology Review, 8,
220 –247.
Todd, R. M., Cunningham, W. A., Anderson,
A. K., & Thompson, E. (2012). Affect- biased
attention as emotion regulation. Trends in
Cognitive Sciences, 16, 365–372.
Watson, D., & Tellegen, A. (1985). Toward a
consensual structure of mood. Psychological
Bulletin, 98, 219–235.
Wilson, T. D., Lindsey, S., & Schooler, T. Y.
(2000). A model of dual attitudes. Psychologi-
cal Review, 107, 101–126.
Wojnowicz, M. T., Ferguson, M. J., Dale, R., &
Spivey, M. J. (2009). The self- organization of
explicit attitudes. Psychological Science, 20,
1428–1435.
Zanna, M. P., & Rempel, J. K. (1988). Attitudes:
A new look at an old concept. In D. Bar-Tal
& A. Kruglanski (Eds.), The social psychol-
ogy of knowledge (pp. 315–334). New York:
Cambridge University Press.

267
Whereas research on emotion regulation in
individuals has been extremely useful, there
are numerous advantages to studying emo-
tion regulation in couples. Couples afford
high ecological validity, provide opportuni-
ties to view the rich panoply of emotion reg-
ulatory strategies, and are ideal for study-
ing the dynamics of emotion regulation as
partners engage in a rich choreography of
emotional expression and regulation that
unfolds in complex ways over time.
In this chapter, we consider several
important aspects of emotion regulation in
couples, discussing its social nature, defin-
ing qualities, consequences, development,
and assessment. We end by considering
the future of this research area, including
unmet needs, unfilled gaps, and unanswered
research questions. Our aim in this review
of the relevant literatures is not intended to
be exhaustive but rather to highlight key
studies that illustrate important issues. We
also draw anecdotally from our experiences
with couples who seek therapy for troubled
relationships (Levenson, Cowan, & Cowan,
2010). These couples almost always have
problems with emotion regulation.
The Social Nature
of Emotion Regulation
Research on emotion regulation in couples
should be booming. After all, most human
emotions occur in decidedly social situations
(Campos, Walle, Dahl, & Main, 2011).
Other people are deeply entwined in the
fabric of our emotional lives. They are the
proximal stimulus for most of our emotions
and the recipients of most of the emotions
we express. When others respond to our
emotions with their own emotional reac-
tions, they provide the fuel needed to sustain
the chains of exchanged emotions that are so
emblematic of our social lives.
With so much of human emotion being
socially situated, it is reasonable to expect
that most emotion regulation would be simi-
larly social. In fact, it has been observed that
up to 98% of emotion regulation episodes
CHAPTER 17
Emotion Regulation in Couples
Robert W. Levenson
Claudia M. Haase
Lian Bloch
Sarah R. Holley
Benjamin H. Seider

268 SOCIAL ASPECTS
may take place in social contexts (Gross,
Richards, & John, 2006). Viewed from a
functionalist perspective, emotion regula-
tion is a critical element for promoting social
cohesion. If we were to discharge our emo-
tions upon others, full-bore, undiluted, and
absent the moderating influence of emotion
regulation, the resultant affective tsunami
would have dire consequences for us and for
our social groupings.
Given its profoundly social nature, we
might expect that studies of emotion regula-
tion would almost always be conducted in
social situations. However, this could not
be further from the truth. In a recent review
of studies since 2001 (Campos et al., 2011),
less than 12% of the studies assessed emo-
tion regulation in the presence of another
person (and this is an optimistic estimate
that includes studies using both imagined
and real others). Clearly, studies of emotion
regulation in individuals have advantages
over those conducted with couples. They are
easier to administer and more amenable to
tight experimental control. However, they
are not optimal for studying the dynamic,
interpersonal aspects of emotion regulation.
Although most theorists would agree that
emotion regulation is profoundly social in
nature and is a fundamental area of concern
in close relationships, the literature on emo-
tion regulation in couples is still surprisingly
immature, with many gaps and unanswered
questions.
Before moving forward with our consid-
eration of emotion regulation in couples in
intimate relationships we should note that
they are only one of many kinds of dyads
for whom emotion regulation is critical
(e.g., new acquaintances, friends, enemies).
Moreover, emotion regulation in social con-
texts scales up from dyads through families,
groups, communities, and nations. Emotion
regulation in these larger social groupings is
fascinating and instructive (e.g., communi-
ties and nations coping with fear, anger, and
grief following collective losses, seen quite
famously in the U.S. and world responses to
the September 11, 2001, terrorist attacks).
So, why focus on couples in intimate rela-
tionships? Dyads are the smallest social unit,
thus providing an excellent starting point
for building a science of socially embedded
emotion regulation. Intimate relationships
assume a central role in the lives of most
individuals. For example, according to the
2009 census, 96% of all Americans over age
65 have been married at least once.
Defining Qualities
There are many ways to define emotion reg-
ulation. Because most theory and research
in the field of emotion regulation have
focused on individuals, popular definitions
of emotion regulation reflect this bias. For
example, Gross (1998b, p. 275) defines emo-
tion regulation as “the processes by which
individuals [emphasis added] influence
which emotions they have, when they have
them, and how they experience and express
these emotions.” Of course, a definition of
this sort could be altered so that “individu-
als” becomes “individuals and couples.”
But does this cover all bases? The answer to
this question depends on whether we think
that emotion regulation in couples can be
fully captured by summing the regulatory
activities of the two individuals involved, or
whether there are emergent qualities of emo-
tion regulation that are found only in the
couples context. Clearly there are aspects
of emotion regulation that are common to
both individuals and couples. For example,
emotion regulation in both can be explicit
(i.e., effortful) or implicit (i.e., automatic)
(Gyurak, Gross, & Etkin, 2011), and suc-
cessful or unsuccessful (Gross & Levenson,
1993). And in both contexts it can be diffi-
cult to determine where emotional reactivity
ends and emotion regulation begins (Gross,
Sheppes, & Urry, 2011). Nonetheless, as we
hope the following sections illustrate, emo-
tion regulation in couples has a number of
characteristics that are quite different from
those found in individuals.
Dynamic and Iterative
Consider two emotion regulatory scenarios.
In the first, a recent PhD recipient is prepar-
ing to give an important job talk and is in
the throes of a bout of stage fright. Fear is
the dominant emotion welling up. He is con-
cerned that the intensity of his fear, should
it reach sufficiently high levels, will compro-
mise the quality of the talk. The standard-
issue emotion regulatory toolbox offers a
number of strategies, including altering the
Emotion Regulation in Couples 269
context, reappraising the situation, or will-
fully controlling aspects of the emotional
response. The speaker chooses one (or
more) of these strategies, applies them in the
moment, successfully down- regulates the
fear, gives an excellent talk, and averts the
crisis.
Now, consider a second scenario involv-
ing emotion regulation in couples. A mar-
ried couple is talking about the husband’s
pending surgery, and he is clearly experienc-
ing high levels of fear. In that moment he
wants calming support from his wife. But
she is reacting with a great deal of sadness,
talking about her concerns that he might die
and that their children would go through
life without a father. Getting from this start-
ing point to a place where both husband
and wife are feeling less distressed is not as
straightforward as the situation in the first
scenario, in large part because there are two
actors involved. Both individuals have to
react to their own and their partner’s emo-
tional state, the impact of each partner’s
regulatory attempts (some well- chosen, oth-
ers misguided), and the unfolding sequence
of action and reaction that will occur over
time as the couple works toward achieving
a state that is more emotionally optimal for
both partners.
The second scenario illustrates some of
the ways that emotion regulation in couples
differs from emotion regulation in indi-
viduals. In regulation in couples there are
always two actors and reactors, each with
his or her own emotional motivations, goals,
strengths, blind spots, and hot buttons. Cou-
ples often find themselves in a complex emo-
tional landscape that changes continuously
as partners express and regulate their own
emotions, respond to each other’s emotions
and regulatory attempts, and try to regulate
each other’s emotions. This extremely fluid
situation, replete with highly dynamic and
iterative sequences of emotion, creates an
extremely challenging, complex landscape
for emotion regulation, one that is quite dif-
ferent from that faced by individuals.
Bidirectional
Although most discussions of emotion reg-
ulation in individuals allow for both up-
regulation and down- regulation of emotion,
the emphasis most often seems to be on
reducing emotional responses. In the canon-
ical examples, individuals seek to reduce
their negative emotions to avoid harmful
consequences for self and others. Consis-
tent with this, our own early experimental
studies of emotion regulation focused exclu-
sively on the consequences of having sub-
jects reduce their behavioral responses to
emotion- inducing films (Gross & Levenson,
1993). It was only later that we began to con-
sider up- regulation of emotion in these kinds
of studies as well (Kunzmann, Kupperbusch,
& Levenson, 2005). In recent years, espe-
cially as researchers have explored the neu-
ral bases of emotion regulation using patient
and neuroimaging models, studies including
both up- regulation and down- regulation
have become more common (e.g., Gyurak,
Goodkind, Kramer, Miller, & Levenson,
2012; Ochsner et al., 2004). However, it is
still the case that when studying emotion
regulation in individuals, the primary focus
is on reducing emotion.
In the realm of emotion regulation in
couples, this emphasis on down- regulation
is less appropriate. There are many times
when couples need to amplify the magnitude
of emotion so that it emerges more clearly
against the backdrop of other aspects of
their interaction. This amplification takes
many forms, ranging from the exaggerated
tonality of emotional speech patterns used
by mothers when they communicate with
and soothe their infants (Fernald, 1991) to
the stylized and exaggerated expressions of
love and affection that are so important in
courtship rituals. Interestingly, among those
who seek couple therapy, it is extremely
common for one partner (usually the woman
in heterosexual couples) to desire the other
partner (usually the man in these couples) to
up- regulate emotion (i.e., expressing emo-
tion more often and more clearly).
Bivalent
The prototypical examples of emotion reg-
ulation in individuals all focus on negative
emotions. This is seen in common parlance
(e.g., “Don’t let them see you sweat” [fear],
“Don’t let it get to you” [anger], “Grown
men don’t cry” [sadness]); in many ado-
lescent rituals in which a calm demeanor
is maintained while viewing, touching, or
ingesting extremely gross things (disgust);
270 SOCIAL ASPECTS
and even in therapeutic contexts (where
cathartic release is often sought for pent up
anger, fear, and sadness). Consistent with
this emphasis, most laboratory research on
emotion regulation has focused on control-
ling negative emotions. Such work is facili-
tated by eliciting negative emotions such as
disgust readily and powerfully, using static
images (Lang, Greenwald, & Bradley, 1988)
and films (Gross & Levenson, 1995). In addi-
tion, although studies comparing the regula-
tion of negative and positive emotions have
been rare, these comparisons have often not
produced dramatic differences (e.g., Gross
& Levenson, 1997).
In couples, however, the regulation of
positive emotion is at least as important
as the regulation of negative emotion. Up-
regulating positive emotion is critical for
building and maintaining intimate relation-
ships throughout the lifespan. This is seen
in parent– infant and parent– child relation-
ships, and continues with childhood friend-
ships, early romantic relationships, mate
selection, and long-term committed rela-
tionships (Carstensen, Graff, Levenson, &
Gottman, 1996). Similarly, down- regulating
positive emotion also plays an important role
for couples. For example, failure to down-
regulate amusement in response to a part-
ner’s failures and insecurities can be critical.
In these contexts, laughing, aggressive teas-
ing, and unrelenting, unsupportive humor
can be experienced as cruel and demeaning,
thus serving to undermine relationship qual-
ity (see Martin, 2007).
Coregulatory
Most of us find managing our own emotions
sufficiently challenging to occupy signifi-
cant segments of our waking (and sleeping)
hours. This challenge increases dramatically
when we take on the additional responsibil-
ity of attempting to manage the emotions
of another person. Whether the interaction
partner is an infant, a friend, a romantic love
interest, or a partner in a long-term relation-
ship, moving the focus of emotion regula-
tion from “my emotions” to “your and our
emotions” takes us into new and complex
realms. A recurrent theme in studies of opti-
mal performance is that individuals function
optimally when they are somewhat, but not
overly, aroused (Yerkes & Dodson, 1908).
We expect that the same is true of couples.
Maintaining an optimal level of emotional
arousal for couples, however, requires moni-
toring and regulating the emotional state
of both partners. The dynamic and itera-
tive nature of emotion exchanges in couples
means that the level of emotional arousal is
constantly changing. Thus, the maintenance
of an optimal state requires continuous
monitoring of arousal levels and continuous
adjustment of regulatory efforts. This situ-
ation has caused us on multiple occasions
to observe that a good marriage requires a
good thermostat (a role most often assumed
by wives in heterosexual couples; Gottman
& Levenson, 1988).
Further complicating matters, in cou-
ples, one partner’s regulatory efforts often
become potent emotional stimuli for the
other partner. Consider this example: A
husband has been trying to soothe his wife’s
anger over a canceled vacation, hoping that
when she is calmed down they will be able
to discuss alternative plans. At this point in
the interaction, the wife is overly aroused
and the husband is relatively calm. The wife
says, “I hate it when you try to manage me.
It’s insulting and belittling. You’re just so
incredibly selfish; if you really loved me, you
wouldn’t do this to me.” Hearing this, the
husband feels unjustly judged and injured.
His anger starts welling up and he becomes
quite defensive. Suddenly, they are both
overly aroused and find themselves casting
hurtful aspersions about each other’s char-
acter defects. Any possibility of having a
constructive discussion about vacation plans
will soon be placed on indefinite hold.
Coregulation in couples would be chal-
lenging enough if both partners always had
the same regulatory goals (e.g., both want-
ing to feel less aroused and more calm, or
both wanting to intensify feelings of pas-
sionate love). However, the emotional
stars are not always so well- aligned. The
“demand– withdraw” pattern, commonly
found in both opposite sex (Christensen,
1987) and same-sex (Holley, Sturm, &
Levenson, 2010) couples, provides a good
example. A couple wants to talk effectively
about a significant relationship issue. How-
ever, one partner (typically the one who
wants change) becomes quite aroused and
engages energetically in complaining and
criticizing the other partner. The criticized

Emotion Regulation in Couples 271
partner (typically the one who wants to
maintain the status quo) increasingly tunes
out and withdraws emotionally from the
interaction, thus achieving some level of
calm. For this couple, the coregulatory chal-
lenge is to calm the overaroused, demanding
partner and at the same time increase the
emotional involvement of the underaroused,
withdrawing partner. However, this must
be done without causing either partner to
overshoot the desired emotional endpoint
(no easy feat). Coregulation of emotion in
couples regularly introduces these kinds of
complexities, which are simply not found in
individual emotion regulation.
Consequences
It is easy to make a case for the importance of
emotion regulation in the lives of individuals.
Greater use of specific emotion regulation
strategies, as measured by self- and other-
reports, has been found to predict higher
levels of well-being, mental health, physical
health, relationship quality, and social func-
tioning, and lower levels of problem behav-
ior (Aldao, Nolen- Hoeksema, & Schweizer,
2010; Caspi, Henry, McGee, Moffitt, &
Silva, 1995; Gross & John, 2003; John &
Gross, 2004; Lopes, Salovey, Côté, Beers,
& Petty, 2005; Nelis et al., 2011). Although
studies of these associations using labora-
tory assessments of emotion regulation (as
opposed to self- and other- reports) are still
rare, we recently found that greater ability
to down- regulate and up- regulate emotional
response (as assessed using well- established
laboratory procedures) was associated with
greater well-being and higher income (Côté,
Gyurak, & Levenson, 2010). If emotion
regulation is broadened to include delay of
gratification, another laboratory- based par-
adigm, research strongly indicates that high
levels of this ability early in preschool years
are associated with a host of positive out-
comes, including greater cognitive and aca-
demic competence and ability to cope with
frustration later in life (Mischel et al., 2011).
Because of a relative dearth of studies
that have directly measured emotion regu-
lation in couples and its consequences, the
case for the importance of emotion regula-
tion in couples must be based on collateral
literatures. One such literature, concerned
with marriage and other committed rela-
tionships, has produced a number of find-
ings that suggest greater ability to regulate
emotion in couples is associated with posi-
tive outcomes. For example, questionnaire
studies have found that couples who report
less frequent use of “control or contain-
ment” (which is similar to suppression) of
negative emotion have higher marital satis-
faction (Feeney, 1999). In the literature on
intimate partner violence, inability to regu-
late negative emotion has been associated
with increased likelihood of partner abuse
(McNulty & Hellmuth, 2008).
A paradigm for studying couples’ interac-
tion developed by Levenson and Gottman
(1983) has been endorsed as an exemplar
for how emotion regulation can be studied
in social contexts (Campos et al., 2011). In
this paradigm, couples (usually married het-
erosexual couples, but also same-sex and
dating couples) come to the laboratory and
engage in a series of unrehearsed 15-minute
conversations on relationship topics (e.g.,
events of the day, a problem area, a pleasant
topic). During these conversations, behavior
is videotaped for subsequent coding of emo-
tional behavior by trained observers, and
in both partners a number of physiological
measures relevant to emotional responding
are monitored continuously. Later, partners
view the videotapes of their conversations
and use a rating dial to provide continu-
ous ratings of the valence of their emotional
experience during the interactions (Gott-
man & Levenson, 1985). These streams of
continuous multimethod data (self- report,
behavior, physiology) can be used to derive
measures of emotion reactivity and emotion
regulation in the individual partners and in
the dyad.
A number of findings from these stud-
ies seem highly relevant when considering
the consequences of emotion regulation
for couples. In this regard, the discussions
about marital problems (which can occa-
sion intense negative emotions and heroic
efforts at emotion regulation) have been
particularly informative, with links found
between measures of emotion regulation (in
subjective experience, behavior, and physiol-
ogy) and important consequences in several
domains.
In terms of subjective emotional experi-
ence, low levels of both negative emotion

272 SOCIAL ASPECTS
and negative emotion reciprocity (i.e., nega-
tive emotional experience by one partner
followed by negative emotional experience
by the other partner) have been associated
with higher levels of marital satisfaction
both concurrently and over time (Leven-
son & Gottman, 1983, 1985). In terms of
emotional behavior, husbands’ inability to
deescalate negative emotion during marital
conflict predicted less marital stability over
time (Gottman, Coan, Carrere, & Swanson,
1998). In a similar vein, “regulated” cou-
ples, operationalized as those who produced
an increasingly high ratio of positive to neg-
ative emotional behaviors over the course
of a 15-minute conflictive interaction, had
higher levels of marital satisfaction, lower
risk for marital dissolution, and better health
measured over a 4-year period (Gottman &
Levenson, 1992). Finally, less escalation of
negative emotional behavior was associated
with higher marital satisfaction in couples in
long-term marriages (Carstensen, Gottman,
& Levenson, 1995).
The continuous measures of peripheral
physiological activity have been particularly
interesting, in part because autonomic ner-
vous system responses are very difficult to
control voluntarily (e.g., Levenson, 1976)
and because they can provide a ready metric
of changing levels of arousal in the couple.
Using these measures, low levels of both
physiological arousal and physiological
linkage (synchrony between partners’ physi-
ology) were associated with higher marital
satisfaction (Levenson & Gottman, 1983,
1985).
Couple Therapy
Not surprisingly, given its critical role in
couple relationships and profound down-
stream consequences, issues with emotion
regulation frequently assume center stage
when couples seek treatment for relationship
problems. Although the specifics differ from
couple to couple, distressed couples almost
always struggle with either down- regulating
negative emotion (e.g., issues involving jeal-
ousy and disagreements over things such
as household duties, relatives, child rear-
ing, and finances) or up- regulating positive
emotions (e.g., issues involving poor and
infrequent communication, not doing things
together, loss of sexual interest and intimacy,
coldness and lack of empathy, and absence
of joy) or both. Historically, couples thera-
pies have focused more on nonemotional
aspects of these problems (e.g., communi-
cation deficits, individual psychopathology,
attachment histories, poor family- of- origin
relationship models), but emotion and emo-
tion regulation are increasingly becoming
important foci in many forms of couple ther-
apy (Gottman & Gottman, 2008; Johnson,
1996; Wile, 2002).
Development
Viewed from a developmental perspective,
several key dyadic relationships assume
prominence at different stages of the lifes-
pan and serve as crucibles for the emergence
and refinement of regulatory skills. Here we
focus on three of these dyads: parents and
infants, early romantic relationships, and
late-life couples.
Parent–Infant Dyads
Parent– infant dyads, and mother– infant
dyads in particular, invest significant efforts
in emotion regulation. The initial focus is
on reducing negative emotion (managing
the infant’s distress), but this quickly engen-
ders efforts to increase positive emotion
(engaging in activities that amuse, distract,
and calm). In this stage of life, infants can
become overwhelmed by their negative emo-
tions and lack the skills to bring them under
control themselves. Thus, infants rely on
their caregivers to regulate their emotions
(Thompson, 1991). The ontological origins
of social emotion regulation clearly reside in
these dyads. If all goes well, coregulation of
emotion in the parent– infant dyad will lead
to infants beginning to develop the ability to
regulate their own emotions.
Attachment theory (Bowlby, 1988) pro-
vides the most influential account of the
transition from coregulation to individual
regulation in infancy, providing elegant
descriptions about the kinds of parent–
infant relationships that are likely to result
in good versus poor emotion regulation in
the infant. Attachment theory was given an
enormous empirical boost with the develop-
ment of observational methods for quantify-
ing mother– infant attachment. For example,
Emotion Regulation in Couples 273
the “Strange Situation” paradigm (Ain-
sworth, Blehar, Waters, & Wall, 1978) uses
close observation of episodes of mother–
infant separation and reunion to classify
attachment styles in ways that have profound
implications for emotion regulation initially
in the dyad and ultimately in the infant (e.g.,
securely attached infants develop greater
ability to regulate their own emotions than
do insecurely attached infants).
The Strange Situation focuses on the
down- regulation of negative emotions (pri-
marily fear and sadness), but the mother–
infant dyad also is involved in a great deal of
coregulation of positive emotion. The social
nature of positive emotion regulation in
infancy is seen vividly in studies of the syn-
chrony and reciprocity of positive emotion
(Cole, Teti, & Zahn- Waxler, 2003; Tron-
ick, 1989). In addition, parent– infant dyads
often engage in elaborate behavioral rituals
designed to increase positive emotions in the
infant (e.g., tickling and peekaboo rituals).
For parents, the infant’s smile is a highly
prized reinforcer; parents will go to great
lengths to evoke smiles in their infants.
We include discussion of these early
parent– infant dyads because we believe they
have important implications for emotion
regulation and other aspects of later inti-
mate relationships. However, one enduring
question about attachment styles (and asso-
ciated emotion regulatory abilities) is how
parent– infant interactions are related to
attachment styles that characterize intimate
relationships later in life (Mikulincer &
Shaver, 2007). In this regard, some theorists
have emphasized discontinuity (e.g., Kagan,
1984), while others have leaned more toward
continuity (e.g., Bowlby, 1988). Conducting
empirical research on these issues is quite
challenging. One approach has been to uti-
lize chain mediation models, in which expe-
riences during one life stage predict expe-
riences in the next, which in turn predict
experiences in the next (Sroufe, Coffino, &
Carlson, 2010). In one such study, which
bridged infancy, adolescence, and adult rela-
tionships, secure attachment in infancy was
found to predict more secure relationships
with close friends in adolescence, which in
turn predicted more positive daily emotional
experiences in adult romantic relationships
and less negative affect in conflict resolution
(Simpson, Collins, Tran, & Haydon, 2007).
Early Romantic Dyads
Selecting a partner and building a roman-
tic bond are critical developmental tasks.
Although often viewed through a lens that
emphasizes mate selection and family build-
ing (Havighurst, 1976), these relationships
are also important vehicles for develop-
ing emotion regulatory skills. In contrast
to parent– infant dyads, where the primary
focus is on mastering down- regulation of
negative emotions, in early romantic rela-
tionships the primary focus is clearly on
up- regulating positive emotions. The emo-
tions that are typically targets for this up-
regulation include passionate love, affection,
joy, excitement, and enthusiasm (Gable,
Gonzaga, & Strachman, 2006).
Several lines of research highlight the
importance of positive emotions in early
romantic relationships. Romantic love is a
phenomenon found across many cultures
(Jankowiak & Fischer, 1998) and has been
termed a “mammalian system for mate
choice” (Fisher, Aron, & Brown, 2006).
It is associated with a complex physiologi-
cal, psychological, and behavioral profile
that includes feelings of euphoria, focused
attention, and obsessive thinking about a
specific individual; craving for emotional
connection with the other person; expanded
sense of self; and greatly increased energy
(A. Aron et al., 2005; E. N. Aron & Aron,
1996). There is good evidence linking the
early stages of intense passionate love with
subcortical reward and goal centers in the
brain that are highly responsive to dopa-
mine (A. Aron et al., 2005; Fisher, Aron,
& Brown, 2006), which may help explain
the almost addictive quality of passionate
love, along with its attendant cravings (e.g.,
intensely missing the partner when absent)
and powerful withdrawal reactions (e.g., the
pain of lost love). From an emotion regula-
tory point of view, these high- intensity posi-
tive emotional states are highly desirable,
and, not surprisingly, lovers engage in quite
elaborate strategies to up- regulate their
positive feelings to extremely high levels
of intensity. There is little doubt that these
intense feelings contribute significantly to
mate selection and reproduction. In many
ways, they are the perfect fuel for launch-
ing romantic dyads along the path to family
formation.
274 SOCIAL ASPECTS
Clearly, down- regulating negative emo-
tion is an important item on the dyadic
agenda at all stages of development. This
often takes the form of managing jeal-
ousy (Shaver & Mikulincer, 2007) in early
romantic relationships, in which real and
imagined infidelities are a source of power-
ful negative emotions (fear, sadness, anger)
that must be controlled if the relationship
is to survive. Emotion regulation continues
to play a critical role as romantic relations
grow and develop. Looming large is the tran-
sition to parenthood, which is highly chal-
lenging for most couples (Cowan & Cowan,
1992; Doss, Rhoades, Stanley, & Markman,
2009). Successful navigation of this transi-
tion requires couples to deploy the full range
of individual, dyadic, and, ultimately, triadic
emotion regulatory skills.
Late‑Life Dyads
In late life, as couples move beyond the
prime reproductive period, life challenges
and life goals change, and emotion regula-
tory needs change accordingly. During this
developmental period, dealing with losses
and finding meaning in life become particu-
larly salient (Erikson, Erikson, & Kivnick,
1986). As individuals get older, they expe-
rience functional losses in domains such
as cognition (Salthouse, 2004), physical
abilities, and health (albeit with consider-
able individual differences, Rowe & Kahn,
1997). They also experience losses in their
social networks as retirement from the
workforce limits daily social contacts and
friends are lost due to relocation, illness,
and death (Charles & Carstensen, 2007;
Wrzus, Hänel, Wagner, & Neyer, 2013).
Lifespan developmental theory emphasizes
the importance of finding new sources of
meaning in late life, because earlier sources
of meaning (e.g., partner selection, family
building, career building) are no longer as
relevant. These can include developing qual-
ities of generativity (caring for future genera-
tions) and integrity (acceptance of one’s life)
(Erikson, 1950), and investing more deeply
in close social relationships (Carstensen,
Isaacowitz, & Charles, 1999).
In late-life couples, emotion regulation
becomes very important and may contrib-
ute to older adults’ relatively preserved lev-
els of well-being even in the face of decline
and loss (Mather, 2012). In survey studies,
older individuals report believing that they
improve in this ability (Gross et al., 1997).
Laboratory studies (e.g., Shiota & Leven-
son, 2009) paint a more complex picture,
with some regulatory strategies remaining
stable with age (i.e., suppressing visible signs
of emotional response), others declining
with age (i.e., using detached appraisals to
down- regulate emotion), and still others in
fact improving (i.e., using positive apprais-
als to down- regulate emotion). In late life,
up- regulating particular kinds of positive
emotion is very important. For example,
reminiscing with others about past accom-
plishments can be particularly rewarding
(Erikson, 1982), and companionate love,
which is characterized by low levels of pas-
sion but high levels of intimacy and com-
mitment (Sternberg, 1986), often assumes
the position of primary importance that
was occupied earlier by romantic love. Also
important is the ability to down- regulate
particular negative emotions such as sad-
ness (in response to interpersonal losses) and
embarrassment (in response to losses in cog-
nitive and physical abilities). Finally, com-
plex emotions such as poignancy, become
increasingly prevalent in late life (Ersner-
Hershfield, Mikels, Sullivan, & Carstensen,
2008), leading to time spent reliving and
savoring memories that have both positive
and negative emotional qualities (e.g., chil-
dren marrying and leaving home).
Importantly, the coregulation of emo-
tion also becomes increasingly important
as older adults spend more time with their
spouses as opposed to friends and acquain-
tances (Charles & Carstensen, 2007). For
those without close friends, down- regulating
negative emotions (e.g., sadness, fear) asso-
ciated with loneliness becomes critically
important. The stakes may be especially
high for failures of emotion regulation in
late life; the negative consequences of lone-
liness, for example, on health, have been
widely documented (Hawkley & Cacioppo,
2010).
Earlier we presented evidence that cou-
ples’ ability to lower levels of physiologi-
cal arousal is an important predictor of
relationship quality and stability over time
(Gottman & Levenson, 1992; Levenson &
Gottman, 1985). These kinds of calming
effects can be produced by touch (Coan,

Emotion Regulation in Couples 275
Schaefer, & Davidson, 2006) and also by
positive emotions, which can reduce levels
of autonomic nervous system arousal pro-
duced by negative emotions in individu-
als (Fredrickson & Levenson, 1998) and
in couples (Yuan, McCarthy, Holley, &
Levenson, 2010). These soothing effects of
positive emotions may contribute to positive
emotions becoming increasingly important,
desired, and salient in late life (Carstensen
et al., 1999).
Assessment
Emotion regulation in couples can be mea-
sured using self- report measures that assess
beliefs (one’s own or those of others who
know us) about emotion regulation. Alter-
natively, emotion regulation in couples can
be measured using performance measures
based on the observation of actual emotion
regulation. In both self- report and perfor-
mance measures, the focus can be on regula-
tory abilities (i.e., what the person or couple
is capable of doing) or regulatory practices
(i.e., what the person or couple typically
does). As noted earlier, there are aspects (e.g.,
coregulation) and qualities (e.g., dynamic) of
emotion regulation that are more prominent
in couples than in individuals. Thus, the
measures used for assessing emotion regula-
tion in couples should be designed to capture
these qualities, in addition to qualities that
are also prominent in emotion regulation in
individuals.
Unfortunately, the state of the art in mea-
suring emotion regulation in couples is not
as advanced as we would wish. As we discuss
below, most existing self- report measures of
emotion regulation clearly focus on indi-
vidual regulation. The self- report measures
that do have items relevant for assessing
emotion regulation in couples were almost
all designed for other purposes (e.g., mea-
suring relationship satisfaction). Progress
in developing methods for assessing actual
regulatory performance in couples is also
hindered by the lack of studies of emotion
regulation that include an actual (or even
an imagined) interaction partner (Campos
et al., 2011). Moreover, when such stud-
ies have been conducted, they have often
used unacquainted dyads (e.g., Butler et al.,
2003). These stranger pairings, although
clearly useful, are a far cry from the kinds of
intimate dyads in which emotion regulation
emerges and is refined during development
(see earlier discussion).
Observational studies of mother– infant,
mother– toddler, and mother– preschooler
dyads (Cole et al., 2003; Denham, 1993;
Dumas, LaFreniere, & Serketich, 1995;
Tronick, 1989) provide one bright spot in
this otherwise sparsely populated land-
scape. Although typically not designed to
study emotion regulation per se, they do
offer some valuable insights as to how emo-
tion regulatory processes function in these
early-life dyads.
Self‑Report Measures
In the emotion regulation domain, most
self- report measures focus on regulation in
the individual, not in the couple. For exam-
ple, the Emotion Regulation Questionnaire
(Gross & John, 2003) assesses two styles of
emotion regulation using a 10-item scale in
which six items assess the dispositional ten-
dency to use cognitive reappraisal strategies
and four items assess the dispositional ten-
dency to use suppression strategies. Another
individual- focused inventory, the Difficul-
ties in Emotion Regulation Scale (Gratz &
Roemer, 2004), comprises 36 items that
assess six dimensions of emotion regulation:
(1) lack of awareness of emotional responses,
(2) lack of clarity of emotional responses, (3)
nonacceptance of emotional responses, (4)
limited access to emotion regulation strat-
egies, (5) difficulties controlling impulses
when experiencing negative emotions, and
(6) difficulties engaging in goal- directed
behavior when experiencing negative emo-
tions.
It is certainly possible to alter an individ-
ual self- report measure of emotion regula-
tion so that items refer to a particular couple
relationship. However, this would essentially
constitute a new instrument, and its reliabil-
ity and validity would need to be established.
Moreover, questionnaires that were origi-
nally developed with a focus on the individ-
ual are unlikely to assess aspects of emotion
regulation that are particularly relevant to
couples (e.g., reciprocity of emotion, reac-
tions to each other’s regulatory styles).
The literature on close relationships has
produced a number of self- report inventories
276 SOCIAL ASPECTS
that were developed to assess relationship
functioning and satisfaction. A subset of
these inventories focuses on couples’ conflict
resolution and communication skills, and
includes items that are relevant to assessing
couples’ emotion regulation. For example, in
the 78-item Revised Conflict Tactics Scales
(Straus, Hamby, Boney-McCoy, & Sugar-
man, 1996), respondents are asked about
how they deal with disagreement, with ques-
tions asking how often they “shouted at or
yelled at my partner,” or how often conflict
escalated into several more violent acts. In
the 109-item Managing Affect and Differ-
ences Scale (Arellano & Markman, 1995),
subscales that are directly relevant to emo-
tion regulation include Negative Escalation
(e.g., “Unable to get out of heated argu-
ments”), Stop Actions (e.g., “When conflicts
get out of hand, agree to stop and talk at a
later time”), and Withdrawal (e.g., “When
discussing issues, my partner usually with-
draws for fear of conflict”). In a brief,
10-item screening inventory for relationship
discord (Whisman, Snyder, & Beach, 2009),
respondents are asked, “Whenever you are
feeling sad, does your partner make you feel
loved and happy again?” and “Do minor
disagreements with your partner often end
up in big arguments?”
There are a few self- report measures that
do focus on individuals’ emotion regulation
vis-à-vis a specific partner (e.g., a roman-
tic partner or a parent). Arguably, the best
known of these are primarily designed to
assess attachment styles, but they do have
items of relevance to emotion regulation.
The Experiences in Close Relationships
Scale (Brennan, Clark, & Shaver, 1998) is a
36-item measure that assesses avoidance and
anxiety in close relationships, with items
that focus on the dyadic context (e.g., “I pre-
fer not to show a partner how I feel deep
down”). The Adult Attachment Interview
(Main & Goldwyn, 1984) is based on 20
questions, some of which focus on impor-
tant aspects of emotion regulation (e.g.,
experiences with parents involving distress,
separation, rejection, or loss). It analyzes
responses to these questions, focusing on not
only what is said but also how it is said (e.g.,
the coherence of the narrative).
All of the measures reviewed thus far yield
scores that are considered to represent trait-
like qualities. Such approaches are ideal for
capturing enduring dispositional qualities of
couples’ regulatory styles but are not well-
suited for capturing the dynamics of emo-
tion regulation. Emotion self- reports can be
obtained in ways that yield more dynamic
information using affect rating dial (Ruef
& Levenson, 2007) and experience sam-
pling (Hektner, Schmidt, & Csikszentmih-
alyi, 2007) methodologies. There have also
been attempts to use self- report measures of
emotion regulation in new ways to capture
dynamic dyadic processes. For example,
in one study (Butner, Diamond, & Hicks,
2007), couples were asked to provide daily
ratings of positive and negative affect for
3 weeks, operationalizing coregulation as
covariation in partners’ daily levels of affect
and coupling of the rates of change of part-
ners’ affective cycles. In a similar vein, Fer-
rer and Nesselroade (2003) assessed emo-
tional experience of partners in one married
dyad, who recorded their experience of 20
emotions over 182 consecutive days.
None of these approaches provides an
off-the-shelf solution for measuring emo-
tion regulation in couples, but they do pro-
vide items and scales (mostly focused on
the management of negative emotion) that
could be useful starting points for build-
ing more comprehensive couple assessment
instruments. Nonetheless, our enthusiasm
for self- report measures of emotion regula-
tion is tempered by the problems that beset
all self- report measures (e.g., social desir-
ability and other self- presentation biases,
vulnerability to wording and context). In
the realm of emotional functioning, these
problems are compounded by individuals
often not being very accurate observers and
reporters of the nuances of their own emo-
tional functioning. Evidence of this comes
from findings of low correlations between
self- report and behavioral measures of emo-
tional functioning (e.g., low correlations
between well- established self- report mea-
sures of empathy and a performance- based
measure of empathic accuracy; Levenson
& Ruef, 1992) and variations among indi-
viduals in the coherence between self- report,
behavioral, and physiological measures of
emotion (Mauss, Levenson, McCarter, Wil-
helm, & Gross, 2005; Sze, Gyurak, Yuan,
& Levenson, 2010). Whether these kinds of
problems do in fact beset particular ques-
tionnaires designed to measure emotion reg-
Emotion Regulation in Couples 277
ulation in couples, and the ultimate utility of
such questionnaires for particular purposes,
are best settled on the basis of actual data.
Performance Measures
Whereas self- report measures of emo-
tion regulation assess the rater’s beliefs
about emotion regulation in self or others,
performance- based measures assess emo-
tion regulation in vivo, evaluating emotion
regulation as it actually occurs. These are
often multimethod performance measures
that include assessment of subjective experi-
ence, emotional behavior, and physiological
activation.
As noted earlier, performance measures
(and self- report measures) can focus on
either regulatory abilities or regulatory
practices. Measures of regulatory abilities
typically provide participants with spe-
cific instructions to alter some aspect of
emotional responding (e.g., “Try not to let
your emotions show”), then assess how well
they do (e.g., measuring the amount of vis-
ible emotional expression using a sensitive
behavioral coding system). Measures of reg-
ulatory practices, in contrast, typically place
participants in a situation in which emo-
tion regulation would be expected to occur
(e.g., discussing an area of conflict with a
relationship partner), then assess how well
participants regulate their emotions (e.g.,
measuring positive and negative emotional
behaviors and physiological arousal).
Each of these approaches has advantages
and disadvantages. For example, measures
of abilities tell us a lot about what people
are capable of doing in the emotion regula-
tory domain but not necessarily what they
actually do in their day-to-day lives (e.g.,
a person might be able to suppress all vis-
ible signs of emotion when watching a sad
movie but be very volatile in interactions
with her romantic partner, or vice versa).
With measures of practices, the lack of
instructions reduces experimental control,
and inferential leaps must be made between
what is measured and its relationship to
emotion regulation (e.g., slowed heart rate
might indicate emotional calming, but it
can also have many different psychological
and physiological causes). We should also
note that recent evidence from neurological
patients indicates that ability and perfor-
mance measures of emotion regulation are
likely subserved by different neural circuits
(Goodkind, Gyurak, McCarthy, Miller, &
Levenson, 2010). For all of these reasons,
measures of abilities and performance
should not be used interchangeably.
Measures of regulatory ability that have
been widely used in studies of emotion regu-
lation in individuals instruct participants to
suppress and amplify behavioral responses
(e.g., Gross & Levenson, 1993, 1997; Hage-
mann, Levenson, & Gross, 2006; Roberts,
Levenson, & Gross, 2008) and use vari-
ous appraisal strategies (e.g., Gross, 1998a;
Shiota & Levenson, 2009). However, these
measures have not been used as much with
couples. One exception is a study by Rich-
ards, Butler, and Gross (2003), in which dat-
ing couples were instructed to reappraise or
suppress their emotions as they discussed a
relationship conflict to determine the effects
on memory for conversation utterances
(which were increased by reappraisal and
decreased by suppression) and emotional
memories (which were increased by suppres-
sion).
Measures of regulatory practices have
been utilized in studies of emotion regula-
tion in adult dyads, with emotion regulation
operationalized in a number of different
ways, including (1) the amount of negative
or positive emotional experience (e.g., feel-
ing positive regard; Murray, 2005); (2) the
amount or ratio of negative and positive
emotional behavior (Gottman, 1993; Gott-
man & Levenson, 1992); (3) autonomic ner-
vous system activation (Levenson & Gott-
man, 1985); and (4) central nervous system
activation (Coan et al., 2006).
Measures of regulatory practices have also
been utilized in mother– infant dyads, most
famously in research using the Strange Situ-
ation (Ainsworth, Blehar, Waters, & Wall,
1978). In this test, children’s behavior is
measured after separation from the attach-
ment figure, a prototypical situation for elic-
iting distress and fear in infants. Another
measure of emotion regulation in infant–
caregiver dyads is the Still-Face Paradigm
(Tronick, Als, Adamson, Wise, & Brazelton,
1978). Here, the primary caregiver becomes
unresponsive and maintains a neutral facial
expression. In response, infants typically
show increased gaze aversion, less smiling,
and heightened negative affect.

278 SOCIAL ASPECTS
Many of the aforementioned approaches
to measuring emotion regulation in couples
result in scores that represent regulation
averaged over some period of time. However,
it is also possible for performance- based
measures to be dynamic, quantifying pat-
terns of emotional reactivity and regulation
that unfold over time. These more dynamic
approaches have been utilized in studies
that track changes in emotional experience
(e.g., Levenson & Gottman, 1983), emo-
tional behavior (see review in Gottman &
Levenson, 1988), autonomic nervous system
physiology (Yuan et al., 2010), hormonal
responses (Laurent & Powers, 2007), and
central nervous system activation (Coan et
al., 2006). Particularly promising are those
techniques that use the responses of both
members of the dyad to characterize quali-
ties of emotional coregulation over time
(Butler, 2011). These include measures of
(1) emotional reciprocity—the exchange of
emotions between partners in continuous
self- ratings of emotional experience (Lev-
enson & Gottman, 1983) or observational
coding of emotional behavior (Carstensen
et al., 1995; Gottman et al., 1998; Gottman
& Levenson, 1992; Julien, Brault, Char-
trand, & Bégin, 2000; Tronick et al., 1978);
(2) emotional linkage—the extent to which
physiological, hormonal, or mood responses
of partners become “synchronized” or can
be predicted from each other (Levenson &
Gottman, 1983; Saxbe & Repetti, 2010);
or (3) physiological soothing—the transi-
tion from high to low levels of autonomic
activation in a couple (Yuan et al., 2010).
Recently, Levenson (2013) described a new
statistical approach that characterizes cycles
of emotion (transitions between high and
low arousal, and between negative and posi-
tive emotion) that incorporates continuous
measurement of behavior, physiology, and
subjective experience.
Agenda for Future Research
Research on emotion regulation in couples
is poised for growth and discovery. Studies
of close social relationships are dramatically
increasing in many areas of psychology.
Clearly, we have only scratched the surface
in terms of both understanding the role that
emotion regulation plays in the functioning
of couples’ relationships and using couples’
relationships as a test bed for increasing our
understanding of what emotion regulation
is, how it operates, and its sources and con-
sequences. For this research area to move
forward and realize its potential, there are
several pressing needs:
1. Needed are sound self- report and per-
formance measures of couples’ emotion reg-
ulation that have been carefully constructed
and have well- established psychometric
qualities of reliability and validity. Mea-
sures are needed that (a) assess both emo-
tion regulatory abilities (what people can
do) and practices (what people do do); (b)
move beyond a primary focus on the down-
regulation of negative emotion to include the
up- regulation and down- regulation of both
positive and negative emotion; (c) assess
multiple aspects of emotion regulation (sub-
jective experience, behavior, physiology);
and (d) allow assessment of emotion regula-
tion in ways that capture its dynamic, itera-
tive, co- regulatory nature.
2. Needed are the development and
refinement of experimental paradigms that
are appropriate for studying emotion regu-
latory abilities and practices in couples. We
believe that observational studies of couple
and parent– child interactions provide good
bases for moving forward, but hope that
new paradigms will also be developed that
stimulate new research in this area.
3. Relationships between self- report and
performance measures of emotion regula-
tion in couples need to be studied and estab-
lished. The existing self- report measures
of emotion regulation in individuals have
generally not been studied in this way; thus,
there is not a firm basis for assuming equiva-
lencies across methods.
4. Issues concerning the distinctions
between emotion reactivity and emotion
regulation need to be addressed in emotion
regulation in couples, in much the same way
as they are being addressed in research on
individual emotion regulation (e.g., Ochsner
et al., 2009). These are challenging issues
with deep theoretical and practical impli-
cations. They should benefit greatly from
expanding knowledge about how emotional
functioning is organized in the central and
peripheral nervous systems.

Emotion Regulation in Couples 279
5. Research is needed on the anteced-
ents of emotion regulation in couples. We
expect that additional progress can be made
in exploring biological (e.g., genetic, temp-
eramental) and psychological (e.g., per-
sonality, attachment history) factors that
predispose couples to develop particular
regulatory styles. Of great interest are the
ways that couples’ emotion regulatory styles
develop over the course of the relationship,
when they are malleable, and when they
solidify.
6. More research is needed on the conse-
quences of emotion regulation, with special
attention given to mapping different kinds of
emotion regulation in relation to outcomes
in multiple domains (including physical and
mental heath, well-being, and relationship
quality). This kind of research would benefit
greatly from longitudinal designs; we expect
that many effects of particular regulatory
styles only emerge over fairly long periods
of time.
7. Research is needed on the similarities
and differences that emerge when socially
situated emotion regulation is scaled up
from dyads to larger groups (e.g., commu-
nity responses to traumatic events). We need
more comparisons of the nature of emotion
regulation in different kinds of dyads (e.g.,
romantic, friendship, coworker dyads). We
also need more research on how communi-
ties deal with tragic and triumphant events,
with a particular focus on the emotion regu-
latory challenges that must be faced in their
aftermath.
8. Research is needed on ways to improve
emotion regulation abilities in individuals,
dyads, and groups. In the realm of adult rela-
tionships, there are a number of therapeutic
approaches that afford particular attention
to emotional functioning (Gottman & Gott-
man, 2008; Johnson, 1996; Wile, 2002).
Unfortunately, research on the efficacy and
effectiveness of these treatments tends to
compare outcomes for one extremely com-
plex, multifaceted treatment package to a
non- treatment condition (or sometimes to
another highly complex treatment pack-
age). More research is needed using designs
that enable honing in on the “active ingredi-
ents” responsible for specific areas of change
and improvement. For parent– child rela-
tionships, despite a huge cottage industry
devoted to proffering parenting advice, the
research needs are similar.
Although the state of the science in emo-
tion regulation in couples is still relatively
immature, the potential is clearly enormous.
With the development of new measurement
tools and sound, ecologically valid experi-
mental paradigms that enable us to study
regulatory dynamics in interpersonal con-
texts, we expect great progress to be made
in many important areas related to emotion
regulation in couples.
References
Ainsworth, M. D. S., Blehar, M. C., Waters, E.,
& Wall, S. (1978). Patterns of attachment: A
psychological study of the Strange Situation.
Oxford, UK: Erlbaum.
Aldao, A., Nolen- Hoeksema, S., & Schweizer, S.
(2010). Emotion- regulation strategies across
psychopathology: A meta- analytic review.
Clinical Psychology Review, 30, 217–237.
Arellano, C. M., & Markman, H. J. (1995).
The Managing Affect and Differences Scale
(MADS): A self- report measure assessing con-
flict management in couples. Journal of Fam-
ily Psychology, 9, 319–334.
Aron, A., Fisher, H., Mashek, D. J., Strong, G.,
Li, H., & Brown, L. L. (2005). Reward, moti-
vation, and emotion systems associated with
early-stage intense romantic love. Journal of
Neurophysiology, 94, 327–337.
Aron, E. N., & Aron, A. (1996). Love and the
expansion of the self: The state of the model.
Personal Relationships, 3, 45–58.
Bowlby, J. (1988). A secure base: Parent–child
attachment and healthy human development.
New York: Basic Books.
Brennan, K. A., Clark, C. L., & Shaver, P. R.
(1998). Self- report measurement of adult
attachment: An integrative overview. In J. A.
Simpson & W. S. Rholes (Eds.), Attachment
theory and close relationships (pp. 46–76).
New York: Guilford Press.
Butler, E. A. (2011). Temporal interpersonal
emotion systems: The “TIES” that form rela-
tionships. Personality and Social Psychology
Review, 15, 367–393.
Butler, E. A., Egloff, B., Wilhelm, F. H., Smith,
N. C., Erickson, E. A., & Gross, J. J. (2003).
The social consequences of expressive suppres-
sion. Emotion, 3, 48 – 67.
280 SOCIAL ASPECTS
Butner, J., Diamond, L. M., & Hicks, A. M.
(2007). Attachment style and two forms of
affect coregulation between romantic part-
ners. Personal Relationships, 14, 431–455.
Campos, J. J., Walle, E. A., Dahl, A., & Main, A.
(2011). Reconceptualizing emotion regulation.
Emotion Review, 3, 26–35.
Carstensen, L. L., Gottman, J. M., & Levenson,
R. W. (1995). Emotional behavior in long-
term marriage. Psychology and Aging, 10,
140–149.
Carstensen, L. L., Graff, J., Levenson, R. W., &
Gottman, J. M. (1996). Affect in intimate rela-
tionships: The developmental course of mar-
riage. In C. Magai & S. H. McFadden (Eds.),
Handbook of emotion, adult development,
and aging (pp. 227–247). San Diego, CA: Aca-
demic Press.
Carstensen, L. L., Isaacowitz, D. M., & Charles,
S. T. (1999). Taking time seriously: A theory
of socioemotional selectivity. American Psy-
chologist, 54, 165–181.
Caspi, A., Henry, B., McGee, R. O., Moffitt,
T. E., & Silva, P. A. (1995). Temperamental
origins of child and adolescent behavior prob-
lems: From age three to fifteen. Child Devel-
opment, 66, 55–68.
Charles, S. T., & Carstensen, L. L. (2007). Emo-
tion regulation and aging. In J. J. Gross (Ed.),
Handbook of emotion regulation (pp. 307–
327). New York: Guilford Press.
Christensen, A. (1987). Detection of conflict pat-
terns in couples. In K. Hahlweg & M. J. Gold-
stein (Eds.), Understanding major mental dis-
order: The contribution of family interaction
research (pp. 250–265). New York: Family
Process Press.
Coan, J. A., Schaefer, H. S., & Davidson, R. J.
(2006). Lending a hand: Social regulation of
the neural response to threat. Psychological
Science, 17, 1032–1039.
Cole, P. M., Teti, L. O., & Zahn- Waxler, C.
(2003). Mutual emotion regulation and the
stability of conduct problems between pre-
school and early school age. Development and
Psychopathology, 15, 1–18.
Côté, S., Gyurak, A., & Levenson, R. W. (2010).
The ability to regulate emotion is associated
with greater well-being, income, and socioeco-
nomic status. Emotion, 10, 923–933.
Cowan, C. P., & Cowan, P. A. (1992). When
partners become parents. New York: Basic
Books.
Denham, S. A. (1993). Maternal emotional
responsiveness and toddlers’ social- emotional
competence. Journal of Child Psychology and
Psychiatry, 34, 715–728.
Doss, B. D., Rhoades, G. K., Stanley, S. M., &
Markman, H. J. (2009). The effect of the tran-
sition to parenthood on relationship quality:
An 8-year prospective study. Journal of Per-
sonality and Social Psychology, 96, 601–619.
Dumas, J. E., LaFreniere, P. J., & Serketich, W.
J. (1995). “Balance of power”: A transactional
analysis of control in mother– child dyads
involving socially competent, aggressive, and
anxious children. Journal of Abnormal Psy-
chology, 104, 104–113.
Erikson, E. H. (1950). Childhood and society.
New York: Norton.
Erikson, E. H. (1982). The life cycle completed:
A review. New York: Norton.
Erikson, E. H., Erikson, J., & Kivnick, H.
(1986). Vital involvement in old age. New
York: Norton.
Ersner- Hershfield, H., Mikels, J. A., Sullivan,
S. J., & Carstensen, L. L. (2008). Poignancy:
Mixed emotional experience in the face of
meaningful endings. Journal of Personality
and Social Psychology, 94, 158 –167.
Feeney, J. A. (1999). Adult attachment, emo-
tional control, and marital satisfaction. Per-
sonal Relationships, 6, 169–185.
Fernald, A. (1991). Prosody in speech to children:
Prelinguistic and linguistic functions. Annals
of Child Development, 8, 43–80.
Ferrer, E., & Nesselroade, J. R. (2003). Model-
ing affective processes in dyadic relations via
dynamic factor analysis. Emotion, 3, 344–
360.
Fisher, H. E., Aron, A., & Brown, L. L. (2006).
Romantic love: A mammalian brain system
for mate choice. Philosophical Transactions of
the Royal Society B: Biological Sciences, 361,
2173–2186.
Fredrickson, B. L., & Levenson, R. W. (1998).
Positive emotions speed recovery from the
cardiovascular sequelae of negative emotions.
Cognition and Emotion, 12, 191–220.
Gable, S. L., Gonzaga, G. C., & Strachman, A.
(2006). Will you be there for me when things
go right?: Supportive responses to positive
event disclosures. Journal of Personality and
Social Psychology, 91, 904 –917.
Goodkind, M. S., Gyurak, A., McCarthy, M.,
Miller, B. L., & Levenson, R. W. (2010).
Emotion regulation deficits in frontotemporal
lobar degeneration and Alzheimer’s disease.
Psychology and Aging, 25, 30 –37.
Gottman, J. M. (1993). A theory of marital dis-
Emotion Regulation in Couples 281
solution and stability. Journal of Family Psy-
chology, 7, 57–75.
Gottman, J. M., Coan, J., Carrere, S., & Swan-
son, C. (1998). Predicting marital happiness
and stability from newlywed interactions.
Journal of Marriage and the Family, 60, 5–22.
Gottman, J. M., & Gottman, J. S. (2008). Gott-
man method couple therapy. In A. S. Gurman
(Ed.), Clinical handbook of couple therapy
(pp. 138–164). New York: Guilford Press.
Gottman, J. M., & Levenson, R. W. (1985). A
valid procedure for obtaining self- report of
affect in marital interaction. Journal of Con-
sulting and Clinical Psychology, 53, 151–160.
Gottman, J. M., & Levenson, R. W. (1988). The
social psychophysiology of marriage. In P.
Noller & M. A. Fitzpatrick (Eds.), Perspectives
on marital interaction: Monographs in social
psychology of language, No. 1. (pp. 182–200).
Clevedon, UK: Multilingual Matters.
Gottman, J. M., & Levenson, R. W. (1992).
Marital processes predictive of later dissolu-
tion: Behavior, physiology, and health. Jour-
nal of Personality and Social Psychology, 63,
221–233.
Gratz, K. L., & Roemer, L. (2004). Multidi-
mensional assessment of emotion regulation
and dysregulation: Development, factor struc-
ture, and initial validation of the Difficulties
in Emotion Regulation Scale. Journal of Psy-
chopathology and Behavioral Assessment, 26,
41–54.
Gross, J. J. (1998a). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Gross, J. J. (1998b). The emerging field of emo-
tion regulation: An integrative review. Review
of General Psychology, 2, 271–299.
Gross, J. J., Carstensen, L. L., Pasupathi, M.,
Tsai, J., Skorpen, C. G., & Hsu, A. Y. C.
(1997). Emotion and aging: Experience,
expression, and control. Psychology and
Aging, 12, 590–599.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., & Levenson, R.W. (1993). Emo-
tional suppression: Physiology, self- report,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64, 970–986.
Gross, J. J., & Levenson, R. W. (1995). Emotion
elicitation using films. Cognition and Emo-
tion, 9, 87–108.
Gross, J. J., & Levenson, R. W. (1997). Hiding
feelings: The acute effects of inhibiting nega-
tive and positive emotion. Journal of Abnor-
mal Psychology, 106, 95–103.
Gross, J. J., Richards, J. M., & John, O. P.
(2006). Emotion regulation in everyday life.
In D. K. Snyder, J. Simpson, & J. N. Hughes
(Eds.), Emotion regulation in couples and
families: Pathways to dysfunction and health
(pp. 13–35). Washington, DC: American Psy-
chological Association.
Gross, J. J., Sheppes, G., & Urry, H. L. (2011).
Taking one’s lumps while doing the splits: A
big tent perspective on emotion generation and
emotion regulation. Cognition and Emotion,
25, 789–793.
Gyurak, A., Goodkind, M. S., Kramer, J. H.,
Miller, B. L., & Levenson, R. W. (2012).
Executive functions and the down- regulation
and up- regulation of emotion. Cognition and
Emotion, 26(1), 103–118.
Gyurak, A., Gross, J. J., & Etkin, A. (2011).
Explicit and implicit emotion regulation: A
dual- process framework. Cognition and Emo-
tion, 25, 400– 412.
Hagemann, T., Levenson, R. W., & Gross, J.
J. (2006). Expressive suppression during an
acoustic startle. Psychophysiology, 43, 104–
112.
Havighurst, R. J. (1976). Developmental tasks
and education. New York: David McKay.
Hawkley, L. C., & Cacioppo, J. T. (2010). Lone-
liness matters: A theoretical and empirical
review of consequences and mechanisms.
Annals of Behavioral Medicine, 40, 218 –227.
Hektner, J. M., Schmidt, J. A., & Csikszentmih-
alyi, M. (2007). Experience sampling method:
Measuring the quality of everyday life. Thou-
sand Oaks, CA: Sage.
Holley, S. R., Sturm, V. E., & Levenson, R. W.
(2010). Exploring the basis for gender differ-
ences in the demand– withdraw pattern. Jour-
nal of Homosexuality, 57, 666–684.
Jankowiak, W. R., & Fischer, E. F. (1998). A
cross- cultural perspective on romantic love. In
J. M. Jenkins, K. Oatley, & N. L. Stein (Eds.),
Human emotions: A reader (pp. 55–62). Mal-
den, MA: Blackwell.
John, O. P., & Gross, J. J. (2004). Healthy and
unhealthy emotion regulation: Personal-
ity processes, individual differences, and life
span development. Journal of Personality, 72,
1301–1333.
282 SOCIAL ASPECTS
Johnson, S. M. (1996). The practice of emotion-
ally focused marital therapy: Creating connec-
tion. Philadelphia: Brunner/Mazel.
Julien, D., Brault, M., Chartrand, É., & Bégin, J.
(2000). Immediacy behaviours and synchrony
in satisfied and dissatisfied couples. Canadian
Journal of Behavioural Science, 32, 84–90.
Kagan, J. (1984). The nature of the child. New
York: Basic Books.
Kunzmann, U., Kupperbusch, C. S., & Leven-
son, R. W. (2005). Behavioral inhibition and
amplification during emotional arousal: A
comparison of two age groups. Psychology
and Aging, 20, 144–158.
Lang, P. J., Greenwald, M. K., & Bradley, M. M.
(1988). The International Affective Picture
System (IAPS) standardization procedure and
initial group results for affective judgments.
Gainesville: Center for the Study of Emotion
and Attention, University of Florida.
Laurent, H., & Powers, S. (2007). Emotion regu-
lation in emerging adult couples: Tempera-
ment, attachment, and HPA response to con-
flict. Biological Psychology, 76, 61–71.
Levenson, R. W. (1976). Feedback effects and
respiratory involvement in voluntary control
of heart rate. Psychophysiology, 13, 108–114.
Levenson, R. W. (2013). Emotion and emo-
tion regulation. In D. Hermans, B. Rimé,
& B. Mesquita (Eds.), Changing emotions
(pp. 105–112). New York: Psychology Press.
Levenson, R. W., Cowan, C. P., & Cowan, P. A.
(2010). A specialty clinic model for clinical
science training: Translating couples research
into practice in the Berkeley Couples Clinic.
In M. C. Schulz, M. K. Pruett, P. K. Kerig, &
D. Ross (Eds.), Strengthening couple relation-
ships for optimal child development: Lessons
from research and intervention (pp. 197–209).
Washington, DC: American Psychological
Association.
Levenson, R. W., & Gottman, J. M. (1983).
Marital interaction: Physiological linkage and
affective exchange. Journal of Personality and
Social Psychology, 45, 587–597.
Levenson, R. W., & Gottman, J. M. (1985). Phys-
iological and affective predictors of change in
relationship satisfaction. Journal of Personal-
ity and Social Psychology, 49, 85–94.
Levenson, R. W., & Ruef, A. M. (1992). Empa-
thy: A physiological substrate. Journal of Per-
sonality and Social Psychology, 63, 234–246.
Lopes, P. N., Salovey, P., Côté, S., Beers, M., &
Petty, R. E. (2005). Emotion regulation abili-
ties and the quality of social interaction. Emo-
tion, 5, 113–118.
Main, M., & Goldwyn, R. (1984). Adult attach-
ment scoring and classification system.
Unpublished manuscript, University of Cali-
fornia, Berkeley.
Martin, R. A. (2007). The psychology of humor:
An integrative approach. Amsterdam, The
Netherlands: Elsevier.
Mather, M. (2012). The emotion paradox in the
aging brain. Annals of the New York Acad-
emy of Sciences, 1251, 33– 49.
Mauss, I. B., Levenson, R. W., McCarter, L.,
Wilhelm, F. H., & Gross, J. J. (2005). The tie
that binds?: Coherence among emotion expe-
rience, behavior, and physiology. Emotion, 5,
175–190.
McNulty, J. K., & Hellmuth, J. C. (2008). Emo-
tion regulation and intimate partner violence
in newlyweds. Journal of Family Psychology,
22, 794–797.
Mikulincer, M., & Shaver, P. R. (2007). Attach-
ment in adulthood: Structure, dynamics, and
change. New York: Guilford Press.
Mischel, W., Ayduk, O., Berman, M. G., Casey,
B. J., Gotlib, I. H., Jonides, J., et al. (2011).
“Willpower” over the life span: Decomposing
self- regulation. Social Cognitive and Affective
Neuroscience, 6, 252–256.
Murray, S. L. (2005). Regulating the risks of
closeness: A relationship- specific sense of felt
security. Current Directions in Psychological
Science, 14, 74–78.
Nelis, D., Kotsou, I., Quoidbach, J., Hansenne,
M., Weytens, F., Dupuis, P., et al. (2011).
Increasing emotional competence improves
psychological and physical well-being, social
relationships, and employability. Emotion, 11,
354–366.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D., et al.
(2004). For better or for worse: Neural sys-
tems supporting the cognitive down- and up-
regulation of negative emotion. NeuroImage,
23, 483– 499.
Ochsner, K. N., Ray, R. R., Hughes, B., McRae,
K., Cooper, J. C., Weber, J., et al. (2009).
Bottom-up and top-down processes in emo-
tion generation: Common and distinct neu-
ral mechanisms. Psychological Science, 20,
1322–1331.
Richards, J. M., Butler, E. A., & Gross, J. J.
(2003). Emotion regulation in romantic rela-
tionships: The cognitive consequences of con-
Emotion Regulation in Couples 283
cealing feelings. Journal of Social and Per-
sonal Relationships, 20, 599–620.
Roberts, N. A., Levenson, R. W., & Gross, J. J.
(2008). Cardiovascular costs of emotion sup-
pression cross ethnic lines. International Jour-
nal of Psychophysiology, 70, 82–87.
Rowe, J. W., & Kahn, R. L. (1997). Successful
aging. The Gerontologist, 37, 433–440.
Ruef, A. M., & Levenson, R. W. (2007). Contin-
uous measurement of emotion. In J. A. Coan
& J. J. B. Allen (Eds.), The handbook of emo-
tion elicitation and assessment (pp. 286–297).
New York: Oxford University Press.
Salthouse, T. A. (2004). What and when of cog-
nitive aging. Current Directions in Psycholog-
ical Science, 13, 140–144.
Saxbe, D., & Repetti, R. L. (2010). For better
or worse?: Coregulation of couples’ cortisol
levels and mood states. Journal of Personality
and Social Psychology, 98, 92–103.
Shaver, P. R., & Mikulincer, M. (2007). Adult
attachment strategies and the regulation of
emotion. In J. J. Gross (Ed.), Handbook of
emotion regulation (pp. 446–465). New York:
Guilford Press.
Shiota, M. N., & Levenson, R. W. (2009).
Effects of aging on experimentally instructed
detached reappraisal, positive reappraisal, and
emotional behavior suppression. Psychology
and Aging, 24, 890–900.
Simpson, J. A., Collins, W. A., Tran, S., & Hay-
don, K. C. (2007). Attachment and the experi-
ence and expression of emotions in romantic
relationships: A developmental perspective.
Journal of Personality and Social Psychology,
92, 355 –367.
Sternberg, R. J. (1986). A triangular theory of
love. Psychological Review, 93, 119–135.
Straus, M. A., Hamby, S. H., Boney-McCoy, S.,
& Sugarman, D. B. (1996). The Revised Con-
flict Tactics Scales (CTS2). Journal of Family
Issues, 17, 283–316.
Sroufe, L. A., Coffino, B., & Carlson, E. A.
(2010). Conceptualizing the role of early
experience: Lessons from the Minnesota lon-
gitudinal study. Developmental Review, 30,
36–51.
Sze, J. A., Gyurak, A., Yuan, J. W., & Levenson,
R. W. (2010). Coherence between emotional
experience and physiology: Does body aware-
ness training have an impact? Emotion, 10,
803–814.
Thompson, R. A. (1991). Emotional regulation
and emotional development. Educational Psy-
chology Review, 3, 269 –307.
Tronick, E., Als, H., Adamson, L., Wise, S., &
Brazelton, T. B. (1978). The infant’s response
to entrapment between contradictory mes-
sages in face-to-face interaction. Journal of the
American Academy of Child and Adolescent
Psychiatry, 17, 1–13.
Tronick, E. Z. (1989). Emotions and emotional
communication in infants. American Psychol-
ogist, 44, 112–119.
Whisman, M. A., Snyder, D. K., & Beach, S. R.
H. (2009). Screening for marital and relation-
ship discord. Journal of Family Psychology,
23, 247–254.
Wile, D. B. (2002). Collaborative couple therapy.
In A. S. Gurman & N. S. Jacobson (Eds.),
Clinical handbook of couple therapy (3rd ed.,
pp. 281–307). New York: Guilford Press.
Wrzus, C., Hänel, M., Wagner, J., & Neyer, F. J.
(2013). Social network changes and life events
across the life span: A meta- analysis. Psycho-
logical Bulletin, 139, 53–80.
Yerkes, R. M., & Dodson, J. D. (1908). The rela-
tion of strength of stimulus to rapidity of habit
formation. Journal of Comparative Neurol-
ogy and Psychology, 18, 459–482.
Yuan, J. W., McCarthy, M., Holley, S. R., &
Levenson, R. W. (2010). Physiological down-
regulation and positive emotion in marital
interaction. Emotion, 10, 467–474.

284
Emotion regulation promotes an individu-
al’s social adjustment. Having an emotion
means to take a stance, to have a particu-
lar relationship with the world (Solomon,
2004), and to have a specific intention to act
(Frijda, 1986). To take anger as an example,
the experience of anger implies an attitude
of nonacceptance, an assessment that one
has a relatively high level of control over
(others in) the situation (Frijda, Kuipers, &
Terschure, 1989), and a readiness to act in
such a way that these other people accom-
modate to your wishes, goals, and values
(Stein, Trabasso, & Liwag, 1993). Emotions
are thus relationship engagements (Mes-
quita, Marinetti, & Delvaux, 2012). Emo-
tion regulation refers to all those processes
involved in fashioning emotions to be most
adaptive within the relationship.
Cultural differences in emotion regulation
are to be expected, because the common and
most valued relationships differ across socio-
cultural contexts (e.g., Markus & Kitayama,
1991). Therefore, the emotions that are most
adaptive within those relationships— the
endpoints of emotion regulation— are likely
to differ cross- culturally as well. For exam-
ple, feelings of cheerfulness and happiness
are conceived as “good” and “desirable” in
European American culture (D’Andrade,
1984; Wierzbicka, 1994), because they sig-
nal that a person has successfully managed
the central cultural tasks of standing out and
accomplishing personal and material goals
(e.g., Hochschild, 1995). Happiness com-
municates to other Americans a “good inner
self” and psychological well-being (Markus
& Kitayama, 1994). Yet, as natural as the
desire to be happy may seem to most Ameri-
can readers, this emotion norm is far from
universal. The anthropologist Catherine
Lutz (1987), herself a European American,
was reprimanded for smiling at a girl who
acted happy during her stay with the Ifaluk
(on a Pacific atoll). The Ifaluk condemn hap-
piness, because they believe it leads a person
to neglect his or her social duties. Whereas
European Americans seek to yield and to
maximize happiness, Ifaluk life is geared
toward minimizing this emotion. An emo-
tion’s fit with the cultural models of self and
relationships will thus determine whether it
is up- or down- regulated.
In this chapter we propose, first, that the
endpoints of emotion regulation in each cul-
ture are those that match the culture’s valued
ideas and norms of how to be a good person
and how to relate to other people— the so-
called cultural models of self and relating
(Bruner, 1990; D’Andrade, 1984; Markus
& Kitayama, 1991). Second, we propose
that cultural regulation of emotions may be
initiated by an individual, but often is an
effect of the way the social environment is
CHAPTER 18
The Cultural Regulation of Emotions
Batja Mesquita
Jozefien De Leersnyder
Dustin Albert

The Cultural Regulation of Emotions 285
organized. Third, we will show that culture
(defined both at the level of the individual
and at the level of the social environment)
plays an important role in all stages of emo-
tion regulation.
Cultural Models
To provide a foundation for the discus-
sion on emotion regulation, we first briefly
review the idea of the cultural model itself.
Dominant cultural models of self and
relating are manifest in two distinct ways
that may be described as “culture in the
world” and “culture in the head” (Adams
& Markus, 2004) On the one hand, “cul-
ture in the world” refers to a culture’s daily
routines and organizational structures, to
the reward structures that are in place, to
social expectancies, and to the common
types of social interaction. “Culture in the
world” thus stands for the affordances and
constraints that implicitly (but powerfully)
shape individual experience. On the other
hand, “culture in the head” refers to inter-
nalized goals, values, meanings, representa-
tions, and behavioral repertoires, and thus
translates into experiential and behavioral
tendencies. Both manifestations of cultural
models— in the world and in the head—
appear to be involved in emotion regulation.
This idea can be illustrated by contrasting
the most widely studied cultural models: the
European American and East Asian cultural
models. According to middle- class Euro-
pean American models of self and relating,
the individual should be independent and
free from others, as well as stand out among
them (e.g., Kim & Markus, 1999). On the
one hand, everyday social arrangements fos-
ter a self that is independent and free. Exam-
ples are sleeping arrangements: In contrast
to infants in many areas of the world, Euro-
pean American infants sleep by themselves
very early on (Morelli, Rogoff, Oppenheim,
& Goldsmith, 1992; Shweder, 1991). On
the other hand, culture is internalized as the
psychological tendency of self- enhancement
and the value attached to choice in Euro-
pean American contexts (Heine, Lehman,
Markus, & Kitayama, 1999; Hochschild,
1995).
In contrast, the dominant goal of the self
in most East Asian cultural contexts is to be
like others and to enhance the fit between
what one is doing and what is expected (e.g.,
Heine et al., 1999; Lebra, 1992). The culture
values a person’s self- improvement, with the
aim of meeting these relational expectations,
fulfilling role-based obligations, and dem-
onstrating one’s loyalty to significant social
ingroups (Heine et al., 1999; Rothbaum
Pott, Azuma, Miyake, & Weisz, 2000). Cul-
ture in the world is organized toward these
goals: Politeness rituals and strictly prescrip-
tive role behavior are cases in point. “Cul-
ture in the head” can be seen from the East
Asian tendency to take the perspective of the
generalized other and thus focus on meeting
expectations (Cohen, Hoshino- Brown, &
Leung, 2007; Masuda et al., 2008).
It should be noted that the theoretical
framework of cultural models does not
assume or argue that people who live in
the same cultural context engage in exactly
the same way. First, people engage in many
models at the same time, such as models
of gender, socioeconomic status, cohort,
ethnicity, religion, and professional status.
These models all structure individuals’ real-
ity, as do family dynamics, close relationship
characteristics, and parenthood. Second,
and perhaps relatedly, people do not inter-
nalize the dominant cultural models in the
same way and to the same extent. Yet even if
people have not completely internalized the
dominant models, they still have to contend
with cultural models in the world (Shweder,
1991).
The “Right” Emotions Match
the Cultural Models
What feels “right” differs substantially
across cultures, yet we propose that every-
where the endpoints of emotion regulation
match the cultural models of self and rela-
tionships (Eid & Diener 2001; Kitayama,
Mesquita, & Karasawa, 2006; Mesquita &
Leu, 2007; Tsai, Knutson, & Fung, 2006).
The “right” emotions may be those consis-
tent with injunctive norms (ought-emotions,
[dis]approved by most others in the culture),
ideal emotions (emotions wanted by most
others in the culture), or descriptive norms
(emotions actually experienced by most oth-
ers in the culture). Cultural differences in
the explicit feeling rules and ideal emotions
286 SOCIAL ASPECTS
illustrate the different endpoints of regula-
tion; cultural differences in the commonly
experienced emotions are taken as evidence
for culture- specific emotion norms, if they
match the respective models of self and relat-
ing.
Feeling Rules and Ideal Emotions
Match the Cultural Models
A culture’s feeling rules—the most desirable
and valued emotional states— are endpoints
of emotion regulation. Differences in feeling
rules can be understood from differences in
the dominant cultural models. For instance,
Eid and Diener (2001) conducted a large
cross- cultural study in which participants
from both independent (European American
and Australian) and interdependent (China
and Taiwan) cultural contexts rated the
desirability of several emotions, both posi-
tive and negative. The largest cultural differ-
ences in desirability were found for “pride”
and “guilt.” Feelings of pride were more
positively valued in independent than in
interdependent cultures, whereas the oppo-
site was true for feelings of guilt. This may
be the case, because pride signals a person’s
autonomy and uniqueness, which are val-
ued in independent cultures but considered
“dangerous” in interdependent cultures that
recognize the potential of pride to disrupt
social harmony. Conversely, guilt may be
desirable from the viewpoint of East Asian
cultural models, because it signals an indi-
vidual’s concern for relational harmony (and
the readiness to take full responsibility for a
violation of this harmony), but undesirable
in Western cultures because it suggests a less
than positive performance of the individual.
There are also systematic cultural dif-
ferences in the emotions people “ideally
would like to feel” (Tsai et al., 2006). Tsai
and her colleagues asked people from dif-
ferent cultures to rate their “ideal feelings”
on emotion scales that represented the
four quadrants of the affective circumplex;
The affective circumplex is defined by the
dimensions of pleasantness (unpleasant–
pleasant) and activation (low–high). They
found consistent differences between Euro-
pean Americans, who “ideally” wanted to
feel more high- activation positive states such
as excitement and elation, and East Asians,
who preferred low- activation positive states
such as peaceful and serene feelings. Further
research showed that ideal emotions prepare
individuals best for the tasks that are cul-
turally central (Tsai, Miao, Seppala, Fung,
& Yeung, 2007). High- activation positive
emotions, promoted in North American
contexts, prepare individuals for influencing
others. In contrast, low- activation positive
emotions, promoted in East Asian contexts,
facilitate social adjustment.
Patterns of Emotional Experience
Match the Cultural Models
That the endpoints of regulation differ may
also be inferred from cultural differences
in the prevalent emotional patterns (i.e.,
the patterns of frequencies and intensities),
which can be understood from the pertinent
cultural models of self and relating. Adopt-
ing a variety of methods, Kitayama and his
colleagues (2006; Kitayama & Markus,
2000) found that European American par-
ticipants reported more socially disengag-
ing emotions— such as feeling pride, anger,
or irritation— and Japanese participants
reported more socially engaging emotions—
such as feeling close, ashamed or indebted.
The dimension of social engagement was
empirically derived, and emotions on the
disengaging end of the dimension underline
an individual’s independence, whereas emo-
tions on the engaging end foreground the
connectedness between people. The studies
made use of the self- reported frequency of
emotions in the past month, the self- reported
emotional experience in response to a pre-
defined set of situation types, and daily dairy
studies. Cultural differences in emotions, as
they appear from these studies, correspond
with other reports of emotions for the same
cultures (see the high frequency and preva-
lence of shame in Japanese cultural contexts
as reported by Benedict, 1946; Heine et al.,
1999). Culturally prevalent patterns of emo-
tions thus appeared to match the cultural
models of self and relating.
That the endpoints of regulation are cul-
turally defined is also suggested by research
on emotional acculturation: changes in the
patterns of emotional experience after peo-
ple move to a different culture. In a series
of studies, we asked immigrant and majority
groups to rate the patterns of emotions expe-
rienced in different types of situations that

The Cultural Regulation of Emotions 287
were defined according to the valence (posi-
tive, negative) and the social engagement
(engaged, disengaged) of the emotion they
tended to elicit. For each type of situation,
we compared immigrants’ pattern of emo-
tion ratings with the average majority mem-
ber’s pattern. The more an immigrant had
been exposed to the new culture, the more
his or her pattern of emotions resembles that
of the average majority member. This finding
held true for two different minority groups
in two different majority cultures: Turkish
immigrants in Belgium, and Korean immi-
grants in the United States (De Leersnyder,
Mesquita, & Kim, 2011). In both immigrant
groups, the number of years spent in the new
culture, as well as the degree of contact with
members of the majority culture, predicted
emotional similarity to the majority culture.
A separate study compared, for the same
type of situations, emotional patterns of Bel-
gian Turks and Belgian natives to those of
Turks living in Turkey (De Leersnyder, Mes-
quita, & Kim, 2013). Emotional patterns of
Belgian Turks were more similar to the Bel-
gian norm than were emotional patterns of
Turks in Turkey, even though the Turks in
Turkey were on average better educated (and
in that respect more similar to the Belgians)
than Belgian Turks. The combined find-
ings suggest that exposure to a new culture
sparks changes in the prevalent emotional
patterns that supposedly match the new cul-
tural models more closely.
That cross- culturally different endpoints
of regulation are rewarding can be inferred
from the link with well-being. The combined
evidence suggests that having the “right”
emotions is associated with higher well-
being. In the diary study reviewed earlier
(Kitayama et al., 2006), emotions that were
consistent with the descriptive norm best pre-
dicted well-being: disengaging emotions in
the European American sample, and engag-
ing emotions in the Japanese sample. Simi-
larly, our own research with U.S., Korean,
and Belgian samples showed that emotional
similarity to the cultural average predicted
higher satisfaction with social relationships
(De Leersnyder, Mesquita, Kim, Eom, &
Choi, 2013).Thus, having the “right” emo-
tions appears to be rewarding, even if the
exact processes involved are as yet unknown.
Conversely, emotions that are discrep-
ant with the culturally ideal emotions have
negative consequences for well-being. Tsai
and her colleagues (2006) found that the
discrepancy between a person’s actual and
ideal emotions predicted that person’s level
of depressive symptoms, but only for the
domain of emotions that was culturally most
focal: The discrepancy between actual and
ideal high- activation positive emotions pre-
dicted depression in European Americans,
whereas the discrepancy between actual and
ideal low- activation positive emotions pre-
dicted depression in East Asians. Therefore,
not having the “right” emotions appears to
have negative personal consequences.
In summary, emotions appear to be regu-
lated in ways that improve their match with
the prevalent cultural models of self and
relating. This is suggested by findings that
(1) actual emotions fit the culture’s models
of self and relating, (2) immigrants’ actual
emotions become more consistent with the
new cultural models, and (3) emotions that
are consistent with either the descriptive
norms or the ideal emotions in a culture
positively predict well-being. The rest of this
chapter is devoted to the question of how the
match between emotions and cultural mod-
els is achieved: This is the domain of emo-
tion regulation proper.
Cultural Regulation of Emotions
Emotion regulation has been defined broadly
as “the processes that influence which emo-
tions we have, when we have them, and how
we experience or express these emotions”
(Gross, 1998; Gross, Sheppes, & Urry, 2011,
p. 767). In this chapter, we subscribe to this
definition, and we note that it is agnostic to
(1) the source of regulation, and (2) whether
the regulation is effortful or conscious. We
propose that culturally driven emotion regu-
lation may be initiated by either individuals
themselves or their social environments, and
they may be either conscious and effortful
or automatic and effortless. We speak of cul-
tural regulation when there is evidence that
emotional processes are fashioned to match
the cultural models of self and relationship.
Most of the literature on emotion regula-
tion has focused on individual emotion reg-
ulation—for example, a person who avoids
seeing a friend who makes her feel miser-
able, who seeks distraction after a fight with
288 SOCIAL ASPECTS
her boss, or who tries to see the situation
in a different light. However, a full under-
standing of the role of culture in emotion
regulation requires that we move beyond
individual emotion regulation to include
what we call social emotion regulation. We
speak of social emotion regulation when the
social environment is the agent of emotion
regulation. Culture “in the world” is clearly
important to this type of regulation. Exam-
ples abound: Sex segregation in some cul-
tures is a means to avoid situations of embar-
rassment (Abu- Lughod, 1986); head hunting
rituals in another culture serve to channel
individuals’ ingroup anger (Rosaldo, 1980).
Closer to home, social sharing with friends
can help to restore a person’s self- esteem
after a painful breakup, thereby alleviating
distress. Thus, culture plays an important
role in both individual and social emotion
regulation.
It should be noted that our approach dif-
fers from accounts that assimilate emotion
regulation to the effortful redirection of
“the spontaneous flow” of emotions (Koole,
2009, p. 6). First, in light of cultural differ-
ences in the most prevalent emotions, we
challenge the notion of spontaneous flow.
Second, we propose that emotion regula-
tion is often automatic (cf. Bargh & Wil-
liams, 2007; Gyurak, Gross, & Etkin, 2011;
see Mauss, Bunge, & Gross, 2008, for a
cultural perspective on automatic emotion
regulation).
It is not always possible (and perhaps not
meaningful either; see Mesquita & Frijda,
2011) to draw a distinction between cultural
emotion regulation and culturally influenced
emotion generation. Regulatory processes
are clearly distinct when individual and
social emotion regulation changes the course
of an ongoing emotion in culture- specific
ways, but in many cases, emotion regulation
is inferred from the fact that emotional out-
comes cross- culturally vary in nonrandom
ways. For example, the types of situations
that individuals encounter may be responsi-
ble for aligning emotions with the endpoints
of regulation (e.g., politeness reduces anger,
and may thus help to achieve relational har-
mony). In this case, the distinction between
emotion generation and emotion regulation
becomes futile (Mesquita & Frijda, 2011).
However, we speak of emotion regulation in
these cases because the social organization
indisputably plays a role in “what emotions
we have” and “when we have them” (Gross
et al., 2011, p. 767). To the extent that
either individual or social processes seem to
increase the match of emotions to norms, we
consider them forms of emotion regulation.
We assume that a culture’s individual and
social emotion regulation strategies go hand
in hand, and supplement each other, in an
attempt to realize the cultural norms (Kita-
yama, Karasawa, & Mesquita, 2004; Mauss
et al., 2008). Below we discuss evidence for
the role of culture in multiple strategies of
emotion regulation; for each strategy we
consider evidence for both individual and
social emotion regulation.
Situation Selection
One major path of emotion regulation has
been termed situation selection, described
as “approaching or avoiding certain people,
places, or objects in order to regulate emo-
tions” (Gross, 1998, p. 283). Cultural dif-
ferences in situation selection are suggested
with regard to individual and social emotion
regulation. Differences in individuals’ moti-
vational focus are taken as evidence for the
role of culture in individual emotion regula-
tion; differences in the social realities that
individuals encounter— the a priori selec-
tion of situations— are considered a form of
social emotion regulation.
Individual Regulation
of Situation Selection
There is some evidence that different cul-
tural models affect individuals’ situation
selection. A number of studies found that
the relative focus of individuals on either
avoiding negative or approaching posi-
tive situations differs across cultures (e.g.,
Elliott, Chirkov, Kim, & Sheldon, 2001;
Lee, Aaker, & Gardner, 2000). Whereas
the dominant focus in American contexts
appears to be on the accomplishment of
positive outcomes (i.e., a promotion focus),
East Asians and Russians have been found
to be more concerned with avoiding failures
to meet social expectations (i.e., a preven-
tion focus). These cultural differences in
motivational focus can be understood from
the respective models of self and relating: An
independent American model emphasizes
The Cultural Regulation of Emotions 289
self- enhancement and the achievement of
positive outcomes, whereas the more inter-
dependent East Asian and Russian models
underline the importance of avoiding social
violations, and may thus be more driven by
avoidance than approach.
That differences in situation selection
may affect emotional experience was sug-
gested by a cross- cultural vignette study by
Lee et al. (2000, Studies 3–5). The research-
ers examined differences in emotional reac-
tions to success and failure between Euro-
pean Americans (promotion focus) and
East Asian Hong Kong Chinese (prevention
focus). They hypothesized that a promo-
tion focus would foster happiness under
conditions of success, and sadness under
conditions of failure, whereas a prevention
focus would lead to reports of relaxation
or relief upon success, and anxiety upon
failure. Consistent with the differences in
motivational focus, the European Ameri-
can participants reported higher intensi-
ties of happiness– depressed emotions than
relief– anxiety emotions, whereas the Chi-
nese participants reported higher intensities
of relief– anxiety than happiness– depressed
emotions. This is some of the first evidence
that cultural differences in situation selec-
tion may be related to differences in the
prevalent types of emotions. In summary,
cultural models of self and relating moti-
vate individual- level selection of situations,
which itself can be understood as a form of
emotion regulation.
Social Regulation of Situation Selection
Social situation selection may render certain
emotions either more frequent or more rare.
For example, European American social
life is characterized by practices that serve
to make individuals feel special and unique,
and that promote happiness and feeling
good about themselves (D’Andrade, 1984).
Contemporary American schools promote
the happiness and pride of their students
with practices that make the children feel
important and special. As early as preschool,
children are the focus of attention during
“show-and-tell” meetings, and throughout
elementary school, children’s accomplish-
ments are marked by smiley faces, stickers,
and gift-box rewards for every achieve-
ment, however small. The importance of
self- esteem is nicely illustrated by anecdotal
evidence. In his book The Geography of
Thought, the American psychologist Rich-
ard Nisbett (2003) describes how the school
board in his hometown even “debated
whether the chief goal of the schools should
be to impart knowledge or inculcate self-
esteem” (p. 55).
In contrast, many of the practices in Japa-
nese cultural contexts promote anticipatory
fear or shame, consistent with a cultural
model emphasizing the continuing obliga-
tion to accommodate others, fulfill one’s
roles, and perfect one’s contributions in
order to approach others’ expectations or
cultural ideals in general (Heine et al., 1999).
For example, at the end of each day Japanese
schoolchildren are encouraged to engage in
self- reflection or self- criticism (i.e., hansei),
so that they can look for ways to improve
their shortcomings or weaknesses in order
to meet the group’s standards (e.g., Lewis,
1995). The constant awareness of one’s
shortcomings is conducive to the experience
of emotions such as anticipated fear and
shame.
Culturally comparative research provides
systematic support for the idea that situa-
tions eliciting culturally condoned emotions,
such as shame in Japan, are relatively more
frequent than culturally condemned emo-
tions, such as anger in Japan (e.g., Boiger,
Mesquita, Uchida, & Barrett, 2013). In a
recent study, we compared the frequency of
shame and anger situations in Japan and the
United States, utilizing carefully selected,
representative elicitors of shame and anger
that had been reported in extensive pilot
studies by either Japanese or U.S. college
students. Shame was chosen because it was
expected to be consistent with an inter-
dependent, but not with an independent,
model of self and relating; anger was selected
because it was thought to have the opposite
connotations. New samples of Japanese and
U.S. students read standardized vignettes
describing these eliciting situations, and
rated (1) their likelihood of occurrence
and (2) the degree to which the situations
would elicit the intended emotion (anger or
shame). In the United States, where shame
violates the cultural model of independence,
we expected people to avoid shame, but
in Japan, where shame is an expression of
the cultural model of interdependence, we
290 SOCIAL ASPECTS
expected them to promote it. In contrast,
we expected anger to be avoided in Japan
(because it interferes with social harmony),
and promoted in the United States (since it
underlines independence). The findings pro-
vided support for culturally different pat-
terns of situation selection. Situations that
elicited higher levels of shame were rated
as more likely to occur in Japan, and as less
likely to occur in the United States. In con-
trast, situations that were thought to elicit
more anger were rated as more frequent in
the United States, and less frequent in Japan.
Thus, the “social” selection of situations
may contribute to the previously reported
differences in the frequencies with which
people in American and Japanese cultural
contexts experience anger (disengaging) and
shame (engaging) emotions (see Kitayama et
al., 2006). One way of understanding these
findings is that Americans simply encoun-
ter more anger- provoking situations, and
Japanese more shame- provoking situations
in their everyday lives. Social conventions
may help to avoid situations of interper-
sonal frustration in Japan, and situations of
interpersonal criticism in the United States,
respectively. In other words, social interac-
tions may be structured in culture- specific
ways that affect emotional experience.
Child- rearing practices provide another
example of social situation selection affect-
ing emotional experience. Several studies
suggest that Japanese mothers structure
their children’s environment in ways that
encourage relative stability and modera-
tion of emotion, whereas American mothers
provide greater situational variability, thus
increasing the probability that their children
will experience a range of both positive and
negative emotions (Rothbaum et al., 2000).
Japanese mothers lull and comfort their
infants more by soothing and maintaining
close proximity to their children. In doing
so, they create a safe and stable environ-
ment, thus decreasing the likelihood that
their children experience strong negative
emotions. In contrast, American mothers
allow their children more exploratory activ-
ity and use more distal proximity strategies
(e.g., eye contact) than do Japanese mothers
(Rothbaum et al., 2000). They offer their
children the opportunity for exploration,
and in doing so create more excitement but
also greater potential for negative emotions.
In summary, cultural differences in the
common forms of social interaction account
for differences in emotion experience. We
speak of social situation selection if these
differences in emotional experience match
the respective cultural models of self and
relating. Several studies suggest the exis-
tence of cultural differences in social situa-
tion selection.
Focus of Attention
Culture may also be instrumental in regu-
lating the focus of attention, or what in the
emotion regulation literature has been called
attentional deployment (Gross, 1998): the
channeling of attention in ways that are con-
ducive to the desired emotional outcome.
There is some evidence that attention may be
channeled in ways that are consistent with
the cultural models.
Individual Regulation of the Focus
of Attention
Cultural models may affect individuals’
focus of attention on relatively different
aspects of emotional situations. In a com-
parative study on emotion perception, Japa-
nese and Americans rated the emotions of
a central person expressing anger, sadness,
or happiness, who was surrounded by four
other people (Masuda et al., 2008). The
facial expressions of the other people var-
ied, independent of the expression of the
central person. Consistent with an indepen-
dent model, Americans exclusively focused
attention on the central person’s emotions,
disregarding the emotions of the surround-
ing people. Consistent with an interdepen-
dent model, however, the Japanese focused
attention on the other people in the picture
as well. For example, Japanese rated the
sadness of the central person to be higher if
the other people in the situation were sad as
well, relative to situations in which the other
people were not sad. Eye tracking confirmed
that, compared to American participants,
Japanese participants spent more time scan-
ning the periphery of the picture for the
facial expressions of the other people. There-
fore, consistent with interdependent models
of self and relating, Japanese focused their
attention on everybody involved in the social
situation.
The Cultural Regulation of Emotions 291
Culture might focus individuals’ atten-
tion on not only different types of social
information but also differently valenced
information. A recent study by Grossmann,
Ellsworth, and Hong (2012) compared Rus-
sian and European American participants’
focus on positive and negative emotional
stimuli, respectively. This was based on eth-
nographic and empirical work that charac-
terized Russian people as “brooders” who
place greater value than European Ameri-
cans on immersing themselves in negative
feelings. The researchers hypothesized that
Russians’ attention would be drawn more to
negative stimuli. In support of this hypoth-
esis, Russians, but not European Americans,
spent significantly more time looking at neg-
ative than at positive stimuli. In a follow- up
study with biculturals, Russian culture was
compared to a European culture (Latvia).
Making use of a technique used to study
cultural frame switching in biculturals, the
authors primed bicultural Russian Latvians’
with either Russian or Latvian culture, by
presenting them with Russian or Latvian
cultural symbols. Priming biculturals with
their Russian heritage led to a faster recogni-
tion of negative words and a slower recogni-
tion of positive words as compared to their
baseline reaction time to these words; also
consistent with the authors’ expectations,
Russian Latvians primed with Latvian cul-
tural symbols failed to focus more attention
on negative information. Interestingly, the
effect of priming Russian culture was more
pronounced for biculturals who reported
more affinity with their Russian heritage; it
is possible that the priming activated a more
elaborate Russian emotional repertoire in
these individuals.
Social Regulation of the Focus
of Attention
Other people are often involved in funnel-
ing an individual’s attention. We refer to
this as social regulation when the changed
focus of attention aligns the individual’s
emotional experiences with the dominant
cultural model of self and relating. Research
on caregiver– child interactions in two Nep-
alese groups illustrates this idea nicely (Cole,
Tamang, & Shrestha, 2006). Tamang and
Chhetri- Brahmin mothers were asked what
they would do if their 4- or 5-year-old child
was angry at them. Both groups of moth-
ers channeled their children’s attention in
ways that were consistent with the cultural
models. The majority of Tamang mothers
reported that they would give the children
food and cajole them in order to calm them
down; this distraction (of attention) strategy
is consistent with Tamang Buddhist models
of social harmony that focus on egalitarian-
ism, compassion, and tolerance. In contrast,
the majority of Chhetri- Brahmin mothers
reported they would respond by telling their
children to behave, to study, and to go to
school. The Chhetri- Brahmin mothers thus
focused their children’s attention on the val-
ues of social order and disciplined action
central to their Hindu cultural models, sup-
posedly inducing more conflictual emotions.
In both cases, the refocusing of attention
had an effect on emotional experience that
was consistent with the cultural model.
Further evidence for the role of others in
focusing attention comes from an observa-
tional study with European American and
Taiwanese mothers of 3-year-olds. Euro-
pean American mothers drew the child’s
attention to his or her independent achieve-
ments, thereby fostering the child’s hap-
piness and pride. In contrast, Taiwanese
mothers shamed their children by focusing
their attention on their transgressions, and
the negative effects these transgressions had
had on the mothers (Miller, Fung, & Mintz,
1996). In both cases, parental regulation of
the child’s attention served to increase the
fit between the child’s emotional experience
and the cultural model.
Although more obvious in child research
(for a review, see Diamond & Aspinwall,
2003), emotion regulation by others does
not cease in childhood or adolescence; even
after children are mature enough to inter-
nalize effective emotion regulation strate-
gies of their own, significant social partners
continue to contribute regulatory influence
across the lifespan. This can be observed in
not only adult interpersonal practices such
as the provision of comfort and support,
the expression of empathy, the redirection
of attention, or suggestions for cognitive
reframing of emotionally salient informa-
tion (Diamond & Aspinwall, 2003), but also
other common practices of social sharing of
emotions in adults (e.g., support groups). In
summary, other people may focus a person’s
292 SOCIAL ASPECTS
attention in ways that render emotional
experience consistent with cultural mod-
els of self and relating. Individuals’ social
environments may be especially likely to
help and channel attention when individuals
focus on aspects of the situation that render
their emotional experience inconsistent with
the cultural model.
Regulation of Appraisal
Another major path of emotion regulation is
through fashioning the individual’s apprais-
als of the situation. Appraisal always occurs
against the backdrop of the cultural models
that constitute reality. These cultural models
may be internalized, in which case they play
a role in individual emotion regulation, or
they may be instantiated by the social con-
text, in which case they may activate in the
individual certain appraisals; the latter may
be seen as a form of social appraisal regula-
tion.
Individual Regulation of Appraisal
Cultural models form the background
against which situations are appraised, and
emotional experience is contingent on an
individual’s interpretation of the situation
(e.g, Ellsworth, 1994; Mesquita & Ells-
worth, 2001). One obvious way in which
cultural models of self and relating are
essential to emotion regulation is by fashion-
ing the appraisal processes to create certain
emotions.
Individual appraisal is fashioned, in the
first place, by the concerns and schemas
against which situations are appraised. Cul-
tures differ with respect to these concerns.
For instance, what it means to a woman
that a man—not her intimate— looks at her
may differ depending on whether her culture
construes such attention as an honor viola-
tion, a sign that she is attractive, or a marker
that she is being objectified in a sexist world.
Receiving male attention has different emo-
tional consequences in each of these cases:
A woman would feel an emotion of shame
or embarrassment if she lived in an honor
culture, an emotion of pride or excitement
if she experienced the male attention as a
sign of her attractiveness, and an emotion
of indignation if she considered it an act of
sexism. Depending on the “availability” or
”salience” of certain interpretations, the
woman would feel a different emotion. We
refer to these cultural differences in appraisal
as forms of cultural emotion regulation, even
if they may not involve attempts at effortful
regulation on the part of the individual. The
salience of concerns that fit the cultural mod-
els is “culture in the head.” It is this culture
in the head that renders the emotion norma-
tive (i.e., “regulates” the emotion).
Differences in daily emotional experi-
ences can often be understood from cultural
differences in individuals’ common world
views or daily preoccupations (also “culture
in the head”). For example, the emotion of
anger is characterized by appraisals of an
event as unpleasant, going against personal
goals, but relatively controllable. In trying to
understand the near absence of frustration
and anger among Tahitians, the American
anthropologist Robert Levy (1978, p. 226)
pointed to “a shared common sense that
individuals have very limited control over
nature and over the behavior of others.”
Thus, as Levy notes, a universe so defined is
“cognitively less frustrating than those cul-
tures which define realities in which almost
anything is possible to individuals” (p. 226).
The general expectation that the world is
minimally rewarding, and that there is no
way to force rewards, leads to a lower preva-
lence of anger. The Tahitian perspective on
life contrasts with middle- class American
models that emphasize and value control
and predictability (Mesquita & Ellsworth,
2001). In middle- class American contexts,
the prevailing view is that the world is mal-
leable. Changing or influencing the environ-
ment rather than adjusting to it tends to be
a dominant response, reflecting the assump-
tion that circumstances can be made to fit
one’s personal goals (Boiger, Mesquita,
Tsai, & Markus, 2012; Morling, Kitayama,
& Miyamoto, 2002; Weisz, Rothbaum, &
Blackburn, 1984). Hence, there is a relative
emphasis on control and agency in middle-
class American appraisals of emotional
situations. As such, people more readily
appraise events in terms of inconsistency
with their goals, the responsibility of other
people, and the possibility of forcing oth-
ers to accommodate to their wishes; hence,
the emotional experiences of frustration and
anger are more likely (Frijda, 1986). Cultural
models thus provide the backdrop against
The Cultural Regulation of Emotions 293
which events are meaningful. A sense that
the world is a place in which agents make
a difference also renders the experience of
a lack of agency more unpleasant. This is
illustrated by a study by Roseman, Dha-
wan, Rettek, Naidu, and Thapa (1995), in
which Indian and American college students
reported instances of anger, sadness, and
fear, and rated their experiences on several
appraisal dimensions. Cultural models for
Indian college students are less likely to make
salient the possibility of successful control of
one’s circumstances than are cultural mod-
els for American students (Savani, Markus,
Naidu, Kumar, & Berlia, 2010). Consistent
with this understanding, Roseman and col-
leagues (1995) found that Indian college stu-
dents rated self- reported emotional events
to be less “incongruent with their motives”
than did their American counterparts. As
expected, Indian students also reported
lower overall intensities of both sadness and
anger; moreover, these cultural differences in
emotion intensity were fully mediated by the
perception that the event was less discrepant
with goals. Although the link between the
cultural emphasis on controllability and the
perception of goal relevance was not mea-
sured, we suggest that cultural representa-
tions of agency affected the perception of a
discrepancy between emotional events and
personal goals, and in turn influenced the
intensity of felt sadness and anger.
A more direct test of the relationship
between concerns and emotional experiences
was provided by two recent studies of Bel-
gian students (De Leersnyder & Mesquita,
2013), who were asked to describe a recently
experienced emotional situation and to indi-
cate if and to what extent the situation had
been either consistent or inconsistent with a
number of different concerns, some of which
were other- focused (e.g., being loyal, helping
others), and others which were self- focused
(personal success, ambition). The frequency
with which these values were judged as rele-
vant to the emotional situations exactly mir-
rored young Belgians’ value hierarchy (i.e.,
most important values as “guiding prin-
ciples in people’s life”), as obtained from a
national representative sample by the Euro-
pean Social Survey (ESS, Round 5; Norwe-
gian Social Science Data Services, 2012).
Moreover, the types of emotions depended
on the types of values considered relevant.
The combined results thus suggest that (1)
culturally salient concerns are more readily
available as standards of evaluation for emo-
tional situations, and (2) the different types
of concerns translate into different patterns
of emotional experience. As such, emotional
experiences are culturally regulated to be
about the most important cultural themes
and concerns.
In summary, cultural models appear to
provide the backdrop of expectations and
point(s) of view with which individuals meet
the world. As such, culture regulates the
meaning and thus the emotional significance
of the world to the individual.
Social Regulation of Appraisal
Other people appear to influence an indi-
vidual’s appraisals in two ways. One is by
example: Other people’s emotions and
appraisals inform an individual’s appraisals
in a given situation. There is developmental
work showing that children look at their
caregivers’ facial expressions in trying to
appraise a situation as, for example, danger-
ous or safe (e.g., Campos & Stenberg, 1981).
Adults also use other people’s appraisals as
input for the meaning of the situation (Par-
kinson & Simons, 2009). We expect that
social appraisal regulation plays a large role
in cultural differences, because the prevalent
emotions in each culture differ. When other
people’s emotions are used as a compass, this
compass will point in different directions
for different cultural environments. There
are some illustrations of this phenomenon
in the parenting literature: Parents’ own
reactions to their children’s emotions may
sometimes model their children’s appraisal.
For instance, Japanese parents rarely express
direct disagreement with their angry chil-
dren; instead, they go through cycles of
mutual perspective taking (Trommsdorff &
Kornadt, 2003) or express negativity indi-
rectly (e.g., by silence). Parents thus model
the interpretation of the situation as one
that requires perspective taking. In contrast,
German parents in the same study (Trom-
msdorff & Kornadt, 2003) were much more
confrontational, and in that way, modeled
the appraisal as one of conflict.
A second social appraisal situation con-
sists of more explicit approval or disapproval
of certain emotions or interpretations. There
294 SOCIAL ASPECTS
is again some cross- cultural work on social
appraisal regulation in the caregiver– child
literature (Boiger & Mesquita, 2012; De
Leersnyder, Boiger, & Mesquita, 2013).
For example, parents may pay attention to
some of their children’s emotions, thus vali-
dating the appraisal of the situation, and
ignore other emotions of the child, thus fail-
ing to endorse the child’s interpretation of
the event. German mothers who witnessed
their children’s mishaps focused on the chil-
dren’s distress, thereby confirming that the
children had a good reason for their negative
emotions. By contrast, Japanese and Indian
mothers ignored their child’s negative emo-
tions, thus challenging their interpretation
of the situation as one of distress (Tromms-
dorff & Friedlmeier, 2010). Japanese care-
givers also helped their children adopt the
outside- in perspective that is also common
for Japanese adults: They drew their chil-
dren’s attention to how their anger makes
others feel (e.g., Conroy, Hess, Azuma, &
Kashiwagi, 1980). Reappraising anger-
ing situations in this way may explain the
lower levels of anger in Japan (Zahn- Waxler,
Radke- Yarrow, & King, 1979). European
American parents, on the other hand,
expected their children to self- assert and
to stand up for themselves (Conroy et al.,
1980). When dealing with an angry child,
they tended to encourage appraisals of frus-
tration in the child, leading to high levels of
anger.
To our knowledge, there is no systematic
empirical evidence that adults help each
other in reappraising situations in ways that
are consistent with their cultural values.
However, there is some anecdotal evidence
for social appraisal regulation beyond child-
hood. For instance, Kitayama and Masuda
(1995) describe how American friends help
each other when one is feeling shameful and
down “by reorienting the person’s atten-
tion . . . to external objects or events the
person can reasonably blame for the imped-
ing problem” (p. 220). This social regula-
tion may explain why shame is frequently
transformed into anger in the American cul-
tural context (Kitayama & Masuda, 1995).
By reappraising the shameful event as being
caused by others rather than by oneself, the
focus of appraisal shifts from one’s own
painful shortcomings to the more empower-
ing experience of self- integrity, and others’
blameworthiness. Maintaining high self-
esteem and avoiding self- critical informa-
tion constitute central goals for the Ameri-
can independent self; anger can thus be seen
as a more desirable endpoint of emotion
regulation than shame.
Behavior Regulation
Cross- cultural differences have tradition-
ally been explained as differences in delib-
erate and effortful suppression of emotional
expressions that did not match the cultural
ideals (e.g., Matsumoto, 1990). The idea has
been that people across different cultural
contexts adhere to the prevalent “display
rules” (i.e., the “cultural norms that dictate
the management and modification of emo-
tional displays depending on social circum-
stances; Ekman & Friesen, 1969),” and that
this type of post hoc regulation is the most
important route to cultural differences in
emotion (Matsumoto et al., 2008, p. 58).
While it is clear that cultural differences in
emotion regulation are not due to differ-
ences in response suppression only, there is
evidence that suggests cultural differences in
this strategy, too. We discuss this evidence
below, but we also suggest that cultural
differences in the regulation of emotional
behavior are not limited to differences in
individual emotion suppression.
Individual Regulation of Behavior
There is evidence of cultural differences in
display rules. Several studies have linked the
use of display rules to the culture’s level on
the individualism dimension; a dimension
that is an important aspect of cultural mod-
els of self and relationship. Cultures with
interdependent models are thought to be low
on individualism (and high on collectivism);
while independent cultures are high on indi-
vidualism. Pertinent studies have found that
there is a greater desire to express emotions,
especially positive, high- activation emotions
in cultures higher on individualism. For
instance, Matsumoto and colleagues (2008)
asked participants from 32 different coun-
tries what they should do if they felt each
of seven emotions (anger, contempt, disgust,
fear, happiness, sadness, and surprise) on a
scale ranging from emotion amplification
(i.e., express more), to deamplification (i.e.,
The Cultural Regulation of Emotions 295
express less) or masking (i.e., hide emo-
tion with smile). The authors found that
the country’s scores on individualism (as
indexed by Hofstede, 2001) predicted the
country’s mean level of emotional expressiv-
ity. However, several qualifications of these
results are in order.
First, much of the relationship between
individualism and expression was “carried
by the relationship between individualism
with happiness and surprise” (Matsumoto
et al., 2008, p. 66). Thus, whereas people
in the United States, Canada, and Australia
expressed a desire to up- regulate happiness
and surprise, people in East Asian countries
indicated that they would actively seek to
avoid these emotional states (see also Tsai et
al., 2006). Second, nearly all of the emotions
included in the current study except sadness
were high- activation (disengaging) emo-
tions. Since the expression of high- activation
disengaged emotions would be more incon-
sistent with the prevalent cultural models,
there may indeed be a higher need to regu-
late these types of emotions in East Asian
than in Western cultural contexts. Conse-
quently, the association between emotion
expressivity and individualism may not have
been found (or even be in the opposite direc-
tion) if only low-activation engaging emo-
tional experiences had been included— an
idea that is supported by the negative (par-
tial) correlation between individualism and
emotional expressivity of sadness (Matsu-
moto et al., 2008). Therefore, rather than
concluding that individuals in collectivist
cultures suppress their emotions more than
individuals in individualist cultures, a bet-
ter conclusion might be that people across
cultural contexts engage in the suppression
of those emotions that are inconsistent with
the pertinent cultural models.
There are also cultural differences in both
the effectiveness and costs of emotion sup-
pression (Cheung & Park, 2010; Mauss &
Butler, 2010; Soto, Perez, Kim, Lee, & Min-
nick, 2011) Convergent results from experi-
mental and questionnaire studies show that
suppression of emotional responses is effort-
ful in European American samples, and
although effective in modifying emotional
behavior, it does not change the emotional
experience. Furthermore, in European
American samples, suppression is associ-
ated with increases in physiological arousal
and with detrimental effects on memory,
felt affect (less positive and no less nega-
tive emotion), and social relationships (e.g.,
Gross, 1998; Richards & Gross, 2000).
Finally, habitual suppression in European
Americans was related to both high levels of
depressive symptoms and low levels of life
satisfaction (Cheung & Park, 2010; Soto et
al., 2011).
In Western cultures, emotional suppres-
sion has therefore been found to be maladap-
tive. However, there are many indications
that this is not the case in Asian American
or East Asian samples. First, emotion sup-
pression may be more effective in these sam-
ples. For example, in a study by Mauss and
Butler (2010), Asian Americans who valued
emotional control were not only coded as
expressing less anger but they also reported
feeling less angry than before. Moreover,
they showed a “challenge pattern” of car-
diovascular responding, suggesting that the
suppression was not that effortful, because
their resources met or exceeded the demands
of the situation. Second, habitually engag-
ing in emotionally suppressive behavior
was unrelated to psychological well-being
in Hong Kong Chinese participants (Soto
et al., 2011) and much less associated with
depression in Asian American compared to
European American respondents (Cheung &
Park, 2010).
The different meanings of suppression can
be understood from the respective cultural
models (see Mesquita & Delvaux, 2012).
Whereas the expression of one’s feeling in
independent cultural contexts defines iden-
tity, it does not do so in interdependent con-
texts. These latter contexts emphasize situ-
ational adjustment, and may thus encourage
suppression of emotions whenever the situa-
tion requires it.
Culturally comparative work has focused
on up- and down- regulation of emotions but
has not paid much attention to the quality
of emotional behavior: What do people usu-
ally do when they feel a certain emotion?
Descriptive, ethnographic work has sug-
gested that individuals in some cultures have
specific behavioral scripts associated with
certain emotions (Mesquita & Frijda, 1992).
These behavioral scripts may be considered
the outcome of regulation to the extent that
they serve the prevalent cultural models of
self and relationships. A good illustration
296 SOCIAL ASPECTS
is the Balinese reaction to socially threat-
ening events, falling asleep (Bateson &
Mead, 1942), which can be understood as
an expression of the emotion of fear that
avoids disrupting the cultural model. Falling
asleep satisfies, at least subjectively, the goal
of reducing one’s exposure to threat, while
avoiding the emotional disruption that other
fear responses are felt to cause. Therefore,
falling asleep can be considered a culturally
effective form of individual emotion regula-
tion.
In summary, cultural models implicate
display rules for emotion regulation: Indi-
viduals tend to suppress emotions that are
inconsistent with the cultural models. Emo-
tional suppression is an individual regula-
tion strategy that requires different efforts,
that differs in effectiveness and is associated
with different psychological costs across dif-
ferent cultural contexts, in ways that can
be understood from the cultural models of
self and relationship. Furthermore, cultures
may model specific behaviors that meet the
cultural models. When individuals engage in
behavior that is culturally modeled, we call
this regulation. We do not think that indi-
viduals necessarily suppress another (more
natural) response before engaging in culture-
specific behavior. Rather, the fact that they
choose a culturally valued, rather than a less
valued response, makes this a meaningful
case of cultural regulation.
Social Regulation of Behavior
Across cultures, the preferred contexts and
practices for behavioral expression differ as
well. Certain social contexts provide oppor-
tunity for emotional expression, whereas
other cultural contexts inhibit the display of
emotions, or of certain emotions. Rituals are
an example: “Rituals consist of prescribed
behavior modes that remove the need to
expose one’s individual feelings. Yet at the
same time, they form opportunities to vent
one’s emotions in a socially acceptable way”
(Mesquita & Frijda, 1992, p. 197). A good
example of a ritual that appears to provide
an institutionalized means for expressing
emotional behaviors that would otherwise
conflict with the prevailing cultural model
was described for the Philippine Ilongots by
the anthropologist Michelle Rosaldo. In this
small community, in-group harmony was
essential for survival of the group. Ilongots,
therefore, saw it as a communal responsibil-
ity to regulate emotions that could threaten
the harmony of the group. Liget—an emo-
tion denoting energy, passion, and anger
simultaneously— was one of those threaten-
ing emotions (Rosaldo, 1980). A ritual of
headhunting was in place, should feelings
of liget arise. When one or more Ilongot
men experienced the heavy feeling of liget,
a group of them would go out to kill an out-
sider. After the beheading, the Ilongot men
came home purged of violence, and the com-
munity celebrated the overcoming of liget
by singing together. The ritual thus directed
the behavioral intentions elicited by liget
toward an out-group target, channeling a
potentially intraculturally disruptive behav-
ior into one that reinforced the solidarity of
the group. Rituals may thus be understood
as prototypes for the mechanisms by which
sociocultural practices afford opportunities
to express (or act on) emotions in culturally
supported ways.
In summary, there are cultural differences
in both individual and social behavioral reg-
ulation. Some of these are differences in sup-
pression versus expressivity. However, the
connotations of suppression are not cultur-
ally the same. Other differences are better
seen as differences in the selection of behav-
ior, often in the function of the models and
practical opportunities for behavior that the
culture provides.
Cultural Differences in the Preference
for Different Strategies
of Emotion Regulation
In a number of studies that have compared
cultural preferences for particular types of
emotion regulation, the suppression of emo-
tional responses was not contrasted with
expression, but rather with reappraisal, a
different type of emotion regulation. In dif-
ferent studies, participants from indepen-
dent cultural contexts expressed a stronger
preference for reappraisal over suppression
than participants in interdependent cultural
contexts, for whom this preference was
sometimes even absent (e.g., Novin, Ban-
jeree, Dadhkah, & Rieffe, 2009; Soto et al.,
2011). These differential preferences may
correspond to different cultural models. In
independent cultural contexts, the expres-

The Cultural Regulation of Emotions 297
sion of one’s feeling is identity defining. A
change in emotional expression achieved by
reappraisal respects the link between feeling
and expression. On the other hand, suppres-
sion of emotions seems fine in interdepen-
dent cultural contexts, where the emphasis
is on situational adjustment; suppression
and reappraisal would be equivalent means
to reaching this goal. Among respondents
from independent cultural contexts, the use
of suppression is inversely related to the use
of reappraisal, suggesting that they more
readily use the one or the other strategy.
In contrast, there is a positive correlation
between the use of suppression and reap-
praisal among respondents from interdepen-
dent cultural contexts, suggesting that they
use both strategies when they attempt to
regulate their emotions (Matsumoto et al.,
2008).
Conclusion
There is substantial evidence of the cultural
regulation of emotions, conceived here as
the combined mechanisms that align an
individual’s emotional experience to the per-
tinent cultural model. Cultural regulation
takes place not only at the level of culture,
by way of the habitual social practices and
interpersonal interactions that constitute
an individual’s lived environment, but also
at the level of individual schemas, goals,
and modes of attention. Moreover, regula-
tion takes place at all the different stages of
the emotion: Both cultural and individual
processes are instrumental in creating and
modifying emotional events, channeling an
individual’s attention, and shaping an indi-
vidual’s appraisals of the situation in ways
that promote culturally desirable emotions;
moreover, both cultural and individual
processes shape emotion expression into
congruence with cultural norms. Cultural
practices and psychological tendencies thus
co- constitute emotional experiences and
expressions that are aligned with prevailing
cultural models of self and relating.
At the level of the individual, emotion
regulation is often implicit and automatic,
although it can also be conscious and effort-
ful. When it is automatic, it can be con-
ceived in terms of psychological tendencies:
the tendency to engage in certain types of
interactions, to focus attention on certain
aspects of the situation, and to appraise a
situation in a certain way. These psychologi-
cal tendencies may originate from culture-
level regulation. Thus, individuals’ tenden-
cies to experience certain emotions may be
a consequence of repeated exposure to a cul-
tural set of daily situations (see Kitayama &
Imada, 2010). For instance, a habitual focus
on controllability may chronically increase
the propensity to appraise a situation as one
of high control, and thus of experiencing
anger. In the same way, caregivers’ repeated
emphasis on the child’s inability to meet
social standards may bias the child’s focus
toward noticing failures, thus enhancing the
tendency to feel shame. The resulting regula-
tion is implicit and antecedent- focused.
The large cultural differences observed in
everyday emotional experience suggest that
emotion regulation is widespread. How-
ever, given the functional role of emotions
in guiding adaptive behavior, we contend
that effortful emotion regulation must be
very limited; it is “culture’s last resort” for
shaping emotions in a culturally normative
fashion, used only when all other means
have failed. Most regulatory processes must
be automatic, as in the culturally different
psychological tendencies that align an indi-
vidual’s experience and expression with the
cultural models.
The cultural perspective contributes sev-
eral insights to emotion regulation generally.
First, it suggests that while the goal of emo-
tion regulation may universally be proper
self- presentation and relationship mainte-
nance, specific cultural models define the
norms for self- presentation, as well as the
ways to maintain a relationship. Cultural
models thus set specific goals for emotion
regulation. In this way, emotion regulation
ties a person to the most important cultural
goals and values, just as it ties a person to his
or her personality (e.g., Tamir, 2005).
Second, the cultural perspective suggests
that emotion regulation is not merely an
intrapersonal process. Rather, emotions are
importantly regulated by the ways in which
our worlds are structured and our lives are
organized. We have illustrated how emotion
is regulated at the level of cultural practices
through the structuring of social situations
and the dynamics of social interactions;
close others’ attempts to modify the indi-

298 SOCIAL ASPECTS
vidual’s focus of attention within a situation;
and the opportunities afforded for emo-
tional behavior. The structure of everyday
life can be seen as the primary way in which
emotions are culturally regulated, although
at this level the distinction between emotion
generation and emotion regulation is mute
(see also Kappas, 2011; Mesquita & Frijda,
2011).
Taken together, the results suggest that
there is “redundancy” in the cultural regu-
lation of emotions (Levy, 1978), such that
regulatory processes at many different loci
of the emotion, and as initiated by many
different sources, converge into a pattern of
emotional experience and expression that
fits the cultural model (more than not).
References
Abu- Lughod, L. (1986). Veiled sentiments.
Berkeley: University of California Press.
Adams, G., & Markus, H. R. (2004). Toward
a conception of culture suitable for a social
psychology of culture. In M. Schaller & C. S.
Crandall (Eds.), Psychological foundations of
culture (pp. 335–360). Mahwah, NJ: Erlbaum.
Bargh, J. A., & Williams, L. E. (2007). The
nonconscious regulation of emotion. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 429–445). New York: Guilford Press.
Bateson, G., & Mead, M. (1942). Balinese char-
acter: A photographic analysis. New York:
New York Academy of Sciences.
Benedict, R. (1946). The chrysanthemum and
the sword. Patterns of Japanese culture. Bos-
ton: Houghton Mifflin.
Boiger, M., & Mesquita, B. (2012). The construc-
tion of emotion in interactions, relationships,
and cultures. Emotion Review, 4, 221–229.
Boiger, M., Mesquita, B., Tsai, A., & Markus,
H. (2012). Influencing and adjusting in daily
emotional situations: A comparison of Euro-
pean and Asian American action styles. Cog-
nition and Emotion, 26, 332–340.
Boiger, M., Mesquita, B., Uchida, Y., & Barrett,
L. F. (2013). Condoned or condemned: The
situational affordance of anger and shame in
Japan and the US. Personality and Social Psy-
chology Bulletin, 39(4), 540–553.
Bruner, J. (1990). Acts of meaning. Cambridge,
MA: Harvard University Press.
Campos, J. J., & Stenberg, C. (1981). Perception,
appraisal, and emotion: The onset of social
referencing. In M. E. Lamb & L. R. Sher-
rod (Eds.), Infant social cognition: Empirical
and theoretical considerations (pp. 273–314).
Hillsdale, NJ: Erlbaum.
Cheung, R. Y. M., & Park, I. J. K. (2010). Anger
suppression, interdependent self- construal,
and depression among Asian American and
European American college students. Cul-
tural Diversity and Ethnic Minority, 16(4),
517–525.
Cohen, D., Hoshino- Browne, E., & Leung, A.
K.-Y. (2007). Culture and the structure of
personal experience: Insider and outsider phe-
nomenologies of the self and social world. In
M. P. Zanna (Ed.), Advances in experimental
social psychology (Vol. 39, pp. 1–67). San
Diego, CA: Academic Press.
Cole, P. M., Tamang, B. L., & Shrestha, S.
(2006). Cultural variations in the socialization
of young children’s anger and shame. Child
Development, 77, 1237–1251.
Conroy, M., Hess, R. D., Azuma, H., & Kashi-
wagi, K. (1980). Maternal strategies for
regulating children’s behavior: Japanese and
American families. Journal of Cross- Cultural
Psychology, 11, 153–172.
D’Andrade, R. G. (1984). Culture meaning sys-
tems. In R. A. Shweder & R. A. Levine (Eds.),
Culture theory: Essays on mind, self, and
emotion (pp. 88–119). Cambridge, UK: Cam-
bridge University Press.
De Leersnyder, J., Boiger, M., & Mesquita, B.
(2013). Cultural regulation of emotion: Indi-
vidual, relational, and structural sources.
Frontiers in Psychology, 4(55). [E-publication
prior to print]
De Leersnyder, J., & Mesquita, B. (2013). What
are emotions about?: The role of cultural val-
ues in emotional experience. Manuscript in
preparation.
De Leersnyder, J., Mesquita, B., & Kim, H.
(2011). Where do my emotions belong?: A
study of immigrants’ emotional acculturation.
Personality and Social Psychology Bulletin,
37, 451–463.
De Leersnyder, J., Mesquita, B., & Kim, H.,
(2013). Emotional acculturation. In D. Her-
mans, B. Mesquita, & B. Rime (Eds.), Chang-
ing emotions (pp. 127–133). Hove, UK: Psy-
chology Press.
De Leersnyder, J., Mesquita, B., & Kim, H.
(2013). Emotional fit with culture: A predic-
tion of individual differences in relational
well-being. Manuscript under review.
Diamond, L. M., & Aspinwall, L. G. (2003).
The Cultural Regulation of Emotions 299
Emotion regulation across the life span: An
integrative perspective emphasizing self-
regulation, positive affect, and dyadic pro-
cesses. Motivation and Emotion, 27, 125–156.
Eid, M., & Diener, E. (2001). Norms for expe-
riencing emotions in different cultures: Inter-
and intranational differences. Journal of
Personality and Social Psychology, 81(5),
869–885.
Ekman, P., & Friesen, W. V. (1969). The reper-
toire of nonverbal behavior: Categories, ori-
gins, usage, and coding. Semiotica, 1, 49–98.
Elliott, A., Chirkov, V., Kim, Y., & Sheldon, K.
(2001). A cross- cultural analysis of avoidance
(relative to approach) personal goals. Psycho-
logical Science, 12, 505–510.
Ellsworth, P. C. (1994). Sense, culture, and sensi-
bility. In S. Kitayama & H. R. Markus (Eds.),
Emotion and culture: Empirical studies of
mutual influence (pp. 23–50). Washington,
DC: American Psychological Association.
ESS Round 5: European Social Survey Round 5
Data. (2012). Data file edition 3.0. Norway:
Norwegian Social Science Data Services, data
archive and distributor of ESS data.
Frijda, N. H. (1986). The emotions. Cambridge,
UK: Cambridge University Press.
Frijda, N. H., Kuipers, P., & Terschure, E. (1989).
Relations between emotion, appraisal and
emotional action readiness. Journal of Person-
ality and Social Psychology, 57, 212–228.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J., Sheppes, G., & Urry, H. L. (2011).
Emotion generation and emotion regulation: A
distinction we should make (carefully). Cogni-
tion and Emotion, 25, 765–781.
Grossmann, I., Ellsworth, P. C., & Hong, Y.
(2012). Culture, attention, and emotion. Jour-
nal of Experimental Psychology: General,
14(1), 31–36.
Gyurak, A., Gross, J. J., & Etkin, A. (2011).
Explicit and implicit emotion regulation: A
dual- process framework. Cognition and Emo-
tion, 25(3), 400–412.
Heine, S. J., Lehman, D. R., Markus, H. R., &
Kitayama, S. (1999). Is there a universal need
for positive self- regard? Psychological Review,
106(4), 766–794.
Hochschild, J. L. (1995). What is the American
dream? In J. L. Hochschild (Ed.), Facing up
to the American dream: Race, class and the
soul of the nation (pp. 15–38). Princeton, NJ:
Princeton University Press.
Hofstede, G. H. (2001). Culture’s consequences:
Comparing values, behaviors, institutions
and organizations across nations (2nd ed.).
Thousand Oaks, CA: Sage.
Kappas, A. (2011). Emotion and regulation are
one! Emotion Review, 3, 17–25.
Kim, H., & Markus, H. R. (1999). Deviance
or uniqueness, harmony or conformity?: A
cultural analysis. Journal of Personality and
Social Psychology, 77(4), 785–800.
Kitayama, S., & Imada, T. (2010). Implicit inde-
pendence and interdependence: A cultural task
analysis. In B. Mesquita, L. F. Barrett, & E. R.
Smith (Eds.), The mind in context (pp. 174–
200). New York: Guilford Press.
Kitayama, S., Karasawa, M., & Mesquita, B.
(2004). Collective and personal processes
in regulating emotions: Emotion and self
in Japan and the US. In P. Philippot & R. S.
Feldman (Eds.), The regulation of emotion
(pp. 251–273). Hillsdale, NJ: Erlbaum.
Kitayama, S., & Markus, H. R. (2000). The pur-
suit of happiness and the realization of sym-
pathy: Cultural patterns of self, social rela-
tions, and well-being. In E. Diener & E. Suh
(Eds.), Subjective well-being across cultures
(pp. 113–161). Cambridge, MA: MIT Press.
Kitayama, S., & Masuda, T. (1995). Reapprais-
ing cognitive appraisal from a cultural per-
spective. Psychological Inquiry, 6, 217–223.
Kitayama, S., Mesquita, B., & Karasawa, M.
(2006). The emotional basis of independent
and interdependent selves: Socially disengag-
ing and engaging emotions in the US and
Japan. Journal of Personality and Social Psy-
chology, 91(5), 890–903.
Koole, S. L. (2009). The psychology of emotion
regulation: An integrative review. Cognition
and Emotion, 23(1), 4 – 41.
Lebra, T. S. (1992). Self in Japanese culture. In
N. E. Rosenberger (Ed.), Japanese sense of
self. New York: Oxford University Press.
Lee, A. Y., Aaker, J. L., & Gardner, W. L.
(2000). The pleasures and pains of distinct
self- construals: The role of interdependence in
regulatory focus. Journal of Personality and
Social Psychology, 78(6), 1122–1134.
Levy, R. I. (1978). Tahitian gentleness and redun-
dant controls. In A. Montagu (Ed.), Learn-
ing non- aggression: The experience of non-
literate societies (pp. 222–235). New York:
Oxford University Press.
Lewis, C. C. (1995). Educating hearts and minds.
New York: Cambridge University Press.
Lutz, C. (1987). Goals, events, and understand-
300 SOCIAL ASPECTS
ing in Ifaluk emotion theory. In N. Quinn &
D. Holland (Eds.), Cultural models in lan-
guage and thought (pp. 290–312). New York:
Cambridge University Press.
Markus, H. R., & Kitayama, S. (1991). Culture
and self: Implications for cognition, emotion,
and motivation. Psychological Review, 98(2),
224–253.
Markus, H. R., & Kitayama, S. (1994). The
cultural construction of self and emotion:
Implications for social behavior. In S. Kita-
yama & H. R. Markus (Eds.), Emotion and
culture: Empirical studies of mutual influence
(pp. 89–130). Washington, DC: American Psy-
chological Association.
Masuda, T., Ellsworth, P. C., Mesquita, B., Leu,
J., Tanida, S., & Veerdonk, E. (2008). Plac-
ing the face in context: Cultural differences
in the perception of facial emotion. Journal
of Personality and Social Psychology, 94(3),
365–381.
Matsumoto, D. (1990). Cultural similarities and
differences in display rules. Motivation and
Emotion, 14(3), 195–214.
Matsumoto, D., Yoo, S. H., Fontaine, J., Anguas-
Wong, A. M., Arriola, M., & Ataca, B. (2008).
Mapping expressive differences around the
world: The relationship between emotional
display rules and individualism versus collec-
tivism. Journal of Cross- Cultural Psychology,
39(1), 55–74.
Mauss, I. B., Bunge, S. A., & Gross, J. J. (2008).
Culture and automatic emotion regulation.
In S. Ismer, S. Jung, S. Kronast, C. van Sch-
eve, & M. Vanderkerckhove (Eds.), Regulat-
ing emotions: Culture, social necessity and
biological inheritance (pp. 39–60). London:
Blackwell.
Mauss, I. B., & Butler, E. A. (2010). Cultural
context moderates the relationship between
emotion control values and cardiovascular
challenge versus threat responses. Biological
Psychology, 84, 521–530.
Mesquita, B., & Delvaux, E. (2012). A cultural
perspective on emotion labor. In A. Grandey,
J. Diefendorff, & D. Rupp (Eds.), Emotional
labor in the 21st century: Diverse perspectives
on emotion regulation at work (pp. 251–272).
New York: Psychology Press/Routledge.
Mesquita, B., & Ellsworth, P. C. (2001). The role
of culture in appraisal. In K. R. Scherer & A.
Schorr (Eds.), Appraisal processes in emotion:
Theory, methods, research (pp. 233–248).
New York: Oxford University Press.
Mesquita, B., & Frijda, N. H. (1992). Cultural
variations in emotions: A review. Psychologi-
cal Bulletin, 112(2), 179–204.
Mesquita, B., & Frijda, N. H. (2011). An emo-
tion perspective on emotion regulation. Cog-
nition and Emotion, 25, 782–784.
Mesquita, B., & Leu, J. (2007) The cultural
psychology of emotions. In S. Kitayama & D.
Cohen (Eds.), Handbook for cultural psychol-
ogy (pp. 734–759). New York: Guilford Press.
Mesquita, B., Marinetti, C., & Delvaux, E.
(2012). The social psychology of emotions. In
S. Fiske & N. Macrae (Eds.), Sage handbook
of social cognition (pp. 290–310). Thousand
Oaks, CA: Sage.
Miller, P. J., Fung, H., & Mintz, J. (1996). Self-
construction through narrative practices: A
Chinese and American comparison of early
socialization. Ethos, 24(2), 237–280.
Morelli, G. A., Rogoff, B., Oppenheim, D., &
Goldsmith, D. (1992). Cultural variation in
infants’ sleeping arrangements: Questions of
independence. Developmental Psychology,
28(4), 604–613.
Morling, B., Kitayama, S., & Miyamoto, Y.
(2002). Cultural practices emphasize influence
in the United States and adjustment in Japan.
Personality and Social Psychology Bulletin,
28(3), 311–323.
Nisbett, R. E. (2003). The geography of thought.
How Asians and Westerners think differ-
ently . . . and why. New York: Free Press.
Novin, S., Banjeree, R., Dadhkah, A., & Rieffe,
C. (2009). Self- reported use of display use in
the Netherlands and Iran: Evidence for socio-
cultural influence. Social Development, 18(2),
397– 411.
Parkinson, B., & Simons, G. (2009). Affecting
others: Social appraisal and emotion contagion
in everyday decision making. Personality and
Social Psychology Bulletin, 35, 1071–1084.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79, 410–424.
Rosaldo, M. Z. (1980). Knowledge and passion:
Ilongot notions of self and social life. New
York: Cambridge University Press.
Roseman, I., Dhawan, N., Rettek, S., Naidu, R.
K., & Thapa, K. (1995). Cultural differences
and cross- cultural similarities in appraisals
and emotional responses. Journal of Cross-
Cultural Psychology, 26(1), 23– 48.
Rothbaum, F., Pott, M., Azuma, H., Miyake,
K., & Weisz, J. (2000). The development of
close relationships in Japan and the United
The Cultural Regulation of Emotions 301
States: Paths of symbiotic harmony and gen-
erative tension. Child Development, 71(5),
1121–1142.
Savani, K., Markus, H. R., Naidu, N. V. R.,
Kumar, S., & Berlia, N. (2010). What counts
as a choice?: U.S. Americans are more likely
than Indians to construe actions as choices.
Psychological Science, 21, 391–398.
Shweder, R. A. (1991). Thinking through cultures.
Cambridge, MA: Harvard University Press.
Solomon, R. C. (2004). Thinking about feeling:
Contemporary philosophers on emotions.
New York: Oxford University Press.
Soto, J. A., Perez, C. R., Kim, Y.-H., Lee, E.
A., & Minnick, M. R. (2011). Is expressive
suppression always associated with poorer
psychological functioning?: A cross- cultural
comparison between European Americans
and Hong Kong Chinese. Emotion, 11(6),
1450–1455.
Stein, N. L., Trabasso, T., & Liwag, M. (1993).
The representation and organization of emo-
tional experience: Unfolding the emotion epi-
sode. In M. Lewis & J. M. Haviland (Eds.),
Handbook of emotions (pp. 279–300). New
York: Guilford Press.
Tamir, M. (2005). Don’t worry, be happy?: Neu-
roticism, trait- consistent affect regulation,
and performance. Journal of Personality and
Social Psychology, 89, 449– 461.
Trommsdorff, G., & Friedlmeier, W. (2010). Pre-
school girls’ distress and mothers’ sensitivity
in Japan and Germany. European Journal of
Developmental Psychology, 7, 350–370.
Trommsdorff, G., & Kornadt, H. (2003). Parent–
child relations in cross- cultural perspective. In
L. Kuczynski (Ed.), Handbook of dynamics in
parent– child relations (pp. 271–305). Thou-
sand Oaks, CA: Sage.
Tsai, J. L., Knutson, B., & Fung, H. H. (2006).
Cultural variation in affect valuation. Jour-
nal of Personality and Social Psychology, 90,
288–307.
Tsai, J. L., Miao, F., Seppala, E., Fung, H. H.,
& Yeung, D. (2007). Influence and adjustment
goals: Mediators of cultural differences in
ideal affect. Journal of Personality and Social
Psychology, 92(6), 1102–1117.
Weisz, J. R., Rothbaum, F. M., & Blackburn, T.
C. (1984). Standing out and standing in: The
psychology of control in America and Japan.
American Psychologist, 39(9), 955–969.
Wierzbicka, A. (1994). Emotion, language,
and cultural scripts. In S. Kitayama & H. R.
Markus (Eds.), Emotion and culture: Empiri-
cal studies of mutual influence (pp. 133–196).
Washington, DC: American Psychological
Association.
Zahn- Waxler, C., Radke- Yarrow, M., & King,
R. A. (1979). Child rearing and children’s pro-
social initiations toward victims of distress.
Child Development, 50, 319–330.

PART VI
PERSONALITY PROCESSES
AND INDIVIDUAL DIFFERENCES

305
In recent years, advances in research on tem-
perament have contributed to many other
areas of psychology, including socioemo-
tional and personality development, psycho-
pathology, emotion, and emotion regulation
(Zentner & Shiner, 2012). Temperament
refers to constitutionally based individual
differences in reactivity and self- regulation,
and historically, has been linked to the biol-
ogy of the person (Rothbart, 2011). Recent
temperament research has employed increas-
ingly sophisticated psychometric methods,
developing a basic taxonomy of tempera-
ment that extends from infancy to adult-
hood and links temperament to neural anat-
omy and function.
In our research, broad factors of Fear,
Frustration, Negative Affectivity, Extraver-
sion/Surgency, Affiliativeness, Orienting/
Perceptual Sensitivity, and Effortful Control
have been extracted from questionnaire data
(Putnam, Ellis, & Rothbart, 2001; Roth-
bart, 2011). These dimensions show strong
similarities to those found in other laborato-
ries, and reactive and self- regulative aspects
of temperament have been observed in both
the laboratory and in parent and self- report
data (Zentner & Shiner, 2012).
In this chapter we describe concepts of
emotion and emotion regulation within a
temperament framework. We first put for-
ward definitions of temperament, personal-
ity, emotion, and emotion regulation. We
then consider how defense and approach
emotion systems regulate each other, fol-
lowed by a review of links between emotion
regulation and individual differences in ori-
enting. We then examine in detail the rela-
tion of emotion regulation to temperamen-
tal effortful control (EC) and describe the
underpinnings of EC in the brain’s executive
attention network. Because temperament
develops, and because EC provides a general
mechanism for the control of emotion that
is not well- developed until early childhood
(Rueda, 2012), we describe emotion regu-
lation in a developmental context. Finally,
we raise empirical issues about the study
of emotional reactivity and regulation, and
describe future directions for research.
Definitions of Key Terms
Rothbart and Bates (2006) define tempera-
ment as “constitutionally based individual
differences in reactivity and self- regulation,
in the domains of affect, activity, and atten-
tion.” This chapter focuses on the tempera-
ment domains of affect and attention, and
the term affect is used synonymously with
emotion. The term constitutional indicates
that temperament is biologically based and
influenced over time by heredity, matura-
CHAPTER 19
Temperament and Emotion Regulation
Mary K. Rothbart
Brad E. Sheese
Michael I. Posner

306 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
tion, and experience. Reactivity and self-
regulation are terms originally used broadly
by Rothbart and Derryberry (1981) to orga-
nize the temperament domain. Reactivity
refers to responses to change in the external
and internal environment, including reac-
tions that can be conceptualized broadly
or narrowly (e.g., fear, motor activity, ori-
enting, negative affectivity, cardiac reactiv-
ity). Reactivity is measured in terms of the
latency, duration, and intensity of affective,
motor, and orienting reactions (Rothbart &
Derryberry, 1981). Self- regulation involves
effortful attention that serves to modulate
reactivity and organize change.
Individual differences in temperament
fall within the more general rubric of per-
sonality. We define personality as individual
differences in dispositional traits, coping
strategies, and cognitions of self and oth-
ers that influence the person’s adjustments
to the environment. Dispositional traits are
patterns of thoughts, emotions, and behav-
ior that show consistency across situations
and stability over time (Allport, 1937), and
temperament traits constitute a subset of
personality traits (Rothbart, 2011). Tem-
perament traits include consistent patterns
of reactivity of the emotions and the self-
regulation of thoughts, emotions, and action
through attention. We note that this defini-
tion relates temperament to personality and
at the same time differentiates temperament
from other personality domains.
Personality goes beyond temperament to
include attitudes, cognitive coping strate-
gies, concepts of self and other, values, mor-
als, and beliefs, but there is also evidence
that the broad personality traits of Extra-
version, Agreeableness, Conscientiousness,
Neuroticism, and Openness may be orga-
nized around the basic temperament traits
that can be observed early in life (McCrae et
al., 2000; Rothbart, 2011; Shiner & Caspi,
2012).
Emotions can be seen as broadly integra-
tive systems that order feelings, thoughts,
and actions (LeDoux, 1989, 2000). They
represent the output of neural information-
processing networks that assess the mean-
ing or affective significance of events for the
individual. Whereas object recognition sys-
tems and spatial processing systems address
the questions “What is it?” and “Where is
it?,” emotion- processing networks address
the questions “Is it good for me?”; “Is it bad
for me?”; and “What shall I do about it?”
(action tendencies). These networks have
been evolutionarily conserved to allow the
organism to deal with environmental and
internally generated threat and opportu-
nity. When we discuss temperamental reac-
tivity, we include individual differences in
emotion- processing networks. Because the
emotions include both action tendencies and
physiological support for these tendencies,
they have regulatory aspects, and one emo-
tion can regulate another. Thus, fear poten-
tiates withdrawal, attack, or inhibition, and
positive affectivity potentiates the speed and
energy of approach.
By emotion regulation, we mean the
modulation of a given emotional reaction,
including its inhibition, activation, or graded
modulation. Down- and up- regulation refer
to general reductions or increases in the acti-
vation of emotion- related neural networks.
Emotion regulation includes attentional
strategies employed through effortful con-
trol (EC), as well as the modulating effects
of other emotions and orienting. Orienting
early in life is chiefly reactive, and when
distractors are presented to infants, orient-
ing modulates the expression of the infants’
emotions (Harman, Rothbart, & Posner,
1997). Later, orienting comes more under
the control of executive attention. A purer
form of self- regulation is seen in the execu-
tive attentional processes underlying EC
that regulate reactivity (Posner & Rothbart,
2007a, 2007b).
Thus when we consider individual differ-
ences in temperament, we are dealing with
a dynamic balance between emotional ten-
dencies, and between emotions and atten-
tion. Again, it is important to recognize
that emotion regulation strategies go far
beyond temperament to reflect cognitive and
experience- dependent behavioral strategies,
although temperamental characteristics are
likely to be involved in their development
(Rueda, 2012).
Temperament and
Emotion–Motivation
Emotion, cognition, and behavior are orga-
nized around the goals of the organism
(Campos, Caplovitz, Lamb, Goldsmith, &
Temperament and Emotion Regulation 307
Stenberg, 1983; Derryberry & Rothbart,
1997). These goals have been evolutionarily
conserved in the nervous system but are fur-
ther programmed by the person’s specific
experiences, plans, and effortful actions.
Affective– motivational systems of emotion
evaluate goal- relevant situations and orga-
nize goal- appropriate behavior. Based on
a review of the literature, Derryberry and
Rothbart (1997) described temperament
systems that include emotional components.
These include systems that support defense
and fear, approach and appetitive behaviors,
and nurturance/affiliation. Here, we con-
centrate on the first two of these, the defense
and approach systems.
Defense and Approach Systems
The term defense describes a system of brain
networks that serve to avoid harm by pro-
moting organized responses to immediate
and long-term threats (Derryberry & Roth-
bart, 1997). Portions of the lateral and cen-
tral amygdala appear to be key neural struc-
tures in the functioning of this network.
Fear, anxiety, and defensive anger are emo-
tions that reflect activation of the defense
system. These emotions are related to
behavioral tendencies to inhibition or with-
drawal from active threats and avoidance of
potentially threatening situations in a flight
response. When withdrawal from severe
threats is blocked, however, the defense sys-
tem also serves to promote defensive aggres-
sion and a fight response. Systems analogous
to the defense system are common to many
psychobiological approaches to emotion,
including LeDoux’s (1989) fear system and
Gray’s behavioral inhibition system (Gray &
McNaughton, 1996; see review by Zucker-
man, 2012).
Fear and anxiety are aversive states com-
monly associated with various forms of
dysfunction and pathology in the clinical
literature (Rothbart & Posner, 2006). Con-
sequently, it might be expected that indi-
viduals exhibiting high levels of fear would
be more prone to pathology than those
exhibiting lower levels, and there is some
evidence that this is the case (Rothbart &
Bates, 2006). A psychobiological approach,
however, notes that fear and anxiety are
evolutionarily conserved to promote poten-
tially adaptive behavior when signals of dan-
ger are detected, so very low levels of fear
and anxiety can also be problematic. There
is a need for some level of fear and anxiety
to promote adaptive patterns of respond-
ing, while under- or overactivation of the
system may promote maladaptive respond-
ing. Although all negative emotions contrib-
ute to general negative affectivity or stress
proneness, we have also found it useful to
differentiate anger from fear (Rothbart,
2011; Rothbart & Bates, 2006). Anger is
positively related to the approach (Extraver-
sion/Surgency) system described next, and
it is particularly linked to the development
of externalizing problems (Rothbart, 2011;
Rothbart & Bates, 2006).
An approach system serves the goal of
resource acquisition by promoting organized
responses to potential rewards (Derryberry
& Rothbart, 1997). Dopaminergic neurons
appear to form the core of this network,
which also extends to the basolateral amgy-
dala, ventral tegmental area, and the nucleus
accumbens. Positive emotional states, such
as joy and elation, as well as feelings of eager
anticipation, are thought to reflect the broad
alterations in neurological and physiologi-
cal responding that occur in the activation
of approach. These serve to bias responding
toward positive emotion and reward acquisi-
tion, and can be seen in behavioral tendencies
to seek out and approach rewards and atten-
tional sensitivity to rewards (Derryberry
& Reed, 1994). When goals are blocked,
anger and possibly aggression may result (see
review by Deater- Deckard & Wang, 2012).
Co‑Occuring Approach
and Defense Reactions
When situations present potential reward
along with risk, both approach and defense
systems can become active and may either
cooperate or compete to influence process-
ing. In some networks, there appears to be
localized competition affecting specific cor-
tical and subcortical processes in a winner-
take-all process, in which the “stronger” sys-
tem dominates (Norman & Shallice, 1986).
However, over time, activation strength may
wax and wane, leading to an alternation of
opposing behaviors or to the appearance of
disorganized or inconsistent responding. In
our laboratory, we have seen such oppos-
ing behaviors in infants, who show abrupt

308 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
alternation between approach- related and
withdrawal- related behaviors, such as smil-
ing directly followed by distress in response
to potentially threatening stimuli.
Inhibitory influences of defense and
approach systems may not be symmetrical.
Instead, defense systems appear to have
preferential status. Observation of a norma-
tive “negativity bias” in humans and other
animal species (e.g., Ito & Cacioppo, 2005)
suggests that defense tends to dominate
approach in situations that present both
risk and reward. While the balance may be
usually skewed toward defense, this bal-
ance may not characterize every individual.
Instead, individuals will differ in the degree
to which they exhibit a negativity bias based
on temperament and life experience (Ito &
Cacioppo, 2005).
Aspects of the approach system, includ-
ing sociability, smiling and laughter, and
approaching novel objects, can be observed
by 3 months of age, and show normative
increases over the first year of life (Rothbart,
Derryberry, & Hershey, 2000). Late in the
first year, some infants also begin to demon-
strate fear and related behavioral inhibition
of approach and positive emotion in response
to unfamiliar and intense stimuli (Rothbart,
1988), and fearful inhibition shows consid-
erable longitudinal stability across child-
hood and into adolescence (Kagan & Fox,
2006). In our longitudinal research, infant
fear was assessed by inhibited approach and
negative emotion in the laboratory, and it
predicted childhood fear, sadness, and shy-
ness at 7 years of age (Rothbart et al., 2000).
Fear also predicted lower approach, impul-
sivity, and aggression. These findings sug-
gest that fear is involved in the regulation of
both approach and aggressive tendencies (as
argued by Gray & McNaughton, 1996).
More fearful infants also showed greater
empathy, guilt, and shame in childhood
(Rothbart, Ahadi, & Hershey, 1994).
These findings suggested that fear might be
involved in the early development of moral
motivation, and Kochanska (1997) has
found that temperamental fearfulness pre-
dicts conscience development in preschool-
age children. Extreme fear, on the other
hand, may lead to problems through chil-
dren’s rigid control of their own behavior,
as in Block’s (2002) description of overcon-
trolled responses that can limit children’s
positive experiences. Thus, although fear
and its component inhibition allows the first
major control system over approach, it is a
reactive one that can lack flexibility.
Other temperament emotion systems also
regulate each other. For example, affiliative-
ness is related to lower anger/aggression,
and affiliativeness is also related to higher
internalizing negative affect, including sad-
ness (Rothbart, 2011). In this chapter, how-
ever, we focus on individual differences in
developing networks of attention and emo-
tion regulation. We begin with individual
differences in orienting.
Attention and Emotion Regulation
Orienting
Much of the early life of the infant is con-
cerned with the regulation of state, includ-
ing regulation of distress (Rothbart, 2011).
Orienting, that is, the selection of informa-
tion from sensory input (Posner, 2012), is a
major mechanism for this regulation (Pos-
ner, Rothbart, Sheese, & Voelker, 2012).
Caregivers report how they use attention
to regulate the state of the infant, distract-
ing their infants by bringing their attention
to other stimuli. As infants orient, they are
often quieted, and their distress appears to
diminish.
We have studied orienting and sooth-
ing in 3- to 6-month-old infants (Harman,
Rothbart, & Posner, 1997). Infants were
first shown a sound and light display that
led to distress in about 50% of the infants.
These infants strongly oriented to interest-
ing visual and auditory soothing events
when they were presented, and during their
orienting, facial and vocal signs of dis-
tress disappeared. As soon as the orienting
stopped (e.g., when the object was removed),
however, infant distress returned to almost
exactly the level shown prior to presentation
of the distractor. An internal system, which
we termed the “distress keeper,” appeared
to hold a computation of the initial level of
distress, so that it returned if the infant’s ori-
entation to the novel event was lost. In later
studies, we found that infants could be qui-
eted by distraction for as long as 1 minute,
without changing the eventual level of dis-
tress reached once the orienting had ended
(Harman et al., 1997).
Temperament and Emotion Regulation 309
For young infants, the control of orienting
is at first largely in the hands of caregiver
presentations. By 4 months, however, infants
have gained considerable control over disen-
gaging their gaze from one visual location
and moving it to another, and greater ori-
enting skill in the laboratory has been asso-
ciated with lower parent- reported negative
emotion and greater soothability (Johnson,
Posner, & Rothbart, 1991). Sustained atten-
tion, as indexed by engagement with low-
intensity toys (blocks) at 9 months, is also
predictive of observed effortful control at
22 months (Kochanska, Murray, & Harlan,
2000). Difficulty with sustaining attention
at 9 months predicts behavioral inhibition in
toddlers and social difficulties in adolescents
(Pérez-Edgar et al., 2010), and Crockenberg,
Leerkes, & Barrig (2008) found that infants
who focused more on frustrating stimuli at 6
months of age were more aggressive as tod-
dlers.
We observed a number of changes in emo-
tion regulation in longitudinal observations
of infants between 3 and 13 months (Roth-
bart, Ziaie, & O’Boyle, 1992). Older infants
increasingly looked to their mothers dur-
ing presentation of arousing stimuli such as
masks and unpredictable mechanical toys.
Infants’ disengagement of attention from
arousing stimuli was also related to lower
levels of negative affect in the laboratory at
13 months. Stability from 10 to 13 months
was found in infants’ use of attentional dis-
engagement, mouthing, hand to mouth (e.g.,
thumb sucking), approach, and withdraw-
ing the hand, suggesting that some of the
infants’ regulatory strategies were becom-
ing habitual. Over the period from 3 to 13
months, passive self- soothing decreased
and more active approach, attack on the
object, and body self- stimulation increased.
Infants who showed the greatest distress at
3 months, however, tended to persist in an
early form of regulation, self- soothing. Once
a mechanism for emotion regulation devel-
ops, it may persist because it has brought
relief, even though more sophisticated emo-
tion regulation mechanisms are now avail-
able, an important consideration in devel-
opmental approaches to social relationships
and clinical problems (Rothbart, 2011).
Other studies have found direct links
between infants’ disengagement of atten-
tion and decreases in their concurrent nega-
tive emotion. In one study of 7-month-olds,
parent reports of infants’ orienting in daily
life were related to higher levels of positive
emotionality and lower levels of negative
emotionality (Sheese, Voelker, Posner, &
Rothbart, 2009). Early mechanisms for cop-
ing with negative emotion may also later be
transferred to the control of cognition and
behavior (Posner & Rothbart, 1998). Cor-
relations have been found, for example,
between infants’ use of regulatory strategies
in anger- inducing situations and their pre-
school ability to delay responses (Calkins &
Williford, 2003). In research by Mischel and
his colleagues (Sethi, Mischel, Aber, Shoda,
& Rodriguez, 2000), toddlers’ use of dis-
traction strategies in an arousing situation
was positively related to their later delay of
gratification at age 5.
Activation of defense or approach may
also alter orienting and the processing of
sensory information (LeDoux, 2000). Selec-
tion and attention to emotion- related stim-
uli may be critically important in situations
related to the health and well-being of the
individual. This selection process can be
viewed as involving multiple potential tar-
gets in competition for attention. Orienting
of attention can produce a bias toward one or
more of these targets, and emotion is related
to attentional bias (see review by Kanske &
Kotz, 2012). For example, more threatening
stimuli are associated with greater vigilance
in a probe detection task (Beaver, Mogg, &
Bradley, 2005), and presentation of aversive
stimuli is associated with increased interfer-
ence during mathematical problem- solving
and line detection tasks (Schimmack, 2005).
The effect of emotions on perceptual
processes varies as temperament systems
develop and differs across individuals,
reflecting each person’s pattern of tempera-
mental reactivity. In adults, negative emo-
tionality, neuroticism, and trait anxiety have
been related to biases in patterns of look-
ing to various kinds of threatening stimuli
(see review by Derryberry & Reed, 2002),
as well as to patterns of attention toward
detecting errors (Paulus, Feinsten, Simmons,
& Stein, 2004). Neuroticism has also been
linked to difficulty in disengaging attention
from sources of threat, and extraversion is
related to similar difficulties in disengag-
ing from rewarding stimuli (Derryberry &
Reed, 2002).
310 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Initial orienting to threatening or reward-
ing stimuli is often accompanied by behav-
ioral quieting and muted physiological
responding, including cardiac deceleration,
but orienting may be quickly followed by
an action phase involving behaviors such as
approach, fight, or flight that require dra-
matic changes in metabolic functioning. The
modulation of autonomic arousal in prepa-
ration for behavioral responding is another
mechanism through which defense and
approach systems can influence respond-
ing. Relevant here are changes in skin con-
ductance, heart and respiration rate, blood
pressure and patterns of blood circulation,
potentiation of the startle response, and
secretion of cortisol and catecholamines
(Bradley, Codispoti, Cuthbert, & Lang,
2001).
We now turn to attention processes under-
lying the development of EC, a flexible and
attention- based form of self- regulation of
thought, action, and emotion. We focus par-
ticularly on this topic because of its impor-
tance to behavior and emotion regulation,
its susceptibility to training, and the many
recent research advances in this area.
EC and Executive Attention
EC is defined as the ability to inhibit a domi-
nant response so as to activate a subdomi-
nant response (Rothbart & Bates, 2006). EC
initially emerged from psychometric temper-
ament research as a higher- order factor that
included lower-order scales of attentional
focusing, inhibitory control, perceptual sen-
sitivity, and low- intensity pleasure in child-
hood (Rothbart, 2011; Rothbart, Ahadi,
Hershey, & Fisher, 2001), and attentional
control, inhibitory control, and activational
control in adulthood (Evans & Rothbart,
2007). We have proposed that EC describes
individual differences in self- regulatory
capacities linked to the functioning of the
executive attention network (Posner &
Rothbart, 1998, 2007a, 2007b).
EC predicts a broad array of outcomes,
including the development of higher proso-
cial and lower antisocial behavior (Lengua
& Wachs, 2012; Rueda, 2012). The direct
regulation of negative emotional reactions,
such as anger, anxiety, or fear, represents
one avenue through which EC can influence
behavior. It has been argued that individuals
with higher EC are better able to regulate the
affective responses that promote antisocial
behavior (e.g., Kochanska et al., 2000). EC
has also been positively linked to empathy
and prosocial behavior (Rothbart & Bates,
2006), and it interacts with contextual vari-
ables to predict behaviors linked to emotion
regulation. For example, early temperament
interacts with parenting practices to predict
conscience development (Kochanska, 1997)
and psychopathology (see review in Roth-
bart, 2011).
Kochanska et al. (2000) have character-
ized the construct of EC as “situated at the
intersection of the temperament and behav-
ioral regulation literatures” (p. 220). What
does EC mean for theories of temperament
and personality? It means that contrary
to earlier theories that emphasized how
behavior is driven by positive and nega-
tive emotions or one’s level of arousal, we
are not always at the mercy of emotion and
the affective– motivational systems (Roth-
bart, 2011). Using EC, we can more flexibly
approach the situations we fear and inhibit
the actions we desire. The efficiency of EC,
however, may depend on the strength of the
emotional processes against which effort is
exerted. For example, when a child must
delay an approach to an appealing toy, the
child with a stronger disposition to approach
is likely to require greater EC to succeed.
EC is also involved in the inhibition
of immediate approach, with the goal of
attaining a larger reward later, as in delay
of gratification research (Shoda, Mischel, &
Peak, 1990), and in Block’s (2002) hedonism
of the future. EC also allows activation of
behavior that would otherwise not be per-
formed, letting one act “on principle” when
the principle opposes one’s otherwise domi-
nant response. EC is not itself a basic moti-
vation; rather, it provides the means to effec-
tively satisfy desired ends. It resembles the
attentional capacities that underlie Block’s
(2002) construct of ego resiliency, that is,
the ability to shift one’s level of control flex-
ibly depending on requirements of the situ-
ation.
There is substantial evidence that during
childhood EC, as measured bytemperament
questionnaires, is correlated with the ability
to resolve conflict in many cognitive tasks
Temperament and Emotion Regulation 311
(Rothbart, 2011; Rueda, 2012). This pro-
vides an opportunity to examine the brain
systems that are related to both conflict res-
olution and EC.
The Executive Attention Network
Posner and colleagues have identified three
brain networks that serve different functions
of attention and have different neural anato-
mies and neuromodulators (Posner, 2012).
The first two networks involve alerting and
orienting, respectively. The third, the execu-
tive attention network, which functions to
monitor and resolve conflict, involves the
anterior cingulate cortex (ACC), anterior
insula, and basal ganglia. Dorsal areas of the
cingulate have been implicated in the regula-
tion of cognitive processing, while more ven-
tral areas have been implicated in emotional
processing (Bush, Luu, & Posner, 2000). In
more recent research, individual differences
in the functioning of the executive attention
network have been theoretically and empiri-
cally linked to the temperament construct of
EC (Chang & Burns, 2005; Posner, 2012;
Rothbart, 2011).
According to one theory, executive atten-
tion, and the ACC in particular, is involved
primarily in the monitoring of conflict
between potentially competing systems
(Botvinick, Braver, Barch, Carter, & Cohen,
2001) whereas the prefrontal cortex, partic-
ularly on the right side, is involved in inhibi-
tion of competing systems (see review by Pos-
ner, 2012). This division of labor between
the ACC and the prefrontal cortex may not
be the full story, but these ideas suggest com-
plementary associations between the ACC
and prefrontal cortex in self- regulation.
The ACC, one of the main nodes of the
executive attention network, has been linked
to specific functions related to self- regulation
(see reviews by Posner, 2012; Rueda, 2012).
These include the monitoring of conflict,
control of working memory, regulation of
emotion, and response to error. In emotion
studies, the cingulate is often seen as part of
a network involving the orbitofrontal cortex
and amygdala that regulates our emotional
response to input. Activation of the ACC is
observed when people are asked to control
their reactions to strong positive (Beaure-
gard, Levesque, & Bourgouin, 2001) and
negative emotions (Ochsner, Bunge, Gross,
& Gabrieli, 2002).
As noted previously, dorsal areas of the
cingulate have been implicated in regulating
cognitive processing and more ventral areas
in emotional processing (Bush et al., 2000).
The portion of the ACC related to emotion
regulation has a very high level of tonic
activity, even at rest. This activity, together
with the widespread connectivity of the cin-
gulate to other brain areas, may be related
to the need to maintain emotional control
even when one is not performing any spe-
cific task. There is also some evidence of an
inhibitory interaction between the more ven-
tral emotion regulation part of the ACC and
the more dorsal areas related to cognitive
control (Drevets & Raichle, 1998).
ACC activation in response to perceived
errors has been reported in infants as young
as 7 months of age (Berger, Tzur, & Pos-
ner, 2006). Adults show ACC activation
and usually slow down following an error.
However, in a study using a Simple Simon
task, we found that slowing down follow-
ing an error did not arise until children were
between 39 and 41 months of age (Jones,
Rothbart, & Posner, 2003). We believe that
the difference between infants who detect
the error and children who modify action
based on the error involves the change in
connectivity of the ACC to other brain
structures that takes place during child-
hood (Fair et al., 2009; Gao et al., 2009).
The connectivity hypothesis may also have
implications for other issues in develop-
ment, for example, the observation that
fear is shown in distress reactions before it
is linked to behavioral inhibition (Rothbart
et al., 2000). Further research is needed to
relate connectivity to behavioral changes
such as slowing.
One assessment of executive attention in
early childhood is the spatial conflict task,
in which object identity and spatial loca-
tion of a stimulus are placed in conflict
(Gerardi- Caulton, 2000). Here the child
has two response keys, labeled with pictures
of stimuli. Stimuli are presented on a com-
puter screen, and the child is instructed to
press the key corresponding to the picture
presented. Conflict trials are those in which
the stimulus appears on the side opposite the
correct key.
312 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Between 30 and 36 months of age, chil-
dren learn to perform this task, which
requires inhibiting the dominant response
toward a spatial location in order to make
a response based on matching identity
(Gerardi- Caulton, 2000; Rothbart, Ellis,
Rueda, & Posner, 2003). At 24 months,
children are only able to carry out this task
when the stimulus is on the same side of the
computer screen as the matching response
key (the congruent condition), but by 30
months, most children can handle incongru-
ent trials in which the matching target is on
the opposite side of the stimulus, although
they are greatly slowed in conflict trials
(adults are also slowed in this condition).
Children with higher levels of perfor-
mance on spatial conflict have also been
reported by their parents as having higher
levels of EC and lower levels of negative
emotionality on the Children’s Behavior
Questionnaire (CBQ; Rothbart et al., 2001,
2003). Two-year-old children who could not
complete the spatial conflict task were also
described by their parents as having lower
EC and higher negative emotionality. These
findings are consistent with the idea that the
capacity to engage in rule-based action in
conflict situations can support responding
to social rules and regulation of emotion in
daily life.
Another important conflict task is the
Attention Networks Task (ANT), which
measures efficiency of the alerting, orient-
ing, and executive attention networks. In the
ANT flanker task, the response to a target is
in conflict with surrounding flanker stimuli
(Rueda et al., 2004). Both spatial conflict
and flanker tasks have been linked in imag-
ing studies to the functioning of the brain’s
executive attention network (Fan, Flom-
baum, McCandliss, Thomas, & Posner,
2003) and have been used as model tasks
for assessing the functioning of this net-
work (Posner, 2012). Using the Child ANT
(Rueda et al., 2004), significant improve-
ment in conflict resolution has been found
up until age 7, but a remarkable similarity in
both reaction time and accuracy was found
from the age of 7 to adulthood.
Throughout childhood, ANT perfor-
mance is related to EC performance (Roth-
bart, 2011; Rueda, 2012), and adults high
in EC show lower conflict scores and lower
negative emotion (Rothbart, 2011). In ado-
lescents and adults, performance on the
ANT has also been linked to a number of
psychopathologies (see review by Posner,
2012). These studies illustrate the close rela-
tion between attention, EC, and emotion
regulation throughout life.
EC in Childhood
Evidence for stability of EC has been found
in research by Mischel and his colleagues
(Shoda et al., 1990). Preschoolers were tested
on their ability to wait for a delayed treat
that was larger than a readily accessible one.
Children better able to delay gratification
were found to have better self- control and
greater ability to regulate reactions to stress
and frustration. Their delay of gratification
in seconds also predicted parent- reported
attentiveness, concentration, emotion regu-
lation, and intelligence during adolescence.
In follow- up studies when the participants
were in their 30s, preschool delay predicted
goal- setting and self- regulatory abilities
(Ayduk et al., 2000), suggesting remarkable
continuity in self- regulatory skills.
Some of the complexity of this continuity
in self- regulation is reflected in longitudi-
nal studies of genetic contributions to early
development. There is substantial evidence
in adult studies that genetic alleles in the
dopamine system are related to executive
attention (Posner, 2012). One of these genes,
the dopamine 4 receptor gene, was found to
influence sensation seeking behavior as early
as 18–20 months in interaction with the qual-
ity of parenting (Sheese, Voelker, Rothbart,
& Posner, 2007). When the 7-repeat allele
was present, relatively low- quality parenting
produced higher sensation- seeking ratings,
but when the 7-repeat was absent, sensa-
tion seeking was moderate or low, regard-
less of parenting quality. The remarkable
susceptibility of children with the 7-repeat
allele to parental and other environmental
influences has been replicated multiple times
(e.g., Belsky & Pluess, 2009). We did not
see any relation of this gene to EC at 18–20
months (Sheese et al., 2007), but by 4 years
there was a gene × parenting quality inter-
action in which EC ratings of children with
the 7-repeat were more positively influenced
by parenting quality than children without
Temperament and Emotion Regulation 313
the 7-repeat (Sheese, Voelker, Rothbart &
Posner, 2012). Adult studies have also indi-
cated that people with the 7-repeat allele are
more influenced by their environment than
those without it (Larsen et al., 2010). Thus,
there appears to be an underlying continu-
ity of genetic influence even though neural
networks may be changing in their connec-
tivity.
EC plays an important role in the develop-
ment of conscience, with greater internalized
conscience in children high in EC (Kochan-
ska et al., 2000). Both the reactive tempera-
mental control system of fearful inhibition
and the attention- based system of EC appear
to regulate the development of socialized
thought and behavior, with the influence of
fear found earlier in development. We have
found that 6- to 7-year-old children who
were high in EC were also high in empathy
and guilt/shame, and low in aggressiveness
(Rothbart et al., 1994). EC may support
empathy by allowing children to attend to
another child’s condition instead of focus-
ing only on their own sympathetic distress.
Eisenberg, Fabes, Nyman, Bernzweig, and
Pinulas (1994) found that 4- to 6-year-old
boys with good attentional control tended to
deal with anger by using nonhostile verbal
methods rather than overt aggression.
During the toddler and preschool years,
development of the executive attention net-
work underlying EC allows children greater
control of stimulation and response, includ-
ing the ability to select responses in a con-
flict situation. Aksan and Kochanska (2004)
found that children who were more fearful
and inhibited at 33 months showed more
volitional inhibitory control at 45 months.
They suggest that more fearful and inhibited
children have a greater opportunity to foster
their own self- control during their periods
of slow approach to novel situations.
Attention and Emotion Regulation
We now consider contributions of execu-
tive attention to emotion regulation via dis-
traction, suppression, and reappraisal. In
the section on orienting we described how
defense and approach systems monitor for
goal- relevant stimuli and, once activated,
bias orienting toward some targets. Levels
of emotional activation are thus maintained
or increased until something occurs to inter-
rupt the cycle. One way to interrupt the
cycle is to perform actions that remove the
stimulus or make it less relevant. For exam-
ple, if a person is afraid of heights, visual
cues indicating precipitous drops may initi-
ate a cycle of anxiety and fear. One way to
interrupt this cycle is to back away from the
ledge. If such an action is not available or
is undesirable, a second approach is to “not
look down.” Information reception can thus
be controlled to reduce the activation of the
system. This kind of regulation can also be
applied to the approach system. Just as one
can look away from things that are feared,
one can also look away from things that are
desired. Consistent with this hypothesis,
children who are most able to resist temp-
tation are those most likely to orient away
from desired stimuli while waiting for them
(e.g., Sethi et al., 2000).
Regulation of orienting may not always
be sufficient to manage emotional reactions.
Not looking at a cookie may reduce the
probability we will eat it, but even when we
are not looking at it, we still know it is there.
Conceptual processing and memory allow us
to maintain an internal representation of the
stimulus over time. Internal representations
may be just as effective, or even more so, in
triggering and maintaining the activation of
affective systems. Consequently, regulating
internal representations becomes an impor-
tant avenue for the regulation of emotional
responding. Executive attention has been
related to regulation of inappropriate cogni-
tions, as in the Stroop effect (Posner, 2012),
and similar mechanisms are likely involved
in emotional regulation.
The same general network involved in
control of emotions is active during the
manipulation of internal representations,
such as generating word associations (Pos-
ner & Raichle, 1994). Because working
memory is finite, focusing attention on other
representations may lead to the exclusion of
“unwanted” representations. The execu-
tive attention system serves to monitor and
resolve conflict among brain networks and
may facilitate the manipulation of internal
representations by allowing for the selection
of one representation over another. We can
thus attempt to control emotional respond-
ing by literally thinking about other things,
314 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
a process that has been referred to as dis-
traction. However, as an emotion regulation
strategy, distraction may have limited util-
ity and ultimately adverse long-term conse-
quences (Gross, this volume).
Research on sensory systems has shown
that attention can work by increasing acti-
vation in an attended sensory system, as well
as by reducing or suppressing activation of
other systems (Posner & Raichle, 1994). To
date, the upregulation of emotions has not
been commonly addressed in either theoreti-
cal treatments or empirical studies of emo-
tion regulation, although Kieras, Tobin,
Graziano, and Rothbart (2005) have found
that children higher in EC skills are more
likely to be able to smile at the presenta-
tion of a disappointing gift, and laboratory
research has linked high executive attention
on the Child ANT to smiling in this para-
digm (Rothbart et al., 2003). It seems likely
that, as in orienting to sensory systems, the
ability to smile in the face of disappointment
involves both activation of smiling and the
suppression of the emotion related to the
disappointing gift.
Links have also been found between the
executive attention network and another
strategy for altering representations to pro-
mote emotion regulation, reappraisal, which
involves reinterpreting the meaning or value
of a representation (Gross, 2002). Ochsner
et al. (2002) found that reappraisal led to
reduced activity in the amgydala, suggest-
ing that reappraisal changes emotion- related
processing. Subsequent research has indi-
cated that prefrontal and anterior cingu-
late regions are involved in the modulation
of emotion processing through reappraisal
(Ochsner et al., 2004). In some studies the
orbitofrontal area that lies adjacent to the
ACC seems to account for the major differ-
ence in brain activity found between dis-
traction and reappraisal (Kanske, Heissler,
Schönfelder, Bongers, & Wessa, 2011).
Reappraisal may thus be another mecha-
nism through which the executive attention
system regulates affective systems, in that
it involves a competition among alterna-
tive internal representations. The executive
attention system facilitates the selection of
a secondary representation over the prepo-
tent representation (Posner, 2012). Recent
studies of depressed patients have found
that emotion regulation through reappraisal
does not result in the same reduction in
amygdala activity in patients as in normal
participants. However, distraction worked
similarly in both groups (Kanske, Heissler,
Schönfelder, & Wessa, 2012).
Controlling attentional orienting, exclud-
ing prepotent representations, and reinter-
pretation are strategies for reducing activa-
tion of reactive systems by altering overt or
internal input into those systems. A differ-
ent avenue for regulation concerns altering
output of the reactive systems. Bidirectional
links between the affective– motivational
systems and areas of output, such as the
autonomic system, allow for feedback loops
that can maintain emotional states (Tang &
Posner, 2009). The executive attention sys-
tem may enable efforts to modulate different
aspects of physiological arousal, including
both up- regulation and down- regulation.
Direct manipulation of aspects of physio-
logical arousal that may be under conscious
control, such as changes in rates of respira-
tion and body muscle tension, is one pos-
sibility. Evidence from imaging studies and
animal models indicates that the ACC plays
a substantial role in the modulation of auto-
nomic reactivity in response to situational
demands (e.g., Critchley et al., 2003).
So far, we have primarily considered
emotion regulation in terms of reduction of
activation. The defense and approach sys-
tems motivate adaptive behavior by induc-
ing emotional states, while the executive
attention system allows for the suppres-
sion of these reactive systems. However, it
is important to note that the same mecha-
nisms through which the executive attention
system produces reductions in activation of
reactive systems can also be used to produce
increases in activation. If we want to induce
an affective state, we can consciously shift
attention to the appropriate affect- inducing
stimuli, recall affect- inducing representa-
tions, or reinterpret neutral representa-
tions to promote affective responses. The
executive attention system may also allow
us to elicit emotional responses by increas-
ing physiological arousal or by produc-
ing behaviors that are consistent with the
desired emotional state.
Since the executive attention network is
important in emotional control, an obvious
question is whether intervention during pre-
school or later can improve the functioning

Temperament and Emotion Regulation 315
of this network and thus alter the ability to
regulate emotion. We now describe efforts
to improve executive attention through
training.
Training Executive Attention
There may be two quite different ways to
train attention (Tang & Posner, 2009). One
involves training by exercising a particular
attention network, and the other, a change
in brain state through meditation or related
procedures. We have examined training of
the executive attention system in children
ages 4–6 years (Rueda, Rothbart, McCan-
dliss, Saccomanno, & Posner, 2005). The
programs we have used were adapted from
those used to train monkeys for space flight
(Rumbaugh & Washburn, 1995). They
involve first training the child to use a joy-
stick to place a cat on grass rather than on
mud. As training proceeds, the grass shrinks
and control becomes more difficult; these
skills are further built on to train anticipa-
tion, improve memory, and exercise the abil-
ity to resolve conflict (Rueda et al., 2005).
Both 4- and 6-year-olds showed greater
improvements in the brain networks related
to executive attention after 5 days of com-
puter training than a control group that
interacted with videos (Rueda et al., 2005).
The group taking attention training also
showed improved IQ scores. A replication
and extension of this study to 10 days of
training was carried out in a Spanish pre-
school (see review in Rueda, 2012). In addi-
tion to replicating previous work, attention
training in this study improved children’s
emotion regulation and self- control, includ-
ing their performance on delay of reward
and children’s gambling tasks. A number
of other methods used in classroom stud-
ies have also been effective in enhancing the
development of executive attention; they are
helpful to children with and without prob-
lems in attention (see Diamond & Lee, 2011,
for a summary).
Studies of mindfulness meditation have
also reported improvements of adults on
the conflict measure of the ANT and more
positive mood after only 5 days of training
in comparison with a control group given
relaxation training (Tang et al., 2007). After
5 days of training, stress levels, as measured
by cortisol secretion, were reduced follow-
ing a cognitive challenge of mental arithme-
tic. After 30 days of training, cortisol levels
were lower even at baseline, suggesting that
the meditation group members experienced
reduced stress in their daily life to a greater
degree than those without this training
(Tang et al., 2007). The mechanism of these
changes appears to be in alterations of the
activation and connectivity of the ACC to
other brain areas (Tang, Lu, Fan, Yang, &
Posner, 2012).
Issues in Temperament Assessment
In temperament studies involving emotion
regulation it has been common to consider
only individual differences in components of
reactive temperament, without considering
either regulatory aspects of temperament,
such as EC, or the interactions between
these components. Studies focusing on only
single traits or looking only at main effects
may be missing important opportunities for
understanding the dynamic contribution of
temperament to outcomes. Research sup-
ports the importance of considering inter-
actions among dimensions of temperament
in predicting external criteria. For example,
Gunnar, Sebanc, Tout, Donzella, and van
Dulmen (2003) found that high levels of sur-
gency (approach) combined with low levels
of EC were associated with higher cortisol
levels and higher peer rejection in childhood
(see reviews by Rothbart, 2011; Rothbart &
Bates, 2006).
It is also important to note that depend-
ing on context, the systems may either act
relatively independently, in direct competi-
tion, or complement one another. Without
considering the person’s goals, it is difficult
to predict his or her particular reactions a
priori. For example, considering fearfulness
in terms of the defense system emphasizes
that the network serves not only to inhibit
behavior in some circumstances but also to
motivate avoidance or attack. In contrast,
EC, considered as an index of individual
differences in the functioning of the execu-
tive attention system, serves to inhibit and
facilitate processing in diverse cognitive and
affective networks, with the goal of modu-
lating prepotent responses. Sometimes, the
goals of the defense and executive atten-
tion systems may agree. In these instances

316 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
we would expect both temperamental fear-
fulness and EC to contribute to predicting
an outcome (e.g., following directions dur-
ing a fire drill). In other circumstances the
goals of the systems may be at odds, and we
would expect behavioral outcomes to reflect
an interaction between the two systems (e.g.,
jumping out of an airplane).
The approach adopted here also suggests
that it is important to consider how each
system contributes to a given temperament
assessment. When we bring a child into the
laboratory and attempt to assess EC using a
variety of game-like tasks, task performance
reflects dynamic interactions among several
systems, not just EC. The approach sys-
tem may respond to engaging activities and
rewards; the defense system may respond to
the novelty of the laboratory, the procedures,
and the experimenters. Finally, the executive
attention system contributes to task per-
formance, and also to keeping emotional
responses in line with the parent’s, experi-
menter’s, and personal expectations. We can
try to get direct assessments of one aspect of
temperament, but outcomes almost always
reflect a mix of reactive and regulatory pro-
cesses. For example, Laptook, Klein, Olino,
Dyson, and Carlson (2010) showed that
children displaying the same low approach/
engagement could be differentiated into chil-
dren with low positive affect and children
with high behavioral inhibition.
Inhibitory control tasks, commonly
employed in behavioral assessments of EC,
present an interesting example of how even
basic assessments may potentially tap into
multiple temperament systems. Assessments
such as the day–night Stroop task focus
on assessing the inhibition of prepotent
responses. However, to perform this task
successfully, children must also sit still, fol-
low instructions, attend to the stimuli, and
activate nonprepotent responses; motivation
to perform the task is also important (Hui-
zenga, van der Molen, Bexkens, Bos, & van
den Wildenbeg, 2012).
Even in the absence of overt feedback or
reward from the experimenter, we might
expect participants’ reactive responses to
vary in the performance of these tasks. Con-
sistent with this idea, Wolfe and Bell (2004)
found that while parent- reported inhibitory
control was a significant predictor of perfor-
mance on inhibitory control laboratory tasks
(r = .36), parent- reported approach/anticipa-
tion was a better predictor (r = –.56). Thus,
while inhibitory control may be properly
considered a facet of EC, inhibitory control
measures may reflect an interaction between
the approach and the executive attention
systems.
Dynamic interaction between brain
areas is also found in imaging studies when
increases in activation of the ACC are accom-
panied by reduced activity in the amygdala
during control of negative emotion (Etkin,
Egner, Peraza, Kandel, & Hirsch, 2006). In
patient populations such as those suffering
from borderline personality disorder (Gold-
stein et al., 2007) or depression (Kanske &
Kotz, 2012), this interaction is disrupted.
This dynamic view of interacting systems
suggests that single- method assessments of
temperament may be problematic. When
possible, researchers should employ batter-
ies of differentiated assessments in which the
common feature is the temperament charac-
teristic of interest. Multimethod assessment
procedures are further complemented when
multiple temperament traits are assessed
(e.g., in questionnaires), particularly when
reactive and regulatory components of tem-
perament are being examined. A multitrait
approach allows us to better assess individ-
ual differences in regulation by controlling
for individual differences in reactivity, and
vice versa.
Future Directions
We suggest that our understanding of emo-
tion regulation will be advanced if we con-
ceptualize temperament constructs in terms
of affective– motivational and attentional
systems, study these systems at multiple lev-
els, including the level of neural networks,
and consider dynamic interactions among
these systems. Considering emotional reac-
tivity and emotion regulation as core compo-
nents of temperament represents a first step
toward a more dynamic, process- oriented
view. The second step leads to considering
reactive and regulative components of tem-
perament together rather than in isolation.
Linking temperament to the study of emo-
tion and emotion regulation also provides
temperament researchers with a rich empiri-
cal base for generating models of tempera-

Temperament and Emotion Regulation 317
ment development, such as models of the
development of social interaction and the
development of coping strategies (Rothbart,
2011). Advances in understanding the basic
processes in emotional reactivity and emo-
tion regulation should inform and enrich
research on temperament. At the same time,
research on temperament offers a unique
perspective for examining emotion and emo-
tion regulation.
References
Aksan, N., & Kochanska, G. (2004). Links
between systems of inhibition from infancy
to preschool years. Child Development, 75,
1477–1490.
Allport, G. W. (1937). Personality: A psychologi-
cal interpretation. Oxford, UK: Holt.
Ayduk, O., Mendoza- Denton, R., Mischel, W.,
Downey, G., Peake, P., & Rodriguez, M.
(2000). Regulating the interpersonal self: Stra-
tegic self- regulation for coping with rejection
sensitivity. Journal of Personality and Social
Psychology, 79, 776–792.
Beauregard, M., Levesque, J., & Bourgouin, P.
(2001). Neural correlates of conscious self-
regulation of emotion. Journal of Neurosci-
ence, 21(RC165), 1–6.
Beaver, J. D., Mogg, K., & Bradley, B. P. (2005).
Emotional conditioning to masked stimuli and
modulation of visuospatial attention. Emo-
tion, 5, 67–79.
Belsky, J., & Pluess, M. (2009). Beyond diathesis
stress: Differential susceptibility to environ-
mental stress. Psychological Bulletin, 135,
895–908.
Berger, A., Tzur, G., & Posner, M. I. (2006).
Infant brains detect arithmetic error. Proceed-
ing of the National Academy of Sciences USA,
103, 12649–12653.
Block, J. (2002). Personality as an affect-
processing system: Toward an integrative the-
ory. Mahwah, NJ: Erlbaum.
Botvinick, M. M., Braver, T. S., Barch, D. M.,
Carter, C. S., & Cohen, J. D. (2001). Conflict
monitoring and cognitive control. Psychologi-
cal Review, 108, 624–652.
Bradley, M. M., Codispoti, M., Cuthbert, B. N.,
& Lang, P. J. (2001). Emotion and motivation
I: Defense and appetitive reactions in picture
processing. Emotion, 1, 276–299.
Bush, G., Luu, P., & Posner, M. I. (2000). Cogni-
tive and emotional influences in anterior cin-
gulate cortex. Trends in Cognitive Sciences, 4,
215–222.
Calkins, S. D., & Williford, A. P. (2003, April).
Anger regulation in infancy: Consequences
and correlates. Paper presented at the meeting
of the Society for Research in Child Develop-
ment, Tampa, FL.
Campos, J. J., Caplovitz, B. K., Lamb, M. E.,
Goldsmith, H. H., & Stenberg, C. (1983).
Socioemotional development. In P. H. Mussen,
M. M. Haith, & J. J. Campos (Eds.), Hand-
book of child psychology (Vol. 2, pp. 783–
915). New York: Wiley.
Chang, F., & Burns, B. M. (2005). Attention in
preschoolers: Associations with effortful con-
trol and motivation. Child Development, 76,
247–263.
Critchley, H. D., Mathias, C. J., Josephs, O.,
O’Doherty, J., Zanini, S., Dewar, B. K., et
al. (2003). Human cingulate cortex and auto-
nomic control: Converging neuroimaging and
clinical evidence. Brain, 126, 2139–2152.
Crockenberg, S. C., Leerkes, E. M. J., & Barrig,
P. S. (2008). Predicting aggressive behavior
in the third year from infant reactivity and
regulation as moderated by maternal behav-
ior. Development and Psychopathology, 20,
37–54.
Deater- Deckard, K., & Wang, Z. (2012). Anger
and irritability. In M. Zentner & R. L. Shiner
(Eds.), Handbook of temperament (pp. 124–
144). New York: Guilford Press.
Derryberry, D., & Reed, M. A. (1994). Tem-
perament and attention: Orienting toward and
away from positive and negative signals. Jour-
nal of Personality and Social Psychology, 66,
1128–1139.
Derryberry, D., & Reed, M. A. (2002). Anxiety-
related attentional biases and their regulation
by attentional control. Journal of Abnormal
Psychology, 111, 225–236.
Derryberry, D., & Rothbart, M. K. (1997). Reac-
tive and effortful processes in the organization
of temperament. Developmental Psychopa-
thology, 9, 633–652.
Diamond, A., & Lee, K. (2011). Interventions
shown to aid Executive Function develop-
ment in children 4–12 years old. Science, 333,
959–964.
Drevets, W. C., & Raichle, M. E. (1998). Recip-
rocal suppression of regional cerebral blood
flow during emotional versus higher cogni-
tive processes: Implications for interactions
between emotion and cognition. Cognition
and Emotion, 12, 353–285.
318 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Eisenberg, N., Fabes, R. A., Nyman, M., Bern-
zweig, J., & Pinulas, A. (1994). The relations
of emotionality and regulation to children’s
anger-
related reactions. Child Development,
65, 109–128.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E.
R., & Hirsch, J. (2006). Resolving emotional
conflict: A role for the rostral anterior cingu-
late cortex in modulating activity in the amyg-
dala. Neuron, 51, 871–882.
Evans, D., & Rothbart, M. K. (2007). Develop-
ing a model for adult temperament. Journal of
Research in Personality, 41, 868–888.
Fair, D. A., Cohen, A. L., Power, J. D., Dosen-
bach, N. U. F., Church, J. A., Miezin, F. M., et
al. (2009). Functional brain networks develop
from a local to distributed organization. PLoS
Computational Biology, 5, e1000381.
Fan, J., Flombaum, J. I., McCandliss, B. D.,
Thomas, K. M., & Posner, M. I. (2003). Cog-
nitive and brain consequences of conflict.
NeuroImage, 18, 42–57.
Gao, W., Zhu, H., Giovanello, K. S., Smith, J.
K., Shen, D., Gilmore, J. H., et al. (2009).
Evidence on the emergence of the brain’s
default network from 2-week-old to 2-year-
old healthy pediatric subjects. Proceedings of
the National Academy of Sciences USA, 106,
6790–6795.
Gerardi-
C
aulton, G. (2000). Sensitivity to spatial
conflict and the development of self-
r
egulation
in children 24–36 months of age. Develop-
mental Science, 3, 397–404.
Goldstein, M., Brendel, G., Tuescheer, O., Pan,
H., Epstein, J., Beutel, M., et al. (2007). Neu-
ral substrates of the interaction of emotional
stimulus process and motor inhibitory control:
An emotional linguistic go/no-go fMRI study.
NeuroImage, 36, 1026–1040.
Gray, J. A., & McNaughton, N. (1996). The
neuropsychology of anxiety: Reprise.
Nebraska Symposium on Motivation, Per-
spectives on anxiety, panic and fear. Vol. 38,
pp.
61–134.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39, 281–291.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp.
3–20). New York:
Guilford Press.
Gunnar, M. R., Sebanc, A. M., Tout, K., Don-
zella, B., & van Dulmen, M. M. (2003). Peer
rejection, temperament, and cortisol activity
in preschoolers. Developmental Psychobiol-
ogy, 43, 346–358.
Harman, C., Rothbart, M. K., & Posner, M. I.
(1997). Distress and attention interactions in
early infancy. Motivation and Emotion, 21,
27–43.
Huizenga, H. M., van der Molen, M. W.,
Bexkens, A., Bos, M. G., & van den Wilden-
berg, W. P. (2012). Muscle or motivation?: A
stop-
s
ignal study on the effects of sequential
cognitive control. Frontiers in Cognition, 3.
[E-publication prior to print]
Ito, T. A., & Cacioppo, J. T. (2005). Variations
on a human universal: Individual differences
in positivity offset and negativity bias. Cogni-
tion and Emotion, 19, 1–26.
Johnson, M. H., Posner, M. I., & Rothbart, M.
K. (1991). Components of visual orienting in
early infancy: Contingency learning, antici-
patory looking, and disengaging. Journal of
Cognitive Neuroscience, 3, 335–344.
Jones, L. B., Rothbart, M. K., & Posner, M. I.
(2003). Development of executive attention in
preschool children. Developmental Science, 6,
498–504.
Kagan, J., & Fox, N. A. (2006). Biology, culture,
and temperamental biases. In W. Damon & N.
Eisenberg (Eds.), Handbook of child psychol-
ogy: Vol. 3. Social, emotional and personal-
ity development (6th ed., pp. 167–225). New
York: Wiley.
Kanske, P., Heissler, J., Schönfelder, S., Bongers,
A., & Wessa, M. (2011). How to regulate emo-
tion?: Neural networks for reappraisal and
distraction. Cerebral Cortex, 21, 1379–1388.
Kanske, P., Heissler, J., Schönfelder, S., & Wessa,
M. (2012). Neural correlates of emotion reg-
ulation deficits in remitted depression: The
influence of regulation strategy, habitual regu-
lation use, and emotional valence. NeuroIm-
age, 61, 686–693.
Kanske, P., & Kotz, S. A. (2012). Effortful con-
trol, depression, and anxiety correlate with the
influence of emotion on executive attentional
control. Biological Psychology, 91, 88–95.
Kieras, J. E., Tobin, R. M., Graziano, W. G.,
& Rothbart, M. K. (2005). You can’t always
get what you want: Effortful control and chil-
dren’s responses to undesirable gifts. Psycho-
logical Science, 16, 391–396.
Kochanska, G. (1997). Multiple pathways to
conscience for children with different temper-
aments: From toddlerhood to age 5. Develop-
mental Psychology, 33, 228–240.
Kochanska, G., Murray, K. T., & Harlan, E. T.
Temperament and Emotion Regulation 319
(2000). Effortful control in early childhood:
Continuity and change, antecedents, and
implications for social development. Develop-
mental Psychology, 36, 220–232.
Laptook, R. S., Klein, D. N., Olino, T. M.,
Dyson, M. W., & Carlson, G. (2010). Low
positive affectivity and behavioral inhibition
in preschool- age children: A replication and
extension of previous findings. Personality
and Individual Differences, 48, 547–551.
Larsen, H., van der Zwaluw, C. S., Overbeek, G.,
Granic, I., Franke, B., & Engels, R. C. (2010).
A variable- number- of- tandem- repeats poly-
morphism in the dopamine D4 receptor gene
affects social adaptation of alcohol use: Inves-
tigation of a gene– environment interaction.
Psychological Science, 21, 1064–1068.
LeDoux, J. E. (1989). Cognitive– emotional inter-
actions in the brain. Cognition and Emotion,
3, 267–289.
LeDoux, J. E. (2000). Emotion circuits in the
brain. Annual Review of Neuroscience, 23,
155–184.
Lengua, L. J., & Wachs, T. D. (2012). Tem-
perament and risk: Resilient and vulnerable
responses to adversity. In M. Zentner & R.
L. Shiner (Eds.), Handbook of temperament
(pp. 519–540). New York: Guilford Press.
McCrae, R. R., Costa, P. T., Jr., Ostendorf, F.,
Angleitner, A., Hrebícková, M., Avia, M. D.,
et al. (2000). Nature over nurture: Tempera-
ment, personality, and life span development.
Journal of Personality and Social Psychology,
78, 173–186.
Norman, D. A., & Shallice, T. (1986). Atten-
tion to action: Willed and automatic control
of behavior. In G. E. Schwartz & D. Shap-
iro (Eds.), Consciousness and self- regulation
(Vol. 4, pp. 1–18). New York: Plenum.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. E. (2002). Rethinking feelings:
An FMRI study of the cognitive regulation of
emotion. Journal of Cognitive Neuroscience,
14, 1215–1229.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D., et al.
(2004). For better or for worse: Neural sys-
tems supporting the cognitive down- and up-
regulation of negative emotion. NeuroImage,
23, 483– 499.
Paulus, M. P., Feinstein, J. S., Simmons, A., &
Stein, M. B. (2004). Anterior cingulate activa-
tion in high trait anxious subjects is related to
altered error processing during decision mak-
ing. Biological Psychiatry, 55, 1179–1187.
Pérez-Edgar, K., McDermott, J. N. M., Korelitz,
K., Degnan, K. A., Curby, T. W., Pine, D. S.,
et al. (2010). Patterns of sustained attention
in infancy shape the developmental trajectory
of social behavior from toddlerhood through
adolescence. Developmental Psychology, 46,
1723–1730.
Posner, M. I. (2012). Attention in a social world.
New York: Oxford University Press.
Posner, M. I., & Raichle, M. E. (1994). Images
of mind. New York: Scientific American
Library.
Posner, M. I., & Rothbart, M. K. (1998). Atten-
tion, self- regulation and consciousness. Philo-
sophical Transactions of the Royal Society of
London B, 353, 1915–1927.
Posner, M. I., & Rothbart, M. K. (2007a). Edu-
cating the human brain. Washington, DC:
American Psychological Association Books.
Posner, M. I., & Rothbart, M. K. (2007b).
Research on attentional networks as a model
for the integration of psychological science.
Annual Review of Psychology, 58, 1–23.
Posner, M. I., Rothbart, M. K. Sheese, B. E., &
Voelker, P. (2012). Control networks and neu-
romodulators in early development. Develop-
mental Psychology, 48, 827–835.
Putnam, S. P., Ellis, L. K., & Rothbart, M. K.
(2001). The structure of temperament from
infancy through adolescence. In A. Eliasz &
A. Angleitner (Eds.), Advances in research on
temperament (pp. 165–182). Lengerich, Ger-
many: Pabst Science.
Rothbart, M. K. (2011). Becoming who we are:
Temperament and personality in develop-
ment. New York: Guilford Press.
Rothbart, M. K. (1988). Temperament and the
development of inhibited approach. Child
Development, 59, 1241–1250.
Rothbart, M. K., Ahadi, S. A., & Hershey, K.
L. (1994). Temperament and social behavior
in childhood. Merrill–Palmer Quarterly, 40,
21–39.
Rothbart, M. K., Ahadi, S. A., Hershey, K. L.,
& Fisher, P. (2001). Investigations of tempera-
ment at three to seven years: The children’s
behavior questionnaire. Child Development,
72, 1394–1408.
Rothbart, M. K., & Bates, J. E. (2006). Tem-
perament in children’s development. In W.
Damon, R. Lerner, & N. Eisenberg (Eds.),
Handbook of child psychology: Vol 3. Social,
emotional, and personality development (6th
ed., pp. 99–166). New York: Wiley.
Rothbart, M. K., & Derryberry, D. (1981). Devel-
320 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
opment of individual differences in tempera-
ment. In M. E. Lamb & A. L. Brown (Eds.),
Advances in developmental psychology (Vol.
1, pp. 37–86). Hillsdale, NJ: Erlbaum.
Rothbart, M. K., Derryberry, D., & Hershey,
K. (2000). Stability of temperament in child-
hood: Laboratory infant assessment to parent
report at seven years. In V. J. Molfese & D. L.
Molfese (Eds.), Temperament and personality
development across the life span (pp. 85–119).
Hillsdale, NJ: Erlbaum.
Rothbart, M. K., Ellis, L. K., Rueda, M. R., &
Posner, M. I. (2003). Developing mechanisms
of temperamental effortful control. Journal of
Personality, 71, 1113–1143.
Rothbart, M. K., & Posner, M. I. (2006). Tem-
perament, attention, and developmental psy-
chopathology. In D. Cicchetti & D. J. Cohen
(Eds.), Developmental psychopathology: Vol.
2. Developmental neuroscience (2nd ed.,
pp. 465–501). New York: Wiley.
Rothbart, M. K., Ziaie, H., & O’Boyle, C. G.
(1992). Self- regulation and emotion in infancy.
In N. Eisenberg & R. A. Fabes (Eds.), Emo-
tion and its regulation in early development
(pp. 7–23). San Francisco: Jossey-Bass.
Rueda, M. R. (2012). Effortful control. In M.
Zentner & R. L.Shiner (Eds.), Handbook of
temperament (pp. 145–167). New York: Guil-
ford Press.
Rueda, M. R., Fan, J., McCandliss, B. D., Hal-
parin, J. D., Gruber, D. B., Lercari, L. P., et
al. (2004). Development of attentional net-
works in childhood. Neuropsychologia, 42,
1029–1040.
Rueda, M. R., Rothbart, M. K., McCandliss, B.
D., Saccomanno, L., & Posner, M. I. (2005).
Training, maturation, and genetic influences
on the development of executive attention.
Proceedings of the National Academy of Sci-
ences USA, 102, 14931–14936.
Rumbaugh, D. M., & Washburn, D. A. (1995).
Attention and memory in relation to learning:
A comparative adaptation perspective. In G.
R. Lyon & N. A. Krasengor (Eds.), Attention,
memory and executive function (pp. 199–
219). Baltimore: Brookes.
Schimmack, U. (2005). Attentional interference
effects of emotional pictures: Threat, negativ-
ity, or arousal? Emotion, 5, 55–66.
Sethi, A., Mischel, W., Aber, J. L., Shoda, Y., &
Rodriguez, M. L. (2000). The role of strate-
gic attention deployment in development of
self- regulation: Predicting preschoolers’ delay
of gratification from mother– toddler interac-
tions. Developmental Psychology, 36, 767–
777.
Sheese, B. E., Voelker, P., Posner, M. I., & Roth-
bart, M. K. (2009). Genetic variation influ-
ences on the early development of reactive
emotions and their regulation by attention.
Cognitive Neuropsychiatry, 14, 332–355.
Sheese, B. E., Voelker, P. M., Rothbart, M. K., &
Posner, M. I. (2007). Parenting quality inter-
acts with genetic variation in dopamine recep-
tor DRD4 to influence temperament in early
childhood. Development and Psychopathol-
ogy, 19, 1039–1046.
Sheese, B. E., Voelker, P. M., Rothbart, M. K.,
& Posner, M. I. (2012). The dopamine recep-
tor D4 gene 7-repeat allele interacts with par-
enting quality to predict effortful control in
four-year-old children. Child Development
Research, 86, 32–42.
Shiner, R. L., & Caspi, A. (2012). Temperament
and the development of personality traits,
adaptations, and narratives. In M. Zentner &
R. L. Shiner (Eds.), Handbook of temperament
(pp. 497–518). New York: Guilford Press.
Shoda, Y., Mischel, W., & Peake, P. K. (1990). Pre-
dicting adolescent cognitive and self- regulatory
competencies from preschool delay of gratifica-
tion: Identifying diagnostic conditions. Devel-
opmental Psychology, 26, 978–986.
Tang, Y.-Y., Lu, Q., Fan, M., Yang, Y., & Pos-
ner, M. I. (2012). Mechanisms of white matter
changes induced by meditation. Proceedings
of the National Academy of Sciences USA,
109, 10570–10574.
Tang, Y.-Y., Ma, Y., Wang, J., Fan, Y., Feng, S.,
Lu, Q., et al. (2007). Short term meditation
training improves attention and self regula-
tion. Proceedings of the National Academy of
Sciences USA, 104, 17152–17156.
Tang, Y., & Posner, M. I. (2009). Attention
training and attention state training. Trends
in Cognitive Science, 13, 222–227.
Wolfe, C. D., & Bell, M. A. (2004). Working
memory and inhibitory control in early child-
hood: Contributions from electrophysiology,
temperament, and language. Developmental
Psychobiology, 44, 68–83.
Zentner, M., & Shiner, R. L. (2012). Handbook
of temperament. New York: Guilford Press.
Zuckerman, M. (2012). Models of adult temper-
ament. In M. Zentner & R. L. Shiner (Eds.),
Handbook of temperament (pp. 41–68). New
York: Guilford Press.

321
Over the past two decades, interest in the
way people can and do regulate their affec-
tive states has increased rapidly. Much of
the research has been experimental (e.g.,
Webb, Miles, & Sheeran, 2012). However,
to understand the antecedents and conse-
quences of affect regulation in everyday life,
outside the laboratory, one must study natu-
rally occurring individual differences (John
& Gross, 2007). Although it is now widely
agreed that individuals differ systematically
and consistently in affect regulation, these
individual differences have been conceptual-
ized and measured in many different ways.
Different conceptualization and measures
arose, in part, because individual differences
in affect regulation are not uniquely associ-
ated with just one field within psychology.
Instead, researchers in several distinct sub-
disciplines in psychology are keenly inter-
ested in this topic.
Personality and social psychologists are
interested in positive and negative mood
states, and how stable individual differ-
ences in self- control influence longer- term
adaptation (e.g., Block & Block, 1980;
Baumeister, Zell, & Tice, 2007). Stress and
coping researchers study the myriad ways
individuals differ when they encounter an
intensely stressful life situation and try to
maintain well-being (e.g., Carver & Scheier,
1994; Folkman & Lazarus, 1980). Clinical
psychologists tend to focus on the dysregu-
lation of affect (i.e., failures and problems
with regulation) and its contribution to
various forms of pathology (e.g., Kring &
Sloan, 2009). Emotion researchers exam-
ine the specific experiential, expressive, and
physiological components of the emotional
response process and the role of regulation
within this process (e.g., Gross & Leven-
son, 1993). Developmental psychologists are
interested in how children learn to regulate
emotional states in family and peer contexts
and to do so in ways that are adaptive and
socially appropriate, thus becoming emo-
tionally competent adults (e.g., Morris, Silk,
Steinberg, Myers, & Robinson, 2007).
With such a wide range of stakehold-
ers and their particular interests, concep-
tual preferences, and measurement tradi-
tions, it is not surprising that research on
individual differences in the regulation of
affective states (e.g., stress, mood, specific
emotions) has not proceeded as a unified
science. Many researchers agree that this is
not a desirable state of affairs. For example,
Weinberg and Klonsky (2009, p. 616) noted,
CHAPTER 20
Three Approaches
to Individual Differences in Affect Regulation:
Conceptualizations, Measures, and Findings
Oliver P. John
Joshua Eng

322 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
“Despite growing consensus regarding the
importance of emotion dysregulation in psy-
chopathology, the field has not yet reached
an agreement on the construct’s definition.
Multiple components of emotion regulation
have been proposed. . . . ” Similarly, Mor-
ris et al. (2007, p. 363) concluded, “Existing
empirical studies of ER [emotion regulation]
differ widely in the measures, methods, and
levels of analyses employed.”
More generally, research on individual
differences
in the emotion regulation domain is still in
its infancy. A consequence is the lack of a
definitive conceptualization and assessment
methods of ER, with significant inconsisten-
cies across studies. . . . In order to contrib-
ute to greater scientific and methodological
rigor, there is a need to empirically examine
these relationships within a valid theoretical
framework that conceptualizes ER in a multi-
faceted way. . . . Utilization of a model, such
as Gross’ (1998), would do good service to this
weakness in the existing literature. (Bariola,
Gullone, & Hughes, 2011, p. 208)
Indeed, the process model proposed by
Gross (1998, this volume; see also Gross &
Thompson, 2007) has attracted much atten-
tion and has proven influential in research on
individual differences as well (e.g., Gross &
John, 2003; John & Gross, 2004), because it
defines core constructs and provides a gen-
eral terminology.
In the first major part of this chapter,
we describe this model and the individual-
differences research it has inspired, focus-
ing on a small set of carefully specified pro-
cesses used to regulate emotions. We begin
with this approach to individual differences
for two reasons, one personal and the other
conceptual. First, having contributed to this
research, we are obviously more familiar
with this work than with other approaches.
Second, Gross’s process model is sufficiently
general to serve as a framework that can help
organize, interpret, and compare the various
ways individual differences in affect regula-
tion have been conceptualized and studied.
Our review of the existing research on
individual differences suggests that, in
addition to work based on the emotion
regulation process model, two other major
approaches address the regulation of affec-
tive states broadly defined, namely, indi-
vidual differences in coping with stress and
individual differences in emotional compe-
tence. We begin with a brief overview of
these three approaches. Then we consider
each approach in turn, focusing on concep-
tualization and major measures. In the final
section we outline limitations, continuing
issues, and future directions for research.
Overview: Three Approaches
to Individual Differences
in Affect Regulation
Humans have multiple kinds of affective
(i.e., valenced) states that they may control
or regulate, such as stress, negative affect,
or distinct emotions (e.g., pride or sadness)
(Gross & Thompson, 2007). Therefore, we
designate individual differences in affect reg-
ulation as the superordinate domain; here
we include all kinds of regulatory attempts
to influence any valenced responses. Figure
20.1 shows that below that broad rubric, we
may consider three somewhat overlapping
subdomains, namely, individual differences
(1) in specific processes used to regulate
emotions, (2) in coping with stress, and (3)
in emotional competence.
Most goal- directed actions and cognitions
can serve to maximize pleasure or minimize
pain and may therefore be said to regu-
late affective states. Nonetheless, the three
approaches to the study of individual dif-
ferences in Figure 20.1 do differ from each
other, in terms of the particular affective
responses subject to regulation, the relevant
situational contexts, and the nature of the
cognitive and behavioral processes included.
Broadly speaking, the coping approach
can be distinguished from the specific emo-
tion regulation process approach by the fact
that coping focuses primarily on reducing
negative affect rather than distinct emo-
tions, and typically extends across longer
periods of time (e.g., coping with loss).
Stress responses also are less clearly defined
than specific emotions in terms of behav-
ioral response tendencies; thus, coping is
more likely to involve changing experiential,
rather than behavioral– expressive, compo-
nents of the affective response.
Whereas the coping approach is narrowly
focused on those situations that are stress-
ful, individual differences in the emotional
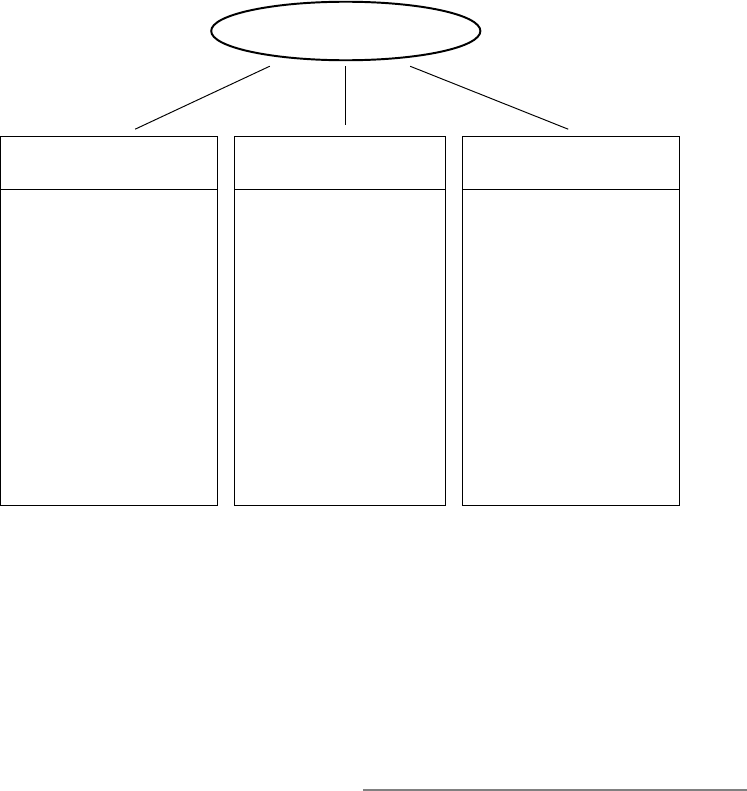
Individual Differences in Affect Regulation 323
competence approach apply to a broader
set of contexts. Furthermore, the emotional
competence approach includes a host of
processes, skills, and competencies (e.g.,
attention to feelings; clarity about feeling
states) that do not directly regulate emo-
tions but make it easier for the individual
to behave in socioemotionally appropriate
ways. Additionally, in the emotional compe-
tence approach, the specific processes used
to regulate feelings or moods are not of core
importance and thus tend to remain undif-
ferentiated; individual differences in strat-
egy use are often summarized in terms of a
single concept, such as whether the individ-
ual makes greater or lesser efforts at “mood
repair” (e.g., Salovey et al., 1995) or has
great or only limited “access to effective reg-
ulation strategies” (e.g., Gratz & Roemer,
2004). Thus, the emotional competence
approach is much broader than the specific
emotion regulation process approach.
Each of these three broad conceptual
approaches has generated a number of
measures. Examples of the major measures
associated with each approach are listed in
Figure 20.1. We now describe each approach
and the associated measures in some detail.
Individual Differences
in Specific Processes Used
to Regulate Emotions
The Process Model
We begin with an approach to individual
differences that is based on the model of
specific emotion regulation processes devel-
oped by Gross (1998). He derived this model
from a generally accepted conception of the
emotion- generative process, which holds
that an emotion begins with an evalua-
tion (or appraisal) of emotion cues. When
attended to and evaluated in certain ways,
emotion cues trigger a coordinated set of
experiential, behavioral, and physiological
response tendencies. Once these response
tendencies arise, they may be modulated
in various ways. Because emotion unfolds
over time, emotion regulation strategies
can be differentiated in terms of when they
have their primary impact on the emotion-
FIGURE 20.1. Three major approaches to studying individual differences in affect regulation via self-
report and some of the major measures.
AFFECT REGULATION
•
Emotion Regulation
Questionnaire
(ERQ; Gross & John,
2003)
•
Emotion Regulation
Questionnaire for
Children and
Adolescents (ERQ-CA;
Gullone & Taffe,
2012)
•
Negative Mood Regulation
Scale (NMR; Catanzaro &
Mearns, 1990)
•
Trait Meta-Mood Scale
(TMMS; Salovey et al.,
1995)
•
Difficulties in Emotion
Regulation Scales (DERS;
Gratz & Roemer, 2004)
•
Mayer–Salovey–Caruso
Emotional Intelligence
Test (MSCEIT
; Mayer,
Salovey, & Caruso, 2002)
•
Ways of Coping (WOC;
Folkman & Lazarus,
1980)
•
COPE Inventory (Carver,
Scheier, & Weintraub,
1989)
•
Cognitive Emotion
Regulation
Questionnaire
(CERQ; Garnefski &
Kraaij, 2007)
Specific Processes to
Regulate Emotion
Coping with Stress
Emotional
Competence
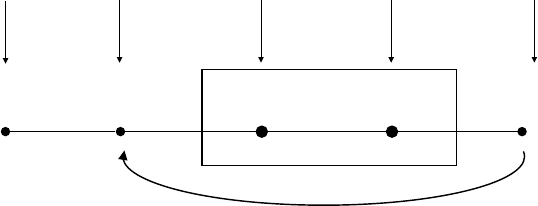
324 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
generative process. As shown in Figure 20.2,
five families of more specific strategies can
be located along the timeline of the emotion
process (Gross, 1998).
Here we illustrate the use of these strat-
egies for the most frequent goal of emo-
tion regulation in everyday life, namely,
down- regulating (decreasing) emotions
that typically have a negative valence, such
as anxiety/fear, sadness, and anger (Gross,
Richards, & John, 2006). Specific forms of
emotion down- regulation are indicated in
Figure 20.2 in parentheses under each fam-
ily name. In particular, situation selection
refers to avoiding certain people, places, or
activities so as to limit one’s exposure to sit-
uations likely to generate negative emotion.
Once a situation has been selected, situation
modification operates to tailor or change a
situation so as to decrease its negative emo-
tional impact. Third, situations have many
different aspects, so attentional deploy-
ment can be used to focus on less negatively
valenced aspects of the situation. Once one
has focused on a particular aspect of the sit-
uation, cognitive change refers to construct-
ing a more positive meaning out of the many
possible meanings that may be attached to
that situation. Finally, response modulation
refers to various kinds of attempts to influ-
ence emotion response tendencies once they
already have been elicited.
Individual Differences
Measured with the Emotion
Regulation Questionnaire
Empirical research on individual differences
has focused on two specific regulatory pro-
cesses (for reviews, see John & Gross, 2004,
2007). Cognitive reappraisal is a form of
cognitive change that involves construing a
potentially emotion- eliciting situation in a
way that changes its emotional impact. For
example, during an admissions interview,
one might view the give and take as an oppor-
tunity to find out how much one likes the
school, rather than as a test of one’s worth.
Expressive suppression is a form of response
modulation that involves inhibiting ongoing
emotion- expressive behavior. For example,
one might keep a poker face while holding
a great hand during an exciting card game.
Antecedent- focused strategies such as reap-
praisal influence whether or not particular
emotion response tendencies are triggered,
and are therefore expected to have generally
positive implications for affective and social
functioning. In contrast, response- focused
strategies such as suppression influence how
emotion response tendencies are modulated
once they have been triggered, and are
therefore expected to have generally more
negative implications for affective and social
functioning (cf. Gross, 1998).
Situation ResponseAttention Appraisal
Situation
Selection
(Avoidance)
Situation
Modification
(Self-Assertion)
Attention
Deployment
(Distraction)
Response
Modulation
(Suppression)
Cognitive
Change
(Reappraisal)
FIGURE 20.2. A process model of emotion regulation. Individual differences in emotion regulation
may arise at five points in the emotion- generative process: (1) selection of the situation; (2) modification
of the situation; (3) deployment of attention; (4) change of cognitions; and (5) modulation of experien-
tial, behavioral, or physiological responses. Specific instantiations of these five families of regulatory
strategies (given in parentheses) may be used for the down- regulation of negative emotion, as described
in the text. From John and Gross (2007). Copyright 2007 by The Guilford Press. Reprinted by permis-
sion.
Individual Differences in Affect Regulation 325
To test whether there are reliable and sys-
tematic individual differences in these two
emotion regulation processes, Gross and
John (2003) developed the Emotion Regu-
lation Questionnaire (ERQ), a brief 10-item
measure of the habitual use of reappraisal
and suppression. Items were derived ratio-
nally, starting with the experimental manip-
ulations used in previous experimental
research, and indicating clearly in each item
the intended emotion regulatory process,
such as “I control my emotions by changing
the way I think about the situation I’m in”
(reappraisal) and “I control my emotions by
not expressing them” (suppression). These
example items refer to emotion in general. In
addition, the Reappraisal and Suppression
scales each include at least one item asking
about regulating negative emotion (illus-
trated for the participants by giving sad-
ness and anger as examples) and one item
about regulating positive emotion (exempli-
fied by joy and amusement). Gross and John
also were careful to limit the item content
to the intended emotion regulatory strategy
in order to avoid potential confounding by
mentioning any positive or negative conse-
quences on affect, social functioning, or
well-being.
The structure of the ERQ is consistent
across samples, ages, and cultures, indicat-
ing a clear two- factor structure. Factor anal-
yses have shown two independent factors in
multiple samples of young and older adults
in the United States (John & Gross, 2004),
Germany (Abler & Kessler, 2009), Italy
(Balzarotti, John, & Gross, 2010), China
(English & John, 2013), Japan (Eng, Akutsu,
Gross, & John, 2013), and more than 15
other language communities in which the
ERQ has been adapted (Matsumoto et al.,
2008). Consistent with factor- analytic
findings, the correlation between the ERQ
Reappraisal and Suppression scales tends
to be close to zero. That is, individuals who
frequently use reappraisal are no more (or
less) likely to use suppression than individu-
als who use reappraisal infrequently. These
findings are important because they provide
clear evidence against a single, general- factor
model, in which some individuals regulate
their emotions a lot using both reappraisal
and suppression, whereas other individuals
regulate their emotions rarely, using neither
strategy frequently. Instead, reappraisal and
suppression are two independent regulatory
strategies that different individuals use to
varying degrees.
In terms of mean differences, gender and
culture differences emerged for suppres-
sion, which is expected to reflect culturally
defined display rules for emotion (Ekman,
1972). The pattern of gender differences was
highly consistent across cultures. Masculin-
ity is generally associated with acting tough
and unemotional, thus avoiding any indica-
tion of weakness or dependency (Knobloch
& Metts, 2013). Indeed, emotion expression
may be costly for men; for example, expres-
sion of negative emotion has been linked
to lower social status for men but not for
women (Anderson, John, Keltner, & Kring,
2001). In general, then, men should use sup-
pression more often than women. As shown
in Figure 20.3, men indeed reported lower
levels of suppression use than women; in
contrast, there were no gender differences in
reappraisal use in any of the cultures stud-
ied.
Figure 20.3 also shows that among both
men and women, the Japanese samples scored
highest in suppression use. This is consistent
with mean suppression scores in 23 nations
(Matsumoto et al., 2008); individuals in
Western countries that value independence
were less likely to suppress their emotions
than individuals in East Asian countries
that value interdependence. However, cross-
national comparisons cannot conclusively
pinpoint the origin of the national– group
differences, so Eng et al. (2013) tested for
acculturation effects (1) between groups
of European Americans and East Asian
Americans and (2) within a group of Asian
American immigrants who differed in their
acculturation to Western culture (indexed
by the length of their residence in the United
States). Figure 20.4A shows that European
Americans scored lower in suppression than
did East Asian Americans. Figure 20.4B
shows parallel suppression differences in
the within- group analysis: The longer East
Asian immigrants had lived in the United
States and been exposed to Western cultural
values and practices, the less suppression
they reported, in effect becoming more like
European Americans. How can these cul-
tural group differences and acculturation
effects be explained? As we will argue in
the final section of this chapter, models of
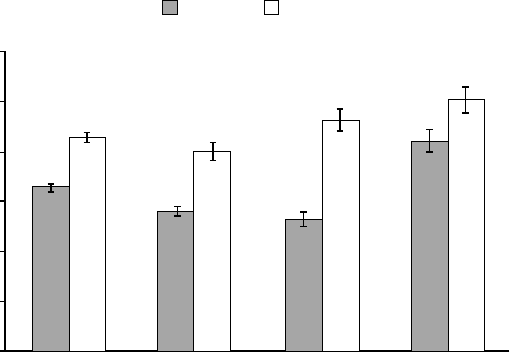
326 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
individual differences in emotion regulation
need to consider values, goals, and other
motivational factors, such as the culturally
transmitted values associated with indepen-
dent and interdependent self- construal.
Finally, it is important to comment on
discriminant validity. The ERQ Reappraisal
and Suppression scales were not related to
various measures of cognitive ability (Gross
& John, 2003; McRae, Jacobs, Ray, John,
& Gross, 2012). Similarly, correlations with
social desirability were small, probably
because the ERQ items are worded fairly
neutrally and do not mention adjustment or
psychological health outcomes. Finally, cor-
relations with the broad personality dimen-
sions defined by the Big Five traits (e.g.,
John, Naumann, & Soto, 2008) showed
only modest relations; highly neurotic indi-
viduals were slightly less likely to use reap-
praisal, and highly extraverted individuals
were somewhat less likely to use suppres-
sion. Overall, these findings are consistent
with a conceptualization of reappraisal
and suppression as rather specific and nar-
rowly defined individual differences in emo-
tion regulation processes. The discriminant
validity findings are particularly important
here, because theory predicts habitual use of
reappraisal should have generally favorable
implications for adjustment and psychologi-
cal health, whereas greater habitual use of
suppression should have less favorable impli-
cations. Thus, it is important that any such
adjustment effects cannot be attributed to
other factors, such as intelligence, socially
desirable responding, or general tempera-
ment and personality traits.
In terms of adjustment outcomes, the
general process model (Gross, 1998) holds
that reappraisal occurs early in the emotion-
generative process, before emotion response
tendencies have been fully generated, and
thus permits the modification of the entire
emotional sequence, including the expe-
rience of more positive and less negative
emotion, without notable physiological,
cognitive, or interpersonal costs. Suppres-
sion, by contrast, comes relatively late in the
emotion- generative process and primarily
modifies behavioral emotion response ten-
dencies, without reducing the experience
of negative emotion. Because suppression
comes late in the emotion- generative pro-
cess, it requires the individual to effortfully
manage emotion response tendencies that
arise continually, thus consuming cognitive
resources that could otherwise be used for
optimal performance in the social contexts
in which the emotions occur. Moreover, in
everyday life, the habitual suppression of
one’s true feelings is expected to create in the
Women Men
Mean Suppression
U.S. Germany Italy Japan
4.5
4
3.5
3
2.5
2
1.5
FIGURE 20.3. Men suppress more than women across four cultures. Mean ERQ suppression scores
are shown for men and women in samples from the United States (Gross & John, 2003), Germany
(Abler & Kessler, 2009), Italy (Balzarotti, John, & Gross, 2010), and Japan (Eng et al., 2013).
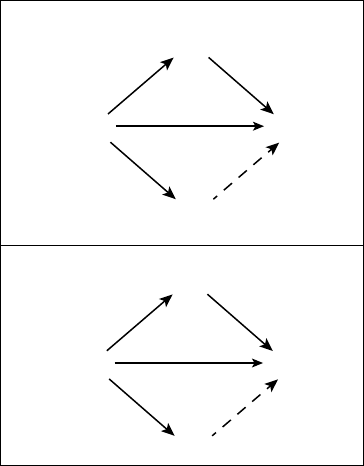
Individual Differences in Affect Regulation 327
individual a sense of discrepancy between
inner experience and outer expression, lead-
ing to negative feelings about the self and
to interpersonal behavior that is distracted,
strained, and avoidant, thus impeding the
development of emotionally close relation-
ships (see also English & John, 2013; Eng-
lish, John, & Gross, 2013; John & Gross,
2004).
In general, empirical evidence has sup-
ported the prediction that more regulation
is not necessarily better but, instead, the
effects of regulation should depend on the
particular regulatory process used: Indi-
vidual differences in the habitual use of
reappraisal and suppression have differen-
tial implications for effective functioning
(e.g., Abler & Kessler, 2009; Balzarotti et
al., 2010; English & John, 2013; English,
John, & Gross, 2013; Gross & John, 2003;
Richards & Gross, 2000; Srivastava, Tamir,
McGonigal, John, & Gross, 2009). Figure
20.5 illustrates typical findings. In terms of
affective implications, habitual use of reap-
praisal was related to more positive emo-
tion and less negative emotion. Cognitively,
reappraisal does not appear to have reliable
effects on social memory. In the domains of
interpersonal functioning and well-being,
reappraisal was generally associated with
better psychological health. However, these
relationships may turn out to be more com-
plex; for example, the positive interpersonal
effects of reappraisal may be more apparent
in peer ratings of relationship quality than
in self- ratings, and the well-being effects
of reappraisal may vary somewhat depend-
ing on measures and samples (e.g., Aldao,
Nolen- Hoeksema, & Schweizer, 2010; Eng-
lish & John, 2013; English, John, Srivastava,
& Gross, 2012).
A rather different picture emerges for
habitual use of suppression. As shown in
Figure 20.5, suppression is related to less
positive and more negative emotion experi-
ence. Cognitively, suppression is associated
with degraded memory for socially relevant
information. Socially, suppression is con-
sistently related to relationships that are
less close, satisfying, and supportive, even
in highly interdependent cultures such as
China (English & John, 2013). In terms of
well-being, suppression again has unfavor-
able effects, although that may be limited
to Western cultures that highly value inde-
pendence and authenticity (English & John,
2013).
Whereas there is now considerable evi-
dence for effects on adjustment and psycho-
logical health, much less is known about
the development of individual differences
in reappraisal and suppression (see John
& Gross, 2004). For example, research on
adults suggests that gender and cultural
socialization processes are likely playing a
role. As discussed earlier (see Figures 20.3
and 20.4), on average men report higher lev-
Independence
Interdependence
SuppressionAsian vs. White
Ethnicity
n.s.
–
–
–
+
(A)
Independence
Interdependence
Suppression
Length of
Residence
in U.S.
n.s.
–
–
–
+
(B)
FIGURE 20.4. Explaining the effects of culture
on emotion regulation: Ethnicity and accul-
turation effects on suppression were mediated
by independent (but not interdependent) self-
construal. Panel (A) shows that in a sample of
Asian and White students, the between- group
effect of ethnicity (coded Asian = 0, White = 1)
on suppression was mediated through indepen-
dence (but not through interdependence). Panel
(B) shows parallel mediation effects within the
group of Asian immigrants: length of accultura-
tion (residence) in the United States was associ-
ated with less suppression use, and this effect was
mediated through greater independence (but not
interdependence) in self- construal. Signs indicate
the relationship between variables: “+” indicates
a positive effect, “-“ a negative effect, and “n.s.”
a null effect.
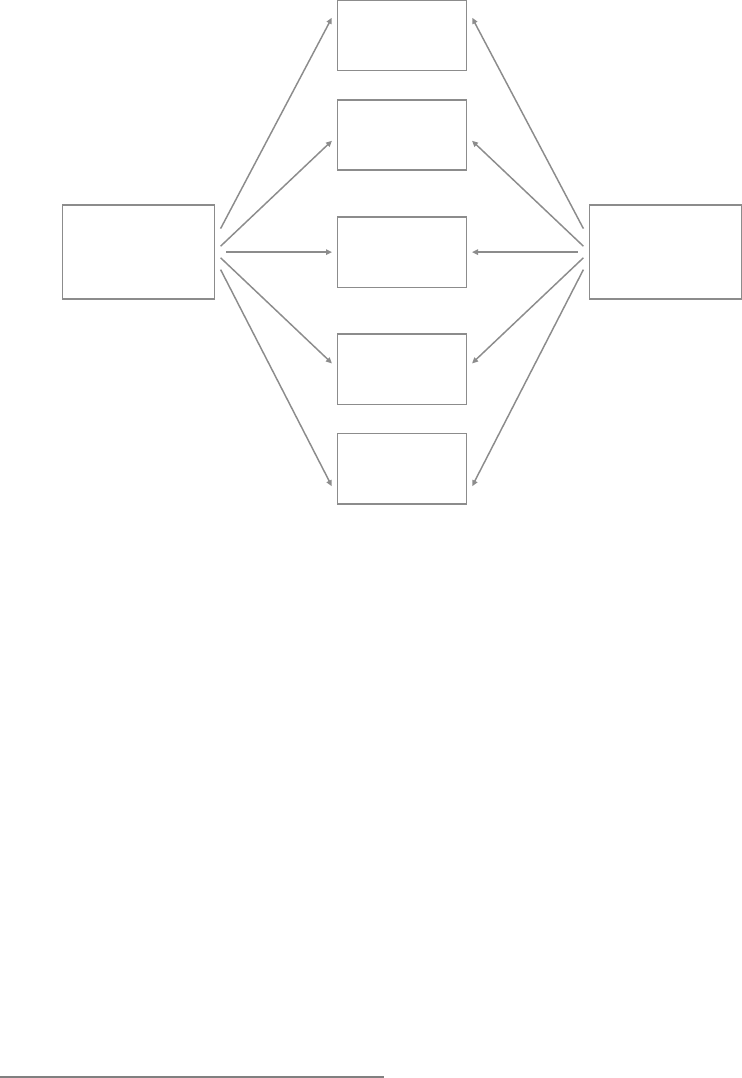
328 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
els of suppression use than women, and East
Asians report higher levels of suppression
use than Westerners. However, at this point
we know little about when and how these
differences in emotion regulation develop.
There is reason to expect this state of
affairs to change for the better in the near
future. A version of the ERQ adapted for
children and adolescents (ERQ-CA) has
recently been published (Gullone & Taffe,
2012). Using this instrument, promising
findings on the development of reappraisal
and suppression are beginning to appear
(e.g., Bariola et al., 2011).
The Coping-with-Stress Approach
and Its Major Measures
The literature on stress and coping origi-
nated from psychodynamic concepts and
ideas on anxiety and defense mechanisms
that have provided an important start-
ing point for much thinking about affec-
tive states (e.g., Shaver & Mikulincer,
2007; Westen & Blagov, 2007). For several
decades, stress researchers have studied
individual differences in coping styles— the
ways individuals attempt to deal with adver-
sity (e.g., Lazarus & Folkman, 1984; see
also Carver & Scheier, 1994). In their pio-
neering work, Folkman and Lazarus (1985)
emphasized that coping has two major func-
tions, namely, “the regulation of distressing
emotions and doing something to change
for the better the problem causing the dis-
tress” (p. 152). That is, coping includes
regulatory efforts directed at internal emo-
tional responses (e.g., anxiety) or at external
problems facing the individual (e.g., a final
exam). What are the major ways, or dimen-
sions, of coping in which individuals differ
from each other?
Ways of Coping Questionnaire
Folkman and Lazarus (1980, 1985, 1988)
reasoned that multiple ways of coping could
REAPPRAISAL
Well-Being
(e.g., life
satisfaction)
Social
(e.g., closeness)
Cognitive
(e.g., memory)
Negative
Emotion
Positive
Emotion
SUPPRESSION
–.47*
.35*
.17
.26*
.30*
.36*
–.58*
–.27*
–.25*
–.34*
FIGURE 20.5. Individual differences in the habitual use of reappraisal and suppression are differen-
tially associated with healthy adaptation in emotion experience, cognition, relationships, and well-
being (adapted from John & Gross, 2004, 2007). Specific correlational findings in the figure are given
only for illustrative purposes, and effect sizes vary across samples and measures used.
Individual Differences in Affect Regulation 329
be distinguished but, lacking an established
theoretical framework, tried to discover
the major dimensions of coping empiri-
cally. They initially assembled in the Ways
of Coping Questionnaire 68 items intended
to capture a wide variety of behavioral and
cognitive coping responses, representing
the things people commonly do when deal-
ing with stress. Some of these items were
inspired by previous theory and literature,
including defense mechanisms (e.g., wish-
ful thinking, denial), whereas others were
added later at the suggestion of subjects in
their studies (e.g., prayer). Exploratory fac-
tor analyses and rational item selection led
eventually to eight scales assumed to mea-
sure distinct ways of coping: Confrontive,
Distancing, Self- Controlling, Seeking Social
Support, Accepting Responsibility, Escape-
Avoidance, Planful Problem Solving, and
Positive Reappraisal (Folkman & Lazarus,
1988). These eight coping styles constituted
the first and “most commonly used measure
of basic coping responses” (Parker, Endler,
& Bagby, 1993, p. 361).
The inclusion of cognitive coping styles
such as Planful Problem Solving and Accept-
ing Responsibility shows that the concep-
tual domain of coping (see Figure 20.1) also
includes processes that are not aimed at the
regulation of affective states per se, such as
“analyzing the problem in order to under-
stand it better.”
Some scales, however, do fit with the
broader notion of affect regulation set out
in Figure 20.1. Consider, for example, the
scale initially labeled Emphasizing the Posi-
tive (Folkman & Lazarus, 1985). Its revised
label, Positive Reappraisal, implies a concep-
tual link to the family of emotion regulation
strategies that Gross (1998) called Cognitive
Change, especially the Cognitive Reappraisal
strategy. Do the seven items on Folkman and
Lazarus’s (1988) Positive Reappraisal coping
scale measure cognitive reappraisal? They
include “I was inspired to do something cre-
ative”; “I changed or grew as a person in a
good way”; “I came out of the experience
better than I went in”; “I found new faith”;
“I rediscovered what is important in life”; “I
changed something about myself”; and “I
prayed.” These items indeed describe a per-
son who emphasizes positive experiences or
behaviors in a stressful situation. However,
they do not directly assess the particular
process of changing the personal meaning
or appraisal of an emotion- eliciting event.
Instead, these items describe diverse, though
generally positive, consequences arising
from or after the stressful experience (e.g.,
“I came out of the experience better than I
went in”), including personal growth, self-
transformation, greater creativity, and even
spiritual renewal.
This complex item content poses serious
issues for research on the correlates and
adaptive consequences of using a particu-
lar coping style. As Lazarus (2000, p. 666)
explained, “The danger of confounding is
that measures of coping could contain some
of the same variables— for example, distress
or psychopathology— as the outcome mea-
sure of mental health. Thus, if the anteced-
ent and consequent measures are essentially
the same, any correlation between them
would represent some degree of tautology.”
This problem— confounding the predictor
or causal variable (i.e., regulation) with sub-
sequent outcomes to be predicted (i.e., better
adjustment) within the same item—is a com-
mon issue in research on individual differ-
ences in affect regulation. It highlights that
in writing items researchers should focus
item content specifically on the regulatory
process of interest. Indeed, among the items
listed earlier, only one (“I prayed”) describes
a potential emotion regulatory activity with-
out mentioning some other, positive out-
comes as well.
In addition to confounding, lack of speci-
ficity is another potential issue in these
items. For example, the item “I prayed” is
vague vis-à-vis the particular regulatory pro-
cess at work: Individuals may pray in order
to gain a new perspective or understanding
of an emotion- eliciting event, but they may
also pray to distract themselves, or to share
their feelings with a greater power, or even
to muster the inner strength to modify the
situation. These uses of prayer and religion
for affect regulation may well be distinct
and show differential correlates and adjust-
ment outcomes.
These issues also apply to items on other
scales (see Folkman & Lazarus, 1985). For
example, “I jog or exercise,” scored on the
Tension Reduction scale, may represent
multiple specific regulation strategies, such
as situation selection (e.g., avoiding the
stressor), attentional deployment (e.g., dis-
330 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
tracting oneself), and even response mod-
ulation (e.g., going off to jog in order to
avoid expressing one’s emotion to another
person). These items could be more specific
regarding the specific regulatory processes
intended.
In summary, most items on the Ways of
Coping Questionnaire are worded broadly
and lack specificity regarding the regulatory
process involved. Many items suffer from
a confounding problem where regulation
and extraneous outcomes (e.g., psychoso-
cial consequences) are combined in the same
item. At the scale level, the lack of a process
model led to a reliance on exploratory factor
analyses of these broad and complex items
to define the conceptual building blocks (i.e.,
the particular coping styles). The resulting
concepts (as represented by the scales) are
heterogeneous and often combine conceptu-
ally distinct regulatory processes. With such
complex and global concepts, the scales are
hard to interpret and have been criticized
for lacking construct validity; discriminant
validity has been a particular problem,
because intercorrelations between some of
the scales tend to be very high, with some
exceeding .70 (e.g., Parker et al., 1993).
COPE Inventory
In an important contribution, Carver,
Scheier, and Weintraub (1989) substan-
tially reworked the original Folkman and
Lazarus scales, using modern psychometric
methods. They subdivided some of the most
heterogeneous scales, added new scales, and
improved the overall conceptual coherence
and internal consistency. The resulting Cop-
ing Orientations to Problems Experienced
Scale (COPE) is a self- report questionnaire
that measures 14 coping styles with four
items each. Even though the COPE scales
are shorter, they are more reliable than the
earlier Ways of Coping scales. The COPE
covers a broad array of diverse coping styles,
such as Planning, Active Coping, Mental
Disengagement, Seeking Social Support—
Instrumental, Seeking Social Support—
Emotional, Positive Reinterpretation and
Growth, Turning to Religion, Focus on and
Venting of Emotion, and Denial.
However, some of the problems of the
Ways of Coping Questionnaire remain. The
lack of a process, structural, or concep-
tual model continues to be a problem. The
scales thus continue to define coping styles
at rather different levels of abstraction, with
some scales (e.g., Turning to Religion) mea-
suring very broad and complex constructs
and others (e.g., Denial) measuring much
more specific processes. It is not clear how
the various coping styles being measured
should be conceptually or causally related.
And many of the scales remain conceptu-
ally heterogeneous; for example, the scale
labeled Focus on and Venting of Emotions
involves both being aware of one’s distress
and “letting it out.”
Before using these scales, it is important
to examine the item content of the COPE
scales to understand what they are mea-
suring (e.g., John & Gross, 2007). Some
of them can be linked to the specific emo-
tion regulation strategies defined in Gross’s
(1998) process model. For example, the
COPE Active Coping and Planning scales
include items that refer to regulatory pro-
cesses involving situation selection and situ-
ation modification. Another example is the
scale that Folkman and Lazarus (1988) had
initially called Emphasizing the Positive and
that Carver et al. (1989) relabeled Positive
Reinterpretation and Growth (e.g., looking
for the silver lining in stressful situations;
trying to learn from difficult experiences).
One would expect this coping style to be
related to the specific emotion regulation
strategy of Cognitive Reappraisal. How-
ever, this COPE scale also includes general
optimism and longer- term positive changes
and adaptations individuals might make
later on, following the stressful experience.
Indeed, the correlation of this COPE scale
with Gross and John’s (2003) Cognitive
Reappraisal scale tends to be moderate (e.g.,
Gross & John, 2003; Balzarotti, John, &
Gross, 2010), consistent with the broader
definition of the COPE scale. That is, the
inclusion of positive processes and long-
term outcomes that have little to do with
the immediate regulatory task at hand make
the scale complex and conceptually hetero-
geneous.
This conceptual heterogeneity makes it
difficult to establish whether and when a
particular coping style is effective or dys-
functional. For example, Carver et al.
(1989) had assumed that the Focus on and
Venting of Emotions scale would be dys-
Individual Differences in Affect Regulation 331
functional but instead found its adjustment
correlates to be complex; the positive cor-
relation with Social Support suggests some
positive adjustment implications. Concep-
tually, we suggest, this scale combines two
distinct processes with potentially different
adjustment implications. On the one hand,
items like “I get upset, and am really aware
of it” are conceptually related to rumina-
tion (Nolen- Hoeksema, Parker, & Larson,
1994); focusing on a negative emotion will
likely intensify the experience of that emo-
tion further and thus make down- regulation
more difficult, leading to lower adjustment
and well-being. On the other hand, items
like “I feel a lot of emotional distress and I
find myself expressing those feelings a lot”
describe an individual whose emotion expe-
rience and expression are congruent (Mauss
et al., 2011); congruence between experience
and expression, and sharing of emotions
with others, can increase authenticity and
generate greater closeness with others, lead-
ing to higher well-being (English & John,
2013). If the research goal is to understand
individual differences relevant to affect reg-
ulation and how these individual differences
are related to important life outcomes, such
as well-being and relationship functioning,
then less complex measures may be needed
to provide conceptual clarity and coherence
in findings.
A similar analysis applies to the two Seek-
ing Social Support scales on the COPE—
after all, talking to another person about
one’s problems requires some sharing and
expression of feelings (i.e., low suppres-
sion) and will serve to focus the individual
on these emotions rather than distract him
or her from them (i.e., low distraction). In
contrast, the Mental Disengagement scale
(e.g., coping by turning to work; going to the
movies) should relate most to distraction;
however, as Carver et al. (1989) noted, these
items represent a rather loose set of diverse
activities. The inclusion of items about sleep
and daydreaming suggest a potential link
to situation selection as well, because these
activities can be performed for reasons other
than distraction (e.g., avoiding the stressful
situation).
Drug and alcohol use “in order to think
about it less” may also be related to distrac-
tion. As noted earlier in our discussion of
prayer use, the Turning to Religion scale
on the COPE could serve several regulatory
strategies; it would be interesting to clarify
empirically the processes that account for
positive effects of religiosity on well-being,
which may include antecedent processes,
such as reappraisal or distraction, or even
response modulation via lowering suppres-
sion, because prayer may offer a safe place
for emotional expression by sharing feelings
with a trusted higher entity.
Consistent with our earlier observation
that coping styles are defined rather broadly,
several COPE scales fall clearly outside
the scope of the affect regulation domain.
For example, the Suppression of Compet-
ing Activities scale and the Restraint Cop-
ing scale involve behavioral responses that
reflect problem- focused coping, showing
that coping efforts are often directed at
problems or events in the external world
that interfere with the individual’s goals,
even though they do not cause a specific
emotional response. Several other COPE
scales refer to long-term adjustments some
individuals make as a consequence of the
stressful experience, a timescale outside the
more limited time frame of the immediate
emotion regulatory episode. This temporal
issue applies to Behavioral Disengagement
(“I just give up trying to reach my goal”),
Acceptance (“I learn to live with it”; “I get
used to the idea that it happened”), Denial
(“I act as though it hasn’t even happened”),
and the Growth component of Positive Rein-
terpretation and Growth (“I try to grow as a
person as a result of the experience”).
Cognitive Emotion
Regulation Questionnaire
Although its name does not refer to stress
and coping, Garnefski and Kraaij’s (2007)
Cognitive Emotion Regulation Question-
naire (CERQ) also originated in the coping
approach. Like the COPE, the goal was to
assess a set of regulatory strategies that help
people “to keep control over their emotions
during or after the experience of threaten-
ing or stressful events” (Garnefski & Kraaij,
2007, p. 141). However, the CERQ focuses
more narrowly than the COPE on cognitive
coping strategies, which are said to represent
“the conscious, cognitive way of handling
the intake of emotionally arousing informa-
tion” (p. 141).
332 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
The CERQ has a format very similar to
the COPE and also consists of relatively brief
4-item scales, “each referring to what some-
one thinks after the experience of threaten-
ing or stressful events” (Garnefski & Kraaij,
2007, p. 141). In other words, just like the
coping styles on the COPE, these cognitive
regulation dimensions focus on stressful
situations and have the longer time horizons
(e.g., after the actual emotional experience)
that differentiate coping styles from the spe-
cific emotion- regulation processes defined in
Gross’s model.
Some of the nine CERQ scales were
adapted from the COPE and other cop-
ing instruments: “These dimensions were
defined either by taking out or reformulat-
ing the cognitive dimensions of existing cop-
ing measures (Carver et al., 1989; de Ridder,
1997), ‘transforming’ non- cognitive coping
strategies into cognitive dimensions or add-
ing new strategies on theoretical grounds”
(Garnefski & Kraaij, 2007, p. 142). Cop-
ing styles that were clearly cognitive, such
as Acceptance, Planning, and Positive Rein-
terpretation and Growth (renamed Positive
Reappraisal) were retained from the COPE.
Behavioral and overly complex coping styles
from the COPE, such as Use of Social Sup-
port, Substance Abuse, Humor, Religious
Coping, and even Focus on and Venting of
Emotions were left out of the CERQ.
In addition, some regulatory processes
from other literatures were added, such as
Focus on Thought/Rumination (e.g., “I
often think about how I feel about what I
have experienced”), Catastrophizing (e.g.,
“I continually think how horrible the situa-
tion has been”), and Self-Blame (e.g., “I feel
that I am the one to blame for it”). Again,
as in the earlier coping measures, many of
the scales do not focus on the regulation
of internal experiences; rather, they focus
on the external, stressful situation, as illus-
trated by the items on the Acceptance scale
(e.g., “I think that I have to accept the situa-
tion”; “I think that I have to accept that this
has happened”).
In our view, the singular focus on “think-
ing” aspects in an instrument with nine dis-
tinct scales has two potential disadvantages.
One is discriminant validity, in the sense that
subsets of the scales become relatively simi-
lar and thus highly correlated. This problem
has been criticized in the two earlier cop-
ing style measures as well. For example, the
three CERQ scales— Positive Reappraisal (“I
think that I can become a stronger person as
a result of what has happened”), Refocus on
Planning (“I think of what I can do best”),
and Putting into Perspective (“I think that
it hasn’t been too bad compared to other
things”)—all share elements of cognitive
change or reappraisal. Indeed, Garnefski
and Kraaij (2007) reported that Positive
Reappraisal and Refocus on Planning corre-
lated close to .70 in their adult participants.
Unfortunately, the ERQ (or COPE) was not
included, thus providing no information
about the convergent validity of these three
new CERQ scales with earlier measures of
cognitive reappraisal.
The second disadvantage of the purely
cognitive focus is that emotion researchers
agree that emotions have not only experi-
ential but also behavioral– expressive com-
ponents (e.g., Mauss et al., 2011), and the
coherence of these emotion response systems
is likely a function of regulatory processes.
Therefore, the omission of any scales related
to individual differences in response modu-
lation, such as expressive suppression, would
seem to make an instrument less useful for
research on individual differences in affect
regulation.
In conclusion, this comparison of three
measures of coping styles and specific emo-
tion regulation processes suggests that there
is some overlap between the two approaches.
Overall, however, the two approaches are
surprisingly distinct. One core difference is
that the constructs measured by all the cop-
ing questionnaires are defined in broader
and more complex terms than the specific
emotion regulatory strategies. Several cop-
ing styles, especially the problem- focused
ones, fall outside the domain of emotion reg-
ulation processes. A great methodological
strength of the coping approach is its clear
focus on stressful situations. Much coping
research has studied individual differences
within the concrete context of a particular
stressful encounter (e.g., preparing for an
upcoming test), focusing on the individu-
al’s behavioral and cognitive responses in
this situation. This contextually grounded
approach emphasizes what individuals actu-
ally do, or try to do, in a specific context
(even when reported on questionnaires or
in interviews) and contrasts with global

Individual Differences in Affect Regulation 333
approaches such as the Big Five trait model
(e.g., John et al., 2008) that pay scant atten-
tion to the context and instead assess broad
trends in usual or typical behavior.
Future coping research may take a more
theoretically guided approach to resolve the
conceptual complexity and heterogeneity
of the coping scales in terms of their affect
regulatory implications. Renewed scale con-
struction efforts may address the confound-
ing of regulatory processes and downstream
adjustment outcomes within the same ques-
tionnaire item. Finally, because the coping
approach and the specific emotion regula-
tion processes approach differ in terms of
their time horizons and relevant situational
contexts, each has unique advantages and
applications. The various measures of cop-
ing styles provide a useful focus on stressful
situations and regulating negative affective
states.
The Emotional Competence
Approach and Its Major Measures
The emotional competence approach origi-
nated in developmental and clinical analyses
of what a child needs to learn to become an
emotionally and socially competent adult.
Saarni (e.g., 1999, 2011) analyzed emotional
functioning from the perspective of how
well it serves the adaptive and instrumental
goals of the individual, then defined emo-
tional competence as a set of affect- oriented
behavioral, cognitive, and regulatory skills.
Simply put, the child needs to learn what it
means to feel something and to do some-
thing about those feelings.
Saarni (1999, 2011) postulated eight broad
skills that she considers to be prerequisites
for emotional competence: (1) awareness of
one’s own emotional state; (2) the skills to
discern and understand the emotions of oth-
ers; (3) skill in using the common vocabu-
lary of emotion and expression; (4) capacity
for empathic and sympathetic involvement
in others’ emotional experiences; (5) ability
to realize that one’s inner emotional state
need not correspond to outer expression; (6)
capacity for adaptive coping with aversive or
distressing emotions by using self- regulatory
strategies that ameliorate the intensity or
temporal duration of such emotional states;
(7) awareness that relationships are defined
by emotional genuineness of expressive dis-
play and reciprocity; and (8) capacity for
emotional self- efficacy (i.e., individuals can
accept their own emotional experiences and
view themselves as generally feeling the way
they want to feel). Although not necessarily
accepted by all, these eight emotional com-
petencies provide a formidable set of com-
plex socioemotional skills that researchers
in this tradition consider to be part of affect
regulation. Note that from the perspective
of the specific emotion regulation process
approach, the capacity for adaptive cop-
ing would relate most directly to individual
differences in affect regulation. The other
competencies seem to specify processes that
make emotion regulation possible or may
help (rather than hinder) the enactment of
effective regulatory strategies. For example,
if an individual has no awareness of his or
her current emotional state, then that person
would hardly proceed with attempts to regu-
late that emotion.
Generalized Expectancies
for Negative Mood Regulation Scale
The Generalized Expectancies for Negative
Mood Regulation Scale (NMR) was devel-
oped by Catanzaro and Mearns (1990) and
is one of the earliest measures to take the
emotional competence approach. It now has
fallen out of use. This 30-item self- report
questionnaire focuses on individuals’ beliefs
that some “behavior or cognition will allevi-
ate a negative state or induce a positive one”
(p. 547), and asks them to indicate the extent
to which they believe their attempts to alter
their negative moods will work.
Many of the items focus on ways to
eliminate or at least avoid negative emo-
tions. Thus, the measure has been criti-
cized for equating mood regulation with
the avoidance of negative affect (e.g., Gratz
& Roemer, 2004); simply avoiding nega-
tive states is assumed to be an indication
of effective regulation, as shown by items
such as “When I’m upset, I believe that I can
forget about what’s upsetting me pretty eas-
ily” versus “When I’m upset, I believe that
I won’t be able to put it out of my mind”
(reverse scored). Moreover, the NMR does
not assess aspects of mood regulation, such
as awareness, clarity, or acceptance of emo-
tions, that later emotional competence
334 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
researchers, such as Saarni (1999), Salovey
et al. (1995), and others, deemed important.
Finally, regulatory processes are not differ-
entiated but are instead measured only in
terms of whether the individual thinks he or
she has access to mood regulation strategies
perceived to be effective.
Trait Meta‑Mood Scales
Consistent with their emotional competence
perspective, Salovey et al. (1995) aimed to
understand the reflective (or meta) processes
that accompany many mood states. These
“meta-mood” processes capture how indi-
viduals reflect on their feelings, including
how they monitor, evaluate, and regulate
them (Mayer & Gaschke, 1988). Salovey et
al. (1995) assumed that emotions serve as an
important source of information, and that
individuals differ in how skilled they are at
processing this kind of information, particu-
larly in “their understanding of and ability
to articulate their affective states” and their
ability to “regulate such feelings and use them
adaptively to motivate behavior” (p. 147).
The Trait Meta-Mood Scales (TMMS) were
designed to measure stable and general atti-
tudes about moods and the degree to which
individuals attempt to manage (or repair)
mood experiences. The TMMS measures
three constructs— people’s tendency to
attend to their moods and emotions (atten-
tion), to discriminate clearly among them
(clarity), and to regulate them (repair)—each
capturing individual differences considered
“fundamental to the self- regulatory domain
of emotional intelligence” (p. 147).
Given that the TMMS focuses on indi-
vidual differences in meta-moods—that
is, thoughts and attitudes that accompany
mood experiences— we would not expect
any links to regulatory strategies that
occur earlier in the emotion process, prior
to the onset of an emotional episode itself,
such as situation selection or modification.
However, attentional processes, cognitive
change, and response modification should
be of considerable relevance.
The TMMS Attention scale refers to pay-
ing close attention to feelings, accepting
feelings, valuing them positively, and letting
oneself experience them fully and inten-
sively, using items such as “I often think
about my feelings” versus “I don’t think it’s
worth paying attention to your emotions or
moods” (reverse scored). As expected, the
Attention scale correlates with the Private
Self- Consciousness scale, which measures
awareness and attention to private aspects
of the self (e.g., thoughts and feelings). One
would expect the Attention scale to relate
negatively to chronic use of the emotion
regulation strategies distraction and sup-
pression. The link to distraction is theoreti-
cally interesting, because paying close atten-
tion to negative mood states has been shown
to magnify and intensify the experience of
negative affect and the risk for depression
(Scheier & Carver, 1977), whereas in our
model, distraction is expected to decrease
negative emotion experience. Indeed, in one
of the studies by Salovey et al. (1995), the
Attention scale was related to higher depres-
sion scores. Regarding the negative link to
suppression, being intensely aware of and
paying close attention to one’s emotions
should interfere with the considerable and
ongoing cognitive effort required to sup-
press one’s emotions effectively (Richards &
Gross, 2000), and that should be especially
true for individuals who experience their
emotions intensely. Moreover, individuals
scoring high on the Attention scale value
their feelings and believe in letting them
guide their behavior. That is the opposite of
individuals who habitually use suppression
and thus show expressive behavior that is
inconsistent with their inner feelings (Gross
& John, 2003). Indeed, the TMMS Atten-
tion scale correlated negatively with the
ERQ Suppression scale.
The TMMS Clarity scale assesses clarity
about one’s feelings, and contrasts aware-
ness and acceptance of feelings with confu-
sion about feelings and their meaning and
implications. True- scored item examples
include “I feel at ease about my emotions”
versus false- scored items such as “I can’t
make sense out of my feelings.” Low clarity
was related not only to ambivalence about
emotional expression but also to vulner-
ability to negative affect, such as neuroti-
cism, distress, and depression. Salovey et al.
(1995) conceptually linked lack of clarity to
ruminative thought processes (e.g., individu-
als who do not know how they feel about a
negative event have to keep thinking about it)
and showed empirically that, after watching
a stressful video, individuals scoring high on
Individual Differences in Affect Regulation 335
the Clarity scale exhibited a decline in rumi-
native thought and recovered their positive
mood to a greater extent than low- scoring
individuals. These findings suggest a positive
relation between clarity and distraction (the
ability to use attentional resources to move
away from a negative emotion stimulus,
rather than ruminating about it), and a nega-
tive relation with suppression. Individuals
who are clear about and comfortable with
their emotions should feel little need to sup-
press their behavioral expression of emotion,
and the evidence supported this predicted
negative relation (Gross & John, 2003).
The TMMS Repair scale assesses attempts
to improve negative mood by thinking posi-
tively and taking an optimistic (rather than
pessimistic) attitude more generally. Item
examples include “Although I am sometimes
sad, I have a mostly optimistic outlook” and
“I try to think good thoughts no matter how
badly I feel,” contrasted with “Although I
am sometimes happy, I have a mostly pes-
simistic outlook” (reverse scored). This scale
seems conceptually similar to coping styles
reviewed earlier, such as Emphasizing the
Positive on the original Ways of Coping
Questionnaire and Positive Reinterpretation
and Growth on the COPE.
As expected, the Repair scale correlated
substantially with general optimism and low
vulnerability to distress and depression; it
also predicted lower levels of distress after
participants watched a stressful video. In
terms of specific emotion regulation pro-
cesses, one would expect the Repair scale to
relate positively to the use of distraction, as
well as reappraisal: The explicit mood repair
efforts included in the scale involve (1) using
thought to focus on something other (e.g.,
pleasant things; good thoughts) than the dis-
tressing stimulus, thus implicating distrac-
tion, and (2) also trying to think differently
(e.g., more positively) about the situation,
thus implicating reappraisal. In contrast,
expressive suppression is hardly an optimis-
tic process (Gross & John, 2003); its habit-
ual use is related not only to negative emo-
tion, as shown in Figure 20.5, but also to
feelings of inauthenticity (English & John,
2013) and the pessimistic expectation that
others will disapprove if shown one’s “real
self,” as shown as in Table 20.1. Consistent
with these considerations, Gross and John
(2003) found that the TMMS Repair scale
was differentially related to the ERQ Reap-
praisal (r = .36) and Suppression (r = –.26)
scales.
TABLE 20.1. Examples of Beliefs and Motivations Predicting Individual Differences
in the Habitual Use of Reappraisal and Suppression as Measured by the ERQ
Construct studied
Emotion regulation strategy
Reappraisal Suppression
Incremental (vs. entity) theory of emotion
a
.35 .04
(If they want to, people can change the emotions they have)
Global emotion regulation self-efficacy
a
.21 .00
(I certainly can control my emotions when running into a former
boyfriend/girlfriend)
Subjective authenticity
b
–.02 –.30
(It’s important to me to be true to myself)
Fear of negative consequences
c
–.05 .31
(I worry that if I express negative emotions, such as fear and anger,
other people will not approve of me)
Low self-efficacy for self-expression
c
–.12 .45
(At times I am just not able to express what I am really feeling)
Note. ERQ, Emotion Regulation Questionnaire (Gross & John, 2003).
a
Tamir, John, Srivastava, and Gross (2007, Study 1).
b
English and John (2013; mean across Studies 1–3).
c
John (2010).
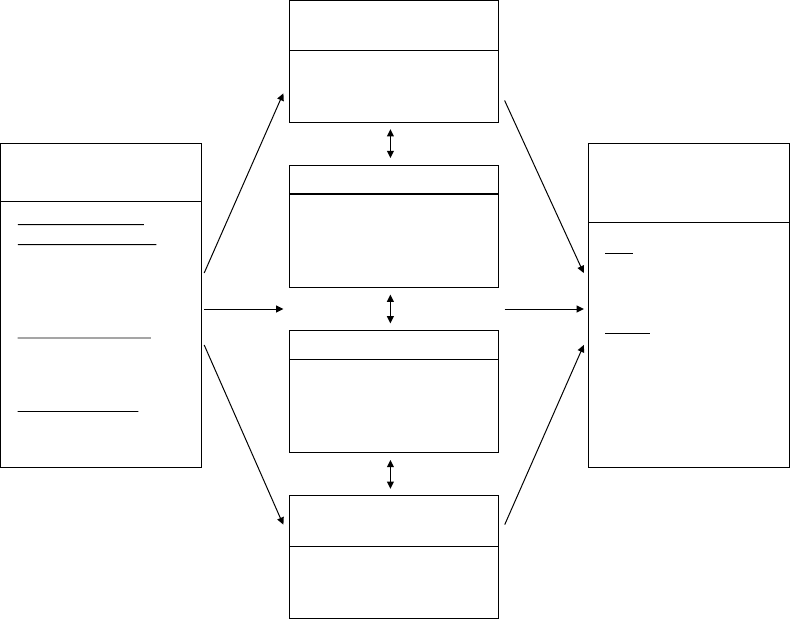
336 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
However, the correlations of the ERQ
scales and the TMMS scales were not large in
size. Suppression was related negatively to all
three TMMS scales, most strongly to Atten-
tion (r = –.41) and least strongly to Repair
(r = –.26). This is interesting and worth fur-
ther study for two reasons. First, we need
to understand better how individual differ-
ences in cognitive variables such as attention
to emotion are linked to behavioral vari-
ables, such as emotion- expressive behavior.
Second, we need to address questions about
causal order: How are cognitive variables
such as attention best conceptualized? In our
view, some may be antecedents (see Figure
20.6 below): Individuals may use suppres-
sion frequently because they have learned to
devalue and ignore their emotions as impor-
tant sources of information, and because
they have the pessimistic belief that others
will respond negatively if they truly express
their feelings. Other meta-mood processes
may represent consequences: Frequent use of
suppression leads to expressive behavior that
is incongruent with internal feeling states,
and may in turn lead to confusion and lack of
clarity about one’s emotions (and eventually
an unclear self- concept and low self- esteem).
These considerations imply that research
on individual differences in beliefs and moti-
vational factors will be critical in helping
us conceptualize how meta-mood processes
and specific emotion regulatory processes
are related. Table 20.1 summarizes the find-
ings from several initial studies. Incremental
beliefs that emotions can be changed, and
self- efficacy beliefs about one’s own ability
to change one’s emotions, predicted greater
use of cognitive reappraisal, whereas sub-
jective inauthenticity, fear of negative con-
sequences, and low self- efficacy predicted
greater use of suppression.
Demographic and
Experiential Factors
Gender socialization
Aging
Acculturation
Personality Factors
Big Five traits
Attachment
Cognitive Factors
IQ
Cognitive control
DISTAL
ANTECEDENTS
Use
Habitual
Situational
Ability
Actual
Perceived
INDIVIDUAL
DIFFERENCES IN
EMOTION REGULATION
Implicit theories
(incremental vs. entity)
Self-efficacy and
control beliefs
BELIEFS
Attention
Awareness
Clarity
EMOTION PERCEPTION
AND UNDERSTANDING
Ideal affect
Hedonic/instrumental
goals
Self-construal goals
VALUES AND GOALS
Feeling rules
Display rules
NORMS AND
EXPECTATIONS
FIGURE 20.6. Examples of several types of individual differences in emotion regulation and their
potential antecedents.
Individual Differences in Affect Regulation 337
Difficulties in Emotion
Regulation Scale
Gratz and Roemer (2004) defined emotion
regulation competencies almost as broadly
as did Saarni (1999), whose influence they
acknowledge: “(a) awareness and under-
standing of emotions, (b) acceptance of emo-
tions, (c) ability to control impulsive behav-
iors and behave in accordance with desired
goals even when experiencing negative emo-
tions, and (d) ability to use situationally
appropriate regulation strategies flexibly to
modulate emotional responses as desired
in order to meet individual goals and situ-
ational demands” (p. 42).
In devising measures of these four compe-
tency constructs, Gratz and Roemer (2004,
see p. 44) were influenced by the content of
Salovey et al.’s (1995) TMMS scales, as well
as by the older NMR, which they used as a
template to structure the format of some of
the items. Specifically, they wrote their items
assessing difficulties with regulating emo-
tions during times of distress using the same
general format as the NMR. Thus, many
of their items begin with the sentence stem
“When I’m upset, I . . . ” This focus on a
global negative affect (upset) is a feature that
both the NMR and the Difficulties in Emo-
tion Regulation Scale (DERS) share with the
coping scales discussed earlier.
The 36-item DERS has six subscales.
Note that because the authors are interested
in dysregulation and its link to psychopa-
thology, they score all the subscales in terms
of difficulties with various competencies
considered important for emotion regula-
tion. Possibly as a result of this naming con-
vention, several of the DERS subscales have
vague or confusing names. It is thus impera-
tive to review the items that make up each
scale to ascertain what is being measured.
Two subscales seem rather similar to
existing TMMS scales but label the opposite
pole: (1) Lack of Emotional Awareness (e.g.,
“I pay attention to how I feel”—reverse
scored; emphasis added) captures the low
pole of the TMMS Attention scale; (2) Lack
of Emotional Clarity (e.g., “I am clear about
my feelings”—reverse scored; emphasis
added) is very similar to the low pole of the
TMMS Clarity scale.
Two other subscales seem to assess cop-
ing styles: (3) Nonacceptance of Emotional
Responses (e.g., “When I’m upset, I feel
ashamed with myself for feeling that way”;
emphasis added) seems similar to ineffective
coping scales such as Self- Blaming, which
might be a simpler yet clearer label for this
subscale; (4) Difficulties Engaging in Goal-
Directed Behavior (e.g., “When I’m upset,
I have difficulty thinking about anything
else”; emphasis added) seems to involve not
goal- directed behavior per se but ineffec-
tive attentional deployment (Gross, 1998)
and resembles what Nolen- Hoeksema et al.
(1994) conceptualized as ruminative coping
(i.e., difficulty shifting attention and disen-
gaging from a preoccupying affective state).
Finally, (5) the Impulse Control Diffi-
culties subscale (e.g., “When I’m upset, I
become out of control”) is similar to Impulse
Strength or Intensity scales (e.g., Gross &
John, 1997, 1998) that describe individu-
als who have very strong feelings that are
hard to control; and (6) the subscale Lim-
ited Access to Effective Emotion Regulation
Strategies (e.g., “When I’m upset, I believe
that there is nothing I can do to make myself
feel better” and “When I’m upset, I believe
that wallowing in it is all I can do”; empha-
ses added) is similar to the earlier NMR
Belief scale and seems to capture the low
pole of Tamir, John, Srivastava, and Gross’s
(2007) measure of Emotion Regulation Self-
Efficacy Beliefs (see Table 20.1).
As with the NMR and the TMMS, these
six subscales do not differentiate between
kinds of regulatory strategies. As with the
coping measures in the previous section,
discriminant validity has been noted as a
potential problem with the DERS, because
some of the scale intercorrelations exceed
.60 and the subscales tend to correlate simi-
larly with indicators of distress or dysfunc-
tion (e.g., Weinberg & Klonsky, 2009). All
six subscales are often simply summed to
obtain an overall dysregulation score.
The DERS (and its Adolescent version)
is increasingly being used in studies of
adult pathology and child disorders; find-
ings show that scores on the DERS are
correlated with rather diverse psychologi-
cal problems, such as depression, anxiety,
suicidal ideation, eating disorders, alcohol
use, and drug use (e.g., Weinberg & Klon-
sky, 2009). Such a sweeping pattern of
maladjustment correlates may indicate that
the DERS assesses a rather undifferenti-
338 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
ated self- presentation as a troubled person:
Such individuals are overwhelmed by strong
affective impulses they do not understand,
and they feel no confidence (self- efficacy)
that they can control these impulses. With-
out some evidence of discriminant validity,
it is difficult to interpret correlations of the
DERS with indicators of psychopathology.
Overall, it is not clear which of these six
subscales was intended to capture the core
regulatory concept defined as the “ability
to use situationally appropriate regulation
strategies flexibly to modulate emotional
responses” (Gratz & Roemer, 2004, p. 42).
Certainly, use of the DERS for individual
cases in clinical and applied contexts seems
premature.
Emotional Intelligence Test
We have seen repeatedly that the emotional
competence approach conceptualizes emo-
tion regulation in terms of a number of spe-
cific abilities. Yet all the measures we have
discussed so far have used self- report ques-
tionnaire methodology: that is, research-
ers assess self- perceptions (including self-
efficacy beliefs) and typical experiences and
behaviors. In fact, we have seen that some
of these “competence” scales can be diffi-
cult to differentiate from coping styles. This
is hardly a foolproof way to assess ability
constructs, which are usually measured in
terms of carefully controlled maximum per-
formance tests of the behavior or process in
question.
Considerable credit is due to Mayer,
Salovey, and Caruso (2002), who acknowl-
edged this methodological inconsistency
and tackled the difficult task of construct-
ing a test of emotional intelligence that
is scored in terms of correct and incorrect
answers, namely, the Mayer– Salovey–
Caruso Emotional Intelligence Test (abbre-
viated MSCEIT). They define emotional
intelligence as a set of skills involved in the
processing of emotion- relevant informa-
tion. Due to space limitations, of the four
MSCEIT components, we briefly address
only the component that is most relevant
here, namely, emotion management ability,
which they define as the capacity to reduce,
increase, or maintain particular emotions
in both oneself and other people. The tasks
they use to measure these abilities require
respondents to react to hypothetical scenar-
ios and evaluate the effectiveness of various
behaviors and subjective construals for emo-
tion management purposes. For example,
participants are asked to judge the effective-
ness of strategies to help a friend enhance a
joyful mood or reduce feelings of sadness.
Unfortunately, scientific evaluation and
research use of the MSCEIT are hindered,
because the test is owned by a commer-
cial “test publisher (who) does not autho-
rize reproduction of actual test items”
(e.g., Lopes, Salovey, Côté, & Beers, 2005,
p. 114); thus, we are allowed to provide here
only two abridged examples that were pub-
lished in this form by Lopes et al. (2005,
pp. 114–115), each consisting of a vignette
paired with separate response options:
Debbie just came back from vacation. She was
feeling peaceful and content. How well would
each action preserve her mood? (1) She started
to make a list of things at home that she needed
to do. (2) She began thinking about where and
when to go on her next vacation. (3) She called
a friend to tell her about the vacation . . .
Ken and Andy have been good friends for over
10 years. Recently, however, Andy was pro-
moted and became Ken’s manager. Ken felt
that the new promotion had changed Andy
in that Andy had become very bossy to him.
How effective would Ken be in maintaining
a good relationship, if he chose to respond in
each of the following ways? (1) Ken tried to
understand Andy’s new role and tried to adjust
to the changes in their interactions. (2) Ken
approached Andy and confronted him regard-
ing the change in his behavior . . .
These examples are from the fourth (Man-
aging Emotions) “branch” of the MSCEIT,
which comprises two distinct tasks. Five
vignettes measure ability in emotion man-
agement, and each describes a person (like
Debbie) who is experiencing a mood or
emotion. For each of the five vignettes, the
respondent rates (on a 5-point scale) how
effective four different actions would be for
obtaining a specified effect on the person’s
experience (here, to preserve Debbie’s good
mood), yielding a total of 20 separate rat-
ings. The second task measures emotional
relationship abilities and comprises three
vignettes describing relationships between
persons (like Ken and Andy). In each

Individual Differences in Affect Regulation 339
vignette, the respondent rates how effective
three different actions would be to maintain
a good relationship between the persons, for
a total of nine separate ratings. Each of the
29 individual ratings is scored according to
normative effectiveness ratings provided by
a panel of emotion experts or the group con-
sensus.
Although abbreviated, these two exam-
ples are very instructive. First, they illustrate
that the total emotion management ability
score includes more than 30% of ratings that
assess not emotional but relational skills,
raising questions about content validity. Sec-
ond, each vignette and action includes a lot
of detailed contextual information specific
to that rating, which adds error and keeps
interitem correlations, and thus reliability,
low; with 29 ratings aggregated into the
total score, reliability in this study was a
measly .63, and that is higher than in other
studies (see Føllesdal & Hagtvet, 2009, for
a thoughtful psychometric analysis and cri-
tique). Third, as the MSCEIT authors read-
ily acknowledge, these vignette ratings do
not actually measure individual differences
in skillful or effective regulation scored or
observed objectively in an emotional situa-
tion; instead they tap the individual’s knowl-
edge, and capacity to reason, about emo-
tions and emotional situations (e.g., Lopes
et al., 2005, p. 114). Fourth, the emphasis
on knowledge and complex reasoning pro-
cesses is likely to introduce correlations with
measures of other abilities, creating dis-
criminant validity problems. Fifth, because
there are interpersonal themes even in the
emotion management vignettes (e.g., calling
a friend to share one’s mood, thus capital-
izing on the experience), performance on
these items may yield surprising correlations
with personality variables, again introduc-
ing potential problems with discriminant
validity. In response to these discriminant
validity concerns, the test authors and their
collaborators have tried to demonstrate that
the MSCEIT predicts social, emotional, and
leadership outcomes even when intelligence
and broad personality traits are controlled.
So far, however, many researchers have
remained unconvinced; the MSCEIT, and
emotional intelligence research more gener-
ally, is viewed with some skepticism among
researchers (e.g., Landy, 2005; Joseph &
Newman, 2010).
For example, Lopes et al. (2005) obtained
eight criterion measures (e.g., interpersonal
sensitivity; socioemotional competence;
friendship nominations) with self- ratings
or peer nominations. When the personal-
ity traits measured by the Big Five Inven-
tory (John et al., 2008) were controlled, the
MSCEIT emotion management ability scale
still significantly predicted two of these cri-
teria. However, with two out of eight signifi-
cant correlations, it is hard to say whether
the predictive validity goblet is a quarter full
or three quarters empty. Of greater interest
to personality researchers is a finding not
highlighted by the authors: By far the high-
est correlation (r = .40) was not found with
any of the eight predicted socioemotional
outcome measures but, somewhat unexpect-
edly, with the Big Five trait of Agreeableness.
In conclusion, the MSCEIT, though an
admirable and conceptually interesting
undertaking, has not proven to be the deci-
sive fix for the less compelling self- report
measures of emotional “competencies” that
have come before it. Even though, as outsid-
ers, we do not know much about the inner
workings of the MSCEIT, it seems unlikely
that a measure consisting of only five emo-
tion management vignettes and three rela-
tionship vignettes can provide the conceptual
building blocks needed to construct a com-
prehensive conception of emotion regulation
in terms of specific competencies. More gen-
erally, the emotional competence approach
(e.g., Saarni, 1999) includes all manner of
cognitive, behavioral, self- perception, and
emotion perception processes under one
broad rubric. This conceptual richness and
reach, and the resulting complexity, is theo-
retically appealing but may be keeping this
approach from achieving its full empirical
potential. Fewer constructs, more narrowly
delineated distinctions, and tighter links
between construct definitions and actual
measures (and items) may prove a fruitful
avenue for future research on the vast dif-
ferences in people’s emotional competence.
Continuing Issues, Limitations,
and Future Directions
In this brief and necessarily limited review,
we have covered almost 50 scales purporting
to measure individual differences in affec-
340 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
tive regulation. Our review suggests that
the most recent arrivals show a great deal
of redundancies with their forebears, such
as the newer CERQ with the older COPE,
and the newer DERS with the older NMR
and TMMS. In our view, the time has come
to stop the proliferation of self- report scales
and to begin a moratorium that refocuses on
solid theory development.
The Need for an Integrative
Framework and for
Mediation Research
In addition to a great number of affect regu-
lation concepts and scales, we have touched
upon a number of factors that predict or
influence individual differences in emotion
regulation. As a first, modest step toward a
broader framework, we have organized these
variables in the first two columns of Figure
20.6. In the first column we have included
broad person factors that we expect to shape
the acquisition and use of particular emo-
tion regulation strategies, such as gender
socialization, acculturation, and aging, all of
which have been shown to predict individual
differences in emotion regulation in adult-
hood. Broad personality factors, attachment,
and cognitive ability factors also need to be
considered, even if only as control variables,
as we have seen in the debate over the valid-
ity and utility of the MSCEIT.
The effects of gender, acculturation,
and aging on regulation are unlikely to be
direct and thus will need to be explained. In
research on naturally occurring individual
differences, explanatory research usually
takes the form of mediation designs, such
as the one depicted in Figure 20.4. In this
example, the increasing adoption of cul-
turally transmitted values associated with
independent (but not interdependent) self-
construal is the mechanism through which
greater acculturation to Western practices
and values leads Asian immigrants to use
suppression less and less frequently the lon-
ger they live in the United States. Another
example is a recent series of studies show-
ing that the link between habitual suppres-
sion and negative social consequences (see
Figure 20.5; consequences of regulation are
not shown in Figure 20.6) can be explained
by individual differences in authenticity in
young and older adults, as well as in samples
from the United States and China (English
& John, 2013).
More generally, we recommend that the
field increase recent efforts (see Table 20.1)
to link values, goals, and other motivational
factors, as well as beliefs, norms, and expec-
tations, to emotion regulation, both concep-
tually and empirically. We have organized
these social- cognitive processing character-
istics into four boxes in the middle column
of Figure 20.6, with arrows indicating that
these concepts have the greatest promise for
explanatory accounts.
Finally, Figure 20.6 also illustrates our
hypotheses about where cognitive meta-
mood processes may belong in such an inte-
grative framework. In particular, attention
to emotional experience, awareness of emo-
tional experience, and clarity of emotional
experience do not seem to us to be isomor-
phic with regulatory processes. Rather, these
cognitive meta-mood processes seem to be
important antecedents that can trigger, help,
or hinder the use of various, more or less
effective regulatory processes. For exam-
ple, Scheier and Carver (1977) showed that
focusing attention on an emotional state can
result in both intensification of that state
and clarification; intensification may make
subsequent regulation efforts less effective,
whereas greater clarity may have the oppo-
site effect. In other words, the nature and
causal direction of the relationships among
the concepts in Figure 20.6 needs to be
tested empirically.
The Need to Expand Beyond Habitual
Measures of Individual Differences
It is time to acknowledge some limitations
in our coverage and in the work presented
here. We have focused almost exclusively on
habitual differences in affective regulation,
which implies that the individual differences
under study show at least some consistency
over time and may in fact be chronic in
nature. It is true that in all three approaches
reviewed here, habitual concepts represent
the lion’s share of the work, but other kinds
of individual differences in emotion regula-
tion have been studied (see the third column
of Figure 20.6). For example, a number of
recent studies have examined situationally
Individual Differences in Affect Regulation 341
flexible and spontaneous use of emotion reg-
ulation as well as abilities. Using a longitudi-
nal design, Srivastava et al. (2009) explicitly
differentiated habitual or stable use from
dynamic use of suppression during the tran-
sition from high school and home town to
college; they found considerable change dur-
ing this transition, with suppression use tem-
porarily increasing as students constructed
new friendship networks. Feinberg, Willer,
Antonenko, and John (2012) studied spon-
taneous, rather than habitual, emotion regu-
lation. In one study, they had subjects make
moral judgments about a variety of dis-
gusting (but not unethical) behaviors; cod-
ing thought protocols, they tested whether
some individuals had spontaneously used
reappraisal to down- regulate their disgust
response and, in turn, made less affectively
driven and therefore less moralistic judg-
ments about the behaviors.
We have also not been able to cover recent
efforts to extend ERQ work to the domain
of individual differences in emotion regula-
tion ability, such as actual differences in the
effectiveness of reappraisal as assessed with
laboratory procedures (e.g., McRae et al.,
2012; Troy, Willhelm, Shallcross, & Mauss,
2010) and self- perceived differences in effec-
tiveness in implementing an instructional
set in the laboratory (e.g., Gruber, Har-
vey, & Gross, 2012). This is exciting work,
and we are looking forward to seeing more
diverse individual- difference measures that
go beyond the existing measures of habitual
emotion regulation.
Limitations and Future Directions
in Research on Specific Emotion
Regulation Processes
Finally, lest we forget, we must acknowledge
some fundamental limitations of previous
research using the ERQ. Whereas much of
the work in the coping approach has focused
specifically on negative affect and stress-
ful situations, work using the ERQ (e.g.,
English et al., 2012) has studied individual
differences more generally, measuring reap-
praisal and suppression at the global regula-
tory process level, shown as Level 2 in the
hierarchical model in Figure 20.7. Certainly,
the level of specific regulatory processes is
preferable to the even more abstract level of
general emotional control (Level 1). How-
ever, the choice of Level 2 (e.g., Gross &
John, 2003) should not be taken as an indi-
cation that theorists believe regulatory pro-
cesses operate exactly in the same way for
positive and negative emotions (Level 3), or
that there are no important regulatory differ-
ences (at Level 4) between discrete negative
emotions, such as anger and fear, or positive
emotion, such as pride and joy (e.g., Shiota,
Keltner, & John, 2006). Indeed, theoreti-
cal accounts of Asian–White differences in
emotion suggest that Asians’ greater use of
suppression is particularly pronounced for
emotions that are positive and highly acti-
vated, such as pride and love. Along similar
lines, Larsen (2000) suggested that suppres-
sion might be “more effective for controlling
affects with clear expressive components,
such as angry moods, compared to affects
that are not clearly expressive, such as lone-
liness” (p. 138). Webb et al. (2012) note that
even in the experimental literature few stud-
ies compared the effectiveness of a particu-
lar strategy in regulating different emotions.
This is certainly an important new direction
in research on individual differences as well.
Indeed, as shown in Figure 20.7, our con-
ceptual framework needs to be extended even
further downward to include contextual fac-
tors (at Level 5) that change the meaning of
the emotion for the individual; for example,
an Asian American feeling pride in a col-
lective context (e.g., feeling proud of the
achievements of a younger sibling) should
suppress much less than he or she would in
an individualistic context (e.g., having won
a prize for personal achievement). Finally,
research on individual differences with the
ERQ has focused on two important regula-
tory processes, reappraisal and suppression.
Future research needs to extend this focus
along the horizontal dimension of Level 2
shown in Figure 20.7 and add exemplars of
the other three families of regulatory pro-
cesses in Gross’s process model (see Figure
20.2), namely, situation selection, situation
modification, and attention deployment. We
have discussed these processes conceptually
throughout this chapter, but we have not yet
measured them in terms of individual differ-
ences. In brief, there is plenty left to do. It is
an exciting time to be working on individual
differences in affect regulation.
342
Emotional Control
Reappraisal Suppression
Negative
Fear Anger
Positive
Pride Joy
Negative
Fear Anger
Positive
Pride Joy
Individual
(e.g., of self)
Collective
(e.g., of family member)
Low Status
(e.g., with boss)
High Status
(e.g., with employee)
Level 1
Global
Regulation
Level 2
Regulatory
Process
Level 3
Emotion
Valence
Level 4
Specific
Emotion
Level 5
Context
FIGURE 20.7. Hierarchical framework for the study of individual differences in emotion regulation. Emotion regulation is conceptualized at five levels of
abstraction: (1) global emotional control, (2) particular regulatory processes, (3) regulation of positive and negative emotion, (4) regulation of specific emotions,
and (5) regulation of specific emotions in particular contexts.

Individual Differences in Affect Regulation 343
Acknowledgments
This work was supported in part by a Retire-
ment Research Foundation grant and a Faculty
Research Grant (University of California, Berke-
ley) to Oliver P. John, and by a National Science
Foundation Graduate Research Fellowship and a
Dissertation Year Fellowship (University of Cali-
fornia, Berkeley) to Joshua S. Eng.
References
Abler, B., & Kessler, H. (2009). Emotion Regu-
lation Questionnaire: A German version of
the ERQ by Gross & John. Diagnostica, 55,
144–152.
Aldao, A., Nolen- Hoeksema, S., & Schweizer, S.
(2010). Emotion- regulation strategies across
psychopathology: A meta- analytic review.
Clinical Psychology Review, 30, 217–237.
Anderson, C., John, O. P., Keltner, D., & Kring,
A. M. (2001). Who attains social status?
Effects of personality and physical attractive-
ness in social groups. Journal of Personality
and Social Psychology, 81, 116–132.
Balzarotti, S., John, O. P., & Gross, J. J. (2010).
An Italian adaptation of the Emotion Regula-
tion Questionnaire. European Journal of Psy-
chological Assessment, 26, 61– 67.
Bariola, E., Gullone, E., & Hughes, E. K. (2011).
Child and adolescent emotion regulation:
The role of parental emotion regulation and
expression. Clinical Child and Family Psycho-
logical Review, 14, 198–212.
Baumeister, R. F., Zell, A. L., & Tice, D. M.
(2007). How emotions facilitate and impair
self- regulation. In J. J. Gross (Ed.), Handbook
of emotion regulation (pp. 408–426). New
York: Guilford Press.
Block, J. H., & Block, J. (1980). The role of ego-
control and ego resiliency in the organization
of behavior. In W. A. Collins (Ed.), Minne-
sota Symposia on Child Psychology (Vol. 13,
pp. 39–101). Hillsdale, NJ: Erlbaum.
Carver, C. S., & Scheier, M. F. (1994). Situ-
ational coping and coping dispositions in a
stressful transaction. Journal of Personality
and Social Psychology, 66, 184–195.
Carver, C. S., Scheier, M. F., & Weintraub, J. K.
(1989). Assessing coping strategies: A theoreti-
cally based approach. Journal of Personality
and Social Psychology, 56, 267–283.
Catanzaro, S. J., & Mearns, J. (1990). Mea-
suring generalized expectancies for negative
mood regulation: Initial scale development
and implications. Journal of Personality
Assessment, 54, 546–563.
de Ridder, D. (1997). What is wrong with coping
assessment?: A review of conceptual and meth-
odological issues. Psychology and Health, 12,
417– 431.
Ekman, P. (1972). Universals and cultural dif-
ferences in facial expression of emotion. In
J. Cole (Ed.), Nebraska Symposium on Moti-
vation (pp. 207–283). Lincoln: University of
Nebraska Press.
Eng, J., Akutsu, S., Gross, J. J., & John, O. P.
(2013). Emotion regulation in East Asian and
European Americans: Effects of regulatory
strategy, emotion, and independent construal.
Unpublished manuscript, Department of Psy-
chology, University of California, Berkeley.
English, T., & John, O. P. (2013). Understanding
the social effects of emotion regulation: The
mediating role of authenticity for individual
differences in suppression. Emotion, 13, 314–
329.
English, T. E., John, O. P., & Gross, J. J. (2013).
Emotion regulation in close relationships. In J.
A. Simpson & L. Campbell (Eds.), The Oxford
handbook of close relationships (pp. 500–
513). Oxford, UK: Oxford University Press.
English, T. E., John, O. P., Srivastava, S., &
Gross, J. J. (2012). Emotion regulation and
peer-rated social functioning: A 4-year longi-
tudinal study. Journal of Research in Person-
ality, 46, 780–784.
Feinberg, M., Willer, R., Antonenko, O., &
John, O. P. (2012). Liberating reasons from
passion: Overriding intuitionist moral judg-
ments through emotion reappraisal. Psycho-
logical Science, 23, 788–795.
Folkman, S., & Lazarus, R. S. (1980). An analy-
sis of coping in a middle- aged community
sample. Journal of Health and Social Behav-
ior, 21, 219–239.
Folkman, S., & Lazarus, R. S. (1985). If it
changes it must be a process: Study of emo-
tion and coping during three stages of a col-
lege examination. Journal of Personality and
Social Psychology, 48, 150–170.
Folkman, S., & Lazarus, R. S. (1988). Manual
for the Ways of Coping Questionnaire. Palo
Alto, CA: Consulting Psychology Press.
Føllesdal, H., & Hagtvet, K. A. (2009). Emo-
tional intelligence: The MSCEIT from the
perspective of generalizability theory. Intelli-
gence, 37, 94–105.
Garnefski, N., & Kraaij, V. (2007). The Cogni-
344 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
tive Emotion Regulation Questionnaire: Psy-
chometric features and prospective relation-
ships with depression and anxiety in adults.
European Journal of Psychological Assess-
ment, 23, 141–149.
Gratz, K. L., & Roemer, L. (2004). Multidi-
mensional assessment of emotion regulation
and dysregulation: Development, factor struc-
ture, and initial validation of the Difficulties
in Emotion Regulation Scale. Journal of Psy-
chopathology and Behavioral Assessment, 26,
41–54.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp.
3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (1997). Revealing
feelings: Facets of emotional expressivity in
self-
r
eports, peer ratings, and behavior. Jour-
nal of Personality and Social Psychology, 72,
435–448.
Gross, J. J., & John, O. P. (1998). Mapping the
domain of emotional expressivity: Multi-
m
ethod evidence for a hierarchical model.
Journal of Personality and Social Psychology,
74, 170–191.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., & Levenson, R. W. (1993). Emo-
tional suppression: Physiology, self-
re
port,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64, 970–986.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp.
3–24). New York: Guilford Press.
Gross, J. J., Richards, J. M., & John, O. P. (2006).
Emotion regulation in everyday life. In D. K.
Snyder, J. A. Simpson, & J. N. Hughes (Eds.),
Emotion regulation in families and close rela-
tionships: Pathways to dysfunction and health
(pp.
13–35). Washington, DC: American Psy-
chological Association.
Gruber, J., Harvey, A. G., & Gross, J. J. (2012).
When trying is not enough: Emotion regula-
tion and the effort-
s
uccess gap in bipolar dis-
order.
Emotion, 12, 997–1003.
Gullone, E., & Taffe, J. (2012). The Emotion
Regulation Questionnaire for Children and
Adolescents (ERQ–CA): A psychometric
evaluation. Psychological Assessment, 24,
409– 417.
John, O. P., & Gross, J. J. (2004). Healthy and
unhealthy emotion regulation: Personality
processes, individual differences, and lifes-
pan development. Journal of Personality, 72,
1301–1334.
John, O. P., & Gross, J. J. (2007). Individual
differences in emotion regulation strategies:
Links to global trait, dynamic, and social cog-
nitive constructs. In J. J. Gross (Ed.), Hand-
book of emotion regulation (pp.
351–372).
New York: Guilford Press.
John, O. P., Naumann, L. P., & Soto, C. J. (2008).
Paradigm shift to the integrative Big Five trait
taxonomy: History, measurement, and con-
ceptual issues. In O. P. John, R. W. Robins,
& L. A. Pervin (Eds.), Handbook of personal-
ity: Theory and research (pp.
114–158). New
York: Guilford Press.
Joseph, D. L., & Newman, D. A. (2010). Emo-
tional intelligence: An integrative meta-
a
nalysis and cascading model. Journal of
Applied Psychology, 95, 54–78.
Knobloch, L. K., & Metts, S. (2013). Emotion in
relationships. In J. A. Simpson & L. Campbell
(Eds.), The Oxford handbook of close rela-
tionships (pp. 514–534). Oxford, UK: Oxford
University Press.
Kring, A. M., & Sloan, D. S. (Eds.). (2009).
Emotion regulation and psychopathology:
A transdiagnostic approach to etiology and
treatment. New York: Guilford Press.
Landy, F. (2005). Some historical and scientific
issues related to research on emotional intel-
ligence. Journal of Organizational Behavior,
26, 411–424.
Larsen, R. J. (2000). Toward a science of mood
regulation. Psychological Inquiry, 11, 129–
141.
Lazarus, R. S. (2000). Toward better research on
stress and coping. American Psychologist, 55,
665–673.
Lazarus, R. S., & Folkman, S. (1984). Stress,
appraisal and coping. New York: Springer.
Lopes, P. N., Salovey, P., Côté, S., & Beers, M.
(2005). Emotion regulation ability and the
quality of social interaction. Emotion, 5, 113–
118.
Matsumoto, D., Yoo, S. H., Nakagawa, S., Alex-
andre, J., Altarriba, J., Anguas-Wong, A. M.,
Individual Differences in Affect Regulation 345
et al. (2008). Culture, emotion regulation, and
adjustment. Journal of Personality and Social
Psychology, 94, 925–937.
Mauss, I. B., Shallcross, A. J., Troy, A. S., John,
O. P., Ferrer, E., Wilhelm, F. H., et al. (2011).
Don’t hide your happiness! Positive emotion
dissociation, social connectedness, and psy-
chological functioning. Journal of Personality
and Social Psychology, 100, 738–748.
Mayer, J. D., & Gaschke, Y. N. (1988). The
experience and meta-
e
xperience of mood.
Journal of Personality and Social Psychology,
55, 102–111.
Mayer, J. D., Salovey, P., & Caruso, D. R. (2002).
MSCEIT user’s manual. Toronto: Author.
McRae, K., Jacobs, S. E., Ray, R. D., John, O.
P., &
Gross, J. J. (2012). Individual differ-
ences in reappraisal ability: Links to reap-
praisal frequency, well-being, and cognitive
control. Journal of Research in Personality, 7,
253–262.
Morris, A. S., Silk, J. S., Steinberg, L., Myers,
S. S., & Robinson, L. R. (2007). The role
of the family context in the development of
emotionregulation. Social Development, 16,
362–388.
Nolen-
H
oeksema, S., Parker, L. E., & Larson,
J. (1994). Ruminative coping with depressed
mood following loss. Journal of Personality
and Social Psychology, 67, 92–104.
Parker, J. D. A., Endler, N. S., & Bagby, R. M.
(1993). If it changes, it might be unstable:
Examining the factor structure of the ways of
coping questionnaire. Psychological Assess-
ment, 5, 361–368.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79, 410–424.
Saarni, C. (1999). The development of emo-
tional competence. New York: Guilford Press.
Saarni, C. (2011). Emotional development in
childhood. In R. E. Tremblay, R. G. Barr, R.
DeV. Peters, & M. Boivin (Eds.), Encyclopedia
on early childhood development: Emotions
(pp.
1–7). Montréal: Centre of Excellence for
Early Childhood Development and Strategic
Knowledge Cluster on Early Child Develop-
ment.
Salovey, P., Mayer, J. D., Golman, S. L., Turvey,
C., & Palfai, T. P. (1995). Emotional attention,
clarity, and repair: Exploring emotional intel-
ligence using the Trait Meta-Mood Scale. In
J. W. Pennebaker (Ed.), Emotion, disclosure,
and health (pp.
125–154). Washington, DC:
American Psychological Association.
Scheier, M. F., & Carver, C. S. (1977). Self-
f
ocused attention and the experience of
emotion: Attraction, repulsion, elation, and
depression. Journal of Personality and Social
Psychology, 35, 625–636.
Shaver, P. R., & Mikulincer, M. (2007). Adult
attachment strategies and the regulation of
emotion. In J. J. Gross (Ed.), Handbook of
emotion regulation (pp.
446–465). New York:
Guilford Press.
Shiota, L., Keltner, D., & John, O. P. (2006).
Positive emotion dispositions differentially
associated with Big Five personality and
attachment style. Journal of Positive Psychol-
ogy, 1, 61–71.
Srivastava, S.,
Tamir, M., McGonigal, K. M.,
John, O. P., & Gross, J. J. (2009). The social
costs of emotional suppression: A prospective
study of the transition to college. Journal of Per-
sonality and Social Psychology, 96, 883 –897.
Tamir, M., John, O. P., Srivastava, S., & Gross, J.
J. (2007). Implicit theories of emotion: Affec-
tive and social outcomes across a major life
transition. Journal of Personality and Social
Psychology, 92, 731–744.
Troy, A. S., Willhelm, F. H., Shallcross, A. J.,
& Mauss, I. B. (2010). Seeing the silver lin-
ing: Cognitive reappraisal ability moderates
the relationship between stress and depressive
symptoms. Emotion, 10, 783–795.
Weinberg, A., & Klonsky, E. (2009). Measure-
ment of emotion dysregulation in adolescents.
Psychological Assessment, 21, 616– 621.
Webb, T. L., Miles, E., & Sheeran, P. (2012).
Dealing with feeling: A meta-
a
nalysis of the
effectiveness of strategies derived from the
process model of emotion regulation. Psycho-
logical Bulletin, 138, 775–808.
Westen, D., & Blagov, P. S. (2007). A clinical–
e
mpirical model of emotion regulation: From
defense and motivated reasoning to emotional
constraint satisfaction. In J. J. Gross (Ed.),
Handbook of emotion regulation (pp.
373–
392). New York: Guilford Press.

346
Desires pervade everyday life. Accordingly,
our language is filled with metaphors of how
we experience and deal with desire:
We burn and are aflame with desire; we are
pierced by or riddled with desire; we are sick or
ache with desire; we are tortured, tormented,
and racked by desire; we are possessed, seized,
ravished, and overcome by desire; . . . our
desire is fierce, hot, intense, passionate, incan-
descent, and irresistible; . . . We battle, resist,
and struggle with, or succumb, surrender to,
and indulge our desires. (Belk, Ger, & Askeg-
aard, 2000, p. 99)
Even though the described struggle with
desire is primarily mental, the downstream
implications of poor desire regulation can
be enormous, such as when political leaders
lose their jobs over a scandalous love affair.
Additionally, public health statistics show
that 40% of deaths in the United States
each year are associated with unhealthy
behaviors— behaviors that are at least par-
tially attributable to the way people deal
with certain appetitive desires, such as
those for unhealthy foods, tobacco, alcohol,
unprotected sex, and illicit drugs (Schro-
eder, 2007). From this perspective, gaining
a better understanding of how and when
desires impact behavior and how unwanted
desires may be successfully regulated is
clearly needed.
With this chapter, we hope to show that
some new insights may be gleaned by treat-
ing desires as emotions and, consequently,
desire regulation as an instance of emo-
tion regulation. In our reading, the issues
of desire and desire regulation have been
studied primarily from the vantage point
of addiction research, clinical research,
and cognitive neuroscience. Applying some
of the insights gained from the burgeoning
emotion regulation literature to the desire
case may broaden our understanding of the
problem at hand and open up new avenues
of inquiry.
We believe that an emotion regulation per-
spective on desire may also benefit the field
of self- control. That is, a focus on desire
and its regulation, may be helpful, wherever
the motivational conflicts under study can
be framed as a struggle between immediate
desires (e.g., for tasty food, alcoholic drinks,
sexual interaction) and self- regulatory goals
and values (e.g., maintaining one’s weight,
not driving drunk, staying faithful). Such
conflicts arguably make up a great deal of
self- control situations in everyday life. Yet
until recently, they have been mainly stud-
ied under the rubric of goal conflicts (e.g.,
a short-term goal to eat tasty chocolate vs.
a long-term goal to lose weight). While such
a terminology is parsimonious and helpful
in many respects, it may not get at a deeper
CHAPTER 21
Desire and Desire Regulation:
Basic Processes and Individual Differences
Wilhelm Hofmann
Hiroki P. Kotabe
Desire and Desire Regulation 347
understanding of the specific characteris-
tics of the two opponents involved in such
motivational struggles (two opponents,
which we argue, are quite different). That
is, by framing the short-term goal as “just”
another goal, we might miss a closer analysis
of the driving force (i.e., of desire) behind
that short-term motivation, the specific laws
that trigger this driving force, and the most
effective strategies to tame it. By treating
desire as an essentially emotional phenom-
enon (involving motivational and cognitive
components), we can begin to ask more spe-
cific questions about how desire waxes and
wanes in close interaction with environmen-
tal characteristics and how it can be strategi-
cally up- or down- regulated.
Accordingly, self- control researchers have
begun to scrutinize the driving forces under-
lying many self- control struggles (Hofmann,
Friese, & Strack, 2009; Hofmann & Van
Dillen, 2012) and to look at how factors
commonly thought to impact people’s con-
trol capacity might also affect the strength
of desires and cravings (Schmeichel, Har-
mon-Jones, & Harmon-Jones, 2010; Vohs
et al., 2012). By and large, the science of
self- control appears to be shifting toward
the more balanced view that for many self-
control situations, the role of desire is just as
important and vital to our understanding as
the role of restraint (Hofmann & Van Dil-
len, 2012).
To advance an emotion regulation per-
spective on desire, we address the following
three major questions in this chapter:
1. What are the main characteristics of
desire?
2. How can desire be successfully regu-
lated?
3. How does personality shape desire expe-
riences and desire regulation?
Desire Characteristics
Colloquially, there are many usages of the
word desire, especially as a verb to express
all kinds of wishes and wants (e.g., “I desire
that there be more rainbows next year”).
Here, we focus exclusively on so- called
appetitive desires, which are those motiva-
tions that propel us to approach and con-
sume objects or otherwise engage in activi-
ties that provide a relative gain in immediate
pleasure (including relief from discomfort).
Appetitive desires, in the narrow sense, are
typically rooted in primary or acquired
physiological need states, such as the desires
for food and drink, alcohol, sex, a cigarette,
and sleep (no story line intended here); how-
ever, we would also include more second-
ary, socially learned desires (e.g., the urge
to check one’s smartphone for new messages
or the desire to watch a favorite TV show)
in a broader definition of appetitive desires.
We therefore use the term desire to refer to
appetitive desires of all sorts. Moreover, we
use the term craving to refer to desires across
domains that are particularly high- intensity
(e.g., drug craving, food craving).
Desire as Emotion
In this chapter, we want to make a strong
case for treating desires as emotions. Why?
Because desires, in our view, share many
of the major hallmark characteristics of an
emotion (see Franken, 2003). Most impor-
tant, like emotions, desires are multifaceted
phenomena combining affective, motiva-
tional, and cognitive components (see Table
21.1).
First, desires have a clearly affective com-
ponent, consisting of a phenomenological
feeling of “wanting” of varying intensity.
On the stimulus side, we perceive objects of
desire as appealing; desires signal to us that
a given thing, person, or activity has high
momentary relevance against the backdrop
of our current goals, bodily need states,
and learning history. Although enacting or
thwarting desires may have various emo-
TABLE 21.1. Components of Desire
Component Description
Affective A feeling of “wanting” toward
certain appealing objects of desire
(things, activities, people).
Motivational A prepotent behavioral driving
force to acquire, consume, or be
close to the object of desire.
Cognitive Accompanying thoughts (e.g.,
expectations, images, and
fantasies) regarding the enactment
of desire.
348 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
tional consequences (more on this later),
desires, intrinsically, have a distinct emo-
tional experience to them, which is that phe-
nomenological feeling of wanting.
Second, like other emotions, desires pre-
pare and motivate behavior. Desiring some-
thing means wanting to have, consume, or
do something that we expect will yield plea-
sure (or reduce discomfort). When we desire
a cupcake, we expect its consumption to
provide us with a highly pleasurable experi-
ence. When a tobacco addict craves a ciga-
rette, he or she expects that smoking it will
alleviate distress (including, somewhat iron-
ically, the discomfort of deprivation created
by the addiction). Desires therefore motivate
us through their more or less explicit prom-
ise of pleasure or relief. Even though hedonic
motives may not be the only reasons we pur-
sue desires, we consider the striving for a
relative gain in immediate pleasure the pri-
mary motivational underpinning of desire.
Because desires are directed toward certain
objects (i.e., things, persons, activities), they
share a sense of “aboutness” (Higgins, 1998)
with all other emotions (e.g., being happy,
sad, angry, disgusted about something).
Their target- oriented nature distinguishes
them, among other things, from more dif-
fuse affective states such as mood.
1
Third, desires also have an important
cognitive component that is strongly inter-
twined with the aforementioned affective–
motivational components: According to
Kavanagh, Andrade, and May’s (2005)
elaboration– intrusion theory of desire,
desire is typically accompanied by intrusive
thoughts about the object of desire. Such
cognitions comprise expectations about the
consequences of desire enactment and the
feasibility of attaining the desired object,
as well as imagination and fantasies. These
cognitions may vary on a continuum, from
very realistic to very unrealistic (i.e., overly
optimistic), with stronger desires typically
leading to more biased and distorted cog-
nitions (Kavanagh et al., 2005). Moreover,
the interplay between cognition and affect
is recursive and dynamical; as a person
mentally elaborates a desire, its strength
increases, and more and more mental
resources are allocated to the desire, thus
lending desire its well-known potential to
escalate. As we elaborate below, this state of
affairs can reach a point where desire can
fully crowd out opposing mental represen-
tations such as those of self- control goals
(Hofmann, Friese, Schmeichel, & Baddeley,
2011; Hofmann & Van Dillen, 2012; Kava-
nagh et al., 2005).
Not All Desires Are Temptations
It is important to clarify that desires and
temptations are not synonyms. In our view,
temptations are a special subset of desires.
To say that somebody is “tempted” by some-
thing means that the person has a desire to do
A on one hand, and simultaneously has rea-
son not to do A (Mele, 2001). (In this chap-
ter we use the term temptation to refer to
the ambivalent psychological state of being
tempted, whereas we use tempting stimuli to
refer specifically refer to the external objects
that give rise to temptation.) Whether a
person has reason not to do A depends on
whether the behavior implied by the desire
conflicts with a person’s background set of
endorsed values and self- regulatory goals
(Hofmann, Baumeister, Förster, & Vohs,
2012). For instance, the desire for mousse
au chocolat seems harmless unless one sub-
scribes to a low-fat diet. Similarly, the desire
for physical closeness with an attractive
acquaintance may be completely unproblem-
atic unless one is subscribed to traditional
Western marriage, and—to add some fur-
ther intricacies—that acquaintance is also
one’s best friend’s spouse. The problem that
our appetitive desires are so strongly con-
nected to whatever provides us with imme-
diate pleasure or relief from discomfort on
a physiological basis, and that their impli-
cations may deviate from what is rationally
regarded as optimal, proper, or virtuous,
renders occasional self- control conflict an
inevitable feature of the human condition.
A recent experience- sampling project
called the “Everyday Temptations Study”
set out to collect base rate information on
the prevalence of desires and temptations
in everyday life (Hofmann, Baumeister,
et al., 2012; Hofmann, Vohs, & Baumeis-
ter, 2012). Participants were equipped with
smartphones for a week. On multiple random
occasions each day, they received a question-
naire via these smartphones and were asked
whether they were currently experiencing
a desire from a list of 15 desire domains,
including food, nonalcoholic drinks, sleep,
Desire and Desire Regulation 349
sex, social contact, leisure, sports, spending,
media, alcohol, tobacco, and other drugs.
Participants reported a current desire about
50% of the time they were signaled. The
lion’s share of desires were reported to be
unproblematic, that is, not in conflict with
other important goals (Hofmann, Baumeis-
ter, et al., 2012). However, more than one-
third of desires were experienced as conflict-
ing with important self- regulatory goals,
such as health goals (e.g., healthy eating,
bodily fitness), abstinence/restraint goals
(e.g., remaining abstinent, saving money,
being faithful), achievement- related goals
(e.g., educational achievements), social goals
(e.g., social recognition, moral integrity),
and time use goals (e.g., not delaying things)
(Hofmann, Vohs, et al., 2012). Although
there were some prominent connections
between specific desires and specific oppos-
ing goals (e.g., desire for tobacco ↔ reduc-
ing health damage; spending ↔ saving
expenses), desire– goal conflicts on average
were very multifaceted. Thus, one and the
same desire can be experienced as a tempta-
tion for many different reasons.
Linked Emotions
As noted earlier, the inherent affective core
of desire (the feeling of “wanting”) needs to
be distinguished from the emotional conse-
quences of desire enactment– nonenactment,
which we call linked emotions. The enact-
ment of desire is typically linked to a relative
short-term gain in pleasure resulting from
need satisfaction or relief from discomfort.
Conversely, the prolonged nonenactment of
desire typically leads to a period of frustra-
tion, until the motivational system man-
ages to disengage from the desire altogether
(Carver & Scheier, 1998). Note, however,
that the primary emotions of pleasure and
frustration need not be the only linked emo-
tions. In the case of temptation, things may
become more complicated: Because enact-
ing a temptation implies that one may have
violated important values relevant for one’s
identity, giving in to temptation may lead
to feelings of guilt or shame (Hofmann &
Fisher, 2012; Shiffman et al., 1997), on top
of pleasure. Conversely, not enacting a temp-
tation may trigger feelings of pride on top
of frustration. Thus, in contrast to unprob-
lematic desires, enacting temptations may at
times yield a combination of (positive and
negative) primary and secondary emotions
(pleasure and guilt; frustration and pride).
As we argue elsewhere (Hofmann, Kotabe,
& Luhmann, in press), the net affective out-
come of such mixed emotional states may be
a function of the relative intensities of the
linked emotions involved. Enacting a certain
temptation (e.g., desire to eat cake when on
a diet) may therefore result in a “spoiled
pleasure” effect, a reduction in the overall
gain in affect as compared with the enact-
ment of the equivalent desire in an unprob-
lematic context (e.g., desire to eat cake when
not on a diet).
Although not a focus of the present chap-
ter, the linked emotions of desire enact-
ment and nonenactment can themselves, of
course, be subject to emotion regulation.
For instance, to increase their affective well-
being, people may try to down- regulate the
frustration ensuing from unproblematic
desires that cannot be enacted because of
external obstacles, or to cherish the pleasure
ensuing from their enactment for as long as
possible. In the case of temptation, a person
may try to down- regulate guilt after a lapse
has occurred. Likewise, people may try to
maintain or up- regulate pride ensuing from
successful resistance—a self- conscious emo-
tion that some have argued is often not “har-
vested” enough for bolstering self- control
(Hofmann & Fisher, 2012).
How Does Desire Emerge
and Impact Behavior?
Desire emerges in a relatively automatic
manner as reward processing centers in
midbrain regions (e.g., the ventral striatum)
evaluate external stimuli (or mental images
thereof
2
) against the backdrop of internal
need states and an individual’s learning his-
tory (Hofmann et al., 2009; Hofmann &
Van Dillen, 2012). This early reward pro-
cessing may have the potential to trigger fast
impulsive, habitual responses (e.g., Mogen-
son, Jones, & Yim, 1980; Winkielman, Ber-
ridge, & Wilbarger, 2005). However, most
typically, reward signals from midbrain
regions are forwarded to prefrontal regions
in the brain involved in reward representa-
tion and integration, with the orbitofrontal
cortex (OFC) being among the regions most
consistently implicated (Van der Laan, de

350 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Ridder, Viergevera, & Smeetsa, 2011). Even
though somewhat speculative, we believe
that the involvement of prefrontal regions
such as the OFC is an important element in
the conscious representation of desires and
cravings.
As desire gains access to consciousness
and, therefore, the global workspace of the
mind, it gains the potential to “broadcast”
its message to a wide range of participat-
ing systems, including those that generate
thoughts and behavioral intentions (Baars
& Franklin, 2003; Baumeister, Masicampo,
& Vohs, 2011; Hofmann & Wilson, 2010).
Like market criers who try to gather atten-
tion of those around them, contents of the
global workspace of consciousness com-
pete for mental processing resources. Once
a desire becomes conscious, it can function
very much like an attractor state (Carver &
Scheier, 2002) that exerts increasing influ-
ence on conscious processing the more sup-
porting working memory resources it is able
to acquire, along the lines of “whoever has
will be given more.” In other words, desire-
related processing can be subject to a vicious
circle of reprocessing and rumination as
people harbor increasingly elaborated, con-
scious mental representations of desire that
bias attentional, affective, and cognitive
processing.
As desire grows in consciousness, so does
its potential to instigate concrete action
plans and behavioral intentions to consume
the object of desire. In the case of tempta-
tion, desires may lead to the inhibition of
self- regulatory values and goals (Hofmann,
Koningsbruggen, Stroebe, Ramanathan,
& Aarts, 2010; Stroebe, Mensink, Aarts,
Schut, & Kruglanski, 2008). Moreover, as
people become increasingly occupied by a
certain desire (Kavanagh et al., 2005), they
may engage in processes of motivated reason-
ing that license and justify indulgence (e.g.,
“I deserve a special treat today because . . .”;
“This is going to be my last cigarette, and
then I’ll quit!”; Kunda, 1990; Sayette &
Griffin, 2011). Unless people manage to
down- regulate desire effectively and/or allo-
cate attention away from desire- related (hot)
cognitions toward competing (cooler) attrac-
tor states such as those of self- control goals,
desires can hijack the very mechanisms that
may otherwise support “reasoned” action.
In extreme cases of desire escalation, a
desire may have gathered so much clout in
working memory that it may be able to fully
crowd out all other opposing, goal- related
representations—a state we describe as “all-
consuming passion” in everyday language.
How Can Desire Be
Successfully Regulated?
Given that desires and cravings may some-
times be experienced as conflicting with
one’s set of self- regulatory goals and values,
the question that emerges is how can tempt-
ing desires be effectively controlled? In the
remainder of this chapter, we therefore focus
on the down- regulation of tempting desires.
Note, however, that the taming of tempta-
tions is only one special case of desire regu-
lation, because desires may be up- or down-
regulated for a variety of other reasons. For
instance, in order to enjoy a special dinner
even more, one may deliberately up- regulate
one’s appetitive desire for food by refrain-
ing from the regular afternoon snack. Or
in order to stay awake on a train ride from
Tokyo to Osaka to view Mt. Fuji from the
window, one may deliberately down- regulate
one’s desire to sleep on the train by getting a
good night’s rest the night before.
As laid out by Gross (this volume), emo-
tion regulation refers to shaping which emo-
tions one has, when one has them, and how
one experiences them. Applied to tempting
desires, we focus on regulatory processes
and strategies affecting (1) whether a given
tempting desire is consciously experienced to
begin with, and, if so, (2) how such a tempt-
ing desire can be effectively down- regulated.
The underlying assumption is that the like-
lihood of a given tempting desire to impact
behavior, all else being equal, is a function of
the desire’s intensity (more intense desire is
more capable of hijacking working memory
resources in its favor). Therefore, all mecha-
nisms that help keep the intensity of tempting
desire below a critical enactment level, either
by preventing tempting desire from emerg-
ing in consciousness in the first place, or by
helping the person down- regulate tempting
desire, can be considered effective desire reg-
ulation strategies. Conversely, mechanisms
that increase desire intensity, contrary to the
desire down- regulation goal, can be consid-
ered ineffective.
Desire and Desire Regulation 351
In close accordance with the process
model of emotion regulation (Gross, 1998),
we review emotion regulation mechanisms
and strategies pertaining to situation and
stimulus control, attention allocation, cog-
nitive reappraisal, and suppression. The
emotion regulation strategies reviewed vary
in the extent to which they may prevent
the emergence of conscious desire (i.e., by
preventing exposure to triggering cues or
interfering with the conscious processing of
tempting stimuli) or aid the down- regulation
of conscious desire experiences.
Situation and Stimulus Control
Availability begets desire (Carter & Tiffany,
2001). Without any doubt, the most effec-
tive strategy to prevent desire is to avoid
exposure to tempting situations or stimuli
altogether through techniques of situation
and stimulus control (Mahoney & Thore-
sen, 1972). Based on the notion that external
stimuli, in their interaction with a person’s
learning history and current need states,
play a seminal role in the generation of
desire as “impellers” (Finkel & Eckhardt, in
press; Hofmann & Van Dillen, 2012), these
techniques can greatly alter the odds that
people will experience temptation. Situation
and stimulus control techniques can either
be learned and applied by a person directly
(e.g., keeping one’s home free of unhealthy
but tempting foods) or be applied through
paternalistic “nudges” (Thaler & Sunstein,
2010) that reduce the availability of temp-
tations in people’s environments (e.g., “no
smoking” bans in restaurants and bars; caf-
eterias that primarily offer healthy choice
options). Because situation and stimulus
control are not always feasible (e.g., when
one is “stuck” in a temptation- rich environ-
ment), however, situation and stimulus con-
trol cannot be the sole answer to effective
desire regulation.
Attention Allocation
Our previous analysis of how desire emerges
suggests a fundamental role of attention in
desire regulation. Attention can be parsed
into both automatic, bottom- up attention
(i.e., attention driven by salient stimulus
properties) and controlled, top-down atten-
tion (i.e., the goal- driven allocation of atten-
tion toward goal- relevant information in
working memory) (Knudsen, 2007). Both
types of attention shape desire experiences;
therefore, both mechanisms may be har-
nessed to regulate desire effectively, though
we argue that top-down attention is ulti-
mately more important.
Regarding bottom- up attention, a grow-
ing number of studies in the addiction
domain have tried to reduce conscious crav-
ings and associated behaviors by modifying
automatic attentional biases toward tempt-
ing stimuli (see also MacLeod & Grafton,
this volume). However, the results of these
attentional retraining studies have been
mixed. Sometimes the effects generalized to
other contexts, but at other times the effects
did not extend much beyond the retrain-
ing task (Attwood, O’Sullivan, Leonards,
Mackintosh, & Munafo, 2008; Fadardi &
Cox, 2009; Field, Duka, Tyler, & Schoen-
makers, 2009; Schoenmakers, Wiers, Jones,
Bruce, & Jansen, 2007). The most encour-
aging results were obtained when several
attentional retraining sessions were used
and participants were explicitly told about
the purpose and theoretical rationale of the
retraining task.
Increasing evidence suggests, however,
that top-down attention may be more deci-
sive in desire regulation. Top-down atten-
tion has been shown to modulate bottom-
up attention (Rauss, Schwartz, & Pourtois,
2011; Van Dillen & Koole, 2009). Top-down
attention enables the selective processing of
information relevant for one’s current goal,
preventing distracting information from
entering awareness (Knudsen, 2007). Hence,
when a current goal directs attention in such
a way that tempting information becomes
irrelevant, the attentional capture of tempt-
ing stimuli should be substantially reduced
or not occur at all. Conversely, people should
process tempting cues more strongly in the
absence of a specific goal (i.e., when their
top-down attention is free to be captured by
salient cues), or when their current goal focus
prompts attentional processing of such cues.
Indeed, recent research has shown that cog-
nitively demanding tasks (unrelated to the
temptation at hand), can actually prevent the
emergence of desire in response to tempting
stimuli (Kemps, Tiggemann, & Christian-
son, 2008; Van Dillen, Papies, & Hofmann,
2013). For instance, in the Van Dillen et al.
352 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
(Study 1), participants categorized pictures
of tempting (e.g., brownies) versus neutral
(e.g., radishes) food stimuli according to their
spatial location on the screen while rehears-
ing either a one-digit number (low load) or
an eight-digit number (high load) during
each task trial. Participants under low cogni-
tive load allocated more attention to tempt-
ing than to neutral stimuli, as evidenced by
slower spatial categorizations of attractive
food pictures compared to neutral food pic-
tures. Participants under high cognitive load
were equally fast to respond to tasty and
neutral food items, suggesting that they may
not have processed the hedonic relevance of
the attractive food (as much). Consequently,
participants in the high load condition
reported lower snack cravings following the
categorization task. Hence, the desire to con-
sume attractive food temptations may only
surface in consciousness to the extent that
people have sufficient attentional resources
to assess their hedonic value.
3
Early power-
ful distraction may therefore facilitate desire
regulation by preventing the emergence of
desire and—by implication—the conscious
pursuit of desire satisfaction.
The powerful role of top-down attention
in desire regulation suggests that teach-
ing people how to distract themselves from
cravings through mentally engaging activi-
ties may be an effective element in craving
management and relapse prevention (Flor-
sheim, Heavin, Tiffany, Colvin, & Hiraoka,
2008). The power of attention is also exem-
plified in recent applied interventions show-
ing how mindfulness- and acceptance- based
interventions can reduce cravings in prob-
lem populations (Alberts, Mulkens, Smeets,
& Thewissen, 2010; Forman et al., 2007).
The concept of mindfulness (see also Farb,
Anderson, Irving, Segal, this volume) has
been tightly linked with effective top-down
attention (Chambers, Lo, & Allen, 2008).
One mechanism through which mindfulness
interventions may work is by increasing peo-
ple’s ability to maintain their self- regulatory
goals in working memory and shield them
from interference through desire- related
processing (Hofmann, Gschwendner, Fri-
ese, Wiers, & Schmitt, 2008; Hofmann,
Schmeichel, & Baddeley, 2012). In addition,
the down- regulation of ongoing desires and
cravings may be facilitated by the acceptance
component contained in these interventions:
Accepting desires and cravings as transient
(i.e., fleeting) states instead of trying to sup-
press them may make it easier for people to
disengage from the maladaptive vicious cir-
cle of reprocessing and rumination.
Reappraisal
Rather than avoid tempting situations and
stimuli or allocate attention in strategic
ways, people can also employ strategies that
modify how they appraise tempting stimuli.
For instance, in On Desire: Why We Want
What We Want, William Irvine (2006)
relates advice provided by a Buddhist monk
on how to deal with tantalizing sexual desire
by thinking about the human body in less
favorable terms: “Don’t think about her full
breasts and flaxen hair; think instead about
her lungs, . . . phlegm, pus, spittle.” Accord-
ing to the monk, the aim of the mediation
“is not to produce aversion and disgust but
detachment, to extinguish the fire of lust by
removing its fuel” (p. 187).
Early pioneering research on delay of grat-
ification by Walter Mischel has shown that
cognitive reappraisal of tempting stimuli can
have a large impact on how well schoolchil-
dren are able to resist immediate rewards,
such as marshmallows, by cognitively trans-
forming the rewards in nonconsummatory
ways (e.g., imagining the marshmallows
as white puffy clouds) (Mischel & Baker,
1975). Recent work applying this idea has
demonstrated that reappraisal can have a
profound impact on affective responses to
tempting stimuli (Fujita & Han, 2009; Hof-
mann, Deutsch, Lancaster, & Banaji, 2010).
Engaging people in an abstract rather than
concrete mindset (Fujita & Han, 2009) or
imagining tempting stimuli in nonconsum-
matory ways (Hofmann, Deutsch, et al.,
2010) appear to interfere with the early
reward processing of these stimuli, thus tak-
ing the edge of temptation.
Findings that cognitive reappraisal can
have far- reaching effects on how people ini-
tially appraise tempting stimuli align well
with recent accounts arguing that emotion
regulation can often operate via powerful
automatic or implicit processes (see Gyurak
& Etkin, this volume). For instance, several
lines of work have suggested that, at least

Desire and Desire Regulation 353
under certain circumstances, mental repre-
sentations of self- control goals may exert an
automatic inhibiting effect on mental rep-
resentations of desire, implying that tempt-
ing stimuli may lose their desire- activating
potential. For instance, Fishbach, Friedman,
and Kruglanski (2003) argued that tempting
stimuli can trigger the automatic activation
of an overriding self- control goal in success-
ful self- regulators (e.g., the sight of a cake
activating a dieting goal).
4
Thus, strong and
easily accessible self- control goals may help
to reduce desire- related processing because
of the automatic inhibition of goal- irrelevant
information (Shah, Friedman, & Kruglan-
ski, 2002), which typically implies a relative
devaluation of the tempting stimulus (Veling,
Holland, & van Knippenberg, 2007, 2008).
Automatic self- regulatory processes may
thus contribute to desire down- regulation
and—depending on their potency—may
even make the need for more effortful self-
control obsolete.
The discovery of automatic self- regulatory
processes has raised the intriguing question
of whether the initial appraisal of tempt-
ing stimuli (i.e., the reward signal triggered
upon exposure) may be altered through
training and intervention techniques. The
underlying rationale is that people should
have an easier time keeping desires at bay
if the tempting stimuli that typically elicit
strong desires and cravings are appraised in
less positive terms. One method to shape the
initial appraisal of tempting stimuli is evalu-
ative conditioning (Hofmann, De Houwer,
Perugini, Baeyens, & Crombez, 2010). Spe-
cifically, pairing a tempting stimulus (e.g.,
alcohol) repeatedly with an affectively nega-
tive unconditioned stimulus can decrease
desire for alcohol among problematic
drinkers (Houben, Havermans, & Wiers,
2010; Van Gucht, Baeyens, Vansteenwe-
gen, Hermans, & Beckers, 2010). A second
promising method is avoidance training, in
which people are trained to respond rou-
tinely to tempting stimuli with avoidance
responses (Wiers, Eberl, Rinck, Becker, &
Lindenmeyer, 2011; Wiers, Rinck, Kordts,
Houben, & Strack, 2010). In one intrigu-
ing study, alcoholic inpatients who under-
went cognitive- behavioral therapy (CBT)
and four 15-minute training sessions to
avoid alcohol stimuli showed reduced crav-
ings after the avoidance treatment, as well
as reduced relapse 1 year after treatment
when compared with control patients who
only did CBT (Wiers et al., 2011). Relatedly,
laboratory work has shown that implemen-
tation intentions to avoid tempting stimuli
can reduce automatic affective reponses
to these stimuli, presumably because such
implementation intentions reprogram the
mind to appraise the tempting stimulus more
negatively (Hofmann, Deutsch, et al., 2010).
Suppression
If accepting a desire may facilitate desire
regulation as noted earlier, how does the
opposite strategy fare? Is the willful sup-
pression or negation of cravings an effective
desire regulation strategy? The bulk of the
literature on appetitive thought suppres-
sion across domains suggests not (Barnes
& Tantleff- Dunn, 2010; Erskine, 2008;
Johnston, Bulik, & Anstiss, 1999; Mann &
Ward, 2001). The problem with the forced
suppression of desire is that even though
suppression may provide some short-term
relief, suppression may often backfire, lead-
ing to ironic rebound effects (Wegner, 1994).
According to Wegner’s theory, when one
tries actively to suppress something, atten-
tion may be repeatedly redirected toward the
very mental content one is trying to avoid.
This feature of suppression may contribute to
keeping the desire– processing loop alive, or
even lead to the hyperaccessibility of desire-
related thoughts. As we argue below, these
(main) effects do not imply that everybody
suffers from ironic rebound effects to the
same extent. Suppression may well be effec-
tive for those individuals who are particu-
larly skilled at directing their attention in a
top-down manner (Brewin & Smart, 2005).
Individual Differences in Desire
and Its Regulation
Our main focus, up to this point, has been
on general mechanisms that facilitate or hin-
der successful desire regulation. In this last
part of this chapter, we address the research
on how individual differences may shape the
way people experience and deal with appeti-
tive desires.
354 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Regarding the intensity of desire experi-
ences, there is strong evidence that indi-
viduals high in behavioral approach system
(BAS) sensitivity experience stronger desires
and cravings than do those low in BAS. This
effect was found both in cue- reactivity para-
digms in the laboratory (Franken, 2002)
and when aggregating average self- reported
desire strength across many desire domains
(Hofmann, Baumeister, et al., 2012). These
findings clearly support Gray’s (1982) rein-
forcement sensitivity theory, arguing that
the BAS is responsible for the reward pro-
cessing in appetitive motivation, and suggest
an important role for related constructs such
as sensation seeking.
The Everyday Temptations Study also
showed that individuals high in trait self-
control (TSC) reported lower average desire
strength (as well as lower average conflict
and less use of active resistance to control
desire) (Hofmann, Baumeister, et al., 2012).
Even though somewhat speculative, we
believe this surprising pattern of findings
may reflect the joint impact of two mech-
anisms. First, individuals with high TSC
may make more use of preventive situation
and stimulus control strategies, thus avoid-
ing tempting desires more often than their
low-TSC counterparts (and hence reduc-
ing the need for effortful control). In sup-
port, independent raters rated the desires
reported by high-TSC participants as less
problematic for the “average person” than
the desires reported by low-TSC partici-
pants (Hofmann, Baumeister, et al., 2012).
In other words, high-TSC individuals seem
to be navigating an objectively less tempt-
ing desire landscape. Second, it is conceiv-
able that those with high TSC have more
powerful automatic desire regulation pro-
cesses at their disposal. Some support for
this assumption comes from studies reveal-
ing that people who report to be success-
ful self- regulators in a given domain, such
as dieting, tend to show more effective
automatic self- regulation than do unsuc-
cessful self- regulators (Papies, Stroebe, &
Aarts, 2008a). These findings are intriguing
because they suggest that, in everyday life,
TSC may not so much be about how well
temptations are resisted (the type of “late-
stage” self- control typically studied in the
laboratory situations) as about how well
temptations are being avoided or appraised
at early stages in the self- control process.
Furthermore, we believe that TSC is dis-
tinct from individual differences in what has
been called the restraint bias (Nordgren, van
Harreveld, & van der Pligt, 2009), which
taps into people’s overoptimistic—and thus
unrealistic—beliefs about their capacity
at self- control. As a consequence of their
inflated beliefs, people high in the restraint
bias tend to overexpose themselves to tempt-
ing situations, leading to more frequent self-
control failures in the heat of the moment
(Nordgren et al., 2009). Thus, we predict
that individuals high in TSC harbor more
realistic impulse control beliefs than do indi-
viduals low in TSC. This more realistic will-
power self- assessment (and a more realistic
appreciation of the power of the situation)
may lead them to prudently avoid high-risk
situations and remove tempting stimuli from
their environment when they can.
Individuals also differ considerably in
their command of top-down attention.
In the cognitive literature on executive
functioning, top-down attention has been
strongly linked with the concept of working
memory capacity (WMC; Kane, Bleckley,
Conway, & Engle, 2001), which represents
the ability to maintain and update relevant
information in working memory, and to
shield it from interfering processing or dis-
traction (Engle, 2002; Kane et al., 2001).
This ability may be highly instrumental to
a variety of strategies important for effective
desire regulation (Hofmann et al., 2011).
First, WMC may help people disengage
faster and be less influenced by attention-
grabbing desire- related cues. Eye- tracking
studies, for instance, have shown that people
low in WMC are prone to bias eye direction
toward tempting cues, with the degree of
bias correlating with their automatic affec-
tive reactions toward those cues (Friese, Bar-
gas-Avila, Hofmann, & Wiers, 2010; Friese
& Hofmann, 2012). Those high in WMC,
in contrast, were much less influenced by
their automatic affective reactions, which
suggests that they were faster at disengaging
attention from salient cues.
Second, WMC may aid a host of emotion
regulation strategies, such as the efficient
use of distraction, reappraisal, and emo-
tion suppression (Hofmann et al., 2008,
Study 3; Hofmann, Schmeichel, et al., 2012;
McRae, Jacobs, Ray, John, & Gross, 2012;

Desire and Desire Regulation 355
Schmeichel, Volokhov, & Demaree, 2008).
Third, individuals high in WMC seem to
be better at controlling their own thoughts,
with fewer thought intrusions (Brewin
& Smart, 2005; Kane et al., 2007). Even
though more direct evidence for the mod-
erating role of WMC in the regulation of
desires and cravings is missing, it does not
appear to be great leap to suggest that indi-
viduals with high WMC are more effective
than those with low WMC at breaking the
desire- processing cycle.
Because executive functions such as work-
ing memory can be trained, at least to some
extent (for a discussion, see Shipstead,
Redick, & Engle, 2012), there is a large
potential for intervention research aimed at
finding ways to improve the management
of unwanted desires and cravings. Further-
more, fascinating new research suggests that
repeated high- frequency (i.e., excitatory)
stimulation of the dorsolateral prefrontal
cortex via transcranial magnetic stimulation
(TMS) or transcranial direct current stimu-
lation (tDCS) may reduce cravings (e.g.,
Amiaz, Levy, Vainiger, Grunhaus, & Zan-
gen, 2009; Boggio et al., 2008; Camprodon,
Martinez- Raga, Alonso- Alonso, Shih, &
Pascual- Leone, 2007). One highly plausible
explanation is that the high- frequency stim-
ulation leads to an additional mobilization
of executive control processes implied in the
down- regulation of cravings.
Regarding further personality traits, the
Everyday Temptations Study showed that a
measure of perfectionism (tapping primarily
into negative, dysfunctional perfectionism)
was associated with stronger desire inten-
sity in daily life (Hofmann, Baumeister, et
al., 2012), more intense feelings of conflict,
and more frequent desire resistance. Even
though somewhat speculative, this find-
ing suggests that people high in this sort of
perfectionism may become overly preoccu-
pied with regulating their desires, perhaps
making too much use of counterproductive
strategies such as suppression, consistent
with research on the trait’s connection with
emotion suppression (Bergman, Nyland, &
Burns, 2007).
Finally, when discussing the positive
effects of mindfulness on desire regulation,
we highlighted the possible mediating role
of desire acceptance as an effective regula-
tion strategy. The case of acceptance raises
the intriguing issue of whether there are reli-
able individual differences in the way people
relate to their desires. A number of personal-
ity characteristics, such as faith in intuition,
trait hedonism, tolerance for ambiguity, as
well as demographic variables, such as reli-
giosity, may moderate the extent to which
people see their appetitive desires as integral
parts of themselves (leading to high accep-
tance) as opposed to “dark and sinister”
forces that lack integration with the self and
thus need to be kept at bay. In the absence
of empirical work that has explored such
possible links between acceptance of appeti-
tive desires and personality traits, we predict
that such personality characteristics should
(1) relate to how often people go about regu-
lating their appetitive desires and (2) facili-
tate regulation of appetitive desires to the
extent that they promote acceptance- based
rather than suppression- based emotion regu-
lation strategies.
Conclusion
As humans we experience all sorts of appeti-
tive desires. Sometimes, these stand in strong
opposition to our self- regulatory goals or
values, forcing us to face the challenge of
how best to “get a grip” on such “unwanted
wants.” In this chapter, we have argued
that desires can be conceptually treated as
emotions, because, like emotions, desires
have affective, motivational, and cognitive
components. Hence, it may be beneficial to
address the issue of desire regulation from
an emotion regulatory perspective.
Our review of the available literature
suggests that, as with emotion regulation,
effective desire regulation can take place at
various phases of desire processing. Desire
regulation strategies can be roughly divided
into those that prevent exposure to desire-
eliciting stimuli (situation and stimulus con-
trol), those that may prevent a desire from
reaching conscious awareness (e.g., atten-
tion allocation), and those that prevent a
desire from becoming overly dominant (e.g.,
reappraisal). Mapping a given strategy to
just one of these different phases may be
overly simplistic, however. For example,
take attention allocation: Sometimes, pow-
erful distractors (resulting in mental preoc-
cupation) may prevent a desire from reach-

356 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
ing conscious awareness; at other times,
attention allocation may be used strategi-
cally to down- regulate a desire only after it
has reached conscious awareness. In a simi-
lar vein, it is possible that automatic down-
regulation may sometimes be so powerful
that it may even prevent the onset of a real
desire, whereas at other times, it may make
a desire less potent, so that it does not take
up as many mental resources as it otherwise
would. Moreover, it is possible that multiple
strategies may complement each other in
important ways that are worthy of future
study. For instance, powerful automatic reg-
ulation should reduce the demand for more
effortful emotion regulation processes.
Finally, people appear to differ as to how
intensely they experience desire and how
they go about regulating it. Individuals high
in TSC seem to make more use of early pre-
ventive strategies such as situation and stim-
ulus control. (Dysfunctional) perfectionists
may focus too much on suppression, thus
becoming overly obsessed with their desires.
Being dispositionally high in executive func-
tions such as WMC may make it a lot easier
to resist the attentional pull of tempting
stimuli. Taking a closer look at what strate-
gies work for what types of people should
help practitioners to devise more effective
customized treatments for those who suffer
from tempting desires and cravings. As tech-
nological possibilities mature, we expect
to see fascinating new developments in the
years to come with regard to one of the cen-
tral challenges of what it means to be human:
how to shape one’s internal landscape of
appetitive desires in a way that strikes a
healthy balance between desire enactment
and the occasional need to curb one’s pas-
sion for what might be a pretty good reason.
Notes
1. Even though the two are often correlated,
desires are also conceptually distinct from
attitudes, because liking does not necessar-
ily imply wanting and vice versa (Berridge,
1996). For instance, one may like lobster in
general but have no desire to eat one right
now. A long-term smoker may dislike ciga-
rettes (“They’re unhealthy and expensive”)
yet have a craving for one.
2. Whereas external stimuli are typically
involved and sometimes strategically
employed by those wanting to instigate cer-
tain desires in others (e.g., companies using
scents to better sell their products; a person
wearing sexy clothing on a date), it is also
possible that desires can be activated in a top-
down fashion in the absence of any external
stimuli by willfully activating certain memo-
ries or simulating certain experiences (Kava-
nagh et al., 2005).
3. Note that the effects of cognitive load are
quite the opposite of the effects of resource
depletion. Recent work by Vohs et al. (2012)
shows that people in a depleted state experi-
ence stronger emotional reactions to valenced
stimuli, intensified pain in a cold pressor task,
and an intensified desire to eat cookies pre-
sented during a taste and rate test.
4. The reverse process (i.e., inhibition of the
self- control goal) may take place among less
successful self- regulators (see Papies, Stroebe,
& Aarts, 2008b; Stroebe, Mensink, Aarts,
Schut, & Kruglanski, 2008), suggesting
important individual differences with regard
to automatic desire- regulation processes.
References
Alberts, H. J. E. M., Mulkens, S., Smeets, M.,
& Thewissen, R. (2010). Coping with food
cravings. Investigating the potential of a
mindfulness- based intervention. Appetite, 55,
160–163.
Amiaz, R., Levy, D., Vainiger, D., Grunhaus,
L., & Zangen, A. (2009). Repeated high-
frequency transcranial magnetic stimulation
over the dorsolateral prefrontal cortex reduces
cigarette craving and consumption. Addic-
tion, 104, 653–660.
Attwood, A. S., O’Sullivan, H., Leonards, U.,
Mackintosh, B., & Munafo, M. R. (2008).
Attentional bias training and cue reactivity
in cigarette smokers. Addiction, 103, 1875–
1882.
Baars, B. J., & Franklin, S. (2003). How con-
scious experience and working memory inter-
act. Trends in Cognitive Sciences, 7, 166–172.
Barnes, R. D., & Tantleff- Dunn, S. (2010).
Food for thought: Examining the relation-
ship between food thought suppression and
weight- related outcomes. Eating Behaviors,
11, 175–179.
Baumeister, R. F., Masicampo, E. J., & Vohs,
K. D. (2011). Do conscious thoughts cause
Desire and Desire Regulation 357
behavior? Annual Review of Psychology, 62,
331–361.
Belk, R. W., Ger, G., & Askegaard, S. (2000).
The missing streetcar named desire. In S. Rat-
neshwar, D. G. Mick, & C. Huffman (Eds.),
The why of consumption: Contemporary
perspectives on consumer motives, goals and
desires (pp. 98–119). London: Routledge.
Bergman, A. J., Nyland, J. E., & Burns, L. R.
(2007). Correlates with perfectionism and the
utility of a dual process model. Personality
and Individual Differences, 43, 389–399.
Berking, M., & Schwarz, J. (this volume, 2014).
Affect regulation training. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 529–547). New York: Guilford Press.
Berridge, K. C. (1996). Food reward: Brain sub-
strates of wanting and liking. Neuroscience
and Biobehavioral Reviews, 20, 1–25.
Boggio, P. S., Sultani, N., Fecteau, S., Merabet,
L., Mecca, T., Pascual- Leone, A., et al. (2008).
Prefrontal cortex modulation using transcra-
nial DC stimulation reduces alcohol craving:
A double- blind, sham- controlled study. Drug
and Alcohol Dependence, 92, 55–60.
Brewin, C. R., & Smart, L. (2005). Working
memory capacity and suppression of intrusive
thoughts. Journal of Behavior Therapy and
Experimental Psychiatry, 36, 61–68.
Camprodon, J. A., Martinez- Raga, J., Alonso-
Alonso, M., Shih, M. C., & Pascual- Leone, A.
(2007). One session of high frequency repeti-
tive transcranial magnetic stimulation (rTMS)
to the right prefrontal cortex transiently
reduces cocaine craving. Drug and Alcohol
Dependence, 86, 91–94.
Carter, B. L., & Tiffany, S. T. (2001). The cue-
availability paradigm: The effects of ciga-
rette availability on cue reactivity in smokers.
Experimental and Clinical Psychopharmacol-
ogy, 9, 183–190.
Carver, C. S., & Scheier, M. F. (1998). On the
self- regulation of behavior. Cambridge, UK:
Cambridge University Press.
Carver, C. S., & Scheier, M. F. (2002). Control
processes and self- organization as comple-
mentary principles underlying behavior. Per-
sonality and Social Psychology Review, 6,
304–315.
Chambers, R., Lo, B. C. Y., & Allen, N. B.
(2008). The impact of intensive mindfulness
training on attentional control, cognitive style,
and affect. Cognitive Therapy and Research,
32, 303–322.
Engle, R. W. (2002). Working memory capacity
as executive attention. Current Directions in
Psychological Science, 11, 19–23.
Erskine, J. A. K. (2008). Resistance can be futile:
Investigating behavioural rebound. Appetite,
50, 415–421.
Fadardi, J. S., & Cox, W. M. (2009). Reversing
the sequence: Reducing alcohol consumption
by overcoming alcohol attentional bias. Drug
and Alcohol Dependence, 101, 137–145.
Farb, N. A. S., Anderson, A. K., Irving, J. A., &
Segal, Z. V. (this volume, 2014). Mindfulness
interventions and emotion regulation. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 548–568). New York: Guilford
Press.
Field, M., Duka, T., Tyler, E., & Schoenmak-
ers, T. (2009). Attentional bias modification
in tobacco smokers. Nicotine and Tobacco
Research, 11, 812–822.
Finkel, E. J., & Eckhardt, C. I. (in press). Inti-
mate partner violence. In J. A. Simpson & L.
Campbell (Eds.), The Oxford handbook of
close relationships. New York: Oxford Uni-
versity Press.
Fishbach, A., Friedman, R. S., & Kruglanski, A.
W. (2003). Leading us not unto temptation:
Momentary allurements elicit overriding goal
activation. Journal of Personality and Social
Psychology, 84, 296–309.
Florsheim, P., Heavin, S., Tiffany, S., Colvin, P.,
& Hiraoka, R. (2008). An experimental test
of a craving management technique for adoles-
cents in substance- abuse treatment. Journal of
Youth and Adolescence, 37, 1205–1215.
Forman, E. M., Hoffman, K. L., McGrath, K.
B., Herbert, J. D., Brandsma, L. L., & Lowe,
M. R. (2007). A comparison of acceptance-
and control- based strategies for coping with
food cravings: An analog study. Behaviour
Research and Therapy, 45, 2372–2386.
Franken, I. H. (2003). Drug craving and addic-
tion: Integrating psychological and neuropsy-
chopharmacological approaches. Progress in
Neuropsychopharmacological Biological Psy-
chiatry, 27, 563–579.
Franken, I. H. A. (2002). Behavioral approach
system (BAS) sensitivity predicts alcohol crav-
ing. Personality and Individual Differences,
32, 349–355.
Friese, M., Bargas-Avila, J., Hofmann, W., &
Wiers, R. W. (2010). Here’s looking at you,
Bud: Alcohol- related memory structures pre-
dict eye movements for social drinkers with
low executive control. Social Psychological
and Personality Science, 1, 143–151.
358 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Friese, M., & Hofmann, W. (2012). Just a little
bit longer: Viewing time of erotic material
from a self- control perspective. Applied Cog-
nitive Psychology, 26, 489–496.
Fujita, K., & Han, H. A. (2009). Moving beyond
deliberative control of impulses: The effect of
construal levels on evaluative associations in
self- control conflicts. Psychological Science,
20, 799–804.
Gray, J. A. (Ed.). (1982). The neuropsychology
of anxiety: An enquiry into the functions of
the septo- hippocampal system. New York:
Oxford University Press.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Higgins, E. T. (1998). The aboutness principle:
A pervasive influence on human inference.
Social Cognition, 16, 173–198.
Hofmann, W., Baumeister, R. F., Förster, G., &
Vohs, K. D. (2012). Everyday temptations: An
experience sampling study of desire, conflict,
and self- control. Journal of Personality and
Social Psychology, 102, 1318–1335.
Hofmann, W., De Houwer, J., Perugini, M.,
Baeyens, F., & Crombez, G. (2010). Evalua-
tive conditioning in humans: A meta- analysis.
Psychological Bulletin, 136, 390–421.
Hofmann, W., Deutsch, R., Lancaster, K., &
Banaji, M. R. (2010). Cooling the heat of
temptation: Mental self- control and the auto-
matic evaluation of tempting stimuli. Euro-
pean Journal of Social Psychology, 40, 17–25.
Hofmann, W., & Fisher, R. R. (2012). How
guilt and pride shape subsequent self- control.
Social Psychological and Personality Science,
3, 682–690.
Hofmann, W., Friese, M., Schmeichel, B. J., &
Baddeley, A. D. (2011). Working memory and
self- regulation. In K. D. Vohs & R. F. Baumeis-
ter (Eds.), The handbook of self- regulation:
Research, theory, and applications (Vol. 2,
pp. 204–226). New York: Guilford Press.
Hofmann, W., Friese, M., & Strack, F. (2009).
Impulse and self- control from a dual- systems
perspective. Perspectives on Psychological Sci-
ence, 4, 162–176.
Hofmann, W., Gschwendner, T., Friese, M.,
Wiers, R. W., & Schmitt, M. (2008). Working
memory capacity and self- regulatory behavior:
Toward an individual differences perspective
on behavior determination by automatic ver-
sus controlled processes. Journal of Personal-
ity and Social Psychology, 95, 962–977.
Hofmann, W., Koningsbruggen, G. M., Stroebe,
W., Ramanathan, S., & Aarts, H. (2010). As
pleasure unfolds: Hedonic responses to tempt-
ing food. Psychological Science, 21, 1863–
1870.
Hofmann, W., Kotabe, H., & Luhmann, M. (in
press). The spoiled pleasure of giving in to
temptation. Motivation and Emotion.
Hofmann, W., Schmeichel, B. J., & Baddeley,
A. D. (2012). Executive functions and self-
regulation. Trends in Cognitive Sciences, 3,
174–180.
Hofmann, W., & Van Dillen, L. F. (2012). Desire:
The new hotspot in self- control research. Cur-
rent Directions in Psychological Science, 21,
317–322.
Hofmann, W., Vohs, K. D., & Baumeister, R.
F. (2012). What people desire, feel conflicted
about, and try to resist in everyday life. Psy-
chological Science, 23, 582–588.
Hofmann, W., & Wilson, T. D. (2010). Con-
sciousness, introspection, and the adaptive
unconscious. In B. Gawronski & B. K. Payne
(Eds.), Handbook of implicit social cogni-
tion: Measurement, theory, and applications
(pp. 197–215). New York: Guilford Press.
Houben, K., Havermans, R. C., & Wiers, R.
W. (2010). Learning to dislike alcohol: Con-
ditioning negative implicit attitudes toward
alcohol and its effect on drinking behavior.
Psychopharmacology, 211, 79–86.
Irvine, W. B. (2006). On desire. Why we want
what we want. New York: Oxford University
Press.
Johnston, L., Bulik, C. M., & Anstiss, V. (1999).
Suppressing thoughts about chocolate. Inter-
national Journal of Eating Disorders, 26,
21–27.
Kane, M. J., Bleckley, M. K., Conway, A. R. A.,
& Engle, R. W. (2001). A controlled- attention
view of working- memory capacity. Journal
of Experimental Psychology: General, 130,
169–183.
Kane, M. J., Brown, L. H., McVay, J. C., Sil-
via, P. J., Myin- Germeys, I., & Kwapil, T.
R. (2007). For whom the mind wanders, and
Desire and Desire Regulation 359
when: An experience- sampling study of work-
ing memory and executive control in daily life.
Psychological Science, 18, 614–621.
Kavanagh, D. J., Andrade, J., & May, J. (2005).
Imaginary relish and exquisite torture: The
elaborated intrusion theory of desire. Psycho-
logical Review, 112, 446–467.
Kemps, E., Tiggemann, M., & Christianson, R.
(2008). Concurrent visuo-
s
patial processing
reduces food cravings in prescribed weight-
l
oss dieters. Journal of Behavior Therapy and
Experimental Psychiatry, 39, 177–186.
Knudsen, E. I. (2007). Fundamental components
of attention. Annual Review of Neuroscience,
30, 57–78.
Kunda, Z. (1990). The case for motivated rea-
soning. Psychological Bulletin, 108, 480–498.
MacLeod, C., & Grafton, B. (this volume, 2014).
Regulation of emotion through modification
of attention. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 508–528).
New York: Guilford Press.
Mahoney, M. J., & Thoresen, C. E. (1972).
Behavioral self-
c
ontrol: Power to the person.
Educational Researcher, 1, 5–7.
Mann, T., & Ward, A. (2001). Forbidden fruit:
Does thinking about a prohibited food lead
to its consumption? International Journal of
Eating Disorders, 29, 319–327.
McRae, K., Jacobs, S. E., Ray, R. D., John, O.
P., & Gross, J. J. (2012). Individual differences
in reappraisal ability: Links to reappraisal
frequency, well-being, and cognitive control.
Journal of Research in Personality, 46, 2–7.
Mele, A. (2001). Autonomous agents. From self-
c
ontrol to autonomy. Oxford, UK: Oxford
University Press.
Mischel, W., & Baker, N. (1975). Cognitive
appraisals and transformations in delay behav-
ior. Journal of Personality and Social Psychol-
ogy, 31, 254–261.
Mogenson, G. J., Jones, D. L., & Yim, C. Y.
(1980). From motivation to action: Functional
interface between the limbic system and the
motor system. Progress in Neurobiology, 14,
69–97.
Nordgren, L. F., van Harreveld, F., & van der
Pligt, J. (2009). The restraint bias: How the
illusion of self-
r
estraint promotes impulsive
behavior. Psychological Science, 20, 1523–
1528.
Papies, E. K., Stroebe, W., & Aarts, H. (2008a).
The allure of forbidden food: On the role of
attention in self-
r
egulation. Journal of Experi-
mental Social Psychology, 44, 1283–1292.
Papies, E. K., Stroebe, W., & Aarts, H. (2008b).
Healthy cognition: Processes of self-
r
egulatory
success in restrained eating. Personality and
Social Psychology Bulletin, 34, 1290–1300.
Rauss, K., Schwartz, S., & Pourtois, G. (2011).
Top-down effects on early visual processing
in humans: A predictive coding framework.
Neuroscience and Biobehavioral Reviews, 35,
1237–1253.
Sayette, M. A., & Griffin, K. M. (2011). Self-
r
egulatory failure and addiction. In R. F. Bau-
meister & K. D. Vohs (Eds.), Handbook of
self-
r
egulation: Research, theory, and applica-
tions (Vol. 2, pp.
505–521). New York: Guil-
ford Press.
Schmeichel, B. J., Harmon-Jones, C., & Har-
mon-Jones, E. (2010). Exercising self-
c
ontrol
increases approach motivation. Journal of
Personality and Social Psychology, 99, 162–
173.
Schmeichel, B. J., Volokhov, R. N., & Demaree,
H. A. (2008). Working memory capacity and
the self-
re
gulation of emotional expression
and experience. Journal of Personality and
Social Psychology, 95, 1526–1540.
Schoenmakers, T., Wiers, R. W., Jones, B. T.,
Bruce, G., & Jansen, A. T. M. (2007). Atten-
tional retraining decreases attentional bias in
heavy drinkers without generalization. Addic-
tion, 102, 399–405.
Schroeder, S. A. (2007). We can do better—
Improving the health of the american peo-
ple. New England Journal of Medicine, 357,
1221–1228.
Shah, J. Y., Friedman, R. S., & Kruglanski, A.
W. (2002). Forgetting all else: On the anteced-
ents and consequences of goal shielding. Jour-
nal of Personality and Social Psychology, 83,
1261–1280.
Shiffman, S., Hickcox, M., Paty, J. A., Gnys, M.,
Kassel, J. D., & Richards, T. J. (1997). The
abstinence violation effect following smoking
lapses and temptations. Cognitive Therapy
and Research, 21(5), 497–523.
Shipstead, Z., Redick, T. S., & Engle, R. W.
(2012). Is working memory training effective?
Psychological Bulletin, 138, 628–654.
Stroebe, W., Mensink, W., Aarts, H., Schut,
H., & Kruglanski, A. (2008). Why dieters
fail: Testing the goal conflict model of eating.
Journal of Experimental Social Psychology,
44, 26–36.
Thaler, R. H., & Sunstein, C. R. (2010). Nudge:
Improving decisions about health, wealth,
and happiness. New York: Penguin Books.
360 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Van der Laan, L. N., de Ridder, D. T. D.,
Viergevera, M. A., & Smeetsa, P. A. M.
(2011). The first taste is always with the eyes:
A meta- analysis on the neural correlates of
processing visual food cues. NeuroImage, 55,
296–303.
Van Dillen, L. F., & Koole, S. L. (2009). How
automatic is oautomatic vigilance?: The role
of working memory in attentional interference
of negative information. Cognition and Emo-
tion, 23, 1106–1117.
Van Dillen, L. F., Papies, E., & Hofmann, W.
(2013). Turning a blind eye to temptation.
How task load can facilitate self- regulation.
Journal of Personality and Social Psychology,
3, 427–443.
Van Gucht, D., Baeyens, F., Vansteenwegen, D.,
Hermans, D., & Beckers, T. (2010). Counter-
conditioning reduces cue- induced craving and
actual cue- elicited consumption. Emotion, 10,
688–695.
Veling, H., Holland, R. W., & van Knippenberg,
A. (2007). Devaluation of distracting stimuli.
Cognition and Emotion, 21, 442–448.
Veling, H., Holland, R. W., & van Knippenberg,
A. (2008). When approach motivation and
behavioral inhibition collide: Behavior regula-
tion through stimulus devaluation. Journal of
Experimental Social Psychology, 44, 1013–
1019.
Vohs, K. D., Baumeister, R. F., Mead, N. L., Hof-
mann, W., Ramanathan, S., & Schmeichel, B.
J. (2012). Engaging in self- control heightens
urges and feelings. Unpublished manuscript.
Wegner, D. M. (1994). Ironic processes of mental
control. Psychological Review, 101, 34–52.
Wiers, R. W., Eberl, C., Rinck, M., Becker, E.
S., & Lindenmeyer, J. (2011). Retraining
automatic action tendencies changes alco-
holic patients’ approach bias for alcohol and
improves treatment outcome. Psychological
Science, 22, 490–497.
Wiers, R. W., Rinck, M., Kordts, R., Houben,
K., & Strack, F. (2010). Retraining automatic
action- tendencies to approach alcohol in haz-
ardous drinkers. Addiction, 105, 279–287.
Winkielman, P., Berridge, K. C., & Wilbarger,
J. L. (2005). Unconscious affective reactions
to masked happy versus angry faces influ-
ence consumption behavior and judgments of
value. Personality and Social Psychology Bul-
letin, 31, 121–135.

361
Much of human behavior is purposeful, or
goal directed (Bandura, 1986; Carver &
Scheier, 2000; Deci & Ryan, 1985; Fish-
bach & Ferguson, 2007; Gollwitzer, 1990;
Mischel, Cantor, & Feldman, 1996). Goals
can target our appearance (e.g., to be thin),
our mind (e.g., to be smart), and our behav-
ior (e.g., to spend more time with family or
to work harder). As we argue in this chap-
ter, some of our most important goals target
emotions (e.g., to be happy). A goal is a “cog-
nitive representation of a desired endpoint”
(Fishbach & Ferguson, 2007, p. 491). There-
fore, we define an emotion goal as the cogni-
tive representation of a particular emotional
state that is the desired endpoint. Although
any goal may indirectly involve desired
emotional endpoints (e.g., “I want to buy a
car in order to feel happy as a result”), we
focus on goals that directly target emotions
as the desired endpoint (e.g., “I want to feel
happy”). Emotion goals are a specific type of
affect goal. Whereas affect goals target states
of pleasure or pain, more generally (e.g., “I
want to feel pleasant”), emotion goals target
specific emotional states (e.g., “I want to feel
joyful, proud, or amused”). Therefore, in
this chapter, we distinguish nonaffect goals
(i.e., goals that do not involve affective states
as the direct desired endpoint), affect goals
(i.e., goals that involve pleasure or pain as
the direct desired endpoint), and emotion
goals (i.e., goals that involve specific emo-
tional states as the direct desired endpoint),
and focus mainly on the latter.
It follows from this definition that peo-
ple’s emotion goals are foundational to emo-
tion regulation. In fact, the activation of an
emotion goal is necessary for emotion regu-
lation (Gross, this volume; Mauss, Bunge,
& Gross, 2007). Emotion goals determine
whether people engage in emotion regula-
tion, which emotions they attempt to regu-
late, when they cease their emotion regula-
tory efforts, and people’s satisfaction with
their emotion regulation attempts. Thus,
understanding emotion goals has crucial
implications for understanding emotion reg-
ulation and its effects on well-being.
To date, research on emotion regulation
has focused on understanding different emo-
tion regulation strategies and their outcomes
(Gross & Thompson, 2007; Webb, Miles, &
Sheeran, 2012). As a function of this focus,
research to date has examined the “how”
(e.g., What strategies do people use to regu-
late their emotions?) more than the “why”
and “what” of emotion regulation (e.g.,
When do people decide to regulate an emo-
tion? What emotional states do people want
to attain?). The goal framework we outline
here emphasizes the importance of under-
standing not only the “how” but also the
“why” and “what” of emotion regulation.
CHAPTER 22
Emotion Goals: How Their Content,
Structure, and Operation Shape
Emotion
Regulation
Iris B. Mauss
Maya Tamir
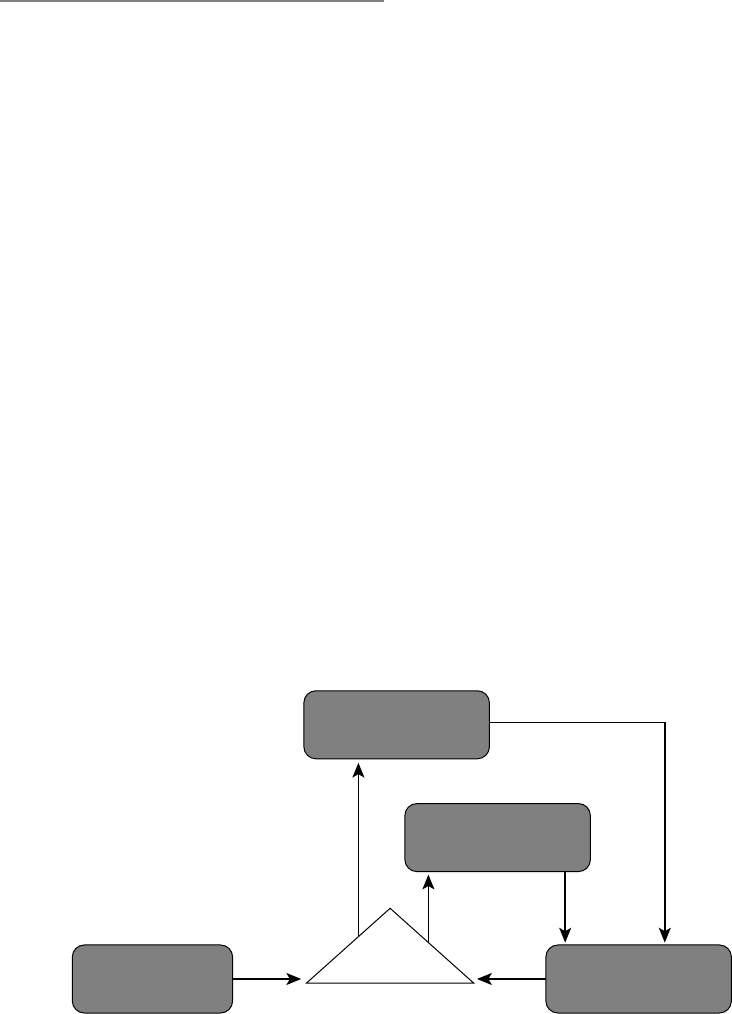
362 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
A Goal Framework
for Emotion Regulation
Although relatively little research has directly
examined emotions as goals, there is ample
research on goals and self- regulation in gen-
eral (e.g., Baumeister & Heatherton, 1996;
Carver & Scheier, 2000; Custers & Aarts,
2010). Applying this body of knowledge to
the emotion domain could greatly advance
the understanding of emotion regulation (cf.
Harmon-Jones, Harmon-Jones, Amodio, &
Gable, 2011; Koole, van Dillen, & Sheppes,
2011; Webb, Schweiger Gallo, Miles, Goll-
witzer, & Sheeran, 2012). People’s goals
determine their actions as they attempt to
decrease perceived discrepancies between
current and desired states. As Figure 22.1
illustrates, when applied to emotions, this
idea can help us understand what determines
the initiation and course of emotion regula-
tion. More specifically, perceived discrepan-
cies between current emotional states (e.g.,
“I feel sad”) and emotion goals (“I want to
feel less sad”) initiate emotion regulation,
which is set in motion to bring current emo-
tional states closer to desired emotion states.
Thus, the emotion goals people hold deter-
mine whether or not they engage in emotion
regulation, which emotions they attempt to
regulate, and in which direction (i.e., increase
or decrease). In this chapter we discuss emo-
tion goals, building on available knowledge
about goal pursuit. We organize this chap-
ter around three features of goals that have
been highlighted in research on goal pursuit
(Fishbach & Ferguson, 2007): their content,
their hierarchical structure, and their opera-
tion. First, we consider the content of emo-
tion goals. We examine what emotion goals
people adopt and highlight two factors that
determine these goals: hedonic benefits (i.e.,
greater pleasure and less pain) and nonhe-
donic benefits (e.g., to prepare an organism
for fight) of particular emotions. Second, we
consider the structure of emotion goals. Mul-
tiple goals, including emotion and nonemo-
tion ones, coexist at any given point in time
and are hierarchically organized. We con-
sider possible features of this organization
and their implications. Third, we consider
the operation of emotion goals that unfolds
as people regulate their emotions, distin-
guishing relatively automatic from deliber-
ate types of emotion regulation. Finally, we
highlight the goal framework’s implications
for understanding the links between emotion
regulation and well-being.
1
FIGURE 22.1. Hypothesized operation of emotion goals. Perceived discrepancy between current
emotional states and emotion goals initiates and directs emotion regulation, which influences cur-
rent emotional states. The evaluation of the difference between current emotional states and emotion
goals is emotional in nature (e.g., contentment when discrepancy decreases, distress when discrepancy
increases). We therefore refer to the output of this evaluation as meta- emotion.
Goal Pursuit
Goal Content
Comparator
Emotion
Regulation
Meta-
Emotion
Current
Emotional State
Emotion Goal

Emotion Goals 363
The Content of Emotion Goals:
Emotions as Desired States
What emotion goals do people have and
what determines these goals? Because all
creatures strive to attain pleasure and avoid
pain, and because emotions are pleasant
or painful subjective states, emotion goals
are often determined by the immediate
hedonic benefits of emotions. For example,
people may be motivated to increase hap-
piness because it is pleasant, and they may
be motivated to decrease fear because it is
unpleasant. Pleasant emotions generally are
preferred to unpleasant emotions by people
from different cultures (Diener, 2000; Tsai,
Knutson, & Fung, 2006) and with different
personality dispositions (Rusting & Larsen,
1995).
The immediate hedonic benefits of emo-
tions are a powerful determinant of emo-
tion goals. However, they are not the sole
determinant. Functional theories of emo-
tions hold that emotions serve to promote a
broad array of nonhedonic benefits (Frijda,
1986; Nesse & Ellsworth, 2009). Accord-
ing to the instrumental approach to emotion
regulation (Bonanno, 2001; Parrott, 1993;
Tamir, 2009), people may be motivated to
experience emotions to attain either hedonic
or nonhedonic benefits. When hedonic ben-
efits are prioritized, people are motivated
to experience pleasant emotions and avoid
unpleasant ones. However, when nonhe-
donic benefits are prioritized, people may
be motivated to experience either pleasant
or unpleasant emotions, depending on their
instrumental implications (Tamir, 2009).
Emotions can offer at least three types
of nonhedonic benefits. First, emotions can
offer performance benefits. By engaging
various physiological, cognitive, and moti-
vational processes, emotions can change
how effectively we deal with situational
demands (Frijda, 1986). Second, emotions
can have epistemic benefits. They provide us
with important information regarding our
state in the world (Clore, Gasper, & Garvin,
2001). Third, emotions can carry cultural
benefits. In group contexts, emotions signal
group membership and support of cultural
values (Keltner & Haidt, 1999).
We believe that people may be motivated
to experience emotions to attain any one
of these benefits. First, emotions can orient
behavior to deal with situational demands
as effectively as possible (Frijda, 1986). For
instance, joy may promote creativity (Fred-
rickson, 2001). Therefore, when perfor-
mance is likely to benefit from increased cre-
ativity, joy might be useful for performance.
People may be motivated to experience an
emotion to attain its performance- related
benefits. In support of this hypothesis, we
have recently shown that people want to
experience emotions they believe would
promote their performance (Tamir, Salerno,
Rhodes, & Schreier, 2012). In a series of stud-
ies, we led participants to expect anger to be
either useful, irrelevant, or harmful for per-
formance on an upcoming task. Participants
were motivated to increase the experience
of anger when they expected it be useful for
performance, even though it was unpleasant
to experience. This effect was obtained even
when beliefs about usefulness were manipu-
lated outside of conscious awareness. These
findings demonstrate that emotion goals can
be determined by the expected benefits of
emotions for performance.
Second, emotions provide people with
important information about themselves
and their state in the world (Clore et al.,
2001). Such information can support or
conflict with core assumptions about who
we are and what the world is like. People are
generally motivated to preserve these core
assumptions (Swann & Schroeder, 1995).
Just as people seek feedback that maintains
their self-image (Swann & Schroeder, 1995),
people may be motivated to experience emo-
tions that maintain their self-image (i.e.,
that have epistemic benefits). In support of
this proposition, Wood and her colleagues
showed that people with low self esteem
are motivated to maintain sad feelings,
because such feelings are familiar to them
and because they reinforce their low sense of
personal value (Wood, Heimpel, Manwell,
& Whittington, 2009).
Third, emotions can reinforce one’s com-
mitment to and investment in particular
cultural values. For instance, pride is tied to
the value of personal achievement, whereas
shame is tied to the value of social harmony
(Kitayama, Mesquita, & Karasawa, 2006).
Because cultures generally seek to preserve
specific values, they prescribe certain emo-
tions as more normative than others (Eid
& Diener, 2001). In support of this notion,
364 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Tsai and her colleagues (2006) have shown
that cultures vary in the extent to which they
value different emotional states. Whereas
pleasant high- arousal states are more highly
valued in individualistic cultures, pleasant
low- arousal states are more highly valued
in collectivistic cultures. Thus, beyond emo-
tions’ hedonic benefits, emotion goals may
be determined by values that are prevalent
in people’s cultural context.
As the research reviewed here demon-
strates, people are often motivated to expe-
rience emotions for their immediate hedonic
benefits, but they can also be motivated to
experience emotions for their performance-
related benefits, epistemic benefits, or cul-
tural benefits. This list of determinants of
emotion goals is not exhaustive (for a more
complete list, see Tamir & Bigman, in press).
But it demonstrates that people can be moti-
vated to experience almost any emotion.
Thus, and perhaps surprisingly, emotion
goals are not limited to pleasant emotions.
The Structure of Emotion Goals
Up to this point, we have treated emotion
goals as though they are singular, isolated
entities. However, people pursue many dif-
ferent goals at any given moment. As Figure
22.2 illustrates, goals can be ordered hier-
archically according to their importance,
centrality, and abstraction, with some goals
assuming superordinate and others assum-
ing subordinate positions (Carver & Scheier,
2000; Fishbach & Ferguson, 2007). To fully
understand emotion goals, it is important to
FIGURE 22.2. Hypothesized organization of emotion goals. Multiple goals are hierarchically orga-
nized, with some goals assuming superordinate and others assuming subordinate positions. Goals can
be emotional or nonaffective in nature, and they can be compatible or in conflict with one another.
As the examples illustrates, goal structure can be characterized by multifinality and equifinality. The
goal of “joy” may serve the superordinate goal to be happy or to feel competent (multifinality). The
superordinate goal to feel competent can be subserved by either joy or anger (equifinality). Conflict can
arise when one goal (e.g., feel anger) subserves one superordinate goal (feel competent) but not another
(be happy).
Superordinate
goals
Subordinate
goals
Joy Worry Love
Spend time
with family
Do well
at work
Feel
pleasant
BE HAPPY
Superordinate
goals
Subordinate
goals
Joy Joy Anger
Win in
confrontations
Feel
pleasant
Make
friends
FEEL COMPETENT
Emotion Goals 365
examine them within the hierarchical struc-
ture of a broader goal system.
Goal systems can be characterized by mul-
tifinality and equifinality (Kruglanski et al.,
2002). Multifinality refers to the idea that
a given subordinate goal may serve multiple
superordinate goals (see Figure 22.2). In the
context of emotion goals, this implies that a
subordinate emotion goal (e.g., to feel joy)
may serve superordinate affect goals (e.g., to
feel pleasant) as well as superordinate non-
affect goals (e.g., to make friends). Equifi-
nality refers to the idea that a given super-
ordinate goal may be subserved by multiple
subordinate goals (see Figure 22.2). In the
context of emotion goals, this implies that a
superordinate goal (e.g., to be happy) may be
served by various affect (e.g., to feel good, to
feel less bad) and nonaffect (e.g., to do well
at work, to spend time with family) goals.
The pursuit of superordinate goals can
automatically activate related subordinate
goals (e.g., Fishbach & Ferguson, 2007).
Adapting this principle to the study of emo-
tion goals, we have recently shown that emo-
tion goals can be activated by priming related
superordinate goals (Tamir, Ford, & Ryan,
2013). Building on the idea that anger can
impair collaboration, we showed that par-
ticipants who were nonconsciously primed
with the goal of collaboration became less
motivated to experience anger before a
social interaction. These findings could not
be explained by concurrent emotional expe-
riences and demonstrate that emotion goals
can operate in the service of superordinate
nonaffect goals.
Multifinality and equifinality of goals can
thus facilitate goal pursuit because they offer
multiple ways to achieve goals. However, as
Figure 22.2 illustrates, the multifinality of
goals can also give rise to goal conflict when
subordinate goals simultaneously promote
the pursuit of some superordinate goals and
impair the pursuit of others. This may be
particularly salient in the context of emotion
goals, because emotions have both hedonic
and nonhedonic implications. Pleasant
emotions can either promote or impair the
attainment of superordinate nonaffect goals
(e.g., joy might help people make friends but
also lead them to spend less time studying
for an exam). However, pleasant emotions
generally promote the attainment of super-
ordinate affect goals (i.e., they feel good).
Thus, goal conflict may be less likely when a
pleasant emotion promotes both affect and
nonaffect superordinate goals, and more
likely when a pleasant emotion promotes an
affect goal but impairs a nonaffect goal.
Goal conflict may be particularly likely
when pursuing unpleasant emotions. Like
pleasant emotions, unpleasant emotions can
either promote or impair the attainment of
superordinate nonaffect goals (e.g., anger
might help people win a fight but it might
also impair friendships). However, unlike
pleasant emotions, unpleasant emotions
often impair the attainment of superordinate
affect goals (i.e., they feel bad). Therefore,
the pursuit of unpleasant emotions depends
on the relative strength of competing super-
ordinate nonaffect and affect goals. People
may be more likely to pursue subordinate
unpleasant emotion goals when superor-
dinate nonaffect goals become salient, and
such pursuits likely involve some degree of
goal conflict.
These ideas lie at the heart of the instru-
mental approach to emotion regulation
(Tamir, 2009). According to this approach,
people can pursue either pleasant or unpleas-
ant emotion goals, to the extent that such
goals serve salient superordinate goals. For
instance, to the extent that anger promotes
successful confrontation (e.g., Frijda, 1986),
people may be motivated to feel angry when
it is important for them to win a fight. In
such cases, people would be motivated to
experience unpleasant emotions, despite the
immediate hedonic cost of doing so.
There is now a body of empirical evidence
in support of these ideas. For instance, Tamir,
Mitchell, and Gross (2008) tested whether
people wanted to increase their anger when
preparing for confrontation. Participants
were given salient confrontational or non-
confrontational goals (e.g., kill enemies or
build an empire in a virtual computer game).
To assess emotion goals, participants indi-
cated the extent to which they preferred to
engage in various emotion- inducing activi-
ties, including those that were neutral, excit-
ing, and anger- inducing. Although partici-
pants acknowledged that the anger- inducing
activities would be unpleasant, they nonethe-
less preferred to engage in them when pursu-
ing the confrontational, but not the noncon-
frontational, goals. A similar pattern was
found in an examination of people’s prefer-

366 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
ences for anger before a face-to-face nego-
tiation task (Tamir & Ford, 2012b). Partici-
pants who thought they were preparing for a
confrontational negotiation showed stronger
preferences for anger before the negotiation.
In contrast, participants who thought they
were preparing for a collaborative negotia-
tion showed weaker preferences for anger
and stronger preferences for happiness. Such
preferences, in turn, were fully mediated
by the belief that anger would promote or
impair successful performance. These stud-
ies suggest that emotion goals can subserve
nonaffect goals, and that people pursue
unpleasant emotions when they expect them
to subserve salient superordinate goals.
Interestingly, the relative importance of
affect and nonaffect goals may shift system-
atically across the lifespan. Specifically, the
importance of affect goals seems to change
with age, with affect goals gaining in relative
importance over nonaffect goals (Charles
& Carstensen, this volume; Carstensen,
Isaacowitz, & Charles, 1999). If people are
likely to pursue unpleasant emotions when
they subserve superordinate nonaffect goals,
unpleasant emotion goals should be more
prevalent in younger than in older adults.
Indeed, wanting to maintain or increase
unpleasant emotions and decrease pleasant
emotions is more prevalent in younger than
in older adults (Riediger, Schmiedek, Wag-
ner, & Lindenberger, 2009).
In this section, we have discussed the
structure of emotion goals, highlighting
the fact that emotion goals operate within
a broader goal system, which includes both
affect and nonaffect goals that are hierarchi-
cally organized. The features of this orga-
nization have important implications for
understanding how emotion goals interact
with one another and with other types of
goals. In the next section, we focus on the
operation of emotion goals.
The Operation of Emotion Goals:
Automatic and Deliberate
Emotion Regulation
As illustrated in Figure 22.1, according
to feedback, or cybernetic, models of self
regulation, people initiate self- regulatory
attempts when they perceive discrepancies
between their current state and their goal
(Baumeister & Heatherton, 1996; Carver
& Scheier, 2000). When applied to emotion
goals, these models direct our attention to
an important element of emotion regula-
tion. Goals can be represented outside of
conscious awareness, as well as consciously
(Custers & Aarts, 2010; Fishbach & Fergu-
son, 2007). People pursue conscious goals
deliberately, whereas they pursue noncon-
scious goals relatively effortlessly and with
little or no conscious awareness (automati-
cally). It follows that emotion goals can
set in motion relatively automatic as well
as deliberate emotion regulation attempts.
Research shows that nonemotion goals, such
as achievement and cooperation, can be pur-
sued automatically (e.g., Bargh, Gollwitzer,
Lee-Chai, Barndollar, & Trötschel, 2001).
Might emotion goals also be pursued in an
automatic manner, with little effort and out-
side of conscious awareness?
While it has long been hypothesized that
people can engage in emotion regulation
unconsciously (Freud, 1936), only recently
has empirical research begun to explore this
possibility (see Gyurak & Etkin, this vol-
ume; Fitzsimons & Bargh, 2004; Gyurak,
Gross, & Etkin, 2011; Mauss, Bunge, et al.,
2007). To examine individual differences in
automatic emotion regulation, we developed
a variant of the Implicit Association Test
(IAT; Greenwald, McGhee, & Schwartz,
1998) that estimates implicit evaluation of
emotion control versus expression, the Emo-
tion Regulation IAT (ER-IAT; Mauss, Evers,
Wilhelm, & Gross, 2006, Study 1). We
reasoned that people who implicitly evalu-
ate emotion control positively would tend
to engage in automatic emotion regulation
(Aarts & Dijksterhuis, 2000; Custers &
Aarts, 2005).
To examine how automatic emotion reg-
ulation predicts emotional responding we
assessed whether ER-IAT scores were asso-
ciated with responses to a laboratory anger
provocation (Mauss et al., 2006, Study 2).
While most participants became angry
during the provocation, those with greater
ER-IAT scores (i.e., those who implicitly
evaluated emotion control more positively)
reported relatively less anger experience. In
addition, and in line with the notion that
regulation attempts had taken place, they
exhibited a challenge cardiovascular acti-
vation pattern, characterized by greater
Emotion Goals 367
cardiac output and lower total peripheral
resistance (cf. Tomaka, Blascovich, Kelsey,
& Leitten, 1993). The relative reduction of
anger experience appeared to have happened
without conscious effort, because asso-
ciations between ER-IAT scores and anger
responding held when researchers controlled
for self- reported effortful emotion control.
In summary, these findings are consistent
with the idea that people who implicitly val-
ued emotion control tended to regulate their
emotion automatically and experienced less
anger.
To examine the possible causal role of
automatic emotion regulation, we (Mauss,
Cook, & Gross, 2007) manipulated noncon-
scious emotion regulation goals by priming
emotion control versus emotion expression
with a sentence- unscrambling task (Srull &
Wyer, 1979). Participants primed with emo-
tion control in the laboratory responded
with less anger to a subsequent anger provo-
cation than did participants primed with
emotion expression. The fact that partici-
pants were not aware of the purpose of the
priming task suggests that these effects were
not conscious. These conclusions were con-
firmed in a study in which participants were
either explicitly instructed or primed outside
of awareness to engage in emotion regula-
tion. Participants in the priming condition
achieved the same decrease in physiological
reactivity to an anxiety induction as those
explicitly instructed to regulate their emo-
tion (Williams, Bargh, Nocera, & Gray,
2009).
Work on implementation intentions also
suggests that emotion regulation can unfold
automatically. An implementation inten-
tion is a plan that links situations to specific
goal- directed behaviors (Gollwitzer, 1999),
such as “After I get up in the morning, I
will run 2 miles.” By putting goal- directed
behavior under the control of the situation
in this way, the execution of the goal is
removed from effortful and conscious con-
trol and rendered relatively automatic (Webb
& Sheeran, 2007). Recent research suggests
that implementation intentions can be used
in the service of emotion regulation (for a
review, see Webb, Schweiger Gallo, et al.,
2012). For example, Schweiger- Gallo, Keil,
McCulloch, Rockstroh, and Gollwitzer
(2009) showed spider phobics images that
included spiders. Participants were given
no instructions, were asked to form a goal
intention (“I will not get frightened!”), or
were asked to form an implementation inten-
tion (“If I see a spider, then I will remain
calm and relaxed!”). Afterward, when view-
ing spider pictures, participants who formed
implementation intentions reported less
negative affect and exhibited less physiologi-
cal arousal compared to both other groups.
This research further supports the idea that
emotion regulation can take place without
conscious awareness.
It appears, then, that the nonconscious
pursuit of emotion goals may be just as
effective as the conscious pursuit of emotion
goals. Unlike conscious goal pursuit, how-
ever, nonconscious goal pursuit “eats up”
less cognitive resources and is less effortful
(Custers & Aarts, 2010; Fishbach, Fried-
man, & Kruglanski, 2003). Automatic emo-
tion regulation, therefore, could help people
cope with powerful negative situations with-
out conscious effort. Given how impor-
tant emotion regulation is to psychological
health (Rottenberg & Johnson, 2007), the
intriguing possibility arises that the auto-
matic pursuit of emotion goals might play
a beneficial role in psychological health. A
recent study tested whether this might be
the case (DeWall et al., 2011). It showed that
after social exclusion, participants low in
depressive symptoms or high in self- esteem
automatically (without conscious intent)
initiated the up- regulation of positive emo-
tion. Similar findings have been obtained
for individuals with healthy traits such as
high action orientation or secure attach-
ment (for review, see Koole & Rothermund,
2011). These findings suggest that automatic
emotion regulation may be part of the psy-
chological immune system: in healthy indi-
viduals a threat sets in motion an automatic
emotion regulation process that leads to
increased positive and decreased negative
emotions.
This does not imply that automatic emo-
tion regulation is always associated with
beneficial outcomes. After all, defensiveness
and repression are based on implicitly rep-
resented goals, yet they are associated with
negative outcomes (Freud, 1936; Vaillant,
1977; Weinberger, 1995). The goal frame-
work may help explain when automatic emo-
tion regulation is beneficial versus harmful.
As we discuss in the next section, automatic

368 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
emotion regulation may be beneficial to the
extent that people (1) use effective regulation
strategies and (2) pursue adaptive emotion
goals (Hopp, Troy, & Mauss, 2011).
Implications for Well-Being
The goal framework leads to several novel
predictions regarding when and why emo-
tion regulation is associated with healthy
versus unhealthy outcomes. Next, we exam-
ine two particularly important sets of pre-
dictions and empirical evidence to support
them.
Emotion Goals and Emotion
Regulation Strategies
People can use a multitude of emotion regu-
lation strategies to attain emotion goals. For
example, to feel less angry, Person A might
think of something else whereas Person B
may vent. The study of emotion regulation
strategies and their relative adaptiveness
(i.e., to what extent different types of emo-
tion regulation strategies are associated with
greater well-being) has paid relatively little
attention to the emotion goals people pur-
sue. We argue that it is fruitful to examine
emotion regulation strategies in the context
of emotion goals. The goal framework offers
three specific hypotheses. First, the adaptive-
ness of emotion regulation strategies should
depend on the extent to which they help a
person achieve their emotion goals (i.e., to
the extent that they are effective). Second,
the adaptiveness of emotion regulation strat-
egies should depend on not only their inher-
ent features but also the extent to which they
are used in a goal- sensitive, flexible manner.
Third, the adaptiveness of emotion regula-
tion strategies should depend on the extent
to which they are used in the service of adap-
tive emotion goals.
Much research has focused on classify-
ing the various emotion regulation strategies
that exist (Gross & Thompson, 2007; Koole,
2009; Totterdell & Parkinson, 1999). One
of the most prominent models distinguishes
different emotion regulation strategies based
on the stage in the emotion process in which
they intervene (Gross & Thompson, 2007).
According to this model, emotion regulation
strategies can be characterized depending
on whether they target the emotional situ-
ation, a person’s attention to it, or appraisal
of it (antecedent- focused) versus a later
component of the emotional response, such
as emotion- expressive behaviors (response-
focused).
Which emotion regulation strategies are
most effective for attaining emotion goals
(e.g., decrease anger)? Antecedent- focused
emotion regulation strategies should be rela-
tively more effective at altering emotional
responses because they have the advantage
of a preventive strategy: they take place
before the emotional response fully unfolds
and thus should be more effective than
response- focused emotion regulation strate-
gies. A recent meta- analysis of 190 studies
is broadly consistent with this hypothesis
(Webb, Miles, et al., 2012). Are the most
effective emotion regulation strategies also
the most adaptive? Research comparing
antecedent- focused emotion regulation (i.e.,
cognitive appraisal) to response- focused
emotion regulation (i.e., expressive suppres-
sion) suggests that, indeed, on average reap-
praisal is associated with better psychologi-
cal health (Garnefski & Kraaij, 2006; Gross
& John, 2003; Troy, Wilhelm, Shallcross, &
Mauss, 2010). Thus, there is some evidence
that some of the most effective emotion reg-
ulation strategies are also relatively adaptive.
Importantly, from a goal perspective, the
adaptiveness of an emotion regulation strat-
egy should be determined by its inherent
properties, but also by how flexibly it is used
to support an individual’s changing emotion
goals (Bonanno & Burton, in press; Brandt-
staedter & Rothermund, 2002; Cheng,
2001; Kashdan & Rottenberg, 2010). Find-
ings in support of this notion have been
obtained from daily diary studies in which
participants reported on stressful life events
and how many different coping strategies
they used (Cheng, 2001). Flexibility was
operationalized as participants’ ability to
vary coping strategies with the demand of
the stressful event. Participants demonstrat-
ing greater flexibility exhibited greater well-
being compared to participants who adhered
more rigidly to particular coping strategies,
regardless of the particular type of coping
strategy.
Bonanno, Papa, Lalande, Westphal, and
Coifman (2004) tested a related idea by
deriving a laboratory measure of how well
Emotion Goals 369
participants were able to match their emo-
tion regulation efforts to changing goals
(either increase or decrease emotional
expression). Participants who were better
able to regulate their emotions in pursuit
of their concurrent goals reported greater
psychological health after the September
11, 2001 attacks (Bonanno et al., 2004)
and greater well-being after high life stress
(Westphal, Seivert, & Bonanno, 2010).
From a goal framework, emotion regula-
tion strategies are therefore adaptive to the
extent that they help people attain their
concurrent emotion goals. However, the
adaptiveness of emotion regulation more
generally depends on which emotion goals
people are trying to achieve. If people hold
maladaptive emotion goals, even the most
effective and flexible emotion regulation
strategies should not be adaptive. Which
emotion goals are adaptive? Perhaps those
that are sensitive to situational demands
and are consistent with superordinate goals
and basic needs (Deci & Ryan, 1985; Troy,
Shallcross, & Mauss, in press) (e.g., increase
joy in the service of successfully collaborat-
ing with others, and increase anger in the
service of successfully confronting others).
Consistent with these ideas, we found that
the more people wanted to feel angry and
the less they wanted to feel happy in con-
frontational situations, the higher their
psychological well-being. The converse pat-
tern was found the more angry and the less
happy people wanted to feel in collaborative
situations (Tamir & Ford, 2012a).
In summary, the goal framework can help
us understand when and why emotion regu-
lation strategies are adaptive or maladaptive.
Emotion regulation strategies are adaptive to
the extent that they help individuals attain
their emotion goals, are used in a goal-
sensitive and flexible manner, and are used
in the context of adaptive emotion goals.
Feedback Processing: Emotion Goals,
Evaluation, and Meta‑Emotion
Emotion regulation does not operate in a
linear, one- directional fashion. Rather, as
Figure 22.1 illustrates, it involves recursive
feedback loops. Feedback models of self-
regulation (Carver & Scheier, 2000) propose
that people monitor the discrepancy between
their current state and the desired end state,
as well as the progress of their efforts to
decrease discrepancies between the two.
Importantly, the output of this monitoring
process is emotional in nature. When people
progress faster than expected toward their
goal, they feel positively (e.g., contentment);
when people progress more slowly than
expected they feel negatively (e.g., distress).
Because these emotional states are about an
emotional state, they can be referred to as
meta- emotion. Considering emotion regu-
lation in the context of this feedback loop
leads to two interesting predictions. First,
decreasing the discrepancy between current
and desired state should yield better well-
being, whether the discrepancy reduction
occurs by changing one’s current emotional
state or by changing one’s desired emotional
state. Conversely, an increased discrepancy
between current and desired state should
yield negative outcomes, whether it is due to
one’s current or desired emotional state. Sec-
ond, meta- emotion may play an important
role in the effects of emotion regulation on
well-being.
The hypothesis that adjusting one’s goals
(in addition to or instead of one’s current
state) plays an important role in well-being
has been supported in the context of cop-
ing with stressors (e.g., Brandtstaedter &
Rothermund, 2002; Wrosch, Scheier, Miller,
Schulz, & Carver, 2003). This research sug-
gests that goal adjustment (e.g., failing to
disengage from impossible goals) is at least
as important to well-being as the effective
pursuit of goals. This principle also appears
to apply to emotion goals. In one experi-
mental study, researchers manipulated par-
ticipants’ emotion goals by instructing them
to make themselves feel as happy as possible
while they listened to emotionally ambigu-
ous music (i.e., their desired emotional end
state was set to a highly positive state). In line
with the idea that unrealistic emotion goals
can lead to negative emotional outcomes,
participants in this condition were less
happy compared to participants who were
not given an emotion goal (Schooler, Ariely,
& Loewenstein, 2003). In another study,
researchers manipulated emotion goals more
subtly by presenting to participants a sham
newspaper article discussing the advantages
of happiness. Participants induced in this
way to assume a happiness goal were less
happy than control participants after subse-

370 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
quently watching a happy film clip (Mauss,
Tamir, Anderson, & Savino, 2011, Study
2). This research converges to support the
hypothesis that holding unrealistic emotion
goals (e.g., high levels of happiness) can lead
to decreased positive emotion.
Do people who chronically hold unreal-
istic emotion goals experience more nega-
tive well-being outcomes? We examined this
question by measuring the extent to which
participants held the goal to be happy, with
items such as “Feeling happy is extremely
important to me.” On average, the more par-
ticipants valued happiness, the lower their
emotional well-being (Mauss et al., 2011,
Study 1), and the higher their likelihood of
being diagnosed with major depressive dis-
order (Ford, Shallcross, Mauss, Floerke, &
Gruber, under review). These studies make
the point that one’s emotion goals play an
important role in well-being and psychologi-
cal health.
The perspective that adjusting one’s emo-
tion goals is one important avenue to well-
being brings insight to a puzzling area of
research: that of emotional acceptance.
Acceptance is defined as the process of non-
judgmentally engaging with negative emo-
tions (Teasdale et al., 2000). Correlational
and experimental research on acceptance
has consistently found it to be negatively
correlated with negative emotion and mood
disorder (Campbell- Sills, Barlow, Brown, &
Hofmann, 2006; Kashdan, Barrios, Forsyth,
& Steger, 2006; Roemer, Salters, Raffa,
& Orsillo, 2005; Shallcross, Troy, Boland,
& Mauss, 2010). The inverse relationship
between acceptance and negative affect may
appear paradoxical at first: How can engag-
ing with negative emotions be associated
with less negative emotion? The goal per-
spective suggests one solution to this appar-
ent paradox: Acceptance may involve more
realistic emotion goals, which in turn lead
to greater well-being. Well-being, therefore,
is a function of not only effective emotion
regulation but also having attainable emo-
tion goals. Or, in the words of the adage,
“Happiness is not having what you want,
but wanting what you have.”
As there are with nonemotion goals, there
are likely costs for inflexibly pursuing emo-
tion goals that are difficult to attain. How-
ever, unlike nonemotion goals, because it
leads to meta- emotion, the pursuit of unat-
tainable emotion goals can be self- defeating.
For instance, in the study discussed earlier
(Mauss et al., 2011, Study 2), compared to
participants in the control condition, par-
ticipants who were led to pursue happiness
goals ended up feeling more disappointed in
their emotional state, which resulted in less
happiness. Interestingly, these effects were
only observed for participants who watched
a happy film clip, not for those who watched
a sad film clip. This may be because in rela-
tively negative situations (e.g., when watch-
ing a sad film clip), people have a good rea-
son not to feel happy, and are less likely to
feel disappointed if they fail to meet their
happiness goal. Conversely, in relatively
positive situations (e.g., when watching a
happy film clip), people have every reason
to feel happy and ironically end up feeling
disappointed when they do not. Whereas
the pursuit of nonemotion goals influences
behavior and results in emotions, the pur-
suit of emotion goals influences emotions
and results in emotions. Thus, when pursu-
ing emotion goals, meta- emotional experi-
ences can interfere with successful goal pur-
suit.
In summary, the goal framework high-
lights the fact that people’s well-being is
determined by not only how they pursue
emotion goals but the goals themselves. Set-
ting exceedingly positive emotion goals or
failing to adjust emotion goals can, ironi-
cally, lead to less positive emotion and to
lower well-being. These effects are due in
part to people’s meta- emotion (how they feel
about their feelings).
Conclusions and Future Directions
In this chapter, we argue that it is crucial
to consider emotion regulation in the con-
text of the emotions people want to feel.
Understanding the content of emotion goals
helps us better understand the initiation and
course of emotion regulation; understand-
ing the structure of emotion goals helps us
better understand how emotion and other
goals interact with one another in goal hier-
archies; and understanding the operation of
emotion goals helps us better understand the
emotion regulation process.
Taking a goal perspective can offer
answers to questions such as the following:

Emotion Goals 371
When do people regulate their emotions?
How do multiple, potentially conflicting
goals interact with one another? What role
do automatic processes play in the pursuit
of emotion goals? How do emotion goals
and pursuit of them affect well-being? In
addition to guiding us to approach these
novel questions, the proposed goal frame-
work helps us critically examine some core
assumptions in research on emotion regula-
tion. For example, one core assumption is
that people want to feel pleasant emotions
and avoid unpleasant emotions. However,
the goal framework challenges this assump-
tion (Tamir, 2009; Tamir & Ford, 2009).
Positioning emotion goals in a broader goal
hierarchy leads us to predict that individuals
will seek more unpleasant emotions in pur-
suit of superordinate nonaffect goals.
Another core assumption in research on
emotion regulation is that there is something
inherently beneficial or harmful about par-
ticular emotion regulation strategies. The
goal framework suggests that emotion regu-
lation and its implications for well-being can
be fully understood only in the context of
a person’s broader goal hierarchy (Bonanno
& Burton, in press; Thompson, 2011; Troy
et al., in press).
While some research has already adopted
a goal framework in emotion regulation,
more work needs to be done on each of the
three domains of emotion goals on which
we have focused (i.e., their content, struc-
ture, and operation). In terms of the content
of emotion goals, it will be important to
develop a systematic approach to measur-
ing these goals, whether they are transient
or more chronically held. More work is
necessary especially to measure implicitly
represented emotion goals (those not read-
ily accessible to introspection). In addition,
important open questions remain about
what biological, psychological, and cultural
factors shape people’s emotion goals.
In terms of the structure of emotion goals,
it will be important to obtain a systematic
and comprehensive understanding of how
emotion and nonaffect goals interact with
one another, and to identify the implica-
tions of different types of goal conflict. For
example, how malleable are the associations
between subordinate and superordinate
emotion and nonaffect goals? What are the
short- and long-term implications of conflict
among goals? Of particular interest here
might be conflict among explicit (relatively
conscious) and implicit (relatively uncon-
scious) emotion goals. Understanding con-
flict among explicit and implicit goals has
been fruitful in domains such as achieve-
ment motives (Brunstein, Schultheiss, &
Grässman, 1998); it would be promising in
the domain of emotion goals as well.
In terms of the operation of emotion
goals, there is still much to learn about their
nonconscious representation and automatic
pursuit. For instance, what gives rise to indi-
vidual differences in automatic emotion reg-
ulation? What are its costs and benefits? In
addition, the goal framework makes several
predictions about emotion goals’ implica-
tions for well-being. For example, it implies
that healthy functioning hinges on the selec-
tion of appropriate emotion goals, interac-
tions among emotion regulation strategies
and emotion goals, and the meta- emotions
that arise as a function of emotion goal pur-
suit. These features and implications of emo-
tion goals have yet to be fully explored.
Note
1. Our review is selective. We do not cover con-
cepts such as emotional effects of goals and
goal pursuit (e.g., feelings of disappoint-
ment when not attaining a nonemotion goal,
positive evaluation of goals) or goals that are
infused with a lot of emotion (e.g., a goal
about which one feels passionate). While these
phenomena are important, they are distinct
from the focus of this chapter.
References
Aarts, H., & Dijksterhuis, A. (2000). Habits as
knowledge structures: Automaticity in goal-
directed behavior. Journal of Personality and
Social Psychology, 78, 53–63.
Bandura, A. (1986). Social foundations of
thought and action: A social cognitive theory.
Upper Saddle River, NJ: Prentice Hall.
Bargh, J. A., Gollwitzer, P. M., Lee-Chai, A.,
Barndollar, K., & Trötschel, R. (2001). The
automated will: Nonconscious activation and
pursuit of behavioral goals. Journal of Person-
ality and Social Psychology, 81, 1014–1027.
Baumeister, R. F., & Heatherton, T. F. (1996).
372 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Self- regulation failure: An overview. Psycho-
logical Inquiry, 7, 1–15.
Bonanno, G. A. (2001). Emotion self- regulation.
In T. J. Mayne & G. A. Bonanno (Eds.), Emo-
tions: Current issues and future directions
(pp. 251–285). New York: Guilford Press.
Bonanno, G. A., & Burton, C. (in press). Regu-
latory flexibility: An individual differences
perspective on coping and emotion regulation.
Perspectives on Psychological Science.
Bonanno, G. A., Papa, A., Lalande, K., West-
phal, M., & Coifman, K. (2004). The impor-
tance of being flexible: The ability to both
enhance and suppress emotional expression
predicts long term adjustment. Psychological
Science, 15, 482–487.
Brandtstaedter, J., & Rothermund, K. (2002).
The life- course dynamics of goal pursuit and
goal adjustment: A two- process framework.
Developmental Review, 22, 117–150.
Brunstein, J. C., Schultheiss, O. C., & Gräss-
man, R. (1998). Personal goals and emotional
well-being: The moderating role of motive dis-
positions. Journal of Personality and Social
Psychology, 75, 494–508.
Campbell- Sills, L., Barlow, D. H., Brown, T. A.,
& Hofmann, S. G. (2006). Effects of suppres-
sion and acceptance on emotional responses
of individuals with anxiety and mood disor-
ders. Behaviour Research and Therapy, 44,
1251–1263.
Carstensen, L. L., Isaacowitz, D. M., & Charles,
S. T. (1999). Taking time seriously: A theory
of socioemotional selectivity. American Psy-
chologist, 54, 165–181.
Carver, C. S., & Scheier, M. F. (2000). On the
structure of behavioral self- regulation. In M.
Boekaerts, P. R. Pintrich, & M. Zeidner (Eds.),
Handbook of self-regulation (pp. 41–84). San
Diego, CA: Academic Press.
Charles, S. T., & Carstensen, L. L. (this volume,
2014). Emotion regulation and aging. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 203–218). New York: Guilford
Press.
Cheng, C. (2001). Assessing coping flexibility
in real-life and laboratory settings: A multi-
method approach. Journal of Personality and
Social Psychology, 80, 814–833.
Clore, G. L., Gasper, K., & Garvin, E. (2001).
Affect as information. In J. P. Forgas (Ed.),
Handbook of affect and social cognition
(pp. 121–144). Mahwah, NJ: Erlbaum.
Custers, R., & Aarts, H. (2005). Positive affect
as implicit motivator: On the nonconscious
operation of behavioral goals. Journal of
Personality and Social Psychology, 89, 129–
142.
Custers, R., & Aarts, H. (2010). The uncon-
scious will: How the pursuit of goals operates
outside of conscious awareness. Science, 329,
47–50.
Deci, E. L., & Ryan, R. M. (1985). Intrinsic
motivation and self- determination in human
behavior. New York: Plenum.
DeWall, C. N., Twenge, J. M., Koole, S. L., Bau-
meister, R. F., Marquez, A., & Reid, M. W.
(2011). Automatic emotion regulation after
social exclusion: Tuning to positivity. Emo-
tion, 11, 623–636.
Diener, E. (2000). Subjective well-being: The sci-
ence of happiness and a proposal for a national
index. American Psychologist, 55, 34–43.
Eid, M., & Diener, E. (2001). Norms for expe-
riencing emotions in different cultures: Inter-
and intranational differences. Journal of Per-
sonality and Social Psychology, 81, 869–885.
Fishbach, A., & Ferguson, M. F. (2007). The
goal construct in social psychology. In A. W.
Kruglanski & E. T. Higgins (Eds.), Social
psychology: Handbook of basic principles
(pp. 490–515). New York: Guilford Press.
Fishbach, A., Friedman, R. S., & Kruglanski, A.
W. (2003). Leading us not unto temptation:
Momentary allurements elicit overriding goal
activation. Journal of Personality and Social
Psychology, 84, 296–309.
Fitzsimons, G. M., & Bargh, J. A. (2004). Auto-
matic self- regulation. In R. F. Baumeister & K.
D. Vohs (Eds.), Handbook of self- regulation:
Research, theory, and applications (pp. 151–
170). New York: Guilford Press.
Ford, B. Q., Shallcross, A. J., Mauss, I. B.,
Floerke, V. A., & Gruber, J. (under review).
Desperately seeking happiness: Valuing hap-
piness is associated with symptoms and diag-
nosis of depression.
Fredrickson, B. L. (2001). The role of positive
emotions in positive psychology: The broaden-
and-build theory of positive emotions. Ameri-
can Psychologist, 56, 218–226.
Freud, A. (1936). The ego and the mechanisms of
defense. New York: International Universities
Press.
Frijda, N. H. (1986). The emotions. New York:
Cambridge University Press.
Garnefski, N., & Kraaij, V. (2006). Relationships
between cognitive emotion regulation strate-
Emotion Goals 373
gies and depressive symptoms: A comparative
study of five specific samples. Personality and
Individual Differences, 40, 1659–1669.
Gollwitzer, P. M. (1990). Action phases and
mind-sets. In E. T. Higgins & R. M. Sorren-
tino (Eds.), Handbook of motivation and cog-
nition: Foundations of social behavior (Vol. 2,
pp. 53–92). New York: Guilford Press.
Gollwitzer, P. M. (1999). Implementation inten-
tions: Strong effects of simple plans. American
Psychologist, 54, 493–503.
Greenwald, A. G., McGhee, D. E., & Schwartz,
J. L. K. (1998). Measuring individual differ-
ences in implicit cognition: The Implicit Asso-
ciation Test. Journal of Personality and Social
Psychology, 74, 1464–1480.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 3–24). New York: Guilford Press.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Gyurak, A., Gross, J. J., & Etkin, A. (2011).
Explicit and implicit emotion regulation: A
dual- process framework. Cognition and Emo-
tion, 25, 400– 412.
Harmon-Jones, E., Harmon-Jones, C., Amo-
dio, D. M., & Gable, P. A. (2011). Attitudes
toward emotions. Journal of Personality and
Social Psychology, 101, 1332–1350.
Hopp, H., Troy, A. S., & Mauss, I. B. (2011). The
unconscious pursuit of emotion regulation:
Implications for psychological health. Cogni-
tion and Emotion, 25, 532–545.
Kashdan, T. B., Barrios, V., Forsyth, J. P., & Ste-
ger, M. F. (2006). Experiential avoidance as a
generalized psychological vulnerability: Com-
parisons with coping and emotion regulation
strategies. Behaviour Research and Therapy,
44, 1301–1320.
Kashdan, T. B., & Rottenberg, J. (2010). Psy-
chological flexibility as a fundamental aspect
of health. Clinical Psychology Review, 30,
865–878.
Keltner, D., & Haidt, J. (1999). Social functions
of emotions at four levels of analysis. Cogni-
tion and Emotion, 13, 505–521.
Kitayama, S., Mesquita, B., & Karasawa, M.
(2006). Cultural affordances and emotional
experience: Socially engaging and disengag-
ing emotions in Japan and the United States.
Journal of Personality and Social Psychology,
91, 890–903.
Koole, S. L. (2009). The psychology of emotion
regulation: An integrative review. Cognition
and Emotion, 23, 4– 41.
Koole, S. L., & Rothermund, K. (2011). “I feel
better but I don’t know why”: The psychology
of implicit emotion regulation. Cognition and
Emotion, 25, 389–399.
Koole, S. L., van Dillen, L. F., & Sheppes, G.
(2011). The self- regulation of emotion. In K.
D. Vohs & R. F. Baumeister (Eds.), Hand-
book of self- regulation: Research, theory, and
applications (2nd ed., pp. 22–40). New York:
Guilford Press.
Kruglanski, A. W., Shah, J. Y., Fishbach, A.,
Friedman, R., Chun, W. Y., & Sleeth- Keppler,
D. (2002). A theory of goal- systems. In M. P.
Zanna (Ed.), Advances in experimental psy-
chology (pp. 331–378). New York: Academic
Press.
Mauss, I. B., Bunge, S. A., & Gross, J. J. (2007).
Automatic emotion regulation. Social and Per-
sonality Psychology Compass, 1, 146 –167.
Mauss, I. B., Cook, C. L., & Gross, J. J. (2007).
Automatic emotion regulation during anger
provocation. Journal of Experimental Social
Psychology, 43, 698–711.
Mauss, I. B., Evers, C., Wilhelm, F. H., & Gross,
J. J. (2006). How to bite your tongue without
blowing your top: Implicit evaluation of emo-
tion regulation predicts affective responding
to anger provocation. Personality and Social
Psychology Bulletin, 32, 589–602.
Mauss, I. B., Tamir, M., Anderson, C. L., & Sav-
ino, N. S. (2011). Can seeking happiness make
people unhappy?: Paradoxical effects of valu-
ing happiness. Emotion, 11, 807–815.
Mischel, W., Cantor, N., & Feldman, S. (1996).
Principles of self- regulation: The nature of
willpower and self- control. In E. T. Higgins
& A. W. Kruglanski (Eds.), Social psychology:
Handbook of basic principles (pp. 329–360).
New York: Guilford Press.
374 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
Nesse, R. M., & Ellsworth, P. C. (2009). Evo-
lution, emotions, and emotional disorders.
American Psychologist, 129–139.
Parrott, W. G. (1993). Beyond hedonism: Motives
for inhibiting good moods and for maintain-
ing bad moods. In D. M. Wegner & J. W. Pen-
nebaker (Eds.), Handbook of mental control
(pp. 278–305). Upper Saddle River, NJ: Pren-
tice Hall.
Riediger, M., Schmiedek, F., Wagner, G. G., &
Lindenberger, U. (2009). Seeking pleasure and
seeking pain: Differences in prohedonic and
contra- hedonic motivation from adolescence
to old age. Psychological Science, 20, 1529–
1535.
Roemer, L., Salters, K., Raffa, S. D., & Orsillo,
S. M. (2005). Fear and avoidance of internal
experiences in GAD: Preliminary tests of a
conceptual model. Cognitive Therapy and
Research, 29, 71–88.
Rottenberg, J., & Johnson, S. L. (2007). Emo-
tion and psychopathology: Bridging affective
and clinical science. Washington, DC: Ameri-
can Psychological Association.
Rusting, C. L., & Larsen, R. J. (1995). Moods as
sources of stimulation: Relationships between
personality and desired mood states. Personal-
ity and Individual Differences, 18, 321–329.
Schooler, J. W., Ariely, D., & Loewenstein, G.
(2003). The pursuit and monitoring of hap-
piness can be self- defeating. In J. Carrillo &
I. Brocas (Eds.), Psychology and economics
(pp. 41–70). Oxford, UK: Oxford University
Press.
Schweiger- Gallo, I. S., Keil, A., McCulloch, K.
C., Rockstroh, B., & Gollwitzer, P. M. (2009).
Strategic automation of emotion regulation.
Journal of Personality and Social Psychology,
96, 11–31.
Shallcross, A. J., Troy, A. S., Boland, M., &
Mauss, I. B. (2010). Let it be: Accepting nega-
tive emotional experiences predicts decreased
negative affect and depressive symptoms.
Behaviour Research and Therapy, 48, 921–
929.
Srull, T. K., & Wyer, R. S. (1979). Role of cat-
egory accessibility in the interpretation of
information about persons— Some determi-
nants and implications. Journal of Personality
and Social Psychology, 37, 1660–1672.
Swann, W. B., & Schroeder, D. G. (1995). The
search for beauty and truth—A framework for
understanding reactions to evaluations. Per-
sonality and Social Psychology Bulletin, 21,
1307–1318.
Tamir, M. (2009). What do people want to feel
and why?: Pleasure and utility in emotion reg-
ulation. Current Directions in Psychological
Science, 18, 101–105.
Tamir, M., & Bigman, Y. (in press). When do peo-
ple want to feel bad?: A taxonomy of motives.
In W. G. Parrott (Ed.), The positive side of
negative emotions. New York: Guilford Press.
Tamir, M., Bigman, Y., Rhodes, E., Salerno, J.,
& Schreier, J. (2013). Utilitarian emotions:
Expected usefulness of emotions shapes emo-
tion regulation, experience, and decision
making. Manuscript under review.
Tamir, M., & Ford, B. Q. (2009). Choosing to
be afraid: Preferences for fear as a function of
goal pursuit. Emotion, 9, 488 – 497.
Tamir, M., & Ford, B. Q. (2012a). Should people
pursue feelings that feel good or feelings that
do good?: Emotional preferences and well-
being. Emotion, 12, 1061–1070.
Tamir, M., & Ford, B. Q. (2012b). When feeling
bad is expected to be good: Emotion regula-
tion and outcome expectancies in social con-
flicts. Emotion, 12, 807–816.
Tamir, M., Ford, B. Q., & Ryan, E. (2013). Non-
conscious goals can shape what people want to
feel. Journal of Experimental Social Psychol-
ogy, 49, 292–297.
Tamir, M., Mitchell, C., & Gross, J. J. (2008).
Hedonic and instrumental motives in anger
regulation. Psychological Science, 19, 324–328.
Teasdale, J. D., Segal, Z. V., Williams, J. M. G.,
Ridgeway, V. A., Soulsby, J. M., & Lau, M.
A. (2000). Prevention of relapse/recurrence in
major depression by mindfulness- based cogni-
tive therapy. Journal of Consulting and Clini-
cal Psychology, 68, 615–623.
Thompson, R. A. (2011). Emotion and emotion
regulation: Two sides of the developing coin.
Emotion Review, 3, 53–61.
Tomaka, J., Blascovich, J., Kelsey, R. M., & Leit-
ten, C. L. (1993). Subjective, physiological,
and behavioral effects of threat and challenge
appraisal. Journal of Personality and Social
Psychology, 65, 248–260.
Totterdell, P., & Parkinson, B. (1999). Use and
effectiveness of self- regulation strategies for
improving mood in a group of trainee teach-
ers. Journal of Occupational Health Psychol-
ogy, 4, 219–232.
Troy, A. S., Shallcross, A. J., & Mauss, I. B.
(in press). A person-by-situation approach to
emotion regulation: Cognitive reappraisal can
either hurt or help depending on the context.
Psychological Science.
Emotion Goals 375
Troy, A. S., Wilhelm, F. H., Shallcross, A. J., &
Mauss, I. B. (2010). Seeing the silver lining:
Cognitive reappraisal ability moderates the
relationship between stress and depression.
Emotion, 10, 783–795.
Tsai, J. L., Knutson, B., & Fung, H. H. (2006).
Cultural variation in affect valuation. Jour-
nal of Personality and Social Psychology, 90,
288–307.
Vaillant, G. (1977). Adaptation to life. Boston:
Little, Brown.
Webb, T. L., Miles, E., & Sheeran, P. (2012).
Dealing with feeling: A meta- analysis of the
effectiveness of strategies derived from the
process model of emotion regulation. Psycho-
logical Bulletin, 138, 775–808.
Webb, T. L., Schweiger Gallo, I., Miles, E., Goll-
witzer, P. M., & Sheeran, P. (2012). Effective
regulation of affect: An action control perspec-
tive on emotion regulation. European Review
of Social Psychology, 23, 143–186.
Webb, T. L., & Sheeran, P. (2007). How do
implementation intentions promote goal
attainment?: A test of component processes.
Journal of Experimental Social Psychology,
43, 295–302.
Weinberger, D. A. (1995). The construct validity
of the repressive coping style. In J. L. Singer
(Ed.), Repression and dissociation: Implica-
tions for personality theory, psychopathology,
and health (pp. 337–386). Chicago: University
of Chicago Press.
Westphal, M., Seivert, N. H., & Bonanno, G.
A. (2010). Expressive flexibility. Emotion, 10,
92–100.
Williams, L. E., Bargh, J. A., Nocera, C. C., &
Gray, J. R. (2009). The unconscious regulation
of emotion: Nonconscious reappraisal goals
modulate emotional reactivity. Emotion, 9,
847–854.
Wood, J. V., Heimpel, S. A., Manwell, L. A., &
Whittington, E. J. (2009). This mood is familiar
and I don’t deserve to feel better anyway: Mech-
anisms underlying self- esteem differences in
motivation to repair sad moods. Journal of Per-
sonality and Social Psychology, 96, 363–380.
Wrosch, C., Scheier, M. F., Miller, G. E., Schulz,
R., & Carver, C. S. (2003). Adaptive self-
regulation of unattainable goals: Goal disen-
gagement, goal reengagement, and subjective
well-being. Personality and Social Psychology
Bulletin, 29, 1494–1508.

376
The evolution of human self- awareness con-
stituted a seismic shift in mammalian psy-
chology. Although some of our prehuman
ancestors may have possessed rudimentary
forms of self- awareness, much like modern
chimpanzees and orangutans, evidence sug-
gests that prehistoric people did not begin
to think consciously about themselves until
around 2 million years ago. Moreover, the
archeological record indicates that people
did not have the capacity to think about
themselves in the abstract and symbolic ways
that characterize modern human beings until
culture began to appear between 40,000
and 60,000 years ago (Leary & Buttermore,
2003). The evolution of self- awareness had
important implications for human beings’
emotional lives, because human emotions
often arise from the ways in which people
think about themselves and the events that
happen to them. Self- awareness and self-
relevant thought have implications for the
stimuli that trigger emotional states, the
specific emotions that people experience,
and people’s efforts to manage and regulate
their emotions.
Evidence suggests that most animals
lack the ability to think consciously about
themselves. Yet animals nonetheless experi-
ence a variety of emotions (Veissier, Boissy,
Désiré, & Greiveldinger, 2009), and people
sometimes experience emotions even when
they are not self-aware. Thus, conscious self-
awareness is not necessary for emotional
experience. The emotional reactions of ani-
mals that lack self- awareness and those of
human beings when they are not self-aware
are controlled by two primary processes that
occur without conscious awareness. First,
many stimuli naturally and automatically
evoke emotions. For example, most spe-
cies instinctively react to certain threat cues
without conscious thought, such as when
the silhouette of a hawk in flight elicits fear
in many birds, or when human beings react
with fear to an object that approaches them
suddenly. Other emotions, such as anger,
sadness, and joy, appear to be similarly
hard-wired.
Second, animals may learn to experience
emotions in response to previously neutral
stimuli through classical conditioning. For
example, human and nonhuman animals
may come to respond with negative emotions
to otherwise neutral stimuli that have been
repeatedly paired with aversive stimuli, or to
respond positively to a neutral stimulus that
has been associated with rewarding events.
Indeed, clinical psychologists have used
conditioning techniques such as systematic
desensitization and counterconditioning to
change people’s emotional responses and
CHAPTER 23
Self‑Awareness and Self‑Relevant Thought
in the Experience and Regulation of Emotion
Mark R. Leary
Dina Gohar

Self‑ Awareness and Self‑ Relevant Thought in Emotion 377
improve their emotion regulation in the
treatment of anxiety problems such as pho-
bias, panic attacks, and posttraumatic stress
disorder (Foa, Huppert, & Cahill, 2006).
Although emotions can occur without
self- awareness, people’s capacity for self-
awareness and self- relevant thought renders
their emotional lives far more complex than
those of animals that lack self- awareness. In
this chapter, we discuss four basic ways in
which self- awareness affects emotional expe-
rience and examine how people intentionally
capitalize on these processes to manage their
emotions. Specifically, due to their ability to
think consciously about themselves, people’s
emotions are affected when they (1) com-
pare themselves to their personal standards,
(2) think about themselves in the past and
future, (3) evaluate their personal charac-
teristics, and (4) think about how they are
perceived by other people. Each of these pro-
cesses requires the ability to self- reflect.
1
Comparisons to Personal Standards
Emotions often result when people assess the
implications of a particular situation, event,
or experience for their personal concerns
(Frijda, 1986). Generally, these assessments
involve a comparison of real or imagined
outcomes to some goal or standard. Decades
of research have shown that people’s goals
and standards influence their reactions to
events, particularly when they are thinking
consciously about them at the time, which
requires self- awareness.
Self‑Awareness
and Personal Standards
According to various theories of self-
awareness (Carver & Scheier, 1981; Duval
& Wicklund, 1972), being self-aware
induces an evaluative process in which peo-
ple compare themselves to whatever stan-
dards are salient at that particular moment.
For example, becoming self-aware while
taking a test may lead people to evaluate
themselves with regard to standards involv-
ing intellectual ability, whereas being self-
aware while trying on new clothes may lead
people to compare themselves to standards
of physical attractiveness. Self- awareness is
not necessary for goal pursuit (Bargh, Goll-
witzer, & Lee-Chai, 2001); nevertheless,
becoming self-aware seems to cause people
to compare themselves and their behaviors
to salient goals.
Becoming self-aware induces an evalua-
tive process that evokes positive emotions
when conditions meet or exceed one’s stan-
dards and negative emotions when they do
not. Because discrepancies between people’s
standards and their behavior are unpleasant,
people who are self-aware try to avoid vio-
lating their personal standards and reduce
such discrepancies when they occur (Duval
& Wicklund, 1972; Macrae, Bodenhausen,
& Milne, 1998; Scheier, Fenigstein, & Buss,
1974). In brief, many emotional experiences
involve a comparison of situations, events,
or experiences to goals and standards, and
self- awareness is intimately involved in peo-
ple’s emotional reactions to events that have
implications for their self- relevant goals.
According to self- discrepancy theory
(Higgins, 1987), the specific emotions that
people experience when they fail to achieve
their goals depend on the nature of the dis-
crepancy involved. When people perceive a
discrepancy between how they think they are
and how they would like to be (i.e., between
their actual and ideal selves), dejection-
related emotions such as disappointment,
sadness, and depression predominate. In
contrast, a discrepancy between how peo-
ple think they are and how they think they
ought to be (i.e., between actual and ought
selves) leads to agitation- related emotions
such as guilt, fear, and anxiety. Research
has generally supported the notion that self-
discrepancies influence emotion, but specific
discrepancies do not always predict distinct
emotions precisely as self- discrepancy theory
predicts (Phillips & Silvia, 2010; Tangney,
Niedenthal, Covert, & Barlow, 1998). For
example, Phillips and Silvia (2010) found
that although actual– ought discrepancies
uniquely predicted anxiety, both actual–
ideal and actual– ought discrepancies pre-
dicted depressed affect. When people think
that they are close to becoming their feared
selves, these actual– feared discrepancies pre-
dict agitation- related emotions better than
do actual– ought discrepancies (i.e., how
people think they ought to be), which more
strongly predict emotions when people think
they are far from assuming a feared identity
(Carver, Lawrence, & Scheier, 1999).
378 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
In any case, discrepancies between how
people think they are and how they want
(and do not want) to be evoke negative
emotions and lower subjective well-being
(Brown & McConnell, 2011; Philips, Silvia,
& Paradise, 2007; Tangney et al., 1998).
When people make internal attributions
for these discrepancies, they are especially
likely to experience strong negative emo-
tions (Petrocelli & Smith, 2005). For exam-
ple, attributing a negative outcome to one’s
own bad decisions or incompetent behav-
ior would likely result in different emo-
tions than attributing the same outcome
to another person’s malevolent actions.
Only when people believe that they played
some role in undesired outcomes do self-
discrepancies come into play. In addition,
increased self- awareness (e.g., induced by
seeing one’s reflection in a mirror; Phillips
& Silvia, 2005) and dispositional private
self- consciousness (Fromson, 2006) both
strengthen the relationship between self-
discrepancies and negative emotions. These
findings suggest that people can reduce the
emotional impact of self- relevant discrepan-
cies both by making external attributions
for them and by reducing self- awareness.
Minimizing Self‑Awareness
One implication of the link between self-
awareness and emotion is that people can
sometimes regulate their emotions by man-
aging their self- awareness. Self- awareness
prolongs and intensifies people’s emotional
states (Bryant & Veroff, 2007; Silvia, 2002).
People who have experienced undesired
events often find it difficult to stop thinking
about them and their self- relevant implica-
tions, even though doing so usually makes
them feel worse (Kross & Ayduk, 2008).
In fact, trying not to think about disturb-
ing events can increase the frequency of
such thoughts and lead to a mood that is the
opposite of the one intended (Wegner, Erber,
& Zanakos, 1993). Therefore, rather than
trying to stop thinking about themselves by
force of will, people sometimes try to reduce
their self- awareness more generally, thereby
diminishing any unpleasant feelings that are
sustained by self- thought. Evidence suggests
that people sometimes try to attenuate their
negative emotions by engaging in activities
that lower their self- awareness (Baumeister,
1991). Although some of these tactics can be
problematic (e.g., frequent substance abuse),
others (e.g., mindfulness and meditation
practices) can help people respond to nega-
tive events with greater equanimity.
One common way that people attenu-
ate self- awareness is through distraction.
People report that they watch television,
socialize, read, engage in hobbies, exercise,
shop, and distract themselves in other ways
to take their mind off of unpleasant events.
Research suggests that such distraction can,
in fact, reduce negative feelings (Joormann,
Siemer, & Gotlib, 2007; Kross & Ayduk,
2008). The effects of distraction on emo-
tion may be mediated by a number of pro-
cesses (e.g., many distracting activities are
inherently pleasurable), but the reduction of
self- thought is likely involved. Not only does
thinking about one’s experiences help to
maintain one’s emotional reactions to them,
but self- awareness also intensifies affective
states (Field, Joudy, & Hart, 2010; Scheier
& Carver, 1977). Therefore, anything that
lowers self- awareness should reduce emo-
tion.
People also try to escape self- awareness
and the aversive emotions that it can elicit in
ways that may be maladaptive, for example,
using alcohol and drugs, binge eating, and
even masochism (Baumeister, 1991). Not all
of the ways that people try to escape self-
awareness are maladaptive, however. For
example, people have used meditation to
reduce their internal self-talk since ancient
times. Although meditative practices are
often promoted within spiritual traditions,
their primary function is to reduce the
amount of time that the practitioner engages
in self- reflection (Leary, 2004). By taming
what Hindus call the “chattering monkey
mind,” people are less likely to respond emo-
tionally to self- related thoughts and more
likely to be relaxed and present- focused.
Indeed, clinical and counseling psycholo-
gists have successfully reduced problematic
emotions in their clients by helping them to
quiet their negative thoughts and to focus
on the present moment, with practices that
include features of mindfulness or medi-
tation (e.g., acceptance and commitment
therapy: Hayes, Strosahl, & Wilson, 1999;
dialectical behavior therapy: Linehan, 1993;
mindfulness- based stress reduction: Kabat-
Zinn, 2003).

Self- Awareness and Self- Relevant Thought in Emotion 379
Reflecting on the Past
and the Future
Unlike most other animals, people can reflect
consciously on themselves over time—that
is, to remember themselves in the past and
imagine themselves in the future. Through
the “eyes” of their cognitive analogue- I
(Jaynes, 1976; Leary, Estrada, & Allen,
2009), people are able not only to remember
or imagine themselves in other situations but
also to plan intentionally for the future, to
consider behavioral options, to imagine ret-
rospectively how events might have turned
out differently, and to learn from their mis-
takes. This ability to think consciously about
the past and future also leads people to react
emotionally to events that exist only in their
minds. Because remembered, anticipated,
and fantasized experiences of the analogue-
I often have the emotional potency of real
events, they can similarly impact people’s
emotions.
Reliving the Past and Imagining
the
Future
Reflecting about oneself in the past and
future has a strong influence on people’s
emotions. Indeed, we suspect that a far
greater proportion of people’s emotional
experiences arise from thinking about them-
selves in the past or future than from events
that are occurring at the present time, and
that people often compromise the positivity
of their current emotional states by unneces-
sarily reflecting on the past and future.
First, people often experience strong emo-
tions by reliving past experiences in their
minds. For example, ruminating about past
mistakes or misfortunes can elicit sadness,
regret, or guilt. Autobiographical memories
involving unfulfilled desires for competence,
autonomy, and relatedness seem to affect
negative emotions particularly strongly
(Philippe, Koestner, Lecours, Beaulieu-
Pelletier, & Bois, 2011). Conversely, recall-
ing positive memories from the past plays
an important role in maintaining positive
moods (Joormann et al., 2007).
Similarly, many emotions, both pleasant
and unpleasant, can be triggered by self-
relevant thoughts about the future. Of these,
anxiety is perhaps the most common. Of
course, anticipating future threats is often
adaptive, because it motivates people to take
steps to avoid problems or plan how to cope
effectively with future threats. However,
people typically imagine far more frequent
and more serious threats than actually occur.
And even when dreaded events do arise,
anticipatory anxiety typically does little
more than to cause suffering in advance. In
fact, many people experience chronic anxi-
ety because they consistently overestimate
the probability of negative events occurring
or the adverse consequences of those events
(Marshall et al., 2007; Miranda & Mennin,
2007). Moreover, believing that negative
events are likely to occur (and that positive
events are not likely to occur) can produce
hopelessness and depression (Joiner, 2001).
Thus, the human capacity to worry about
things that have not yet happened— and that
might, in fact, never even happen— takes a
heavy emotional toll (Roemer & Borkovec,
1993).
If anxiety about the future is so often
unnecessary, if not maladaptive, then why
are people so prone to experiencing anxiety?
Martin (1999) speculated that self- generated
anxiety about the future became prevalent
only with the emergence of agriculture
around 10,000 years ago. Although our
prehistoric ancestors were self-aware before
this time (Leary & Buttermore, 2003), their
hunting– gathering– scavenging lifestyle did
not evoke much rumination about the dis-
tant future. With the emergence of agricul-
ture, however, people moved to a delayed-
return lifestyle in which many important
outcomes— both good and bad—lay weeks
or months in the future. The agricultural
revolution led people to settle in commu-
nities, accumulate possessions, and plan
for planting and harvesting of crops, and,
thus, to fret about future outcomes that lay
months or years ahead. Modern industrial-
ized societies are profoundly delayed- return
environments in which people devote con-
siderable effort now for uncertain outcomes
in the future. As a result, we are plagued by
far more self- generated anxiety than were
our hunter– gatherer ancestors.
When people imagine future events, they
often think about how they are likely to feel
when those events occur, which requires
reflection about oneself in the future.
Although people’s affective forecasts are
often inaccurate (Wilson & Gilbert, 2003),
380 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
imagining how they will feel in the future
often helps people behave in ways that lead
to desired emotional outcomes (e.g., “I will
regret it later if I don’t do something now”).
Moreover, people’s expectations about how
they will feel when an event occurs can
influence their emotional reactions to the
event when it actually transpires (Wilson &
Gilbert, 2003).
How people feel when an event occurs also
depends on how they think about the alter-
natives. Concocting imaginary scenarios
about how events might have turned out dif-
ferently can make people feel better or worse
about their lot depending on whether the
alternatives they imagine are worse or bet-
ter than what actually happened. Medvec,
Madey, and Gilovich’s (1995) finding that
Olympic bronze medal winners (i.e., third-
place finishers) appeared happier than silver
medal winners (i.e., second- place finishers)
is a good example of this effect. Presumably,
bronze medalists compare finishing third
to not getting a medal at all, whereas silver
medalists compare their second- place finish
to the imagined alternative of winning the
gold. Similarly, people may become discon-
tent even when current circumstances are
good if they think that being somewhere else
would be even better. Therefore, people can
regulate their emotional responses to events
by managing the ways in which they con-
strue alternative outcomes.
In addition, cognitive representations of
desired future states can motivate and guide
people’s behaviors (Emmons, 1986; Markus
& Nurius, 1986). The capacity for self-
thought again enters the picture, because
thinking about progress toward one’s goals
evokes positive emotions, while lack of prog-
ress (and particularly movement away from
those goals) evokes negative emotions (Hig-
gins, 1987; Markus & Nurius, 1986). Thus,
emotions are experienced not only because
the current state of affairs is favorable or
unfavorable to one’s well-being but also
because events reflect movement toward or
away from one’s goals.
Predictive Control
As noted, some tactics for improving peo-
ple’s emotion regulation involve teaching
them to remain focused on the present situa-
tion as much as possible, turning to thoughts
about the past and future only as needed to
deal with practical issues. People can also
regulate their emotional reactions to future
events by managing their expectations. Peo-
ple’s emotional reactions to events depend
in part on what they expect to happen. For
instance, expected negative outcomes tend
to evoke less distress than unexpected ones,
and events that fail to occur as anticipated
may evoke stronger reactions than if they
had not been expected. Self- awareness is
not necessary for animals to develop expec-
tations or to react emotionally to fulfilled
and unfulfilled expectancies (e.g., the fam-
ily pet may become distressed when daily
routines are disrupted). However, the ability
to self- reflect allows people to manage their
feelings about upcoming events by exerting
deliberate “predictive control” (Rothbaum,
Weisz, & Snyder, 1982) in which they tell
themselves what to expect.
For example, people can use positive
self-talk to allay their anxiety about future
outcomes. By telling themselves that things
will turn out OK, people can sometimes
positively influence how they feel. Such opti-
mism is sometimes costly, though. Sweeny
and Shepperd (2010) found that after con-
trolling for actual exam performance, stu-
dents who expected higher exam scores did
not feel any better before receiving feedback
and actually felt worse after learning their
scores.
At other times, people manage their
expectations to avoid being disappointed.
For example, people try to soften the blow
of failures, bad news, disappointments,
and other aversive events by lowering their
expectations and telling themselves that bad
outcomes are likely to occur. This may not
be an optimal tactic for maximizing positive
affect, however, because the costs of nega-
tive expectations can outweigh the benefits
for many people (Golub, Gilbert, & Wilson,
2009). On average, participants felt worse
when they expected a negative rather than
a positive outcome; moreover, their pessi-
mistic expectations did not serve as a buf-
fer against negative emotions once the event
occurred. However, anticipating negative
outcomes appears to lower anxiety among
dispositionally anxious people who use this
strategy regularly (i.e., defensive pessimists;
Norem & Illingworth, 1993). In fact, pre-
venting defensive pessimists from thinking

Self‑ Awareness and Self‑ Relevant Thought in Emotion 381
about anticipated negative events typically
increases their negative emotions. Thus,
defensive pessimism may be a useful means
of emotional self- regulation for some people
but not for others.
Evaluation of One’s
Personal Characteristics
People’s self- relevant beliefs and evalua-
tions often evoke emotional responses and
are also involved in emotion regulation.
Human infants develop a conception of
themselves slowly over the first 2 years of
life (Kagan, 1998) and characterize them-
selves in increasingly complex and abstract
ways in a developmental progression that
continues into late adolescence (Harter,
2012). As their self- concepts develop, chil-
dren begin to experience myriad emotions
by thinking about their own characteris-
tics and behavior. Once people develop the
capacity to label, characterize, and evaluate
themselves, they react emotionally to these
self- representations, as well as to events and
information that validate or threaten them.
Self‑Evaluations and Emotion
People react negatively to events that threaten
their self-views and positively to events that
affirm them, even when such events have no
real consequences for their well-being. Thus,
by virtue of being self-aware, people become
emotionally invested in whether reality
conforms to their desired self-views. Two
kinds of symbolic satisfactions and threats
to people’s self- construals have received
the most attention from researchers— self-
enhancement and self- verification.
People prefer to evaluate themselves posi-
tively rather than negatively, and a great deal
of research has examined the ways in which
people try to maintain views of being good
and effective individuals. Thus, events that
support a favorable view of oneself tend to
evoke positive emotions, such as pride, hap-
piness, and satisfaction; events that contra-
dict one’s favorable self-view, on the other
hand, are associated with negative emotions,
such as anxiety, despondency, frustration,
shame, and rage (Baumeister, 1998). Because
people’s identities include significant others
in their lives, people also feel good or bad
because of the successes or failures of people
with whom they are associated.
Although many theorists have assumed
that people desire positive feedback about
themselves because they have a need to
maintain or enhance their self- esteem, self-
enhancement may be more parsimoniously
viewed as a way to regulate emotion. People
seek positive information about themselves,
because being capable, attractive, socially
skilled, or likable elicits positive emotions
by increasing the perceived likelihood of
desired outcomes. Conversely, receiving neg-
ative self- relevant feedback typically indi-
cates a lower likelihood of desired outcomes
and a greater likelihood of undesired ones.
Sociometer theory carries this notion
a step further by suggesting that people’s
affective reactions to self- enhancing and
self- depreciating events serve an important
interpersonal function by providing feed-
back about the degree to which they are
valued and accepted by others (for a review,
see Leary, 2006). Events that threaten a
person’s positive self-image evoke negative
emotions, because they cast that person in
an undesirable light in other people’s eyes,
thereby undermining his or her relation-
ships with them (Leary, Tambor, Terdal, &
Downs, 1995). From this perspective, the
feelings that arise from positive and negative
self- relevant events are part of a monitoring
system that keeps people apprised of their
relational value and motivates behaviors to
help them avoid social rejection.
Several perspectives suggest that people
want to maintain not only a positive view
of themselves but also a stable and consis-
tent self-view (Swann & Buhrmester, 2012).
As a result, information suggesting that
people are not who or what they think they
are evokes negative feelings. Cognitive dis-
sonance theory was among the first per-
spectives to describe the emotional effects
of having inconsistent beliefs. Any pair of
contradictory beliefs can potentially induce
the unpleasant affective state of dissonance,
but inconsistent beliefs involving aspects of
oneself seem to be particularly unpleasant
(Aronson, 1968).
Swann and his colleagues have specifi-
cally examined people’s responses to events
that verify or disconfirm their self-views (for
a review, see Swann & Buhrmester, 2012).
People tend to be troubled by information
382 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
that discredits their self- perceptions, pre-
sumably because such information under-
mines their certainty in their beliefs about
themselves and lowers their sense of predict-
ability and control (Swann, Stein- Seroussi,
& Giesler, 1992). As a result, receiving
information that is inconsistent with one’s
self- concept is often troubling, even if it is
favorable (Kwang & Swann, 2010).
Although self- verification and self-
enhancement have often been pitted against
one another as theoretical perspectives on
motivated self- cognition, evidence shows
that both processes occur, and research
has begun to uncover the conditions under
which each effect is obtained (Kwang, &
Swann, 2010). Importantly, the possibility
exists that both processes serve the goal of
promoting one’s social acceptance. That is,
people often increase their chances of social
acceptance by either portraying themselves
in positive ways or seeking to interact with
people who see them as they see themselves.
Regulating Emotion
by Managing Self‑Evaluations
Because self- evaluations influence emotions,
people can regulate their emotions by man-
aging how they perceive and evaluate them-
selves. Two of the most widely researched
tactics for doing so involve social compari-
son and attribution.
Social Comparison
People’s self- evaluations— and their
emotions— are affected by how they com-
pare to others, particularly in domains where
no objective standard for self- evaluation
exists (e.g., judgments of physical attractive-
ness or popularity). Therefore, people some-
times regulate their emotions through their
choices of social comparison targets (Suls &
Wheeler, 2012). Although people sometimes
use comparison targets that provide an
accurate indication of their own attributes,
their selections more often appear designed
to promote positive feelings (Buunk, Col-
lins, Taylor, VanYperen, & Dakof, 1990;
Morrell et al., 2012). At times, people seek
downward comparisons to feel better about
themselves by contrast; at other times, they
seek upward comparisons for inspiration or
hope.
Theorists once assumed that comparing
ourselves to those who are worse off than
we are (i.e., downward social comparison)
generally leads to more positive emotions
than comparing ourselves to people who
are better off (upward social comparison).
However, whether downward or upward
comparisons lead to positive or negative
affect depends on how people interpret the
comparative information. When downward
comparisons make people feel better or
more fortunate than others, they induce pos-
itive emotions. In contrast, when downward
social comparisons raise the specter that one
might also fare poorly (e.g., when a newly
diagnosed cancer patient compares him- or
herself to a patient in later stages of the ill-
ness), they lead to negative emotions (Buunk
et al., 1990; Morrell et al., 2012). Similarly,
comparing oneself to outstanding role mod-
els evokes positive affect if the superstar’s
accomplishments seem personally attain-
able but negative affect when they do not
(Lockwood & Kunda, 1997). The effects
of upward and downward social compari-
son are quite complex and are also moder-
ated by variables such as self- esteem, mood,
neuroticism, and optimism (Lyubomirsky
& Ross, 1997). Nevertheless, people often
choose and interpret social comparisons in
ways that help them regulate their emotions.
Attributions
In the same vein, people can regulate their
emotions through their attributions for
events that occur. People’s beliefs about
why particular emotion- inducing events
occurred in the first place (e.g., “Why was
I passed over for the promotion?”) and why
they reacted as they did to those events (e.g.,
“Why did I lose my temper?”) influence both
their self- evaluations and their emotions.
Since Schachter and Singer’s (1962) initial
demonstration that people’s interpretations
of arousing events affect their emotional
experiences, research has shown that attri-
butions have a strong effect on emotion. The
same event can evoke drastically different
emotions depending on the person’s attribu-
tions for it (Siemer, Mauss, & Gross, 2007),
and such attributions necessarily involve
self- focused thought.
Weiner’s (1985) attribution theory of
emotion most fully describes the relation-
Self‑ Awareness and Self‑ Relevant Thought in Emotion 383
ship between patterns of attributions and
specific emotions. From this perspective,
people’s emotional responses to positive and
negative events depend on three aspects of
the attributions that they make—causal
locus (whether the cause of the event was
internal or external to the individual), sta-
bility (whether the cause is short-lived or
long- lasting), and controllability (whether
the cause is under the person’s control). For
example, if people attribute success to their
own efforts (an internal, stable, and control-
lable cause), they experience positive self-
agency emotions such as pride, satisfaction,
and confidence, whereas attributing that
same success to other people (an external,
stable, uncontrollable cause) results in posi-
tive other- agency emotions such as gratitude,
obligation, and appreciation toward those
who helped. And, if the success is attributed
to impersonal circumstances (an external,
unstable, and uncontrollable causes), people
feel lucky and happily surprised (positive
situation- agency emotions).
The attribution of agency has proven to
be particularly important in distinguishing
among negative emotions, such as anger
(other- agency), guilt (self- agency), and sor-
row (circumstance- agency; Ellsworth &
Smith, 1988). Additionally, agency attribu-
tions, regardless of their accuracy, influence
people’s appraisals of their ability to deal
with negative events and their consequences
(Ellsworth & Scherer, 2003). The emotional
effects of attributions are also mediated
by people’s appraisals of the implications
of those attributions for their well-being
(David, Schnur, & Belloiu, 2002).
Research on both student and clinical
samples demonstrates that people who char-
acteristically explain negative events in terms
of internal, stable, and global causes (i.e.,
a pessimistic explanatory style) are more
likely to develop symptoms of depression
(in particular, the subtype “hopeless depres-
sion”) than those who tend to make exter-
nal, unstable, and specific attributions for
negative outcomes (Abramson, Metalsky, &
Alloy, 1989; Alloy et al., 2000). In addition,
attributing positive events to unstable and
specific sources is associated with depres-
sion, albeit less strongly than attributions
for negative events (Ahrens & Haaga, 1993).
In fact, negative attributional styles contrib-
ute to a variety of common emotional dif-
ficulties, including depression, anxiety, and
frequent anger and guilt (Hilt, 2004).
In light of the link between people’s
attributions and their emotions, interven-
tions and psychotherapy techniques have
been developed that focus on attributional
reframing to ameliorate emotional distress.
Such approaches involve teaching people
to identify and challenge their inaccurate
causal attributions and to develop more
accurate, positive ones. For example, when
people engage in excessive self-blame about
negative events (i.e., unrealistically attribut-
ing negative events to a stable internal fac-
tor), an approach known as attributional
retraining has been used with beneficial
effects on depression, anxiety, and guilt.
Attributional retraining involves reviewing
the negative event that led to the attribution,
searching for alternative explanations that
are at least equally plausible, and alleviating
some or all of the person’s responsibility for
the negative outcome. Conversely, attribu-
tional retraining can also involve fostering
internal attributions for positive outcomes
when appropriate, such as learning to attri-
bute one’s successes to effort and skill rather
than luck, in order to increase positive emo-
tions and self- efficacy (Hilt, 2004).
Techniques to help clients reframe their
attributions are often used within the con-
text of cognitive- behavioral therapy, which
makes it difficult to assess their effectiveness
as a unique mechanism for change. Even so,
research generally supports the efficacy of
attributional reframing for reducing emo-
tional distress (Hilt, 2004). For example,
decreased negative attributions in couples
mediated increases in relationship satis-
faction (Hrapczynski, Epstein, Werlinich,
& LaTaillade, 2012), and a reduction in
patients’ negative inferences lowered their
depression and anxiety (DeFronzo Dobkin
et al., 2007). In addition, an integral compo-
nent of treatment for panic disorder involves
modifying patients’ attributions for their
physiological symptoms of anxiety (e.g.,
learning that they are not harmful) in order
to reduce anxiety and the frequency of panic
attacks.
Attributional retraining has also been
used successfully in nonclinical settings,
such as schools and the workplace, as both
a method for preventing emotional distress
and an intervention for emotional problems.

384 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
For instance, a study that focused on chang-
ing employees’ negative attributional styles
resulted in significant increases in job sat-
isfaction and subjective well-being (Proud-
foot, Corr, Guest, & Dunn, 2009). Overall,
research suggests that attributional refram-
ing can be an effective technique for improv-
ing emotional outcomes in both clinical and
nonclinical populations. And, again, all of
these effects are mediated by people’s self-
relevant thoughts.
Awareness of Other People’s
Perceptions and Evaluations
A great deal of human emotion arises from
people’s beliefs about how they are perceived
and evaluated by other people. Other peo-
ple’s judgments of us have important impli-
cations for our social, occupational, roman-
tic, financial, and other outcomes, so we
understandably react differently when we
are viewed favorably rather than unfavorably
by other people. Self- awareness is needed to
infer other people’s thoughts, and some the-
orists have even proposed that the ability to
reflect on one’s private experiences evolved
to help people infer what others might be
thinking about them (Gallup, 1997). In fact,
in young children the ability to imagine
other people’s perspectives emerges at about
the same time as self- awareness (Focquaert,
Braeckman, & Platek, 2008).
Self‑Awareness and Social Emotions
Around this time, children also begin to
experience self- conscious emotions— such as
embarrassment, pride, shame, and guilt—in
response to the real or imagined evaluations
of other people (Miller, 1996). This particu-
lar subset of emotions is often labeled “self-
conscious” because these emotions typically
involve acute feelings of self- consciousness,
as people think a great deal about them-
selves and what other people might be think-
ing about them (Tangney & Tracy, 2012).
Yet the central feature of these emotions is
that they occur when people believe that
others have formed, or might form, particu-
lar positive or negative impressions of them.
These emotions inherently involve reactions
to social- evaluative events or perceived vio-
lations of social standards (see Dickerson,
Grunewald, & Kemeny, 2004), even if those
standards have been internalized and people
are no longer consciously aware of their ori-
gin (Baldwin & Baccus, 2004).
People’s perceptions of how others view
and evaluate them (often called reflected
appraisals) can elicit a variety of emotions.
For example, people experience social anxi-
ety when they think that others will not
form desired impressions of them, even if
they know that they possess positive attri-
butes. Research has shown that people who
score high in social anxiety tend to be highly
self- focused and think about themselves
negatively during social interactions. In fact,
negative self- relevant imagery is causally
involved in both trait social anxiety and the
experience of state social anxiety in people
who are not typically anxious (Makkar &
Grisham, 2011). Similarly, people become
embarrassed when they believe that other
people have formed undesired impressions
of them (Miller, 1996) and experience hurt
feelings when they think that others do not
sufficiently value them (Leary, Springer,
Negel, Ansell, & Evans, 1998). In each of
these cases, self- reflection is needed to infer
what others might be thinking and to elicit
the emotional response.
Guilt and shame also involve concerns
about other people’s evaluations. Guilt
involves a negative evaluation of a specific
behavior, whereas shame involves a nega-
tive evaluation of oneself (Tangney & Tracy,
2012). People often feel guilty or ashamed
simply from knowing that others regard
their behavior or character unfavorably,
even if they personally believe that they did
nothing wrong. Of course, people also expe-
rience positive emotions from imagining
other people’s reactions to them. People may
experience pride when they think that others
admire them, and joy when they think that
others love them deeply, even in the absence
of explicit indications of admiration or love.
Furthermore, people’s beliefs about how
others expect them to feel also affect their
emotions. For example, when people feel
sad but believe that others think they should
be happy, they experience stronger negative
emotions and lower subjective well-being. In
fact, perceived social expectancies predict
people’s subsequent emotions and well-being
more consistently than—and independently
of—their personal expectancies. Research

Self‑ Awareness and Self‑ Relevant Thought in Emotion 385
has shown this is the case because perceived
social expectancies (e.g., to be happy) pro-
mote negative self- evaluations in people
when their emotions (e.g., sadness) violate
these expectations (Bastian et al., 2012).
Regulating Social Emotions
Given the role of self- relevant thought in
social emotions, another way that people
can regulate their emotions is by managing
their thoughts about what others might be
thinking about them. Such strategies fall
into two broad categories.
First, people sometimes reduce undesired
social emotions by reevaluating the impor-
tance of other people’s impressions and eval-
uations of them. Although people tend to
react rather automatically to indications that
others are judging them unfavorably, they
may later decide that others’ judgments are
not actually important or consequential in
a particular instance (Leary, 2006). Reduc-
ing the motivation to make a desired impres-
sion or the importance of being evaluated in
a particular way can attenuate or minimize
emotions such as social anxiety, embarrass-
ment, guilt, and hurt feelings.
Second, to the extent that unpleasant
social emotions arise from believing that
one is being viewed unfavorably, people can
reevaluate others’ likely evaluations of them.
For example, people who frequently experi-
ence social anxiety can be taught that other
people’s evaluations of them are usually not
as negative as they imagine. Similarly, peo-
ple whose feelings are easily hurt can learn
that others’ seemingly hurtful actions—
such as minor slights, snubs, and signs of
disinterest— are usually more benign than
they might first appear.
Conclusion
Our focus in this chapter has been on the
ways in which the human ability to self-
reflect affects emotional experience and
regulation. As we have seen, self- awareness
allows people to experience emotions by
comparing themselves to their personal stan-
dards, thinking about themselves in the past
and future, assessing their personal charac-
teristics, and thinking about how they are
perceived by other people. By virtue of being
self-aware, human beings live in an emo-
tional world that differs greatly from that of
other animals. Indeed, it is difficult to imag-
ine what human subjective experience would
be like without self- awareness and the inter-
nal self-talk that underlies so much human
emotion.
Self- awareness was perhaps the pivotal
psychological adaptation that put human
beings on such a distinctly different path
than that of other animals. From the stand-
point of emotion, however, the capacity
to think consciously about oneself is both
a blessing and a curse (Leary, 2004). Self-
awareness and self- relevant thought are ben-
eficial in planning for the future in ways that
maximize gains and minimize losses, and
the emotions that arise when people look
forward and backward are often useful in
informing judicious decisions and effective
behavior. Yet, as we have seen, people suf-
fer far more from temporal emotions such
as anxiety and regret than is necessary for
successful self- regulation. Similarly, people’s
ability to compare themselves to standards
and evaluate themselves accordingly pro-
vides important input into their behavioral
choices. But people are often distressed by
unfavorable self- evaluations even when they
do not matter in a practical sense. Likewise,
people could not interact successfully with
others without an ability to infer what oth-
ers are thinking about them, and the self-
conscious emotions that sometimes arise are
often beneficial in steering their behavior in
ways that are socially advantageous. How-
ever, people are frequently more concerned
about others’ evaluations than they need to
be, resulting in unnecessary social anxiety,
embarrassment, guilt, and shame that can
sometimes interfere with effective behavior
and self- regulation.
People obviously prefer to feel positive
emotions more than negative emotions, and
they can change how they feel by changing
how they think about themselves. As we have
seen, people regulate their emotions in many
ways, including attenuating self- awareness,
predictive control, social comparison, and
attributional reframing, among others.
But even this hedonically desirable ability
has both good and bad effects. Minimiz-
ing unwanted emotions certainly promotes
subjective well-being, and managing one’s
emotions often facilitates effective behavior

386 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
and better self- regulation. However, when
unpleasant emotions serve an important
purpose in keeping people attuned to threats
to their well-being or motivating them to
engage in needed behavioral change, the use
of self-talk to dampen such emotions might
be counterproductive. We hope that future
research will result in a better understand-
ing of the conditions under which regulating
emotions via self- relevant thought is benefi-
cial rather than detrimental to people’s well-
being.
Note
1. Although our focus is on the implications of
self- awareness for emotion, we should note
that affective experiences can also influence
the operation of self- related processes. For
example, emotions can affect people’s self-
perceptions, self- efficacy, and ability to self-
regulate (DeSteno & Salovey, 1997; Leith &
Baumeister, 1996). Emotional experiences
also influence where people focus their atten-
tion (Fredrickson & Branigan, 2005) and
how they make decisions (Kugler, Connolly,
& Ordóñez, 2012). Thus, not only is self-
awareness intimately involved in the experi-
ence and regulation of emotion, but emotions
also reciprocally affect self- related attention
and thought.
References
Alloy, L. B., Abramson, L. Y., Hogan, M. E.,
Whitehouse, W. G., Rose, D. T., Robinson,
M. S., et al. (2000). The Temple– Wisconsin
Cognitive Vulnerability to Depression (CVD)
Project: Lifetime history of Axis I psychopa-
thology in individuals at high and low cogni-
tive risk for depression. Journal of Abnormal
Psychology, 109, 403– 418.
Abramson, L. Y., Metalsky, F. I., & Alloy, L.
B. (1989). Hopelessness depression: A theory
based subtype of depression. Psychological
Review, 96, 358–372.
Ahrens, A. H., & Haaga, D. A. F. (1993). The
specificity of attributional style and expec-
tations to positive and negative affectivity,
depression, and anxiety. Cognitive Therapy
and Research, 17, 83–98.
Aronson, E. (1968). Dissonance theory: Prog-
ress and problems. In R. P. Abelson, E. Aron-
son, W. J. McGuire, T. M. Newcomb, M. J.
Rosenberg, & P. H. Tannenbaum (Eds.), The-
ories of cognitive consistency: A sourcebook
(pp. 5–27). Chicago: Rand McNally.
Baldwin, M. W., & Baccus, J. R. (2004). Main-
taining a focus on the social goals underly-
ing self- conscious emotions. Psychological
Inquiry, 15, 139–144.
Bargh, J. A., Gollwitzer, P. M., & Lee-Chai, A.
(2001). The automated will: Nonconscious
activation and pursuit of behavioral goals.
Journal of Personality and Social Psychology,
81, 1014–1027.
Bastian, B., Kuppens, P., Hornsey, M. J., Park,
J., Koval, P., & Uchida, Y. (2012). Feeling bad
about being sad: The role of social expectan-
cies in amplifying negative mood. Emotion,
12, 69–80.
Baumeister, R. F. (1991). Escaping the self. New
York: Basic Books.
Baumeister, R. F. (1998). The self. In D. T. Gil-
bert, S. T. Fiske, & G. Lindzey (Eds.), Hand-
book of social psychology (4th ed., Vol. 2,
pp. 680–740). Boston: McGraw-Hill.
Brown, C. M., & McConnell, A. R. (2011).
Discrepancy- based and anticipated emotions
in behavioral self- regulation. Emotion, 11,
1091–1095.
Buunk, B. P., Collins, R. L., Taylor, S. E., VanY-
peren, N. W., & Dakof, G. A. (1990). The
affective consequences of social comparison:
Either direction has its ups and downs. Jour-
nal of Personality and Social Psychology, 59,
1238–1249.
Bryant, F. B., & Veroff, J. (2007). Savoring: A
new model of positive experience. Mahwah,
NJ: Erlbaum.
Carver, C. S., Lawrence, J. W., & Scheier, M. F.
(1999). Self- discrepancies and affect: Incorpo-
rating the role of fear selves. Personality and
Social Psychology Bulletin, 25, 783–792.
Carver, C. S., & Scheier, M. F. (1981). Atten-
tion and self- regulation: A control theory
approach to human behavior. New York:
Springer- Verlag.
David, D., Schnur, J., & Belloiu, A. (2002).
Another search for the “hot” cognitions:
Appraisal, irrational beliefs, attributions, and
their relation to emotion. Journal of Rational-
Emotive and Cognitive- Behavior Therapy,
20, 93–132.
DeFronzo Dobkin, R., Allen, L. A., Alloy, L. B.,
Menza, M., Gara, M. A., & Panzarella, C.
Self‑ Awareness and Self‑ Relevant Thought in Emotion 387
(2007). Adaptive inferential feedback partner
training for depression: A pilot study. Cogni-
tive and Behavioral Practice, 14(4), 350–363.
DeSteno, D. A., & Salovey, P. (1997). The effects
of mood on the structure of the self- concept.
Cognition and Emotion, 11, 351–372.
Dickerson, S. S., Grunewald, T. L., & Kemeny,
M. E. (2004). When the social self is threat-
ened: Shame, physiology, and health. Journal
of Personality, 72, 1189–1216.
Duval, S., & Wicklund, R. A. (1972). A theory of
objective self awareness. New York: Academic
Press.
Ellsworth, P. C., & Scherer, K. R. (2003).
Appraisal processes in emotion. In R. J. David-
son, K. R. Scherer, & H. H. Goldsmith (Eds.),
Handbook of affective sciences (pp. 575–595).
New York: Oxford University Press.
Ellsworth, P. C., & Smith, C. A. (1988a). From
appraisal to emotion: Differentiating among
unpleasant feelings. Motivation and Emotion,
12, 271–302.
Emmons, R. A. (1986). Personal strivings: An
approach to personality and subjective well-
being. Journal of Personality and Social Psy-
chology, 51, 1058–1068.
Field, N. P., Joudy, R., & Hart, D. (2010). The
moderating effect of self- concept valence on
the relationship between self- focused atten-
tion and mood: An experience sampling
study. Journal of Research in Personality, 44,
70 –77.
Foa, E. B., Huppert, J. D., & Cahill, S. P.
(2006). Emotional processing theory: An
update. In B. O. Rothbaum (Ed.), Pathologi-
cal anxiety: Emotional processing in etiology
and treatment (pp. 3–24). New York: Guil-
ford Press.
Focquaert, F., Braeckman, J., & Platek, S. M.
(2008). An evolutionary cognitive neurosci-
ence perspective on human self- awareness and
theory of mind. Philosophical Psychology, 21,
47–68.
Fredrickson, B. L., & Branigan, C. (2005). Posi-
tive emotions broaden the scope of attention
and thought– action repertoires. Cognition
and Emotion, 19, 313–332.
Fromson, P. M. (2006). Self- discrepancies and
negative affect: The moderating roles of pri-
vate and public self- consciousness. Social
Behavior and Personality, 34, 333–350.
Frijda, N. H. (1986). The emotions. London:
Cambridge University Press.
Gallup, G. G., Jr. (1997). On the rise and fall of
self- conception in primates. In J. G. Snodgrass
& R. L. Thompson (Eds.), The self across psy-
chology (pp. 78–82). New York: New York
Academy of Sciences.
Golub, S. A., Gilbert, D. T., & Wilson, T. D.
(2009). Anticipating one’s troubles: The costs
and benefits of negative expectations. Emo-
tion, 9, 277–281.
Harter, S. (2012). The construction of the self:
A developmental perspective (2nd ed.). New
York: Guilford Press.
Hayes, S. C., Strosahl, K., & Wilson, K. G.
(1999). Acceptance and commitment therapy:
An experiential approach to behavior change.
New York: Guilford Press.
Higgins, E. T. (1987). Self- discrepancy: A theory
relating self and affect. Psychological Review,
94, 319–340.
Hilt, L. M. (2004). Attribution retraining for
therapeutic change: Theory, practice, and
future directions. Imagination, Cognition and
Personality, 23(4), 289–307.
Hrapczynski, K. M., Epstein, N. B., Werlinich,
C. A., & LaTaillade, J. J. (2012). Changes in
negative attributions during couple therapy for
abusive behavior: Relations to changes in sat-
isfaction and behavior. Journal of Marital and
Family Therapy, 38(1), 117–132.
Jaynes, J. (1976). The origin of consciousness in
the breakdown of the bicameral mind. Boston:
Houghton- Mifflin.
Joiner, T. E. (2001). Negative attributional style,
hopelessness depression and endogenous
depression. Behaviour Therapy and Research,
39, 139–149.
Joormann, J., Siemer, M., & Gotlib, I. H. (2007).
Mood regulation in depression: Differential
effects of distraction and recall of happy mem-
ories on sad mood. Journal of Abnormal Psy-
chology, 116, 484–490.
Kabat-Zinn, J. (2003). Mindfulness- based stress
reduction (MBSR). Constructivism in the
Human Sciences, 8, 73 –107.
Kagan, J. (1998). Is there a self in infancy? In
M. Ferrari & R. J. Sternberg (Eds.), Self-
awareness: Its nature and development
(pp. 137–147). New York: Guilford Press.
Kross, E., & Ayduk, O. (2008). Facilitating
adaptive emotional analysis: Distinguishing
distanced- analysis of depressive experiences
from immersed- analysis and distraction. Per-
sonality and Social Psychology Bulletin, 34,
924–938.
Kugler, T., Connolly, T., & Ordóñez, L. D.
388 PERSONALITY PROCESSES AND INDIVIDUAL DIFFERENCES
(2012). Emotion, decision, and risk: Betting
on gambles versus betting on people. Journal
of Behavioral Decision Making, 25, 123–134.
Kwang, T., & Swann, W. B. (2010). Do people
embrace praise even when they feel unworthy?:
A review of critical tests of self- enhancement
versus self- verification. Personality and Social
Psychology Review, 14, 263–280.
Leary, M. R. (2004). The curse of the self: Self-
awareness, egotism, and the quality of human
life. New York: Oxford University Press.
Leary, M. R. (2006). Sociometer theory and the
pursuit of relational value: Getting to the root
of self- esteem. European Review of Social
Psychology, 16, 75–111.
Leary, M. R., & Buttermore, N. E. (2003). Evo-
lution of the human self: Tracing the natural
history of self- awareness. Journal for the The-
ory of Social Behaviour, 33, 365–404.
Leary, M. R., Estrada, M. J., & Allen, A. B.
(2009). The analogue- I and the analogue- me:
The avatars of the self. Self and Identity, 8,
147–161.
Leary, M. R., Springer, C., Negel, L., Ansell, E.,
& Evans, K. (1998). The causes, phenomenol-
ogy, and consequences of hurt feelings. Jour-
nal of Personality and Social Psychology, 74,
1225–1237.
Leary, M. R., Tambor, E. S., Terdal, S. K., &
Downs, D. L. (1995). Self- esteem as an inter-
personal monitor: The sociometer hypothesis.
Journal of Personality and Social Psychology,
68, 518–530.
Leith, K. P., & Baumeister, R. F. (1996). Why do
bad moods increase self- defeating behavior?:
Emotion, risk- taking, and self- regulation.
Journal of Personality and Social Psychology,
71, 1250–1267.
Linehan, M. M. (1993). Cognitive behavioral
treatment of borderline personality disorder.
New York: Guilford Press.
Lockwood, P., & Kunda, Z. (1997). Superstars
and me: Predicting the impact of role models
on the self. Journal of Personality and Social
Psychology, 73, 91–103.
Lyubomirsky, S., & Ross, L. (1997). Hedonic
consequences of social comparison: A con-
trast of happy and unhappy people. Journal
of Personality and Social Psychology, 73,
1141–1157.
Macrae, C. N., Bodenhausen, G. V., & Milne,
A. B. (1998). Saying no to unwanted thoughts:
Self-focus and the regulation of mental life.
Journal of Personality and Social Psychology,
75, 578–589.
Makkar, S. R., & Grisham, J. R. (2011). Social
anxiety and the effects of negative self- imagery
on emotion, cognition, and post-event pro-
cessing. Behaviour Research and Therapy, 49,
654–664.
Markus, H., & Nurius, P. (1986). Possible selves.
American Psychologist, 41, 954–969.
Marshall, R. D., Bryant, R. A., Amsel, L., Suh,
E., Cook, J. M., & Neria, Y. (2007). The
psychology of ongoing threat: Relative risk
appraisal, the September 11 attacks, and
terrorism- related fears. American Psycholo-
gist, 62, 304–316.
Martin, L. (1999). I-D compensation the-
ory: Some implications of trying to satisfy
immediate- return needs in a delayed- return
culture. Psychological Inquiry, 10, 195–208.
Medvec, V. H., Madey, S. E., & Gilovich, T.
(1995). When less is more: Counterfactual
thinking and satisfaction among Olympic
medalists. Journal of Personality and Social
Psychology, 69, 603–610.
Miller, R. S. (1996). Embarrassment: Poise and
peril in everyday life. New York: Guilford Press.
Miranda, R., & Mennin, D. S. (2007). Cognitive
Therapy and Research, 31, 71–82.
Morrell, B., Jordens, C. C., Kerridge, I. H., Har-
nett, P., Hobbs, K., & Mason, C. (2012). The
perils of a vanishing cohort: A study of social
comparisons by women with advanced ovar-
ian cancer. Psycho- Oncology, 21, 382–391.
Norem, J. K., & Illingworth, K. S. S. (1993).
Strategy- dependent effects of reflecting on self
and tasks: Some implications of optimism and
defensive pessimism. Journal of Personality
and Social Psychology, 65, 822–835.
Petrocelli, J. V., & Smith, E. R. (2005). Who I
am, who we are, and why: Links between
emotions and causal attributions for self- and
group discrepancies. Personality and Social
Psychology Bulletin, 31, 1628–1642.
Phillips, A. G., & Silvia, P. J. (2005). Self-
awareness and the emotional consequences of
self- discrepancies. Personality and Social Psy-
chology Bulletin, 31, 703–713.
Phillips, A. G., & Silvia, P. J. (2010). Individual
differences in self- discrepancies and emotional
experience: Do distinct discrepancies predict
distinct emotions? Personality and Individual
Differences, 49, 148–151.
Phillips, A. G., Silvia, P. J., & Paradise, M. J.
(2007). The undesired self and emotional
experience: A latent variable analysis. Journal
of Social and Clinical Psychology, 26, 1035–
1047.
Self- Awareness and Self- Relevant Thought in Emotion 389
Philippe, F. L., Koestner, R., Lecours, S.,
Beaulieu-
P
elletier, G., & Bois, K. (2011). The
role of autobiographical memory networks in
the experience of negative emotions: How our
remembered past elicits our current feelings.
Emotion, 11, 1279–1290.
Proudfoot, J. G., Corr, P. J., Guest, D. E., &
Dunn, G. (2009). Cognitive-
b
ehavioural
training to change attributional style improves
employee well-being, job satisfaction, produc-
tivity, and turnover. Personality and Individ-
ual Differences, 46(2), 147–153.
Roemer, L., & Borkovec, T. D. (1993). Worry:
Unwanted cognitive activity that controls
unwanted somatic experience. In D. M. Weg-
ner & J. W. Pennebaker (Eds.), Handbook of
mental control (pp.
220–238). Englewood
Cliffs, NJ: Prentice Hall.
Rothbaum, F., Weisz, J. R., & Snyder, S. S.
(1982). Changing the world and changing the
self: A two-
p
rocess model of perceived control.
Journal of Personality and Social Psychology,
42, 5–37.
Schachter, S., & Singer, J. E. (1962). Cognitive,
social, and physiological determinants of emo-
tional state. Psychological Review, 69, 379–
399.
Scheier, M. F., & Carver, C. S. (1977). Self-
f
ocused attention and the experience of
emotion: Attraction, repulsion, elation, and
depression. Journal of Personality and Social
Psychology, 35, 625–636.
Siemer, M., Mauss, I., & Gross, J. J. (2007). Same
situation—
d
ifferent emotions: How appraisals
shape our emotions. Emotion, 7, 592–600.
Silvia, P. J. (2002). Self-
a
wareness and emotional
intensity. Cognition and Emotion, 16, 195–
216.
Scheier, M. F., Fenigstein, A., & Buss, A. H.
(1974). Self-
a
wareness and physical aggres-
sion. Journal of Experimental Social Psychol-
ogy, 10, 264–273.
Suls, J., & Wheeler, L. (2012). Social comparison
theory. In P. M. Van Lange, A. W. Kruglanski,
& E. Higgins (Eds.), Handbook of theories
of social psychology (Vol. 1, pp.
460–482).
Thousand Oaks, CA: Sage.
Swann, W. B., Jr., & Buhrmester, M. D. (2012).
Self-
v
erification: The search for coherence. In
M. R. Leary & J. P. Tangney (Eds.), Hand-
book of self and identity (2nd ed., pp. 405–
424). New York: Guilford Press.
Swann, W. B., Jr., Stein-
S
eroussi, A., & Giesler,
R. B. (1992). Why people self-
v
erify. Journal
of Personality and Social Psychology, 62,
392–401.
Sweeny, K., & Shepperd, J. A. (2010). The costs
of optimism and the benefits of pessimism.
Emotion, 10, 750–753.
Tangney, J. P., Niedenthal, P. M., Covert, M.
V., & Barlow, D. H. (1998). Are shame and
guilt related to distinct self-
d
iscrepancies?:
A test of Higgins’s (1987) hypotheses. Jour-
nal of
P
ersonality and Social Psychology, 75,
256–268.
Tangney, J. P., & Tracy, J. L. (2012). Self-
c
onscious emotions. In M. Leary & J. P. Tang-
ney (Eds.), Handbook of self and identity (2nd
ed., pp.
446–478). New York: Guilford Press.
Veissier, I. I., Boissy, A. A., Désiré, L. L., &
Greiveldinger, L. L. (2009). Animals’ emo-
tions: Studies in sheep using appraisal theo-
ries. Animal Welfare, 18, 347–354.
Wegner, D. M., Erber, R., & Zanakos, S. (1993).
Ironic processes in the mental control of mood
and mood-
r
elated thought. Journal of Person-
ality and Social Psychology, 65, 1093–1104.
Weiner, B. (1985). An attributional theory of
achievement motivation and emotion. Psycho-
logical Review, 92, 548–573.
Wilson, T. D., & Gilbert, D. T. (2003). Affec-
tive forecasting. In M. Zanna (Ed.), Advances
in experimental social psychology (Vol. 35,
pp.
345–411). New York: Elsevier.

PART VII
PSYCHOPATHOLOGY

393
The relationship between emotion regula-
tion (ER) and anxiety disorders has received
considerable attention since the publication
of the first edition of this handbook. ER
features more prominently in conceptual
accounts of anxiety disorders, and empiri-
cal investigations that focus on the intersec-
tion of ER and anxiety have increased dra-
matically. It is no longer justified to assert
that the role of emotion and ER is under-
recognized in conceptualizations and treat-
ments for anxiety disorders. Nor is it nec-
essary to rely on extrapolation of findings
from healthy samples to posit relationships
between ER and anxiety disorders: In an
exciting development, we are now able to
review behavioral and neurobiological lit-
eratures that elucidate the role of ER in the
development, phenomenology, and treat-
ment of anxiety disorders.
This recent acceleration of research has
generated an array of potentially important
observations regarding the relationship of
ER to anxiety disorders, which we aim to
integrate in this chapter. One area of partic-
ular interest is the use of specific ER strate-
gies (e.g., suppression, cognitive reappraisal)
by individuals with anxiety disorders: how
frequently they use certain strategies, how
successful they are at implementing them,
and—most importantly— how this may
relate to the development and maintenance
of anxiety disorder symptoms. We contend
that the use of specific ER strategies by anx-
ious individuals is best understood within
the broader context of the basic emotional
processing that characterizes anxiety disor-
ders. The first section of the chapter there-
fore reviews evidence supporting the view
that ER in anxiety disorders derives in large
part from biological and psychological vul-
nerabilities that confer increased emotional
reactivity, attentional biases toward threat
and other negative information, and global
tendencies to experience emotions as aver-
sive and to engage in avoidant processing
and behavior. These features of anxiety dis-
orders influence anxious individuals’ use of
the specific ER strategies that constitute the
focus of the latter portion of the chapter. In
discussing these strategies, we consider sev-
eral potential pathways to the ER difficulties
reported by individuals with anxiety disor-
ders, including overreliance on maladaptive
ER, less frequent use or compromised effec-
tiveness of adaptive ER, and dysfunction of
neural systems supporting ER.
Defining Our Terms
Before we turn to our main topics, it will be
useful to clarify the definitions of terms used
throughout this chapter. Our definitions of
CHAPTER 24
Emotion Regulation in Anxiety Disorders
Laura Campbell-Sills
Kristen K. Ellard
David H. Barlow

394 PSYCHOPATHOLOGY
emotion and emotion regulation closely par-
allel those articulated by Gross (this volume).
We consider emotions to be multimodal phe-
nomena that involve changes in subjective
experience, physiology, and action tenden-
cies. Emotions occur in response to internal
or external stimuli that are meaningful to
the organism’s survival, well-being, or other
goals. In many situations, the subjective
experience, physiological changes, or behav-
ioral tendencies associated with an emo-
tion increase the chances of attaining a goal
(e.g., sympathetic arousal and the impulse
to flee help achieve the goal of survival in
a life- threatening situation). However, by
definition, the emotions that characterize
anxiety disorders are excessive, often caus-
ing the individual both subjective distress (in
the form of intense emotions, catastrophic
thoughts, and/or uncomfortable physical
sensations) and interference with adaptive
functioning (e.g., when patterns of extreme
avoidance develop).
When using the term emotion regula-
tion, we refer to processes that influence
the occurrence, intensity, duration, and
expression of emotion. These processes may
support up- regulation or down- regulation
of positive or negative emotions, and can
be placed on a continuum of automatic to
effortful (Gross, this volume). While all
forms of ER are likely relevant to anxiety
disorders, much of this chapter focuses on
effortful down- regulation of negative emo-
tion. This choice is partly based on typical
anxiety disorder presentations, which are
largely characterized by excessive negative
emotions such as fear and anxiety. It also
reflects the predominant focus of empiri-
cal work to date, because most research has
focused on a limited number of effortful ER
strategies.
Throughout the chapter we also refer to
maladaptive and adaptive ER. By maladap-
tive we mean that an ER strategy is either
unsuccessful in reducing the unwanted emo-
tional response (as reflected by subjective
report, physiological arousal, behavioral
data, or brain imaging data) or associated
with costs that likely outweigh any benefits
of short-term reduction of acute emotion.
In contrast, “adaptive” strategies promote
(1) decreased subjective distress, physiologi-
cal arousal, maladaptive behavior, and/
or activation of emotion- generating brain
regions; and (2) maintenance of abilities to
pursue short- and long-term goals that are
important to the individual. Effective ER
likely involves a combination of selecting
“good” strategies and being able to apply
these methods flexibly depending on contex-
tual demands (e.g., Kashdan & Rottenberg,
2010).
A Conceptual Framework
for Examining ER
in Anxiety Disorders
Consideration of the biological and psycho-
logical factors that underlie anxiety disor-
ders provides an important framework for
understanding ER in these disorders. Barlow
(1988, 2002) proposed that anxiety disor-
ders emerge out of a “triple vulnerability,”
wherein generalized biological vulnerabili-
ties toward greater emotional reactivity, and
a generalized psychological vulnerability
arising from early developmental events that
engender a sense of the world as uncontrol-
lable or unpredictable (hence, threatening),
represent an etiological diathesis toward
psychopathology. When combined with
life stress and learned experiences (specific
vulnerabilities), symptoms associated with
anxiety and related emotional disorders are
likely to result. Specific diagnostic presenta-
tions (e.g., social anxiety disorder [SAD],
panic disorder [PD]) are determined by learn-
ing experiences that focus the diathesis on
particular contexts (e.g., social evaluation,
bodily sensations; Barlow, 2002; Suárez,
Bennett, Goldstein, & Barlow, 2009). In this
way, the DSM anxiety diagnoses represent
the endpoint of the convergence of the triple
vulnerabilities. However, at the core of each
specific disorder lies a common tendency to
experience intense emotions that are viewed
as threatening or unwanted, often prompt-
ing efforts to diminish or avoid the aversive
emotional experience (Barlow, 1988, 2002).
Various lines of research lend support
to the different components of this model.
Neurobiological findings are broadly con-
sistent with the notion of a generalized bio-
logical vulnerability to increased emotional
reactivity in anxiety and related emotional
disorders. Specific genetic polymorphisms
have been associated with anxiety- related
traits (e.g., Montag, Fiebach, Kirsch, &
Emotion Regulation in Anxiety Disorders 395
Reuter, 2011) and shown to interact with
stressors to predict anxiety sensitivity (an
established risk factor for PD; Stein, Schork,
& Gelernter, 2008) and onset of symptoms
of emotional disorders (e.g., Caspi et al.,
2003). Genetic findings have been linked to
neural models of anxiety disorders, in that
associations have been observed between
these polymorphisms and both activation
of neural substrates of emotion generation
(e.g., Drabant et al., 2012; Munafo, Brown,
& Hariri, 2008) and functional connectiv-
ity between emotion- generating regions and
structures implicated in their inhibitory
control (Pezawas et al., 2005). Importantly,
hyperactivation of limbic structures impli-
cated in emotion generation (e.g., amygdala,
insula) coupled with reduced inhibitory cor-
tical control of these structures has been
found across anxiety disorders (e.g., Etkin
& Wager, 2007; Shin & Liberzon, 2010).
Empirical support for a generalized psy-
chological vulnerability to anxiety disorders
is also evident. Anxious individuals con-
sistently display attentional biases toward
negative or threatening cues, as well as dif-
ficulty disengaging from negative stimuli
(Beck & Clark, 1997; MacLeod, Mathews,
& Tata, 1986; Olantunji & Wolitzky- Taylor,
2009; Pineles & Mineka, 2005). These cog-
nitive biases toward negative information
have been shown to predict elevated anxi-
ety symptoms in response to stress (e.g.,
MacLeod & Hagan, 1992; van den Hout,
Tenney, Huygens, Merckelbach, & Kindt,
1995). Additionally, traits reflecting dimin-
ished tolerance for distress, physical sensa-
tions, and other types of discomfort (e.g.,
uncertainty) are associated with a range
of anxiety and mood disorders (Boswell,
Thompson- Holland, Farchione, & Barlow,
2013; Keough, Riccardi, Timpano, Mitch-
ell, & Schmidt, 2010; McEvoy & Mahoney,
2012; Olatunji & Wolitzky- Taylor, 2009)
and in some cases have been shown to pre-
dict onset of anxiety disorders following
stress (Schmidt, Lerew, & Jackson, 1999) or
to mediate change in anxiety symptoms over
time (Dugas, Laugesen, & Bukowski, 2012).
Implications for Emotion Regulation
in Anxiety Disorders
The underlying features of anxiety disorders
described earlier (i.e., heightened emotional
reactivity, hypersensitivity to threat, and
tendencies to experience emotions as aver-
sive) likely have a considerable impact on
the ER of anxious individuals. These influ-
ences may occur at multiple points along
the temporal continuum of ER (see Gross,
this volume), beginning at the earliest stages
when automatic processes related to atten-
tion and awareness are engaged. Attention
and automatic responses to threat occur
within milliseconds and influence further
information processing almost immediately
(e.g., Garner, Mogg, & Bradley, 2006). As
noted earlier, an extensive literature sug-
gests that attentional biases toward threat
and other negative information are key fea-
tures of anxiety disorders (Beck & Clark,
1997; MacLeod et al., 1986; Olantunji &
Wolitzky- Taylor, 2009; Pineles & Mineka,
2005). Such biases in the early processing of
emotional stimuli may provoke a range of
subsequent responses, including behavioral
efforts to dampen responding (e.g., gaze
aversion; Garner et al., 2006) and attempts
to escape threatening information via cog-
nitive control (e.g., Leutgeb, Schäfer, &
Schienle, 2009; Paquette et al., 2003).
According to the “hypervigilance–
avoidance” model, the early attentional
biases toward threat that characterize anxi-
ety disorders are paradoxically followed
by reduced processing of threat (e.g., via
cognitive avoidance) and increased avoid-
ant responding. This model converges with
behavioral and neuroimaging findings (e.g.,
Bishop, Duncan, Brett, & Lawrence, 2004;
Koster, Crombez, Verschuere, Van Damme,
& Wiersema, 2006; see Hofmann, Ellard,
& Siegle, 2012, for a review), and with the
clinical consensus that cognitive and behav-
ioral avoidance are integral to the psycho-
pathology of anxiety disorders. Examples
from clinical observation include avoid-
ance of situational and interoceptive (i.e.,
bodily sensation) cues in PD; avoidance of
eye contact and social interactions in SAD;
avoidance of contamination through wash-
ing, doubting thoughts through checking, or
intrusive images through repetitive phrases
in obsessive– compulsive disorder (OCD);
avoidance of trauma reminders in posttrau-
matic stress disorder (PTSD); and avoidance
of anxious arousal and uncertainty through
worry and reassurance seeking in general-
ized anxiety disorder (GAD).
396 PSYCHOPATHOLOGY
Tendencies toward cognitive and behav-
ioral avoidance likely have broad impacts on
anxious individuals’ ER. Available evidence
suggests that engagement with emotion-
ally salient stimuli generally promotes more
effective ER than avoidance or disengage-
ment. For instance, a study that employed
eye- tracking demonstrated that regardless of
the specific ER strategy used (cognitive reap-
praisal or suppression), subjects who focused
visual attention more on the emotionally
salient aspects of a stimulus were more likely
to regulate emotions successfully (Bebko,
Franconeri, Ochsner, & Chiao, 2011). In
addition, simply labeling emotions (which
involves awareness and acknowledgment
of emotions) has been shown to reduce dis-
tress as effectively as “active” ER strategies
such as cognitive reappraisal and distraction
(Lieberman, Inagaki, Tabibnia, & Crockett,
2011). Anxious subjects’ tendencies to avoid
deeper processing of emotional stimuli may
preclude them from experiencing the ben-
efits of engagement- oriented responses to
emotional stimuli.
Relationship of Anxiety Disorders
to ER: General or Specific?
Thus far we have discussed anxiety as a
global trait, without distinguishing among
the anxiety disorders (cf. Barlow, Sauer-
Zavala, Carl, Bullis, & Ellard, 2013). As
we move toward discussion of specific ER
strategies, we will for the most part continue
to refer to anxiety disorders collectively. We
should, however, note that there has been
particular interest in the relationship of
ER to GAD (e.g., McLaughlin, Mennin, &
Farach, 2007; Mennin, Heimberg, Turk, &
Fresco, 2005; Mennin, McLaughlin, & Fla-
nagan, 2009; Salters- Pedneault, Roemer,
Tull, Rucker, & Mennin, 2006; Turk, Heim-
berg, Luterek, Mennin, & Fresco, 2005). The
emotion dysregulation model of GAD posits
that this disorder results from a convergence
of high levels of emotional reactivity and
intensity, dysfunctional meta- emotion (e.g.,
decreased clarity and understanding of emo-
tions), and maladaptive efforts to regulate
emotions (Mennin et al., 2005; Mennin &
Fresco, this volume). GAD has been con-
ceptualized as the “basic” anxiety disorder
given that its defining features (e.g., anxious
apprehension) reflect processes that contrib-
ute to the manifestation of symptoms in all
anxiety disorders (Barlow, 1988; Brown,
Barlow, & Liebowitz, 1994). Thus, findings
related to emotion dysregulation in GAD
may reflect processes found in anxiety dis-
orders more generally.
In support of this view, several analogous
findings emerge when considering emotional
experience and ER across specific anxiety
disorders. For instance, decreased emotional
clarity has been associated with symptoms
of GAD, PD, PTSD, and SAD (Baker, Hol-
loway, Thomas, Thomas, & Owens, 2004;
McLaughlin et al., 2007; Tull & Roemer,
2007; Weiss et al., 2012). Decreased accep-
tance of emotions has been found in GAD,
PD, and PTSD (McLaughlin et al., 2007;
Tull & Roemer, 2007; Weiss et al., 2012),
and in one study individuals with SAD were
not clearly differentiated from individuals
with GAD on this variable (Mennin et al.,
2009). Individuals with symptoms of PTSD
(e.g., Ehring & Quack, 2010; Weiss et al.,
2012), GAD (Mennin et al., 2005, 2009;
Salters- Pedneault et al., 2006), and SAD
(Mennin et al., 2009; Turk et al., 2005) also
endorse difficulties with repairing negative
mood and accessing effective ER strategies
when distressed (these dimensions of ER
were not assessed in available studies of PD).
Recent meta- analytic work also supports
the view that maladaptive ER represents
a transdiagnostic feature of anxiety and
related emotional disorders. An analysis of
114 studies suggested comparable relation-
ships of ER strategies to anxiety and mood
disorder symptoms, which tended to display
stronger relationships to ER than did eat-
ing and substance use disorder symptoms
(Aldao, Nolen- Hoeksema, & Schweizer,
2010). Avoidance, rumination, and suppres-
sion were positively related to anxiety and
depression, whereas problem solving and
cognitive reappraisal were negatively associ-
ated with both types of symptoms (though
the reappraisal– anxiety relationship was
only marginally significant, it was similar
in magnitude to the reappraisal– depression
relationship). Another investigation found
that a latent “cognitive ER” factor (defined
by cognitive reappraisal, problem solving,
suppression, and rumination) was signifi-
cantly associated with symptoms of three
classes of disorder: anxiety, mood, and eat-
ing disorders (Aldao & Nolen- Hoeksema,
2010). The strongest association of the cog-
nitive ER factor was with depressive symp-

Emotion Regulation in Anxiety Disorders 397
toms, followed by anxiety symptoms, then
eating symptoms.
Sum mary
Anxiety disorders are understood to emerge
from a combination of biological vulner-
abilities, psychological vulnerabilities, and
stress. The vulnerabilities that underlie
anxiety disorders have numerous potential
impacts on the ER process. At the earliest
stages, hypersensitivity to threat constrains
attention, thereby limiting perceptual infor-
mation and increasing threat salience. This
appears to set the stage for attempts to reg-
ulate away from the threat either through
cognitive control strategies or behavioral
strategies that limit ongoing contact with
the threatening stimuli. These types of
avoidant responses may preclude effective
ER that derives from engagement with emo-
tional stimuli and recognition of emotions.
Available evidence suggests that these basic
emotion- processing and ER tendencies are a
common factor across anxiety disorders.
Investigating Use of Specific ER
Strategies in Anxiety Disorders
Having provided an overview of our concep-
tual framework for considering ER in rela-
tion to anxiety disorders, we now turn to
discussion of specific ER strategies that have
been the focus of most empirical work on
this topic. We have chosen to highlight three
ER strategies: cognitive reappraisal, suppres-
sion, and acceptance. These strategies have
been strongly represented in recent empirical
work and in development of treatments for
anxiety disorders. For our purposes in this
chapter, we examine acceptance alongside
the “straightforward” ER strategies (cog-
nitive reappraisal and suppression), while
recognizing that acceptance is perhaps more
precisely characterized as an “orientation”
or “attitude” toward emotions rather than
a regulation strategy per se (i.e., by defini-
tion, acceptance involves a relinquishing of
efforts to modify emotional experience). We
also should note that many other ER strate-
gies are potentially relevant to anxiety; some
of these (e.g., rumination, distraction) have
been more extensively studied in relation to
mood disorders and are covered by Joor-
mann and Siemer (this volume).
Suppression
Suppression is a response- focused ER strat-
egy that occurs late in the temporal unfold-
ing of the emotional response (Gross, this
volume). Suppression can be directed spe-
cifically toward inhibition of the behav-
ioral expression of emotion (expressive sup-
pression) and/or toward the dampening of
subjective feelings (emotion suppression).
Studies of healthy subjects indicate that
expressive suppression (relative to passive
viewing of emotional stimuli) effectively
reduces expressive behavior; however, it also
increases sympathetic arousal and fails to
reduce the intensity of the subjective experi-
ence of emotion (Gross & Levenson, 1993,
1997). Neuroimaging work further suggests
that expressive suppression increases acti-
vation of emotion- generating brain regions
(e.g., amygdala and insula; Goldin, McRae,
Ramel, & Gross, 2008).
Investigations of suppression with anxiety-
prone subjects report similar results. Several
studies have employed biological challenges
involving inhalation of carbon dioxide–
enriched air, which can provoke panic
symptoms. In these studies, instructions to
suppress emotions did not lead to reduced
subjective distress in subjects with high anx-
iety sensitivity (Eifert & Heffner, 2003) or in
patients diagnosed with PD (Levitt, Brown,
Orsillo, & Barlow, 2004). A study using a
mixed anxiety and mood disorder sample
also found that instructions to suppress
emotions during an emotion- provoking film
did not reduce the subjective experience of
emotion, and were associated with increased
heart rate when compared to instructions to
accept emotions during the film (Campbell-
Sills, Barlow, Brown, & Hofmann, 2006a).
This study also measured recovery of emo-
tional responses, and showed that subjects
instructed to suppress emotions during the
film reported higher levels of distress during
the postfilm recovery period than did sub-
jects who had accepted emotions. This lat-
ter finding raises the possibility that another
detrimental effect of suppression could be
interference with recovery of emotions fol-
lowing termination of an emotion- provoking
stimulus.
While these results suggest that suppres-
sion is generally an ineffective ER strategy
for both healthy and anxious individuals,
it should be noted that suppression is likely
398 PSYCHOPATHOLOGY
beneficial under certain circumstances. For
example, suppression after exposure to
traumatic scenes was shown to reduce both
subjective distress during exposure and later
intrusions (Dunn, Billotti, Murphy, & Dal-
gleish, 2009). It may not be the engagement
of suppression per se, but the rigid or habit-
ual use of this ER strategy, that is maladap-
tive (cf. Bonanno, Papa, Lalande, Westphal,
& Coifman, 2004).
Cognitive Reappraisal
Cognitive reappraisal entails thinking about
stimuli in a way that diminishes the inten-
sity of emotions. For example, while view-
ing mourners at a funeral, a person using
cognitive reappraisal might think that the
deceased person is at peace and that the
mourners will recover with time. This strat-
egy is conceptualized as an antecedent-
focused strategy (i.e., one that is deployed
before the full-scale emotional response has
occurred; Gross, this volume). Use of cogni-
tive reappraisal to manage negative emotions
is associated with a range of beneficial effects
in healthy subjects. These include reduced
intensity of the subjective experience of emo-
tion (Bebko et al., 2011; Gross, 1998; Hof-
mann, Heering, Sawyer, & Asnaani, 2009),
reduced expressive behavior (Gross, 1998),
attenuation of startle (Jackson, Malmstadt,
Larson, & Davidson, 2000), enhanced para-
sympathetic tone (Aldao & Mennin, 2012),
reduced behavioral avoidance (Wolgast,
Lundh, & Viborg, 2011), and reduced amyg-
dala activation (e.g., Goldin et al., 2008;
Ochsner, Bunge, Gross, & Gabrieli, 2002;
Ochsner, Ray, et al., 2004).
Several of these outcomes have not been
measured in studies of cognitive reappraisal
that included anxious subjects. However, the
most frequently reported benefit of cognitive
reappraisal— reduced intensity of the subjec-
tive experience of emotion— has been dem-
onstrated in individuals high in trait anxiety
(Campbell- Sills et al., 2011) and in subjects
diagnosed with anxiety disorders (Aldao &
Mennin, 2012; Ball, Ramsawh, Campbell-
Sills, Paulus, & Stein, 2012). Available evi-
dence suggests divergent effects of cognitive
reappraisal on the physiological responses
and brain function of anxious and nonanx-
ious individuals; these findings are discussed
in detail below (“Pathways to Ineffective ER
in Anxiety Disorders”).
Acceptance
Acceptance entails allowing oneself to
experience emotions without attempting to
alter or suppress them. Acceptance- based
interventions promote accepting attitudes
toward emotions (e.g., emotions have a nat-
ural trajectory and will dissipate if allowed
to run their course) and explicitly discour-
age suppression. The concept of acceptance
shares features with the concept of mind-
fulness, which promotes nonjudgmental,
present- moment awareness of emotions and
other internal experiences. Acceptance and
mindfulness are intended to increase sub-
jects’ awareness of their emotions (including
habitual impulses to suppress or avoid dis-
comfort), which may provide them greater
opportunity to respond to emotions adap-
tively (Roemer, Orsillo, & Salters- Pedneault,
2008; Hill & Updegraff, 2012).
Experimental studies of healthy and anx-
ious samples have shown that instructions to
accept emotions are associated with reduc-
tions in subjective distress and behavioral
avoidance (Eifert & Heffner, 2003; Levitt et
al., 2004; Wolgast et al., 2011). For exam-
ple, studies employing carbon dioxide chal-
lenges have shown that anxious subjects who
accepted emotions reported less intense fear,
fewer catastrophic thoughts, and greater
willingness to complete another biological
challenge when compared to subjects who
either suppressed emotions or received no
instructions (Eifert & Heffner, 2003; Levitt
et al., 2004). Another study of a mixed anxi-
ety and mood disorder sample showed that,
compared to suppression, engaging in accep-
tance resulted in reduced heart rate during
emotion- provoking films and faster recovery
of subjective distress (Campbell- Sills et al.,
2006a).
Summary
Results of experiments instructing subjects
to use specific ER strategies support the view
that reappraisal and acceptance are generally
adaptive strategies, whereas suppression is
adaptive under a more restricted range of cir-
cumstances (and can often be maladaptive).
The available literature (though still limited)
suggests that, like healthy subjects, individ-
uals with anxiety disorders derive benefits
from cognitive reappraisal and acceptance
(e.g., reduced subjective distress) and experi-

Emotion Regulation in Anxiety Disorders 399
ence detrimental effects of suppression (e.g.,
increased physiological arousal).
Pathways to Ineffective ER
in Anxiety Disorders
Individuals with anxiety disorders report
wide- ranging difficulties related to emo-
tional experience and ER (Baker et al., 2004;
Ehring & Quack, 2010; McLaughlin et al.,
2007; Mennin et al., 2005, 2009; Salters-
Pedneault et al., 2006; Tull & Roemer,
2007; Turk et al., 2005; Weiss et al., 2012).
Consideration of the literature focused on
specific ER strategies such as suppression,
cognitive reappraisal, and acceptance raises
a number of possible explanations for these
ER difficulties. In this section, we consider
the following potential pathways to ineffec-
tive ER in anxiety disorders:
1. Strategy selection. Anxious individuals
might select maladaptive strategies more
often, or adaptive strategies less often,
than nonanxious individuals.
2. ER ability. Anxious individuals may be
less competent in applying ER strategies.
3. Divergent effects of ER strategies. The
same ER strategy, applied with a similar
level of competence, may have different
effects for individuals with and without
anxiety disorders.
4. Neurobiological differences. Function-
ing of neural substrates supporting spe-
cific ER strategies may be compromised
in anxious individuals.
Strategy Selection
Neurobiology, temperament, learning his-
tory, and other factors likely interact to
determine individual differences in propensi-
ties to use specific ER strategies (e.g., see, in
this volume, John & Eng; Rothbart, Sheese,
& Posner; and Thompson). Consistent with
experimental results, questionnaire studies
suggest that habitual use of reappraisal to
manage emotions is associated with a range
of positive outcomes, including higher lev-
els of overall positive affect and well-being,
lower levels of overall negative affect, better
interpersonal functioning, and fewer symp-
toms of anxiety and mood disorders (Aldao
& Nolen- Hoeksema, 2010; Aldao et al.,
2010; Eftekhari, Zoellner, & Vigil, 2009;
Gross & John, 2003). In contrast, habitual
use of suppression is related to lower levels
of overall positive affect and well-being;
higher levels of overall negative affect;
poorer interpersonal functioning; and more
symptoms of anxiety, mood, and eating dis-
orders (Aldao & Nolen- Hoeksema, 2010;
Aldao et al., 2010; Gross & John, 2003).
Though effects of habitual use of acceptance
have not been investigated, habitual mind-
fulness has been associated with lower lev-
els of daily anxiety and depression, reduced
emotional reactivity to laboratory stressors,
improved abilities to differentiate emotions,
decreased emotional lability, and fewer self-
reported ER difficulties (Arch & Craske,
2010; Hill & Updegraff, 2012).
When selecting strategies to regulate emo-
tional experiences, there is evidence that
individuals with anxiety disorders rely more
on maladaptive strategies, such as suppres-
sion, and less on adaptive strategies, such
as reappraisal and acceptance. For instance,
studies using both survey- based and experi-
mental methods have found that individuals
with anxiety disorders utilize suppression
more than do nonanxious individuals (Baker
et al., 2004; Campbell- Sills et al., 2006a;
Levitt et al., 2004; Ball et al., 2012). With
regard to adaptive strategy use, subjects
with GAD have been found to endorse less
frequent use of cognitive reappraisal (Ball et
al., 2012) and acceptance (Salters- Pedneault
et al., 2006). Children and adolescents diag-
nosed with anxiety disorders also report
less frequent use of reappraisal in everyday
life than do nonanxious subjects (Carthy,
Horesh, Apter, Edge, & Gross, 2010).
A critical issue related to ER strategy
selection in anxiety disorders is the role of
emotional intensity. Empirical work has now
shown that for healthy controls, ER strategy
preference depends on situation intensity.
Engagement of reappraisal is preferred in
lower intensity situations, whereas disen-
gagement/distraction is preferred in higher
intensity situations (Sheppes, Scheibe, Suri,
& Gross, 2011). Due to differences in base-
line emotional state, emotional reactivity,
and attentional biases, the same situation
may be significantly more intense for anx-
ious than for nonanxious individuals. The
more intense emotions elicited by the situa-
tion may in turn create different conditions
for selection of ER strategies; for instance,
facilitating a disengagement- oriented strat-
400 PSYCHOPATHOLOGY
egy (e.g., suppression) in a situation where
the lower intensity emotions of healthy
individuals would prompt an engagement-
oriented strategy (e.g., cognitive reappraisal).
A variety of other factors may contribute
to the observed differences between anxious
and nonanxious subjects in the selection of
ER strategies. With respect to reappraisal,
an expanded awareness is necessary to allow
for the processing of alternative interpreta-
tions. The narrowed attention fostered by
attentional biases associated with anxiety
disorders may preclude processing of alter-
nate stimulus meanings, a necessary compo-
nent of successful reappraisal. Similarly, the
propensity toward hypervigilance followed
by avoidant processing may preclude engage-
ment of mindfulness and acceptance, both
of which require openness toward all experi-
ence, including that which is uncomfortable.
By contrast, suppression and behavioral
avoidance naturally emanate from avoid-
ant processing, and may therefore be more
readily recruited by individuals with anxiety
disorders.
ER strategy selection also may relate to
individual differences in abilities to use
executive control to mitigate the impact of
emotional stimuli. Recent work suggests
that individuals who habitually use cogni-
tive reappraisal may have an enhanced abil-
ity to use executive control in the service
of regulating emotions (Cohen, Henik, &
Moyal, 2012; Gyurak, Goodkind, Kramer,
Miller, & Levenson, 2012). By contrast,
individuals with anxiety disorders may have
compromised abilities in this regard. This
view is consistent with findings of neuro-
imaging studies (discussed in more detail
below) that show reduced prefrontal cortex
(PFC) activation in individuals with anxi-
ety disorders using reappraisal to regulate
emotions (Goldin, Manber-Ball, Werner,
Heimberg, & Gross, 2009; Goldin, Manber,
Hakimi, Canli, & Gross, 2009; Ball et al.,
2012; New et al., 2009), and the observa-
tion that nonclinical, anxiety- prone subjects
require greater PFC resources than healthy
controls to down- regulate negative emo-
tions with reappraisal (Campbell- Sills et al.,
2011). Diminished abilities to recruit execu-
tive control networks early in the processing
of emotional stimuli could also lead to over-
reliance on suppression (a strategy deployed
later in the temporal continuum of ER) to
manage negative emotions.
Another factor relevant to ER strategy
selection is subjects’ “meta- experience” of
emotion, which includes dimensions such
as attitudes toward emotions, understand-
ing of emotions, emotional clarity, and
acceptability of emotions. As noted earlier,
individuals with anxiety disorders exhibit
higher levels of traits reflecting diminished
tolerance for emotional distress and other
types of discomfort, as well as decreased
clarity and acceptability of emotions. While
studies directly examining the relationships
between meta- experiences of emotion and
selection of ER strategies have been sparse,
one study using a mixed anxiety and mood
disorder sample showed that less accepting
attitudes toward current emotions mediated
the relationship between negative emotion
intensity and spontaneous use of suppression
(Campbell- Sills et al., 2006a). Thus, more
intense negative emotion prompted subjects
to engage in suppression only when the emo-
tions were experienced as less acceptable.
This finding suggests that meta- experience
of emotion may influence strategy selection
in individuals with anxiety disorders.
ER Ability
Individual differences in abilities to imple-
ment ER strategies undoubtedly exist
and may be relevant to the ER difficulties
reported by anxious subjects. However,
very few studies have evaluated the levels
of competence that subjects have in apply-
ing specific ER strategies. This research aim
requires methods for assessing the quality
of the process by which individuals engage
in ER strategies. One exception is Carthy et
al.’s (2010) analysis of cognitive reappraisal
abilities demonstrated by children with and
without anxiety disorders during a labora-
tory ER task. In this study, subjects recited
their reappraisals out loud during the task
(which utilized negative images) and their
responses were coded as reappraisal suc-
cesses or failures. While children with anxi-
ety disorders were able to reappraise the
majority of stimuli successfully (87%), they
demonstrated slightly but significantly lower
reappraisal abilities than healthy controls
(93%). One other study reported on the pro-
cess of training subjects to use reappraisal to
down- regulate emotions elicited by negative
images (Campbell- Sills et al., 2011). Anxious
and healthy control groups did not differ in
Emotion Regulation in Anxiety Disorders 401
their need for extra training in reappraisal
prior to performing the experimental task.
More work is needed to elucidate whether
there are important differences in abilities
of anxious and nonanxious individuals to
apply cognitive reappraisal and other ER
strategies.
Divergent Effects of ER Strategies
Even when the same ER strategy is selected
and applied with a similar level of compe-
tence, its effects may vary for anxious and
nonanxious individuals. In particular, the
beneficial effects of adaptive strategies could
be attenuated, or the negative effects of mal-
adaptive strategies could be amplified, lead-
ing to greater ER difficulties in individuals
with anxiety disorders.
Two studies suggest that the costs of sup-
pression may be higher for anxious individu-
als. In one study, the effects of suppression
and no instructions were compared in sub-
jects high and low in trait negative affect
(a trait strongly associated with anxiety
and mood disorders; Barlow, 2002; Brown
& Barlow, 2009). Individuals with high
negative affect who engaged in suppression
showed a paradoxical increase in negative
emotions when writing about a negative
personal memory, whereas those with low
negative affect did not (Dalgleish, Yiend,
Schweizer, & Dunn, 2009). Another study
found that instructions to suppress emo-
tions were associated with increased subjec-
tive distress in subjects with elevated scores
on a measure of experiential avoidance (a
trait also associated with anxiety disorders;
Feldner, Zvolensky, Eifert, & Spira, 2003).
The increased sympathetic arousal found
to accompany suppression may render this
strategy particularly ineffective for anxiety-
prone individuals, who are sensitive to and
often fear physical symptoms.
Research suggests that under controlled
experimental conditions (i.e., when sub-
jects are trained to reappraise standard-
ized emotional stimuli), anxious subjects
can achieve reductions in subjective distress
that are comparable to those observed in
healthy subjects (Campbell- Sills et al., 2011;
Ball et al., 2012). However, another recent
study suggests that even when the subjec-
tive outcomes of cognitive reappraisal are
equivalent, the physiological benefits of this
strategy may be compromised for anxious
individuals. In this study, subjects with
GAD and healthy controls were assigned
to cognitive reappraisal, acceptance, or
passive viewing conditions (Aldao & Men-
nin, 2012). Their subjective experience and
heart rate variability (HRV) were measured
while they viewed films that elicited anxi-
ety, sadness, and disgust. HRV, an index
of parasympathetic influence on the heart,
reflects the capacity of the autonomic ner-
vous system to respond adaptively to chang-
ing environmental conditions. HRV tends
to decrease under conditions of threat and
to increase under conditions of flexible and
adaptive regulation (Friedman, 2007).
Results revealed that subjects who used
cognitive reappraisal reported lower subjec-
tive negative affect in response to the films
compared to subjects who used acceptance
or who engaged in passive viewing (which
did not differ). This pattern was consistent
across the GAD and healthy control groups.
With respect to physiological effects, sub-
jects with GAD displayed significantly
lower HRV than healthy controls across
all film types and regulation conditions.
These findings were consistent with previ-
ous reports of low parasympathetic tone
in GAD (Thayer, Friedman, & Borkovec,
1996). Moreover, during recovery from anx-
iety- and disgust- provoking films, subjects
with GAD showed an HRV pattern that was
opposite to that displayed by healthy con-
trols. Healthy subjects who used reappraisal
and acceptance showed higher HRV than
those who had received no instructions,
suggesting enhanced parasympathetic tone
with adaptive ER. In contrast, subjects with
GAD who used reappraisal and acceptance
showed lower HRV than those who received
no instructions. This pattern of parasym-
pathetic response may indicate diminished
efficacy of reappraisal and acceptance in
GAD that is only observed at the physiologi-
cal level.
Aldao and Mennin’s (2012) HRV find-
ings converge with those of another study of
reappraisal in healthy individuals classified
as high or low on neuroticism, a personality
trait strongly related to anxiety and mood
disorders (e.g., Brown, Chorpita, & Barlow,
1998; Barlow et al., 2013; Wilamowska et
al., 2010). In this study (Di Simplicio et al.,
2012), “low neuroticism” subjects showed
the expected pattern of higher HRV when
regulating emotions (vs. maintaining emo-
402 PSYCHOPATHOLOGY
tions), while “high neuroticism” subjects
showed no significant difference in HRV
across the two conditions. These results sig-
nify low parasympathetic tone in the high
neuroticism group, as well as reduced physi-
ological benefits of cognitive reappraisal for
anxiety- prone individuals.
Finally, a recent ecological study of anx-
ious and healthy children found differences
in the efficacy of ER strategies that were
related to anxiety disorder status (Tan et al.,
2012). This study assessed emotional reac-
tions to negative events in the past hour, use
of ER strategies (distraction, reappraisal,
problem solving, acceptance, avoidance, and
rumination), and current emotional experi-
ence. Interestingly, children with anxiety
disorders endorsed using the various ER
strategies with the same frequency as non-
anxious youth. Examination of strategy use
in relation to peak and current emotional
intensity ratings revealed that some adaptive
strategies (e.g., reappraisal) were equally
effective in down- regulating subjective dis-
tress in children with and without anxiety
disorders. However, the data suggested that
acceptance was only effective for decreasing
subjective distress in nonanxious children.
Neurobiological Differences
Another factor that may contribute to inef-
fective ER in anxiety disorders is dysfunc-
tion of neural systems supporting ER. The
majority of research on this topic has focused
on cognitive reappraisal, and these studies
have revealed several potentially important
neural correlates of anxious subjects’ use of
this strategy.
One pattern that has now been observed
in participants diagnosed with several dis-
tinct anxiety disorders (GAD, SAD, PD,
and PTSD) is hypoactivation of PFC regions
implicated in “top-down” control of emo-
tions during reappraisal of emotional stim-
uli (Ball et al., 2012; Goldin, Manber, et al.,
2009; Goldin, Manber-Ball, et al., 2009;
New et al., 2009). This suggests that less
robust engagement of PFC may contribute
to ER difficulties across anxiety disorders.
One recent study (Ball et al., 2012) showed
that subjects with GAD and PD who were
trained to use cognitive reappraisal achieved
similar reductions in subjective distress com-
pared to healthy controls who received the
same training. However, the subjects with
GAD and PD displayed decreased dorsome-
dial and dorsolateral PFC activation while
implementing this strategy. Moreover, PFC
activation during reappraisal was inversely
related to anxiety severity (e.g., subjects with
the highest levels of anxiety severity dis-
played the lowest levels of left dorsolateral
PFC activation), providing additional evi-
dence of a significant relationship between
anxiety and PFC function during ER.
Functional abnormalities also were
observed when subjects with SAD were
instructed to use cognitive reappraisal to
reduce emotions elicited by personally rel-
evant statements (Goldin, Manber-Ball,
et al., 2009). In this study, potentiated
responses in regions such as the medial PFC
and amygdala delayed normal cognitive pro-
cessing in the dorsolateral PFC, which only
occurred after a temporal lag relative to that
in healthy controls. One possible interpreta-
tion of this pattern is that anxious individu-
als must overcome an initial increased aver-
sive response (reflected in medial PFC and
amygdala hyperactivity) before implement-
ing reappraisal (reflected in delayed dorso-
lateral PFC processing).
Dysfunction of neural circuitry support-
ing cognitive reappraisal (and other forms of
ER) may be context dependent. In particu-
lar, dysfunction may be exacerbated in the
presence of emotional cues that represent the
focus of the individual’s anxiety disorder. A
study of individuals with SAD found differ-
ences in neural activation that were related
to stimulus type (Goldin, Manber, et al.,
2009). When regulating responses to images
depicting physical threat, no differences
emerged between subjects with SAD and
healthy controls. However, when regulating
reactions to social threat, subjects with SAD
displayed reduced activation in brain regions
implicated in cognitive control (dorsolat-
eral PFC, dorsal anterior cingulate cortex),
visual attention (medial cuneus, posterior
cingulate), attention (bilateral dorsal pari-
etal), and visual feature detection (bilateral
fusiform, superior temporal gyrus). These
findings suggest that in SAD the coordina-
tion of cognitive control circuitry may be
selectively compromised during the regula-
tion of social threat.
Few neuroimaging studies have exam-
ined anxious subjects’ use of ER strategies
Emotion Regulation in Anxiety Disorders 403
other than reappraisal. An exception, a
recent investigation of subjects with GAD,
compared the effects of acceptance, sup-
pression, and worry on subjective and neu-
ral responses to personally relevant worry
statements (Ellard, 2012). Both acceptance
and suppression were associated with less
subjective distress and amygdala activation
than worry. However, engagement of PFC
structures to meet these regulatory goals
differed for the two conditions. Suppression
was associated with increased activation
of insula and of right lateral PFC regions
implicated in working memory and the gen-
eration of inner speech, which suggested
paradoxically greater engagement of brain
regions implicated in somatic and linguistic
representations of emotional stimuli. On the
other hand, acceptance was associated with
reduced insula activation and greater left
dorsomedial PFC activation. The dorsome-
dial PFC has been implicated in representa-
tions of meta- states of self- awareness, such
as making inferences about moment- to-
moment feelings (Ochsner, Knierim, et al.,
2004), intentions for action (Lau, Rogers,
Haggard, & Passingham, 2004), and tacti-
cal response selection (Matsuzaka, Akiya-
maa, Tanjib, & Mushiake, 2012). Addition-
ally, left lateralization of PFC activation has
been associated with approach motivation,
whereas right lateralized activation has been
associated with avoidance (Speilberg et al.,
2011). Importantly, acceptance also engaged
ventromedial PFC regions implicated in
extinction learning, which may relate to
behavioral data suggesting greater willing-
ness to reengage with aversive stimuli fol-
lowing acceptance (Eifert & Heffner, 2003;
Levitt et al., 2004).
Studies of functional connectivity mea-
sured during resting states also contribute
to our understanding of ER in anxiety dis-
orders. Deviations in functional connectiv-
ity between subregions of the amygdala and
a broad network of somatosensory asso-
ciation cortices, motor regions, memory
regions, and regions associated with self-
referential processing have been observed
in individuals with GAD (Etkin, Prater,
Schatzberg, Menon, & Greicius, 2009).
Increased amygdala– dorsolateral PFC con-
nectivity was also found in patients with
GAD, whereas no such functional connec-
tivity was found in healthy controls. This
finding is intriguing given the consistent
implication of dorsolateral PFC in down-
regulation of negative emotions via cogni-
tive reappraisal (e.g., Goldin et al., 2008;
Ochsner et al., 2002; Ochsner, Ray, et al.,
2004). The greater amygdala– dorsolateral
PFC coupling in GAD may represent a com-
pensatory mechanism that functions as an
attempt to dampen emotional responding
through cognitive control (cf. Borkovec,
Alcaine, & Behar, 2004). Such a compen-
satory mechanism may in fact be counter-
productive for anxious individuals given the
observation that worry is associated with
increased amygdala activation in subjects
with GAD (Ellard, 2012).
Finally, it should be noted that more
complex and fine- grained analyses are
needed to further elucidate the neural bases
of ER difficulties in anxiety disorders. A
recent pathway mapping analysis identified
subcortical regions that mediated the rela-
tionship between PFC activation and reap-
praisal success in healthy subjects (Wager,
Davidson, Hughes, Lindquist, & Ochsner,
2008). Two distinct pathways— one through
the amygdala (associated with reduced
reappraisal success) and one through the
nucleus accumbens and ventral striatum
(associated with greater reappraisal suc-
cess)—were identified. This implies that
successful reappraisal may involve both the
dampening of amygdala responses by the
PFC and an increase in nucleus accumbens/
ventral striatum activation, which may
facilitate approach toward the emotion-
provoking stimulus. Extrapolating to the
case of ER in anxiety disorders, these
results raise the possibility that dysfunction
may extend beyond regions implicated in
cognition and emotion to include regions
implicated in processing sensory informa-
tion and choosing an adaptive behavioral
response. Specifically, ER difficulties across
anxiety disorders may be characterized by
not only deficits in cortical control of affec-
tive responses but also a failure to bring
appropriate approach- and reward- related
behaviors online (e.g., Etkin, Prater, Hoeft,
Menon, & Schatzberg, 2010). These find-
ings also suggest that cortical control of
limbic responses represents only one aspect
of widespread processing necessary in order
to achieve regulatory goals through reap-
praisal.

404 PSYCHOPATHOLOGY
Summary
The available evidence suggests that the
problems with ER reported by individuals
with anxiety disorders may be partly due to
strategy selection, because anxious individu-
als appear to select the maladaptive strategy
of suppression more frequently and adap-
tive strategies such as cognitive reappraisal
and acceptance less frequently. This may be
attributable to the more intense emotions
experienced by anxiety- prone individuals or
to their more negative meta- experiences of
emotions, both of which may favor avoid-
ant and less cognitively demanding methods
for regulating emotions (e.g., suppression).
Biases against use of reappraisal may relate
to diminished abilities of anxious individu-
als to use executive control to modulate emo-
tions, or to narrowed (i.e., threat- focused)
attention that does not allow for process-
ing of alternative appraisals. Overreliance
on suppression may be particularly costly
for anxious individuals given evidence that
they are more susceptible to paradoxical
increases in distress when using suppression
to manage emotions.
More research is needed to determine
whether individuals with anxiety disorders
are less competent in applying adaptive
strategies (e.g., cognitive reappraisal) once
they have been prompted to use them. Avail-
able evidence suggests that when trained to
use reappraisal, anxious and nonanxious
subjects achieve similar reductions in subjec-
tive distress. However, reappraisal may be
less effective in promoting enhanced para-
sympathetic tone in anxious individuals.
The growing neuroimaging literature on ER
and anxiety disorders further suggests a fail-
ure to recruit PFC effectively and efficiently
during cognitive reappraisal. Dysfunction
of neural substrates of ER likely constitutes
another basis of the regulatory difficulties
reported by individuals with anxiety disor-
ders.
Maladaptive ER:
Risk Factor or Epiphenomenon
of Anxiety Disorders?
An important issue we have yet to address
is the directionality of relationships
between anxiety- and ER-related constructs.
Although the reviewed associations between
ER strategies and anxiety disorders are com-
pelling, it is not possible to ascertain causal
relationships due to the cross- sectional
nature of virtually all available studies. For
instance, the findings that anxious subjects
use suppression more than healthy controls
could be explained by habitual suppression
being a risk factor for anxiety disorders.
However, it is also plausible that the intense
emotions that characterize anxiety disorders
could overwhelm the subject to the extent
that more cognitively demanding ER strate-
gies (e.g., reappraisal) are less available and
suppression becomes the chosen strategy by
default.
Fortunately, two recent longitudinal stud-
ies provide data that permit discrimination
between the “risk” and “epiphenomenon”
hypotheses. The data so far suggest that use
of maladaptive ER conveys risk for psycho-
pathology. In a prospective study of ado-
lescents, structural equation modeling was
used to evaluate the relationship between
ER (a latent factor defined by indicators
of emotional understanding, dysregulated
expression of sadness and anger, and rumi-
native responses to distress) and symptoms
of anxiety, depression, aggression, and eat-
ing pathology (McLaughlin, Hatzenbuehler,
Mennin, & Nolen- Hoeksema, 2011). Ado-
lescents were assessed at baseline and again
seven months later. Analyses showed that
after researchers controlled for baseline
symptoms, poor ER at baseline predicted
increased symptoms of anxiety, aggression,
and eating pathology (but not depression)
measured 7 months later. Importantly, psy-
chopathology symptoms measured at base-
line did not predict worse ER at the follow-
up.
A large (N > 1,000), prospective,
community- based study also investigated
the relationship between use of adaptive and
maladaptive ER strategies and psychopa-
thology (Aldao & Nolen- Hoeksema, 2012).
Composite ER scores were used, such that
maladaptive ER reflected use of behavioral
disengagement, denial, suppression, and the
“brooding” facet of rumination, and adap-
tive ER reflected use of positive reframing
(similar to reappraisal) and acceptance. The
overall index of psychopathology that was
used included anxiety symptoms, depres-
sive symptoms, and alcohol use. Regression
Emotion Regulation in Anxiety Disorders 405
analyses controlling for baseline symptoms
showed that self- reported use of maladap-
tive strategies at baseline predicted global
psychopathology 1 year later. The authors
noted that the same results emerged when
only internalizing symptoms (depression
and anxiety) were considered, though results
pertaining to anxiety symptoms alone were
not reported. Although measurement of anx-
iety was not optimal in this study (composite
of a single clinician rating and questionnaire
that mainly assesses autonomic arousal), the
results contribute to the evidence that use of
maladaptive strategies is not simply an epi-
phenomenon or consequence of anxiety dis-
orders or other psychopathology.
If overreliance on maladaptive ER strate-
gies constitutes a risk factor for anxiety and
related emotional disorders, it is plausible
that habitual use of adaptive strategies could
impart resilience to psychopathology. How-
ever, the only available longitudinal data
do not support this hypothesis. In Aldao
and Nolen- Hoeksema’s (2012) longitudinal
study, self- reported use of adaptive strategies
(cognitive reappraisal and acceptance) at
baseline did not significantly predict psycho-
pathology symptoms 1 year later. Nor did
the interaction of adaptive and maladaptive
strategies at baseline predict psychopathol-
ogy at follow- up. The lack of a significant
longitudinal relationship between adaptive
ER and psychopathology converges with
cross- sectional research showing that mal-
adaptive strategies have stronger associations
with symptoms of anxiety and mood disor-
ders than adaptive strategies (e.g., Aldao &
Nolen- Hoeksema, 2010; Aldao et al., 2010;
Moore, Zoellner, & Mollenholt, 2008). For
example, a cross- sectional investigation of
ER in trauma- exposed women found that
expressive suppression was strongly posi-
tively associated with stress- related symp-
toms, whereas cognitive reappraisal was
only weakly negatively associated with these
symptoms (Moore et al., 2008).
Despite these negative findings, it is still
possible that adaptive ER relates in impor-
tant ways to anxiety disorders and other
psychopathology. For example, one longi-
tudinal study of an undergraduate sample
reported divergent outcomes for individuals
high in neuroticism who endorsed low and
high levels of “emotional repair,” a construct
related to use of adaptive ER (Auerbach,
Abela, & Ringo Ho, 2007). Highly neurotic
individuals with low levels of emotional
repair endorsed increased engagement in
risky behaviors following increases in anxi-
ety and depression symptoms. In contrast,
highly neurotic individuals who endorsed
high levels of emotional repair displayed
decreased engagement in risky behaviors fol-
lowing increases in anxiety and depression.
These results suggest that adaptive ER may
curtail emotional responses that could lead
to clinical worsening or complications.
Cross- sectional evidence also suggests
that adaptive ER strategies may have a pal-
liative effect on anxiety disorder symptoms.
For example, a study of male veterans with
PTSD found that the combination of higher
levels of emotional clarity and more fre-
quent use of cognitive reappraisal was asso-
ciated with lower overall PTSD severity and
greater positive affect (Boden, Bonn- Miller,
Kashdan, Alvarez, & Gross, 2012). Another
study showed that trauma- exposed under-
graduates with “probable PTSD” endorsed
greater difficulties related to ER such as
decreased emotional acceptance and diffi-
culties engaging in goal- directed behavior,
controlling impulsive behavior, and access-
ing adaptive ER strategies when distressed.
In contrast, trauma- exposed subjects with-
out PTSD had fewer difficulties controlling
impulsive behaviors and greater access to
adaptive ER strategies when distressed (rela-
tive to both trauma- exposed subjects with
probable PTSD and those without trauma
exposure; Weiss et al., 2012). Aldao and
Nolen- Hoeksema (2012) also reported a
significant interaction effect of adaptive and
maladaptive ER strategies on psychopathol-
ogy symptoms at the baseline assessment of
their study. Level of adaptive strategy use
was negatively correlated with psychopa-
thology for subjects endorsing high usage of
maladaptive strategies, suggesting that adap-
tive ER mitigated the detrimental effects of
maladaptive ER.
It is also possible that aspects of adap-
tive ER that have not yet been examined in
longitudinal investigations are important to
resilience to psychopathology. Although fre-
quent use of adaptive ER strategies was not
shown to predict psychopathology symp-
toms (Aldao & Nolen- Hoeksema, 2012),
frequent and competent use may do so.
Though cross- sectional, one community-

406 PSYCHOPATHOLOGY
based study showed that cognitive reap-
praisal ability (measured as success in reduc-
ing subjective and physiological indicators
of emotion on a standardized task) was
associated with lower levels of depressive
symptoms in women experiencing high lev-
els of stress (Troy, Wilhelm, Shallcross, &
Mauss, 2010).
Finally, it is noteworthy that treatment-
related changes in ER are associated with
reductions in anxiety disorder symptoms.
For example, a recent study showed that
changes in reappraisal self- efficacy mediated
the effects of cognitive- behavioral therapy
for SAD (Goldin et al., 2012). Initial efficacy
data from several treatments for anxiety dis-
orders that directly target ER further sug-
gest that facilitating adaptive ER can lead to
resolution of anxiety disorder symptoms. In
addition to ER-focused treatments for spe-
cific anxiety disorders (e.g., emotion regu-
lation therapy for GAD: Mennin & Fresco,
this volume; acceptance- based behavior
therapy for GAD: Roemer et al., 2008),
our group has developed and tested a uni-
fied protocol for transdiagnostic treatment
of emotional disorders, designed to reduce
maladaptive emotional processing, promote
increased engagement of adaptive ER, and
facilitate extinction of distressing reactions
to intense negative emotions (Barlow et al.,
2011). Results of the initial randomized con-
trolled trial demonstrated that this interven-
tion led to significant reductions in anxiety
focused on emotional experience, anxiety
sensitivity, and emotional avoidance strate-
gies (Ellard et al., 2011), while another study
showed large treatment effect sizes across
disorders (Farchione et al., 2012). Empirical
support for the efficacy of the unified pro-
tocol and other ER-focused treatments for
anxiety disorders reinforces the notion that
ER is an important factor that may contrib-
ute to the onset and/or maintenance of anxi-
ety disorders.
Summary
The results of two recent longitudinal inves-
tigations suggest that maladaptive ER may
temporally precede and increase risk for
psychopathology, including anxiety disor-
ders, consistent with the etiological model to
which we have referred in this chapter. While
adaptive ER does not appear to confer pro-
tection against later anxiety or related symp-
toms, preliminary research suggests that
adaptive ER is associated with decreased
severity of anxiety disorder symptoms and
may reduce clinical complications (which
often result from maladaptive/avoidant ER).
Interventions designed to facilitate adaptive
ER also have shown promising results. More
longitudinal research is needed to determine
whether competent use of adaptive strategies
protects individuals from developing anxi-
ety and related emotional disorders when
exposed to stressors or in the presence of
other risk factors for disorder.
Conclusions and Future Directions
A dramatic increase in research over the last
decade has led to important observations
regarding the relationship of ER to the etiol-
ogy, phenomenology, and treatment of anxi-
ety disorders. We have attempted to synthe-
size these important data and to consider
them within the broader context of basic
features of anxiety disorders that influence
ER.
Individuals with anxiety disorders report
wide- ranging difficulties related to emo-
tional experience and ER. We have posited
that these difficulties emanate from underly-
ing features of anxiety disorders that include
heightened emotional reactivity, hypersen-
sitivity to threat, and increased tendencies
toward avoidant processing and behavior.
These basic characteristics of anxiety dis-
orders may contribute to problematic ER
strategy selection, because the emotional
processing of anxious individuals appears
to facilitate maladaptive strategies, such as
suppression, and impede adaptive strategies,
such as cognitive reappraisal and accep-
tance. ER-related difficulties also may be
exacerbated by differences in consequences
of ER strategies for anxious individuals
(e.g., negative effects of suppression may be
potentiated; physiological benefits of adap-
tive strategies may be attenuated). Further-
more, mounting evidence of dysfunction of
neural systems supporting adaptive ER sug-
gests that this may be a key factor underly-
ing the difficulties reported by individuals
with anxiety disorders.
Despite the substantial expansion of inter-
est and research related to ER and anxiety

Emotion Regulation in Anxiety Disorders 407
disorders, it is clear that much more empiri-
cal work is needed to refine our understand-
ing of the topics addressed in this chapter.
Basic questions related to measurement of
ER and construct validity remain impor-
tant areas for further study and, in general,
replication of many of the findings summa-
rized in this chapter is needed. Although we
believe the evidence points to maladaptive
ER being a transdiagnostic feature of anxi-
ety and related emotional disorders, more
empirical work is needed to confirm this
view or, alternatively, to establish the speci-
ficity of relationships among ER-related
constructs and distinct anxiety symptoms
and disorders. Additionally, because longi-
tudinal studies have been sparse, more of
this type of research is needed to clarify the
directionality of relationships between ER-
and anxiety- related variables, and to iden-
tify the most optimal ER-related targets for
treatment and prevention of anxiety disor-
ders. The recent longitudinal work suggest-
ing that maladaptive ER contributes to risk
for anxiety disorders offers further support
for the view that ER is an important factor
to consider in etiological models of anxi-
ety disorders, as well as in future treatment
development and prevention efforts.
References
Aldao, A., & Mennin, D. S. (2012). Paradoxical
cardiovascular effects of implementing adap-
tive emotion regulation strategies in general-
ized anxiety disorder. Behaviour Research
and Therapy, 50, 122–130.
Aldao, A., & Nolen-
H
oeksema, S. (2010). Speci-
ficity of cognitive emotion regulation strate-
gies: A transdiagnostic examination. Behav-
iour Research and Therapy, 48, 974–983.
Aldao, A., & Nolen-
Hoeksema, S. (2012). When
are adaptive strategies most predictive of psy-
chopathology? Journal of Abnormal Psychol-
ogy, 121, 276–281.
Aldao, A., Nolen-
H
oeksema, S., & Schweizer, S.
(2010). Emotion-
r
egulation strategies across
psychopathology: A meta-
a
nalytic review.
Clinical Psychology Review, 30, 217–237.
Arch, J. J., & Craske, M. G. (2010). Labora-
tory stressors in clinically anxious and non-
a
nxious individuals: The moderating role of
mindfulness. Behaviour Research and Ther-
apy, 48, 495–505.
Auerbach, R. P., Abela, J. R., & Ringo Ho,
M. H. (2007). Responding to symptoms of
depression and anxiety: Emotion regulation,
neuroticism, and engagement in risky behav-
iors. Behaviour Research and Therapy, 45,
2182–2191.
Baker, R., Holloway, J., Thomas, P. W., Thomas,
S., & Owens, M. (2004). Emotional process-
ing and panic. Behaviour Research and Ther-
apy, 42, 1271–1287.
Ball, T. M., Ramsawh, H. J., Campbell-
S
ills, L.,
Paulus, M. P., & Stein, M. B. (2013). Prefron-
tal dysfunction during emotion regulation in
generalized anxiety and panic disorder. Psy-
chological Medicine, 43(7), 1475–1486.
Barlow, D. H. (1988). Anxiety and its disorders:
The nature and treatment of anxiety and
panic (1st ed.). New York: Guilford Press.
Barlow, D. H. (2002). Anxiety and its disorders:
The nature and treatment of anxiety and
panic (2nd ed.). New York: Guilford Press.
Barlow, D. H., Ellard, K. K., Fairholme, C. P.,
Farchione, T. J., Boisseau, C. L., Allen, L. B.,
et al. (2011). Unified protocol for the trans-
diagnostic treatment of emotional disorders:
Therapist guide. New York: Oxford Univer-
sity Press.
Barlow, D. H., Sauer-
Z
avala, S. E., Carl, J. R.,
Bullis, J. R., & Ellard, K. K. (in press). The
nature, diagnosis, and treatment of neuroti-
cism: Back to the future. Clinical Psychologi-
cal Science.
Bebko, G. M., Franconeri, S. L., Ochsner, K. N.,
& Chiao, J. Y. (2011). Look before you regu-
late: Differential perceptual strategies underly-
ing expressive suppression and cognitive reap-
praisal. Emotion, 11, 732–742.
Beck, A. T., & Clark, D. A. (1997). An informa-
tion processing model of anxiety: Automatic
and strategic processes. Behaviour Research
and Therapy, 35, 49–58.
Bishop, S., Duncan, J., Brett, M., & Lawrence,
A. D. (2004). Prefrontal cortical function and
anxiety: Controlling attention to threat-
r
elated
stimuli. Nature Neuroscience, 7, 184–188.
Boden, M. T., Bonn-
M
iller, M. O., Kashdan,
T. B., Alvarez, J., & Gross, J. J. (2012). The
interactive effects of emotional clarity and
cognitive reappraisal in posttraumatic stress
disorder. Journal of Anxiety Disorders, 26,
233–238.
Bonanno, G. A., Papa, A., Lalande, K., West-
phal, M., & Coifman, K. (2004). The impor-
tance of being flexible: The ability to both
enhance and suppress emotional expression
408 PSYCHOPATHOLOGY
predicts long-term adjustment. Psychological
Science, 15, 482–487.
Borkovec, T. D., Alcaine, O., & Behar, E. (2004).
Avoidance theory of worry and generalized
anxiety disorder. In R. G. Heimberg, C. L.
Turk, & D. S. Mennin (Eds.), Generalized anx-
iety disorder: Advances in research and prac-
tice (pp. 77–108). New York: Guilford Press.
Boswell, J. F., Thompson- Holland, J., Farchione,
T. J., & Barlow, D. H. (2013). Intolerance of
uncertainty: A common change factor in the
treatment of emotional disorders. Journal of
Clinical Psychology, 69, 630–645.
Brown, T. A., & Barlow, D. H. (2009). A pro-
posal for a dimensional classification system
based on the shared features of the DSM-IV
anxiety and mood disorders: Implications
for assessment and treatment. Psychological
Assessment, 21, 256–271.
Brown, T. A., Barlow, D. H., & Liebowitz, M.
(1994). The empirical basis of GAD. Ameri-
can Journal of Psychiatry, 151, 1272–1280.
Brown, T. A., Chorpita, B. F., & Barlow, D. H.
(1998). Structural relationships among dimen-
sions of the DSM-IV anxiety and mood disor-
ders and dimensions of negative affect, posi-
tive affect, and autonomic arousal. Journal of
Abnormal Psychology, 107, 179–192.
Campbell- Sills, L., Barlow, D. H., Brown, T. A.,
& Hofmann, S. G. (2006a). Effects of suppres-
sion and acceptance on emotional responses of
individuals with anxiety and mood disorders.
Behaviour Research and Therapy, 44, 1251–
1263.
Campbell- Sills, L., Barlow, D. H., Brown, T. A.,
& Hofmann, S. G. (2006b). Acceptability and
suppression of negative emotion in anxiety
and mood disorders. Emotion, 6, 587–595.
Campbell- Sills, L., Simmons, A. N., Lovero, K.
L., Rochlin, A. A., Paulus, M. P., & Stein, M.
B. (2011). Functioning of neural systems sup-
porting emotion regulation in anxiety- prone
individuals. NeuroImage, 54, 689–696.
Carthy, T., Horesh, N., Apter, A., Edge, M. D.,
& Gross, J. J. (2010). Emotional reactivity
and cognitive regulation in anxious children.
Behaviour Research and Therapy, 48, 384–
393.
Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A.,
Craig, I. W., Harrington, H., et al. (2003).
Influence of life stress on depression: Modera-
tion by a polymorphism in the 5-HTT gene.
Science, 301, 386–389.
Cohen, N., Henik, A., & Moyal, N. (2012).
Executive control attenuates emotional
effects— for high reappraisers only? Emotion,
12, 970–979.
Dalgleish, T., Yiend, J., Schweizer, S., & Dunn,
B. D. (2009). Ironic effects of emotion sup-
pression when recounting distressing memo-
ries. Emotion, 9, 744–749.
Di Simplicio, M., Costoloni, G., Western, D.,
Hanson, B., Taggart, P., & Harmer, C. J.
(2012). Decreased heart rate variability dur-
ing emotion regulation in subjects at risk for
psychopathology. Psychological Medicine, 42,
1775–1783.
Drabant, E. M., Ramel, W., Edge, M. D., Hyde,
L. W., Kuo, J. R., Goldin, P. R., et al. (2012).
Neural mechanisms underlying 5-HTTLPR-
related sensitivity to acute stress. American
Journal of Psychiatry, 169, 397–405.
Dugas, M. J., Laugesen, N., & Bukowski, W. M.
(2012). Intolerance of uncertainty, fear of anx-
iety, and adolescent worry. Journal of Abnor-
mal Child Psychology, 40, 863–870.
Dunn, B. D., Billotti, D., Murphy, V., & Dal-
gleish, T. (2009). The consequences of effortful
emotion regulation when processing distress-
ing material: A comparison of suppression and
acceptance. Behaviour Research and Therapy,
47, 761–773.
Eftekhari, A., Zoellner, L. A., & Vigil, S. A.
(2009). Patterns of emotion regulation and
psychopathology. Anxiety, Stress and Coping,
22, 571–586.
Ehring, T., & Quack, D. (2010). Emotion regula-
tion difficulties in trauma survivors: The role
of trauma type and PTSD symptom severity.
Behavior Therapy, 41, 587–598.
Eifert, G. H., & Heffner, M. (2003). The effects
of acceptance versus control contexts on
avoidance of panic- related symptoms. Journal
of Behavior Therapy and Experimental Psy-
chiatry, 34, 293–312.
Ellard, K. K. (2012). An examination of the neu-
ral correlates of emotion acceptance versus
worry in generalized anxiety disorder. Unpub-
lished doctoral dissertation, Boston Univer-
sity, Boston, MA.
Ellard, K. K., Fairholme, C. P., Thompson-
Hollands, J., Carl, J. R., Farchione, T. J., &
Barlow, D. H. (2011, November). Early reduc-
tions in distress and anxiety related to emo-
tional experiences in the Unified Protocol:
Effect on treatment response. Paper presented
at the 45th annual meeting of the Associa-
tion for Behavioral and Cognitive Therapies,
Toronto, Canada.
Etkin, A., Prater, K. E., Hoeft, F., Menon, V.,
Emotion Regulation in Anxiety Disorders 409
& Schatzberg, A. F. (2010). Failure of anterior
cingulate activation and connectivity with the
amygdala during implicit regulation of emo-
tional processing in generalized anxiety dis-
order. American Journal of Psychiatry, 167,
545–554.
Etkin, A., Prater, K. E., Schatzberg, A. F., Menon,
V., & Greicius, M. D. (2009). Disrupted amyg-
dalar subregion functional connectivity and
evidence of a compensatory network in gen-
eralized anxiety disorder. Archives of General
Psychiatry, 66, 1361–1372.
Etkin, A., & Wager, T. D. (2007). Functional
neuroimaging of anxiety: A meta- analysis of
emotional processing in PTSD, social anxiety
disorder, and specific phobia. American Jour-
nal of Psychiatry, 164, 1476–1488.
Farchione, T. J., Fairholme, C. P., Ellard, K. K.,
Boisseau, C. L., Thompson- Hollands, J., Carl,
J. R., et al. (2012). Unified protocol for trans-
diagnostic treatment of emotional disorders:
A randomized controlled trial. Behavior Ther-
apy, 43, 666–678.
Feldner, M. T., Zvolensky, M. J., Eifert, G. H., &
Spira, A. P. (2003). Emotional avoidance: An
experimental test of individual differences and
response suppression using biological chal-
lenge. Behaviour Research and Therapy, 41,
403–411.
Friedman, B. H. (2007). An autonomic
flexibility– neurovisceral integration model
of anxiety and cardiac vagal tone. Biological
Psychology, 74, 185–199.
Garner, M., Mogg, K., & Bradley, B. P. (2006).
Orienting and maintenance of gaze to facial
expressions in social anxiety. Journal of
Abnormal Psychology, 115, 760–770.
Goldin, P. R., Manber, T., Hakimi, S., Canli, T.,
& Gross, J. J. (2009). Neural bases of social
anxiety disorder: Emotional reactivity and
cognitive regulation during social and physi-
cal threat. Archives of General Psychiatry, 66,
170–180.
Goldin, P. R., Manber-Ball, T., Werner, K.,
Heimberg, R., & Gross, J. J. (2009). Neural
mechanisms of cognitive reappraisal of nega-
tive self- beliefs in social anxiety disorder. Bio-
logical Psychiatry, 66, 1091–1099.
Goldin, P. R., McRae, K., Ramel, W., & Gross, J.
J. (2008). The neural bases of emotion regula-
tion: Reappraisal and suppression of negative
emotion. Biological Psychiatry, 63, 577–586.
Goldin, P. R., Ziv, M., Jazaieri, H., Werner, K.,
Kraemer, H., Heimberg, R. G., et al. (2012).
Cognitive reappraisal self- efficacy mediates
the effects of individual cognitive- behavioral
therapy for social anxiety disorder. Journal of
Consulting and Clinical Psychology, 80(6),
1034–1040.
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85, 348–362.
Gross, J. J., & Levenson, R. W. (1993). Emo-
tional suppression: Physiology, self- report,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64, 970–986.
Gross, J. J., & Levenson, R. W. (1997). Hiding
feelings: The acute effects of inhibiting nega-
tive and positive emotion. Journal of Abnor-
mal Psychology, 106, 95–103.
Gyurak, A., Goodkind, M. S., Kramer, J. H.,
Miller, B. L., & Levenson, R. W. (2012).
Executive functions and the down- regulation
and up- regulation of emotion. Cognition and
Emotion, 26, 103–118.
Hill, C. L., & Updegraff, J. A. (2012). Mindful-
ness and its relationship to emotional regula-
tion. Emotion, 12, 81–90.
Hofmann, S. G., Ellard, K. K., & Siegle, G. J.
(2012). Neurobiological correlates of cog-
nitions in fear and anxiety: A cognitive–
neurobiological information- processing model.
Cognition and Emotion, 26, 282–299.
Hofmann, S. G., Heering, S., Sawyer, A. T., &
Asnaani, A. (2009). How to handle anxiety:
The effects of reappraisal, acceptance, and
suppression strategies on anxious arousal.
Behaviour Research and Therapy, 47, 389–
394.
Jackson, D. C., Malmstadt, J. R., Larson, C.
L., & Davidson, R. J. (2000). Suppression
and enhancement of emotional responses to
unpleasant pictures. Psychophysiology, 37,
515–522.
John, O. P., & Eng, J. (this volume, 2014). Three
approaches to individual differences in affect
regulation: Conceptualization, measures, and
findings. In J. J. Gross (Ed.), Handbook of
410 PSYCHOPATHOLOGY
emotion regulation (2nd ed., pp. 321–345).
New York: Guilford Press.
Joormann, J., & Siemer, M. (this volume, 2014).
Emotion regulation in mood disorders. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 413–427). New York: Guilford
Press.
Kashdan, T. B., & Rottenberg, J. (2010). Psy-
chological flexibility as a fundamental aspect
of health. Clinical Psychology Review, 30,
865–878.
Keough, M. E., Riccardi, C. J., Timpano, K. R.,
Mitchell, M. A., & Schmidt, N. B. (2010).
Anxiety symptomatology: The association
with distress tolerance and anxiety sensitivity.
Behavior Therapy, 41, 567–574.
Koster, E. H., Crombez, G., Verschuere, B.,
Van Damme, S., & Wiersema, J. R. (2006).
Components of attentional bias to threat in
high trait anxiety: Facilitated engagement,
impaired disengagement, and attentional
avoidance. Behaviour Research and Therapy,
44, 1757–1771.
Lau, H. C., Rogers, R. D., Haggard, P., & Pass-
ingham, R. E. (2004). Attention to intention.
Science, 303, 1208–1210.
Leutgeb, V., Schäfer, A., & Schienle, A. (2009).
An event- related potential study on exposure
therapy for patients suffering from spider pho-
bia. Biological Psychology, 82, 293–300.
Levitt, J. T., Brown, T. A., Orsillo, S. M., & Bar-
low, D. H. (2004). The effects of acceptance
versus suppression of emotion on subjective
and psychophysiological response to carbon
dioxide challenge in patients with panic disor-
der. Behavior Therapy, 35, 747–766.
Lieberman, M. D., Inagaki, T. K., Tabibnia, G., &
Crockett, M. J. (2011). Subjective responses to
emotional stimuli during labeling, reappraisal,
and distraction. Emotion, 11, 468–480.
MacLeod, C., & Hagan, R. (1992). Individual
differences in the selective processing of threat-
ening information, and emotional responses to
a stressful life event. Behaviour Research and
Therapy, 30, 151–161.
MacLeod, C., Mathews, A., & Tata, P. (1986).
Attentional bias in emotional disorders. Jour-
nal of Abnormal Psychology, 95, 15–20.
Matsuzaka, Y., Akiyama, T., Tanji, J., & Mush-
iake, H. (2012). Neuronal activity in the pri-
mate dorsomedial prefrontal cortex contrib-
utes to strategic selection of response tactics.
Proceedings of the National Academy of Sci-
ences, 109, 4633–4638.
McEvoy, P. M., & Mahoney, A. E. (2012). To
be sure, to be sure: Intolerance of uncertainty
mediates symptoms of various anxiety disor-
ders and depression. Behavior Therapy, 43,
533–545.
McLaughlin, K. A., Hatzenbuehler, M. L., Men-
nin, D. S., & Nolen- Hoeksema, S. (2011).
Emotion dysregulation and adolescent psy-
chopathology: A prospective study. Behaviour
Research and Therapy, 49, 544–554.
McLaughlin, K. A., Mennin, D. S., & Farach, F.
J. (2007). The contributory role of worry in
emotion generation and dysregulation in gen-
eralized anxiety disorder. Behaviour Research
and Therapy, 45, 1735–1752.
Mennin, D. S., & Fresco, D. M. (this volume,
2014). Emotion regulation therapy. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 469–490). New York: Guilford
Press.
Mennin, D. S., Heimberg, R. G., Turk, C. L., &
Fresco, D. M. (2005). Preliminary evidence
for an emotion dysregulation model of gener-
alized anxiety disorder. Behaviour Research
and Therapy, 43, 1281–1310.
Mennin, D. S., McLaughlin, K. A., & Flanagan,
T. J. (2009). Emotion regulation deficits in
generalized anxiety disorder, social anxiety
disorder, and their co- occurrence. Journal of
Anxiety Disorders, 23, 866–871.
Montag, C., Fiebach, C. J., Kirsch, P., & Reuter,
M. (2011). Interaction of 5-HTTLPR and a
variation on the oxytocin receptor gene influ-
ences negative emotionality. Biological Psy-
chiatry, 69, 601–603.
Moore, S. A., Zoellner, L. A., & Mollenholt, N.
(2008). Are expressive suppression and cogni-
tive reappraisal associated with stress- related
symptoms? Behaviour Research and Therapy,
46, 993–1000.
Munafo, M. R., Brown, S. M., & Hariri, A. R.
(2008). Serotonin transporter (5-HTTLPR)
genotype and amygdala activation: A meta-
analysis. Biological Psychiatry, 63, 852–857.
New, A. S., Fan, J., Murrough, J. W., Liu, X.,
Liebman, R. E., Guise, K. G., et al. (2009). A
functional magnetic resonance imaging study
of deliberate emotion regulation in resilience
and posttraumatic stress disorder. Biological
Psychiatry, 66, 656–664.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. (2002). Rethinking feelings:
An FMRI study of the cognitive regulation of
emotion. Journal of Cognitive Neuroscience,
14, 1215–1229.
Ochsner, K. N., Knierim, K., Ludlow, D. H.,
Emotion Regulation in Anxiety Disorders 411
Hanelin, J., Ramachandran, T., Glover, G.,
& Mackey, S. C. (2004). Reflecting upon feel-
ings: An fMRI study of neural systems sup-
porting the attribution of emotion to self or
other. Journal of Cognitive Neuroscience, 16,
1746–1772.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Rob-
ertson, E. R., Chopra, S., Gabrieli, J. D., et al.
(2004). For better or for worse: Neural sys-
tems supporting the cognitive down- and up-
regulation of negative emotion. NeuroImage,
23, 483– 499.
Olatunji, B. O., & Wolitzky- Taylor, K. B. (2009).
Anxiety sensitivity and the anxiety disorders:
A meta- analytic review and synthesis. Psycho-
logical Bulletin, 135, 974–999.
Paquette, V., Lévesque, J., Mensour, B., Ler-
oux, J. M., Beaudoin, G., Bourgouin, P., et al.
(2003). “Change the mind and you change the
brain”: Effects of cognitive- behavioral therapy
on the neural corrleates of spider phobia. Neu-
roImage, 18, 401–409.
Pezawas, L., Meyer- Lindenberg, A., Drab-
ant, E. M., Verchinski, B. A., Munoz, K. E.,
Kolachana, B. S., et al. (2005). 5-HTTLPR
polymorphism impacts human cingulate–
amygdala interactions: A genetic susceptibility
mechanism for depression. Nature Neurosci-
ence, 8, 828–834.
Pineles, S. L., & Mineka, S. (2005). Attentional
biases to internal and external sources of
potential threat in social anxiety. Journal of
Abnormal Psychology, 114, 314–318.
Roemer, L., Orsillo, S. M., & Salters- Pedneault,
K. (2008). Efficacy of an acceptance- based
behavior therapy for generalized anxiety dis-
order: Evaluation in a randomized controlled
trial. Journal of Consulting and Clinical Psy-
chology, 76, 1083–1089.
Rothbart, M. K., Sheese, B. E., & Posner, M. I.
(this volume, 2014). Temperament and emo-
tion regulation. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 305–320).
New York: Guilford Press.
Salters- Pedneault, K., Roemer, L., Tull, M. T.,
Rucker, L., & Mennin, D. S. (2006). Evidence
of broad deficits in emotion regulation associ-
ated with chronic worry and generalized anxi-
ety disorder. Cognitive Therapy and Research,
30, 469–480.
Schmidt, N. B., Lerew, D. R., & Jackson, R. J.
(1999). Prospective evaluation of anxiety sen-
sitivity in the pathogenesis of panic: Replica-
tion and extension. Journal of Abnormal Psy-
chology, 108, 532–537.
Sheppes, G., Scheibe, S., Suri, G., & Gross, J. J.
(2011). Emotion regulation choice. Psycholog-
ical Science, 22, 1391–1396.
Shin, L. M., & Liberzon, I. (2010). The neuro-
circuitry of fear, stress, and anxiety disorders.
Neuropsychopharmacology, 35, 169–191.
Speilberg, J. M., Miller, G. A., Engels, A. S., Her-
rington, J. D., Sutton, B. P., Banich, M. T., et
al. (2011). Trait approach and avoidance moti-
vation: Lateralized neural activity associated
with executive function. NeuroImage, 54,
661–670.
Stein, M. B., Shorck, N. J., & Gelernter, J. (2008).
Gene-by- environment (serotonin transporter
and childhood maltreatment) interaction for
anxiety sensitivity, an intermediate phenotype
for anxiety disorders. Neuropsychopharma-
cology, 33, 312–319.
Straube, T., Glauer, M., Dilger, S., Mentzel, H. J.,
& Miltner, W. H. (2006). Effects of cognitive-
behavioral therapy on brain activation in spe-
cific phobia. NeuroImage, 29, 125–135.
Suárez, L. M., Bennett, S. M., Goldstein, C.
R., & Barlow, D. H. (2009). Understanding
anxiety disorders from a “triple vulnerabil-
ity” framework. In M. M. Antony & M. B.
Stein (Eds.), Oxford handbook of anxiety and
related disorders (pp. 153–172). New York:
Oxford University Press.
Tan, P. Z., Forbes, E. E., Dahl, R. E., Ryan,
N. D., Siegle, G. J., Ladouceur, C. D., et al.
(2012). Emotional reactivity and regulation in
anxious and nonanxious youth: A cell-phone
ecological momentary assessment study. Jour-
nal of Child Psychology and Psychiatry, 53,
197–206.
Thayer, J. F., Friedman, B. H., & Borkovec, T.
D. (1996). Autonomic characteristics of gener-
alized anxiety disorder and worry. Biological
Psychiatry, 39, 255–266.
Thompson, R. A. (this volume, 2014). Socializa-
tion of emotion and emotion regulation in the
family. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 173–186). New
York: Guilford Press.
Tolin, D. F., Abramowitz, J. S., Brigidi, B. D., &
Foa, E. B. (2003). Intolerance of uncertainty
in obsessive– compulsive disorder. Journal of
Anxiety Disorders, 17, 233–242.
Troy, A. S., Wilhelm, F. H., Shallcross, A. J., &
Mauss, I. B. (2010). Seeing the silver lining:
Cognitive reappraisal ability moderates the
relationship between stress and depressive
symptoms. Emotion, 10, 783–795.
Tull, M. T., & Roemer, L. (2007). Emotion regu-
412 PSYCHOPATHOLOGY
lation difficulties associated with the experi-
ence of uncued panic attacks: Evidence of
experiential avoidance, emotional nonaccep-
tance, and decreased emotional clarity. Behav-
ior Therapy, 38, 378–391.
Turk, C. L., Heimberg, R. G., Luterek, J. A.,
Mennin, D. S., & Fresco, D. M. (2005). Emo-
tion dysregulation in generalized anxiety dis-
order: A comparison with social anxiety dis-
order. Cognitive Therapy and Research, 29,
89–106.
van den Hout, M., Tenney, N., Huygens, K.,
Merckelbach, H., & Kindt, M. (1995).
Responding to subliminal threat cues is related
to trait anxiety and emotional vulnerability: A
successful replication of Macleod and Hagan
(1992). Behaviour Research and Therapy, 33,
451–454.
Wager, T. D., Davidson, M. L., Hughes, B. L.,
Lindquist, M. A., & Ochsner, K. N. (2008).
Prefrontal- subcortical pathways mediating
successful emotion regulation. Neuron, 59,
1037–1050.
Weiss, N. H., Tull, M. T., Davis, L. T., Dehon,
E. E., Fulton, J. J., & Gratz, K. L. (2012).
Examining the association between emotion
regulation difficulties and probable posttrau-
matic stress disorder within a sample of Afri-
can Americans. Cognitive Behavior Therapy,
41, 5–14.
Wilamowska, Z. A., Thompson- Hollands, J.,
Fairholme, C. P., Ellard, K. K., Farchione, T.
J., & Barlow, D. H. (2010). Conceptual back-
ground, development, and preliminary data
from the unified protocol for transdiagnostic
treatment of emotional disorders. Depression
and Anxiety, 27, 882–890.
Wolgast, M., Lundh, L. G., & Viborg, G. (2011).
Cognitive reappraisal and acceptance: An
experimental comparison of two emotion
regulation strategies. Behaviour Research and
Therapy, 49, 858–866.

413
Imagine receiving a negative evaluation at
work, having an argument with your part-
ner, discovering you have forgotten to pay a
bill and have incurred a late fee; and imagine
experiencing all of this on a gray, rainy, win-
ter day. Situations like these, which have the
potential to induce lasting negative affect,
are part of everyday life. Perhaps not sur-
prisingly, researchers have found that peo-
ple generally do not endure negative affect
passively; rather, they actively use strategies
to try to regulate their negative moods and
emotions. It is important to note, however,
that there is a wide range of individual dif-
ferences in the ability to regulate affect:
Whereas some individuals can successfully
regulate their negative affect, others cannot,
and still others may respond to their emo-
tions in ways that further exacerbate their
negative affect. Importantly, it is becoming
increasingly clear that these individual dif-
ferences in the regulation of negative moods
and emotions play a significant role in the
onset and maintenance of mood disorders.
Less frequently discussed is the regulation
of positive moods and emotions and of dif-
ficulties that may stem from an inability to
respond effectively to positive affect. Exces-
sive bathing in the glory of initial posi-
tive feedback, for example, may lead to an
overestimation of the ability to complete
the project in a timely manner, whereas an
inability to savor a success and the use of
strategies that down- regulate the ensuing
positive mood may interfere with the moti-
vation to start the next project. Therefore,
both difficulties in the regulation of positive
and negative affect have been implicated in
mood disorders.
Mood disorders are among the most
prevalent of all psychiatric disorders, with
major depressive disorder (MDD) affecting
almost 20% of Americans at some point in
their lives (Kessler & Wang, 2009). Given
the high prevalence and the substantial per-
sonal and societal costs of mood disorders,
efforts to identify risk factors and underly-
ing mechanisms, as well as effective inter-
vention strategies, are particularly pressing.
As implied by the label, the hallmark fea-
ture of mood disorders is disordered affect.
MDD is defined by sustained negative affect
and difficulties experiencing positive affect.
Bipolar disorder (BD) is characterized by
episodes of depressed but also elevated and/
or irritable mood. Despite the fact that dis-
ordered affect is the central feature of mood
disorders, theories of the onset, mainte-
nance, and recurrence of these disorders
have traditionally focused on cognition and
CHAPTER 25
Emotion Regulation in Mood Disorders
Jutta Joormann
Matthias Siemer
414 PSYCHOPATHOLOGY
behavior but not as much on affect. Indeed,
cognitive- behavioral interventions have
proven successful in treating these disorders
by focusing on the modification of maladap-
tive cognitions and behaviors (Beck, Rush,
Shaw, & Emery, 1979). Despite these suc-
cesses, many researchers have pointed out
that there is room for improvement in our
theoretical models and in our treatment
approaches. Specifically, a closer look at
the concept of emotion regulation and at
mechanisms that allow us to understand
individual differences in the important abil-
ity to regulate affective states may help us
better understand vulnerability to affective
disorders and thereby improve our treatment
approaches.
Indeed, models of both depression and BD
have emphasized the role of emotion regu-
lation. It has been proposed, for example,
that individuals who experience episodes of
depression do not differ from their nonde-
pressed counterparts in the degree to which
they become sad but instead are character-
ized by an inability to repair or regulate their
emotions, resulting in longer episodes of
sadness and depressed mood for depression-
prone individuals (Teasdale, 1988; Nolen-
Hoeksema, Wisco, & Lyubomirsky, 2008).
In depression, preferential use of maladap-
tive strategies, such as rumination, as well
as difficulties using adaptive strategies, such
as reappraisal, may explain why negative
emotions in response to life events quickly
spiral into sustained negative mood (e.g.,
Nolen- Hoeksema et al., 2008). In BD, we
find similar difficulties regulating negative
affect. In addition, maladaptive responding
to positive affect and events may explain
how elevated mood leads into a manic
episode (Gruber, 2011; Johnson, 2005).
Indeed, Gruber (2011) points out that “BD
involves a tendency to increase or amplify
positive emotions” (p. 219). Likewise, John-
son (2005) has described how increases in
positive affect after an initial success and
individual differences in responding to posi-
tive mood are related to the development of
mania. Even though these predictions seem
plausible, however, empirical evidence is still
largely missing, particularly when it comes
to BD. A first goal of this chapter is there-
fore to summarize empirical findings that
support the proposition that people who are
diagnosed with affective disorders do indeed
differ from their nondisordered counterparts
in emotion regulation.
At least two different but related aspects
of emotion regulation are crucial in this con-
text. First, it is possible that people who are
prone to experience affective disorders and
their nondisordered counterparts differ in
the selection of specific emotion regulation
strategies because of wrong expectations
about the usefulness of a strategy in a given
situation, or because of acquired preferences
for the selection of ineffective or maladap-
tive strategies. Indeed, numerous studies
show that depression is associated with a
tendency to ruminate when experiencing
negative affect, and that the use of this strat-
egy is perceived as helpful in coping with
negative events even though it in fact inter-
feres with recovery (Papageorgiou & Wells,
2001). In addition, it is possible that affec-
tive disorders are characterized by a more
limited repertoire of strategies that may
interfere with the flexible use of multiple
strategies that fit the situation at hand. This
flexibility has been proposed as particularly
adaptive (Kashdan & Rottenberg, 2010). It
is also possible, however, that depression-
related difficulties in emotion regulation are
due not to strategy selection but to prob-
lems implementing adaptive strategies such
as reappraisal. Of course, these two aspects
of emotion regulation in affective disorders
may be related. Difficulties implementing an
adaptive strategy, for example, may result in
preferential selection of maladaptive strate-
gies.
In this chapter, we review studies examin-
ing the first aspect of emotion regulation in
affective disorders by summarizing research
on the use of different emotion regulation
strategies, then examine evidence for the sec-
ond, that people with affective disorders have
difficulties implementing adaptive strategies.
We also examine underlying mechanisms of
emotion dysregulation in affective disorders
by focusing on basic cognitive processes that
may help or hinder emotion regulation and
discuss how these may be affected by mood
disorders. Our discussion focuses on studies
that have used clinically diagnosed samples,
but we also review studies with analogue
samples if they are particularly informative
or if clinical studies are missing.
Emotion regulation is defined here as
strategic and automatic processes that influ-

Emotion Regulation in Mood Disorders 415
ence the occurrence, magnitude, duration,
and expression of an emotional response
(see Gross, this volume). The focus of most
studies on emotion regulation is the down-
regulation of negative affect. This aspect of
emotion regulation is also the primary focus
of this chapter when it comes to MDD. How-
ever, we also discuss how depression may be
associated with problems savoring positive
affect and that a risk factor for BD is rumi-
nation on positive events (e.g., in response
to successful goal achievement), which may
result in a failure to down- regulate positive
affect that may then spiral into a full-blown
manic episode (Johnson, 2005).
Strategy Selection
in Mood Disorders
Recent research suggests that it is overly
simplistic to differentiate adaptive from mal-
adaptive strategies. Indeed, the adaptiveness
of a particular strategy will likely depend on
the context in which it is used, as well as
the flexibility of its use (Bonnano, Papa, Lal-
ande, Westphal, & Coifman, 2004). Still,
previous research has identified specific
strategies that are more likely to incur addi-
tional costs (e.g., increase in cognitive load
or physiological arousal) and that seem less
likely to regulate affect effectively (Gross &
John, 2003). Emotion regulation is a broad
concept that comprises a number of different
processes, and it is beyond the scope of this
chapter to provide an exhaustive review of
the literature. We therefore focus on strat-
egies that have consistently been linked to
mood disorders and have been the focus of
empirical studies on depression and/or BD.
Rumination
A particularly detrimental response to nega-
tive affect that has been associated with
various emotional disorders is rumination,
which is bringing an idea back to mind
over and over again. Originally the word
referred to the way cows and certain other
animals eat, storing partially digested food
in a stomach called a rumen, bringing that
food up later to chew over more thoroughly.
Even in the original Latin, however, it took
on a vivid figurative meaning, describing the
practice of bringing an idea back to mind
to work over further. Many of us mull over
important matters in this way, digesting
them a little at a time. But in depression,
rumination frequently features negative,
self- deprecating statements and pessimistic
ideas about the world and the future.
Depressive rumination has been shown
in numerous studies to exacerbate and pro-
long depressed mood (see review by Nolen-
Hoeksema et al., 2008). It may seem coun-
terintuitive to think that someone would
use rumination as an emotion regulation
strategy given that numerous studies show
that it increases negative affect. However,
it is a frequently used response when peo-
ple experience negative affect or encoun-
ter a negative event, and studies show that
depressed individuals perceive many benefits
of rumination, such as feelings of increased
self- awareness and understanding (Papa-
georgiou & Wells, 2001). Lyubomirksy
and Nolen- Hoeksema (1993), for example,
reported that rumination in dysphoric par-
ticipants (i.e., participants with subclini-
cal levels of depressive symptoms usually
assessed via self- report) was associated with
an enhanced sense of insightfulness. Empiri-
cal evidence has shown that people who tend
to engage in rumination when distressed are
more likely to develop depressive disorders
and tend to experience more prolonged peri-
ods of depression (e.g., Nolen- Hoeksema,
2000). Indeed, considerable evidence has
linked higher trait rumination with the
onset and maintenance of depression (see
Nolen- Hoeksema et al., 2008, for a review).
Nolen- Hoeksema (2000), for example,
examined a sample of approximately 1,300
adults randomly selected from the com-
munity. Among nondepressed individuals,
rumination scores at first assessment pre-
dicted the onset of new major depressive epi-
sodes over the following year. Researchers
have also examined the effects of an experi-
mental induction of rumination on people’s
mood and behaviors. Rumination in these
studies is usually contrasted with distrac-
tion, another emotion regulation strategy
that we discuss later. In general, compared
with distraction, rumination leads to sus-
tained negative mood, increases in negative
cognitions and overgeneral autobiographical
memory, and decreases in effective problem
solving in depressed participants (e.g., Wat-
kins & Moulds, 2005).
416 PSYCHOPATHOLOGY
Although much less studied, individuals
with BD have also been shown to ruminate
about negative emotion. In fact, some studies
indicate that people with BD ruminate even
more than people with unipolar depression
(Kim, Yu, Lee, & Kim, 2012). However, BD
is associated with not only increased depres-
sive rumination but also rumination about
positive emotion. Indeed, positive rumina-
tion, defined as dwelling on the content,
consequences, and causes of positive feelings
(Feldman, Joormann, & Johnson, 2008),
has been identified as an important risk
factor for BD. Gruber (2011), for example,
proposes that positive rumination may inter-
fere with the processing of relevant external
information that could help terminate the
emotional state. Compared to control par-
ticipants, college students diagnosed with
BD or MDD endorsed heightened rumina-
tion in response to negative affect, but only
those with BD endorsed elevated rumina-
tion in response to positive affect (Johnson,
McKenzie, & McMurrich, 2008). Moreover,
within BD, ruminative responses to negative
affect were explained by depressive symp-
toms. Gruber, Eidelman, Johnson, Smith,
and Harvey (2011) also examined rumina-
tion about positive and negative emotions:
Compared to the control group, the BD
group endorsed greater trait rumination for
positive as well as negative emotions. Inter-
estingly, participants with BD in this sample
were not at the time experiencing episodes
of depression or mania, which suggests that
elevated rumination is not just a correlate of
the depressive or manic state. Importantly,
trait rumination about negative and posi-
tive emotion was associated with greater
lifetime depression frequency, whereas trait
rumination about positive emotion was
associated with greater lifetime mania fre-
quency. Similarly, Alloy et al. (2009) found
that trait rumination about positive emotion
was associated with greater lifetime mania
frequency in their BD group. Although few
rumination inductions have been used in the
BD literature, researchers found heightened
positive emotion in a ruminative compared
with reflective (i.e., third- person perspec-
tive) induction in participants with BD com-
pared with healthy controls (Gruber, Har-
vey, & Johnson, 2009). In contrast, Gruber
et al. (2011) exposed their participants with
interepisode BD and control participants to
a rumination induction in response to the
recall of a positive autobiographical memory
and reported no group differences in self-
reported affect or physiological responding.
It seems interesting that whereas positive
rumination is associated with negative out-
comes in BD, depressed participants may
have difficulties savoring positive events.
Thus, although rumination on negative
content clearly seems present and maladap-
tive in both MDD and BD, the maladap-
tive response may be rumination on posi-
tive content in BD, whereas lack of savoring
may be the maladaptive response in MDD.
Raes, Smets, Nelis, and Schoofs (2012), for
example, found in two nonclinical student
samples that a self- reported dampening
response to positive affect predicted depres-
sive symptoms in 3- and 5-month follow- up
assessments.
Reappraisal
In his fascinating book on depression,
Andrew Solomon (2001) writes about one
of his depressive episodes, “I started to fear,
every time my dog left the room, that it was
because he was not interested in me” (p. 86).
The tendency to interpret emotion- eliciting
situations in a negative way is a defining
feature of depressive disorders. Altering
these interpretations is obviously a power-
ful way to regulate affect and a main com-
ponent of cognitive therapy for depression.
Reappraisal involves changing a situation’s
meaning to alter one’s emotional response to
the situation (Gross, 1998; Gross & John,
2003). Reappraisal has been studied exten-
sively in nonclinical populations and has
been shown to reduce negative affect (John
& Gross, 2004; Urry, 2009). In addition,
reappraisal does not entail the social and
cognitive costs associated with rumination
and other less adaptive strategies (Richards
& Gross, 2000). Furthermore, it is associ-
ated with reduced physiological activation in
response to negative emotion (Ray, McRae,
Ochsner, & Gross, 2010; Urry, 2009).
Not surprisingly, studies in clinical and
nonclinical samples have tied less frequent
habitual use of reappraisal to greater depres-
sion severity (Garnefski & Kraaij, 2006;
Joormann & Gotlib, 2010). It should be
noted, however, that most of these studies
have used a cross- sectional design, which
Emotion Regulation in Mood Disorders 417
makes it difficult to examine whether
decreased reappraisal use is a symptom of
depression or indeed a risk factor. Garnef-
ski, Legerstee, Kraaij, van den Kommer, and
Teerds (2002), for example, found reduced
use of reappraisal in a clinical sample diag-
nosed with depression and anxiety. Few
researchers have examined the habitual use
of reappraisal in BD. One exception is Gru-
ber, Harvey, and Gross (2012), who exam-
ined the spontaneous use of reappraisal
in BD in response to emotional film clips.
They found that individuals with BD were
more likely than control individuals to use
reappraisal in response to not only positive
and negative but also neutral film clips. The
authors interpreted this result as showing
that BD is associated with more regulation
effort.
It is important to note that habitual reap-
praisal is not beneficial in all contexts. Some
studies have found habitual use of reappraisal
to be unrelated to symptoms or correlated
with poorer outcomes. Nezlek and Kuppens
(2008), for example, who examined daily
measures of affect and emotion regulation,
found that daily use of reappraisal to upreg-
ulate positive emotions was associated with
increases in positive affect and self- esteem,
whereas no relation was found between the
use of reappraisal to down- regulate nega-
tive affect and the experience of negative
affect. In addition, in a recent meta- analysis,
Aldao, Nolen- Hoeksema, and Schweizer
(2010) reported that whereas greater reap-
praisal use is related to lower depression
and anxiety symptoms, reappraisal is more
inconsistently related to symptoms than
other strategies (rumination, suppression).
In a follow- up study, Aldao and Nolen-
Hoeksema (2012) found that the degree to
which reappraisal is adaptive depends on
the frequency of maladaptive strategy use;
reappraisal was related to lower depression
symptoms but only for individuals who fre-
quently use maladaptive strategies (rumina-
tion, suppression, etc.). The authors argued
that reappraisal serves as a compensatory
strategy to counteract the problems that
come with greater maladaptive strategy use.
Suppression
Suppression has long been regarded as a
particularly maladaptive regulation strat-
egy. Suppression is conceptualized as an
emotion regulation strategy by which an
individual attempts to inhibit the effects of
external cues on internal (e.g., physiological)
and external (e.g., emotional expression)
states. The inhibition of emotion expression
is frequently referred to as expressive sup-
pression. Research on emotion suppression
indicates that habitual use of this strategy
is largely ineffective in reducing negative
emotions. Findings indicate that expressive
suppression, for example, is associated with
increased depression symptoms (Joormann
& Gotlib, 2010). Moreover, emotion sup-
pression is associated with increased use of
rumination (Liverant, Kamholz, Sloan, &
Brown, 2011) and decreased inhibitory con-
trol (Joormann & Gotlib, 2010). However,
one study indicates that providing instruc-
tions on the use of expressive suppression in
response to negative stimuli may be effica-
cious in reducing acute emotional respond-
ing among depressed participants (Liverant,
Brown, Barlow, & Roemer, 2008). Though
virtually no studies have examined the use
of emotion suppression in individuals with
BD, one recent study found that, compared
to healthy controls, interepisode partici-
pants with BD demonstrate increased use
of spontaneous expressive suppression in
response to both positive and negative mood
inductions (Gruber et al., 2012). In a clini-
cal sample that included depressed and anx-
ious participants, Campbell- Sills, Barlow,
Brown, and Hofmann (2006) examined
emotion suppression, using a mood induc-
tion film and the assessment of spontane-
ous use of emotion regulation strategies.
They reported that clinical participants used
more suppression, and that suppression was
related to higher levels of negative affect.
Use of Multiple Strategies
Few studies have examined the use of more
than one strategy in psychopathology. A
noteworthy exception is the aforementioned
meta- analysis by Aldao et al. (2010), which
examined the relation between the habit-
ual use of six emotion regulation strate-
gies (rumination, reappraisal, suppression,
acceptance, problem solving, avoidance) and
four groups of symptoms of psychopathol-
ogy (depression, anxiety, substance- related
disorders, and eating disorders) in studies
418 PSYCHOPATHOLOGY
that used clinical or normative samples.
They obtained a large effect size for the
association of depression with rumination
and avoidance, a medium to large effect size
for problem solving and suppression, and
small effect sizes for acceptance and reap-
praisal. The authors summarized their find-
ings by pointing out that, overall, adaptive
emotion regulation strategies (reappraisal,
acceptance) showed a weaker association
with psychopathology than maladaptive
strategies. In a follow- up study using a com-
munity sample, Aldao et al. (2010) further
found an interaction between the use of
adaptive and maladaptive strategies cross-
sectionally. High use of adaptive strategies
was associated with lower psychopathol-
ogy only in participants who also reported
high levels of use of maladaptive strategies.
The authors further reported that the use of
adaptive strategies did not predict psychopa-
thology in a follow- up assessment, whereas
the use of maladaptive strategies signifi-
cantly predicted psychopathology 6 months
later.
Studies that use clinical samples and
examine the use of a range of different
emotion regulation strategies are particu-
larly informative. D’Avanzato, Joormann,
Siemer, and Gotlib (2013), for example,
compared habitual use of emotion regula-
tion strategies in control participants and
in samples of participants diagnosed with
depression or social anxiety disorder. These
authors reported that depressed participants
were more likely to ruminate and less likely
to use reappraisal than control participants,
whereas participants with social anxiety dis-
order reported more expressive suppression
compared to the two other groups. In addi-
tion, whereas elevated rumination was also
found in previously depressed participants
who were not currently depressed, healthy
controls and the previously depressed group
did not differ on use of reappraisal or expres-
sive suppression. Interestingly, only use of
reappraisal and rumination were related to
symptom severity across all samples; expres-
sive suppression use was unrelated to depres-
sive or anxiety symptoms.
These results are in line with a recent
study by Ehring, Tuschen- Caffier, Schnülle,
Fischer, and Gross (2010) that compared
formerly depressed to never- depressed par-
ticipants. Like D’Avanzato et al. (2013),
they reported that formerly depressed par-
ticipants used more rumination but did not
differ from never- depressed participants in
reported use of reappraisal or suppression.
In addition, the formerly depressed group
reported more emotion nonacceptance.
Interestingly, even though the groups did
not differ on habitual use of suppression,
the formerly depressed group reported more
spontaneous use of suppression in response
to a negative film clip. No group differences
were found in reappraisal use in response
to the film clip. The authors interpret this
finding to suggest that depression may not
be associated with suppression of all emo-
tional experiences but rather with the spe-
cific suppression of sadness. Further studies
are needed to examine this possibility more
closely.
Few studies have examined the use of
multiple strategies in BD. Green et al. (2011)
showed that the BD group reported more
frequent use of rumination and less frequent
use of reappraisal in response to negative life
events compared to unaffected relatives, but
this study did not examine responses to posi-
tive events. Abnormal response styles to neg-
ative mood have previously been observed
in students with hypomanic traits (Thomas
& Bentall, 2002) and in patients with BD
(Thomas, Knowles, Tsai, & Bentall, 2007).
Thomas and Bentall (2002) found that hypo-
manic traits were associated with not only
rumination but also increased distraction
and risk taking. Thomas et al. (2007) found
that patients with mania reported high levels
of risk taking and active coping (distraction
and problem solving), whereas patients with
remitted BD reported high levels of rumina-
tion. Clearly more research is needed on the
interplay of emotion regulation strategies in
this disorder.
Summary of Strategy Selection
In summary, the results clearly show that
depression is characterized by increased use
of rumination and less use of reappraisal.
There is some indication that rumination
use may be a stable feature of depression
risk even outside of acute episodes of the
disorder, whereas reduced use of reappraisal
is observed only in people who are currently
depressed (see D’Avanzato et al., 2013;
Ehring et al., 2010). This finding suggests

Emotion Regulation in Mood Disorders 419
that rumination may be a risk factor for
not only the recurrence but also the onset of
depression, whereas changes in reappraisal
use may be an epiphenomenon of experienc-
ing a depressive episode. Results for expres-
sive suppression and other forms of suppres-
sion are less clear-cut, as are findings for the
use of acceptance in depression.
It is interesting to note that, in contrast
to MDD, BD seems to be characterized by
problems regulating negative and positive
affect, and many studies now suggest an
important role for positive rumination in the
onset of manic episodes. Indeed, it is possi-
ble that acute mood state plays an important
role here, with patients with BD in a depres-
sive episode resembling patients with MDD
in their use of rumination and reduced use
of reappraisal. At the same time, positive
rumination when experiencing hypomania
may play a critical role in the onset of manic
episodes (Johnson, 2005). Other studies
have shown that participants with BD delib-
erately choose self- calming strategies during
early phases of hypomania to try to prevent
the emergence of manic symptoms (Lam
& Wong, 1997). The use of other emotion
regulation strategies in BD, however, has
either not been studied or studies have led to
inconclusive results. It should be noted that
BD is also characterized by irritable affect,
yet no studies have examined the relation of
this aspect of the disorder to emotion regula-
tion.
It is further important to note that most
studies reviewed here examine individuals
who are currently in episode. It is not clear,
therefore, whether the use of maladaptive
strategies precedes the onset of the disor-
der or is merely another symptom of being
depressed or manic. Gaining more insight
into whether the use of emotion regulation
strategies is a vulnerability factor will be
important for intervention and prevention
programs. In addition, we should note that
the majority of studies of habitual strategy
use are cross- sectional and have relied on
self- report only. This is problematic, because
it is not clear to what extent people can accu-
rately report on their use of strategies, and
the self- report of emotion regulation may be
affected by current mood state. More stud-
ies that examine strategy use in response
to a mood manipulation (e.g., Ehring et al.
2010) are clearly needed. Importantly, it is
not clear what underlies the preference and
dispositional use of specific emotion regu-
lation strategies in affective disorders. It is
likely that difficulties implementing certain
strategies also shape the selection of emo-
tion regulation strategies. In the second part
of this chapter, we therefore examine the
implementation of emotion regulation strat-
egies in mood disorders and try to identify
cognitive processes that may underlie diffi-
culties in emotion regulation.
Implementation
of Adaptive Strategies
Whereas the majority of studies discussed in
the previous section used self- report to exam-
ine the frequency of use of different emotion
regulation strategies, other studies have
focused on whether affective disorders are
characterized by less effective implementa-
tion of adaptive strategies. These researchers
typically instruct participants to use a strat-
egy such as reappraisal during an emotion-
eliciting event, then assess affect, as well as
psychophysiological indicators of arousal,
before and after the use of the strategy.
Distraction
Perhaps the best studied adaptive strategy
in depression is distraction. Distraction is
frequently used as a control condition in
rumination studies, and many studies have
shown that distraction seems to regulate
affect effectively in depressed participants
(see Nolen- Hoeksema et al., 2008, for a
review). Whereas depressed participants
instructed to ruminate show increased nega-
tive affect and recall of negative memories,
those instructed to distract do not differ
from the control group on these measures.
For example, in a study that examined the
recall of positive memories in depression,
currently and formerly depressed partici-
pants did not differ from control partici-
pants in their affect recovery after a nega-
tive mood induction that was followed by
a distraction induction (Joormann, Siemer
& Gotlib, 2007). Thus, studies suggest
that depressed participants are able to use
distraction to regulate negative affect effec-
tively. Research on the use of distraction in
BD is currently nonexistent.
420 PSYCHOPATHOLOGY
Reappraisal
Only a handful of studies has examined the
effectiveness of reappraisal in affective dis-
orders, and results are much more mixed.
Indeed, to our knowledge, no study thus
far has examined whether current MDD is
associated with less effective reappraisal use.
Troy, Wilhelm, Shallcross and Mauss (2010)
recruited a nonclinical sample of individu-
als who had recently experienced a stressful
life event and found that elevated depression
symptoms were related to less effective reap-
praisal in response to an emotion- eliciting
film clip. Similarly, McRae, Jacobs, Ray,
John, and Gross (2012) reported that indi-
viduals with lower well-being (composite of
lower positive affect, higher negative affect,
and lower life satisfaction) exhibited reduced
effectiveness of reappraisal in response to
negative emotional pictures, and that per-
formance on this task was related to reduced
habitual use of reappraisal. Thus, this study
suggests a relation between the effectiveness
of strategies and habitual strategies use as
proposed earlier. In contrast, Ehring et al.
(2010) reported that even though formerly
depressed participants were less likely to
endorse the use of reappraisal on self- report,
they did not differ from the control group
in their ability to use reappraisal effectively
when explicitly instructed to do so. Indeed,
these authors summarized their results
by saying that formerly depressed partici-
pants choose ineffective strategies but can
use more functional ones if so instructed.
Given that no studies have examined reap-
praisal effectiveness in current depression,
however, it is unclear whether this result is
specific to a previously depressed sample or
would generalize to current depression. Very
few studies have looked at the effectiveness
of different emotion regulation strategies
in BD. A noteworthy exception is the pre-
viously mentioned study by Gruber et al.
(2012), who found that participants with
BD reported more effort when using reap-
praisal and suppression but also less success.
It should be noted, however, that effort and
success were assessed using two self- report
items; these results therefore clearly require
replication using alternative operationaliza-
tions of these constructs.
Flexibility of use and the timing over the
course of an emotional response are key
variables that may influence the effective-
ness of reappraisal. Reappraisal, initiated
once an emotional response is underway
(often referred to as online reappraisal),
appears to be more difficult to implement
and more cognitively taxing than anticipa-
tory reappraisal or other strategies, such as
distraction, implemented at early processing
stages of an emotional episode (Sheppes &
Meiran, 2007; Sheppes, Catran, & Meiran,
2009; Urry, 2009). Online reappraisal may
be particularly important to examine in
MDD. Given the persistent negative affect
characterizing this disorder, individuals with
depression are likely to need to implement
emotion regulation strategies in response to
an existing emotional state of relatively high
intensity. If implemented online, reappraisal
probably requires levels of cognitive control
that may not be available to people with
affective disorders. This may explain the
mixed findings on the effectiveness of reap-
praisal in mood disorders. We discuss this
point in more detail later, when we examine
cognitive processes that underlie effective
emotion regulation.
Mood‑Incongruent Recall
Other studies have examined the recall of
mood- incongruent material in depression
(Joormann et al., 2007). In these studies,
participants did not receive explicit instruc-
tions to regulate affect. Instead, they were
informed that the project examined memory
processes and were asked to recall mood-
incongruent memories (i.e., recall of a posi-
tive autobiographical memory) immediately
following a negative mood induction. Recall
of mood- incongruent memories was com-
pared to a distraction condition. The results
showed that not only dysphoric but also
currently depressed and formerly depressed
participants compared to controls did not
experience a recovery of their negative affect
when recalling mood- incongruent memories
(see also Werner- Seidler & Moulds, 2012).
Distraction, however, repaired negative
affect in all groups. These results suggest
that depression is associated with an inabil-
ity to focus on positive material to offset
negative affect, which may be an important
automatic mood repair mechanism. The
results are well aligned with the aforemen-
tioned finding that depression is associated
with reduced savoring after experiencing
positive events.
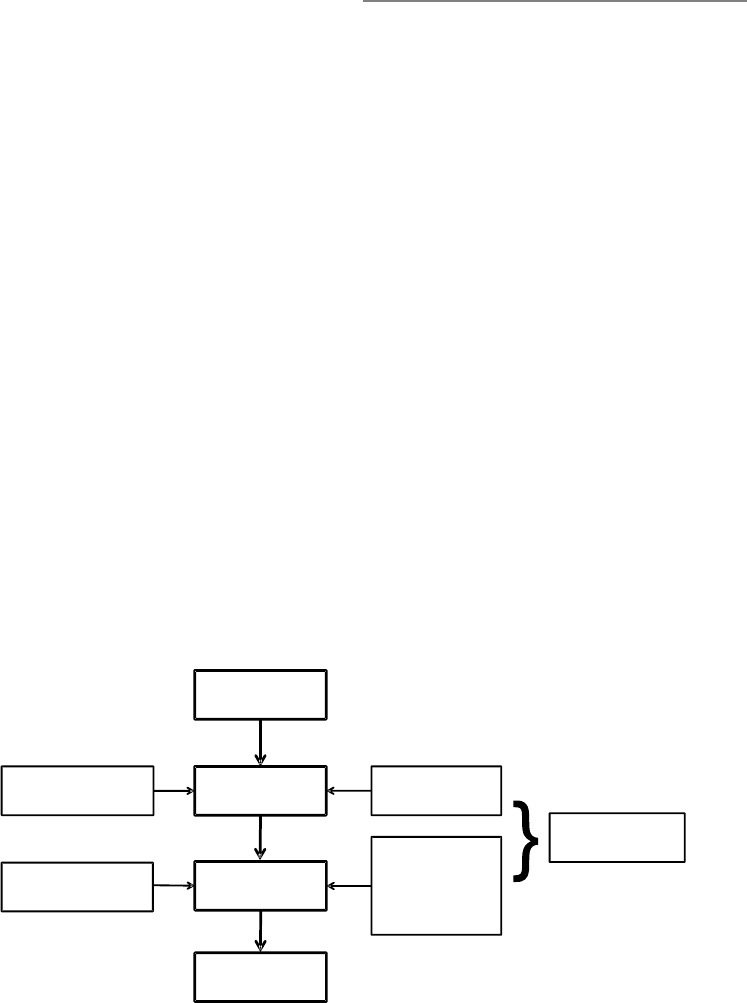
Emotion Regulation in Mood Disorders 421
Summary of Strategy Implementation
In summary, the studies presented so far
suggest that affective disorders are associ-
ated with more frequent use of maladap-
tive strategies, as well as difficulties imple-
menting adaptive strategies. Specifically,
even though distraction seemed to work
for depressed participants, recall of positive
material worked well in nondisordered par-
ticipants but did not repair negative affect in
the clinical sample. Results of studies focus-
ing on reappraisal are more mixed and it
may be important to take the timing of reap-
praisal into account.
Why do depressed people show a prefer-
ence for the use maladaptive strategies, and
why do they find it difficult to use adaptive
strategies? Cognitive theories of emotional
disorders have identified mood- congruent
biases in the processing of emotional infor-
mation, and recent research has examined
deficits in cognitive control that may inter-
fere with emotion regulation (Joormann,
2010). These studies may improve our
insight into mechanisms that underlie emo-
tion and mood regulation difficulties and
thereby provide critical information for
interventions. However, given the scarcity of
research on this topic in BD, in the following
section we focus primarily on MDD.
Cognitive Processes and Difficulties
in Emotion Regulation
Cognitive Biases
Individual differences in cognitive processes
such as attention, memory, and interpre-
tation may affect the attention toward
emotion- eliciting aspects of a situation,
the initial appraisal of a situation, and the
ability to use regulation strategies such as
reappraisal or distraction effectively (see
Figure 25.1). Cognitive models of depres-
sion posit that depressed individuals exhibit
mood- congruent biases in all aspects of
information processing, including atten-
tion, memory, and interpretation (Mathews
& MacLeod, 2005). Biased memory for
negative, relative to positive, information
represents perhaps the most robust cogni-
tive finding associated with MDD and may
be related to the difficulties using mood-
incongruent recall to repair negative affect,
as we discussed earlier. Other studies have
reported interpretation biases in depression
(Lawson, MacLeod, & Hammond, 2002).
Indeed, a recent meta- analysis by Hal-
lion and Ruscio (2012) showed that biased
interpretation is one of the most consis-
tently reported findings in the depression
literature. Interpretation biases may lead
FIGURE 25.1. Cognitive processes and emotion dysregulation in depression. Cognitive biases such as
difficulties disengaging from negative material and biases in interpretation and memory, affect both
attention to emotion-
e
liciting aspects of events and appraisals of the event, which in turn affect the emo-
tion response. Through their effect on attention and appraisal processes, cognitive biases also interfere
with emotion regulation. In addition, cognitive control deficits further impair the effective implemen-
tation of emotion regulation strategies such as reappraisal, distraction, and mood- incongruent recall,
increasing the likelihood of maladaptive responding, for example, in the form of rumination.
Negative Event
Attention
Appraisal
Emotional
Response
Attention Bias
Interpretation and
Memory Bias
Cognitive Control
Deficits
Reappraisal/
Mood-
Incongruent
Recall
Distraction/
Rumination
422 PSYCHOPATHOLOGY
to inflexible, automatic, and unconscious
appraisals that make it difficult to regulate
emotions by using deliberate reappraisal of
the situation (Siemer & Reisenzein, 2007).
Whereas some studies have failed to find
attentional biases in depression, others
suggest that stimulus exposure duration is
critical (e.g., Mogg, Bradley, Williams, &
Mathews, 1993). Specifically, biases have
been found when stimuli are presented for
longer durations (see Mathews & MacLeod,
2005, for a review). Recent studies therefore
suggest that depressed individuals do not
direct their attention to negative informa-
tion more frequently than do control partic-
ipants, but once it captures their attention,
they exhibit difficulties disengaging from it
(e.g., Joormann & Gotlib, 2007). Difficul-
ties disengaging from negative material may
result in maintenance of attention on mood-
congruent information, which may eventu-
ally lead to recurrent ruminative thoughts.
Cognitive Control
In addition to cognitive biases, depression-
related deficits in cognitive control may
affect emotion regulation (see Figure 25.1).
Negative mood is generally associated with
the activation of mood- congruent represen-
tations in working memory (Siemer, 2005).
The ability to control the contents of work-
ing memory (WM) might therefore play an
important role in recovery from negative
affect. WM is a limited- capacity system
that reflects the focus of attention and the
temporary activation of representations
that are the content of awareness. Cognitive
inhibition is part of executive control pro-
cesses that select and update WM content
(Hasher, Zacks, & May, 1999). Deficits in
cognitive inhibition may make it difficult to
discard mood- congruent content from WM,
keeping attention focused on the emotion-
eliciting aspects and the initial appraisals of
the event. The effectiveness of distraction
and reappraisal may directly depend on the
ability to inhibit mood- congruent content in
WM, and difficulties with inhibition may
result in rumination.
In a series of studies using a negative
priming task, dysphoric participants, clini-
cally depressed participants, and partici-
pants with a history of depressive episodes
exhibited reduced inhibition of negative but
not positive material (Joormann & Gotlib,
2010; Goeleven, De Raedt, Baert, & Koster,
2006). These findings suggest that depres-
sion involves difficulties keeping irrelevant
emotional information from entering WM.
Other studies suggest that depression is also
associated with difficulties removing previ-
ously relevant negative material from WM
(Joormann & Gotlib, 2008). Difficulties
inhibiting the processing of negative mate-
rial that was, but is no longer, relevant might
explain why people respond to negative
mood states and negative life events with
recurring, uncontrollable, and unintentional
negative thoughts.
It is likely that deficits in cognitive con-
trol not only affect people’s ability to disen-
gage attention from irrelevant material but
also make it difficult for them intentionally
to forget unwanted material. Investigators
have consistently documented such memory
biases in depression. Deficits in inhibition
have been tested in directed forgetting tasks,
in which participants are instructed to for-
get previously studied material at some point
during the experiment. Later, however,
recall is tested, of both material that was to
be remembered and material that was to be
forgotten. Using emotional material, Power,
Dalgleish, Claudio, Tata, and Kentish (2000)
reported that depressed participants showed
less forgetting of negative material. Using
a similar task with depressed participants,
Joormann, Hertel, LeMoult, and Gotlib
(2009) replicated these difficulties in the
intentional forgetting of negative material.
A subset of participants, however, was pro-
vided a strategy to improve suppression by
using thought substitutes. In this condition
depressed participants were able to inhibit
and forget negative words. This finding has
intriguing implications for interventions.
Deficits in disengaging attention from
negative material and difficulties controlling
the content of WM may affect emotion regu-
lation in various ways. An inability to appro-
priately expel mood- congruent items from
WM as they become irrelevant may result
in rumination. Deficits in cognitive control
may also lead to difficulties attending to and
processing new information, thereby hin-
dering the use of more adaptive strategies.
Effective reappraisal, for example, depends
on a person’s ability to override (automatic)
interpretation biases that lead to unwanted
appraisals of the emotion- eliciting cues.
Replacing automatic appraisals with alter-

Emotion Regulation in Mood Disorders 423
native evaluations of the situation requires
cognitive control. Finally, deficits in cogni-
tive control can make it difficult to access
mood- incongruent material. People fre-
quently recruit pleasant memories to repair
sad mood. Difficulties inhibiting salient but
irrelevant thoughts could therefore reduce
the use of more effective emotion regulation
strategies and/or render these strategies less
effective. Few studies so far have investigated
the association between inhibition and the
use and effectiveness of emotion regulation
strategies such as reappraisal and distrac-
tion. Numerous findings, however, support
the proposition that inhibition deficits are
related to the use of increased rumination.
For example, Joormann (2006) reported a
correlation between rumination and defi-
cits in cognitive inhibition, as assessed by
negative priming, and Joormann and Gotlib
(2008) found a correlation between rumi-
nation and the ability to remove irrelevant
negative material from WM. Finally, Joor-
mann and Gotlib (2010) reported that an
inability to inhibit the processing of negative
material was related to an increased likeli-
hood of rumination and a decreased likeli-
hood of using reappraisal, in both healthy
and depressed participants.
Summary and Future Directions
Given that sustained affective states are the
hallmark feature of mood disorders, basic
research on the regulation of mood states
and emotions provides important informa-
tion for an improved understanding of risk
factors for the development and mainte-
nance of MDD and BD. Research on emo-
tion regulation may thereby contribute to
the development of improved prevention and
intervention efforts. Our review of the litera-
ture provides evidence of the importance of
both aspects of emotion regulation presented
at the beginning of this chapter: individual
differences in the habitual use of specific
emotion regulation strategies and disorder-
related difficulties in the implementation
of adaptive strategies. Specifically, MDD is
associated with frequent use of rumination
and less use of reappraisal. BD is also related
to increased rumination use, although, inter-
estingly, in response to positive and negative
events. Results for other emotion regulation
strategies were either missing or inconsistent,
so more work is needed to clarify further the
role of suppression– avoidance and accep-
tance in both disorders, as well as the role
of reappraisal in BD. Given methodological
issues associated with the use of self- report
in research on emotion regulation, future
studies should include additional measures
such as experience sampling or experimental
manipulations of mood to understand strat-
egy selection better in affective disorders.
Fewer studies have examined difficulties
in the implementation of adaptive strate-
gies. Initial findings suggest that whereas
depressed participants can use distraction
effectively, reappraisal is more challenging.
Importantly, studies suggest that depression
is associated with difficulties using positive
material to offset negative affect. Whereas
some studies suggest that these difficulties
in strategy implementation do not just char-
acterize currently depressed participants,
more research is needed on the question of
whether we find similar deficits prior to the
first episode of the disorder, and whether
these difficulties predict future episodes of
depression or mania. Specifically, longitudi-
nal studies in high-risk samples are needed
to answer the important question of whether
these difficulties in emotion regulation are
indeed a risk factor for the onset of the dis-
order or simply an epiphenomenon of mood
disorders.
We further examined cognitive processes
that may underlie difficulties in emotion
regulation. Biased processing in depres-
sion may affect the initial appraisal of an
emotion- eliciting event, as well as the abil-
ity to reappraise. Cognitive control defi-
cits may affect response- focused strategies
such as distraction and online reappraisal
(see Figure 25.1). Whereas both strategies
require cognitive control, online reappraisal
is frequently seen as the more effortful strat-
egy, which may explain why distraction
works but reappraisal may fail in depressed
participants. Importantly, recent work by
Sheppes, Scheibe, Suri, and Gross (2011) has
shown that reappraisal becomes more dif-
ficult when affect is intense, and that non-
disordered participants prefer distraction
over reappraisal when dealing with intense
emotions. As discussed, mood disorders are
associated with deficits in cognitive con-
trol, and emotion regulation in these disor-
ders frequently requires modifying intense
affective states. Indeed, Sheppes and Gross
424 PSYCHOPATHOLOGY
(2011) have pointed out that engaging emo-
tion regulation strategies at a later point in
the emotion episode typically results in the
regulation of more intense affect. Mood dis-
orders probably involve many attempts at
regulation of high- intensity affect at later
points in time, because they frequently
require the regulation of global moods with
unclear or unknown antecedents. It is there-
fore not surprising that reappraisal is diffi-
cult to implement in mood disorders. Future
research should focus more on identifying
differences between emotion and mood reg-
ulation, and on implications of these differ-
ences for affective disorders.
Most studies on emotion regulation have
focused on the down- regulation of negative
affect, but our review of the literature shows
that the processing of mood- congruent
material and the regulation of positive affect
may also play an important role in mood
disorders, specifically in BD. Difficulties
savoring positive events and recalling posi-
tive material when feeling sad, however,
may also play a role in MDD. Therefore, the
down- regulation of negative affect seems a
very narrow focus, and studies in BD and
depression should pay more attention to the
regulation of positive affect. In addition, at
numerous points during the chapter, we have
recommended flexibility in the use of emo-
tion regulation strategies as an important
topic for future studies. Very few studies
examining affective disorders have assessed
more than one strategy, and it is currently
not known whether the primary problem
in emotion regulation in these disorders is
inflexibility or of habitual use of a specific
strategy, or difficulties implementing a strat-
egy.
Finally, there are some important treat-
ment implications of research on emotion
regulation in mood disorders. Cognitive-
behavioral therapy has focused on the iden-
tification and modification of maladaptive
cognitions, and recent additions to this
intervention have started to focus more sys-
tematically on training reappraisal skills and
preventing emotional avoidance (Campbell-
Sills & Barlow, 2007). If inflexibility is
a problem, it may be helpful to develop a
broader repertoire of strategies and to iden-
tify habitual emotion regulation strategies.
Given the earlier discussion of cognitive
processes that underlie emotion regulation,
recent studies on cognitive bias modification
and cognitive control training seem particu-
larly promising. Most of these studies have
involved anxiety disorders, but at least two
recent articles provide encouraging data to
indicate that training to modify attention
biases may also apply to depression (Wells &
Beevers, 2010; Baert, Koster, & De Raedt,
2011). Other studies have focused on modi-
fying memory and interpretation biases, but
these trainings have not yet been tested with
depressed participants (Raes, Williams, &
Hermans, 2009). Of special importance is
a recent study that showed improvement in
cognitive control after training in executive
control. Specifically, cognitive control train-
ing yielded transferable gains to improve
control over affective stimuli (Schweizer,
Hampshire, & Dalgleish, 2011). A similar
training showed effects on thought control
over intrusive memories (Bomyea & Amir,
2011). These findings are exciting and repre-
sent first steps toward a more comprehensive
model of how cognitive factors facilitate or
hinder emotion regulation, thereby affect-
ing individuals’ vulnerability to experience
depressive episodes.
At this point, however, a lot of questions
remain unanswered. Why do people diag-
nosed with affective disorders prefer certain
emotion regulation strategies, and are they
aware of these preferences? How do people
learn to use these strategies, and how can we
help them unlearn them? Are individual dif-
ferences in attention, interpretation, mem-
ory, and cognitive control indeed related to
the selection and effectiveness of regulation
strategies? Does modifying cognitive biases
and cognitive control affect emotional
responding, stress reactivity, and emotion
regulation? Are the outlined associations
among depression, cognitive processes, and
emotion regulation specific to depressive
disorders or a feature of psychopathology in
general? These and other questions should
be the main focus of future studies in this
exciting area of research. A deeper under-
standing of the ways that cognitive pro-
cesses help and hinder emotion regulation
may allow us to better address some of the
difficulties depressed individuals encounter
in developing new and more flexible ways
of thinking, a central goal of cognitive ther-
apy. Understanding the relation of cognitive
processes and emotion regulation may also
help us understand how we can get these
new ways of thinking to “stick” and lead to

Emotion Regulation in Mood Disorders 425
permanent changes in emotional response,
facilitating the use of these new skills in the
face of stress.
References
Aldao, A., & Nolen- Hoeksema, S. (2012). When
are adaptive strategies most predictive of psy-
chopathology? Journal of Abnormal Psychol-
ogy, 121(1), 276–281.
Aldao, A., Nolen- Hoeksema, S., & Schweizer, S.
(2010). Emotion- regulation strategies across
psychopathology: A meta- analytic review.
Clinical Psychology Review, 30(2), 217–237.
Alloy, L. B., Abramson, L. Y., Flynn, M., Liu,
R. T., Grant, D. A., Jager-Hyman, S., et al.
(2009). Self- focused cognitive styles and bipo-
lar spectrum disorders: Concurrent and pro-
spective associations. International Journal of
Cognitive Therapy, 2(4), 354–372.
Baert, S., Koster, E. H. W., & De Raedt, R.
(2011). Modification of information process-
ing biases in emotional disorders: Clinically
relevant developments in experimental psy-
chopathology. International Journal of Cog-
nitive Therapy, 4(2), 208–222.
Beck, A. T., Rush, A. J., Shaw, B. F., & Emery,
G. (1979). Cognitive therapy of depression: A
treatment manual. New York: Guilford Press.
Bomyea, J., & Amir, N. (2011). The effect of
an executive functioning training program
on working memory capacity and intrusive
thoughts. Cognitive Therapy and Research,
35(6), 529–535.
Bonanno, G. A., Papa, A., Lalande, K., West-
phal, M., & Coifman, K. (2004). The impor-
tance of being flexible. Psychological Science,
15, 482–487.
Campbell- Sills, L., & Barlow, D. H. (2007).
Incorporating emotion regulation into con-
ceptualizations and treatments of anxiety and
mood disorders. In J. J. Gross (Ed.), Hand-
book of emotion regulation (pp. 542–559).
New York: Guilford Press.
Campbell- Sills, L., Barlow, D. H., Brown, T. A.,
& Hofmann, S. G. (2006). Acceptability and
suppression of negative emotion in anxiety
and mood disorders. Emotion, 6(4), 587–595.
D’Avanzato, C., Joormann, J., Siemer, M., &
Gotlib, I. H. (2013). Emotion regulation in
depression and anxiety: Examining diagnos-
tic specificity and stability. Cognitive Therapy
and Research. [E-publication prior to print]
Ehring, T., Tuschen- Caffier, B., Schnülle, J.,
Fischer, S., & Gross, J. J. (2010). Emotion
regulation and vulnerability to depression:
Spontaneous versus instructed use of emotion
suppression and reappraisal. Emotion, 10(4),
563–572.
Feldman, G. C., Joormann, J., & Johnson, S. L.
(2008). Responses to positive affect: A self-
report measure of rumination and dampen-
ing. Cognitive Therapy and Research, 32(4),
507–525.
Garnefski, N., & Kraaij, V. (2006). Cogni-
tive Emotion Regulation Questionnaire—
development of a short 18-item version
(CERQ-short). Personality and Individual
Differences, 41(6), 1045–1053.
Garnefski, N., Legerstee, J., Kraaij, V., van den
Kommer, T., & Teerds, J. (2002). Cognitive
coping strategies and symptoms of depres-
sion and anxiety: A comparison between ado-
lescents and adults. Journal of Adolescence,
25(6), 603–611.
Goeleven, E., De Raedt, R., Baert, S., & Koster,
E. H. W. (2006). Deficient inhibition of emo-
tional information in depression. Journal of
Affective Disorders, 93(1–3), 149 –157.
Green, M. J., Lino, B. J., Hwang, E. J., Sparks,
A., James, C., & Mitchell, P. B. (2011). Cogni-
tive regulation of emotion in bipolar I disorder
and unaffected biological relatives. Acta Psy-
chiatrica Scandinavica, 124(4), 307–316.
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85(2), 348–362.
Gruber, J. (2011). Can feeling too good be bad?:
Positive emotion persistence (PEP) in bipolar
disorder. Current Directions in Psychological
Science, 20(4), 217–221.
Gruber, J., Eidelman, P., Johnson, S. L., Smith,
B., & Harvey, A. G. (2011). Hooked on a feel-
ing: Rumination about positive and negative
emotion in inter- episode bipolar disorder.
Journal of Abnormal Psychology, 120(4),
956–961.
Gruber, J., Harvey, A. G., & Gross, J. J. (2012).
When trying is not enough: Emotion regula-
426 PSYCHOPATHOLOGY
tion and the effort– success gap in bipolar dis-
order. Emotion, 12(5), 997–1003.
Gruber, J., Harvey, A. G., & Johnson, S. L.
(2009). Reflective and ruminative process-
ing of positive emotional memories in bipo-
lar disorder and healthy controls. Behaviour
Research and Therapy, 47(8), 697–704.
Hallion, L. S., & Ruscio, A. M. (2011). A meta-
analysis of the effect of cognitive bias modifi-
cation on anxiety and depression. Psychologi-
cal Bulletin, 137(6), 940–958.
Hasher, L., Zacks, R. T., & May, C. P. (1999).
Inhibitory control, circadian arousal, and age.
In D. Gopher & A. Koriat (Eds.), Attention
and performance XVII: Cognitive regulation
of performance: Interaction of theory and
application (pp. 653–675). Cambridge, MA:
MIT Press.
John, O. P., & Gross, J. J. (2004). Healthy and
unhealthy emotion regulation: Personality
processes, individual differences, and life span
development. Journal of Personality, 72(6),
1301–1333.
Johnson, S. L. (2005). Mania and dysregulation
in goal pursuit: A review. Clinical Psychology
Review, 25(2), 241–262.
Johnson, S. L., McKenzie, G., & McMurrich, S.
(2008). Ruminative responses to negative and
positive affect among students diagnosed with
bipolar disorder and major depressive disor-
der. Cognitive Therapy and Research, 32(5),
702–713.
Joormann, J. (2006). Differential effects of rumi-
nation and dysphoria on the inhibition of
irrelevant emotional material: Evidence from a
negative priming task. Cognitive Therapy and
Research, 30(2), 149–160.
Joormann, J. (2010). Cognitive inhibition and
emotion regulation in depression. Current
Directions in Psychological Science, 19(3),
161–166.
Joormann, J., & Gotlib, I. H. (2007). Selective
attention to emotional faces following recov-
ery from depression. Journal of Abnormal
Psychology, 116(1), 80 –85.
Joormann, J., & Gotlib, I. H. (2008). Updating
the contents of working memory in depres-
sion: Interference from irrelevant negative
material. Journal of Abnormal Psychology,
117(1), 182–192.
Joormann, J., & Gotlib, I. H. (2010). Emotion
regulation in depression: Relation to cognitive
inhibition. Cognition and Emotion, 24(2),
281–298.
Joormann, J., Hertel, P. T., LeMoult, J., & Got-
lib, I. H. (2009). Training forgetting of nega-
tive material in depression. Journal of Abnor-
mal Psychology, 118(1), 34 – 43.
Joormann, J., Siemer, M., & Gotlib, I. H. (2007).
Mood regulation in depression: Differential
effects of distraction and recall of happy mem-
ories on sad mood. Journal of Abnormal Psy-
chology, 116(3), 484–490.
Kashdan, T. B., & Rottenberg, J. (2010). Psy-
chological flexibility as a fundamental aspect
of health. Clinical Psychology Review, 30(4),
467–480.
Kessler, R. C., & Wang, P. S. (2009). Epidemi-
ology of depression. In I. H. Gotlib & C. L.
Hammen (Eds.), Handbook of depression
(2nd ed., pp. 5–22). New York: Guilford Press.
Kim, S., Yu, B. H., Lee, D. S., & Kim, J.-H.
(2012). Ruminative response in clinical
patients with major depressive disorder, bipo-
lar disorder, and anxiety disorders. Journal of
Affective Disorders, 136(1–2), e77–e81.
Lam, D., & Wong, G. (1997). Prodromes, cop-
ing strategies, insight and social functioning
in bipolar affective disorders. Psychological
Medicine, 27(5), 1091–1100.
Lawson, C., MacLeod, C., & Hammond, G.
(2002). Interpretation revealed in the blink
of an eye: Depressive bias in the resolution of
ambiguity. Journal of Abnormal Psychology,
111(2), 321–328.
Liverant, G. I., Brown, T. A., Barlow, D. H.,
& Roemer, L. (2008). Emotion regulation in
unipolar depression: The effects of acceptance
and suppression of subjective emotional expe-
rience on the intensity and duration of sadness
and negative affect. Behaviour Research and
Therapy, 46(11), 1201–1209.
Liverant, G. I., Kamholz, B. W., Sloan, D. M.,
& Brown, T. A. (2011). Rumination in clini-
cal depression: A type of emotional suppres-
sion? Cognitive Therapy and Research, 35(3),
253–265.
Lyubomirsky, S., & Nolen- Hoeksema, S. (1993).
Self- perpetuating properties of dysphoric
rumination. Journal of Personality and Social
Psychology, 65(2), 339–349.
Mathews, A., & MacLeod, C. (2005). Cognitive
vulnerability to emotional disorders. Annual
Review of Clinical Psychology, 1(1), 167–195.
McRae, K., Jacobs, S. E., Ray, R. D., John, O.
P., & Gross, J. J. (2012). Individual differences
in reappraisal ability: Links to reappraisal
frequency, well-being, and cognitive control.
Journal of Research in Personality, 46(1), 2–7.
Mogg, K., Bradley, B. P., Williams, R., &
Emotion Regulation in Mood Disorders 427
Mathews, A. (1993). Subliminal process-
ing of emotional information in anxiety and
depression. Journal of Abnormal Psychology,
102(2), 304–311.
Nezlek, J. B., & Kuppens, P. (2008). Regulating
positive and negative emotions in daily life.
Journal of Personality, 76(3), 561–579.
Nolen- Hoeksema, S. (2000). Further evidence
for the role of psychosocial factors in depres-
sion chronicity. Clinical Psychology: Science
and Practice, 7(2), 224–227.
Nolen- Hoeksema, S., Wisco, B. E., & Lyu-
bomirsky, S. (2008). Rethinking rumination.
Perspectives on Psychological Science, 3(5),
400–424.
Papageorgiou, C., & Wells, A. (2001). Positive
beliefs about depressive rumination: Devel-
opment and preliminary validation of a self-
report scale. Behavior Therapy, 32(1), 13–26.
Power, M. J., Dalgleish, T., Claudio, V., Tata, P.,
& Kentish, J. (2000). The directed forgetting
task: Application to emotionally valent mate-
rial. Journal of Affective Disorders, 57(1–3),
147–157.
Raes, F., Smets, J., Nelis, S., & Schoofs, H.
(2012). Dampening of positive affect prospec-
tively predicts depressive symptoms in non-
clinical samples. Cognition and Emotion,
26(1), 75–82.
Raes, F., Williams, J. M. G., & Hermans, D.
(2009). Reducing cognitive vulnerability to
depression: A preliminary investigation of
MEmory Specificity Training (MEST) in inpa-
tients with depressive symptomatology. Jour-
nal of Behavior Therapy and Experimental
Psychiatry, 40(1), 24–38.
Ray, R. D., McRae, K., Ochsner, K. N., & Gross,
J. J. (2010). Cognitive reappraisal of negative
affect: Converging evidence from EMG and
self- report. Emotion, 10(4), 587–592.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79(3), 410–424.
Schweizer, S., Hampshire, A., & Dalgleish,
T. (2011). Extending brain- training to the
affective domain: Increasing cognitive and
affective executive control through emotional
working memory training. PLoS ONE, 6(9),
e24372.
Sheppes, G., Catran, E., & Meiran, N. (2009).
Reappraisal (but not distraction) is going to
make you sweat: Physiological evidence for
self- control effort. International Journal of
Psychophysiology, 71(2), 91–96.
Sheppes, G., & Gross, J. J. (2011). Is timing
everything? Temporal considerations in emo-
tion regulation. Personality and Social Psy-
chology Review, 15(4), 319–331.
Sheppes, G., & Meiran, N. (2007). Better late
than never?: On the dynamics of online regu-
lation of sadness using distraction and cogni-
tive reappraisal. Personality and Social Psy-
chology Bulletin, 33(11), 1518–1532.
Sheppes, G., Scheibe, S., Suri, G., & Gross, J. J.
(2011). Emotion- regulation choice. Psycholog-
ical Science, 22(11), 1391–1396.
Siemer, M. (2005). Mood- congruent cognitions
constitute mood experience. Emotion, 5(3),
296–308.
Siemer, M., & Reisenzein, R. (2007). The pro-
cess of emotion inference. Emotion, 7, 1–20.
Solomon, A. (2001). The noonday demon. New
York: Scribner.
Teasdale, J. D. (1988). Cognitive vulnerability
to persistent depression. Cognition and Emo-
tion, 2(3), 247–274.
Thomas, J., & Bentall, R. P. (2002). Hypomanic
traits and response styles to depression. Brit-
ish Journal of Clinical Psychology, 41(3),
309–313.
Thomas, J., Knowles, R., Tai, S., & Bentall, R. P.
(2007). Response styles to depressed mood in
bipolar affective disorder. Journal of Affective
Disorders, 100(1–3), 249–252.
Troy, A. S., Wilhelm, F. H., Shallcross, A. J., &
Mauss, I. B. (2010). Seeing the silver lining:
Cognitive reappraisal ability moderates the
relationship between stress and depressive
symptoms. Emotion, 10(6), 783–795.
Urry, H. L. (2009). Using reappraisal to regu-
late unpleasant emotional episodes: Goals and
timing matter. Emotion, 9(6), 782–797.
Watkins, E., & Moulds, M. (2005). Distinct
modes of ruminative self-focus: Impact of
abstract versus concrete rumination on prob-
lem solving in depression. Emotion, 5(3),
319–328.
Wells, T. T., & Beevers, C. G. (2010). Biased
attention and dysphoria: Manipulating selec-
tive attention reduces subsequent depressive
symptoms. Cognition and Emotion, 24(4),
719–728.
Werner- Seidler, A., & Moulds, M. L. (2012).
Mood repair and processing mode in depres-
sion. Emotion, 12(3), 470–478.

428
Have you ever had coffee or tea? A glass of
wine? Smoked even a single cigarette? Virtu-
ally all adults report consuming psychoac-
tive drugs
1
at some point in their lives, sug-
gesting that casual drug use is quite common
(Substance Abuse and Mental Health Ser-
vices Administration [SAMHSA], 2011). On
the other end of the drug use spectrum, sub-
stance use disorders (SUDs; or addictions)
are complex illnesses, encompassing a host
of severe negative physical, economic, and
social consequences, and contributing to
worldwide disability. With a lifetime preva-
lence of 35.3% in the general population,
individuals with SUDs constitute a relatively
small proportion of casual drug users, yet
they also represent the most prevalent and
costly of psychiatric disorders (National
Institute of Mental Health [NIMH], 2007;
SAMHSA, 2011).
Defined as “a problematic pattern of drug
use, leading to clinically significant impair-
ment or distress” (American Psychiatric
Association, 2013, p. 481), SUDs are both
personally and socially devastating in that
they are often chronic and can severely impair
even basic life functioning. In the fifth edi-
tion of the Diagnostic and Statistical Man-
ual of Mental Disorders (DSM-5), SUDs are
characterized by the presence of symptoms
including tolerance, withdrawal, continued
use despite wishes to stop, continued use
despite known negative consequences, and
importantly, a loss of regulatory control over
drug cravings, as well as further drug use.
As such, loss of regulatory control is a key
feature of SUDs. The addition of drug crav-
ing (strong desire for drugs) as a diagnostic
criterion for SUDs in DSM-5 emerged from
a wealth of accumulated research over the
last decade directly linking craving to drug
use and relapse (return to drug use follow-
ing abstinence; e.g., Shiffman et al., 2013;
see later sections for additional discussion).
This suggests that craving is also a key fea-
ture in SUDs, and that regulation of craving
is a specific form of emotion regulation that
can directly reduce drug use.
This chapter focuses on the crucial and
complex role of emotion regulation in SUDs
(see Figure 26.1 for a schematic summary).
In the first section, I discuss the role of acute
drug intoxication as a means of emotion
regulation, arguing specifically that people
use drugs in part to regulate their current
emotional state. This may include increasing
positive affect, ameliorating a preexisting
negative state, or decreasing craving. In the
next section, I explore the role of emotion
dysregulation in SUDs, both as a possible
cause for and a possible consequence of drug
use. In this section, I make several specific
arguments. First, I argue that emotion dys-
regulation in childhood and adolescence may
be an early risk factor and/or distal causal
factor in the later development of SUDs. Sec-
ond, I argue that an inability to effectively
regulate emotions in specific moments may
CHAPTER 26
Emotion Regulation
in Substance Use Disorders
Hedy Kober

Emotion Regulation in Substance Use Disorders 429
be a proximal causal factor for instances of
drug use in individuals who are already suf-
fering from SUDs. Third, I posit that SUDs
are marked by deficits in regulation of a spe-
cific appetitive state, namely, drug craving,
which is at the core of these disorders. I then
review evidence that suggests differences in
the structure and function of the prefrontal
cortex (PFC) may be the neural mechanisms
underlying emotion dysregulation in SUDs.
This section further highlights that although
some PFC abnormalities may precede drug
use, the long-term effect of chronic drug use
on PFC may further impair emotion regula-
tion in SUDs. In this way, drug use may lead
to further emotion dysregulation. The chap-
ter concludes with a section on treatments
for SUDs, many of which focus on increas-
ing emotion regulation skills geared specifi-
cally toward regulation of craving as means
of reducing substance use.
Drug Use as Emotion Regulation
Drugs can regulate emotion by pharmaco-
logically altering one’s current state. For
example, although the exact pharmacologi-
cal profiles of individual drugs differ, and
these differences have both theoretical and
neurobiological implications (e.g., Badiani,
Belin, Epstein, Calu, & Shaham, 2011),
many drugs are ultimately described as
euphoric, increasing positive emotion (Jaffe
& Jaffe, 1989). In human laboratory experi-
ments, self- administration of drugs, includ-
ing alcohol, methamphetamine, cocaine,
and marijuana, significantly increase feel-
ings of “high” and “good drug effects” (e.g.,
Hart, Ward, Haney, Foltin, & Fischman,
2001; see Figure 26.1A). Consistently, it is
has been proposed that these positive effects
of drugs lead to positive reinforcement and
increase the likelihood of future drug use
(Kober, Turza, & Hart, 2009). Further-
more, drug users often develop positive
expectancies regarding drug use (e.g., “If I
drink, I will feel good”) that are associated
with increased drug use and increased risk
of developing SUDs (e.g., Jones, Corbin, &
Fromme, 2001).
In addition to increasing positive emo-
tion, various drugs are known to alleviate
negative emotional states, including anxi-
ety (e.g., alcohol, and anxiolytic medication
such as Valium and Xanax), sadness and
A. Before SUD B. With SUD
PFC
Drugs
PFC
Drugs
Negative Appetitive
(high, craving)
Negative Appetitive
(craving)
Regulation
Emotion
FIGURE 26.1. A simplified model of emotion regulation in SUDs. Panel A: Before SUDs. Prefrontal
cortex (PFC) and drugs can both serve to regulate emotion. It is thought that PFC implements regula-
tion over negative emotion and craving (indicated by downward blunted arrows). In turn, unregulated
negative emotion and craving are associated with increased drug use (upward arrows). Here I propose
that drugs can be seen as a form of emotion regulation as well (indicated by downward blunted arrows),
increasing feelings of high, and decreasing negative emotion and craving. In this context, deficient emo-
tion regulation or PFC control may serve as risk factors for SUDs. Panel B: After development of SUDs.
Chronic drug use affects PFC (indicated by blunted arrow), diminishing its ability to regulate negative
emotion, as well as drug craving (dashed downward blunted arrows). In turn, unregulated negative
emotion and craving further lead to increased drug use (upward arrows). Drug use itself continues to
regulate both negative emotion and drug craving (though perhaps less effectively). This results in a
vicious cycle of reduced PFC-based emotion regulation, negative affect, craving, and increased drug
use. Therefore, treatments for SUDs often focus on enhancing emotion regulation skills, especially
regulation of craving, which has been linked to reduced drug use.

430 PSYCHOPATHOLOGY
depression (e.g., stimulants such as cocaine
and amphetamines), and pain (e.g., heroin,
morphine, and other synthetic prescrip-
tion opiates such as Vicodin). Consistently,
it has been proposed that these negativity-
reducing effects of drugs lead to negative
reinforcement, thus increasing the likeli-
hood of future drug use (Koob & Le Moal,
2008). This idea was initially popularized
by the “self- medication hypothesis” pro-
posed by Khantzian (1985), which has two
main components: (1) Unpleasant affective
states predispose individuals to drug use,
and (2) the choice of drug is not random;
rather, it is the nature of the drug’s effects
in ameliorating the preexisting negative
state that renders a particular drug more
or less reinforcing. In other words, those
with a particular predisposition to negative
affect states are more likely to develop an
SUD for a drug that reverses those particu-
lar affective states. To illustrate, Khantzian
suggested that individuals with strong rage
and aggression use opiate drugs to regu-
late these emotions. In contrast, individu-
als with preexisting depression and melan-
choly develop cocaine use disorders due to
cocaine’s ability to relieve these symptoms.
The self- medication hypothesis is consistent
with patients’ reports that “they got hooked
not because they had taken the drug, but
because they were not normal before in such
a way that the drugs were . . . not the prob-
lem but a solution” (Le Moal, 2009, p. 542).
It is further consistent with the observation
that the expectancy that drugs will allevi-
ate negative affect (e.g., “Drinking will calm
me down”) is associated with increased drug
use and increased risk for SUDs (Jones et al.,
2001).
Although the self- medication hypothesis
has been challenged, several lines of evidence
support the hypothesis that drug use serves
to regulate negative emotion. First, SUDs
frequently co-occur with a number of other
psychiatric disorders, especially mood and
anxiety disorders. Moreover, preexisting
psychiatric diagnoses increase the likelihood
of an individual to subsequently develop an
SUD (e.g., Kessler et al., 2005). This suggests
that individuals who already experience dif-
ficult emotions are more likely to seek and
use drugs, and to develop problematic habits
of drug use that presumably ameliorate their
affective symptoms. A related point is that
treatment for such comorbid disorders fre-
quently reduces drug use (Nunes & Levin,
2004). Second, and similarly, those with
chronic pain are far more likely to develop
SUDs relative to the general, pain-free pop-
ulation, especially to pain- reducing drugs
such as opiates (Morasco et al., 2011).
Third, even normal- range trait levels of
negative affect are related to drug use. For
example, trait depression and neuroticism
correlate negatively with time to relapse
in cigarette smokers (Gilbert, Crauthers,
Mooney, McClernon, & Jensen, 1999),
while trait levels of anger and anxiety corre-
late with craving to drink in alcoholics (Litt,
Cooney, & Morse, 2000). Fourth, negative
affective states are known triggers for crav-
ing in the context of both casual and prob-
lematic substance use (e.g., Shiffman, Paty,
Gnys, Kassel, & Hickcox, 1996). This phe-
nomenon ranges from the common epithet
“I had such a hard day, I need a beer or a
stiff drink” to instances of relapse to drug
use after experiencing a strong life stressor
(e.g., death in family). Indeed, it has been
well documented that both naturally occur-
ring and experimentally induced negative
affect and stress increase drug craving, drug
use, and relapse (e.g., Sinha & Li, 2007).
Finally, drug use also serves to regulate
the experience of craving, which is one of
the most common motivators for drug use
(Childress et al., 1993; Shiffman et al.,
2013). That is, individuals with SUDs use
drugs to temporarily alleviate their experi-
ence of craving, thus generating a vicious
cycle of increasing craving and use. Taken
together, the evidence reviewed in this sec-
tion suggests that drug taking can be a form
of emotion regulation. Specifically, the acute
effects of drugs may regulate preexisting
emotions in both casual and problem drug
users, including increasing positive emotion,
decreasing negative emotion, and decreas-
ing craving for drugs themselves (see Figure
26.1A).
Emotion (dys)Regulation Is a Causal
Factor in SUDs
Although many people casually use drugs
and alcohol, only a small percentage develop
SUDs, highlighting the need to identify risk
and causal factors for the initiation, develop-
Emotion Regulation in Substance Use Disorders 431
ment, and maintenance of these severe dis-
orders. Of course, because SUDs are com-
plex disorders, they are likely caused and
maintained by an intricate combination of
factors, including genetic, cognitive, behav-
ioral, individual- difference, and environ-
mental variables, that likely interact across
multiple levels of analysis. At this time, emo-
tion regulation abilities are already emerging
as one important contributor in the etiology
and maintenance of SUDs, although in the
next decade it is likely that larger longitu-
dinal studies will allow us to identify addi-
tional factors.
Emotion (dys)Regulation as an Early
Risk Factor
As reviewed below, SUDs are frequently
associated with emotion regulation deficits.
The specific question here is: Do these defi-
cits precede the development of the disorder
so that they may be considered a risk fac-
tor? The answer appears to be yes. Begin-
ning with the classic “marshmallow test”
experiments in the 1960s by Mischel and
colleagues, it has been proposed that the
ability to delay gratification, and regulate
emotions like desire, is crucial to children’s
developmental trajectories (for review, see
Mischel, Ayduk, Berman, Casey, Gotlib, et
al., 2011 and Luerssen & Ayduk, this vol-
ume). In these studies, preschool children
were typically presented with a tasty treat
and told that they could have it now, or
alternatively, wait to receive two treats at a
later time—if they could delay gratification.
In his seminal work, Mischel reported that
children vary in their ability to delay gratifi-
cation, ranging from not being able to wait
at all to waiting as long as the experimenter
allowed (and using a variety of spontaneous
strategies to facilitate delay). In his follow-
up work, Mischel (2011) reported that those
preschool children who were able to delay
gratification the longest (by waiting for a
larger treat rather than indulging immedi-
ately in a smaller treat) later achieved higher
Standard Achievement Test (SAT) scores,
had better social- cognitive and emotional
coping in adolescence, and importantly,
were least likely to use crack cocaine in
adulthood. This body of work highlights
how individual differences in emotion regu-
lation (which manifest as early as preschool)
may predate the development of SUDs and
could therefore be conceptualized as a risk
factor predicting illness onset.
In the years since this work was published,
additional data has accumulated to further
suggest that poor self- control
2
in childhood
is indeed a risk factor for drug use and the
development of SUDs. For example, Mof-
fitt and colleagues (2011) followed 1,000
children from birth to age 32. In childhood,
participants were assessed on various self-
control measures related to emotion regula-
tion, including emotional lability, frustra-
tion tolerance, and persistence. The authors
report that individual differences in self-
control were significantly predictive of adult
health outcomes, including substance use
and dependence, as much as 30 years later.
Importantly, the contribution of self- control
factors was distinct from the contribution of
intelligence, social class, and other family-
life variables. Strikingly, the highest and
lowest one-fifth of the sample on measured
self- control were associated with a preva-
lence of 3 and 10%, respectively, of polysub-
stance dependence in adulthood.
In addition, in childhood, the related
construct of trait impulsivity—the ten-
dency to act without thought or regard for
consequences— has been repeatedly asso-
ciated with the development of SUDs in
later adolescence and adulthood (see Iva-
nov, Newcorn, Morton, & Tricamo, 2011;
Verdejo- García, Lawrence, & Clark, 2008,
for reviews). Furthermore, longitudinal
studies suggest that children who suffer
from childhood disorders such as attention-
deficit/hyperactivity disorder and conduct
disorder, which are associated with poor
emotional/behavioral regulation and impul-
sivity, are far more likely to use drugs and
to receive an SUD diagnosis by late adoles-
cence or young adulthood (e.g., August et
al., 2006). It has also been suggested that
the association between childhood disrup-
tive behavior and adolescent- onset sub-
stance use may be mediated by early defi-
cits in emotion regulation and inhibitory
control (Ivanov et al., 2011). A similar con-
struct used by Tarter and colleagues (2003),
termed neurobehavioral disinhibition, is
indexed by measures of emotion regulation,
executive cognitive functioning, and behav-
ior control. This construct distinguishes
between 10- to 12-year-old boys who are at
432 PSYCHOPATHOLOGY
low vs. high risk for development of SUDs
(determined by parental SUD diagnosis). In
addition, this construct was found to predict
substance use at age 16, as well as SUDs in
early adulthood.
The mechanisms by which early emotion
dysregulation leads to later SUDs are a tar-
get of current investigation. One prevailing
hypothesis is that emotion regulation abili-
ties (and cognitive control more generally)
depend on the function of PFC regions (see
Ochsner & Gross, this volume; Johnstone
& Walter, this volume) that are not yet fully
developed in children and adolescents (see
Riediger & Klipker, this volume). Indeed,
adolescence represents a period of both
reduced emotion regulation abilities (Silvers
et al., 2012) as well as substantial neural
development (Giedd et al., 1999). Specifi-
cally, regions of lateral PFC have been found
to be relatively hypoactive during emotion-
related tasks in adolescents as compared to
adults (e.g., Pfeifer, Lieberman, & Dapretto,
2007), with regulation- related activation in
this area increasing with age (McRae et al.,
2012).
Given this developmental trajectory, emo-
tion dysregulation in adolescence may con-
tribute to SUD risk via two parallel routes.
First, immature emotion regulation capaci-
ties in adolescence may result in higher lev-
els of stress and negative emotion, which has
been shown to lead to the initiation of drug
use in animal models (e.g., Haney, Maccari,
Le Moal, Simon, & Vincenzo Piazza, 1995)
and in human adults (see Sinha & Li, 2007,
for review). Second, self- regulation fail-
ures in adolescence may underlie increased
impulsivity and risk- taking behaviors that
may also lead to initiation of drug use (e.g.,
Ivanov et al., 2011). Ultimately, the develop-
mental trajectory of self- regulatory function
suggests that at least some adolescents may
be less able to recruit the neural circuitry
needed to regulate their emotions optimally
and to ultimately avoid substance use.
The idea that adolescents as a group may
in fact be less able to recruit the necessary
neurocircuitry to regulate emotions and
avoid substance use—along with the obser-
vation that individual differences in the
ability to do so are predictive of future sub-
stance use—is especially important, because
adolescence is a period of heightened risk
taking (SAMHSA, 2011) and peer influence
(Steinberg, 2005), both of which expose ado-
lescents to drugs. Thus, increased exposure
to drugs, coupled with increased emotional
reactivity and decreased regulatory capaci-
ties (rooted in ongoing brain development),
make adolescence a particularly vulnerable
period for substance use.
Indeed, drug use is most often initiated
in adolescence. For example, 82.4% of first
uses of alcohol occur in individuals under
the age of 21 (the legal drinking age), and
58.8% of smokers had their first cigarette
under the age of 18 (SAMHSA, 2011). These
early use statistics are especially important,
because earlier age of onset is associated with
higher rates of SUDs and worse outcomes.
For example, those who initiated alcohol
use prior to age 14 are more than four times
more likely to receive an SUD diagnosis in
adulthood (16.2 vs. 3.8%; similar rates are
reported for illicit drugs). Similarly, earlier
age of smoking onset predicts a higher num-
ber of cigarettes smoked in adulthood (Tai-
oli & Wynder, 1991). Taken together, these
data support the notion that emotion (dys)
regulation is an early risk factor for SUDs.
Next I discuss how emotion regulation may
operate as an ongoing causal factor that may
contribute to and exacerbate existing SUDs.
Emotion (dys)Regulation
in
Current
SUDs
Several models of SUDs directly implicate
deficient regulation as a key and primary
motive for ongoing drug use and relapse,
including the relapse prevention model
(Marlatt & Witkiewitz, 2005), the affective
processing model (Baker, Piper, McCarthy,
Majeskie, & Fiore, 2004), and the aforemen-
tioned self- medication hypothesis (Khant-
zian, 1985), among others. Indeed, whether
or not emotion regulation deficits are a pre-
existing risk factor for SUDs (as proposed
in the previous section), those who currently
suffer from SUDs frequently display such
deficits, which may contribute to the clinical
course of the disorder. Several lines of evi-
dence support this association (Figure 26.1B
for schematic illustration).
First, self- reported emotion regulation
skills are lower in individuals with SUDs
than in healthy controls (e.g., Fox, Hong,
& Sinha, 2008). In addition, greater diffi-
culties in regulating emotion is associated
Emotion Regulation in Substance Use Disorders 433
with more drug use (e.g., Berking et al.,
2011) possibly as a means of emotion regu-
lation (Bonn- Miller, Vujanovic, & Zvolen-
sky, 2008). Second, less effective styles of
emotion regulation (e.g., suppression vs.
reappraisal) are related to increased drug
use (Fucito, Juliano, & Toll, 2010). Third,
individual differences in negative affect have
been repeatedly associated with drug use
and relapse in clinical (e.g., Gamble et al.,
2010), as well as laboratory studies (e.g.,
Sinha & Li, 2007). Fourth, as reviewed ear-
lier, SUDs are highly comorbid with affective
disorders, such as depression, which feature
impaired regulation of negative affect as a
key diagnostic feature (American Psychiatric
Association, 2013). Furthermore, those with
co- occurring symptoms of SUDs and affec-
tive disorders show significantly higher rates
of relapse to drug use after treatment (e.g.,
Bradizza, Stasiewicz, & Paas, 2006) offer-
ing additional support for the link between
emotion regulation deficits and SUDs.
Additional evidence links constructs
related to emotion regulation and SUDs. For
example, emotional intelligence—defined as
the ability to be aware of emotions, identify
emotions correctly, interpret them appro-
priately, and regulate them effectively— is
inversely associated with alcohol and drug-
related problems (Riley & Schutte, 2003).
Moreover, emotional intelligence moderated
the association between negative emotion
and alcohol craving in alcohol- dependent
individuals (Cordovil de Sousa Uva et al.,
2010). Furthermore, a recent meta- analysis
of this construct suggests that not only is it
inversely related to smoking, alcohol, and
drug use, but also that individual differences
in particular components, namely, “iden-
tification of emotion” and “regulation of
emotion,” are particularly related to SUDs
(Kun & Demetrovics, 2010). Similarly,
distress tolerance—the ability to persist in
goal- directed activity when experiencing
psychological distress— is related to emo-
tion regulation and is inversely associated
with substance use frequency and SUDs
(Marshall- Berenz, Vujanovic, & MacPher-
son, 2011), as well as SUD treatment drop-
out and eventual relapse (e.g., Daughters et
al., 2005). In addition, impulsivity is report-
edly higher in those with SUDs (see Verdejo-
García et al., 2008, for review). Finally, it
has been suggested that those with SUDs
exhibit relative deficits in nonaffective forms
of self- regulation and executive function,
including working memory and response
inhibition, which may also relate to PFC
function (Goldstein & Volkow, 2011).
Emotion (dys)Regulation in Current
SUDs: Regulation of Craving
In the previous section, I reviewed evidence
suggesting that those with SUDs have dif-
ficulties regulating emotions. Notably, the
evidence overwhelmingly centers on regula-
tion of negative emotions. However, in the
context of SUDs, it is critical to consider
not only regulation of negative emotion but
also an additional and very specific form of
emotion regulation, namely, the regulation
of craving.
Craving, defined here as “intense desire
for drugs,” has long been considered a key
contributor to drug use (e.g., O’Brien, Chil-
dress, Ehrman, & Robbins, 1998). Although
this view has been challenged (Perkins, 2009)
substantial evidence links drug craving to
drug- taking behavior, and it has been sug-
gested that loss of control over cue- induced
craving is at the root of compulsive drug tak-
ing (e.g., Goldstein & Volkow, 2011). For
example, levels of reported craving predict
drug use as well as relapse to drug taking
following abstinence (e.g., Crits- Christoph
et al., 2007; Epstein, Marrone, Heishman,
Schmittner, & Preston, 2010; Galloway, Sin-
gleton, & the Methamphetamine Treatment
Project Corporate Authors, 2008). Con-
versely, the ability to use various strategies
to regulate craving is associated with lower
craving (Kober, Kross, Mischel, Hart, &
Ochsner, 2010; Kober, Mende- Siedlecki, et
al., 2010; Westbrook et al., 2013) and lower
drug use (O’Connell, Hosein, Schwartz, &
Leibowitz, 2007). Further, the acquisition
of strategies during cognitive- behavioral
therapy (as discussed below) is associated
with better long-term outcomes (Carroll,
Nich, Frankforter, & Bisighini, 1999). Fur-
thermore, the use of cognitive strategies to
regulate craving both during and after treat-
ment is associated with reduced craving and
reduced relapse over time (O’Connell et al.,
2007; Shiffman et al., 1996). These findings
suggest that craving is a key motivator of
substance use, and that effective regulation
of craving is associated with lower drug use

434 PSYCHOPATHOLOGY
and better outcomes for those with SUDs.
This in turn suggests that regulation of crav-
ing is a specific form of regulation that is
particularly important in the maintenance
of drug use behavior in SUDs.
The neural mechanism by which regula-
tion of craving operates to reduce drug use
is a topic of current research. It has been
shown previously that exposure to drug
cues (e.g., drug- related images, movies, or
paraphernalia) increases craving, as well as
drug use (e.g., Shiffman et al., 2013). Fur-
thermore, several meta- analyses have shown
that such cue- induced craving is consistently
associated with neural activity in a network
of regions including the ventral striatum,
the subgenual anterior cingulate, and the
amygdala (e.g., Chase, Eickhoff, Laird, &
Hogarth, 2011). These regions, which are
thought to relate to learning, salience, and
value encoding, previously have been associ-
ated with the acute effects of drugs. We have
recently shown that when cigarette smokers
use cognitive strategies in instances of crav-
ing (e.g., when they think about the long-
term negative consequences of smoking),
they report lower craving (Kober, Kross,
et al., 2010), and exhibit lowered activity
in the neural systems that underlie craving,
such as the ventral striatum (Kober, Mende-
Siedlecki, et al., 2010). Importantly, the reg-
ulation of craving with cognitive strategies
is associated with concurrently increased
activity in PFC regions including the dor-
solateral (dlPFC) and ventral PFC—regions
previously associated with regulation of
negative emotion (see Ochsner & Gross, this
volume). These findings have since been rep-
licated with positron emission tomography
in cocaine users (Volkow et al., 2010) and
electrophysiological measurements in ciga-
rette smokers (Littel & Franken, 2011).
Interestingly, we’ve recently shown that
use of mindfulness- based strategies to regu-
late cue- induced craving is also associated
with reductions in reported craving, and
with reduced neural activity in “craving
regions,” including the subgenual cingulate.
However, the use of such mindfulness- based
strategies was not associated with concur-
rent increase in PFC activity (Westbrook et
al., 2013). Taken together, these findings are
consistent with the hypothesis that, across
strategies, regulation of craving operates by
reducing neural activity in regions that are
thought to instantiate the experience of crav-
ing. Preliminarily, it further appears that the
use of cognitive strategies to regulate crav-
ing may depend on increased activity in PFC
but that mindfulness- based strategies may
not, although additional data are required
to confirm this pattern of results.
To summarize, this section has reviewed
evidence suggesting that emotion regula-
tion is implicated in SUDs, both as an early
risk factor and as an ongoing motivator of
drug use. For example, individual differ-
ences in emotion regulation and impulsivity
during development are predictive of drug
use initiation and SUDs. Furthermore, indi-
viduals with ongoing SUDs exhibit deficits
in emotion regulation compared to healthy
controls, and negative affect in such indi-
viduals is associated with instances of drug
use. Importantly, most of the available evi-
dence centers around regulation of negative
emotion. However, regulation of craving is
emerging as another form of regulation that
is important in the etiology and mainte-
nance of these disorders, and may constitute
one key route by which targeted treatments
can ameliorate SUDs, as discussed further
below.
PFC in SUDs: Mechanism
for Emotion (dys)Regulation?
In the prior sections I have reviewed evi-
dence suggesting that PFC development may
underlie the role of emotion dysregulation
as a distal causal factor for development of
SUDs in adolescence. But is this the neural
mechanism that underlies general deficits in
emotion regulation present in SUDs? Indeed,
many current models of SUDs propose that
the loss of control over craving and drug
taking (as evident in the diagnostic criteria
for the disorder) is a result of reduced or
compromised PFC function (e.g., Everitt &
Robbins, 2005; Goldstein & Volkow, 2011;
Potenza, Sofuoglu, Carroll, & Rounsaville,
2011; Volkow, Wang, Fowler, & Tomasi,
2011). And this “PFC hypothesis” is con-
sistent with the already- established link
between cognitive control generally— and
emotion regulation specifically— and the
function of PFC in healthy adults (see Och-
sner & Gross, this volume). However, neu-
Emotion Regulation in Substance Use Disorders 435
roimaging studies directly testing emotion
regulation abilities in SUDs are scarce. Nev-
ertheless, in the following sections, I review
findings that individuals with SUDs exhibit
structural abnormalities in various PFC
regions, as well as functional differences
in studies of nonaffective forms of cogni-
tive control (for a brief review of neuroim-
aging methodologies used in such studies,
see Kober & DeLeone, 2011). Importantly,
although some of these PFC abnormalities
may precede the development of SUDs, I
review evidence suggesting that chronic
drug use is associated with both structural
and functional changes in PFC. Such drug-
induced changes, in turn, suggest that SUDs
may also lead to decrements in PFC that may
underlie further emotion dysregulation (Fig-
ure 26.1B).
In reviewing this evidence, it is important
to note that the PFC is a very large struc-
tural division in the brain, and that different
subregions within the PFC perform very dif-
ferent computations and subserve different
functions— even within the general “cogni-
tive control” framework (Miller & Cohen,
2001). However, at this stage, there are not
yet sufficient data to make finer distinctions
about the functional role of PFC subdivisions
in SUDs, or to begin to speculate about the
role each subregion might have in the neu-
ropathology of these disorders. I hope that
data collected in the next decade will allow
us to answer such questions with far greater
specificity than we can today.
Structural Differences in the PFC
Differences in brain structure have been
reported between those with SUDs and
healthy controls, using several different
methodologies, especially in various subre-
gions of PFC. For example, using voxel-based
morphometry, cigarette smokers exhibited
reduced PFC gray matter density compared
to healthy controls, and PFC thickness was
negatively correlated to reported smoking
(measured in packs-per-year; Brody et al.,
2004). In cocaine- dependent individuals,
relatively reduced gray matter density in
orbitofrontal cortex (OFC) and anterior cin-
gulate cortex (ACC) was reported (Franklin
et al., 2002). Similarly, lower thickness and
volume were reported for other stimulant
users in various prefrontal regions (e.g.,
Daumann et al., 2011) and in right ven-
trolateral PFC (vlPFC) specifically, where
thickness was inversely correlated with drug
craving (Tabibnia et al., 2011). In alcohol-
dependent subjects compared to controls,
lower gray matter volume was reported
across the PFC (Fein, Di Sclafani, & Mey-
erhoff, 2002) and more specifically in the
lateral and superior PFC and OFC (Durazzo
et al., 2011) and medial and lateral PFC
(Rando et al., 2011). In these latter studies,
lower medial PFC volume was associated
with more drinking posttreatment or shorter
time to relapse. In addition, in some studies
(but not all) PFC volume was inversely asso-
ciated with cognitive control measures. For
example, PFC gray matter volume correlated
inversely with executive function measures
in cocaine- dependent individuals (Fein et
al., 2002).
Consistent with these gray matter find-
ings in PFC, diffusion tensor imaging (DTI)
measures of PFC white matter integrity dis-
tinguish between individuals with alcohol
use disorders and controls (e.g., Pfeffer-
baum, Rosenbloom, Rohlfing, & Sullivan,
2009) and further differ between individu-
als who relapsed and those who sustained
abstinence following treatment (Sorg et al.,
2012). In cocaine- dependent participants,
lower measurements of white matter integ-
rity are consistently found in various PFC
regions (e.g., Romero, Asensio, Palau, San-
chez, & Romero, 2010). Similar findings
were reported in methamphetamine (Ali-
cata, Chang, Cloak, Abe, & Ernst, 2009)
and in opiate users (Bora et al., 2012; Liu et
al., 2008).
Taken together, this body of structural neu-
roimaging work suggests that there are con-
sistent anatomical differences between those
with SUDs and healthy controls. A caution-
ary note here is that it is not yet clear what
these differences mean. While it is tempting
to interpret these differences as indicating
impairment in individuals with SUDs, this
link has not yet been consistently demon-
strated. For instance, although reportedly
lower than that in controls, cortical thick-
ness and cognitive function are often within
normal range in SUDs (see Hart, Marvin,
Silver, & Smith, 2011, for extended discus-
sion). Nevertheless, these differences are
consistently reported across PFC and across
types of SUDs. Furthermore, although indi-
436 PSYCHOPATHOLOGY
vidual studies differ with respect to the local-
ization of these differences (possibly due to
sample characteristics, drug pharmacology,
drug use patterns, and methodological and
statistical differences), and only some stud-
ies find association with clinical outcomes,
the PFC is repeatedly implicated, especially
lateral portions. Reported differences are
especially salient given the known role for
PFC in emotion regulation and cognitive
control in healthy adults. Taken together,
these structural findings are consistent with
the hypothesized mechanism by which PFC
abnormalities may contribute to or underlie
deficient emotion regulation in SUDs. How-
ever, future work could more directly link
structural findings in PFC with emotion
regulation in SUDs.
Functional Differences in PFC
Differences in measures of PFC function
between individuals with SUDs and healthy
controls have been consistently reported
since the early days of functional neuroim-
aging. For example, using various forms of
positron emission tomography (PET), it has
been established that those with SUDs often
exhibit relative reductions in “D2” type
receptors of the neurotransmitter dopamine
in striatum and PFC, with some evidence that
these reductions persist even after months
of abstinence (see Volkow et al., 2011, for
review). PET measures of glucose metabo-
lism have repeatedly shown decreased activ-
ity in OFC, ACC, and dlPFC. In stimulant
users decreased activity is further related to
relatively decreased D2 receptor availability
in striatum (Volkow et al., 2011). Notably,
in alcohol- dependent individuals, striatal
D2 availability is linked to not only to PFC
activity but also to self- reported alcohol
craving, suggesting that all three processes
may be functionally related (i.e., OFC func-
tion, D2 receptor availability, and craving;
Heinz et al., 2005).
More recently, several studies have spe-
cifically investigated brain function dur-
ing performance of non- affective cognitive
control tasks comparing individuals with
SUDs and healthy controls, typically with
functional magnetic resonance imaging
(fMRI). One frequently used task is the go/
no-go response inhibition task, in which
participants are asked to respond to all
letters except X with a button press, and
to withhold responding to X. Using this
task, a series of studies reported relatively
worse performance in cocaine- and heroin-
dependent individuals compared to healthy
controls, along with reduced activity in
several PFC regions, including the dorsal
ACC (dACC), dlPFC, and vlPFC (e.g., Fu et
al., 2008; Hester & Garavan, 2004). Such
findings suggest at least some functional
alterations in PFC circuits in SUDs, even in
the absence of emotion regulation demand.
Similarly, Li, Luo, Yan, Bergquist, and
Sinha (2009) used the stop- signal response
inhibition task, and reported lower activ-
ity in dlPFC in alcohol dependence, which
further related to higher alcohol craving
self- reports. In cocaine- dependent individu-
als, dACC activity was lower than that in
controls and negatively correlated with self-
reported difficulties in emotion regulation
(Li et al., 2008).
The Stroop color–word task has also been
used to probe inhibitory control in SUDs by
comparing neural activity during incongru-
ent (BLUE written in red ink) and congruent
(BLUE written in blue ink) trials. Using PET,
both marijuana- and cocaine- dependent
participants showed reduced “Stroop effect”
activity in dACC and dlPFC (Bolla et al.,
2004; Eldreth, Matochik, Cadet, & Bolla,
2004). In the cocaine- dependent sample
only, dlPFC activity negatively correlated
with cocaine use (least “Stroop effect” activ-
ity for the heaviest users; Bolla et al., 2004).
Similarly, DeVito, Kober, Carroll, and
Potenza (in preparation) recently used the
Stroop task and fMRI in cocaine- dependent
participants, and found reduced Stroop-
related PFC activity compared to controls.
Similar findings have been reported with
methamphetamine users (Nestor, Ghahre-
mani, Monterosso, & London, 2011). In
marijuana- dependent individuals who were
about to begin treatment, Kober, DeVito,
DeLeone, Carroll, and Potenza (under
review) found reduced “Stroop effect” activ-
ity in dlPFC compared to healthy controls,
and positive correlations between PFC activ-
ity and treatment success. Similarly, Berk-
man, Falk, and Lieberman (2011) related
neural activity during go/no-go task perfor-
mance to treatment outcome and reported
that increased PFC activity during the task
was related to a weaker association between
Emotion Regulation in Substance Use Disorders 437
craving and cigarette smoking during subse-
quent abstinence.
Taken together, these studies suggest that
those with SUDs exhibit poorer performance
in cognitive control tasks, as well as lower
activity in PFC regions typically associated
with emotion regulation and executive func-
tion more generally, including dlPFC and
dACC. Some studies reported direct asso-
ciation between lower PFC function and
less cognitive control or emotion regulation,
while others link greater PFC activity to bet-
ter treatment outcomes (e.g., Berkman et
al., 2011; Kober et al., under review). These
findings are therefore consistent with the
hypothesis that PFC abnormalities in struc-
ture or function underlie emotion dysregula-
tion in SUDs.
Effects of Drug Use on PFC
Data reviewed thus far suggests that those
with SUDs exhibit deficits in emotion regu-
lation, and both structural and functional
differences in PFC compared to healthy con-
trols. Notably, most of the reviewed data
were generated in the context of case– control
studies— measured at a single point in time,
in individuals with active SUDs. Therefore,
it is not clear whether some of these reported
abnormalities precede the development of
SUDs (and may serve as a risk factor, as
discussed previously), whether they are the
result of chronic drug use (and reflect the
effects of drug exposure) or an interaction
of both. Evidence for PFC abnormalities as
a preexisting risk factor includes a recent
study of individuals with SUDs (cocaine or
amphetamine dependence) and their unaf-
fected siblings compared to healthy adults.
Both individuals with SUDs and their unaf-
fected siblings shared a neurological pheno-
type of reduced structural connectivity in
the right vlPFC, which was further related
to performance on the stop- signal response
inhibition task (Ersche et al., 2012). These
findings suggest that potential abnormalities
in lateral PFC may underlie regulatory defi-
cits that in fact predate the onset of SUDs.
On the other hand, there is ample evi-
dence, mostly from animal studies, that
chronic/regular drug use alters both func-
tion and structure of PFC and other brain
circuits (for an excellent recent review, see
Lüscher & Malenka, 2011). Although it is
outside of the scope of this chapter to discuss
the unique mechanism of action or pharma-
cological effects of individual drugs, one
now- classic finding is that all drugs that are
abused by humans share one common effect.
That is, all drugs of abuse— either directly
or indirectly— increase concentrations of the
neurotransmitter dopamine in the “meso-
corticolimbic” pathway, which includes the
ventral tegmental area, the ventral striatum,
and the PFC (e.g., Dichiara & Imperato,
1988; Volkow et al., 2011).
3
In turn, this
drug- induced increase in dopamine is asso-
ciated with long-term changes or adapta-
tions to neurons in this pathway, including
in PFC (Lüscher & Malenka, 2011). These
changes are thought to facilitate associa-
tions between drugs and drug- related cues
(e.g., alcohol and the bar where one drinks;
cigarettes and the lighter one uses for smok-
ing), lead to future cue- induced drug crav-
ing, and reduce cognitive control (Volkow et
al., 2011).
Furthermore, it is thought that some of
the effects of acute as well as chronic drug
use are neurotoxic—damaging to neural
tissue (Weiss & Koob, 2001). Such claims
emerge primarily from an animal literature
experimentally documenting various forms
of neuronal damage following heavy drug
administration (e.g., Gouzoulis- Mayfrank
& Daumann, 2009). Although it is not
clear that such findings translate to human
drug users (Hart et al., 2011), some studies
in humans have linked length of drug use
with measures of structural or functional
integrity, which is consistent with animal
findings. For example, in opiate users, PFC
white matter integrity correlated negatively
with length of opiate use (Bora et al., 2012;
Liu et al., 2008). Similarly, some human
studies have shown that various functional
and structural abnormalities normalize fol-
lowing drug abstinence, implicating drug
use itself in the originally observed differ-
ences in PFC (e.g., Gouzoulis- Mayfrank &
Daumann, 2009). Taken together, the evi-
dence suggests that even if some PFC abnor-
malities precede the development of SUDs,
drug use itself is associated with long-term
changes to many brain circuits, including
PFC. Furthermore, these changes may cre-
ate or exacerbate deficits in emotion regula-
tion in SUDs. In essence, this suggests that
chronic drug use may lead to a vicious cycle,

438 PSYCHOPATHOLOGY
in which impaired emotion regulation leads
to drug use, and drug use may further lead
to impaired emotion regulation.
Treatment for SUDs:
The Role of Emotion Regulation
Treatments for SUDs are varied and com-
plex, as is appropriate given the hetero-
geneous and complex nature of the disor-
ders that they treat. At this time, despite
repeated scientific efforts, there are few
FDA-approved pharmacological treatments
for SUDs. Therefore, nonpharmacological
(e.g., psychological) treatments are most
common. While the goal of treatment may
be conceptualized as reductions in drug use
and in drug- related harm, and increases in
psychosocial functioning, treatment suc-
cess is most often measured in abstinence,
or complete cessation of drug use. As such,
the available treatments are only moderately
effective; indeed, across all treatments for
SUDs, the most common outcome is relapse
(Dutra et al., 2008). This suggests that while
some individuals successfully remain absti-
nent, the majority of patients either do not
achieve abstinence or return to drug use
within a year, even with the best of treat-
ments. These grim findings underscore the
need to better understand the mechanisms
of action behind the treatments that do
work, in order to improve them further.
From a clinical perspective, treatment
for SUDs can be divided into three phases:
detoxification, recovery, and relapse preven-
tion (e.g., Potenza et al., 2011). The goal of
the detoxification stage is to achieve absti-
nence and undergo withdrawal symptoms
safely, until they abate. The onset, charac-
ter, and length of this stage depend on the
pharmacological properties of the individual
drug, as well as treatment type (some treat-
ments begin with recovery elements, then set
a “quit date” to begin detoxification). The
main stage of treatment is recovery, which
can last from 1 week to many weeks. The
goal of the recovery stage is to develop moti-
vation to avoid drug use and relapse, as well
as learn the skills to do so successfully. In
that sense, what does recovery entail? The
data reviewed in this chapter suggest that
difficulties regulating emotions are a core
feature of SUDs. Specifically, I have argued
that difficulties regulating both negative
(stress, anxiety, or depression) and appeti-
tive (drug craving) states are associated with
drug use and with relapse to drug use fol-
lowing abstinence. Therefore, it is no sur-
prise that at this recovery phase, many of the
leading treatments include training of emo-
tion regulation skills in general, and regula-
tion of craving in particular (e.g., Potenza et
al., 2011; see Figure 26.1B). Indeed, learning
to tolerate or regulate cravings and not to
act on them is the cornerstone of many of
the available treatments, as discussed below.
Finally, the last phase of treatment, relapse
prevention, focuses on implementing long-
term strategies for maintaining abstinence,
which includes replacing old behaviors with
a new and healthy, drug-free lifestyle. Over-
all, there are many types of treatments for
SUDs, and each type has many unique fea-
tures. The following sections focus on two
types of treatment that are related to the role
of emotion regulation in SUDs: cognitive-
behavioral and mindfulness- based treat-
ments.
Cognitive‑Behavioral Therapies
Cognitive- behavioral therapies (CBTs) are
considered the most effective treatment for
many psychiatric disorders (e.g., depres-
sion and anxiety). A version developed spe-
cifically for SUDs (Carroll, 1998) has been
empirically validated in multiple randomized
controlled trials and is considered by many
to be the “gold standard” (e.g., Dutra et al.,
2008; Potenza et al., 2011). CBT for SUDs
has two critical components: functional
analysis and skills training. Functional
analysis is used to identify and assess the
individualized circumstances that are likely
to lead to drug use, and provides insights
into some of the reasons the individual
may be using drugs. These “high-risk situa-
tions” are those in which new skills may be
applied to avoid drug use during and after
treatment. Therefore, in a complementary
fashion, skills training (including emotion
regulation) is individualized to help those
with SUDs “unlearn old habits associated
with [drug] . . . abuse and learn or relearn
healthier skills and habits” (Carroll, 1998,
p. 2). More specifically, these skills initially
include regulating thoughts about drugs,
learning strategies to regulate cravings for
Emotion Regulation in Substance Use Disorders 439
drugs, and managing situations related to
drug-use opportunities (e.g., refusing offers
of drugs).
Subsequent treatment sessions (modules)
focus on problem solving, tolerating and
regulating negative affect, and improv-
ing social skills more generally. Ultimately,
individuals who undergo CBT (compared to
other treatments) are more likely to decrease
drug use and/or achieve abstinence during
and even after treatment has ended (i.e.,
“the sleeper effect”; Potenza et al., 2011).
Although the treatment includes many mod-
ules and stages, one important mechanism
of action is thought to be via enhancing cog-
nitive control over negative affect that may
lead to drug craving, and over drug crav-
ing and drug taking behavior (e.g., Kiluk,
Nich, Babuscio, & Carroll, 2010; Potenza et
al., 2011). This hypothesis is supported by
the finding that the number and quality of
strategies for regulation of craving increase
from pre- to post-CBT treatment, and pre-
dict relapse (e.g., Carroll et al., 1999), and
formally mediate the relationship between
treatment and duration of abstinence (Kiluk
et al., 2010). In turn, this increase in regula-
tion skills is hypothesized to be mediated by
improved PFC function from pre- to post-
treatment (Potenza et al., 2011; see Figure
26.1B for a schematic illustration). Consis-
tent with this hypothesis, Kober, Kross, et al.
(2010) have shown that use of CBT-like cog-
nitive strategies during cue- induced craving
is associated with decreases in self- reported
craving, as well as increased activity in
dlPFC and vlPFC (Kober, Mende- Siedlecki,
et al., 2010). In addition, DeVito et al. (2011)
recently reported that those who underwent
CBT exhibited increased efficiency in vlPFC
and dlPFC during the Stroop task from pre-
to post-CBT treatment, which is consistent
with improvements in cognitive control and
emotion regulation. However, future studies
should test that hypothesis more directly.
Mindfulness‑Based Treatments
Mindfulness- based treatments (MBTs) for
a variety of psychiatric conditions have
emerged in recent years, beginning with
mindfulness- based stress reduction (MBSR).
Mindfulness has been defined as a two-
component construct: (1) self- regulation of
attention to the present moment, coupled
with (2) an attitude of acceptance toward
the present moment (Bishop et al., 2004). As
such, mindfulness is often practiced through
mindfulness meditation, which consists of
focusing attention on one’s immediate expe-
rience (e.g., sensations, breathing, thoughts,
emotion), and regarding it nonjudgmen-
tally. This is thought to cultivate the abil-
ity to observe— rather than be caught up
in—one’s own experience, and to further
facilitate more skillful or mindful respond-
ing (as opposed to automatic reaction;
Zgierska et al., 2009). Importantly, medita-
tion and MBTs have been associated with
beneficial effects on stress, anxiety, pain,
cardiac health, immune functions, psycho-
logical well-being, cognitive functioning,
and several psychiatric disorders (including
mood and anxiety disorders; see Hölzel et
al., 2011, for review). Therefore, it is not
difficult to extrapolate how MBTs could be
beneficial for fostering better emotion regu-
lation in SUDs.
Indeed, several mindfulness- based treat-
ments have recently been adapted for SUDs.
Unlike the well- established CBTs, these
treatments have just shown preliminary effi-
cacy and are now the focus of rigorous ran-
domized controlled trials. MBTs for SUDs
typically include training in mindfulness
meditation, and a focus on attention to and
acceptance of present- moment experience
(including negative emotion and drug crav-
ing). The modal instruction is to regard inter-
nal experiences (e.g., drug craving) as tran-
sient, and to observe and accept them as-is,
without reacting (e.g., without engaging in
drug use). For example, both mindfulness-
based relapse prevention (MBRP; Bowen,
Chawla, & Marlatt, 2010) and mindfulness
training for smoking (MTS; Brewer et al.,
2011) make use of the concept of “urge surf-
ing,” the practice of regarding craving like a
wave that rises, reaches a peak, and subsides
naturally. Patients are instructed to attend to
and accept the sensations as they rise, fall,
and finally disappear— and this technique
is likened to tolerating cravings rather than
actively regulating them, as in CBT (Brewer
et al., 2011). Consistently, Westbrook et al.
(2013) have shown that mindful attention to
smoking cues is associated with lower self-
reported craving and lower neural activity in
regions previously associated with craving,
without concomitant increases in PFC activ-

440 PSYCHOPATHOLOGY
ity. As such, mindful attention and accep-
tance may be regulatory, by preventing the
amplification of craving rather than damp-
ening them down.
Clinically, MBRP, typically administered
as a follow- up to inpatient treatment, is
reportedly efficacious in reducing drug use
and relapse across several different popu-
lations with SUDs, including alcohol and
polysubstance users (e.g., Witkiewitz &
Bowen, 2010). In addition, Brewer et al.
(2011) have recently shown in a pilot ran-
domized controlled trial that MTS admin-
istered as a stand-alone treatment was effec-
tive in achieving smoking cessation. Finally,
similar elements of mindful attention and
acceptance are parts of dialectical behav-
ior therapy and acceptance and commit-
ment therapy, both of which have shown
preliminary efficacy for SUDs (Hernández-
López, Luciano, Bricker, Roales-Nieto, &
Montesinos, 2009; Linehan et al., 2002).
Taken together, these data show substantial
promise for MBTs in the treatment of SUDs,
although the research is still in its infancy.
Nevertheless, one prominent hypothesis sug-
gests that these treatments work by enhanc-
ing emotion regulation, as patients learn to
practice mindfulness in the face of craving
as well as negative emotion. This is sup-
ported by several findings, including consis-
tent reductions in craving post-MBRP (e.g.,
Witkiewitz & Bowen, 2010), and a negative
correlation after MTS between amount of
meditation practice and smoking (Brewer et
al., 2011).
Concluding Remarks
Casual drug use is quite prevalent, and a
percentage of drug users develop SUDs,
which are severe psychiatric conditions with
staggering social, economic, and personal
costs. This underscores the need to identify
risk factors that render specific individu-
als more vulnerable to the development of
SUDs. Furthermore, once established, SUDs
are chronic, relapsing, and very difficult to
treat psychiatric conditions; this underscores
the need to better characterize the proximal
causal factors that lead to continued drug
use, and to better understand the mecha-
nisms that underlie effective treatments for
these disorders. In this chapter, I reviewed
data suggesting that emotion regulation is
one such crucial factor. Indeed, difficulties
in emotion regulation in childhood and ado-
lescence serve as predictive factors for future
drug use and the development of SUDs. Sub-
sequently, those with SUDs report greater
difficulty regulating negative emotions than
do healthy controls, which contributes to
ongoing drug use. Furthermore, I reviewed
evidence suggesting that craving for drugs is
one of the key predictors of drug use, and
that the ability to regulate craving effec-
tively is directly related to reduced drug use
in SUDs.
Consistent with these observations, psy-
chosocial treatments for SUDs often focus
on emotion regulation and on the regula-
tion of craving as means for reducing drug
use. Indeed, improvement in those skills fol-
lowing treatments such as CBT and MBTs
is associated with improved abstinence.
Finally, I reviewed data suggesting that dif-
ferences in PFC structure, as well as function,
may underlie impaired emotion regulation in
those with SUDs, and that ongoing drug use
leads to adaptations in PFC that may further
impair emotion regulation. However, it will
be critical to focus future research on more
precisely characterizing the neural mecha-
nisms behind observed PFC deficits in SUDs
and behind treatment- related improvements.
Indeed, it is my sincere hope that in the com-
ing years, additional data will allow us to
establish these links more firmly. This could
lead to the development of better treatments
that improve emotion regulation in individ-
uals suffering from these devastating disor-
ders.
Acknowledgments
I would like to thank Danielle Bolling, Cameron
DeLeone, and Maggie Mae Mell for their invalu-
able help preparing this chapter. Thanks also go
to Alan Anticevic, Rebecca Boswell, Kathleen
Carroll, Ralph DiLeone, James Gross, and one
anonymous reviewer, for very helpful comments.
And finally, thanks to all those who investigate
emotion regulation in substance use disorders.
Your work inspired this chapter and will con-
tinue to inspire research in this area for years to
come.

Emotion Regulation in Substance Use Disorders 441
Notes
1. Psychoactive drugs are those that primarily
act on the brain and change thinking, mood,
and behavior. These include legal drugs (e.g.,
alcohol, nicotine, caffeine, and opioid pain
medications), as well as illicit drugs (e.g., her-
oin, cocaine, amphetamines, and marijuana).
2. Self- control is often defined as the process of
inhibition of an otherwise imminent thought,
emotion, or action— and as such, it includes
emotion regulation. Related to this is the
construct of cognitive control, which more
broadly includes goal maintenance, selective
attention, conflict monitoring and resolution,
response inhibition, and emotion regulation.
See Gross (this volume) for discussion.
3. It is now known that many other neurotrans-
mitter systems are involved in drug taking
and in the development of SUDs, and the next
decade will likely bring additional investiga-
tions into other neurotransmitter systems and
their relation to SUDs.
References
American Psychiatric Association. (2013). Diag-
nostic and statistical manual of mental disor-
ders (5th ed.). Arlington, VA: Author.
Alicata, D., Chang, L., Cloak, C., Abe, K., &
Ernst, T. (2009). Higher diffusion in striatum
and lower fractional anisotropy in white mat-
ter of methamphetamine users. Psychiatry
Research: Neuroimaging, 174(1), 1–8.
August, G. J., Winters, K. C., Realmuto, G. M.,
Fahnhorst, T., Botzet, A., & Lee, S. (2006).
Prospective study of adolescent drug use
among community samples of ADHD and
non-ADHD participants. Journal of Ameri-
can Academy of Child and Adolescent Psy-
chiatry, 45(7), 824–832.
Badiani, A., Belin, D., Epstein, D., Calu, D.,
& Shaham, Y. (2011). Opiate versus psycho-
stimulant addiction: The differences do mat-
ter. Nature Reviews Neuroscience, 12(11),
685–700.
Baker, T. B., Piper, M. E., McCarthy, D. E.,
Majeskie, M. R., & Fiore, M. C. (2004).
Addiction motivation reformulated: An affec-
tive processing model of negative reinforce-
ment. Psychological Review, 111(1), 33–51.
Berking, M., Margraf, M., Ebert, D., Wupper-
man, P., Hofmann, S. G., & Junghanns, K.
(2011). Deficits in emotion- regulation skills
predict alcohol use during and after cognitive-
behavioral therapy for alcohol dependence.
Journal of Consulting and Clinical Psychol-
ogy, 79(3), 307–318.
Berkman, E. T., Falk, E. B., & Lieberman, M.
D. (2011). In the trenches of real-world self-
control: Neural correlates of breaking the link
between craving and smoking. Psychological
Science, 22(4), 498–506.
Bishop, S. R., Lau, M., Shapiro, S., Carlson, L.,
Anderson, N. D., Carmody, J., et al. (2004).
Mindfulness: A proposed operational defini-
tion. Clinical Psychology: Science and Prac-
tice, 11(3), 230–241.
Bolla, K., Ernst, M., Kiehl, K., Mouratidis, M.,
Eldreth, D., Contoreggi, C., et al. (2004).
Prefrontal cortical dysfunction in abstinent
cocaine abusers. Journal of Neuropsychiatry
and Clinical Neurosciences, 16(4), 456–464.
Bonn- Miller, M. O., Vujanovic, A. A., & Zvo-
lensky, M. J. (2008). Emotional dysregulation:
Association with coping- oriented marijuana
use motives among current marijuana users.
Substance Use and Misuse, 43(11), 1656–1668.
Bora, E., Yücel, M., Fornito, A., Pantelis, C.,
Harrison, B. J., Cocchi, L., et al. (2012).
White matter microstructure in opiate addic-
tion. Addiction Biology, 17(1), 141–148.
Bowen, S., Chawla, N., & Marlatt, G. A. (2010).
Mindfulness- based relapse prevention for
addictive behaviors: A clinician’s guide. New
York: Guilford Press.
Bradizza, C. M., Stasiewicz, P. R., & Paas, N. D.
(2006). Relapse to alcohol and drug use among
individuals diagnosed with co- occurring men-
tal health and substance use disorders: A
review. Clinical Psychology Review, 26(2),
162–178.
Brewer, J. A., Mallik, S., Babuscio, T. A., Nich,
C., Johnson, H. E., Deleone, C. M., et al.
(2011). Mindfulness training for smoking ces-
sation: Results from a randomized controlled
trial. Drug and Alcohol Dependence, 119(1–
2), 72–80.
Brody, A. L., Olmstead, R. E., London, E. D.,
Farahi, J., Meyer, J. H., Grossman, P., et al.
(2004). Smoking- induced ventral striatum
dopamine release. American Journal of Psy-
chiatry, 161(7), 1211–1218.
Carroll, K. M. (1998). Therapy manuals for drug
addiction: A cognitive- behavioral approach:
Treating cocaine addiction. Bethesda, MD:
National Institute of Drug Abuse.
442 PSYCHOPATHOLOGY
Carroll, K. M., Nich, C., Frankforter, T. L., &
Bisighini, R. M. (1999). Do patients change
in the way we intend?: Treatment- specific
skill acquisition in cocaine- dependent patients
using the Cocaine Risk Response Test. Psy-
chological Assessment, 11, 77–85.
Chase, H. W., Eickhoff, S. B., Laird, A. R., &
Hogarth, L. (2011). The neural basis of drug
stimulus processing and craving: An activation
likelihood estimation meta- analysis. Biologi-
cal Psychiatry, 70(8), 785–793.
Childress, A. R., Hole, A. V., Ehrman, R. N.,
Robbins, S. J., McLellan, A. T., & O’Brien,
C. P. (1993). Cue reactivity and cue reactiv-
ity interventions in drug dependence. NIDA
Research Monograph, 137, 73–73.
Cordovil de Sousa Uva, M., Timary, P., Cortesi,
M., Mikolajczak, M., Blicquy, P. R., & Lumi-
net, O. (2010). Moderating effect of emotional
intelligence on the role of negative affect in
the motivation to drink in alcohol- dependent
subjects undergoing protracted withdrawal.
Personality and Individual Differences, 48(1),
16–21.
Crits- Christoph, P., Gibbons, M. B. C., Barber, J.
P., Hu, B., Hearon, B., Worley, M., & Gallop,
R. (2007). Predictors of sustained abstinence
during psychosocial treatments for cocaine
dependence. Psychotherapy Research, 17(2),
240–252.
Daughters, S. B., Lejuez, C., Bornovalova, M.
A., Kahler, C. W., Strong, D. R., & Brown,
R. A. (2005). Distress tolerance as a predic-
tor of early treatment dropout in a residential
substance abuse treatment facility. Journal of
Abnormal Psychology, 114(4), 729–734.
Daumann, J., Koester, P., Becker, B., Wagner,
D., Imperati, D., Gouzoulis- Mayfrank, E.,
& Tittgemeyer, M. (2011). Medial prefron-
tal gray matter volume reductions in users
of amphetamine- type stimulants revealed by
combined tract-based spatial statistics and
voxel-based morphometry. NeuroImage,
54(2), 794–801.
DeVito, E. E., Kober, H., Carroll, K. M., &
Potenza, M. N. (in preparation). Sex differ-
ences in the neural correlates of cognitive con-
trol in cocaine dependence.
DeVito, E. E., Worhunsky, P. D., Carroll, K. M.,
Rounsaville, B. J., Kober, H., & Potenza, M.
N. (2011). A preliminary study of the neural
effects of behavioral therapy for substance use
disorders. Drug and Alcohol Dependence,
122(3), 228–235.
Dichiara, G., & Imperato, A. (1988). Drugs
abused by humans preferentially increase syn-
aptic dopamine concentrations in the meso-
limbic system of freely moving rats. Proceed-
ings of the National Academy of Sciences
USA, 85(14), 5274–5278.
Durazzo, T. C., Tosun, D., Buckley, S., Gazdz-
inski, S., Mon, A., Fryer, S. L., & Meyerhoff,
D. J. (2011). Cortical thickness, surface area,
and volume of the brain reward system in alco-
hol dependence: Relationships to relapse and
extended abstinence. Alcoholism: Clinical and
Experimental Research, 35, 1187–1200.
Dutra, L., Stathopoulou, G., Basden, S. L.,
Leyro, T. M., Powers, M. B., & Otto, M. W.
(2008). A meta- analytic review of psychoso-
cial interventions for substance use disorders.
American Journal of Psychiatry, 165(2), 179–
187.
Eldreth, D. A., Matochik, J. A., Cadet, J. L., &
Bolla, K. I. (2004). Abnormal brain activity
in prefrontal brain regions in abstinent mari-
juana users. NeuroImage, 23(3), 914–920.
Epstein, D. H., Marrone, G. F., Heishman, S.
J., Schmittner, J., & Preston, K. L. (2010).
Tobacco, cocaine, and heroin: Craving and use
during daily life. Addictive Behaviors, 35(4),
318–324.
Ersche, K. D., Jones, P. S., Williams, G. B., Tur-
ton, A. J., Robbins, T. W., & Bullmore, E.
T. (2012). Abnormal brain structure impli-
cated in stimulant drug addiction. Science,
335(6068), 601–604.
Everitt, B. J., & Robbins, T. W. (2005). Neural
systems of reinforcement for drug addiction:
From actions to habits to compulsion. Nature
Neuroscience, 8(11), 1481–1489.
Fein, G., Di Sclafani, V., Cardenas, V., Gold-
mann, H., Tolou-Shams, M., & Meyerhoff,
D. J. (2002). Cortical gray matter loss in
treatment- naive alcohol dependent individu-
als. Alcoholism: Clinical and Experimental
Research, 26(4), 558–564.
Fein, G., Di Sclafani, V., & Meyerhoff, D. J.
(2002). Prefrontal cortical volume reduction
associated with frontal cortex function deficit
in 6-week abstinent crack- cocaine dependent
men. Drug and Alcohol Dependence, 68(1),
87–93.
Fox, H. C., Hong, K. A., & Sinha, R. (2008).
Difficulties in emotion regulation and impulse
control in recently abstinent alcoholics com-
pared with social drinkers. Addictive Behav-
iors, 33(2), 388–394.
Franklin, T. R., Acton, P. D., Maldjian, J. A.,
Gray, J. D., Croft, J. R., Dackis, C. A., et al.
Emotion Regulation in Substance Use Disorders 443
(2002). Decreased gray matter concentration
in the insular, orbitofrontal, cingulate, and
temporal cortices of cocaine patients. Biologi-
cal Psychiatry, 51(2), 134–142.
Fu, L., Bi, G., Zou, Z., Wang, Y., Ye, E., Ma,
L., et al. (2008). Impaired response inhibition
function in abstinent heroin dependents: An
fMRI study. Neuroscience Letters, 438(3),
322–326.
Fucito, L. M., Juliano, L. M., & Toll, B. A.
(2010). Cognitive reappraisal and expressive
suppression emotion regulation strategies
in cigarette smokers. Nicotine and Tobacco
Research, 12(11), 1156–1161.
Galloway, G. P., Singleton, E. G., & the Meth-
amphetamine Treatment Project Corporate
Authors. (2008). How long does craving pre-
dict use of methamphetamine?: Assessment of
use one to seven weeks after the assessment
of craving. Substance Abuse: Research and
Treatment, 1, 63–79.
Gamble, S. A., Conner, K. R., Talbot, N. L., Yu,
Q., Tu, X. M., & Connors, G. J. (2010). Effects
of pretreatment and posttreatment depressive
symptoms on alcohol consumption following
treatment in project MATCH. Journal of Stud-
ies on Alcohol and Drugs, 71(1), 71–77.
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Cas-
tellanos, F. X., Liu, H., Zijdenbos, A., et al.
(1999). Brain development during childhood
and adolescence: A longitudinal MRI study.
Nature Neuroscience, 2, 861–862.
Gilbert, D. G., Crauthers, D. M., Mooney, D.
K., McClernon, F. J., & Jensen, R. A. (1999).
Effects of monetary contingencies on smoking
relapse: Influences of trait depression, person-
ality, and habitual nicotine intake. Experi-
mental and Clinical Psychopharmacology,
7(2), 174–181.
Goldstein, R. Z., & Volkow, N. D. (2011). Dys-
function of the prefrontal cortex in addiction:
Neuroimaging findings and clinical implica-
tions. Nature Reviews Neuroscience, 12(11),
652– 669.
Gouzoulis- Mayfrank, E., & Daumann, J. (2009).
Neurotoxicity of drugs of abuse—the case of
methylenedioxy amphetamines (MDMA,
ecstasy), and amphetamines. Dialogues in
Clinical Neuroscience, 11(3), 305–317.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Haney, M., Maccari, S., Le Moal, M., Simon,
H., & Vincenzo Piazza, P. (1995). Social
stress increases the acquisition of cocaine self-
administration in male and female rats. Brain
Research, 698(1–2), 46–52.
Hart, C. L., Marvin, C. B., Silver, R., & Smith,
E. E. (2011). Is cognitive functioning impaired
in methamphetamine users?: A critical review.
Neuropsychopharmacology, 37(3), 586–608.
Hart, C. L., Ward, A. S., Haney, M., Foltin, R.
W., & Fischman, M. W. (2001). Methamphet-
amine self- administration by humans. Psycho-
pharmacology, 157(1), 75–81.
Heinz, A., Siessmeier, T., Wrase, J., Buchholz, H.
G., Gründer, G., Kumakura, Y., et al. (2005).
Correlation of alcohol craving with striatal
dopamine synthesis capacity and D2/3 recep-
tor availability: A combined [18F]DOPA and
[18F]DMFP PET study in detoxified alcoholic
patients. American Journal of Psychiatry,
162(8), 1515–1520.
Hernández-López, M., Luciano, M. C., Bricker,
J. B., Roales-Nieto, J. G., & Montesinos, F.
(2009). Acceptance and commitment therapy
for smoking cessation: A preliminary study of
its effectiveness in comparison with cognitive
behavioral therapy. Psychology of Addictive
Behaviors, 23(4), 723–730.
Hester, R., & Garavan, H. (2004). Executive
dysfunction in cocaine addiction: Evidence
for discordant frontal, cingulate, and cerebel-
lar activity. Journal of Neuroscience, 24(49),
11017–11022.
Hölzel, B. K., Lazar, S. W., Gard, T., Schuman-
Olivier, Z., Vago, D. R., & Ott, U. (2011).
How does mindfulness meditation work?:
Proposing mechanisms of action from a con-
ceptual and neural perspective. Perspectives
on Psychological Science, 6(6), 537–559.
Ivanov, I., Newcorn, J., Morton, K., & Tri-
camo, M. (2011). Inhibitory control deficits
in childhood: Definition, measurement, and
clinical risk for substance use disorders. In M.
T. Bardo (Ed.), Inhibitory control and drug
abuse prevention: From research to trans-
lation (pp. 125–144). New York: Springer
Science+Business Media.
Jaffe, J. H., & Jaffe, F. K. (1989). Historical per-
spectives on the use of subjective effects mea-
sures in assessing the abuse potential of drugs.
NIDA Research Monographs, 92, 43–72.
Johnstone, T., & Walter, H. (this volume, 2014).
The neural basis of emotion dysregulation. In
J. J. Gross (Ed.), Handbook of emotion regu-
lation (2nd ed., pp. 58–75). New York: Guil-
ford Press.
444 PSYCHOPATHOLOGY
Jones, B. T., Corbin, W., & Fromme, K. (2001).
A review of expectancy theory and alcohol
consumption. Addiction, 96, 57–72.
Kessler, R. C., Berglund, P., Demler, O., Jin, R.,
Merikangas, K. R., & Walters, E. E. (2005).
Lifetime prevalence and age-of-onset distri-
butions of DSM-IV disorders in the National
Comorbidity Survey Replication. Archives of
General Psychiatry, 62(6), 593–602.
Khantzian, E. J. (1985). The self- medication
hypothesis of addictive disorders: focus on
heroin and cocaine dependence. American
Journal of Psychiatry, 142(11), 1259–1264.
Kiluk, B. D., Nich, C., Babuscio, T., & Carroll,
K. M. (2010). Quality versus quantity: Acqui-
sition of coping skills following computerized
cognitive behavioral therapy for substance use
disorders. Addiction, 105(12), 2120–2127.
Kober, H., & DeLeone, C. M. (2011). Smoking
and neuroimaging: A review. Current Cardio-
vascular Risk Reports, 5(6), 484–491.
Kober, H., DeVito, E. E., DeLeone, C. M., Car-
roll, K. M., & Potenza, M. N. (under review).
Pre- treatment neural activity in cannabis
users differs from controls and relates to long
term treatment outcome.
Kober, H., Kross, E. F., Mischel, W., Hart, C. L.,
& Ochsner, K. N. (2010). Regulation of crav-
ing by cognitive strategies in cigarette smok-
ers. Drug and Alcohol Dependence, 106(1),
52–55.
Kober, H., Mende- Siedlecki, P., Kross, E. F.,
Weber, J., Mischel, W., Hart, C. L., et al.
(2010). Prefrontal– striatal pathway underlies
cognitive regulation of craving. Proceedings of
the National Academy of Sciences, 107(33),
14811–14816.
Kober, H., Turza, A. C., & Hart, C. L. (2009).
Risk factors for substance use, abuse, and
dependence: Learning. In H. Kranzler & P.
Korsmeyer (Eds.), Encyclopedia of drugs,
alcohol, and addictive behavior (3rd ed.).
Macmillan Reference USA.
Koob, G. F., & Le Moal, M. (2008). Neurobio-
logical mechanisms for opponent motivational
processes in addiction. Philosophical Transac-
tions of the Royal Society B: Biological Sci-
ences, 363(1507), 3113–3123.
Kun, B., & Demetrovics, Z. (2010). Emotional
intelligence and addictions: A systematic
review. Substance Use and Misuse, 45(7–8),
1131–1160.
Le Moal, M. (2009). Drug abuse: Vulnerability
and transition to addiction. Pharmacopsychi-
atry, 42(S1), 542–555.
Li, C. S. R., Huang, C., Yan, P., Bhagwagar, Z.,
Milivojevic, V., & Sinha, R. (2008). Neural
correlates of impulse control during stop sig-
nal inhibition in cocaine- dependent men. Neu-
ropsychopharmacology, 33(8), 1798–1806.
Li, C. S. R., Luo, X., Yan, P., Bergquist, K., &
Sinha, R. (2009). Altered impulse control in
alcohol dependence: Neural measures of stop
signal performance. Alcoholism: Clinical and
Experimental Research, 33(4), 740–750.
Linehan, M. M., Dimeff, L. A., Reynolds, S. K.,
Comtois, K. A., Welch, S. S., Heagerty, P., et
al. (2002). Dialectical behavior therapy versus
comprehensive validation therapy plus 12-step
for the treatment of opioid dependent women
meeting criteria for borderline personality dis-
order. Drug and Alcohol Dependence, 67(1),
13–26.
Litt, M. D., Cooney, N. L., & Morse, P. (2000).
Reactivity to alcohol- related stimuli in the
laboratory and in the field: Predictors of crav-
ing in treated alcoholics. Addiction, 95(6),
889–900.
Littel, M., & Franken, I. H. A. (2011). Inten-
tional modulation of the late positive potential
in response to smoking cues by cognitive strat-
egies in smokers. PloS ONE, 6(11), e27519.
Liu, H., Li, L., Hao, Y., Cao, D., Xu, L.,
Rohrbaugh, R., et al. (2008). Disrupted white
matter integrity in heroin dependence: A con-
trolled study utilizing diffusion tensor imag-
ing. American Journal of Drug and Alcohol
Abuse, 34(5), 562–575.
Luerssen, A., & Ayduk, O. (this volume, 2014).
The role of emotion and emotion regulation in
the ability to delay gratification. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., 111–125). New York: Guilford Press.
Lüscher, C., & Malenka, R. C. (2011). Drug-
evoked synaptic plasticity in addiction: From
molecular changes to circuit remodeling. Neu-
ron, 69(4), 650–663.
Marlatt, G. A., & Witkiewitz, K. (2005). Relapse
prevention for alcohol and drug problems. In
G. Marlatt & D. Donovan (Eds.), Relapse
prevention: Maintenance strategies in the
treatment of addictive behaviors (2nd ed.,
pp. 1–44). New York: Guilford Press.
Marshall- Berenz, E. C., Vujanovic, A. A., &
MacPherson, L. (2011). Impulsivity and alco-
hol use coping motives in a trauma- exposed
sample: The mediating role of distress toler-
ance. Personality and Individual Differences,
50(5), 588–592.
McRae, K., Gross, J. J., Weber, J., Robertson, E.
Emotion Regulation in Substance Use Disorders 445
R., Sokol- Hessner, P., Ray, R. D., et al. (2012).
The development of emotion regulation: An
fMRI study of cognitive reappraisal in chil-
dren, adolescents and young adults. Social
Cognitive and Affective Neuroscience, 7(1),
11–22.
Miller, E. K., & Cohen, J. D. (2001). An inte-
grative theory of prefrontal cortex function.
Annual Review of Neuroscience, 24(1), 167–
202.
Mischel, W., Ayduk, O., Berman, M. G., Casey,
B., Gotlib, I. H., Jonides, J., et al. (2011).
‘Willpower’ over the life span: Decomposing
self- regulation. Social Cognitive and Affective
Neuroscience, 6(2), 252–256.
Moffitt, T. E., Arseneault, L., Belsky, D., Dick-
son, N., Hancox, R. J., Harrington, H. L., et
al. (2011). A gradient of childhood self- control
predicts health, wealth, and public safety.
Proceedings of the National Academy of Sci-
ences, 108(7), 2693–2698.
Morasco, B. J., Gritzner, S., Lewis, L., Oldham,
R., Turk, D. C., & Dobscha, S. K. (2011). Sys-
tematic review of prevalence, correlates, and
treatment outcomes for chronic non- cancer
pain in patients with comorbid substance use
disorder. Pain, 152(3), 488– 497.
National Institute of Mental Health (NIMH).
(2007). National Comorbidity Survey: Life-
time prevalence estimates. Available from
www.hcp.med.harvard.edu/ncs.
Nestor, L. J., Ghahremani, D. G., Monterosso,
J., & London, E. D. (2011). Prefrontal hypoac-
tivation during cognitive control in early absti-
nent methamphetamine- dependent subjects.
Psychiatry Research: Neuroimaging, 194(3),
287–295.
Nunes, E. V., & Levin, F. R. (2004). Treatment
of depression in patients with alcohol or other
drug dependence. Journal of the American
Medical Association, 291(15), 1887–1896.
O’Brien, C. P., Childress, A. R., Ehrman, R.,
& Robbins, S. J. (1998). Conditioning fac-
tors in drug abuse: Can they explain compul-
sion? Journal of Psychopharmacology, 12(1),
15–22.
Ochsner, K. N., & Gross, J. J. (this volume, 2014).
The neural bases of emotion and emotion regu-
lation: A valuation perspective. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp. 23–42). New York: Guilford Press.
O’Connell, K. A., Hosein, V. L., Schwartz, J. E.,
& Leibowitz, R. Q. (2007). How does coping
help people resist lapses during smoking cessa-
tion? Health Psychology, 26(1), 77–84.
Perkins, K. A. (2009). Does smoking cue- induced
craving tell us anything important about nico-
tine dependence? Addiction, 104(10), 1610–
1616.
Pfefferbaum, A., Rosenbloom, M., Rohlfing,
T., & Sullivan, E. V. (2009). Degradation of
association and projection white matter sys-
tems in alcoholism detected with quantitative
fiber tracking. Biological Psychiatry, 65(8),
680–690.
Pfeifer, J. H., Lieberman, M. D., & Dapretto, M.
(2007). “I know you are but what am I?”: Neu-
ral bases of self-and social knowledge retrieval
in children and adults. Journal of Cognitive
Neuroscience, 19(8), 1323–1337.
Potenza, M. N., Sofuoglu, M., Carroll, K. M.,
& Rounsaville, B. J. (2011). Neuroscience of
behavioral and pharmacological treatments
for addictions. Neuron, 69(4), 695–712.
Rando, K., Hong, K. I., Bhagwagar, Z., Li, C. S.
R., Bergquist, K., Guarnaccia, J., et al. (2011).
Association of frontal and posterior corti-
cal gray matter volume with time to alcohol
relapse: A prospective study. American Jour-
nal of Psychiatry, 168(2), 183–192.
Riediger, M., & Klipker, K. (this volume, 2014).
Emotion regulation in adolescence. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., 187–202). New York: Guilford Press.
Riley, H., & Schutte, N. S. (2003). Low emo-
tional intelligence as a predictor of substance-
use problems. Journal of Drug Education,
33(4), 391–398.
Romero, M. J., Asensio, S., Palau, C., Sanchez,
A., & Romero, F. J. (2010). Cocaine addiction:
Diffusion tensor imaging study of the inferior
frontal and anterior cingulate white matter.
Psychiatry Research: Neuroimaging, 181(1),
57–63.
Shiffman, S., Dunbar, M., Kirchner, T., Li, X.,
Tindle, H., Anderson, S., et al. (2013). Smoker
reactivity to cues: Effects on craving and on
smoking behavior. Journal of Abnormal Psy-
chology, 122(1), 264–280.
Shiffman, S., Paty, J. A., Gnys, M., Kassel, J. A.,
& Hickcox, M. (1996). First lapses to smok-
ing: Within- subjects analysis of real-time
reports. Journal of Consulting and Clinical
Psychology, 64(2), 366–379.
Silvers, J. A., McRae, K., Gabrieli, J. D. E.,
Gross, J. J., Remy, K. A., & Ochsner, K. N.
(2012). Age- related differences in emotional
reactivity, regulation, and rejection sensitiv-
ity in adolescence. Emotion, 12(6), 1235–
1247.
446 PSYCHOPATHOLOGY
Sinha, R., & Li, C. S. R. (2007). Imaging stress-
and cue- induced drug and alcohol craving:
Association with relapse and clinical impli-
cations. Drug and Alcohol Review, 26(1),
25–31.
Sorg, S. F., Taylor, M. J., Alhassoon, O. M.,
Gongvatana, A., Theilmann, R. J., et al.
(2012). Frontal white matter integrity predic-
tors of adult alcohol treatment outcome. Bio-
logical Psychiatry, 71, 262–268.
Steinberg, L. (2005). Cognitive and affective
development in adolescence. Trends in Cogni-
tive Sciences, 9(2), 69–74.
Substance Abuse and Mental Health Services
Administration (SAMHSA). (2011). Results
from the 2010 National Survey on Drug Use
and Health (NSDUH): Summary of national
findings (NSDUH Series H-41). Rockville,
MD: Author.
Tabibnia, G., Monterosso, J. R., Baicy, K., Aron,
A. R., Poldrack, R. A., Chakrapani, S., et al.
(2011). Different forms of self- control share a
neurocognitive substrate. Journal of Neurosci-
ence, 31(13), 4805–4810.
Taioli, E., & Wynder, E. L. (1991). Effect of the
age at which smoking begins on frequency of
smoking in adulthood. New England Journal
of Medicine, 325(13), 968–969.
Tarter, R. E., Kirisci, L., Mezzich, A., Cornelius,
J. R., Pajer, K., Vanyukov, M., et al. (2003).
Neurobehavioral disinhibition in childhood
predicts early age at onset of substance use
disorder. American Journal of Psychiatry,
160(6), 1078–1085.
Verdejo- García, A., Lawrence, A. J., & Clark, L.
(2008). Impulsivity as a vulnerability marker
for substance- use disorders: Review of find-
ings from high-risk research, problem gam-
blers and genetic association studies. Neuro-
science and Biobehavioral Reviews, 32(4),
777–810.
Volkow, N. D., Fowler, J., Wang, G., Telang, F.,
Logan, J., Jayne, M., et al. (2010). Cognitive
control of drug craving inhibits brain reward
regions in cocaine abusers. NeuroImage, 49,
2536–2543.
Volkow, N. D., Wang, G. J., Fowler, J. S., &
Tomasi, D. (2011). Addiction Circuitry in the
Human Brain. Annual review of pharmacol-
ogy and toxicology, 52(1), 321–336.
Weiss, F., & Koob, G. F. (2001). Drug addiction:
Functional neurotoxicity of the brain reward
systems. Neurotoxicity Research, 3(1), 145–
156.
Westbrook, C., Creswell, J. D., Tabibnia, G., Jul-
son, E., Kober, H., & Tindle, H. A. (2013).
Mindful attention reduces neural and self-
reported cue- induced craving in smokers.
Social Cognitive and Affective Neuroscience,
8(1), 73–84.
Witkiewitz, K., & Bowen, S. (2010). Depres-
sion, craving, and substance use following a
randomized trial of mindfulness- based relapse
prevention. Journal of Consulting and Clini-
cal Psychology, 78(3), 362–374.
Zgierska, A., Rabago, D., Chawla, N., Kushner,
K., Koehler, R., & Marlatt, A. (2009). Mind-
fulness meditation for substance use disorders:
A systematic review. Substance Abuse, 30(4),
266–294.
447
For the last several centuries, philosophers,
and later, psychologists have assumed that
the mind works like a machine— a printing
press, a switchboard, a computer. According
to the machine metaphor, the mind consists
of a number of functionally distinct pro-
cesses (mental “modules” or “faculties”) that
interact with one another; if separated, these
processes would still retain their identity and
function. The machine metaphor dictates a
particular view of mental causation: “Psy-
chological process A” localized in one swath
of brain tissue (a region or a network) causes
a change in a separate and distinct “psycho-
logical process B” localized in another swath
of tissue (see Figure 27.1). A good example
of the machine metaphor at work can be
found in the science of emotion regulation.
It is largely assumed, for example, that an
emotion, like fear, is created by one process
computed in one part of the brain (usually
in subcortical limbic or paralimbic cortex)
that is regulated by executive or other cogni-
tive processes located elsewhere in the brain
(typically somewhere in prefrontal cortex).
In the process model of emotion regulation
(Gross, this volume), an emotion can be trig-
gered first, then subsequently is regulated
(e.g., you are walking in the woods, and a
fuzzy bee buzzing around your head triggers
a state of fear, which you then regulate by
suppressing the urge to run and by distract-
ing yourself with a close examination of the
local scenery, such as an interestingly shaped
rock or tree). Regulation might also occur
before the response occurs, preempting the
CHAPTER 27
A Psychological Construction Account
of Emotion Regulation and Dysregulation:
The Role of Situated Conceptualizations
Lisa Feldman Barrett
Christine D. Wilson-Mendenhall
Lawrence W. Barsalou
FIGURE 27.1. A depiction of the machine meta-
phor of brain function.
448 PSYCHOPATHOLOGY
emotion from ever taking place (e.g., before
you start your walk, you might remind your-
self that bees are a part of nature; they pol-
linate beautiful flowers and make delicious
honey). Regardless of which comes first, the
emotion is separate from its regulation.
In the last several years, scientists have
come to question whether the mind and
brain work like a machine with separate,
interacting bits and pieces (e.g., Barrett,
2009), and assumptions about modularity,
even in sensory cortices, are strongly in ques-
tion. As a consequence, other working meta-
phors for the mind and the brain are more
apt—say, molecules that are constructed of
atoms, chamber music emerging from the
interplay of instruments, or recipes from
a well- stocked pantry full of ingredients.
These metaphors begin with a deceptively
simple observation: During every moment
of waking life, the brain takes in sensory
input captured from the world outside the
skin (light, vibrations, odors, etc.) and sen-
sations captured from within the body that
holds the brain (the internal “milieu”), and
uses knowledge from prior experience (also
variously called concepts, memories, associ-
ations, beliefs, predictions, etc.)—stored in
association cortex, and in sensory neurons
and subcortical regions— and makes those
sensory inputs meaningful. This occurs by
creating situated conceptualizations (Bar-
rett, 2006, 2012; Barsalou, 2003, 2009;
Barsalou, Niedenthal, Barbey, & Ruppert,
2003; Wilson- Mendenhall, Barrett, Sim-
mons, & Barsalou, 2011). A situated con-
ceptualization initially is like a prediction
of what sensory input stands for in the
world (i.e., object or event identification),
which properties are salient (i.e., deserving
of attention), what to do about that sensory
input (i.e., a predicted action), and what the
homeostatic and metabolic consequences
will be (i.e., affective changes). From our
perspective, the brain’s architecture can be
thought of as a situated conceptualization
generator producing the individual brain
states that correspond to each individual
mental state, such as an individual instance
of fear or an instance of regulation.
Building on the kitchen metaphor (in Bar-
rett, 2009), we have proposed that each
brain state, each situated conceptualization,
can be understood in terms of more core sys-
tems (i.e., the ingredients), which can them-
selves be characterized at both the psycho-
logical level (e.g., Barrett, 2006, 2012) and
the level of brain networks (e.g., Barrett &
Satpute, 2013; Lindquist & Barrett, 2012).
These core systems are like the “mental state
variables” (see Salzman & Fusi, 2010), fac-
ets or core systems that describe the brain
state. As basic “ingredients” of the mind,
they are necessary for but not specific to
emotion generation, or to emotion regula-
tion per se, just as flour and salt are nec-
essary for but not specific to bread. As the
brain transitions from one state to another,
mental states ebb and flow, and people give
special names to these different states. We
refer to this as a psychological construction-
ist, or merely a constructionist, approach to
the mind and brain. From this construction-
ist point of view, emotions are not unique
mental states that are caused by dedicated
mechanisms, to be modified by another set of
dedicated regulatory mechanisms. Instead,
emotions emerge, and regulation occurs, as
the consequence of an ongoing, continually
modified constructive process that makes
sensory inputs meaningful. Every mental
state, including an emotion both before and
after regulation is said to have occurred, is
a situated conceptualization, constructed
from assemblies of neurons that perform
sensory, conceptual, attentional, and action
functions.
In this chapter, we examine in more detail
the concept of emotion regulation as result-
ing from the never ending sequence of situ-
ated conceptualizations that occur as the
brain transitions from one state to another.
First, we introduce the general idea that
knowledge (as reactivation and recombina-
tion of prior experience) gives meaning to
incoming sensory input and is itself enac-
tive (i.e., adds novel features via perceptual
inference). Next, we link these notions to the
idea of situated conceptualizations from the
literature on concepts and categories, and
discuss how we have broadened it into a gen-
eral proposal of constructed mental states
that involve making meaning of sensory
input and even modifying sensations during
the process. We then discuss how emotions
might be understood as situated conceptual-
izations, and how emotion regulation might
be reconceptualized as shifting from one sit-
uated conceptualization to another. Finally,
we use this framework to consider how emo-

Emotion Regulation as Situated Conceptualizations 449
tional dysregulation might be understood in
terms of the situated conceptualizations that
are constructed.
Making Meaning of Sensory Input
Take a look at the image in Figure 27.2. As
you look at this image, your brain is try-
ing to make sense of the visual input it is
receiving. If you are having difficulty mak-
ing sense of the visual input from the image
(e.g., you cannot recognize an object in it),
then you are in a state of experiential blind-
ness (e.g., Fine, Wade, Brewer, May, Good-
man, et al., 2003). This is because, usually,
in the blink of an eye, quite automatically
and with no effort whatsoever, your brain
is able to integrate impinging sensory stim-
ulation seamlessly with its vast amount of
stored knowledge (from prior experience),
allowing you to construct a visual represen-
tation of the object. Such knowledge is not
merely helpful— it is necessary to normal
perception. Without prior experience, the
sensations are meaningless, and if this were
an object before you, in three- dimensional
space, you would not know how to act on
it. The definition of adaptive behavior is the
use of past experience to guide future action.
Now look at the image at the end of the
chapter (on page 465). Then return to look
again at Figure 27.2.
We hope you can now see the object,
because you have had an experience to help
you make sense of the visual input. The first
lesson here is that it very difficult to “unsee”
the object in the original “blobby” black-
and-white image. A second lesson is that no
matter how hard you try, you cannot gain
introspective access to the processes in your
brain that underlie using stored knowledge
to make incoming sensations meaningful.
Experimental methods are necessary to
unmask its workings. The third lesson from
this example is that your brain infers ele-
ments of the experience that are not imme-
diately present (e.g., the lines that link the
black and gray blobs together into the shape
of a bee). Although you cannot gain intro-
spective access to these inferred features, you
can get a sense that this inference process
works by conjuring some additional percep-
tual detail— the soft drone of buzzing, or the
delicate flutter of wings. In your mind’s eye,
you might see the object nose around as it
searches for pollen. You might even be able
to smell the sweet fragrance of the flower.
Inference is considered one of the primary
purposes of memory and is how experiences
of the past help to inform situated action
in the present. You could not survive in the
world without this capacity. Some scientists
refer to this inference process as simulation
(e.g., Barsalou, 1999, 2009), where you can
connect immediate sensory input with a vast
body of sensory, motor, affective, and other,
related information stored in memory; we
also refer to it as categorization (Barrett,
2006).
The fourth lesson is that these inferences
prepare you for situated action. For some
people, perhaps, who have experienced
bees as part of a beautiful garden and/or
as producing a sweet, tasty delight (honey),
the image of a bee is calming and bucolic.
FIGURE 27.2. An illustration of experiential blindness.
450 PSYCHOPATHOLOGY
For them, seeing a bee might mean moving
in to get a closer look, with an associated
reduction in heart rate, blood pressure, and
skin conductance. For other people, perhaps
who have been stung, resulting in pain and
swelling, the image of a bee is terrifying. For
these people, seeing a bee might mean freez-
ing, with an associated increase in heart
rate, blood pressure, and skin conductance.
Or, it might mean waving their arms or run-
ning away, with an increase in heart rate and
skin conductance but a decrease in blood
pressure. These are the sorts of physiologi-
cal changes that we scientists record when
we show study participants images from
the International Affective Picture System
(IAPS; Lang, Bradley, & Curthbert, 2008)
stimulus set (e.g., Bradley, Codispoti, Cuth-
bert, & Lang, 2001). They arise during a
prediction of how the body should respond
in a specific situation (what we have previ-
ously referred to as an “affective predic-
tion”; Barrett & Bar, 2009).
The fifth lesson from this example is that
the process of making meaning of external
sensations will always produce some kind of
automatic change in your physical state. It
is these internal sensations that likely form
the basis of your pleasant or unpleasant core
affective tone that accompanies any men-
tal states of which it is a part (Barrett &
Bliss- Moreau, 2009; Russell, 2003; Wundt,
1897); the actual visceral changes are not
necessary for feeling, of course, although
some representation of them in the brain is
required. In the same way that your brain
used prior experience to make meaning of
the visual sensations in Figure 27.2, it will
also use such knowledge to make mean-
ing of these bodily sensations. These two
meaning- making achievements are not hap-
pening in quick succession— they are occur-
ring simultaneously, as a function of how
the brain understands the current sensory
array to create a unified conscious moment
(cf. Barrett, 2009). They are not occurring
in a single instant, but they are evolving over
time. This meaning making rarely happens
deliberately, but more often occurs as instan-
taneously, continuously, and effortlessly for
internal sensations as it does for external
sensations. These insights form the basis of
our conceptual act theory of emotion (Bar-
rett, 2006). You experience a perception of
the situation versus an emotion as a function
of your attentional focus. When sensations
from the visual world are foregrounded (and
sensations from the body are in the back-
ground), you experience the bee as friendly
or wicked because you are focused on the
bee, and not on how your body is respond-
ing to the bee (e.g., Anderson, Siegel, White,
& Barrett, 2012). When sensations from
the body are foregrounded, either because
they are particularly intense, because such
focus has been useful and reinforced in a
prior situation like this one, or because you
focus explicitly on them, you will experience
either tranquility or distress.
The sixth lesson from this example is
that prior experiences seed the construction
of present and future experiences by shap-
ing the meaning of momentary, incoming
sensory input. Why might you automati-
cally experience the calm of a bee buzzing
in a bucolic garden, whereas another person
might automatically experience the terror of
a bee attacking and stinging the body? The
answer lies in the nature of prior experience.
Actual experiences with bees, movie scenes
that involve bees, stories, or simply instruc-
tion about bees constitute the knowledge
that is used to make sensations meaning-
ful. Your learning history predisposes you
to experience sensations from the world and
from your own body in particular modal
ways. All things being equal, you have
developed experiential “habits”—what you
have experienced in the past is very likely
what you will experience in the present,
because stored representations of the past
help to constitute the present (hence, the
phrase “the remembered present”; Edelman,
1998). It is now well known that the same
brain network (termed the default mode or
mentalizing network) involved in long-term
memory is also important for imagining the
future (Andrews- Hanna, Reidler, Sepul-
cre, Poulin, & Buckner, 2010), and recent
evidence suggests that these networks are
also important for constructing emotions in
the present (Kober et al., 2008). It is very
likely that when faced with the visual input
in Figure 27.2 your brain reconstituted a
number of different associations that were
in competition with one another, and that
via a variety of selection processes (Barrett,
Tugade, & Engle, 2004; Sporns, Tononi, &
Edelman, 2000a, 2000b), only one was fully
realized (perhaps according to the logic of

Emotion Regulation as Situated Conceptualizations 451
constraint satisfaction) (e.g., Barrett, Och-
sner, & Gross, 2007; Barsalou et al., 2003).
The same logic might hold for making mean-
ing of internal sensations. When is a high
arousal state fear, or anger, or excitement? It
might depend on your prior experience with
these sensations in different contexts, and in
particular, how those various instances were
paired with emotion words such as “fear,”
“anger,” or “excitement” (cf. Barrett, 2006;
Barrett, Lindquist, & Gendron, 2007). With
additional learning or training, it should be
possible to change your experiential habits.
By deliberately cultivating certain types of
experiences, it should be possible to modify
the population of representations that are
available for use in the present.
Situated Conceptualizations
In our prior writing, we have focused on
the process of meaning making as a psy-
chological construction of emotion that
involves creating situated conceptualiza-
tions of internal bodily sensations that are
highly context- dependent and coordinated
with the immediate situation (cf. Barrett,
2006; Wilson- Mendenhall et al., 2011). In
fact, however, the notion of a situated con-
ceptualization implies that people are mak-
ing meaning of both internal and external
sensations at the same time, to create a uni-
fied conscious field (cf. Barrett, 2009). Con-
ceptual knowledge is distributed throughout
the brain’s modal systems for perception and
action in the form of simulators that reenact
sensory, action, affect, and other elements
of situations captured through experience
(e.g., Barsalou, 1999; Simmons & Barsalou,
2003). From this perspective, knowledge is
central to not only the cognition involved in
thinking and imagining offline (reenacting
or simulating a situation that is not present)
but also to perceiving external sensations
from the world and internal sensations from
the body (in which case knowledge fuses
with impinging sensory input—this fusion
occurs seamlessly because knowledge is
stored and represented in the same format
or “language” as the sensations), both of
which are involved in predicting and guid-
ing one’s actions. In this sense, conceptual
knowledge is enactive. When your brain
was foregrounding your bodily sensations
while viewing the bee a few paragraphs ear-
lier, for example, perhaps you experienced
the moment as an emotion; when the focus
was on the visual sensations, perhaps you
experienced it merely as a perception of the
bee. In each case, the visual input was the
same—what differed was the situated con-
ceptualization.
In the past, we have referred to the cre-
ation of situated conceptualizations as an
act of categorization, because situated con-
ceptualizations are enactments that develop
for categories of experience (a variety of
situated conceptualizations develop for the
categories fear, anger, etc.; Barrett, 2006).
To understand how situated conceptualiza-
tions work, then, it is important to under-
stand how concepts and categories work.
Categorization is not a narrow, limited
process— it does not happen only when you
explicitly attempt to assign an object to one
grouping or another. Categorization plays a
central role in all cognitive activity, includ-
ing the sorts of high-level perceptions that
are involved in emotion and emotion regula-
tion. Categorizing is a fundamental cogni-
tive activity. To categorize sensory input is
to determine what it is, why it is, and what
to do with it.
A central property of human knowledge
is that it is organized categorically. Unlike
a recording device that simply stores each
individual, holistic, bitmapped image of
the world, the human brain is constantly
interpreting aspects of experience, using
concepts in memory to make sensations
meaningful. A concept can be viewed as
aggregated memories that accumulate
for a category across experiences with its
instances. By focusing attention on some
aspect of experience repeatedly, a concept
develops over time from instances of the
respective category experienced across situ-
ations (Barsalou, 1999; Barsalou & Hale,
1993; Murphy, 2002). The concept of bee,
for example, aggregates diverse information
about the category of bees across a variety
of situations into a loosely organized repre-
sentation that includes properties (e.g., yel-
low and black, with wings), relations (e.g.,
flowers), rules (for something to be a bee, it
must have black and yellow stripes, it must
fly, etc.), and exemplars (e.g., instances of
honey bees, carpenter bees, a queen bee,
etc.).
1
Concepts develop for all aspects of
452 PSYCHOPATHOLOGY
human experience related to bee, including
objects, settings, and actions (e.g., flowers,
honey, gardens, freezing, running, swatting,
flying, buzzing, stinging). From simpler con-
cepts, more complex concepts emerge for
events (e.g., strolling in a garden, fear of the
bee). Concepts also develop for a wide vari-
ety of internal states (e.g., aroused, quiet), as
well as for the properties and relations that
describe instances of concepts (e.g., yellow,
fast, sweet, above, after, cause). Although
concepts reflect experience to a considerable
extent, they undoubtedly have biological
bases that scaffold learning (Barsalou, 1999,
2008; Carey, 2009; Rips, 2010; Simmons &
Barsalou, 2003).
Extensive evidence now exists that an
instance of conceptual knowledge (an
instance of a concept, or a conceptualiza-
tion) emerges from different multimodal sys-
tems in the brain (e.g., McClelland, 2010).
Depending on the modalities relevant for
processing a concept’s instances, particu-
lar modal areas of the brain store informa-
tion about the category and can later rep-
resent the category in the absence of actual
instances. Martin (2001, 2007) reviews
evidence, for example, that different multi-
modal profiles represent living versus non-
living things. Other research has similarly
established the multimodal profiles that
represent the self and others (cf. Legrand
& Ruby, 2009; Northoff et al., 2006; Van
Overwalle, 2009), people, buildings, and
tools (e.g., Simmons, Reddish, Bellgowan,
& Martin, 2010), the external world versus
internal states (Golland, Golland, Bentin, &
Malach, 2008), and so forth.
Category instances (e.g., a bee) are never
encoded alone into conceptual knowledge,
even though their context may not explic-
itly be the focus of attention. Initially, when
encoding a category instance of a bee, for
example, from actual prior experience with
bees, observational learning about bees,
hearing stories about bees, or being told
rules about bees, the brain captures the ele-
ments of the setting in which the bee occurs
(i.e., other agents and objects), internal
sensory (i.e., somatovisceral) cues from the
body, as well as actions, instructions from
others (in the form of rules) and words (e.g.,
the phonological form for “bee”). Over time,
these situated conceptualizations create a
heterogeneous population of information
that is available to represent new instances
of the category “bee.”
2
Later, when your
brain requires conceptual knowledge to pro-
cess some incoming sensory input, it samples
from the populations of situated conceptual-
izations, associated with relevant concepts,
to create a novel situated conceptualiza-
tion, integrating current sensory input and
retrieved (modal) conceptual knowledge
(Barsalou, 2009). From this perspective,
conceptual processing is actually more like
scene perception, because the brain pro-
duces a conceptual state using multimodal
information about entire situations. In this
way, a situated conceptualization allows an
experiencer to interpret incoming informa-
tion and draw inferences that go beyond the
information given.
It is impossible to have conscious access
to the processes that create situated concep-
tualizations, because they are initiated in
the first milliseconds of perception (or per-
haps even before sensory input is actually
encountered), and evolve over time, but it is
possible to demonstrate the brain’s computa-
tional power in creating them by engaging in
a little imagination. For example, close your
eyes and create an image of a yellow and
black bee in your mind (i.e., simulate a bee).
In doing this, your brain is creating a rep-
resentation that includes the sights, sounds,
smells, and so forth, of the bee, along with
a situation in which the bee occurred, all of
which would prepare you for a certain type
of action (to run, to peer closer, to swat, to
freeze). This representation involves the acti-
vation of neurons throughout your brain,
including sensory and motor neurons, as
well as neurons that regulate and represent
an internal body (somatovisceral) state. All
these elements (activation of sensory and
motor neurons, changes in the physical
state of the body, preparations for action)
are examples of what it means to say that
a representation is “embodied” (Barrett &
Lindquist, 2008). This is also what it means
to say that the brain is making a predic-
tion about an object and how to act on it
(Lindquist, Wager, Kober, Bliss- Moreau, &
Barrett, 2012) or, as we noted before, to say
that the brain is simulating a bee. This sort
of simulation would occur if you were pre-
sented with an image of a bee and asked to
recognize it (as in the prior section of this
chapter), to categorize it explicitly (assign it

Emotion Regulation as Situated Conceptualizations 453
to one stimulus grouping over another), to
judge it in some way, or to perform any kind
of cognitive task with it. It would occur if
you were being asked to remember a bee,
talk about a bee, think about a bee, or when
perceiving bees during an outdoor walk.
Once concepts become established in
memory, they play central roles throughout
cognition and perception (e.g., Barsalou,
2003; Murphy, 2002), and, as we suggest,
emotion. As people experience incoming
sensory input from the world and the body,
they use prior experience to categorize the
agents, objects, setting, behaviors, events,
properties, relations, and bodily states
that are present. As described in Wilson-
Mendenhall et al. (2011), a situated concep-
tualization is the conceptualization of the
current situation across parallel streams of
conceptual processing for all of these ele-
ments. As information from the current situ-
ation registers simultaneously in these pro-
cessing streams, conceptual systems on each
of them categorize the respective informa-
tion and draw inferences. At a more global
level, abstract relational concepts integrate
conceptualizations on the individual pro-
cessing streams into coherent interpretations
of larger events taking place across the situ-
ation as a whole. Categorical inferences (i.e.,
predictions) follow, including inferences
about how an object, or entity, is likely to
behave, how one can best interact with it,
the likely value to be obtained from inter-
acting with it, and so forth, and on a larger
scale, about how situations may unfold
during an event. From the perspective of
grounded cognition, situated conceptual-
izations are responsible for producing the
action, internal states, and perceptual con-
struals that underlie goal- related activity in
the current situation. Because modalities for
action, internals states, and perceptual con-
struals are typically active when a concept is
learned, situated conceptualizations gener-
ate activity in these systems as they become
active on later occasions. On activating the
concept for bee, a situated conceptualization
might activate representations of situation-
specific approach– avoid actions (e.g., swat-
ting the bee), representations of internal
states such as pleasure or displeasure, and
perceptual construals that direct the body
toward a particular instance of pleasure or
displeasure. Not only does bee represent per-
ceptual instances of the concept, it also con-
trols interactions and predicts the resultant
events.
Emotions and Emotion Regulation
as Situated Conceptualizations
Initial work on situated conceptualizations
focused on how this theory of concepts can
be applied to perceiving or interacting with
concrete objects in relevant situations (for a
review, see Barsalou, 2009), with concep-
tual knowledge represented using the brain’s
modal systems for perception, action, and
internal bodily states. We further developed
these ideas into a theory of emotion (Barrett,
2006) and a broader theory of mental states
more generally (Barrett, 2009), although in
the present discussion we focus on emotion.
We hypothesize that situated conceptual-
izations have relevance for not only under-
standing the nature of emotion but also
presenting a computational framework for
understanding emotion regulation.
Emotions as
Situated Conceptualizations
In our view, an emotion concept typically
forms when a given emotion word (e.g.,
“fear”) is explicitly uttered (e.g., by a care-
giver or teacher) during many different
instances involving a variety of changes in
feelings, physiology, and actions, becoming
the statistical regularity that holds the con-
cept together across instances involving dif-
ferent sensory input and actions (cf. Barrett,
Lindquist, et al., 2007). Selectively attend-
ing with some consistency to components
of experience results in category knowledge
that is captured in memory (Schyns, Gold-
stone, & Thibaut, 1998). Because emotions
are abstract, language appears to guide
selective attention to the changes in inter-
nal states that characterize an emotion in
a given situation. For example, each time a
parent (or some other person) labels a child’s
internal state or behavior with an emotion
term, or a child observes the emotion term
being used to label someone else’s behavior,
the child extracts information about that
instance (including the phonological form of
the word) and integrates it with past infor-
mation associated with the same term that
454 PSYCHOPATHOLOGY
is stored in memory. In this way, the pho-
nological form for “fear” could become a
perceptual regularity that occurs repeatedly
across situations, and a concept fear forms
(it is certainly the case that young infants
can use abstract words to make conceptual
inferences about objects that differ in their
sensory properties; Dewar & Xu, 2009).
The consequence is that accumulating con-
ceptual knowledge for fear, for example,
will vary within a person over instances as
context and situated action demand. No sin-
gle situated conceptualization for fear need
give a complete account of the category fear.
There is not one script for fear or one abstract
representation for fear.
3
For example, fear
might occur when excitedly declaring a risky
bet, when lethargically sensing the first signs
of flu, when frantically fleeing a blazing fire,
or when casually flirting with an attractive
stranger. On any given occasion, the content
of a situated conceptualization for fear will
be constructed to contain mainly those prop-
erties of fear that are contextually relevant,
and it therefore contains only a small sub-
set of the knowledge available in long-term
memory about the category fear.
4
In a given
instance, then, the situated conceptualiza-
tion for fear has the potential to change the
internal state of the perceiver, because when
retrieving information about fear, sensory,
motor, and interoceptive states are partially
reinstated in the relevant aspects of cortex,
simulating an instance.
We have hypothesized that concepts and
categories for emotion work in essentially
the same way as other kinds of abstract con-
cepts in the conceptual system, where each
individual situated conceptualization for a
specific emotion (e.g., fear) refers to an entire
situation, including both the internal and
external sensations (Wilson- Mendenhall
et al., 2011). In this way, emotions can be
thought of as affective changes that are
linked to the situation in some way (cf. Bar-
rett, 2006; Clore & Ortony, 2008) and emo-
tions can be said to reflect the structure of
situations. The key hypothesis can be stated
as follows: A momentary array of sensations
from the world (light, sound, smell, touch,
and taste) combined with sensations from the
body (X) counts as perception of emotion (Y)
during a situated conceptualization (C) (Bar-
rett, 2012). Here, a “perception” is meant
to indicate perceiving an instance of sensa-
tions in the self as the experience of emo-
tion, or sensory input (from facial actions,
voice, etc.) coming from others as emotional
expressions. The meaning acquired by the
sensations is not based solely on the physical
properties of sensations alone (as body states
or actions, as represented in the physiol-
ogy of the body and/or in neural activations
within the brain). Conceptual knowledge is
required to give it additional functionality
and meaning. For example, an increase in
heart rate (X
1
) counts as feeling afraid (Y
1
)
when category knowledge about fear is acti-
vated as a specific, embodied representation
of fear, such as when a bee is attempting to
sting you (C
1
). In this example, the increase
in heart rate takes on a meaning and allows
a predicted behavior that it would not oth-
erwise have alone. Emotion regulation
might be characterized in the same way. A
decrease in heart rate (X
2
) counts as evidence
of reappraisal (Y
2
) when another embodied,
situation- specific representation of a bee is
activated, such as when it is floating above
a brightly colored flower petal (C
2
). In these
examples, the concept fear might be applied
to internal sensations from the body, because
they are in the focus of attention, or because
fear is part of a situated conceptualization
that is part of the concept bee. It is a mis-
nomer to refer to conceptual knowledge as
merely psychological or social. For physical
actions and body states (X) to count as an
emotion (Y), some kind of physical change
has to take place somewhere in the brain of
a perceiver to complete a situated conceptu-
alization in that perceiver at that moment
in time (C). So a psychological construc-
tion approach makes predictions about the
brain basis of emotion (and emotion regula-
tion), but one that is different from the typi-
cal machine metaphor illustrated in Figure
27.1 and found in most natural kind mod-
els of emotion (also see Jones, Kirkland, &
Cunningham, this volume). An instance of
emotion is hypothesized to correspond to an
entire brain state—or a series of states chang-
ing over time— including representations of
the body and/or actions and the additional
information that is necessary to create the
new meanings that make emotions real, that
is, the parts that are crucial for creating the
situated conceptualizations.
In our view, then, changes in heart rate
or blood pressure, facial actions such as
Emotion Regulation as Situated Conceptualizations 455
smiles or frowns, and behaviors such as cry-
ing or freezing, are not evidence of emotions
in and of themselves. Instead, they become
part of an emotional episode when they take
on a certain meaning in a certain situation
(Barrett, 2012). Via situated conceptualiza-
tions, physical changes acquire the ability to
perform functions that they do not have on
their own (creating social meaning, prescrib-
ing actions, allowing communication, aid-
ing social influence). In this view, category
knowledge about emotions does not cause
emotions per se—it constitutes emotions by
adding epistemologically novel functions to
sensory input and action. Said another way,
an emotion is constructed when embodied
conceptual knowledge is enacted to shape
the perception of sensory information from
the body and the world, binding a physical
state to an event in the world (as opposed to
being merely a physical sensation or action).
A body state or an action has a certain
physical function (e.g., changes in respira-
tion might regulate autonomic reactivity, or
widened eyes might increase the size of the
visual field), but these events do not intrinsi-
cally have certain functions as an emotion;
events are assigned those functions in the act
of categorizing them as emotion during the
construction of a situated conceptualization.
If a situated conceptualization is repre-
sented as a distributed brain state (with both
cortical and subcortical contributions), or
even a series of brain state transitions across
time, then mental causation is not mechanis-
tic per se, but probabilistic, such that Brain
State A at Time T (bee in the forest) increases
the probability of Brain State B at Time T
+ 1 (fear of the bee expressed as a racing
heart and sweaty hands and the perception
of a stick as a weapon), making swatting
more likely (but perhaps also a bee sting
more likely) (Figure 27.3). Alternatively, the
encounter with the bee might be followed
by situated conceptualization (an image of
bees making honey) as Brain State C at Time
T + 1, decreasing the probability of a rac-
ing heart and sweating, and so forth. From
this perspective, an emotion, such as fear,
is itself not a process but instead represents
a category of phenomena— a collection of
instances of probabilistic situated conceptu-
alizations. This example also illustrates that
situated conceptualizations are not indepen-
dent from one another in time—each occurs
in a context of what came before, and what
is predicted in the future.
Furthermore, we hypothesize that each
situated conceptualization (as a brain state
or series of states) can be understood as a
construction of more basic, domain- general
operations and their interactions. These
operations can themselves be characterized
at both the psychological level (e.g., Barrett,
FIGURE 27.3. A depiction of the probabilistic state-space metaphor of brain function.
456 PSYCHOPATHOLOGY
2006, 2012) and at the level of brain net-
works that emerge from neural integration
across time and space within the brain (e.g.,
Barrett & Satpute, 2013; Lindquist & Bar-
rett, 2012). Such basic operations are like
the “mental state variables” (see Salzman
& Fusi, 2010), facets or core systems that
describe the brain state, or, to return to our
kitchen metaphor, these can be thought of as
the mind’s “basic ingredients.” Rather than
presuming that these ingredients function
in a modular, mechanistic way, each oper-
ation can be thought of as a set of “func-
tional motifs” arising from the structural
motif that undergirds each network (e.g.,
Sporns & Kotter, 2004). Moreover, if these
operations serve as the functional architec-
ture for how mental events and behaviors
are constructed, then this implies that the
science of emotion should focus on mod-
eling emotions as high dimensional brain
states (reflecting the engagement of domain-
general networks, their internal operations,
and their interactions). Such a componen-
tial, constructionist functional architecture
of the human brain would not only reveal
the distinctions between social, affect, and
cognitive neuroscience to be artificial (Bar-
rett & Satpute, 2013), but it would also pres-
ent a set of hypotheses for how the phenom-
ena that we refer to as emotion and emotion
regulation are derived within a common
mechanistic framework.
Emotion Regulation as Changing
Situated Conceptualizations
To the extent that emotions are situated
conceptualizations grounded in the modal
systems of the brain, then shifting from
one situated conceptualization to another
intensifies, diminishes, or alters the auto-
nomic and endocrine responses that underlie
actions and feelings. We propose that a situ-
ated conceptualization framework offers an
account of emotion regulation that under-
girds the process model (Gross, this volume)
at a different level of analysis, which has the
potential to inspire new scientific research
and practical applications. Our hypoth-
esis is that stages of emotion regulation
are often describing the difference between
two consecutive situated conceptualizations
rather than individual processes that can be
chained together by a series of linear causal
linkages in which cognitive systems in the
brain modulate separate and anatomically
distinct affective or emotional systems.
Emotion regulation strategies describe the
changes from one mental state to another,
but these changes can themselves be decom-
posed into more basic facets (i.e., the men-
tal operations and their associated networks
that create the situated conceptualizations).
As a consequence, the process model (Gross,
this volume) can be thought of as offering
a more abstract description of what occurs
during emotion regulation, whereas the core
systems that implement situated conceptu-
alizations are a more mechanistic approach
that produces reappraisals, distraction, sup-
pression, and other instances of emotion
regulation.
This distinction between levels of analy-
sis maps onto the three levels of analysis
described by Marr (1982), which include: a
computational level that describes the phe-
nomenon at hand (What problem does the
system try to solve? What is the goal when
transforming input to output?), an algorith-
mic level that describes how the transforma-
tion from input to output is achieved (How
does the system do what it does? What are
the representations used by the system? What
processes act on these representations?) and
an implementation or physical level (How
is the system physically realized?). Our
hypothesis is that the process model, with
its emphasis on situation selection, situa-
tion modification, and so on, describes emo-
tion regulation at Marr’s computational
level, whereas our situated conceptualiza-
tion account describes emotion regulation
at Marr’s algorithmic and implementational
levels. Each class of regulatory strategies
discussed within the process model of emo-
tion regulation can be understood as situ-
ated conceptualizations that are constructed
from more basic domain- general core sys-
tems. The componential, constructionist
functional architecture of the human brain
for emotion is also the architecture that cre-
ates instances of emotion regulation.
Situation selection can be understood as a
case in which situated conceptualizations are
constructed to anticipate what will happen in
the future. Elsewhere, Barrett and colleagues
(2009; Barrett & Satpute, 2013; Lindquist &
Barrett, 2012; Lindquist et al., 2012) have
hypothesized that the nodes within the “men-
Emotion Regulation as Situated Conceptualizations 457
talizing” network (e.g., Andrews- Hanna et
al., 2010) interact to help guide the construc-
tion of situated conceptualizations by inte-
grating elements of prior experience (which
are represented modally across the brain).
These regions help to produce the multi-
modal simulations that are strongly situated
in a particular background context, mak-
ing sensory input meaningful and support-
ing specific courses of situated action. Using
prior knowledge to simulate possible future
situations may guide the decision making
that underlies situation selection. More spe-
cifically, situated conceptualizations support
simulating what it would be like to experi-
ence specific situations (by reenacting and
reassembling prior knowledge) that produce
information about their value and potential
outcomes (e.g., deciding whether to walk in
a meadow or a forest depending on the prob-
ability of encountering a bee). This hypoth-
esis is consistent with the idea that the “men-
talizing” network constructs mental models
or simulations that facilitate future behavior
(Buckner, 2011).
Because situated conceptualizations are
dynamically constructed when thinking
about future events, their dynamic assembly
is likely influenced by a number of factors,
including the current state of the individual
(and the situated conceptualization that is
being used to interpret this state) and the
executive control resources available (e.g.,
via the “salience” and “frontoparietal con-
trol” networks) to help guide the situated
conceptualization. These factors can influ-
ence elements of the situation that become
the focus of the simulation and how detailed
or vague the simulation becomes, both of
which would impact the inferred outcomes
and thus the decision making that underlies
situation selection. The complexity inherent
to situation selection is often acknowledged
in the literature on emotion regulation (e.g.,
Gross, 2008), but the dynamics involved in
creating such complexity remain unclear,
perhaps because these discussions tend
draw on traditional approaches to emotion
that overlook such dynamics. In contrast,
our approach highlights investigating the
dynamic integration of multimodal facets
involved in situated conceptualization as an
important goal for future research.
The predictive and inferential capacities
provided by situated conceptualizations also
allow for situation modification (i.e., they
provide a perceiver with the ability to pre-
dict what actions in the present will facili-
tate a change in mental state in the future;
for example, using a branch as a weapon
to swat a bee). A situated conceptualiza-
tion approach draws attention to the under-
lying processes that facilitate or detract
from taking actions to modify the external
environment to alter its emotional impact.
Because a situation is already in place dur-
ing situation modification, an individual is
drawing on prior knowledge about specific
facets present in the situated conceptual-
ization to modify the situation itself. For
example, when the individual focuses on
a nearby tree branch with the goal of kill-
ing the bee to avoid being stung, he or she
now infers that the branch could be used a
weapon because it can be manipulated much
like a baseball bat or a fly swatter (i.e., prior
knowledge about using a bat to strike an
object is being dynamically applied within
the situated conceptualization). In this way,
the situated conceptualization used to inter-
pret the environment is shifting dynamically
as its multimodal facets change (e.g., height-
ened arousal and hyperfocus on the environ-
ment), which guides action.
Cognitive change (e.g., a stinging bee
transformed into a flower- loving honey pro-
ducer) and response modulation (e.g., to keep
walking forward rather than to run away)
naturally unfold as the brain shifts from
one situated conceptualization to another,
making predictions about how to act (i.e., a
predicted action) and what the homeostatic
and metabolic consequences will be (i.e.,
affective changes). Cognitive framing and
response modulation are perhaps two of the
most obvious goals of the brain’s functional
architecture— knowing what the current sen-
sory array means and how to act on it. They
are not unique to emotion regulation; they
describe what happens during the construc-
tion of every mental state. During emotion
regulation, though, an individual is often
more aware of these changes because he or
she has an explicit goal to regulate through
cognitive change or response modulation.
An important prediction of a situated con-
ceptualization approach is that these types
of changes can also occur without aware-
ness if they become habitual. Bringing situ-
ated conceptualizations into awareness and
458 PSYCHOPATHOLOGY
manipulating them with effort, intention,
and a feeling of agency appears to be one key
characteristic that distinguishes the mental
events people refer to as “emotion regula-
tion” (vs. those they refer to as “emotion”).
If conceptual knowledge is enactive, and if
situated conceptualizations actually have the
capacity to shape the physiology and actions
that are observed in any mental state, then
changing a conceptualization via any core
system can modify said physiology and
action (as well as the feelings to which they
give rise). Such regulation might occur when
the same physical sensations and actions are
conceptualized as a different emotion (e.g.,
tears are not sadness but anger; a racing
heart is not fear but excitement), or when the
intensity of physical activation is enhanced
or reduced by changing the conceptualiza-
tion (e.g., a bee means the pain of a sting or
the tranquility of a meadow). In such cases,
our hypothesis is that emotion regulation
is the result of conceptual knowledge being
activated as part of a situated conceptualiza-
tion. At the level of subjective experience, it
may feel as if a special mechanism is being
used to down- regulate fear, such as reap-
praisal or suppression, or to up- regulate
anger. Our hypothesis is that these emo-
tion regulation constructs reflect changes in
emotions (as mental states) that result from
successive situated conceptualizations, using
the same operations that constitute an emo-
tion in the first place. Thus, an instruction
to reappraise, to distract by shifting atten-
tion, and so forth, actually manipulates the
underlying core systems of situated concep-
tualizations, which in turn alters the biologi-
cal signals that produce sensations, not just
how bodily sensations are understood (i.e., it
alters the experience of them).
A situated conceptualization framework
also suggests a novel hypothesis that is not
discussed within the process model: Decon-
structing an emotion, by attempting to undo
its situated conceptualization, is another
form of emotion regulation. When the per-
ception and experience of physical sensations
from the body are decoupled from the con-
cept knowledge (e.g., a deactivated unpleas-
ant feeling conceptualized more basically as
fatigue or glucose depletion as opposed to
sadness in a specific situation), they become
less potent and result in less suffering. This
suspension of conceptualization can be diffi-
cult to achieve, and it usually requires train-
ing. If you were to attempt to learn to paint
an image of a bee on a flower, you would
have to train yourself over a series of months
not to see objects (a bee and flower) but to
“undo” this perception and paint pieces of
light. Only by doing this can you render a
reasonable three- dimensional image on
a two- dimensional page. Similarly, when
learning to deconstruct emotion, you would
have to train yourself not to experience emo-
tions, but to experience physical sensations
instead. Meditation practices offer a variety
of tools for deconstructing emotion experi-
ence that may work in this manner (e.g., Hol-
zel et al., 2011; Papies, Barsalou, & Custers,
2012). In other meditation approaches, an
existing emotion is deconstructed and then
is replaced with an alternative, more positive
experience (Lutz, Brefczynski- Lewis, John-
stone, & Davidson, 2008; Lutz, Greischar,
Perlman, & Davidson, 2009).
Recent empirical evidence from our labo-
ratory also suggests that this may be a pro-
ductive strategy. During a neuroimaging
study (Wilson- Mendenhall et al., 2011),
participants immersed themselves in affec-
tively charged situations that involved either
physical danger or social evaluation. After
immersing in a situation, a word cued partic-
ipants to emote in the situation (experience
fear or anger) or to observe in the situation.
Significantly greater activity in visual cor-
tex and significantly less activity in medial
prefrontal cortex occurred when partici-
pants experienced situations by observing
the scenes from a third- person perspec-
tive rather than experiencing them as first-
person, self- relevant emotions. These results
suggest that sensations became the focus of
the affectively charged situation when par-
ticipants were observing in the situation. In
this study, observing was an explicit goal,
but a situated conceptualization approach
suggests that this process of observing could
eventually occur automatically (without
effort) if repeatedly used in specific situa-
tions (e.g., potential anger- inducing situa-
tions).
Finally, psychotherapy— particularly cognitive-
behavioral therapy (CBT) approaches—
might be understood as helping clients to
construct new situated conceptualizations
(thereby modifying their conceptual sys-
tem) that either reduce the intensity of their

Emotion Regulation as Situated Conceptualizations 459
physical responses, or better calibrate the
constructed meaning of those responses to
the situation at hand. CBT interventions
also appear to provide training for when to
use these alternative situated conceptualiza-
tions. The psychotherapeutic process might
be thought of as creating a new population
of learned neural assemblies (for the same
emotion categories, or for new categories)
that would be available to create new or dif-
ferent emotional meaning for the same sen-
sations, or that would modify those sensa-
tions (particularly those related to the body).
The emotional changes that occur with psy-
chotherapy, then, might result from changes
in the conceptual system, or how conceptual
knowledge is used to construct the situated
conceptualizations that are emotion.
Psychopathology
and Dysregulation from a Situated
Conceptualization Framework
From a situated conceptualization frame-
work, psychopathology would result from
two classes of problems. First, we hypoth-
esize that emotional deficits within certain
types of psychopathology (e.g., schizophre-
nia, autism spectrum disorders), and neuro-
degenerative diseases (e.g., frontotemporal
dementia) arise due to damage to the brain’s
structural architecture for situated concep-
tualizations, making it difficult to construct
meaning for sensory inputs that constitute
normal mental states. Perhaps deficits in
white matter connectivity, such as those
seen in autism (Zikopoulous & Barbas,
2010), compromise the ability to construct
and use the distributed conceptual structure
that underlies situated conceptualizations.
Similarly, autism is related to deficits in con-
nectivity that develop during the final stages
of cortical development in paralimbic areas,
particularly within the supragranular layers
of cortext (Zikopoulous & Barbas, 2010).
These layers mainly contain the corticocor-
tical connections that are important for syn-
chronizing the distributed neuronal assem-
blies responsible for constructing normal
situated conceptualizations.
In addition to structural consider-
ations, psychopathology could result from
entrenched conceptualizations that are overly
ritualized and not sufficiently situation-
specific. Deficits in the vocabulary or con-
tent of emotion concepts (perhaps due to
poor socialization), or problems in accessing
and using this knowledge (perhaps associ-
ated with problems with long-term memory
or executive function) might result in a fail-
ure to regulate autonomic (and therefore
affective) reactivity using situated concep-
tualizations, as in alexithymia (for a dis-
cussion, see Lindquist & Barrett, 2008), or
might produce inappropriate or ritualized
situated conceptualizations. To the extent
that repeated processing of a situated con-
ceptualization omits situational details and
focuses instead on general abstract themes,
chronic emotional responses develop that
operate inappropriately across too many sit-
uations. For example, imagine that a situated
conceptualization develops for shame asso-
ciated with performing poorly on math tests
during elementary school. Subsequently, if
attention focuses too much on shame associ-
ated with poor performance and omits the
situational details associated with elemen-
tary school math classes, a decontextualized
shame for poor intellectual performance
could develop that pervades experience
inappropriately. Psychopathology also arise
from problems in forming situated concep-
tualizations that are not well calibrated to
the immediate situation, resulting in dys-
regulated autonomic responses and less
effective actions. To the extent that situated
conceptualizations are learned assemblies of
perceptual, conceptual, interoceptive, and
action processes, psychological disorders of
one type or another might appear to be dis-
orders of specific emotions (e.g., posttrau-
matic stress disorder [PTSD] as a disorder
of fear) because a person’s learning history
has created a “conceptual habit” or regular-
ity of certain situated conceptualizations,
resulting in a sort of entrenchment of cer-
tain changes in bodily state, central repre-
sentations of that state, and meanings that
emerge regardless of the immediate situation
(e.g., even when no actual threat is present).
More generally, psychopathology might
also occur when sensations from the body
are overly personalized and inaccurately
construed to be self- evaluative as a func-
tion of how situated conceptualizations are
constructed. Even an overreliance on such
conceptual knowledge (e.g., conceptualizing
interoceptive cues as psychological instead of
460 PSYCHOPATHOLOGY
physical) would also result in psychopathol-
ogy, and might help explain sex differences
in certain disorders such as depression and
anxiety. Problems might also arise when sit-
uated conceptualizations are too internally
driven (from the body and prior experience)
and insufficiently incorporate external sen-
sory input, as in depression. For example,
the subgenual portion of the anterior cin-
gulate cortex (Brodmann Area 25), which
is an important cortical site for regulating
homeostasis and other autonomic functions
(Ongur, Ferry, & Price, 2003), appears to
keep the mentalizing network in a state of
continual engagement via its connections
to BA 10 in ventromedial prefrontal cortex;
this region is part of the mentalizing net-
work core (Andrews- Hanna et al., 2010) and
is a hub in a range of phenomena, including
homeostatic- and allostatic- related phenom-
ena (e.g., subjective emotional experiences,
autonomic and neuroendocrine function,
reward, pain), social cognition and self-
relevance, and memory- related phenomena
(e.g., memory, mind- wandering, etc.) (Roy,
Shohamy, & Wager, 2012). It is a region
that allows brainstem autonomic function
to be guided by conceptual information
about past, present and future outcomes,
and is important to the creation of affec-
tive meaning (Roy et al., 2012; Lindquist et
al., 2012). This connection between BA 25
and BA 10 keeps depressed people locked
“inside their own heads” and suffering a
constant state of anguish that is marked
by all consuming self-focus that paralyzes
them. Deep brain stimulation to the white
matter tract that connect these two regions
in effect releases the mentalizing network,
allowing people to experience relief and to
focus more on events in the world (May-
berg, personal communication; also see
Holtzheimer, Kelly, Gorss, Filkowski, Gar-
low, et al., 2012; Lujan, Chaturvedi, Choi,
Holdzheimer, Gross, et al., 2013). From
this research, it is easy to hypothesize that
overly internally focused situated concep-
tualizations might occur because a person
possesses a very reactive autonomic nervous
system, producing frequent and intense
internal sensations that demand conceptu-
alization, or because of limited executive
control resources. Moreover, conceptualiza-
tions that are not well- tailored to the situa-
tion in terms of sociocultural norms (which
could happen in a variety of ways) could
produce actions that are not effective in a
particular cultural context.
To understand how the content of disor-
dered situated conceptualizations emerge, it
is important to focus on the mechanisms of
the core systems or “ingredients” that cre-
ate instances of emotion or implement the
moment- to- moment changes that are expe-
rienced as emotion regulation, such as an
overly active autonomic nervous system that
is experienced as affective reactivity, and
other related problems with attention, work-
ing memory, and context insensitivity (e.g.,
Kring & Moran, 2008; Poch & Campo,
2012; Williamson & Allman, 2012). These,
in turn, can be understood in terms of the
dynamics and structure of core networks,
such as the “salience” network, the “fronto-
parietal control” network, and the “mental-
izing” network (Barrett & Satpute, 2013).
Indeed, these networks, which are intrin-
sic to the human brain and are structured
by anatomical connectivity (and which we
understand as the core systems that imple-
ment situated conceptualizations; Barrett
& Satpute, 2013), are implicated in a range
of psychopathologies, including not only
schizophrenia, autism, and frontotemporal
dementia, but also depression and anxi-
ety disorders (Menon, 2011). For example,
instead of understanding PTSD as an exag-
gerated activation of fear circuitry, it is pos-
sible that PTSD symptoms are the result of
hyperaffective reactivity (associated with the
“salience” network) combined with prob-
lems in conceptualization (associated with
the “mentalizing” network) related to work-
ing memory deficits (associated with the
“frontoparietal” control network) (Suvak &
Barrett, 2011).
These ideas present an alternative to the
traditional approach to mental illness, in
which all forms of psychopathology (and
many forms of physical illness, e.g., cardio-
vascular disease and cancer) are conceived
of as involving either excessive or deficient
amounts of one emotion or another. For
example, various anxiety disorders, such as
PTSD and panic disorder, are presumed to
be disorders of fear, thought to arise from
a hyperreactivity of fear processing. Depres-
sion is presumed to be a disorder of sadness
and guilt. Hypertension is thought to involve
an excess amount of anger. And so on. From

Emotion Regulation as Situated Conceptualizations 461
the perspective of the traditional machine
metaphor, each type of illness would arise
from problems with emotions being trig-
gered too frequently or not enough. Also, the
development and maintenance of psychiatric
disorders involve problems in emotion regu-
lation (Kring & Sloan, 2010), so psychopa-
thology might also arise from an inability
to regulate said emotions once they erupt.
Our situated conceptualization approach
connects these insights with transdiagnos-
tic approaches that attempt to identify psy-
chological and biological processes that are
common to many types of psychological
disorders (e.g., Fairholme, Boisseau, Ellard,
Ehrenreich, & Barlow, 2010; Harvey, Wat-
kins, Mansell, & Shafran, 2004; Haslam,
2002; Kendler, 2008; Kring, 2008; Krueger,
Watson, & Barlow, 2005; Millan, 2003;
Sanislow et al., 2010). Just as science is com-
ing to the conclusion that emotion categories
are not natural kinds (Barrett, 2006), clini-
cal science is also coming to the conclusion
that categories for disorders of emotion do
not cut nature at its joints (Haslam, 2002;
Kendler, 2008).
Summary
Situated conceptualizations can be thought
of as cognitive tools used by the human
brain to modify and regulate the body (i.e.,
homeostasis and allostasis, metabolism,
and/or inflammatory processes), to create
feelings, and to create dispositions toward
action. In this sense, they provide an alter-
native framework for describing how mental
states arise, and how actions and feelings,
and the physiological changes that support
them, are formulated and regulated. A focus
on how situated conceptualizations are con-
structed from patterns (or functional motifs)
across the brain’s core systems (or structural
motifs) adds utility to existing explana-
tory frameworks for emotion and emotion
regulation by focusing on the mechanistic
changes that produce emotional and regula-
tory phenomena to which we give abstract
names.
Perhaps the final lesson of the bee exam-
ple is that states and processes are easy to
confuse when it comes to meaning making.
Regardless of whether you automatically
experience the calm of a bee buzzing in a
bucolic garden or the terror of a bee attack-
ing and stinging the body, it is possible to
retrieve different associations of bees in the
next instance, which in turn has the capac-
ity to change the sensations that your brain
receives from your body. Our hypothesis is
that the same processes that were engaged
during the initial instance of meaning mak-
ing (creating tranquility or fear) are engaged
again, and again, and again. When your
bodily response changes, along with the cor-
responding feelings and actions, you experi-
ence this as emotion regulation. If this is cor-
rect, then what we call “emotion regulation”
is grounded in the more basic meaning-
making processes that are operating all the
time to create the flow of mental states that
constitutes your mind. Reappraisal, distrac-
tion, and other terms might refer not to
processes per se, but to changes that occur
as one mental state flows into another (and
one physical state transitions to another)
as meaning changes. A series of sequential
mental states that are experientially distinct
is easy to understand as distinct psychologi-
cal processes, even though scientists have
known for a long time that experiences don’t
reveal the processes that make them.
Notes
1. Hereafter in this chapter, we use italics to
indicate a concept (e.g., fear) and quotes to
indicate the word or phrase associated with it
(e.g., “fear”).
2. Theory and research strongly suggest that
concepts do not have conceptual cores (i.e.,
information that is necessary and sufficient
for membership in the associated category).
Instead, concepts are represented with loose
collections of situated exemplars that are
related by family resemblance. Exemplar
theories of categorization further illustrate
that loose collections of memories for cat-
egory members can produce sophisticated
classification behavior, demonstrating that
abstractions for prototypes and rules are not
necessary. Neural net systems similarly dem-
onstrate that only loose statistical coherence
is necessary for sophisticated categorization.
To the extent that abstraction does occur for
a category, it may only occur partially across
small sets of category instances, reflect the
abstraction of nondefining properties and

462 PSYCHOPATHOLOGY
relations that can be used to describe category
members in a dynamical manner, or reflect
an online abstraction at retrieval rather than
stored abstractions in memory. Nevertheless,
people often believe mistakenly that catego-
ries do have cores, perhaps because a word
can lead people to essentialize.
3. As goal- directed categories that develop to
guide action, the most typical member of a
category such as fear is not the one that is most
frequently encountered, but rather the one
that maximally achieves the theme or goal of
the category (Barsalou, 2003). As a result, the
most typical instances of a category contain
properties that represent the ideal form of the
category, that is, whatever is ideal for meeting
the goal around which the category is orga-
nized, not those that most commonly appear
as instances of the category. From a situated
conceptualization viewpoint, prototypes do
not exist as stored representations in memory,
but they can be constructed (or simulated)
when needed (Barsalou et al., 2003).
4. Highly different instances for the same cat-
egory can become integrated over time, and
become available to construct novel simula-
tions that have never been experienced before.
This, in part, may help to explain why people
believe that emotions such as anger, sadness,
fear, and so on, have specific response signa-
tures, even though the available data do not
support this view. A simulation of fear could
allow a person to go beyond the information
given to fill in aspects of a internal sensa-
tion that are not present at a given percep-
tual instance. In such a case, the simulation
essentially produces an illusory correlation
between response outputs, helping to explain
why researchers continue to search for coordi-
nated autonomic, behavioral, and experiential
aspects of a fear response.
References
Anderson, E., Siegel, E. H., White, D., & Bar-
rett, L. F. (2012). Out of sight but not out of
mind: Unseen affective faces influence evalu-
ations and social impression. Emotion, 12,
1210–1221.
Andrews- Hanna, J. R., Reidler, J. S., Sepulcre, J.,
Poulin, R., & Buckner, R. L. (2010). Functional–
anatomic fractionation of the brain’s default
network. Neuron, 65(4), 550–562.
Barrett, L. F. (2006). Solving the emotion para-
dox: Categorization and the experience of
emotion. Personality and Social Psychology
Review, 10(1), 20 – 46.
Barrett, L. F. (2009). The future of psychology:
Connecting mind to brain. Perspectives on
Psycholical Science, 4(4), 326–339.
Barrett, L. F. (2012). Emotions are real. Emo-
tion, 12(3), 413–429.
Barrett, L. F., & Bar, M. (2009). See it with feel-
ing: Affective predictions in the human brain.
Philosphical Transactions of the Royal Soci-
ety B: Biological Sciences, 364, 1325–1334.
Barrett, L. F., & Bliss- Moreau, E. (2009). Affect
as a psychological primitive. Advances in
Experimental Social Psychology, 41, 167–218.
Barrett, L. F., & Gross, J. J. (2001). Emotion rep-
resentation and regulation: A process model
of emotional intelligence. In T. Mayne & G.
Bonnano (Eds.), Emotion: Current issues and
future directions (pp. 286–310). New York:
Guilford Press.
Barrett, L. F., & Lindquist, K. (2008). The
embodiment of emotion. In G. Semin & E.
Smith (Eds.), Embodied grounding: Social,
cognitive, affective, and neuroscience
approaches (pp. 237–262). New York: Cam-
bridge University Press.
Barrett, L. F., Lindquist, K. A., & Gendron, M.
(2007). Language as context for the percep-
tion of emotion. Trends in Cognitive Sciences,
11(8), 327–332.
Barrett, L. F., Ochsner, K. N., & Gross, J. J.
(2007). On the automaticity of emotion. In J.
Bargh (Ed.), Social psychology and the uncon-
scious: The automaticity of higher mental
processes (pp. 173–217). New York: Psychol-
ogy Press.
Barrett, L. F., & Satpute, A. (2013). Large-scale
brain networks in affective and social neuro-
science: Towards an integrative architecture of
the human brain. Current Opinion in Neuro-
biology, 23, 1–12.
Barrett, L. F., Tugade, M. M., & Engle, R. W.
(2004). Individual differences in working
memory capacity and dual- process theories
of the mind. Psychological Bulletin, 130(4),
553–573.
Barsalou, L. W. (1999). Perceptual symbol sys-
tems. Behavioral and Brain Sciences, 22(4),
577–609.
Barsalou, L. W. (2003). Situated simulation in
the human conceptual system. Language and
Cognitive Processes, 18, 513–562.
Barsalou, L. W. (2008). Grounded cognition.
Annual Review of Psychology, 59, 617–645.
Emotion Regulation as Situated Conceptualizations 463
Barsalou, L. W. (2009). Simulation, situated con-
ceptualization, and prediction. Philosophical
Transactions of the Royal Society B: Biologi-
cal Sciences, 364(1521), 1281–1289.
Barsalou, L. W., & Hale, C. R. (1993). Compo-
nents of coceptual representation: From fea-
ture lists to recursive frames. In J. Hampton,
R. Michalski, & P. Theuns (Eds.), Categories
and concepts: Theoretical views and inductive
data analysis (pp. 97–144). San Diego, CA:
Academic Press.
Barsalou, L. W., Niedenthal, P. M., Barbey, A.,
& Ruppert, J. (2003). Social embodiment. In
B. Ross (Ed.), The psychology of learning and
motivation (Vol. 43, pp. 43–92). San Diego,
CA: Academic Press.
Bradley, M. M., Codispoti, M., Cuthbert, B. N.,
& Lang, P. J. (2001). Emotion and motivation
I: Defensive and appetitive reactions in picture
processing. Emotion, 1(3), 276–298.
Buckner, R. L. (2011). The serendipitous discov-
ery of the brain’s default network. NeuroIm-
age, 62, 1137–1145.
Carey, S. (2009). The origin of concepts. New
York: Oxford University Press.
Clore, G. L., & Ortony, A. (2008). Appraisal
theories: How cognition shapes affect into
emotion. In M. Lewis, J. M. Haviland- Jones,
& L. F. Barrett (Eds.), Handbook of emotions
(3rd ed., pp. 628–642). New York: Guilford
Press.
Dewar, K. M., & Xu, F. (2009). Do early nouns
refer to kinds or distinct shapes?: Evidence
from 10-month old infants. Psychological Sci-
ence, 20, 252–257.
Edelman, G. M. (1998). The remembered pres-
ent: A biological theory of consciousness.
New York: Basic Books.
Fairholme, C. P., Boisseau, C. L., Ellard, K. K.,
Ehrenreich, J. T., & Barlow, D. H. (2010).
Emotions, emotion regulation, and psycholog-
ical treatment: A unified perspective. In A. M.
Kring & D. M. Sloan (Eds.), Emotion regu-
lation and psychopathology (pp. 283–309).
New York: Guilford Press.
Fine, I., Wade, A. R., Brewer, A. A., May, M.
G., Goodman, D. F., Boynton, G. M., et al.
(2003). Long-term deprivation affects visual
perception and cortex. Nature Neuroscience,
6, 915–916.
Golland, Y., Golland, P., Bentin, S., & Malach,
R. (2008). Data- driven clustering reveals a
fundamental subdivision of the human cortex
into two global systems. Neuropsychologia,
46(2), 540–553.
Gross, J. J. (2008). Emotion regulation. In M.
Lewis, J. M. Haviland- Jones, & L. F. Bar-
rett (Eds.), Handbook of emotions (3rd ed.,
pp. 497–512). New York: Guilford Press.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Harvey, A., Watkins, E., Mansell, W., & Shafran,
R. (2004). Cognitive behavioural processes
across psychological disorders: A transdiag-
nostic approach to research and treatment.
New York: Oxford Univeristy Press.
Haslam, N. (2002). Kinds of kinds: A conceptual
taxonomy of psychiatric categories. Philoso-
phy, Psychiatry, and Psychology, 9, 203–217.
Holzel, B. K., Lazar, S. W., Gard, T., Schuman-
Oliver, Z., Vago, D. R., & Ott, U. (2011).
How does mindful meditation work?: Propos-
ing mechanisms of action from a conceptual
and neural perspective. Perspectives on Psy-
chological Science, 6(6), 537–559.
Holtzheimer, P. E., Kelley, M. E., Gross, R. E.,
Filkowski, M. M., Garlow, S. J., Barrocas, A.,
et al. (2012). Subcallosal cingulate deep brain
stimulation for treatment- resistant unipolar
and bipolar depression. Archives of General
Psychiatry, 69(2),150–158.
Jones, C. R., Kirkland, T., & Cunningham, W.
A. (this volume, 2014). Attitudes, evaluation,
and emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 251–266). New York: Guilford Press.
Kendler, K. S. (2008). Explanatory models for
psychiatric illness. American Journal of Psy-
chiatry, 165(6), 695–702.
Kober, H., Barrett, L. F., Joseph, J., Bliss-
Moreau, E., Lindquist, K., & Wager, T. D.
(2008). Functional grouping and cortical–
subcortical interactions in emotion: A meta-
analysis of neuroimaging studies. NeuroIm-
age, 42(2), 998–1031.
Kring, A. M. (2008). Emotion disturbances as
transdiagnostic processes in psychopathology.
In M. Lewis, J. M. Haviland- Jones, & L. F.
Barrett (Eds.), Handbook of emotion (3rd ed.,
pp. 691–705). New York: Guilford Press.
Kring, A. M., & Moran, E. K. (2008). Emotional
response deficits in schizophrenia: Insights
from affective science. Schizophrenia Bulletin,
34(5), 819–834.
Kring, A. M., & Sloan, D. M. (Eds.). (2010).
Emotion regulation and psychopathology.
New York: Guilford Press.
464 PSYCHOPATHOLOGY
Krueger, R. F., Watson, D., & Barlow, D. H.
(2005). Introduction to the special section:
Toward a dimensionally based taxonomy of
psychopathology. Journal of Abnormal Psy-
chology, 114(4), 491–493.
Lang, P. J., Bradley, M. M., & Curthbert, B. N.
(2008). International affective picture sys-
tem (IAPS): Affective ratings of pictures and
instruction manual. Gainsville: University of
Florida.
Legrand, D., & Ruby, P. (2009). What is self-
specific?: Theoretical investigation and critical
review of neuroimaging results. Psychological
Review, 116(1), 252–282.
Lindquist, K., & Barrett, L. F. (2008). Emotional
complexity. In M. Lewis, J. M. Haviland- Jones
& L. F. Barrett (Eds.), Handbook of emotion
(pp. 513–530). New York: Guilford Press.
Lindquist, K. A., & Barrett, L. F. (2012). A
functional architecture of the human brain:
Insights from the science of emotion. Trends
in Cognitive Sciences, 16, 533–540.
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-
Moreau, E., & Barrett, L. F. (2012). The brain
basis of emotion: A meta- analytic review.
Behavioral and Brain Sciences, 35(3), 121–
143.
Lujan, J. L., Chaturvedi, A., Choi, K. S., Hold-
zheimer, P. E., Gross, R. E., Mayberg, H. S.,
et al. (2013). Tractography– activation models
applied to subcallosal cingulate deep brain
stimulation. Brain Stimulation.
Lutz, A., Brefczynski- Lewis, J., Johnstone, T., &
Davidson, R. J. (2008). Regulation of the neu-
ral circuitry of emotion by compassion medi-
tation: Effects of meditative expertise. PLoS
ONE, 3(3), e1897.
Lutz, A., Greischar, L. L., Perlman, D. M., &
Davidson, R. J. (2009). BOLD signal in insula
is differentially related to cardiac function
during compassion meditation in experts vs.
novices. NeuroImage, 47(3), 1038–1046.
Martin, A. (2001). Functional neuroimaging of
semantic memory. In R. Cabeza & A. King-
stone (Eds.), Handbook of functional neu-
roimaging of cognition (pp. 153–186). Cam-
bridge, MA: MIT Press.
Martin, A. (2007). The representation of object
concepts in the brain. Annual Review of Psy-
chology, 58, 25–45.
Marr, D. (1982). Vision: A computational inves-
tigation into the human representation and
processing of visual information. New York:
Freeman.
McClelland, J. L. (2010). Emergence in cogni-
tive science. Topics in Cognitive Science, 2,
751–770.
Menon, V. (2011). Large-scale brain networks
and psychopathology: A unifying triple net-
work model. Trends in Cognitive Sciences,
15(10), 483–506.
Mesquita, B., Barrett, L. F., & Smith, E. (Eds.).
(2010). The mind in context. New York: Guil-
ford Press.
Millan, M. J. (2003). The neurobiology and con-
trol of anxious states. Progress in Neurobiol-
ogy, 70(2), 83–244.
Murphy, G. L. (2002). The big book of concepts.
Cambridge, MA: MIT Press.
Neumeister, A., Henry, S., & Krystal, J. H.
(2007). Neural circuitry and neuroplasticity
in PTSD. In M. J. Friedman, T. M. Keane, &
P. A. Resick (Eds.), Handbook of PTSD: Sci-
ence and practice (pp. 151–165). New York:
Guilford Press.
Northoff, G., Heinzel, A., de Greck, M., Berm-
pohl, F., Dobrowolny, H., & Panksepp, J.
(2006). Self- referential processing in our
brain—A meta- analysis of imaging studies on
the self. NeuroImage, 31(1), 4 40 – 457.
Ongur, D., Ferry, A. T., & Price, J. L. (2003).
Architectonic subdivision of the human orbital
and medial prefrontal cortex. Journal of Com-
parative Neurology, 460, 425–449.
Papies, E. K., Barsalou, L. W., & Custers, R.
(2012). Mindful attention prevents mindless
impulses. Social Psychological and Personal-
ity Science 3, 291–299.
Poch, C., & Campo, P. (2012). Neocortical–
hippocampal dynamics of working memory
in healthy and diseased brain states based on
functional connectivity. Frontiers in Human
Neuroscience, 6, 36.
Rips, L. J. (2010). Lines of thought. New York:
Oxford University Press.
Roy, M., Shohamy, D., Wager, T. D. (2012). Ven-
tromedial prefrontal– subcortical systems and
the generation of affective meaning. Trends in
Cognitive Science, 16, 147–156.
Russell, J. A. (2003). Core affect and the psycho-
logical construction of emotion. Psychological
Review, 110, 145–172.
Salzman, C. D., & Fusi, S. (2010). Emotion,
cognition, and mental state representation
in amygdala and prefrontal cortex. Annual
Review of Neuroscience, 33, 173–202.
Sanislow, C. A., Pine, D. S., Quinn, K. J., Kozak,
M. J., Garvey, M. A., Heinssen, R. K., et al.

Emotion Regulation as Situated Conceptualizations 465
(2010). Developing constructs for psychopa-
thology research: research domain criteria.
Journal of Abnormal Psychology, 119(4),
631–639.
Schell, T. L., Marshall, G. N., & Jaycox, L. H.
(2004). All symptoms are not created equal:
The prominent role of hyperarousal in the
course of posttraumatic psychological dis-
tress. Journal of Abnormal Psychology,
113(2), 189–197.
Schyns, P. G., Goldstone, R. L., & Thibaut, J. P.
(1998). The development of features in object
concepts. Behavioral and Brain Sciences,
21(1), 1–17.
Simmons, W. K., & Barsalou, L. W. (2003). The
similarity- in- topography principle: Reconcil-
ing theories of conceptual deficits. Cognitive
Neuropsychology, 20(3), 451–486.
Simmons, W. K., Reddish, M., Bellgowan, P. S.,
& Martin, A. (2010). The selectivity and func-
tional connectivity of the anterior temporal
lobes. Cerebral Cortex, 20(4), 813–825.
Sporns, O., & Kotter, R. (2004). Motifs in brain
networks. PLoS Biology, 2, e369.
Sporns, O., Tononi, G., & Edelman, G. M.
(2000a). Connectivity and complexity: The
relationship between neuroanatomy and brain
dynamics. Neural Networks, 13(8–9), 909–
922.
Sporns, O., Tononi, G., & Edelman, G. M.
(2000b). Theoretical neuroanatomy: Relat-
ing anatomical and functional connectivity
in graphs and cortical connection matrices.
Cerebral Cortex, 10(2), 127–141.
Suvak, M. K., & Barrett, L. F. (2011). Consider-
ing PTSD from the perspective of brain pro-
cesses: A psychological construction approach.
Journal of Traumatic Stress, 24(1), 3–24.
Van Overwalle, F. (2009). Social cognition and
the brain: A meta- analysis. Human Brain
Mapping, 30(3), 829–858.
Williamson, P. C., & Allman, J. M. (2012). A
framework for interpreting functional net-
works in schizophrenia. Frontiers in Human
Neuroscience, 6, 184.
Wilson- Mendenhall, C. D., Barrett, L. F., &
Barsalou, L. W. (in preparation). Emoting vs.
observing: Conceptualizing affective situa-
tions as self- relevant emotions or sensory-
focused mental states.
Wilson- Mendenhall, C. D., Barrett, L. F., Sim-
mons, W. K., & Barsalou, L. W. (2011).
Grounding emotion in situated conceptualiza-
tion. Neuropsychologia, 49(5), 1105–1127.
Wundt, W. (1897). Outlines of psychology (C. H.
Judd, Trans.). Leipzig: Wilhelm Engelmann.
Zikopoulous, B., & Barbas, H. (2010). Changes
in prefrontal axons may disrupt the network
in autism. Journal of Neuroscience, 30,
14595–14609.

PART VIII
INTERVENTIONS

469
Over the past 25 years, considerable prog-
ress has been achieved in treating adult
psychiatric disorders (e.g., Butler, Chap-
man, Forman, & Beck, 2006). Despite these
significant advances, a sizable subgroup of
individuals with commonly co- occurring
disorders such as generalized anxiety dis-
order (GAD) and major depressive disorder
(MDD) fail to make sufficient treatment
gains, thereby prolonging their deficits in life
functioning and satisfaction. For example, in
a meta- analysis of cognitive- behavioral ther-
apy (CBT) for GAD, Borkovec and Ruscio
(2001) found that only 50–60% of treated
individuals demonstrated clinically mean-
ingful change. Furthermore, although more
efficacious, the difference between current
psychological treatments for MDD and non-
directive supportive therapy reflects only
a small effect size (Cuijpers, van Straten,
Andersson, & van Oppen, 2008). Also,
among individuals who have GAD with
comorbid MDD, gains in treating depres-
sion are not especially durable (Newman,
Przeworski, Fisher, & Borkovec, 2010).
Finally, in the recently concluded Sequenced
Treatment Alternatives to Relieve Depres-
sion Study,funded by the National Institute
of Mental Health (NIMH), the subgroup of
individuals with mixed anxiety– depressive
disorder (e.g., MDD + apprehensive anxious
symptoms) was most treatment refractory
(Farabaugh et al., 2010).
One characteristic common to these dis-
orders, which are often comorbid (Kessler,
Berglund, Demler, Jin, & Walters, 2005), is
that they both strongly (more so than other
conditions) reflect heightened emotional
experience (often referred to as emotional-
ity, neuroticism, or emotional intensity; see
Barlow, 2002; Mennin, Heimberg, Turk,
& Fresco, 2005; Mennin, Holaway, Fresco,
Moore, & Heimberg, 2007). Factor- analytic
evidence demonstrates that these disorders
jointly reflected a higher order factor of
prolonged negative affect or distress that
has been distinguished from a “fear” factor
that relates to disorders more characterized
by acute arousal (e.g., Krueger & Markon,
2006; Watson, 2005). Furthermore, both
GAD and MDD possess heritability factors
reflective of increased emotionality (e.g.,
Kendler, Gardner, Gatz, & Pedersen, 2006).
One possibility is that the “distress” in these
disorders reflects an inherent dispositional
tendency for heightened emotional salience
related to motivational impetuses such as the
need to avoid threat. These underlying emo-
tional and motivational factors may be more
difficult to ameliorate and change (Brown,
2007). Indeed, these higher order emotional
factors, in particular, have been found to
CHAPTER 28
Emotion Regulation Therapy
Douglas S. Mennin
David M. Fresco
470 INTERVENTIONS
account for the underperformance of other-
wise efficacious treatments (Olatunji, Cisler,
& Tolin, 2010).
Repetitive or perseverative thought (Wat-
kins, 2008) represents another characteristic
common to the distress disorders that may
arise as a desperate means of coping or com-
pensating with strongly felt emotional expe-
riences. For instance, pathological worry
(Borkovec, Alcaine, & Behar, 2004) func-
tions as a regulatory strategy aimed at reduc-
ing distress that arises from conflicting emo-
tional and motivational states (e.g., Mennin
& Fresco, 2009; Newman & Llera, 2011).
Similarly, depressive rumination represents
a perseverative cognitive process commonly
defined as repetitive thinking about past mis-
takes and failures, and is strongly associated
with worry (e.g., Nolen- Hoeksema, Wisco,
& Lyubomirsky, 2008; Watkins, 2008). In
essence, individuals with distress disorders
may be more prone to utilize perseverative
cognitive strategies (e.g., worry, rumination)
to escape or dampen emotionality at the cost
of accurately gleaning the motivational mes-
sage that is being conveyed, undermining
immediate behavioral action in response to
their emotions, and ultimately, losing sight
of the enriching and fulfilling aspects of life.
Thus, given the emerging profile of this
difficult to treat, distress- disordered patient
(e.g., strong motivational impetuses accom-
panied by excessive reliance on compen-
satory strategies to escape or avoid these
impetuses reactively, and impoverished
contextual learning repertoires), there is
increasing interest in understanding the
role of emotions and how this knowledge
might generate new targets for intervention,
particularly for more treatment- resistant
cases. The affective science field provides an
opportunity to expand paradigms regard-
ing the role of emotion- related processes in
conceptualizing and treating psychopathol-
ogy. Increasingly, treatments have adopted a
functional approach to emotions to improve
existing interventions a variety of disorders
(e.g., Barlow et al., 2011; Linehan, 1993).
Articulating a model of distress disorders
predicated on underlying emotional and
motivational mechanisms and processes
aligns with two recent initiatives promoted
by the NIMH with the goal of accelerat-
ing the payoff from basic and translational
research into treatment application and
identifying factors that promote or dimin-
ish the effectiveness of evidence- based treat-
ments. The first of these, called the Research
Domain Criteria Initiative (RDoC; Craske,
2012), aims to understand what is expected
in these domains at different levels of
inquiry, so that these normative findings can
be contrasted with disordered subgroups to
identify mechanistic regions of interest that
may in turn become the targets of treatment
development. The second initiative comes
from a recent NIMH’s National Advisory
Mental Health Council report emphasizing
“treatment personalization” as a means to
identify factors that predict who will ben-
efit from a given intervention process, then
to determine systematically ways of match-
ing patients to a particular treatment, as
well as to optimize and augment care. Taken
together, these initiatives highlight the need
to specify transdiagnostic mechanisms
within individuals to further the develop-
ment of treatment processes that specifically
target these mechanisms.
Congruent with current directions in
affect science, these NIMH initiatives, and
advances in the science of behavior therapy,
we have developed an emotion regulation
therapy (ERT; Mennin & Fresco, 2009)
to improve treatment of distress disorders
including GAD and MDD. ERT integrates
traditional and contemporary CBT principles
and practices, and emotion- focused inter-
ventions within a framework that reflects
basic and translational findings in affect sci-
ence. The result is a theoretically derived,
evidence- based treatment that builds on the
solid foundation of CBT by identifying and
targeting putative mechanisms common to
the distress disorders (e.g., motivational–
emotional activation, perseverative think-
ing, and resultant narrowed learning reper-
toires) while striving to normalize emotion
generation and regulation functioning. In
the remainder of this chapter, we (1) describe
and review relevant work related to norma-
tive and disordered motivation, regulation,
and contextual learning consequences; (2)
demonstrate how ERT reflects an approach
to treatment that draws from common prin-
ciples of CBT and affective science to target
proposed mechanisms directly and provide
preliminary efficacy and mechanism find-
ings; and (3) provide an overview of future
directions for ERT.

Emotion Regulation Therapy 471
Normative and Disordered
Emotional Processing
and Learning Consequences
An affect science perspective on adult psy-
chopathology and its treatment includes (1)
motivational mechanisms, reflecting the
functional and directional properties of an
emotional response tendency; (2) regula-
tory mechanisms, reflecting the alteration of
response trajectories to be more congruent
with contextual demands and constraints,
as well as one’s personal values or goals;
and (3) contextual learning consequences,
reflecting, optimally, the promotion of
broad and flexible behavioral repertoires.
In distress disorders, dysfunction may occur
via strong motivational salience and con-
flict, as well as deficits in systems of regula-
tion, which may result in diminished, nar-
row, and rigid behavioral repertoires. In the
remainder of this section, we discuss both
a normative account of these characteristics
and how these normative processes may go
awry in the emotional dysfunction of dis-
tress disorders.
Normative Emotional Functioning
Motivation
One of our most basic, primary directives
is to bring balance with respect to engag-
ing reward and minimizing loss, while
seeking safety and avoiding threat (Dol-
lard & Miller, 1950). As we increasingly
become creatures of habit over the course
of our lives, we are continually pushed and
pulled by motivations to maintain security
and gain reward. Our actions and prepara-
tion for actions are guided by the motiva-
tional salience of the stimuli we encounter
(Gray & McNaughton, 2000). In particu-
lar, a reward system mobilizes a behavioral
approach toward rewarding or appetitive
stimuli or to minimize loss. Reward can be
further specified in relation to consumma-
tory pleasure (i.e., liking, the hedonic impact
that a reward produces) and anticipatory
pleasure (i.e., wanting, the incentive salience
associated with a particular reward; Ber-
ridge, Robinson, & Aldridge, 2009). These
two facets of the reward system have shared
(e.g., nucleus accumbens, comprising a large
part of a region called the ventral striatum;
Stein & Paulus, 2009) and dissociable (e.g.,
the orbitofrontal cortex [OFC], involved in
assigning reward values to stimuli; Berridge
et al., 2009) neurobehavioral correlates. In
terms of neurochemistry, liking is primar-
ily associated with endogenous opioids and
endorphins (Stein & Paulus, 2009), whereas
wanting chiefly involves dopamine, which is
involved in marshaling organisms to exert
the effort required to obtain a reward (Ber-
ridge et al., 2009; Stein & Paulus, 2009).
By contrast, a security system instigates
avoidance of novel, potentially threatening,
or painful stimuli or end states, as well as
engagement of safety stimuli that protect
an individual from such perceived threats
and can reinstate a state of quiescence and
calm. This system encompasses both reflex-
ive motivational behavior and reflective,
goal- setting, behavior (Carver, Avivi, &
Laurenceau, 2008; Gray & McNaughton,
2000; Higgins, 1997) aimed toward avoid-
ance of threats and engagement of safety
signals. At the neural level, whereas the
amygdala is activated in response to novel
and immediate threats, the bed nucleus of
the stria terminalis is activated in response
to more distal or prolonged threats (Lang,
Davis, & Öhman, 2000). Furthermore, the
ventromedial prefrontal cortex (vmPFC) is
involved in higher order processing of goals
reflective of safety seeking and threat avoid-
ance. Neurochemically, norepinephrine and
gamma- aminobutyric acid (GABA) are cen-
trally involved in threat detection and safety
signaling (Stein & Paulus, 2009).
The security and reward systems are
relatively independent and can be activated
alone or in unison, in response to a prompt
(Stein & Paulus, 2009). In essence, norma-
tive functioning represents a constant state
of engaging and resolving situations that
provoke conflicts of motivational systems
in the service of taking effective behavioral
action. Stein and Paulus suggest that the
insula, in relation to the vmPFC, may be
important for detecting motivational con-
flict and helping to shape, and be shaped
by, goal intentions and implementation.
Similarly, Aupperle and Paulus (2010) pro-
pose that optimal balancing of approach
and avoidance systems is coordinated by a
network consisting of the OFC (valuation of
stimuli in service of making choices; inhibit-
ing limbic regions and behavioral responses
472 INTERVENTIONS
during fear processing), the ventral striatum
(orientation toward reward), amygdala (pro-
viding salience/intensity of fear and avoid-
ance stimuli), and the insula (monitoring
current and predicting future interoceptive
states in relation to reward and fear). Cor-
respondingly, engaging behavioral actions
to achieve or restore motivational balance
likely consists of responding in a manner
reflecting a contextually and situationally
appropriate balance of reward and safety–
threat systems, while also informing one’s
actions with higher order, values- based deci-
sion making (Wilson & Murrell, 2004).
Attaining goals that reflect motivational
salience and one’s personal values provides
feedback relating one’s behavioral effort to
an outcome.
Regulation
Emotions are part of a larger self- regulation
system that allows us to respond flexibly to
events in our lives in accordance with both
personal goals/values and changing con-
texts. In some instances, the optimal tuning
in a given situation results in the accentua-
tion (i.e., up- regulation) of the emotional
salience of the situation; in other instances,
toning down (i.e., dampening) the emotional
aspects of the situation is warranted. Neuro-
biological evidence supports the notion that
there are multiple pathways to emotion gen-
eration, including automated, “hard-wired”
or lower order systems (largely involving
physiological responses and their subcortical
control) and more controlled, higher order
systems (largely involving subjective, corti-
cal responses) that are separate but inter-
active and mutually essential for differing
aspects of emotional experience (LeDoux,
1996). These higher order and lower order
neural systems also actively regulate each
other (LeDoux, 1996). A functional systems
approach to emotion regulation argues that
these systems work together to maintain
dynamic homeostasis between bodily sys-
tems and internal and external stimuli in a
context- appropriate manner.
Gross (2002) highlights the importance of
the temporal unfolding of emotional expe-
rience in understanding how individuals
self- regulate. Specifically, one can impact an
emotional event via early selection or modi-
fication of a situation, early stages of emo-
tional processing chiefly involving attention,
later stages of emotional processing chiefly
involving verbal representation and cogni-
tive change, or, once an emotional response
has occurred, by attempting to impact the
quality of that response. Influencing the
information- processing aspects of an emo-
tion (i.e., antecedent- focused strategies) is
more effective than acting on the products
of that processing, such as physiological
responses (i.e., response- focused strategies).
Building on these structural and temporal
characteristics, regulation efforts can be dif-
ferentiated by degree of elaboration, which
essentially refers to the degree of cognitive
effort required to engage a particular capac-
ity, from less elaborative regulatory compo-
nents, such as attention, to more elaborative
regulatory components, such as working
memory and verbal representations (Badre
& D’Esposito, 2007), which, at the neural
level, are associated with greater recruit-
ment of dorsal and lateral areas of the PFC
(Ochsner, Bunge, Gross, & Gabrieli, 2002).
Activation of these elaborative areas is asso-
ciated with greater mental effort and can
result in greater resource depletion given
competition for these resources (Muraven &
Baumeister, 2000). Optimal emotion regu-
lation may begin by engaging less elabora-
tive capacities, followed by more elabora-
tive capacities as needed (Sheppes & Gross,
2011). Nonetheless, both less elaborative
and more elaborative regulation efforts
modify security- and reward- based moti-
vational patterns according to contextual
demands (e.g., Delgado, Gillis, & Phelps,
2008; Sokol- Hessner, Camerer, & Phelps,
2013).
Broad and Flexible Contextual Learning
Adaptation refers to the process by which
an organism becomes better suited to pros-
pering in their habitats (Dobzhansky, 1970).
Knowing and providing the contextually
appropriate behavioral response may mean
the difference between life and death, and
between love and loss. Adaptive and flexible
behavioral responses are dependent on the
ability to increase awareness of cues and con-
tingencies in the environment and respond in
ways to promote survival and success. Adap-
Emotion Regulation Therapy 473
tive motivational responses and regulatory
capacities provide a foundation for behav-
ioral flexibility in that they help us attain
maximal emotional clarity (e.g., Gohm &
Clore, 2002) and subsequently implement
effective and goal- relevant responses for
optimal behavioral outcomes.
Optimal reward learning requires us to
take behavioral actions that are informed by
the assignment of value to possibly reward-
ing stimuli and subsequent predictions about
when and where we might encounter these
stimuli (O’Doherty, 2004). Bogdan and Piz-
zagalli (2006) have examined factors such
as reward sensitivity (increased likelihood
of responding to “rich” rewarding stimuli
based on past learning history) as evidence
of the influence of emotion and accurate cue
detection on reward learning and behavior.
Similarly, reliably detecting and respond-
ing to cues that signal a clear and present
danger are crucial for survival (LeDoux,
1996). However, equally important is learn-
ing to detect safety cues accurately and dif-
ferentiate these signals from threat, so that
we do not expend valuable resources (e.g.,
time and energy) in attempts to escape from
“nonthreats.” Contemporary models of
threat and safety learning are predicated on
principles of Pavlovian conditioning and the
knowledge that successful fear extinction
represents new, inhibitory learning (Bouton,
Mineka, & Barlow 2001). Emotion regula-
tion plays an important role in inhibitory
learning via selection of optimal responses
that promote abolishment of a conditioned
emotional response. Commensurately, acti-
vation in the vmPFC, an area central to
implementation of emotion regulation inten-
tions, has been found to decrease during
acquisition of conditioned responses and
increase during fear extinction (Schiller &
Delgado, 2010).
Dysfunctional Emotional Functioning
Motivational Dysfunction
Individuals with distress disorders may be
subject to frequent conflicting pulls from
reward and safety– threat systems. How-
ever, unlike healthy individuals, they may
be relatively less effective in resolving these
motivation conflicts. Drawing upon the
Aupperle and Paulus (2010) model of moti-
vation balancing, individuals with anxi-
ety and mood disorders likely struggle to
overcome motivational conflicts because
they possess one or more of the follow-
ing deficits: (1) an over- representation of
safety– threat valuation as reflected in lim-
bic (amygdala, insula) overactivation; (2)
over- or underrepresentation of approach
valuation as a function of either attenuated
or exaggerated striatal activation, respec-
tively; and (3) insufficient mediation or arbi-
tration of approach and/or avoidance valu-
ations resulting from attenuated OFC and
vmPFC activation. Klenk, Strauman, and
Higgins (2011) offer a similar view of moti-
vational conflict, especially with respect to
GAD and MDD, by drawing from regula-
tory focus theory (RFT; Higgins, 1997), a
normative model of promotion (i.e., reward)
and prevention (i.e., security) motivations,
in which these two systems are conceptual-
ized as separate and mutually inhibitory of
one another. In accounting for GAD, espe-
cially when it co- occurs with MDD, Klenk
et al. (2011) postulate primary failure in the
prevention system (i.e., hyperactivation) that
in turn can lead to failure (e.g., hypoactiva-
tion) in the promotion system. One possibil-
ity is that salience in one or both of these
motivational systems may increase levels of
subjective intensity and corresponding dis-
tress. Although more rigorous experimental
and biobehavioral research is needed, pre-
liminary findings support a role for both
motivational dysfunction (i.e., Campbell-
Sills, Liverant, & Brown, 2004) and subjec-
tive intensity (i.e., Mennin, D. S., Heimberg,
R. G., Turk, C. L., & Fresco, D. M., 2005;
Mennin, Holaway, Fresco, Moore, & Heim-
berg, 2007) in the distress disorders.
Regulatory Dysfunction
Rather than processing emotion informa-
tion and utilizing its motivational value,
individuals with distress disorders often fail
to enhance or diminish emotional experi-
ences in a manner appropriate to a par-
ticular environmental context. Emotion
regulation deficits commonly occur in GAD
(Etkin, Prater, Hoeft, Menon, & Schatz-
berg, 2010; Etkin & Schatzberg, 2011; Men-
nin et al., 2005; Mennin et al., 2007) and
474 INTERVENTIONS
MDD (Johnstone, van Reekum, Urry, Kalin,
& Davidson, 2007). Rottenberg and Gross
(2003) recommend that investigators need
to parse emotion- generative processes from
regulation efforts and to recognize that,
similar to conceptualizations of healthy reg-
ulation, dysregulation occurs dynamically
throughout different points in the emotion-
generative process. Individuals with distress
disorders demonstrate deficits earlier in less
elaborative, attentional regulation, as well as
later in more verbally elaborative, regulation
in response to motivationally salient stimuli.
At a less elaborative level, these individu-
als are characterized by attentional rigidity
in processing both interoceptive and extero-
ceptive emotional stimuli (Clasen, Wells,
Ellis, & Beevers, 2013; Mogg & Bradley,
2005). For example, GAD with and with-
out MDD is characterized by a failure to
regulate emotional conflict spontaneously
by shifting attention in response to a moti-
vationally salient emotional stimulus in con-
flict adaptation tasks (Etkin et al., 2010;
Etkin & Schatzberg, 2011). Each day, we
are confronted with the simultaneous occur-
rence of emotionally conflicting information
that may perturb our goal- directed behavior,
requiring us to attend to, consider, and pos-
sibly inform our actions before completing
the task at hand. The ability to handle these
instances efficiently is called conflict moni-
toring (Botvinick, Braver, Barch, Carter, &
Cohen, 2001). This capacity represents a
facet of normative emotion regulation and
consistently corresponds to activation of the
anterior cingulate cortex (ACC), a region
associated with attentional and motivational
processes. By contrast, individuals with
GAD + MDD struggle to regulate emotional
conflict, fail to engage the ventral ACC in
ways that dampen amygdala activity, and
do not show regulation- related connectivity
between the ventral ACC and the amygdala
(Etkin et al., 2010).
Individuals with distress disorders also
struggle to implement more verbally elabo-
rative strategies. Aldao and Mennin (2012)
found that control individuals, while watch-
ing emotionally evocative films, demon-
strated increased heart rate variability
(HRV; an index of parasympathetic flex-
ibility; Porges, 2001) when instructed to
reappraise (i.e., employ a different cognitive
vantage point regarding an emotionally pro-
vocative event; see Gross, 2002) or accept
(i.e., defined as openly turning toward,
allowing, and remaining in personal contact
with an emotional experience; see Hayes,
Strosahl, & Wilson, 2012). Participants
with GAD showed a paradoxical pattern of
decreased HRV when trying to implement
these more elaborative strategies, suggesting
a failure of efficiency of these strategies for
these individuals. Furthermore, studies com-
paring individuals with MDD (Johnstone et
al., 2007) to healthy control participants
found dissociable patterns of neural activity
when participants were instructed to reap-
praise. Whereas control participants demon-
strated a negative relationship between acti-
vation in the vlPFC and the amygdala that
was mediated by the vmPFC, MDD partici-
pants showed a positive association between
the vmPFC and the amygdala as well as no
vlPFC activation. Rather than effectively
engaging adaptive strategies, there is consid-
erable evidence that individuals with distress
disorders alternatively employ perseverative
strategies to compensate for a negative emo-
tional state, chiefly by enveloping it in elab-
orative self- conscious processing (Borkovec
et al., 2004; Nolen- Hoeksema et al., 2008).
However, cognitively elaborative process-
ing, such as repetitive thought, is depleting
and requires an expenditure of resources
to employ (Muraven & Baumeister, 2000).
This depletion may come at the cost of not
only effective emotion management and
maintenance of distress but it also appears
to preclude effective emotional processing
and learning.
Narrow and Rigid Contextual Learning
Individuals with distress disorders often
exhibit impoverished and inflexible reper-
toires of behavior in response to the situ-
ations that typically function to promote
escape, avoidance, or inactivity as a means
of attempting to manage emotional–
motivational signals (e.g., Ferster, 1973).
These behavioral patterns negatively impact
reward learning. For example, in contrast to
healthy controls, depressed individuals are
less responsive to future opportunities for
reward, despite a firsthand learning history
for the availability of reward (Bogdan & Piz-

Emotion Regulation Therapy 475
zagalli, 2006). Similarly, depressed individu-
als, when given the choice between rewards
of different sizes, fail to distinguish between
options yielding large versus small rewards
(Forbes, Shaw, & Dahl, 2006). Bar (2009)
proposes a model of optimal functioning
characterized by broad and contextual asso-
ciative processing of historical and environ-
mental factors to imagine future events and
outcomes accurately. Depressive rumination
is one strategy common to distress disorders
that narrows associative processing, and in
turn decreases the likelihood of new reward-
based learning and obfuscates focusing on
purposeful action (Bar, 2009). In support of
this assertion, Whitmer and Gotlib (2012)
found that instructing depressed individuals
to ruminate during a laboratory- based task
reduced sensitivity to stimuli associated with
reward and punishment.
With respect to threat and safety learning,
adaptively attending to motivational and
emotional signals can facilitate inhibitory
learning. By contrast, one factor important
to achieving durable inhibitory learning is
the degree of stimulus generalization that an
individual displays in relation to the acquisi-
tion of a conditioned stimulus (CS; Lissek,
2012). In particular, individuals prone to
anxiety disorders are less successful in dis-
criminating the properties of stimuli that
share characteristics with a training CS,
thereby resulting in stimulus overgeneral-
ization and eliciting fear in response to a
broader array of stimuli. Similarly, for most
organisms, signals or cues in the environ-
ment of unambiguous safety from fear leads
to new inhibitory learning that helps to
abolish the conditioned emotional response.
Individuals prone to anxiety disorders are
less likely to achieve a durable and broad-
based abolishment of a conditioned fear
response because of deficits in detecting cues
of unambiguous safety. Instead, their search
for safety is often characterized by hyper-
vigilance and overactivity, thereby result-
ing in an inferior and less durable acquisi-
tion of inhibitory learning (Lohr, Olatunji,
& Sawchuck, 2007; Woody & Rachman,
1994). Further, resorting to worry to regu-
late perceived threat experiences has been
shown to encourage avoidance of emotional
processing (Borkovec et al., 2004; Newman
& Llera, 2011).
Clinical Application of the Emotion
Regulation Perspective
As we have argued elsewhere (Mennin,
Ellard, Fresco, & Gross, 2013), one impor-
tant step in advancing behavioral treatments
for refractory conditions such as distress
disorders is the delineation of core principles
that underlie various efficacious therapeutic
processes and corresponding mechanistic
targets refined from our growing knowledge
of normative behavioral, biological, and
social functioning. Change principles can
be defined as broad frameworks for guiding
both what is being targeted in a given inter-
vention recipient and characteristics of the
intervention itself. Target mechanisms refer
to the characteristics within the recipient of
the intervention that are the focus of change
for a given principle (Mennin et al., 2013).
ERT reflects these common principles and
target mechanisms by drawing therapeutic
processes from (1) CBT (e.g., psychoeduca-
tion, self- monitoring, cognitive perspective
taking, problem solving, relaxation and dia-
phragmatic breathing exercises; Borkovec et
al., 2004; Dugas & Robichaud, 2007); (2)
acceptance-, dialectic-, and mindfulness-
based behavioral treatments (e.g., mindful-
ness exercises to broaden awareness of sen-
sations, bodily responses, and emotions in
the present moment; exercises to increase
willingness to accept emotions, commitment
to action related to personal values; Hayes et
al., 2012; Linehan, 1993; Roemer & Orsillo,
2009; Segal, Williams, & Teasdale, 2002);
and (3) experiential therapy (e.g., a focus
on empathic attunement, the importance
of agency, delineation of emotion function,
engagement of experiential tasks, see Elliot,
Watson, Goldman, & Greenberg, 2004).
ERT also deliberately seeks to integrate find-
ings from basic and translational affect sci-
ence, while simultaneously being responsive
to emerging NIMH priorities. The net effect
is an integrated, mechanism- targeted CBT
that strives to improve the acute and endur-
ing treatment efficacy for individuals suffer-
ing from distress disorders such as GAD and
MDD, especially when the conditions are
comorbid.
ERT is organized around core principles
and target mechanisms related to an affect
science framework, which, specifically seeks
476 INTERVENTIONS
to (1) increase motivational awareness,
(2) develop regulatory capacities, and (3)
engage new contextual learning repertoires.
ERT comprises 16 weekly sessions and uti-
lizes a phasic structure that helps clients
build skills in the first half of treatment that
are deployed in the second half of therapy
during exposure exercises. This phasic
structure draws from Gross’s (2002) emo-
tion regulation model, which distinguishes
between efforts to regulate emotions ear-
lier (i.e., antecedent- focused strategies) and
later (i.e., response- focused strategies) in
the emotion- generative process. Specifically,
clients progress through ERT first by learn-
ing skills to increase mindful awareness of
emotional and motivational states; learning
skills to increase adaptive emotion regula-
tory responses congruent with contextual
demands (“counteractive” as an alternative
to reactive response- focused regulation);
and increasing values- informed behavioral
actions that strike a balance between secu-
rity and reward motivational pulls (“proac-
tive” regulation akin to antecedent- focused
regulation).
Motivational Awareness Skills Training
The first component of ERT is the cultiva-
tion of skills to increase motivational aware-
ness (i.e., the accurate and rapid detection of
cues that signal the arrival of motivational
pulls). The benefit of emotional and motiva-
tional clarity is a consistent finding across
numerous studies. For instance, Lanaj,
Chang, and Johnson (2012) offer meta-
analytic support for RFT (Higgins, 1997)
in relation to workplace performance, such
that a promotion (reward) focus was posi-
tively associated with task performance and
innovativeness in the workplace, and nega-
tively associated with counterproductive
workplace behaviors. In contrast, a preven-
tion (security) focus that was unrelated to
task performance but positively associated
with engaging in safe workplace practices
was also associated with counterproductive
workplace behaviors. Furthermore, Gohm
and Clore (2002), in a series of studies, have
consistently shown that emotional clarity,
assessed via self- report, is associated with a
variety of beneficial wellness characteristics
and the tendency to approach life circum-
stances with planful, active coping.
From a treatment perspective, motiva-
tional interviewing (MI) represents a stand-
alone or adjunctive intervention that strives
to bring awareness to one’s motivations, and
the discrepancies between one’s stated goals
and actions as a means of enhancing one’s
willingness to change. Westra, Arkowitz,
and Dozois (2009) reported that the addi-
tion of MI to traditional CBT was associated
with greater clinical efficacy in GAD, espe-
cially among clients with higher pretreat-
ment levels of pathological worry. Finally,
training in mindfulness meditation may be
one means of enhancing emotional aware-
ness and clarity. For example, Farb et al.
(2010) found that a sadness provocation is
associated with activation of self- referencing
neural circuits (e.g., medial PFC), but train-
ing in mindfulness meditation is associated
with activation of additional neural regions
associated with visual attention (e.g., pulvi-
nar nucleus), as well as visceral and somato-
sensory areas (e.g., right insula). Greater
activation of these neural regions was also
negatively correlated with concurrent levels
of depression.
Drawing from this empirical basis, ERT
helps clients develop motivational awareness
initially through psychoeducation aimed at
increasing understanding of emotions and
underlying motivations in the context of
personally relevant historical and proximal
events. Clients receive a rationale for the
benefits of observing emotions with greater
granularity and clarity (i.e., better differen-
tiation of each emotion even when multiple
emotions are present). For instance, clients
imagine a musical orchestra in which each
musical instrument represents a different
emotion. The orchestral composition rep-
resents the motivational pulls in their lives.
Ideally, the composition is harmonious and
the music moves clients to take some action
in their lives. Clients are encouraged to lis-
ten in such a way that each and every part of
the orchestra can be heard and discerned for
its contribution to the overall composition.
As noted by Aupperle and Paulus (2010),
individuals with distress disorders struggle
to resolve motivational conflicts that occur
in their lives, via overrepresentation of
threat valuation, inconsistent representa-
tion of approach valuation, and impaired
arbitration of these motivation systems. The
net result of this imbalance of motivational
Emotion Regulation Therapy 477
pulls is that the discrepancy becomes the
salient signal, and individuals with distress
disorders often respond reactively in a des-
perate attempt to resolve the discrepancy.
Thus, building on the orchestra metaphor,
clients also engage in a motivational anchor-
ing exercise that begins their training in cul-
tivating greater motivational and emotional
awareness. Specifically, clients imagine an
important but difficult situation in their lives
that likely is marked by pulls for approach
and avoidance. As clients imagine this situ-
ation, they envision the kinds of behavioral
responses they are likely to take from that
particular balance of approach and avoid-
ance motivations. ERT therapists then sys-
tematically help clients to envision this situ-
ation from various motivational stances,
with the goal of helping them see that most
life circumstances comprise both approach
and avoidance motivations, and different
responses are possible when one attends and
follows to differing degrees one’s emotions
and motivations.
As clients gain an appreciation and under-
standing for how emotions and motivations
can effectively inform their lives, they are
introduced to a metaphor designed to help
them improve the accuracy and rapidity of
detecting cues in their lives. This work is
predicated on the well- validated behavioral
interventions of self- monitoring (e.g., Line-
han, 1993) and functional analysis (e.g.,
Ferster, 1973), in which clients are taught to
increase their self- awareness of life situations
as a means of better identifying antecedents
(triggers), thoughts, feelings, and behaviors
that are prompted by these antecedents, and
the consequences of these behavioral actions.
Self- monitoring helps individuals identify
patterns of responding reactively, often with
little awareness of the emotional and moti-
vational pulls as a first step in bringing more
conscious, deliberate, and ideally effective
action when certain cues arise. In ERT, the
therapist begins self- monitoring and cue
detection by asking clients to imagine a pris-
tine snowball (i.e., a pure emotional experi-
ence) rolling down a hill, and in the course
of its travel, picking up dirt and twigs, and
becoming hard and icy (i.e., the unfolding
of emotional experience, particularly in the
aftermath of failures in nonelaborative and
elaborative emotion capacities). Essentially,
the snowball metaphor informs clients about
the temporal model of emotion regulation
and how less effort is required when emo-
tions are handled closer to their arrival in
the temporal unfolding.
Drawing from this snowball metaphor,
ERT clients are then encouraged to practice
a form of self- monitoring called Catch Your-
self Reacting (CYR). This practice begins as
a simple exercise to help identify triggers of
clients’ emotions, the actual emotions them-
selves (fear, anxiety, disgust, etc.), and the
intensity of each emotion they listed. Later
in ERT, the CYR is also utilized to practice
the emotion regulation skills clients will
learn in the course of treatment (see below).
CYRs are completed at home, then reviewed
in the subsequent session with the therapist.
When clients experience difficulties with
CYR, therapists may choose to conduct a
“Do-Over.” This activity resembles both the
cognitive rehearsal task, derived from tradi-
tional cognitive therapy of depression (Beck,
Rush, Shaw, & Emery, 1979), and evocative
unfolding in experiential therapy (Elliott et
al., 2004). The CYR “Do-Over” essentially
represents a therapist- supervised opportu-
nity to shape and solidify self- monitoring
and cue detection.
Regulatory Skills Training
A principle of regulatory skills training
assumes that individuals with psychopa-
thology do not have immutable regulation
deficits; rather, with appropriate training,
these capacities can be grown and utilized in
their lives. Based on Gross’s model and the
extant clinical and experimental literature,
ERT targets different regulatory capacities
by utilizing a number of CBT intervention
processes that vary in their entry points
along the trajectory of unfolding emotional
experience. Specifically, we promote four
increasingly elaborative (i.e., that use work-
ing memory/verbal representations and are
resource intensive; Badre & D’Esposito,
2007) regulatory capacities that rely on
antecedent- focused processing: attending
(i.e., the ability to focus, sustain, and flexibly
move attention); allowance (i.e., the ability
to openly turn toward, allow, and remain in
personal contact with an emotional experi-
ence); distancing (i.e., the ability to identify,
observe, and generate psychological perspec-
tive from inner experiences); and reframing
478 INTERVENTIONS
(i.e., the ability to change one’s evaluation of
an event such that the event is altered in its
emotional significance).
Attending
A capacity for directed attention implies
flexible responsivity to different contexts.
Whereas constricted, narrowly focused
attention is adaptive during times of poten-
tial threat, broadened attention facilitates
exploratory behavior, thereby allowing for
the possibility of increased detection of new
information and novel incentives (Friedman
& Forster, 2010). One facet of directed atten-
tion is focused attention, which involves
actively choosing the stimulus to which one
will attend (Kabat-Zinn, 1990). Similarly,
sustained attention refers to maintaining
this focus on the target stimulus (see Posner
& Rothbart, 1992), as well as actively redi-
recting attention back to the target stimulus
when attention has wandered (e.g., Small-
wood & Schooler, 2006). Finally, flexible
attention entails deliberately attending to
various aspects of an experience (Kabat-
Zinn, 1990). Converging lines of research
suggest that reduced attentional flexibility
and rigid attentional biases distinguish indi-
viduals with distress disorders from healthy
controls (Clasen et al., 2013; Mogg & Brad-
ley, 2005).
ERT targets directed attention by promot-
ing skills to encourage greater awareness
of emotions, including sensations, bodily
responses, and subjective experience. Mind-
ful attention training is utilized to increase
a healthy awareness of motivations and
emotional responding, with a particular
emphasis on less elaborative, less linguistic
processing of one’s experience (Kabat-Zinn,
1990). Both in- session and daily practices
are derived from mindfulness- based stress
reduction (MBSR; Kabat-Zinn, 1990)
and mindfulness- based cognitive therapy
(MBCT; Segal et al., 2002). ERT promotes
directed attention toward external stimuli by
increasing mindfulness of senses. A broad-
ened awareness of appetitive and aversive
stimuli alike is encouraged through practices
that increase mindful ingestion of senses
(e.g., eating a raisin mindfully; Kabat-Zinn,
1990). ERT also promotes directed attention
to internal, nonverbal stimuli or stimuli that
can be felt in the body (e.g., Craig, 2009),
thereby enhancing the ability to disengage
from elaborative processes about sensory
experiences. Clients are taught diaphrag-
matic breathing as a brief, nonelaborative
practice that focuses attention on sensa-
tions in the body (e.g., Roemer & Orsillo,
2009). Clients also learn a practice adapted
from the body scan work common in MBSR
(Kabat-Zinn, 1990) and MBCT (Segal et
al., 2002), coupled with an awareness- based
progressive muscle relaxation approach that
has been effectively utilized in the treatment
of GAD (Roemer & Orsillo, 2009). After
experiencing some success with directed
attention practices, clients learn an “on the
spot” skill of detecting tension and imple-
menting relaxation techniques to promote
greater flexibility in musculature response.
Finally, although not every emotion expe-
rienced is necessarily adaptive, broadening
awareness to the full spectrum of emotional
experience can help clients move through
“cloudy” (i.e., secondary emotional reac-
tions) toward “clear” (i.e., primary emo-
tions that convey initial action tendencies
and their associated meanings for behav-
ior) emotions that better reflect the array of
motivational cues that may be present (Elliot
et al., 2004; Linehan, 1993). Specifically, cli-
ents learn a mindfulness of emotions exer-
cise in which the goal is to bring to mind a
situation that possesses conflicting emotions
and motivations (i.e., simultaneous security
and reward impetuses), and they are invited
to sit with the experience until they can hold
and more clearly delineate the emotions and
motivational pulls in the situation.
Allowing
Emotional allowance involves maintaining
contact with feeling states, without being
dissuaded by elaborative thought processes,
such as judgments about the experience
(see Hayes et al., 2012). Individuals with
highly developed capacities to accept their
emotions are less likely to avoid certain
endeavors wherein difficult emotions could
arise and are more likely to stay in contact
with emotions associated with aversiveness,
increasing the possibility of updating con-
tingencies related to their valuation (Hayes
et al., 2012). Individuals with psychopathol-
Emotion Regulation Therapy 479
ogy demonstrate difficulties acknowledging
their emotional experiences, which includes
being dissuaded by negative beliefs about
difficult emotions, aversion toward difficult
emotional experiences, engaging in mal-
adaptive elaborative responses when diffi-
cult emotions arise (e.g., worrying, brood-
ing, self- criticizing), attempting to reduce
their awareness and limit their experience
of difficult emotions, and avoiding situa-
tions and activities that could provoke dif-
ficult emotions, even when such activities
are important to them (Barlow et al., 2011;
Hayes et al., 2012; Linehan, 1993; Roemer
& Orsillo, 2009; Segal et al., 2002).
By accepting and exploring emotions,
clients gain an ability to be present with
emotions and learn how to determine their
functional utility in guiding actions. Spe-
cifically, clients conduct an “offline” prac-
tice that involves imaginally engaging and
remaining in contact with sensations, emo-
tions, and cognitions that arise in pursuit
of a difficult to achieve but motivationally
enhancing action— in effect, a personally
salient approach– avoidance conflict. The
on-the-spot version of this practice involves
encouraging clients to “pause” by sustaining
their connection to a cloudy, diffuse situa-
tion until they are able to connect to the pri-
mary emotions that reflect both motivations
for reward and safety. Another borrowed,
on-the-spot practice, designed to sustain or
regain allowance is the 3-minute breathing
space (Segal et al., 2002), which helps clients
anchor themselves in an event with strong
pulls to respond reactively.
Distancing
Various theorists (e.g., Hayes et al., 2012;
Kross & Ayduk, 2009; Segal et al., 2002)
have emphasized the importance of distanc-
ing or decentering, the metacognitive abil-
ity to observe items that arise in the mind
(thoughts, feelings, memories, etc.) with
healthy psychological distance, greater self-
awareness, and perspective taking. Cogni-
tive distancing helps individuals disengage
from an intense emotion, its corresponding
motivational impetus, and associated mal-
adaptive self- referential processing, in favor
of adopting a more experiential perspec-
tive. This ability also involves recognizing
that one’s thoughts, feelings, and urges are
transient internal events rather than inher-
ent, permanent aspects of the self or accu-
rate representations of reality (Fresco et
al., 2007; Segal et al., 2002). Studies reveal
psychological benefits from promoting dis-
tance from the self in time (e.g., viewing
inner experiences as temporary; Watkins,
Teasdale, & Williams, 2000) and in space
(e.g., viewing inner experiences as physi-
cal objects that are separate from oneself;
Kalisch et al., 2005). Distress disorders have
been associated with deficits in cognitive
distancing (e.g., Fresco al., 2007) and can
be reduced by experimental techniques that
promote an observational distance from the
self (e.g., Kross & Ayduk, 2009).
Distancing is targeted through two offline
mindfulness practices and their correspond-
ing on-the-spot versions. In the mountain
meditation, derived from MBSR (Kabat-
Zinn, 1994), clients are invited to internal-
ize a living, breathing mountain to provide
solidarity and permanence in their lives to
help them “weather” the transient emotional
upheavals in their lives. The mountain medi-
tation provides a decentered perspective
through the lens of time— helping clients
tell themselves “This too will pass.” Clients
then practice a brief version of the mountain
meditation designed to be utilized on the
spot in moments that call for a security- first
response to help sustain or regain a decen-
tered stance. Similarly, drawing primarily
from acceptance and commitment therapy
(ACT; Hayes et al., 2012), ERT promotes
a focus on mental spatial distance and is
designed to teach clients to bring situations to
mind, then to granulize the constituent parts
of the situation by placing them externally
on objects in the room. This offline practice
is an invitation to create healthy distance in
one’s mind’s eye so that these products of
mind are more readily observable, and in
turn, can inform our deliberate actions from
a decentered perspective. This practice also
has a corresponding on-the-spot skill, where
clients imaginally place products of their
mind on objects that they ordinarily carry in
their daily lives. One of our clients actually
carried around a shoehorn he crafted, which
he used to “pry” himself away from prod-
ucts of his mind to promote better observa-
tion and nonjudgment.
480 INTERVENTIONS
Reframing
Reframing refers to the ability to change
one’s evaluation of an event so as to alter its
emotional significance (Gross, 2002). Three
of the most common methods include real-
istic reappraisal (e.g., reevaluating an event
in a more accurate, objective, factual man-
ner, while remaining sensitive to contextual
factors; Ray, Wilhelm, & Gross, 2008),
positive reappraisal (e.g., reevaluating an
event in a manner that emphasizes possible
desired, rewarding, or beneficial aspects of
the event or consequences of the event that
may have been overlooked in the original
appraisal; Ray et al., 2008), and compas-
sionate reappraisal (e.g., reevaluating an
event in a manner that appreciates and vali-
dates the presence of emotional pain and
desires to alleviate it, and identifies the pain
as a natural aspect of the human experience;
Leary, Tate, Adams, Batts, & Hancock,
2007). Researchers who have manipulated
changing cognitions in the laboratory have
found psychological benefits from instruct-
ing participants before they encounter a
stimulus or task to reappraise it in a manner
designed to promote personal detachment
or objectivity (e.g., Gross, 2002). A capac-
ity to change cognitions and relinquish one’s
original interpretation may undermine pas-
sive, repetitive elaborative processes central
to transdiagnostic psychopathology (e.g.,
rumination; Nolen Hoeksema et al., 2008)
and promote flexibility that allows more
effective, rather than habitual, behavioral
reactions. Indeed, reappraisal has been
found to be positively associated with a
number of indices of well-being, including
effective interpersonal functioning (Gross,
2002).
ERT employs several strategies to develop
cognitive change capacities to provide tem-
porary relief from emotional distress, facili-
tate a restoration of emotional clarity, and
promote effective action. Once clients have
obtained a decentered perspective from their
emotional and motivational pulls, they are
encouraged to adopt a courageous perspec-
tive wherein they can address any security-
driven responses and provide alternative
statements that reflect their strength in the
face of uncertainty. Clients are also encour-
aged to adopt a self- compassionate reap-
praisal stance wherein they can imagine
telling a very caring, interested, compassion-
ate individual about their difficult thoughts
and feelings, and reminding themselves of
their strengths and coping ability (Gilbert,
2009; Segal et al., 2002). Noticing one’s self-
critical thoughts is encouraged and “soften-
ing” them when they arise is accomplished
by invoking alternative, self- validating state-
ments. These courageous and compassionate
statements are typically written down and
carried with the patient in a pocket or put on
a smartphone as a recurrent reminder. After
committing these statements “to heart,” cli-
ents are then encouraged simply to tap their
pocket or bag containing the written state-
ment or smartphone to regain quickly this
sense of perspective.
Experiential Exposure
A hallmark goal of CBT is to assist clients
in “new learning” to promote the imagin-
ing or enacting of novel experiences that
counteract old and well-worn patterns of
maladaptive associations and reinforcement.
With respect to anxiety disorders, exposure
therapy, derived from principles of classical
conditioning, has demonstrated consider-
able acute and enduring treatment efficacy
(Craske et al., 2008). Exposure therapy is
often coupled with treatment components
that reduce the reliance on escape and avoid-
ance behaviors while cultivating repertoires
of behaviors designed to pursue and obtain
rewards (Craske et al., 2008; Ferster, 1973).
Additionally, informed by important basic
findings about the nature of classical extinc-
tion and inhibitory learning (e.g., Bouton,
Mineka, & Barlow, 2001) implementations
of exposure therapy have moved beyond
sustained fear reduction and habituation
accounts of extinction to promote supe-
rior inhibitory learning and extinction
retrieval (e.g., Craske, & Vervliet, 2013).
Similarly, behavioral accounts of depres-
sion depict depressed individuals as no lon-
ger able to engage the behavioral repertoires
capable of delivering positive reinforcement
(Lewinsohn, 1974) while they inordinately
orient their lives in service of escape and
avoidance of aversive situations (Ferster,
1973; Kanter et al., 2010). To overcome these
deficits, treatment typically consists of inter-
ventions that include activity scheduling,
contingency management, and skills training
Emotion Regulation Therapy 481
as ways to help individuals gain awareness
of the availability of positive reinforcement,
engage in behaviors capable of obtaining
the positive reinforcement contingently, and
ideally, effecting change in the environment
so that access to positive reinforcers remain
available when certain behavioral responses
are provided (Ferster, 1973; Kanter et
al., 2010; Lewinsohn, 1974). In addition,
recent innovative treatments for depression
have benefited from basic and translational
findings (e.g., Bouton et al., 2001) about
developing exposure- based treatments for
depression that deliberately provoke and
activate historical negative content, so that
this material can be explored alongside
information that is dissonant and serve to
facilitate broad-based change in maladap-
tive cognitive– affective– behavioral– somatic
patterns (e.g., Hayes et al., 2007).
Exposure therapy is most effective when
the fear stimulus is focal and difficult to
avoid (e.g., specific phobia). Traditional
exposure therapy may be less efficacious in
distress disorders. Whereas individuals with
uncomplicated presentations of anxiety have
relatively more discrete and circumscribed
fears, individuals with distress disorders
have difficulties confronting emotional
arousal irrespective of what external cue
provokes it. That is, conducting exposure
therapy in response to external fear cues
does not effectively target the source of the
difficulty for individuals with distress disor-
ders. Consistent with an emotion regulation
framework, some approaches promote expo-
sure to emotional experiences themselves to
increase acceptance of these experiences and
diminish the need to utilize strategies such
as worry or rumination in an attempt to
escape aversively perceived emotional states
(e.g., Barlow et al., 2011). However, this
approach may not fully address dysfunction
given that distress- disordered individuals
commonly resort to cognitively elaborative
responses such as worry and rumination to
help them escape emotional processing and
to preclude new inhibitory learning. Fur-
thermore, a focus on negative emotional
exposures may do less to expose individu-
als to core idiographic and schematic themes
that certain emotions may convey. Also, this
approach does not necessarily engage the
reward system by directly encouraging pro-
active behavior toward desired outcomes.
In other words, exposure in distress disor-
ders may be most fruitful when it involves
contexts that have recurrent themes such as
threat (i.e., security motivation system) and
encourage activation (i.e., reward motiva-
tion system).
In support of this contention, recent
work by Newman and Llera (2011) demon-
strates that worry is reinforced by creating
a fixed, invariable, and predictive defensive
emotional state that inevitably precludes
emotional processing. For individuals with
GAD, they argue, what they fear is a stark
emotional contrast, such as moving from a
positive state to a negative state, which may
disincline these individuals to engage a posi-
tive state if it means increased possibility for
a negative state. This conceptualization is
consistent with the emotion regulation model
in which individuals with distress disorders
resort to worry and rumination in response
to security motivations, which overshadow
reward motivations. Furthermore, it is con-
sistent with behavioral accounts of MDD
that view depressed individuals as withdraw-
ing to protect themselves from the aversion
of loss, thus gaining no new rewards to help
them counteract their depression. Taken as a
whole, these perspectives suggest that expo-
sure to rewarding contexts with the possi-
bility of high risk may be most ameliorative
for these individuals, since they simultane-
ously engage both the security and reward
systems.
In ERT, exposure to threat– reward con-
trasts are accomplished by focusing clients’
attention on their personal values (i.e., their
highest priorities and most cherished princi-
ples; Hayes et al., 2012; Wilson & Murrell,
2004). Values-based exposure involves turn-
ing a problem on its head: Rather than being
exposed to feared outcomes, one is exposed
to the way he or she would like to be living,
and the expected arrival of perceived fears,
disappointments, and judgments are treated
as obstacles to being able to live a valued life
(Hayes et al., 2012). ERT expands values-
based exposure commonly utilized in ACT
to address not only “top-down” decisions
about life goals but also “bottom– up” influ-
ences of security and reward motivational
pulls, as well as their interaction. During an
experiential exposure phase of ERT, clients
explore acting in accordance with their val-
ues and confronting any accompanying per-
482 INTERVENTIONS
ceived obstacles that arise both within and
between sessions. Specifically, new learning
is targeted with three main exposure inter-
ventions to promote valued living: (1) ima-
ginal exercises related to values- informed
goals; (2) experiential dialogue exercises
to explore perceived internal motivational
conflicts that impede engaging in valued
actions (Elliot et al., 2004); and (3) planned
between- session exercises wherein clients
engage valued actions outside of session. Cli-
ents also utilize regulatory capacity skills to
help in engagement of in- session experiential
tasks and to facilitate valued action outside
of session.
Proactive Valued Action
In ERT, therapists and clients collaborate
to identify cherished values in life domains
(e.g., family, friends, relationships, work,
personal care), with clients reporting dis-
crepancies between the importance they
place on this value and how consistently
they have been living accordingly (Wilson &
Murrell, 2004). Therapists then encourage
clients to think about a salient value with a
large discrepancy and how they want their
actions to reflect this value today, even if
it involves only a small action step. Specifi-
cally, imaginal exposure tasks that focus on
engaging in specific valued actions are con-
ducted to (1) provide clients with an experi-
entially rich rehearsal of the steps that might
be necessary to live by their values and (2)
confront the emotional challenges that are
likely to come up as clients imagine engage-
ment of valued action. In this imagery expo-
sure task, therapists help clients imagine
each step involved in engaging this action,
while noting changes in motivational lev-
els and encouraging utilization of skills to
address difficulties in awareness and bal-
ancing of emotional responses. Utilizing
imagery to consolidate skills and promote
functional action is also congruent with
traditional interventions such as cognitive
rehearsal (Beck et al., 1979).
Exploring Conflict Themes in Obstacles
to Valued Living
The second component of exposure work
in ERT involves addressing perceived obsta-
cles to taking valued action. Obstacles are
addressed through the lens of “conflict
themes” and include primarily (1) a motiva-
tional conflict (e.g., security motivations are
blocking or interrupting efforts that engage
the reward system) and (2) self- critical reac-
tive responses to emotions (i.e., judgmen-
tal negative beliefs about one’s emotional
responses and associated motivations are
interrupting self- acceptance and engage-
ment of reward). These conflict themes
are addressed within the session using an
experiential dialogue exercise derived from
emotion- focused therapy (Elliot et al.,
2004). The motivational conflict is most
central to interrupting valued action and is
addressed by encouraging clients to engage
in a dialogue between the part of themselves
strongly motivated to obtain security, and the
part motivated toward self- actualization, to
arrive at a more unified motivational stance
that is conducive to valued action. Resolution
comes when expressions from both sides of
the dialogue demonstrate acknowledgment
of the needs of the other and an agreement
to commit to the valued action while allow-
ing a place for a softened obstacle voice to be
present without total control. The purpose
of these tasks is to reduce negative emotional
responses that are activated when obstacles
reflecting these conflicts are perceived (i.e.,
exposure), to generate a new perspective
(i.e., new meaning) on these obstacles, and
to engage more adaptive emotions that are
facilitative of valued action engagement.
Engaging Proactive Valued Action
Outside of Session
Valued action is also promoted between ses-
sions by building upon the in- session valued
action exploration and obstacles confronta-
tion exposure tasks. Therapists encourage
clients to engage both planned (i.e., spe-
cific valued actions related to salient val-
ues explored in session and to which clients
commit in the presence of therapists) and
spontaneous (i.e., any other valued actions
in which clients notice themselves engaging)
valued actions outside of session (Hayes et
al., 2012). Furthermore, clients are encour-
aged to utilize skills both proactively (in
an antecedent- focused manner) when they
are planning to engage valued actions and
counteractively (in a response- focused man-
ner) when they notice themselves getting
Emotion Regulation Therapy 483
unexpectedly distressed and feel pulled to
respond reactively with worry, reassurance
seeking, self- criticism, or behavioral avoid-
ance. Finally, external barriers (i.e., obstacles
in the environment that are outside the cli-
ent’s control) that might have been deferred
during exposure tasks can also be addressed
more actively in between- session exercises.
Therapists can help clients problem- solve
these obstacles or utilize skills such as accep-
tance to further facilitate valued action.
Research Findings
To date, we have tested the efficacy and pur-
ported mechanisms of ERT in a number of
trials at various sites. These findings have
been presented in greater detail at a number
of recent conference sessions (e.g., Mennin
& Fresco, 2011; Mennin, Fresco, & Aldao,
2012) and are the focus of submitted manu-
scripts currently under review.
Preliminary Outcome Results
To date, ERT’s efficacy has been demon-
strated in a recently concluded NIMH-
funded project that comprises an open trial
(N = 19) and a randomized control trial
(RCT; N = 60). In both trials, clients toler-
ated ERT, as evidenced by high ratings of
personal satisfaction and low rates of attri-
tion in the course of treatment. For instance,
18 of 19 open-trial clients and 26 of 30
RCT clients completed treatment. In terms
of clinical outcomes, open-trial clients evi-
denced reductions in both clinician- assessed
and self- report measures of GAD severity,
worry, and trait- anxious and depression
symptoms, and corresponding improve-
ments in quality of life, with within- subjects
effect sizes well exceeding conventions for
large effects (Cohen’s d’s = 1.5 to 4.5). These
gains were maintained for 9 months follow-
ing treatment.
The RCT study compared ERT to a modi-
fied attention control (MAC) condition,
which involved clients periodically speak-
ing via telephone to a clinician who pro-
vided supportive listening. Clients received
assessments at baseline, midtreatment, and
postacute treatment. MAC clients were
offered ERT in an open-label fashion follow-
ing the immediate RCT period. RCT find-
ings revealed that ERT clients, compared to
MAC clients, evidenced significantly greater
reductions in GAD severity, worry, trait
anxiousness, and depression symptoms, and
corresponding improvements in functional-
ity and quality of life, with between- subjects
effect sizes in the medium to large range (d
= 0.50 to 2.0). These gains were maintained
for 9 months following the end of treatment.
In addition, these effect size estimates also
take into account clients who dropped out
of treatment.
Finally, a sizable subgroup of clients with
GAD and comorbid MDD (N = 30) were
enrolled and treated. Within- subjects effect
sizes in both clinician- assessed and self-
report measures of GAD severity, worry,
trait anxiousness, and depression symptoms,
and corresponding improvements in func-
tionality and quality of life, were comparable
to overall trial findings— thereby suggesting
that MDD comorbidity did not interfere
with treatment efficacy (Cohen’s d’s = 1.5 to
4.0). Furthermore, rumination and anhedo-
nia also decreased (Cohen’s d’s = 1.5 to 2.0).
Preliminary Mechanisms Results
We argue that efforts to demonstrate the
mechanisms by which one’s treatment pro-
duces clinical improvement are best tied
to common target mechanisms such as
those that reflect the capacities described
earlier (e.g., directed attention, emotional
acceptance, cognitive distancing, cognitive
change). Furthermore, target mechanism
change should be demonstrated with biobe-
havioral marker assessments (e.g., behavioral
tasks, functional magnetic resonance imag-
ing [fMRI], psychophysiology) that have
established reliability and validity in labora-
tory and analogue studies. This approach is
aligned with the growing multidisciplinary
field of intervention science and with NIMH
priorities (e.g., RDoC; Craske, 2012), which
seek to elucidate biobehavioral markers that
are reliably dissociable in patient subgroups
compared to healthy controls. Also, assess-
ing common target mechanisms in various
approaches may help us better understand
patient characteristics that predict treatment
success and failure (e.g., treatment match-
ing, treatment optimization/augmentation;
NIMH Council’s treatment personalization
initiative; Kraemer, Wilson, Fairburn, &
Agras, 2002).

484 INTERVENTIONS
Commensurate with this viewpoint, ERT
has been designed to target mechanisms of
affective dysfunction that we have argued
are central to refractory distress disorders.
Thus, we have been interested in examining
whether these treatment outcomes are the
result of changes in purported motivational,
regulatory, and contextual learning mecha-
nisms. One promising preliminary finding
is related to emotional conflict adaptation
(Etkin & Schatzberg, 2011). A subset of our
ERT clients (N = 15) completed the Etkin
emotional conflict task (described earlier)
at pretreatment and at the midpoint of ERT.
Findings indicate that by midtreatment, cli-
ents improved in ability to shift their atten-
tion in the face of emotional conflict (pre- to
midtreatment d = 0.74) to levels comparable
to healthy controls (Etkin et al., 2010). Fur-
thermore, clients who showed the greatest
gains in conflict adaptation by midtreat-
ment, showed the greatest pre- to posttreat-
ment response in anxiety, anhedonic depres-
sion, and worry. These preliminary data are
supportive of our hypotheses that ERT may,
in part, exert its therapeutic impact through
normalization of less elaborative emotion
regulatory mechanisms such as ability to
shift attention by adapting to conflict.
Although we have not yet tested changes in
more elaborative regulatory mechanisms, we
have examined responses to more complex
emotional stimuli, which likely require more
effort and elaboration to manage. Specifi-
cally, we assessed HRV during a fearful film
paradigm at pre- and midtreatment. Eigh-
teen clients were assessed during the neutral
film, the fearful film, and, a recovery period.
At pretreatment, these clients displayed a
flattened response throughout the experi-
mental period— suggesting reduced flex-
ibility. At midtreatment, clients displayed a
quadratic pattern of vagal withdrawal (i.e.,
reactivity) and vagal rebound (d
pre to mid-tx
=
0.81) reflecting a more normative response
to these changing emotional contexts.
Period- averaged HRV levels at midtreatment
also increased to within 1 SD of levels in a
healthy control sample. Furthermore, clients
who showed the greatest increases in para-
sympathetic flexibility from pre- to midtreat-
ment showed the greatest pre to posttreat-
ment gains in diagnostic severity, anxiety,
worry, anhedonic depression, impairment,
and life quality. These data suggest that ERT
normalizes emotional response patterns,
and that this normalization plays a role in
the therapeutic effects of ERT. However, it
is unclear how clients were able to improve
their response to the film, because spontane-
ous utilization of ERT-related or other strate-
gies was not assessed. It will be important in
future research to determine whether these
improvements are indeed due to implementa-
tion of the skills learned in ERT.
Conclusions and Future Directions
Individuals with distress disorders (i.e.,
GAD, MDD) are commonly comorbid and
appear to be characterized by higher order
negative emotional factors (e.g., Krueger &
Markon, 2006; Watson, 2005) that reflect
activation of underlying motivational sys-
tems related to threat– safety and reward–
loss (Campbell- Sills et al., 2004; Klenk
et al., 2011; Woody & Rachman, 1994).
Furthermore, they tend to perseverate (i.e.,
worry, ruminate) as a way to manage this
motivationally relevant distress (Borkovec
et al., 2004; Nolen- Hoeksema et al., 2008)
and often utilize these self- conscious pro-
cesses to the detriment of engaging new
learning repertoires. ERT (Mennin &
Fresco, 2009) integrates principles from tra-
ditional and contemporary CBT (e.g., skills
training and exposure) with basic and trans-
lational findings from affect science to offer
a blueprint for improving intervention by
focusing on the motivational responses and
corresponding regulatory characteristics
of individuals with distress disorders. This
emphasis on affect science permits identifi-
cation of mechanisms of treatment in terms
of core disruptions of normative cognitive,
emotional, and motivational systems, which
in turn helps generate more targeted solu-
tions to help clients utilize adaptive ways to
cope or compensate for these core deficits.
In essence, contrasting a client’s difficulties
with what we understand as normative func-
tioning allows us to generate theory- driven
hypotheses that form that basis of our case
conceptualization and treatment planning
(e.g., Craske, 2012).
A summary of the conceptual model of
ERT as presented in this chapter appears in
Figure 28.1. However, ERT continues to be
developed and tested. For up-to-date infor-
485
Motivation
security system; reward system
Regulation
Less Elaborative → More Elaborative
Contextual Learning
flexible responding to simultaneous
risk and reward context
(i.e., approach–avoidance conflict)
Target Mechanisms
Change Principles and
Therapeutic Processes
Regulation Skills Training
mindfulness of sensations, body, and emotions;
allowance metaphors and practices; distancing in
time and space; courageous and
compassionate self-statements
Awareness Skills Training
psychoeducation of motivations/emotions;
improved detection of and attendance to
motivational cues
Experiential Exposure
values delineation; experiential imagery and conflict
dialogue exercises; valued action homework
FIGURE 28.1. Conceptual model of target mechanisms, change principles, and therapeutic processes in emotion regulation therapy.

486 INTERVENTIONS
mation about ERT, please visit www.emo-
tionregulationtherapy.com. Our emotion
regulation model is based on current find-
ings in affect science and the psychopathol-
ogy of distress disorders. However, many
of the central tenets of our model await
further careful experimental inquiry using
multimethod biobehavioral approaches.
Nonetheless, preliminary data are support-
ive of our hypotheses that ERT may exert its
therapeutic impact in part through normal-
ization of emotional processes, and there-
fore be most effective for individuals with
distress disorders in whom this system is
most impaired. Building on these pilot find-
ings on biobehavioral mechanisms of ERT,
we are currently examining neural changes
related to ERT utilizing fMRI procedures
while administering paradigms examining
motivational engagement and regulatory
responses at less and more elaborate levels.
Furthermore, we are currently developing a
portable, computer- based “emotion regula-
tion training” to target mechanisms of ERT
in a briefer, more rapid, and cost- effective
method of intervention, which may be par-
ticularly useful for certain populations and
geographical locations in which standard
treatment efforts are less feasible (Kazdin
& Blase, 2011). Through this work, we are
eager to continue testing motivational and
regulatory mechanisms in the distress dis-
orders and to demonstrate their role in tar-
geted treatments such as ERT.
References
Aldao, A., & Mennin, D. S. (2012). Paradoxical
cardiovascular effects of implementing adap-
tive emotion regulation strategies in general-
ized anxiety disorder. Behaviour Research
and Therapy, 50, 122–130.
Aupperle, R. L., & Paulus, M. P. (2010). Neural
systems underlying approach and avoidance in
anxiety disorders. Dialogues in Clinical Neu-
roscience, 12, 517–531.
Badre, D., & D’Esposito, M. (2007). Functional
magnetic resonance imaging evidence for a
hierarchical organization of the prefrontal
cortex. Journal of Cognitive Neuroscience,
19, 2082–2099.
Bar, M. (2009). A cognitive neuroscience hypoth-
esis of mood and depression. Trends in Cogni-
tive Sciences, 13, 456–463.
Barlow, D. H. (2002). Anxiety and its disorders
(2nd ed.). New York: Guilford Press.
Barlow, D. H., Farchione, T. J., Fairholme, C.
P., Ellard, K. K., Boisseau, C. L., Allen, L. B.,
et al. (2011). The unified protocol for trans-
diagnostic treatment of emotional disorders:
Therapist guide. New York: Oxford Univer-
sity Press.
Barlow, D. H., Gorman, J. M., Shear, M. K., &
Woods, S. W. (2000). Cognitive- behavioral
therapy, imipramine, or their combination for
panic disorder. Journal of the American Medi-
cal Association, 283, 2529–2536.
Beck, A. T., Rush, J., Shaw, B. F., & Emery, G.
(1979). Cognitive therapy of depression: A
treatment manual. New York: Guilford Press.
Berridge, K. C., Robinson, T. E., & Aldridge,
J. W. (2009). Dissecting components of reward:
“Liking,” “wanting,” and learning. Current
Opinion in Pharmacology, 9(1), 65–73.
Bogdan, R., & Pizzagalli, D. A. (2006). Acute
stress reduces reward responsiveness: Impli-
cations for depression. Biological Psychiatry,
60, 1147–1154.
Borkovec, T. D., Alcaine, O., & Behar, E. (2004).
Avoidance theory of worry and generalized
anxiety disorder. In R. G. Heimberg, C. L.
Turk, & D. S. Mennin (Eds.), Generalized
anxiety disorder: Advances in research and
practice (pp. 77–108). New York: Guilford
Press.
Borkovec, T. D., & Ruscio, A. M. (2001). Psy-
chotherapy for generalized anxiety disorder.
Journal of Clinical Psychiatry, 62(Suppl. 11),
37–42.
Botvinick, M., Braver, T., Barch, D., Carter, C.,
& Cohen, J. (2001). Conflict monitoring and
cognitive control. Psychological Review, 108,
624–652.
Bouton, M. E., Mineka, S., & Barlow, D. H.
(2001). A modern learning theory perspective
on the etiology of panic disorder. Psychologi-
cal Review, 108, 4–32.
Brown, T. A. (2007). Temporal course and struc-
tural relationships among dimensions of tem-
perament and DSM-IV anxiety and mood
disorder constructs. Journal of Abnormal
Psychology, 116(2), 313–328.
Butler, A. C., Chapman, J. E., Forman, E. M.,
& Beck, A. T. (2006). The empirical status
of cognitive- behavioral therapy: A review of
meta- analyses. Clinical Psychology Review,
26, 17–31.
Campbell- Sills, L., Liverant, G. I., & Brown,
T. A. (2004). Psychometric evaluation of the
Emotion Regulation Therapy 487
behavioral inhibition/behavioral activation
scales in a large sample of outpatients with
anxiety and mood disorders. Psychological
Assessment, 16, 244–254.
Carver, C. S., Avivi, Y. E., & Laurenceau, J.-P.
(2008). Approach, avoidance, and emotional
experiences. In A. Elliot (Ed.), Handbook of
approach and avoidance motivation (pp. 385–
397). New York: Psychology Press.
Clasen, P. C., Wells, T. T., Ellis, A. J., & Beev-
ers, C. G. (2013). Attentional biases and the
persistence of sad mood in major depressive
disorder. Journal of Abnormal Psychology,
122(1), 74–85.
Craig, A. D. (2009). How do you feel—now?:
The anterior insula and human awareness.
Nature Reviews Neuroscience, 10, 59–70.
Craske, M. G. (2012). THE R-DOC Initiative:
Science and practice. Depression and Anxiety,
29, 253–256.
Craske, M. G., Kircanski, K., Zelikowsky, M.,
Mystkowski, J., Chowdhury, N., & Baker, A.
(2008). Optimizing inhibitory learning during
exposure therapy. Behaviour Research and
Therapy, 46, 5–27.
Craske, M. G., & Vervliet, B. (2013). Extinction
learning and its retrieval. In D. Hermans, B.
Rimé, & B. Mesquita (Eds.), Changing emo-
tions (pp. 53–59). Hove, UK: Psychology
Press.
Cuijpers, P., van Straten, A., Andersson, G.,
& van Oppen, P. (2008). Psychotherapy for
depression in adults: A meta- analysis of com-
parative outcome studies. Journal of Consult-
ing and Clinical Psychology, 76, 909–922.
Delgado, M. R., Gillis, M. M., & Phelps, E. A.
(2008). Regulating the expectation of reward
via cognitive strategies. Nature Neuroscience,
11, 880–881.
Dobzhansky, T. (1970). Genetics of the evolu-
tionary process. New York: Columbia Univer-
sity Press.
Dollard, J., & Miller, N. E. (1950). Personality
and psychotherapy. New York: McGraw-Hill.
Dugas, M. J., & Robichaud, M. (2007).
Cognitive- behavioral treatment for general-
ized anxiety disorder: From science to prac-
tice. New York: Routledge.
Elliott, R., Watson, J. C., Goldman, R., & Green-
berg, L. (2004). Learning emotion- focused
therapy: The process– experiential approach
to change. Washington, DC: American Psy-
chological Association.
Etkin, A., Prater, K. E., Hoeft, F., Menon, V.,
& Schatzberg, A. F. (2010). Failure of anterior
cingulate activation and connectivity with the
amygdala during implicit regulation of emo-
tional processing in generalized anxiety dis-
order. American Journal of Psychiatry, 167,
545–554.
Etkin, A., & Schatzberg, A. F. (2011). Common
abnormalities and disorder- specific compen-
sation during implicit regulation of emotional
processing in generalized anxiety and major
depressive disorders. American Journal of
Psychiatry, 168, 968–978.
Farabaugh, A. H., Bitran, S., Witte, J., Alpert, J.,
Chuzi, S., Clain, A. J., Baer, L., et al. (2010).
Anxious depression and early changes in the
HAMD-17 anxiety- somatization factor items
and antidepressant treatment outcome. Inter-
national Clinical Psychopharmacology, 25,
214–217.
Farb, N. A. S., Anderson, A. K., Mayberg, H.,
Bean, J., McKeon, D., & Segal, Z. V. (2010).
Minding one’s emotions: Mindfulness train-
ing alters the neural expression of sadness.
Emotion, 10, 25–33.
Ferster, C. B. (1973). A functional analysis of
depression. American Psychologist, 28, 857–
870.
Forbes, E. E., Shaw, D. S., & Dahl, R. E. (2006).
Alterations in reward- related decisions in boys
with depressive and anxiety disorders. Biolog-
ical Psychiatry, 59, 126S–126S.
Fresco, D. M., Moore, M., van Dulmen, M.,
Segal, Z., Ma, S., Teasdale, J., et al. (2007).
Initial psychometric properties of the Expe-
riences Questionnaire: Validation of a self-
report measure of decentering. Behavior Ther-
apy, 38, 234–246.
Friedman, R. S., & Forster, J. (2010). Implicit
affective cues and attentional tuning: An inte-
grative review. Psychological Bulletin, 136,
875–893.
Gilbert, P. (2009). The compassionate mind: A
new approach to life’s challenges. Oakland,
CA: New Harbinger.
Gohm, C. L., & Clore, G. L. (2002). Four
latent traits of emotional experience and their
involvement in well-being, coping, and attri-
butional style. Cognition and Emotion, 16,
495–518.
Gray, J. A., & McNaughton, N. (2000). The
neuropsychology of anxiety: An enquiry into
the functions of the septo- hippocampal system
(2nd ed.). Oxford, UK: Oxford University Press.
Gross, J. (2002). Emotion regulation: Affective,
cognitive, and social consequences. Psycho-
physiology, 39(3), 281–291.
488 INTERVENTIONS
Hayes, A. M., Feldman, G. C., Beevers, C. G.,
Laurenceau, J.-P., Cardaciotto, L., & Lewis-
Smith, J. (2007). Discontinuities and cognitive
changes in an exposure- based cognitive ther-
apy for depression. Journal of Consulting and
Clinical Psychology, 75, 409–421.
Hayes, S. C., Strosahl, K. D., & Wilson, K. G.
(2012). Acceptance and commitment therapy:
The process and practice of mindful change
(2nd ed.). New York: Guilford Press.
Higgins, E. T. (1997). Beyond pleasure and pain.
American Psychologist, 52, 1280–1300.
Johnstone, T., van Reekum, C. M., Urry, H. L.,
Kalin, N. H., & Davidson, R. J. (2007). Failure
to regulate: Counterproductive recruitment of
top-down prefrontal– subcortical circuitry in
major depression. Journal of Neuroscience,
27, 8877–8884.
Kabat-Zinn, J. (1990). Full catastrophe living:
Using the wisdom of your body and mind to
face stress, pain, and illness. New York: Delta
Trade.
Kabat-Zinn, J. (1994). Wherever you go, there
you are. New York: Hyperion Press.
Kalisch, R., Wiech, K., Critchley, H. D., Sey-
mour, B., O’Doherty, J. P., Oakley, D. A., et
al. (2005). Anxiety reduction through detach-
ment: Subjective, physiological, and neural
effects. Journal of Cognitive Neuroscience,
17, 874–883.
Kanter, J. W., Manos, R. C., Bowe, W. M.,
Baruch, D. E., Busch, A. M., & Rusch, L.
C. (2010). What is behavioral activation?: A
review of the empirical literature. Clinical Psy-
chology Review, 30, 608–620.
Kazdin, A. E., & Blase, S. L. (2011). Rebooting
psychotherapy research and practice to reduce
the burden of mental illness. Perspectives on
Psychological Science, 6, 21–37.
Kendler, K. S., Gardner, C. O., Gatz, M., &
Pedersen, N. L. (2006). The sources of co-
morbidity between major depression and gen-
eralized anxiety disorder in a Swedish national
twin sample. Psychological Medicine, 37,
453–462.
Kessler, R., Berglund, P., Demler, O., Jin, R., &
Walters, E. E. (2005). Lifetime prevalence and
age-of-onset distributions of DSM-IV disor-
ders in the National Comorbidity Survey Rep-
lication. Archives of General Psychiatry, 62,
593–602.
Klenk, M. M., Strauman, T. J., & Higgins, E.
T. (2011). Regulatory focus and anxiety: A
self- regulatory model of GAD–depression
comorbidity. Personality and Individual Dif-
ferences, 50, 935–943.
Kraemer, H., Wilson, G., Fairburn, C., & Agras,
W. (2002). Mediators and moderators of treat-
ment effects in randomized clinical trials.
Archives of General Psychiatry, 59, 877–883.
Kross, E., & Ayduk, Ö. (2009). Boundary con-
ditions and buffering effects: Does depressive
symptomology moderate the effectiveness of
self- distancing for facilitating adaptive emo-
tional analysis? Journal of Research in Person-
ality, 43(5), 923–927.
Krueger, R. F., & Markon, K. E. (2006). Reinter-
preting comorbidity: A model-based approach
to understanding and classifying psychopa-
thology. Annual Review of Clinical Psychol-
ogy, 2, 111–133.
Lanaj, K., Chang, C.-H., & Johnson, R. E.
(2012). Regulatory focus and work- related
outcomes: A meta- analysis. Psychological Bul-
letin, 138, 998–1034.
Lang, P. J., Davis, M., & Öhman, A. (2000).
Fear and anxiety: Animal models and human
cognitive psychophysiology. Journal of Affec-
tive Disorders, 61, 137–159.
LeDoux, J. E. (1996). The emotional brain: The
mysterious underpinnings of emotional life.
New York: Simon & Schuster.
Leary, M. R., Tate, E. B., Adams, C. E., Allen,
A. B., & Hancock, J. (2007). Self- compassion
and reactions to unpleasant self- relevant
events: The implications of treating oneself
kindly. Journal of Personality and Social Psy-
chology, 92, 887–904.
Lewinsohn, P. M. (1974). A behavioral approach
to depression. In R. M. Friedman & M. M.
Katz (Eds.), The psychology of depression:
Contemporary theory and research (pp. 157–
185). Washington, DC: Winston- Wiley.
Linehan, M. (1993). Skills training manual for
treating borderline personality disorder. New
York: Guilford Press.
Lissek, S. (2012). Toward an account of clinical
anxiety predicated on basic, neurally mapped
mechanisms of Pavlovian fear- learning: The
case for conditioned overgeneralization.
Depression and Anxiety, 29, 257–263.
Lohr, J. M., Olatunji, B., & Sawchuk, C. (2007).
A functional analysis of danger and safety sig-
nals in anxiety disorders. Clinical Psychology
Review, 27, 114–126.
Mennin, D. S., Ellard, K. K., Fresco, D. M., &
Gross, J. J. (2013). United we stand: Emphasiz-
ing commonalities across cognitive- behavioral
Emotion Regulation Therapy 489
therapies within a broadening field of inter-
vention science. Behavior Therapy, 44(2),
234–238.
Mennin, D. S., & Fresco, D. M. (2009). Emotion
regulation as an integrative framework for
understanding and treating psychopathology.
In A. M. Kring & D. M. Sloan (Eds.), Emo-
tion regulation in psychopathology: A transdi-
agnostic approach to etiology and treatment
(pp. 356–379). New York: Guilford Press.
Mennin, D. S., & Fresco, D. M. (2011, Novem-
ber). Emotion regulation therapy for complex
and refractory presentations of anxiety and
depression. A spotlight presentation at the
annual meeting of the Association for Behav-
ioral and Cognitive Therapies, Toronto, Can-
ada.
Mennin, D. S., Fresco, D. M., & Aldao, A.
(2012, November). Increasing vagal flexibility
predicts improved clinical outcomes in emo-
tion regulation therapy for GAD. A paper
delivered at the annual meeting of the Associa-
tion for Behavioral and Cognitive Therapies,
National Harbor, MD.
Mennin, D. S., Heimberg, R. G., Turk, C. L., &
Fresco, D. M. (2005). Preliminary evidence
for an emotion dysregulation model of gener-
alized anxiety disorder. Behaviour Research
and Therapy, 43, 1281–1310.
Mennin, D. S., Holaway, R. M., Fresco, D. M.,
Moore, M. T., & Heimberg, R. G. (2007).
Delineating components of emotion and its
dysregulation in anxiety and mood psycho-
pathology. Behavior Therapy, 38(3), 284–
302.
Mogg, K., & Bradley, B. P. (2005). Attentional
bias in generalized anxiety disorder versus
depressive disorder. Cognitive Therapy and
Research, 29, 29–45.
Muraven, M. R., & Baumeister, R. F. (2000).
Self- regulation and depletion of limited
resources: Does self- control resemble a mus-
cle? Psychological Bulletin, 126, 247–259.
Newman, M. G., & Llera, S. (2011). A novel
theory of experiential avoidance in general-
ized anxiety disorder: A review and synthesis
of research supporting a contrast avoidance
model of worry. Clinical Psychology Review,
31, 371–382.
Newman, M. G., Przeworski, A., Fisher, A. J.,
& Borkovec, T. D. (2010). Diagnostic comor-
bidity in adults with generalized anxiety dis-
order: Impact of comorbidity on psychother-
apy outcome and impact of psychotherapy on
comorbid diagnoses. Behavior Therapy, 41,
59–72.
Nolen- Hoeksema, S., Wisco, B. E., & Lyubomir-
sky, S. (2008). Rethinking rumination. Per-
spectives on Psychological Science, 3, 400–
424.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. (2002). Rethinking feelings: An
fMRI study of the cognitive regulation of emo-
tion. Journal of Cognitive Neuroscience, 14,
1215–1229.
O’Doherty, J. P. (2004). Reward representations
and reward- related learning in the human
brain: Insights from neuroimaging. Current
Opinion in Neurobiology, 14, 769–776.
Olatunji, B. O., Cisler, J. M., & Tolin, D. F.
(2010). A meta- analysis of the influence of
comorbidity on treatment outcome in the anx-
iety disorders. Clinical Psychology Review,
30, 642–654.
Porges, S. (2001). The polyvagal theory: Phylo-
genetic substrates of a social nervous system.
International Journal of Psychophysiology,
42, 123–146.
Posner, M. I., & Rothbart, M. K. (1992). Atten-
tional regulation— from mechanism to cul-
ture. International Journal of Psychology, 27,
41–57.
Ray, R. D., Wilhelm, F. H., & Gross, J. J. (2008).
All in the mind’s eye?: Anger rumination and
reappraisal. Journal of Personality and Social
Psychology, 94, 133–145.
Roemer, L., & Orsillo, S. M. (2009). Mindful-
ness- and acceptance- based behavioral thera-
pies in practice. New York: Guilford Press.
Rottenberg, J., & Gross, J. J. (2003). When emo-
tion goes wrong: Realizing the promise of
affective science. Clinical Psychology Science
and Practice, 10, 227–232.
Schiller, D., & Delgado, M. R. (2010). Over-
lapping neural systems mediating extinction,
reversal and regulation of fear. Trends in Cog-
nitive Sciences, 14, 268–276.
Segal, Z. V., Williams, J. M. G., & Teasdale, J.
D. (2002). Mindfulness- based cognitive ther-
apy for depression: A new approach to pre-
venting relapse. New York: Guilford Press.
Sheppes, G., & Gross, J. J. (2011). Is timing
everything?: Temporal considerations in emo-
tion regulation. Personality and Social Psy-
chology Review, 15(4), 319–331.
Smallwood, J., & Schooler, J. W. (2006). The
restless mind. Psychological Bulletin, 132,
946–958.
490 INTERVENTIONS
Sokol- Hessner, P., Camerer, C. F., & Phelps, E.
A. (2013). Emotion regulation reduces loss
aversion and decreases amygdala responses to
losses. Social Cognitive and Affective Neuro-
science, 8(3), 341–350.
Stein, M. B., & Paulus, M. P. (2009). Imbalance
of approach and avoidance: The yin and yang
of anxiety disorders. Biological Psychiatry,
66(12), 1072–1074.
Watkins, E., Teasdale, J. D., & Williams, R. M.
(2000). Decentering and distraction reduce
overgeneral autobiographical memory in depres-
sion. Psychological Medicine, 30, 911–920.
Watkins, E. R. (2008). Constructive and uncon-
structive repetitive thought. Psychological
Bulletin, 134, 163–206.
Watson, D. (2005). Rethinking the mood and
anxiety disorders: A quantitative hierarchical
model for DSM-V. Journal of Abnormal Psy-
chology, 114(4), 522–536.
Westra, H., Arkowitz, H., & Dozois, D. (2009).
Adding a motivational interviewing pretreat-
ment to cognitive behavioral therapy for gen-
eralized anxiety disorder: A preliminary ran-
domized controlled trial. Journal of Anxiety
Disorders, 23, 1106–1117.
Whitmer, A. J., & Gotlib, I. H. (2012). Switching
and backward inhibition in major depressive
disorder: The role of rumination. Journal of
Abnormal Psychology, 121(3), 570–578.
Wilson, K. G., & Murrell, A. R. (2004). Values
work in acceptance and commitment therapy.
In S. C. Hayes, V. M. Follette, & M. Linehan
(Eds.), Mindfulness and acceptance: Expand-
ing the cognitive- behavioral tradition. New
York: Guilford Press.
Woody, S., & Rachman, S. (1994). Generalized
anxiety disorder (GAD) as an unsuccessful
search for safety. Clinical Psychology Review,
14, 745–753.

491
Our aim in this chapter is to describe a set of
emotion regulation skills developed within
the context of dialectical behavior therapy
(DBT; Linehan, 1993a, 1993b) and their
potential to serve as a transdiagnostic inter-
vention. DBT is a comprehensive cognitive-
behavioral treatment originally developed
for suicidal individuals and later expanded
to suicidal individuals meeting criteria for
borderline personality disorder (BPD). DBT
has since been adapted to treat BPD with
several comorbidities and other psychologi-
cal disorders in which problems in emotion
regulation lead to psychopathology. Data for
the efficacy of DBT are extensive, including
43 clinical trials conducted across 21 inde-
pendent research teams (for a review, see
Neacsiu & Linehan, in press).
DBT conceptualizes difficulties with emo-
tion regulation as a consequence of biosocial
transactions: A biological sensitivity to emo-
tions interacts with aversive or invalidating
experiences during childhood and adoles-
cence, and leads to neurobiological malfunc-
tion and to insufficient skills to manage the
emotional system (Linehan, 1993a; Crowell,
Beauchaine, & Linehan, 2009). Therefore, a
primary focus in DBT is to teach clients how
to regulate emotional responses actively.
In this chapter we present a model of
emotion regulation that we teach in DBT.
We argue that BPD is a disorder of perva-
sive emotion dysregulation, and also provide
examples of how this model is relevant to
people without BPD who have difficulties
managing emotions. We then present how
the DBT skills map onto the model of emo-
tion regulation, and how they can be used
systematically to change dysregulation. We
conclude by describing the research needed
to evaluate the model proposed.
Emotion and Emotion Dysregulation
The DBT Model of Emotion Regulation
Similar to many others (e.g., Ekman & David-
son, 1994), in DBT we consider emotions to
be complex, brief, involuntary, patterned,
full- system responses to internal and exter-
nal stimuli. DBT emphasizes the importance
of the evolutionary adaptive value of emo-
tions (Tooby & Cosmides, 1990). Although
emotional responses are viewed as systemic,
we present them to clients as comprising six
transacting subsystems that are practical in
both understanding and learning to regu-
late emotions: (1) emotion vulnerability fac-
tors; (2) internal and/or external events that
serve as emotional cues; (3) interpretations
CHAPTER 29
Dialectical Behavior Therapy: An Intervention
for Emotion Dysregulation
Andrada D. Neacsiu
Martin Bohus
Marsha M. Linehan
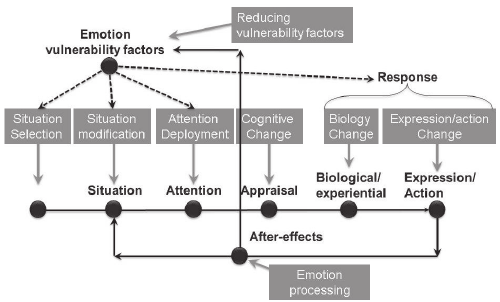
492 INTERVENTIONS
of cues; (4) emotional response tendencies,
including physiological responses, cogni-
tive processing, experiential responses, and
action urges; (5) nonverbal– verbal expres-
sive responses and actions; and (6) afteref-
fects of the initial emotion, including sec-
ondary emotions (see Linehan, 1993a).
Although the DBT emotion model was
developed to serve a clinical population, it
is interesting to note the similarities it has to
the modal model of emotions that evolved
in basic science (Gross & Thompson, 2007).
Briefly, in the modal model, emotions origi-
nate from person– situation transactions that
are relevant to one’s goals and values. Such
a situation acts as a cue and draws the indi-
vidual’s attention, gives rise to an appraisal
of the event, and leads to an emotional
response. This response is associated with
behavioral displays and action tendencies,
and is malleable, in that the course of the
emotion is not fixed when it starts (Gross &
Thompson, 2007).
Both the DBT and the modal model
include the importance of attending to a
cue within a relevant context, highlight
appraisals as potentially influencing the
course of the emotion and present how
emotions directly affect context. Never-
theless, DBT places more emphasis on dif-
ficulties regulating the emotional response
after it has already been initiated, espe-
cially when it is past the point at which it
could be suppressed. Therefore, one differ-
ence between the models is that in DBT the
emotional response is broken into experien-
tial and expressive responses. Research sup-
porting the importance of this distinction
for clinical populations suggests that pro-
cesses occurring after the emotional firing
are key for psychopathology (Aldao, Nolen-
Hoeksema, & Schweizer, 2010).
An additional difference is the inclusion
of emotion vulnerability factors. The con-
struct of emotion vulnerability refers to the
effects of distal and proximal prior events
on the initiation, course, and intensity of
emotional responses. For example, individu-
als diagnosed with BPD who meet diagnos-
tic criteria for co- occurring posttraumatic
stress disorder (PTSD; a distal vulnerability
factor) report significantly higher emotion
dysregulation (Harned, Rizvi, & Linehan,
2010). Similarly, sufficient sleep (a proximal
vulnerability factor) leads to less emotional
intensity when compared to lack of sleep
(Gujar, Yoo, Hu, & Walker, 2011).
In this chapter, we reorganize Linehan’s
original model (1993a) to be applicable to a
wider range of disorders and to use termi-
nology consistent with basic research mod-
els (Figure 29.1; Table 29.1). Briefly, in this
model, emotions start within the context of
a situation, where a cue grabs the individu-
al’s attention. The cue is appraised or inter-
preted, which triggers an emotional response
that comprises biological– experiential
changes (including urges or response ten-
dencies) and expressions– actions changes
(including body language, facial expression,
and actions). All components are affected by
proximal and distal emotion vulnerability
FIGURE 29.1. DBT extended model of emotion regulation. Adapted to be consistent with Gross and
Thompson (2007).

Dialectical Behavior Therapy 493
factors. The emotional response is followed
by aftereffects, including secondary emo-
tions.
To give an example: A depressed woman
has a fight with her partner over house
chores, followed by a night of poor sleep.
Within the context of these emotion vulner-
ability factors, she walks into the kitchen
and a pile of dirty dishes [situation] in the
sink captures her attention. She appraises
the situation by thinking “The dishes are
dirty”; “I should have washed them”; and “I
didn’t so I’m a terrible wife.” The emotion
continues, with her heart beating faster, her
body slumping lower; she has the urge to go
to bed and hide under the covers [biological
response– action urge] and she starts crying
[expression– action response]. Her atten-
tion narrows [aftereffect] and, as she walks
around the house, she sees the clothes [situ-
ation] she did not iron [appraisal], which
refires her emotion of shame. She goes to
bed and covers her head with the blanket
[action response] and subsequently becomes
angry with herself [secondary emotion].
Figure 29.1 and Table 29.1 present types
of strategies that can be used to change each
emotion component. Like Davidson (1998)
we contend that emotion regulation can be
both automatic and effortful, and that regu-
latory processes are an integral part of emo-
tional responding.
Pervasive Emotion Dysregulation
Emotion dysregulation is the inability,
even when one’s best efforts are applied,
to change in a desired way emotional cues,
experiences, actions, verbal responses, and/
or nonverbal expressions under norma-
tive conditions. Characteristics of emotion
dysregulation include an excess of aversive
emotional experiences, an inability to regu-
late intense physiological arousal, problems
turning attention away from stimuli, cogni-
tive distortions and failures in information
TABLE 29.1. Emotion Regulation Tasks and Corresponding DBT Skills
Emotion components Regulation strategies DBT skills
A. Emotion Vulnerability Factors Managing Vulnerability Factors
(Biological and Contextual)
Change Biological Sensitivity
(PLEASE skills)
Accumulate Positives
Build Mastery
Cope Ahead by Covert Rehearsal
[Mindfulness Skills]
B. Situation (emotional cue) Situation Selection
Situation Modification
Problem Solving
Interpersonal Effectiveness Skills
[Mindfulness Skills]
C. Attention Attention Deployment Distract
Crisis Survival Skills
[Mindfulness Skills]
D. Appraisal Cognitive Change Check the Facts
Reality Acceptance
[Mindfulness Skills]
E. Biological/ Experiential
Response
Biological Change Change Physiology (TIP skills)
Self-Soothe
Half-Smile/Willing Hands
[Mindfulness Skills]
F. Expression/ Action Response Expression and Action Change Opposite Action
[Mindfulness Skills]
G. Emotional After-Effects
(including emotional awareness)
Emotional Processing Identify and Label Emotions
[Mindfulness Skills]
Note. See text for explanation of acronyms.

494 INTERVENTIONS
processing, insufficient control of impulsive
behaviors related to strong emotions, diffi-
culties organizing and coordinating activi-
ties to achieve non-mood- dependent goals
when emotionally aroused, and a tendency
to “freeze” or dissociate under very high
stress (Ray et al., 2006). Pervasive emo-
tion dysregulation refers to an inability to
regulate emotions that occurs across a wide
range of emotions and situational contexts.
BPD: A Disorder of Pervasive
Emotion Dysregulation
The Disorder
BPD is a severe mental disorder with a seri-
ous dysregulation of the emotion system at
its core. Clients show a characteristic pat-
tern of instability in emotion regulation,
impulse control, interpersonal relationship,
and self-image. The often severe functional
impairment leads to substantial treatment
utilization and a mortality rate by suicide
of almost 10%, which is 50% higher than
the rate in the general population (American
Psychiatric Association, 2001). BPD affects
approximately 3% of the general popula-
tion, up to 10% of outpatients treated for
mental disorders, and up to 20% of inpa-
tients (Trull, Jahng, Tomko, Wood, & Sher,
2010). Because of the severity of the distur-
bance and the intensive treatment use, cli-
ents with BPD constitute a disproportion-
ately large subset of psychiatric patients,
consuming considerably more mental health
resources than most other psychiatric groups
(Soeteman, Hakkaart- van Roijen, Verheul,
& Busschbach, 2008).
BPD as a Disorder
of Emotion Regulation
Based on clinical experience, Linehan
(1993a) proposed that pervasive emo-
tion dysregulation in BPD is caused by an
interplay between biological (e.g., genetic,
intrauterine factors; trauma to the biologi-
cal system) vulnerability and aversive socio-
biographical experiences. According to this
biosocial theory, a child born with height-
ened biological sensitivity to emotional
cues encounters emotionally aversive expe-
riences (e.g., interpersonal violence, social
rejection, emotional neglect, invalidation)
and consequently develops biological and
psychological alterations of the emotion
regulation system (Linehan, 1993a; Distel
et al., 2011). The neurobiological alterations
in the emotion circuitry manifest in adult-
hood as heightened emotional sensitivity
(low threshold for recognition of/response
to emotional stimuli), heightened reactivity
(high amplitude of emotional responses),
and a slow return to baseline after emotion
induction (Linehan, 1993a; Crowell et al.,
2009). The psychological alterations involve
maladaptive or insufficient learning in how
to understand, label, regulate, or tolerate
emotional responses effectively. A BPD diag-
nosis is hypothesized to emerge from such
biological alterations, coupled with insuffi-
cient knowledge about emotion regulation.
Existing evidence provides emerging
support for this theory. First, 60% of cli-
ents with BPD report sexual abuse and
severe interpersonal violence during child-
hood (Hernandez, Arntz, Gaviria, Labad,
& Gutiérrez-Zotes, 2012; Bornovalova
et al., 2013), which leads to significantly
higher suicidality and emotion dysregula-
tion (Harned et al., 2010). Second, animal
research shows that traumatization during
early life stages leads to morphological alter-
ations of the central frontolimbic system and
to behavioral and epigenetic modifications
(Pryce & Feldon, 2003; Cirulli et al., 2009).
Third, preliminary data connect genetic fac-
tors with the development of borderline fea-
tures (Distel et al., 2009).
Neurobiological Dysfunction in BPD
Numerous studies have tested biological
alterations in the emotion circuitry in BPD,
with the majority of studies assessing reac-
tivity and return to baseline. Findings are
extensive and somewhat mixed; next, we
present some highlights of this body of lit-
erature.
Functional and structural data support
enhanced reactivity and slow return to
baseline in BPD. In this population, volume
reduction of the amygdala (Nunes et al.,
2009) was correlated with amygdala hyper-
reactivity to emotional stimuli (e.g., Her-
pertz et al., 2001; Niedtfeld et al., 2012).
This heightened amygdala activation was
more prominent in BPD and took longer to
return to baseline when compared to clini-
Dialectical Behavior Therapy 495
cal and nonclinical controls (Hazlett et al.,
2012). In addition to amygdala dysfunction,
volume reductions in brain areas hypoth-
esized to serve emotion regulation func-
tions were also found (Tebartz van Elst et
al., 2003; Minzenberg, Fan, New, Tang,
& Siever, 2008). Recent studies also report
reduced neural connectivity between such
brain areas and the amygdala at baseline
(New et al., 2007) or during emotional dis-
tress (Niedtfeld et al., 2012). Furthermore,
research indicates a reduced activation of
prefrontal areas after emotional induction
(Minzenberg, Fan, New, Tang, & Siever,
2007; Schulze et al., 2011). These findings
suggest that in BPD amygdala hyperactivity
in the presence of emotional stimuli takes
longer to return to baseline, partly because
of insufficient modulation from brain cen-
ters responsible for emotion regulation.
Self- report and psychophysiological find-
ings are less clear with regard to heightened
reactivity in individuals diagnosed with BPD
(for a detailed review, see Rosenthal et al.,
2008). Across several samples, people diag-
nosed with BPD self- report being more reac-
tive, having more negative emotions, and
experiencing more emotional instability than
non-BPD controls (Rosenthal et al., 2008;
Lobbestael & Arntz, 2010). Additional stud-
ies using heart rate as a psychophysiologi-
cal measures of distress (Gratz, Rosenthal,
Tull, Lejuez, & Gunderson, 2010; Reitz et
al., 2012), or electrophysiological recordings
(Marissen, Meuleman, & Franken, 2010)
also found greater emotional reactivity for
participants with BPD. Nevertheless, studies
using different psychophysiological indices
of distress (i.e., skin conductance response)
failed to show enhanced reactivity (e.g., Kuo
& Linehan, 2009; Rosenthal et al., 2008).
Thus, additional research is needed to better
understand the mixed findings on reactivity.
Self- report and psychophysiological data
support a prolonged return to baseline.
Applying ambulatory assessments under daily
life conditions, Stiglmayr, Shapiro, Stieglitz,
Limberger, and Bohus (2001) reported signif-
icantly longer intervals of activated aversive
emotions but no nonspecific arousal for peo-
ple with BPD compared to healthy controls.
In addition, in a laboratory study participants
diagnosed with BPD evidenced prolonged
return to baseline after induced anger (Jacob
et al., 2008) and stress (Reitz et al., 2012).
Emotion Dysregulation in BPD
It is important to highlight that the con-
struct of emotion dysregulation is indepen-
dent from neurobiological alterations in
the emotion circuitry that are thought to
underlie BPD. Having increased sensitiv-
ity, reactivity and a slow return to baseline
may make it more difficult for individuals
to regulate emotions. At the same time,
persistent use of dysfunctional regulation
strategies may lead to continued biological
alteration. Whether neurobiological altera-
tions precede dysregulation, or vice versa,
remains an empirical question. Neverthe-
less, both constructs are crucial for under-
standing difficulties with emotions as pre-
sented in DBT.
We propose that individuals with BPD
have pervasive emotion dysregulation. In
support, below we summarize evidence
suggesting that individuals diagnosed with
BPD have problems with each set of emotion
regulation strategies described in the DBT
model. Difficulties with reducing emotion
vulnerability are highlighted by findings
suggesting dysregulated sleep patterns in
BPD individuals (Schredl et al., 2012), high
prevalence of abuse history and substance
use disorders (Trull et al., 2010; Distel et al.,
2012), as well as chronic health problems
and poor lifestyle choices (Frankenburg &
Zanarini, 2004).
Self- inflicted injuries (including suicide
attempts) and most other dysfunctional
behaviors (i.e., suicide threats, impulsive
behaviors, dissociation) are hypothesized
to be maladaptive problem- solving strate-
gies (Reitz et al., 2012), an escape mecha-
nism (Chapman, Gratz, & Brown, 2006), or
a way to communicate distress (Koerner &
Linehan, 1997). These behaviors may sug-
gest problems with regulating the biological
and expressive components of the emotion,
or with selecting effective situation modifi-
cation strategies. Problems with emotion-
induced dissociation have also consistently
been shown and suggest impairments in
the attention regulation component (Ebner-
Priemer et al., 2009). In addition, research
has documented problems with cognitive
flexibility (Ruocco, 2005), cognitive change
(Selby & Joiner, 2009), emotional aware-
ness (Levine, Marzialli, & Hood, 1997),
and aftereffects (Korfine & Hooley, 2000),

496 INTERVENTIONS
suggesting difficulties with regulation of all
the remaining emotion components.
The DBT Model and Other Disorders
Emotional dysregulation has been reported
to underlie etiological and maintenance
mechanisms for a large number of mental
health problems (Kring & Sloan, 2010).
Literature reviews have demonstrated that
mood disorders, anxiety disorders, sub-
stance use disorders (SUDs), eating disor-
ders, schizophrenia, and even psychotic
disorders are directly linked to emotion
dysregulation (Cisler, Olatunji, Feldner, &
Forsyth, 2010; Harrison, Sullivan, Tchan-
turia, & Treasure, 2009; Kring & Werner,
2004; Thorberg, Young, Sullivan, & Lyvers,
2009).
Individuals diagnosed with a variety of
Axis I disorders also appear to reveal emo-
tion dysregulation patterns. Increased reac-
tivity when compared to controls has been
connected to generalized anxiety disorder
(GAD; Mennin, Heimberg, Turk, & Fresco,
2005), substance dependence (Thorberg et
al., 2009), social anxiety disorder (SAD),
and specific phobias (Etkin & Wager, 2007).
Furthermore, the transaction between neu-
robiological dysfunction (e.g., anxiety sen-
sitivity) and lack of skills to manage it is
similar to the etiological theories presented
in panic disorder (Barlow, Allen, & Choate,
2004), GAD (Mennin et al., 2005), and some
specific phobias (Cisler et al., 2010). It can
therefore be hypothesized that in cases where
emotion dysregulation is involved, reactivity
to emotional cues coupled with insufficient
regulation strategies may result in psychopa-
thology. Thus, emotion dysregulation may
be a transdiagnostic phenomenon.
People diagnosed with BPD have difficul-
ties with regulating all of the emotion sub-
systems described in the DBT model. Similar
difficulties can be found in many additional
Axis I disorders, as described in the fifth edi-
tion of the Diagnostic and Statistical Man-
ual of Mental Disorders (DSM-5). Difficul-
ties managing emotion vulnerability factors
are common in mood and anxiety disorders
and in SUDs (e.g., Monti & Monti, 2000;
Wong, Brower, Fitzgerald, & Zucker, 2004).
Individuals diagnosed with anxiety and
mood disorders also make negative inter-
pretations (e.g., catastrophizing) that lead to
dysregulated emotions, and use maladaptive
situation selection and situation modifica-
tion strategies (e.g., avoidance, use of safety
cues; Kring & Werner, 2004; Aldao et al.,
2010). Experimental evidence also makes
a strong link between depression and anxi-
ety, and difficulties regulating attention in
emotional contexts. For example, depressed
individuals have a reduced capacity to sus-
tain positive emotions (Heller et al., 2009)
and more difficulties finding attentional
distracters in the context of a distressing
emotion (Koole, 2009) when compared with
controls. Similarly, when presented with
social threat stimuli, participants with SAD
report more negative emotions than do con-
trols, suggesting attention deployment and/
or cognitive change dysregulation (Goldin,
Manber, Hakimi, Canli, & Gross, 2009).
Finally, difficulties with emotion pro-
cessing have been linked to PTSD (Frewen,
Dozois, Neufeld, & Lanius, 2012).
DBT Emotion Regulation Skills
Rationale
Linehan (1993a) included in DBT a set of
concrete skills translated from behavioral
research and other evidence- based treat-
ments, aimed to address emotion dysregula-
tion in BPD. It was therefore hypothesized
that increases in skills use led to improve-
ments in emotion regulation, which in turn
led to positive outcomes in treated popula-
tions. Empirical findings indeed suggest that
use of DBT skills explains changes in depres-
sion, anger regulation, and suicidal behavior
across BPD treatments (Neacsiu, Rizvi, &
Linehan, 2010). Therefore, behavioral skills
are likely a potent mechanism of change for
emotion dysregulation.
In addition to the five sets of regulatory
processes proposed by Gross and Thomp-
son (2007), DBT targets five additional
processes: managing emotion vulnerability
factors, biological change, expression and
action change, and emotional processing (at
the point of emotional aftereffects). We next
present the skills that directly map onto the
DBT model of emotions, organizing them
according to regulation process to which
they primarily relate, although the functions
Dialectical Behavior Therapy 497
of each set of skills can be applied across
many of the regulation processes (see Table
29.1). This is particularly the case for mind-
fulness skills, which can be viewed as criti-
cal at every juncture in the emotion regula-
tion process.
Strategies for Managing Emotion
Vulnerability Factors
DBT teaches clients to decrease emotion
vulnerability factors by increasing happi-
ness and resilience (“building a life worth
living”) through a set of skills that target
biological homeostasis and influence emo-
tional reactivity. The PLEASE skills target
treating Physical iLlness (Anderson, Hack-
ett, & House, 2004), balancing nutrition
and Eating (Smith, Williamson, Bray, &
Ryan, 1999), staying off nonprescribed
mood-Altering drugs, getting sufficient but
not excessive Sleep (Gujar et al., 2011), and
getting adequate Exercise (Cox, Thomas,
Hinton, & Donahue, 2004).
In addition, DBT promotes resilience by
teaching skills for accumulating positive life
events and for building a sense of general-
ized mastery. Increasing the number of plea-
surable events in one’s life is one approach
to increasing positive emotions. In the short
term, this involves increasing daily posi-
tive experiences. In the long term, it means
working on goals related to important life
values, so that pleasant events will occur
more often. Building mastery is achieved by
engaging in activities that increase a sense
of competence and self- efficacy. The focus is
very similar to activity and mastery schedul-
ing in psychotherapy for depression (Martell,
Addis, & Jacobson, 2001). Both skills have
been shown to predict decreased vulner-
ability to negative emotional states (Joiner,
Lewinsohn, & Seeley, 2002; de Beurs et al.,
2005).
Coping ahead (Linehan, in press) is an
additional skill that promotes contextual
resiliency. Individuals learn to use imaginal
exposure and rehearsal to cope successfully
with a difficult situation ahead of time. For
people who are prone to dysregulation, cop-
ing ahead via covert rehearsal can be help-
ful in building the coping skills necessary
for problem solving (see Fourkas, Avenanti,
Urgesi, & Aglioti, 2006). Thus, this skill is
likely to increase peoples’ appraisal of their
own ability to cope with an emotional event,
effectively increasing a sense of mastery and
self- efficacy.
Situation Selection–
Modification Strategies
Situation selection and modification are
two classes of emotion regulation strategies
through which emotions are modulated via
stimulus control (i.e., avoiding or modify-
ing situations that generate unwanted emo-
tions). To promote effective situation selec-
tion skills, DBT teaches how to generate a
list of pros and cons to guide a course of
action. To improve situation modification,
DBT teaches a simple set of problem- solving
skills (Linehan, in press) aimed at chang-
ing or developing strategies for eliminating,
reducing, or avoiding emotionally problem-
atic situations. The focus here is on defining
those situations that cue unwanted emotions,
then applying standard problem- solving
steps, such as those outline by D’Zurilla and
Nezu (1999) and others.
Because many problems are interpersonal
(and even if they are not may require inter-
personal interactions to solve), DBT also
includes a set of interpersonal effectiveness
skills. These skills focus on how to obtain
a wanted objective without hurting the
interpersonal relationship or one’s own self-
respect. Coping ahead as a way to practice
these skills and to manage intense emotional
arousal before it happens is often a helpful
addition to problem solving.
DBT also includes a set of mindfulness
skills that emphasize observing, describing,
and participating in the present moment
effectively and without judgment. These
skills may also promote adaptive situation
selection by nonjudgmentally expanding
awareness regarding situations that in the
past have evoked emotional experience.
This awareness is hypothesized to increase
sensitivity to the current contingencies in
the environment, allowing the opportunity
for new learning. Thus, by seeing reality
“as it is” (i.e., being in the present moment
without historical filters), mindfulness may
enhance the ability of an individual to decide
what situations to avoid, when to attempt to
problem- solve, or when to cope ahead.
498 INTERVENTIONS
Attentional Deployment Strategies
Mindfulness skills are often used in DBT
to promote attentional control, which can
reduce problems with attentional deploy-
ment. Mindfulness involves learning to con-
trol the focus of attention, not the object to
which one attends (e.g., observing a thought
as a thought or emotion as emotion, with-
out attempting to change the thought or
emotion). Being able to disengage from
emotional stimuli may reduce the tendency
to experience negative affect (Ellenbogen,
Schwartzman, Stewart, & Walker, 2002),
and redeploying attention has been postu-
lated to lead to a “flexibility of attention”
(Teasdale, Segal, & Williams, 1995) needed
for successful attention modulation in emo-
tional contexts. Thus, mindfulness may help
modulate emotional experience by enhanc-
ing the practioner’s ability to turn his or her
attention away from that which is not useful
(or effective) and attend to what is (Lynch,
Rosenthal, et al., 2006).
Cognitive Change Strategies
DBT focuses on analyzing and correcting
situation appraisals by checking the facts
(Linehan, in press). These skills focus on
discriminating assumptions, interpretations,
ruminative thoughts, and worries from the
actual observed facts of situations. Support
for this set of skills comes from a number
of studies comparing different reappraisal
strategies, including nonappraisal control
conditions, following presentation of emo-
tional cues (e.g., Lazarus & Abramovitz,
1962).
An additional set of strategies (reality
acceptance) targets changing one’s appraisal
of emotions as experiences that cannot be
tolerated or experienced willingly. Emo-
tion acceptance, when compared to emotion
suppression, or a control, has been shown
to result in less subjective anxiety or avoid-
ance in clients diagnosed with panic disor-
der undergoing a carbon dioxide challenge
(Levitt, Brown, Orsillo, & Barlow, 2004). In
addition, coaching in an acceptance mind-
set, compared to coaching in a control- your-
emotions mindset or a placebo condition,
significantly increased the amount of time a
subject was willing to spend in a cold pres-
sor task (Hayes et al., 1999). With respect
to emotions, DBT reality acceptance skills
(“turning the mind” toward acceptance,
radical acceptance, and willingness over
willfulness) focus on radical (meaning full
and complete) acceptance of the current
emotion and willingness to experience even
aversive emotions.
Mindfulness skills may also alter situa-
tion appraisal by reducing literal belief in
emotional appraisals. Mindfulness teaches
individuals to observe appraisals as only
thoughts that are not necessarily literally
true. This is hypothesized to increase sen-
sitivity to the current contingencies in the
environment, allowing the opportunity for
new learning. In this context, mindfulness
in DBT would not be predicted to reduce the
frequency of distressing thoughts but instead
to decrease the influence these thoughts have
on subsequent behavior and emotions.
Biological–Experiential
Change Strategies
In DBT, an important part of the biologi-
cal component of an emotion is the action
tendency, or urge, to act in a specific man-
ner. DBT provides a range of distress toler-
ance skills whose aim is to inhibit acting on
maladaptive urges that interfere with long-
term emotion regulation. These skills are
also designed to down- regulate the extreme
physiological arousal that often accompa-
nies intense emotions. The function of these
skills is to impact high arousal quickly, with-
out requiring a high level of cognitive pro-
cessing to complete. Grouped under the term
TIP skills (Linehan, in press) these skills tar-
get activation of the parasympathetic ner-
vous system.
The first skill (Temperature change with
ice water) has to do with using cold, icy
water on the face to trigger the human dive
reflex (which is typically elicited to aid sur-
vival when falling into a frozen lake). This
reflex can be triggered by a combination of
breath holding and face immersion in cold
water. The physiological response that fol-
lows involves both branches of the auto-
nomic nervous system and reduces emotional
arousal for a short period of time (Hurwitz
& Furedy, 1986).
Intense exercise is also recommended if
arousal is very high. Most important here
is the intensity of the exercise. Cox and col-
Dialectical Behavior Therapy 499
leagues (2004) compared intensity of bouts
of exercise and found that while intensity
of exercise conditions did not differ in state
anxiety immediately after exercise, a sig-
nificant difference favoring the most intense
exercise condition over the control condition
emerged at 30 minutes postexercise.
Additional distress tolerance strategies are
Paced breathing and Progressive relaxation,
soothing one of the five senses, adopting an
opened posture with palms facing the ceiling
(willing hands), or adopting a serene facial
expression (half smile).
Expression and Action
Change Strategies
Changing expression and action components
of emotions implies preventing emotional
actions, or acting in a way that opposes or
is inconsistent with the emotion. The DBT
skill of opposite action is based on the idea
that not only is changing action tendencies
essential for reducing emotional disorders,
but also that deliberate actions opposite to
those associated with unwanted emotions
can effectively change emotions as well as
action tendencies. Others have made this
same point (e.g., Barlow, 1988, p. 313). Izard
(1977) stated that treatment for anxiety dis-
orders involves “the individual learn[ing] to
act his way into a new feeling” (cited in Bar-
low, 1988, p. 410).
Opposite action in DBT applies principles
of exposure- based treatments for anxiety
disorders across the entire domain of emo-
tions. All exposure- based interventions
include this one common element: Individu-
als have to approach the object/situation
that is fearful, thus acting counter to (and
inhibiting) their prominent urges to avoid.
Effective treatments for anger also require
individuals to act counter to the urges asso-
ciated with anger (attack physically or ver-
bally) by leaving the situation. Anger inter-
ventions also focus on taking the opposite
perspective, and shifting from aggression
and blame to gentleness and forgiveness
(e.g., Tafrate, Kassinove, & Dundin, 2002).
A number of researchers have observed that
effective therapies for depression also share
a common thread: They activate behavior.
For example cognitive therapy (Beck, Rush,
Shaw, & Emery, 1979) and behavioral acti-
vation (BA; Martell et al., 2001) require that
the individual galvanize him- or herself to
engage in activities that result in a sense of
mastery or pleasure. This engagement runs
counter to the depression urge to withdraw
and shut down.
Opposite action “all the way” (Linehan,
in press) targets changing the entire range of
physical responses that accompany action,
including visceral responses, body postures,
facial expressions, and movements. A large
literature has demonstrated that the activa-
tion of specific physical states activates the
other facets of the corresponding emotion
responses, whether via the face (e.g., Duclos
& Laird, 2001), posture (Stepper & Strack,
1993) or respiration. To the contrary, there
is ample empirical evidence that modulat-
ing one’s physical state alters one’s emo-
tional state (Philippot, Baeyenes, Douilliez,
& Francart, 2004). Also implied by “all the
way” are emotion- linked thought patterns
and verbal responses. The idea here is to act
contrary to an emotion, not to mask or hide
emotions.
In part, opposite action is hypothesized to
work by influencing classically conditioned
emotional responses (Lynch, Chapman,
Rosenthal, Kuo, & Linehan, 2006). Oppo-
site action may also create sensory feedback
from facial muscles and skin that can be
transformed directly into emotional expe-
rience without cognitive mediation (Izard,
1977). Finally, self- perception of expressive
behavior, action, and appraisals regarding
proprioceptive sensations has been proposed
to influence subjective emotional experience
(Laird, 1974). Opposite action may influence
emotion by changing the perception of the
emotional event. Thus, behavior that is the
opposite of the automatic response or action
urge of an emotion is intended to alter the
meaning of the emotional event automati-
cally and without conscious effort (Lynch,
Rosenthal, et al., 2006). In essence, people
conclude that they feel safe because they are
“acting as if” all is safe.
Strategies for Changing
Emotional Aftereffects
Aftereffects of emotions, which include
changes in attention, memory, and reason-
ing, are fairly well established (see Dolan,
2002, for a review). These aftereffects can
increase the probability that the emotion

500 INTERVENTIONS
will reoccur. Interrupting the cycle can be
enhanced if the individual notices and iden-
tifies a current, ongoing emotion, which can
then guide application of relevant change
strategies.
Therefore, DBT included a skill (Observe
and Describe Emotions) through which
increased awareness of the emotional expe-
rience is promoted. This skill is supported
by research showing that processing emo-
tional experience with greater specificity has
advantages for improved emotion regulation
over emotional processing that is overgen-
eral or nonspecific (e.g., Williams, Stiles, &
Shapiro, 1999). Indeed, recent research has
demonstrated that priming individuals with
overgeneral emotional memories results in
more intense emotional experience com-
pared to priming specific emotional memo-
ries or a control condition (Schaefer et al.,
2003). In addition, experimentally manipu-
lated social anxiety has been shown to be
reduced by observing and describing spe-
cifically the fear producing cues, in contrast
to general impressions regarding cues that
resulted in higher fear (Philippot, Burgos,
Verhasselt, & Baeyens, 2002).
Drawing from the work of many, includ-
ing both Shaver (e.g., Shaver, Schwartz, Kir-
son, & O’Connor, 1987) and Hupka (e.g.,
Hupka, Lenton, & Hutchinson, 1999), Line-
han (in press) expanded the original list of
six emotions to a taxonomy of 10 basic emo-
tions: anger, disgust, envy, fear, jealousy,
joy, love, sadness, shame, and guilt. For
each emotion the following characteristics
are listed: (1) family of emotion names asso-
ciated with the basic emotion, (2) typical
prompting events (cues), (3) interpretations
or appraisals, (4) biological changes and
experiences, (5) expressions and actions,
(6) aftereffects, and (7) secondary emotions
associated with each family of emotions.
Using the taxonomy, clients are coached in
learning to observe and describe their emo-
tions relative to various events.
Evidence for DBT Skills as a
Treatment for Emotion Dysregulation
Problems with regulating each component of
the emotion system can be connected with
BPD and with other disorders. As we argued
in the previous section, there are DBT skills
to target each of the types of emotion dys-
regulation our model presented. Therefore,
DBT skills training is a promising candidate
for treating emotion dysregulation in BPD
and other Axis I disorders.
Treatment trials, albeit fraught with
methodological flaws, offer some support
that DBT skills training is effective in reduc-
ing emotion dysregulation in various clinical
presentations. A DBT skills training inter-
vention improved pre- to posttreatment rat-
ings of depression in abuse victims (Iverson,
Shenk, & Fruzzetti, 2009), and depression
and anger in vocational rehabilitation cli-
ents (Koons et al., 2006). When compared
to treatment as usual (TAU) or a wait-list
condition, DBT skills training decreased
depression in treatment- resistant depressed
individuals (Harley, Sprich, Safren, Jacobo,
& Fava, 2008) and self- reported anger in a
forensic sample (Evershed et al., 2003). DBT
skills training was also superior to standard
group therapy in improving depression,
anger and affect instability in a BPD sample
(Soler et al., 2009).
Therefore, although originally developed
to be part of a comprehensive intervention,
skills training by itself may be the mecha-
nism through which change occurs in a vari-
ety of populations with emotion regulation
difficulties. Indeed, skills training has been
linked to the reduction of emotion dysreg-
ulation indices (Neacsiu et al., 2010) and
emotion dysregulation has, in turn, been
related to a variety of mental health prob-
lems (Kring & Sloan, 2010). Although this
research area is in its infancy and more find-
ings are needed, the evidence suggests that
DBT skills are a promising intervention for
emotion dysregulation across psychopathol-
ogy.
Directions for Future Research
Psychopathology Research
Construct Validity of Emotion Regulation
We have refined the construct of emotion
regulation as applicable to psychopathology
and presented a testable model. Although we
have defined dysregulation as dysfunction
at either level in the emotion generation–
regulation process, we do not know whether
one or more “tipping” points differentiate
Dialectical Behavior Therapy 501
normative difficulties regulating extreme
emotional arousal versus non- normative
difficulties that predict serious emotional
disturbance. In addition, how difficulties
as emphasized by this model vary with each
mental disorder is also not yet clear.
Construct Validity of Pervasive
Emotion Dysregulation
We have proposed the construct of pervasive
emotion dysregulation and conceptualized it
as a combination of a tendency to high emo-
tionality across a wide array of both posi-
tive and negative emotions, together with an
inability to regulate intense emotion- linked
responses. The validity of this construct
has not been evaluated, nor are there mea-
sures of the construct. The high incidence
of comorbidity across emotional disorders
suggests that the construct may be a useful
one. Research is needed both to validate and
identify the parameters of the construct. We
further have proposed BPD as a model of
pervasive emotion dysregulation. Research
designed specifically to evaluate this conten-
tion, particularly research comparing BPD
to other emotional disorders, is needed.
Research on BPD and emotions outside of
anxiety, depression, and anger is also needed
to support or refute the pervasiveness of
emotion dysregulation in this disorder.
Neurobiological Dysfunction
of the Emotion Circuitry in BPD
We have defined neurobiological dysfunc-
tion of the emotion system in BPD as sensi-
tivity to emotional stimuli; intense reactions
to such stimuli; and a slow, delayed return to
an emotional baseline. First, more research
is needed to assess whether individuals with
BPD do have a heightened sensitivity to emo-
tional stimuli. Second, although it appears
evident that the intensity of all emotions is
enhanced in BPD, the empirical evidence that
clients with BPD are reactive to emotion-
eliciting cues is mixed. Additional research
clarifying the inconsistent findings on emo-
tional reactivity in BPD is sorely needed.
Furthermore, it is unclear whether mode of
stimulation (visual, auditory, somatic, etc.)
makes a difference.
We have highlighted clear evidence for
both structural and functional alterations in
the frontolimbic circuits of clients with BPD.
However, it remains unclear whether these
findings are specific to clients with BPD or
characteristic of individuals with emotion
regulation difficulties in general. It is also
unclear how neurobiological alterations
interact with pervasive emotion dysregula-
tion, especially in the case of social emo-
tions such as shame or guilt. There is only
beginning research on restitution of these
neurobiological alterations after successful
treatment.
Emotion Dysregulation
as a Transdiagnostic Phenomenon
As we have highlighted, emotion dysregula-
tion and alterations of the biological archi-
tecture of the emotion system are issues not
entirely unique to BPD. Therefore, future
research should further examine the rela-
tionship between problems with emotions
and other disorders. Furthermore, much of
the research assessing emotion dysregula-
tion in BPD has compared people with BPD
to healthy controls (for a review, see Rosen-
thal et al., 2008). There is a great need for
research examining the specificity of emo-
tion dysregulation in BPD, by comparing
people with BPD to those with other mental
disorders. This could help refine nuances
of how emotion dysregulation manifests in
psychopathology and how it can be more
effectively targeted.
Intervention Research
As noted previously, emerging data indicate
that the skills training component of DBT
is a successful stand-alone intervention for
emotion dysregulation in a variety of clini-
cal samples. What we do not know yet is
whether we can use DBT skills training as a
transdiagnostic treatment for emotion dys-
regulation.
In addition, we do not know whether some
DBT skills are more useful than others, nor
are there data regarding the role of compe-
tence of skills application (i.e., whether appli-
cation of the “right skill at the right time” is
important). It is also not clear which skills
are the right skills for various situations.
Given the propensity for emotional avoid-
ance in many emotionally disturbed indi-
viduals, it is extremely important to find out

502 INTERVENTIONS
when to teach clients to distract themselves
from unwanted emotions and cues and when
to expose themselves to emotions and emo-
tional stimuli. In DBT we make a distinction
between moderate and extreme emotional
responses. Extreme emotional responses are
defined as those accompanied by cognitive
processing that is so compromised that skills
requiring high use of cognitive resources
(e.g., problem solving, checking the facts)
are unlikely to be successful. With extreme
responses, skills that more directly impact
somatic arousal (e.g., deep breathing, using
ice water) or attention (e.g., distraction) are
recommended. Data verifying the wisdom of
these recommendations are sorely lacking.
This is particularly important in light of the
increasing use of mindfulness- based treat-
ment interventions, which teach individu-
als to notice and accept ongoing emotional
responses. The question might be reframed
as follows: When is mindfulness of current
emotions (a DBT skill) more or less impor-
tant?
Although there is a fair amount of basic
research supporting the specific skills taught
in DBT, there is little evidence on their indi-
vidual effectiveness as treatment interven-
tions in clinical populations. The systematic
examination of the DBT skills, both indi-
vidually and in combination, is an essen-
tial first step in improving treatment for
deregulated individuals. This is particularly
important for the skill of opposite action.
Linehan (1993a) has suggested that opposite
action will be effective across a wide range
of both dysfunctional positive (e.g., loving
the wrong person) and negative emotions.
One study found promising results for oppo-
site action with shame (Rizvi & Linehan,
2006), but other emotions have not been
studied explicitly. Thus, although DBT has
been thoroughly evaluated in efficacy stud-
ies, there has been substantially less empha-
sis on the treatment mechanisms of change,
and future research must work to narrow
this gap.
Conclusion
To sum up, we propose a framework for
emotion dysregulation that is applicable to
BPD and in theory can be extended to other
psychological disorders. We argue that emo-
tion dysregulation is prevalent beyond BPD
and invite research to support our model
in other disorders in which problems with
emotions have been identified. Furthermore,
we offer a set of skills aimed at addressing
directly common components of dysregu-
lation and illustrate how such skills have
been used with individuals diagnosed with
BPD. We propose additional ways in which
intervention research, as directly relevant
to emotion dysregulation, can advance our
knowledge and help make treatments for
this problem more effective.
Acknowledgment
The authors would like to thank Thomas R.
Lynch, PhD, for his contribution to the previous
version of this chapter.
References
Aldao, A., Nolen- Hoeksema, S., & Schweizer, S.
(2010). Emotion- regulation strategies across
psychopathology: A meta- analytic review.
Clinical Psychology Review, 30, 217–237.
American Psychiatric Association. (2001). Prac-
tice guideline for the treatment of patients
with borderline personality disorder. Ameri-
can Journal of Psychiatry, 158(Suppl. 10),
1–52.
Anderson, C. S., Hackett, M. L., & House, A. O.
(2004). Interventions for preventing depres-
sion after stroke. Cochrane Database Systems
Review, 2, CD003689.
Barlow, D. H. (1988). Anxiety and its disorders:
The nature and treatment of anxiety and
panic. New York: Guilford Press.
Barlow, D. H., Allen, L. B., & Choate, M. L.
(2004). Toward a unified treatment for emo-
tional disorders. Behavior Therapy, 35, 205–
230.
Beck, A. T., Rush, A. J., Shaw, B. F., & Emery, G.
(1979). Cognitive therapy of depression. New
York: Guilford Press.
Bornovalova, M. A., Huibregtse, B. M., Hicks,
B. M., Keyes, M., McGue, M., & Iacono, W.
(2013). Tests of a direct effect of childhood
abuse on adult borderline personality disorder
traits: A longitudinal discordant twin design.
Journal of Abnormal Psychology, 122(1),
180–194.
Chapman, A. L., Gratz, K. L., & Brown, M.
Dialectical Behavior Therapy 503
Z. (2006). Solving the puzzle of deliberate
self-harm: The experiential avoidance model.
Behaviour Research and Therapy, 44, 371–
394.
Cirulli, F., Francia, N., Berry, A., Aloe, L.,
Alleva, E., & Suomi, S. J. (2009). Early life
stress as a risk factor for mental health: Role
of neurotrophins from rodents to non-human
primates. Neuroscience and Biobehavioral
Review, 33, 573–585.
Cisler, J. M., Olatunji, B. O., Feldner, M. T., &
Forsyth, J. P. (2010). Emotion regulation and
the anxiety disorders: An integrative review.
Journal of Psychopathology and Behavioral
Assessment, 32, 68–82.
Cox, R. H., Thomas, T. R., Hinton, P. S., &
Donahue, O. M. (2004). Effects of acute 60
and 80% VO2max bouts of aerobic exercise
on state anxiety of women of different age
groups across time. Research Quarterly for
Exercise and Sport, 75, 165–175.
Crowell, S. E., Beauchaine, T. P., & Linehan, M.
M. (2009). A biosocial developmental model
of borderline personality: Elaborating and
extending Linehan’s theory. Psychological
Bulletin, 135, 495–510.
Davidson, R. J. (1998). Anterior electrophysio-
logical asymmetries, emotion, and depression:
Conceptual and methodological conundrums.
Psychophysiology, 35, 607–614.
de Beurs, E., Comijs, H., Twisk, J. W., Sonnen-
berg, C., Beekman, A. T., & Deeg, D. (2005).
Stability and change of emotional functioning
in late life: Modelling of vulnerability profiles.
Journal of Affective Disorders, 84, 53–62.
Distel, M. A., Middeldorp, C. M., Trull,
T. J., Derom, C. A., Willemsen, G., &
Boomsma, D. I. (2011). Life events and bor-
derline personality features: The influence
of gene– environment interaction and gene–
environment correlation. Psychological Medi-
cine, 4(4), 849–860.
Distel, M. A., Trull, T. J., de Moor, M. M., Vink,
J. M., Geels, L. M., et al. (2012). Borderline
personality traits and substance use: Genetic
factors underlie the association with smoking
and ever use of cannabis, but not with high
alcohol consumption. Journal of Personality
Disorders, 26(6), 867–879.
Distel, M. A., Trull, T. J., Willemsen, G., Vink, J.
M., Derom, C. A., Lynskey, M., et al. (2009).
The five- factor model of personality and bor-
derline personality disorder: A genetic analysis
of comorbidity. Biological Psychiatry, 66(12),
1131–1138.
Dolan, R. J. (2002). Emotion, cognition, and
behavior. Science, 298, 1191–1194.
Duclos, S. E., & Laird, J. D. (2001). The delib-
erate control of emotional experience through
control of expressions. Cognition and Emo-
tion, 15, 27–56.
D’Zurilla, T. J., & Nezu, A. M. (1999). Problem-
solving therapy: A social competence
approach to clinical intervention (2nd ed.).
New York: Springer.
Ebner- Priemer, U. W., Mauchnik, J., Kleindienst,
N., Schmahl, C., Peper, M., Rosenthal, Z., et
al. (2009). Emotional learning during disso-
ciative states in borderline personality disor-
der. Journal of Psychiatry and Neuroscience,
34(3), 214–222.
Ekman, P., & Davidson, R. J. (1994). The nature
of emotion. New York: Oxford University
Press.
Ellenbogen, M. A., Schwartzman, A. E., Stewart,
J., & Walker, C. D. (2002). Stress and selec-
tive attention: The interplay of mood, cortisol
levels, and emotional information processing.
Psychophysiology, 39, 723–732.
Etkin, A., & Wager, T. D. (2007). Functional
neuroimaging of anxiety: A meta- analysis of
emotional processing in PTSD, social anxiety
disorder, and specific phobia. American Jour-
nal of Psychiatry, 164, 1476–1488.
Evershed, S., Tennant, A., Boomer, D., Rees, A.,
Barkham, M., & Watson, A. (2003). Practice-
based outcomes of dialectical behaviour
therapy (DBT) targeting anger and violence,
with male forensic patients: A pragmatic and
non- contemporaneous comparison. Criminal
Behavior and Mental Health, 13, 198–213.
Fourkas, A. D., Avenanti, A., Urgesi, C., &
Aglioti, S. M. (2006). Corticospinal facilita-
tion during first and third person imagery.
Experimental Brain Research, 168(1–2), 143–
151.
Frankenburg, F. R., & Zanarini, M. C. (2004).
The association between borderline person-
ality disorder and chronic medical illnesses,
poor health- related lifestyle choices, and
costly forms of health care utilization. Journal
of Clinical Psychiatry, 65(12), 1660–1665.
Frewen, P. A., Dozois, D. A., Neufeld, R. J., &
Lanius, R. A. (2012). Disturbances of emo-
tional awareness and expression in posttrau-
matic stress disorder: Meta-mood, emotion
regulation, mindfulness, and interference
of emotional expressiveness. Psychological
Trauma: Theory, Research, Practice, and Pol-
icy, 4(2), 152–161.
504 INTERVENTIONS
Goldin, P. R., Manber, T., Hakimi, S., Canli, T.,
& Gross, J. J. (2009). Neural bases of social
anxiety disorder: Emotional reactivity and
cognitive regulation during social and physi-
cal threat. Archives of General Psychiatry, 66,
170–180.
Gratz, K. L., Rosenthal, M. Z., Tull, M. T.,
Lejuez, C. W., & Gunderson, J. G. (2010).
An experimental investigation of emotional
reactivity and delayed emotional recovery
in borderline personality disorder: The role
of shame. Comprehensive Psychiatry, 51(3),
275–285.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 3–24). New York: Guilford Press.
Gujar, N., Yoo, S., Hu, P., & Walker, M. P.
(2011). Sleep deprivation amplifies reactiv-
ity of brain reward networks, biasing the
appraisal of positive emotional experiences.
Journal of Neuroscience, 31(12), 4466–4474.
Harley, R., Sprich, S., Safren, S., Jacobo, M.,
& Fava, M. (2008). Adaptation of dialecti-
cal behavior therapy skills training group
for treatment- resistant depression. Journal of
Nervous and Mental Disease, 196(2), 136–
143.
Harned, M. S., Rizvi, S. L., & Linehan, M. M.
(2010). Impact of co- occurring posttraumatic
stress disorder on suicidal women with border-
line personality disorder. American Journal of
Psychiatry, 167(10), 1210–1217.
Harrison, A., Sullivan, S., Tchanturia, K., &
Treasure, J. (2009). Emotion recognition and
regulation in anorexia nervosa. Clinical Psy-
chology and Psychotherapy, 16, 348–356.
Hayes, S. C., Bissett, R. T., Korn, Z., Zettle,
R. D., Rosenfarb, I. S., Cooper, L. D., et al.
(1999). The impact of acceptance versus con-
trol rationales on pain tolerance. Psycholog-
cial Reports, 49, 33 – 47.
Hazlett, E. A., Zhanga, J., New, A. S., Zel-
manovaa, Y., Goldstein, K. E. M., Haznedar,
M. M., et al. (2012). Potentiated amygdala
response to repeated emotional pictures in
borderline personality disorder. Biological
Psychiatry, 72(6), 448–456.
Heller, A. S., Johnstone, T., Shackman, A. J.,
Light, S. N., Peterson, M. J., Kolden, G. G., et
al. (2009). Reduced capacity to sustain positive
emotion in major depression reflects dimin-
ished maintenance of fronto- striatal brain
activation. Proceedings of the National Acad-
emy of Sciences USA, 106, 22445–22450.
Hernandez, A., Arntz, A., Gaviria, A. M., Labad,
A., & Gutiérrez-Zotes, J. A. (2012). Relation-
ships between childhood maltreatment, par-
enting style, and borderline personality disor-
der criteria. Journal of Personality Disorders,
26(5), 727–736.
Herpertz, S. C., Dietrich, T. M., Wenning, B.,
Krings, T., Erberich, S. G., Willmes, K., et al.
(2001). Evidence of abnormal amygdala func-
tioning in borderline personality disorder: A
functional MRI study. Biological Psychiatry,
50, 292–298.
Hupka, R. B., Lenton, A. P., & Hutchinson, K.
A. (1999). Universal development of emotion
categories in natural language. Journal of Per-
sonality and Social Psychology, 77, 247–278.
Hurwitz, B. E., & Furedy, J. J. (1986). The
human dive reflex— an experimental, topo-
graphical and physiological analysis. Physiol-
ogy and Behavior, 36, 287–294.
Iverson, K. M., Shenk, C., & Fruzzetti, A.
E. (2009). Dialectical behavior therapy for
women victims of domestic abuse: A pilot
study. Professional Psychology: Research and
Practice, 40, 242–248.
Izard, C. E. (1977). Distress— Anguish, grief,
and depression. In C. Izard (Ed.), Human
emotions (pp. 285–288). New York: Plenum
Press.
Jacob, G. A., Guenzler, C., Zimmermann, S.,
Scheel, C. N., Rüsch, N., Leonhart, R., et al.
(2008). Time course of anger and other emo-
tions in women with borderline personal-
ity disorder: A preliminary study. Journal of
Behavior Therapy and Experimental Psychia-
try, 39(3), 391–402.
Joiner, T. E., Jr., Lewinsohn, P. M., & Seeley, J.
R. (2002). The core of loneliness: Lack of plea-
surable engagement— more so than painful
disconnection— predicts social impairment,
depression onset, and recovery from depres-
sive disorders among adolescents. Journal of
Personality Assessment, 79, 472–491.
Koerner, K., & Linehan, M. M. (1997). Case
formulation in dialectical behavior therapy
for borderline personality disorder. In T. Eells
(Ed.), Handbook of psychotherapy case for-
mulation (pp. 340–367). New York: Guilford
Press.
Koole, S. (2009). The psychology of emotion reg-
ulation: An integrative review. Cognition and
Emotion, 23, 4–41.
Koons, C. R., Chapman, A. L., Betts, B. B.,
O’Rourke, B., Morse, N., & Robins, C. J.
(2006). Dialectical behavior therapy adapted
Dialectical Behavior Therapy 505
for the vocational rehabilitation of signifi-
cantly disabled mentally ill adults. Cognitive
and Behavioral Practice, 13, 146–156.
Korfine, L., & Hooley, J. M. (2000). Directed
forgetting of emotional stimuli in borderline
personality disorder. Journal of Abnormal
Psychology, 109(2), 214–221.
Kring, A. M., & Sloan, D. M. (2010). Emotion
regulation and psychopathology: A transdi-
agnostic approach to etiology and treatment.
New York: Guilford Press.
Kring, A. M., & Werner, K. H. (2004). Emotion
regulation and psychopathology. In P. Philip-
pot & R. S. Feldman (Eds.), The regulation
of emotion (pp. 359–408). Mahwah, NJ: Erl-
baum.
Kuo, J. R., & Linehan, M. M. (2009). Disentan-
gling emotion processes in borderline person-
ality disorder: Physiological and self- reported
assessment of biological vulnerability, baseline
intensity, and reactivity to emotionally evoca-
tive stimuli. Journal of Abnormal Psychology,
118(3), 531–544.
Laird, J. D. (1974). Self- attribution of emotion:
The effects of expressive behavior on the qual-
ity of emotional experience. Journal of Per-
sonality and Social Psychology, 29, 475–486.
Lazarus, A. A., & Abramovitz, A. (1962). The
use of “emotive imagery” in the treatment of
children’s phobias. Journal of Mental Science,
108, 191–195.
Levine, D., Marziali, E., & Hood, J. (1997).
Emotion processing in borderline personal-
ity disorders. Journal of Nervous and Mental
Disease, 185(4), 240–246.
Levitt, J. T., Brown, T. A., Orsillo, S. M., & Bar-
low, D. H. (2004). The effects of acceptance
versus suppression of emotion on subjective
and psychophysiological response to carbon
dioxide challenge in patients with panic disor-
der. Behavior Therapy, 25, 747–766.
Linehan, M. M. (1993a). Skills training manual
for treating borderline personality disorder.
New York: Guilford Press.
Linehan, M. M. (1993b). Cognitive- behavioral
treatment of borderline personality disorder.
New York: Guilford Press.
Linehan, M. M. (in press). Skills training for dia-
lectical behavior therapy. New York: Guilford
Press.
Lobbestael, J., & Arntz, A. (2010). Emotional,
cognitive and physiological correlates of
abuse- related stress in borderline and antiso-
cial personality disorder. Behaviour Research
and Therapy, 48(2), 116–124.
Lynch, T. R., Chapman, A. L., Rosenthal, M. Z.,
Kuo, J. R., & Linehan, M. M. (2006) Mecha-
nisms of change in dialectical behavior ther-
apy: Theoretical and empirical observations.
Journal of Clinical Psychology, 62, 459–480.
Lynch, T. R., Rosenthal, M. Z., Kosson, D.,
Cheavens, J. S., Lejuez, C. W., & Blair, R.
J. R. (2006). Heightened sensitivity to facial
expressions of emotion in borderline personal-
ity disorder. Emotion, 6, 647–655.
Marissen, M. E., Meuleman, L., & Franken,
I. A. (2010). Altered emotional information
processing in borderline personality disor-
der: An electrophysiological study. Psychiatry
Research: Neuroimaging, 181(3), 226–232.
Martell, C. R., Addis, M., & Jacobson, N. S.
(2001). Depression in context: Strategies for
guided action. New York: Norton.
Mennin, D. S., Heimberg, R. G., Turk, C. L., &
Fresco, D. M. (2005). Preliminary evidence
for an emotion dysregulation model of gener-
alized anxiety disorder. Behaviour Research
and Therapy, 43, 1281–1310.
Minzenberg, M. J., Fan, J., New, A. S., Tang, C.
Y., & Siever, L. J. (2007). Fronto- limbic dys-
function in response to facial emotion in bor-
derline personality disorder: An event- related
fMRI study. Psychiatry Research, 155(3),
231–243.
Minzenberg, M. J., Fan, J., New, A. S., Tang,
C. Y., & Siever, L. J. (2008). Frontolimbic
structural changes in borderline personality
disorder. Journal of Psychiatric Research, 42,
727–733.
Monti, J. M., & Monti, D. (2000). Sleep distur-
bance in generalized anxiety disorder and its
treatment. Sleep Medicine Reviews, 4, 263–
276.
Neacsiu, A. D., Rizvi, S. L., & Linehan, M. M.
(2010). Dialectical behavior therapy skills use
as a mediator and outcome of treatment for
borderline personality disorder. Behaviour
Research and Therapy, 48(9), 832–839.
Neacsiu, A. D., & Linehan, M. M. (in press).
Dialectical behavior therapy for borderline
personality disorder. In D. Barlow (Ed.), Clini-
cal handbook of psychological disorders (5th
ed.). New York: Guilford Press.
New, A. S., Hazlett, E. A., Buchsbaum, M. S.,
Goodman, M., Mitelman, S. A., Newmark,
R., et al. (2007). Amygdala– prefrontal dis-
connection in borderline personality disorder.
Neuropsychopharmacology, 32, 1629–1640.
Niedtfeld, I., Kirsch, P., Schulze, L., Herpertz, S.
C., Bohus, M., & Schmahl, C. (2012). Func-
506 INTERVENTIONS
tional connectivity of pain mediated affect
regulation in borderline personality disorder.
PLoS ONE, 7(3), e33293.
Nunes, P. M., Wenzel, A., Borges, K. T., Porto,
C. R., Caminha, R. M., & de Oliveira., I.
R. (2009). Volumes of the hippocampus and
amygdala in patients with borderline person-
ality disorder: A meta- analysis. Journal of Per-
sonality Disorders, 23, 333–345.
Philippot, P., Baeyenes, C., Douilliez, C., & Fran-
cart, B. (2004). Cognitive regulation of emotion:
Application to clinical disorders. In P. Philippot
& R. S. Feldman (Eds.), The regulation of emo-
tion (pp. 71–100). New York: Erlbaum.
Philippot, P., Burgos, A. I., Verhasselt, S., &
Baeyens, C. (2002). Specifying emotional
information: Modulation of emotional inten-
sity via executive processes. Paper presented
at the 2002 International Society for Research
on Emotion Conference, La Cuenca, Spain.
Pryce, C. R., & Feldon, J. (2003). Long-term
neurobehavioural impact of the postnatal
environment in rats: Manipulations, effects
and mediating mechanisms. Neuroscience and
Biobehavioral Review, 27, 57–71.
Ray, W. J., Odenwald, M., Neuner, F., Schauer,
M., Ruf, M., Wienbruch, C., et al. (2006).
Decoupling neural networks from reality:
Dissociative experiences in torture victims
are reflected in abnormal brain waves in left
frontal cortex. Psychological Science, 17(3),
825–829.
Reitz, S., Krause-Utz, A., Pogatzki- Zahn, E. M.,
Ebner- Priemer, U., Bohus, M., & Schmahl, C.
(2012). Stress regulation and incision in bor-
derline personality disorder— a pilot study
modeling cutting behavior. Journal of Person-
ality Disorders, 26(4), 605–615.
Rizvi, S. L., & Linehan, M. M. (2006). The
treatment of maladaptive shame in borderline
personality disorder: A pilot study of “Oppo-
site Action.” Cognitive and Behavioral Prac-
tice, 12, 437– 4 47.
Rosenthal, M. Z., Gratz, K. L., Kosson, D. S.,
Cheavens, J. S., Lejuez, C. W., & Lynch,
T. R. (2008). Borderline personality disor-
der and emotional responding: A review of
the research literature. Clinical Psychology
Review, 28, 75–91.
Ruocco, A. C. (2005). The neuropsychology
of borderline personality disorder: A meta-
analysis and review. Psychiatry Resource,
137(3), 191–202.
Schaefer, A., Collette, F., Philippot, P., van der,
L. M., Laureys, S., Delfiore, G., et al. (2003).
Neural correlates of “hot” and “cold” emo-
tional processing: A multilevel approach to the
functional anatomy of emotion. NeuroImage,
18, 938–949.
Schredl, M., Paul, F., Reinhard, I., Ebner- Priemer,
U., Schmahl, C., & Bohus, M. (2012). Sleep
and dreaming in patients with borderline per-
sonality disorder: A polysomnographic study.
Psychiatry Research, 200(2–3), 430–436.
Schulze, L., Domes, G., Krüger, A., Berger, C.,
Fleischer, M., Prehn, K., et al. (2011). Neuro-
nal correlates of cognitive reappraisal in bor-
derline patients with affective instability. Bio-
logical Psychiatry, 69(6), 564–573.
Selby, E. A., & Joiner, T. E. (2009). Cascades of
emotion: The emergence of borderline person-
ality disorder from emotional and behavioral
dysregulation. Review of General Psychology,
13, 219–229.
Shaver, P., Schwartz, J., Kirson, D., & O’Connor,
C. (1987). Emotion knowledge: Further explo-
ration of a prototype approach. Journal of Per-
sonality and Social Psychology, 52, 1061–1086.
Smith, C. F., Williamson, D. A., Bray, G. A., &
Ryan, D. H. (1999). Flexible vs. rigid dieting
strategies: Relationship with adverse behav-
ioral outcomes. Appetite, 32, 295–305.
Soeteman, D. I., Hakkaart- van Roijen, L., Ver-
heul, R., & Busschbach, J. J. (2008). The eco-
nomic burden of personality disorders in men-
tal health care. Journal of Clinical Psychiatry,
69(2), 259–265.
Soler, J., Pascual, J. C., Tiana, T., Cebria, A.,
Barrachina, J., Campins, M. J., et al. (2009).
Dialectical behaviour therapy skills training
compared to standard group therapy in bor-
derline personality disorder: A 3-month ran-
domised controlled clinical trial. Behaviour
Research and Therapy, 47, 353–358.
Stepper, S., & Strack, F. (1993). Proprioceptive
determinants of emotional and nonemotional
feelings. Journal of Personality and Social
Psychology, 64, 211–220.
Stiglmayr, C. E., Shapiro, D. A., Stieglitz, R. D.,
Limberger, M. F., & Bohus, M. (2001). Expe-
rience of aversive tension and dissociation in
female patients with borderline personality
disorder— a controlled study. Journal Psychi-
atric Research, 35, 111–118.
Tafrate, R. C., Kassinove, H., & Dundin, L.
(2002). Anger episodes in high- and low-trait-
anger community adults. Journal of Clinical
Psychology, 58, 1573–1590.
Teasdale, J. D., Segal, Z., & Williams, J. M.
(1995). How does cognitive therapy prevent
Dialectical Behavior Therapy 507
depressive relapse and why should attentional
control (mindfulness) training help? Behav-
iour Research and Therapy, 33, 25–39.
Tebartz van Elst, L., Hesslinger, B., Thiel, T.,
Geiger, E., Haegele, K., Lemieux, L., et al.
(2003). Frontolimbic brain abnormalities in
patients with borderline personality disorder:
A volumetric magnetic resonance imaging
study. Biological Psychiatry, 54, 163–171.
Thorberg, F. A., Young, R. M., Sullivan, K. A.,
& Lyvers, M. (2009). Alexithymia and alco-
hol use disorders: A critical review. Addictive
Behaviors, 34, 237–245.
Tooby, J., & Cosmides, L. (1990). The past
explains the present: Emotional adaptations
and the structure of ancestral environment.
Ethology and Sociobiology, 11, 375–424.
Trull, T. J., Jahng, S., Tomko, R. L., Wood, P.
K., & Sher, K. J. (2010). Revised NESARC
personality disorder diagnoses: Gender, preva-
lence, and comorbidity with substance depen-
dence disorders. Journal of Personality Disor-
ders, 24(4), 412–426.
Williams, J. B. W., Stiles, W. B., & Shapiro,
D. A. (1999). Cognitive mechanisms in the
avoidance of painful and dangerous thoughts:
Elaborating the assimilation model. Cognitive
Therapy and Research, 23, 285–306.
Wong, M. M., Brower, K. J., Fitzgerald, H. E.,
& Zucker, R. A. (2004). Sleep problems in
early childhood and early onset of alcohol and
other drug use in adolescence. Alcoholism:
Clinical and Experimental Research, 28(4),
578–587.

508
Emotions tend to be triggered by situations
and generally operate to facilitate adaptive
functioning (Werner & Gross, 2010). How-
ever, the nature and intensity of emotional
experience is not determined by situational
factors alone. As clearly evidenced by the
extensive work reviewed in this handbook, a
range of psychological operations can mod-
erate emotional responses to environmental
circumstances. Collectively these have been
termed emotion regulation processes. Gross
and others have emphasized that emotion
regulation results from a heterogeneous
array of processes exerting their influence
at differing points in the emotion generation
continuum (Gross, 1998; Gross & Thomp-
son, 2007). Antecedent- focused components
operate prior to the generation of emotion,
and response- focused components operate
subsequent to emotion generation, while
other emotion regulation processes exert
a direct influence on emotion generation
itself. The combined impact of these emo-
tion regulation processes shapes the nature
of emotional experience, with individual dif-
ferences in emotional regulation giving rise
to variation in emotional disposition. Thus,
emotional resilience has been attributed to
the effective use of adaptive emotion regu-
lation (Troy & Mauss, 2011), while height-
ened vulnerability to negative emotions and
to emotional pathology have been attributed
to maladaptive emotional regulation (Moses
& Barlow, 2006). Such accounts carry the
implication that a better understanding of
the specific processes that functionally con-
tribute to emotion regulation, coupled with
the ability to systematically influence these
processes, could potentially enhance such
regulation in ways that ameliorate emo-
tional vulnerability and dysfunction.
Since variation in emotion regulation gives
rise to individual differences in emotional
disposition, it follows that information-
processing biases theoretically implicated
in the determination of emotional disposi-
tion represent plausible emotion regulation
mechanisms. Numerous influential mod-
els have proposed that biased attentional
selectivity contributes both to dispositional
emotional vulnerability and emotional
pathology (cf. Mathews & MacLeod, 2005).
Hence, it is unsurprising that selective atten-
tion has been widely implicated in models of
emotion regulation (Gross & Barrett, 2011).
Kring and Werner (2004) contend that
selective attentional deployment is a criti-
cal emotion regulation process that causally
contributes both to variability in normal
emotional experience and to emotional dys-
function. Thompson and Goodman (2010)
echo this view, arguing that certain types
of attentional selectivity contribute to good
emotional regulation and therefore to emo-
CHAPTER 30
Regulation of Emotion
through Modification of Attention
Colin MacLeod
Ben Grafton

Regulation of Emotion through Modification of Attention 509
tional resilience, while other patterns of
attentional deployment compromise emo-
tional regulation in ways that give rise to
emotional pathology. Many other theorists
have similarly proposed that attentional bias
to negative information makes a maladap-
tive contribution to emotion regulation that
elevates emotional vulnerability and the risk
of emotional pathology (White, Helfinstein,
Reeb- Sutherland, Degnan, & Fox, 2009),
while attentional avoidance of negative
information serves to enhance emotional
resilience (Troy & Mauss, 2011).
This idea that selective attentional
response to negative information makes a
causal contribution to emotional regula-
tion, and therefore functionally influences
emotional vulnerability and dysfunction, is
our focus in this chapter. Here we examine
how use of cognitive technologies that have
proven capable of systematically training
attentional change has served to illumi-
nate the causal role of selective attentional
deployment in the regulation of emotion. We
also review evidence that the enhancement
of emotion regulation, through the applica-
tion of such attentional bias modification
procedures, can yield tangible benefits for
people experiencing emotional difficulties.
Before describing the attentional bias modi-
fication approach, we first briefly summa-
rize the evidence that attentional preference
for negative information represents a reli-
able characteristic of emotional vulnerabil-
ity and dysfunction. This also enables us to
communicate how the types of cognitive–
experimental tasks previously employed to
assess attentional bias recently have been
adapted to create attentional bias modifica-
tion procedures.
Assessing the Association
between Attentional Bias
and Emotion Regulation
Some investigators have sought to assess
attention simply by asking people to intro-
spectively reflect upon and self- report the
manner in which their attention operates
(e.g., Derryberry & Reed, 2002). However,
the limitations of relying on verbal reports
to assess cognitive processes have been well
documented (cf. Nisbett & Wilson, 1977).
In response to these limitations, many
researchers investigating whether individ-
ual differences in emotion regulation are
systematically associated with variation in
selective attentional responding to negative
information have drawn upon cognitive–
experimental methodologies, to appraise
more objectively the attentional processes of
interest (Mathews & MacLeod, 2005). The
resulting assessment procedures infer atten-
tional selectivity not from self- report mea-
sures but from performance measures, such
as response latencies to make particular
decisions, that necessarily will be influenced
in particular ways by underlying patterns of
attentional selectivity. Several such atten-
tional assessment tasks have been widely
used in studies designed to establish whether
biased attention to negative information is
characteristic of people who display evi-
dence of poor emotion regulation. We now
briefly describe these assessment techniques
and illustrate how each has lent support to
the veracity of this hypothesis.
One of the earliest methodologies devel-
oped to objectively assess the association
between selective attentional responding to
negative information and emotional vulner-
ability is the emotional Stroop task. In this
assessment approach, the participant must
rapidly name the color of emotionally toned
words displayed in differing ink colors, while
ignoring their semantic content. The degree
to which color naming is disproportionately
slow on negative words compared to neutral
words is taken as a measure of attentional
bias to negative information, as it suggests
particular difficulty ignoring the negative
content of these words. Individuals who
display heightened emotional vulnerabil-
ity, or who suffer from emotional dysfunc-
tion, commonly show this pattern of emo-
tional Stroop performance (e.g., Williams,
Mathews, & MacLeod, 1996), leading to
the conclusion that variation in the capac-
ity to regulate emotion is indeed related to
differential attentional responding to nega-
tive information (Ashley & Swick, 2009).
Another technique commonly used to assess
the association between selective attentional
response to negative information and indi-
vidual difference in emotion regulation is
the visual search task. In this approach, par-
ticipants are exposed to an array of stimuli
and are required to locate a specified target
stimulus as quickly as possible. An index of

510 INTERVENTIONS
attentional bias to negative information is
provided by their relative speeding to detect
emotionally negative targets compared to
neutral or positive targets. It has repeatedly
been found that participants with height-
ened emotional vulnerability or dysfunction
display greater evidence of such speeding
(c.f. Cisler & Koster, 2010). Investigators
employing this visual search approach have
concluded that variation in attentional
responding to emotional information is sys-
tematically related to variation in the ability
to regulate emotion effectively (Leclerc &
Kensinger, 2008).
Probably the most widely used method
of assessing biased attention to negative
information is the attentional probe task
(MacLeod, Mathews, & Tata, 1986). In
this task, the participant is briefly exposed
to stimulus pairs whose members differ in
emotional valence. These stimuli can be
words or images, and most commonly one
member of each pair is emotionally nega-
tive, while the other is neutral in emotional
tone. Immediately following stimulus offset,
a small visual probe is presented in the locus
of one the previously displayed stimuli, and
the participant is required to rapidly execute
a discriminatory response to this probe. It
is assumed that the probe will be processed
fastest when it appears in the locus where
participants already are attending. Hence,
the degree to which this discrimination
response is speeded when the probe appears
in the locus of the negative information,
rather than the locus of neutral informa-
tion, provides a measure of attentional bias
toward negative information. A reliable
finding from studies using this task is that
emotionally vulnerable individuals, and
those suffering from emotional pathology,
display an attentional bias to negative infor-
mation (c.f. Bar-Haim, Lamy, Pergamin,
Bakermans- Kranenburg, & van IJzendoorn,
2007). Once again, this has led theorists to
conclude that an attentional bias to nega-
tive information is characteristic of deficient
emotion regulation (e.g., Bradley, Mogg,
White, Groom, & de Bono, 1999).
While the weight of evidence leaves little
room for doubt that impaired emotion regu-
lation, as evidenced by heightened emotional
vulnerability or the presence of emotional
pathology, is reliably characterized by an
attentional bias favoring negative informa-
tion, this does not permit the conclusion that
such attentional deployment makes a direct
functional contribution to emotion regula-
tion. This association could instead reflect
the influence of emotion regulation on
attentional selectivity. Alternatively, it could
be that attentional selectivity and emotion
regulation represent the independent conse-
quence of some third individual- difference
variable. To determine whether selective
attention exerts a causal influence on emo-
tion regulation, it is necessary to system-
atically alter attentional selectivity. Adop-
tion of the attentional bias modification
approach has served both to confirm, and to
capitalize on, the contribution of attentional
selectivity to emotion regulation.
The Attentional Bias
Modification Approach
The most powerful way to evaluate the
hypothesis that patterns of selective atten-
tional response to emotional information
make a functional contribution to individ-
ual differences in emotion regulation is to
systematically manipulate such attentional
selectivity, in order to test the prediction
that the modification of attentional bias
will alter emotion regulation, as revealed by
changes in emotional reactivity and dysfunc-
tion. Confirmation of this prediction would
lend powerful support to the contention that
attentional bias is causally involved in emo-
tion regulation. It also would introduce the
possibility that dysfunctional patterns of
emotional symptomatology, resulting from
deficiencies in emotion regulation, might be
therapeutically attenuated by clinical inter-
ventions that appropriately exploit such
attentional bias modification procedures.
A number of early studies designed to
appraise the possibility that emotion regu-
lation may be influenced by altering atten-
tional selectivity have employed only ver-
bal instruction as the intended method
of attentional manipulation. Participants
instructed to alter their attentional style not
uncommonly report that this alters their
emotional experience (Richards & Gross,
2006). However, while such findings are
certainly encouraging, reliance on verbal
instruction to modify attentional selectiv-
ity has a number of significant limitations
Regulation of Emotion through Modification of Attention 511
(MacLeod & Bucks, 2011). For one thing,
such an approach at best could only influ-
ence attentional processes amenable to voli-
tional control, and it is well established that
the patterns of attentional bias that char-
acterize emotional vulnerability and dys-
function commonly operate automatically
within the cognitive system (Mathews &
MacLeod, 2005). Relatedly, while partici-
pants may report that they comply with such
attentional instructions, in the absence of
objective attentional measures it is difficult
to exclude the potential influence of demand
effects. Hence, it would be highly desirable
to induce attentional change in a manner
that does not assume the targeted atten-
tional process to be under voluntary control,
and to objectively verify the efficacy of this
attentional manipulation without relying on
self- report measures of attentional selectiv-
ity.
Recent years have witnessed the develop-
ment and implementation of new cognitive
technologies designed to achieve these objec-
tives by redesigning cognitive– experimental
tasks previously employed to assess patterns
of attentional bias relevant to emotion regu-
lation, in ways that transform them into pro-
cedures capable of instead training change
in such attentional selectivity (Mathews &
MacLeod, 2002). Collectively referred to
as cognitive bias modification for attention
(CBM-A), or more simply as attentional
bias modification (ABM) tasks, this general
approach has involved introducing system-
atic contingencies into the original assess-
ment tasks, such that task performance will
be enhanced by the acquisition of a biased
attentional response to a target category of
emotional information. Following extensive
practice in the execution of such tasks, sub-
sequent performance on conventional atten-
tional bias assessment procedures has served
to confirm that these ABM procedures can
systematically alter selective attentional
responding to emotional information.
MacLeod, Rutherford, Campbell,
Ebsworthy, and Holker (2002) developed
the earliest and most widely used ABM
task by transforming the attentional probe
task into a training procedure. The assess-
ment version of this task presents probes
with equal frequency in the screen locations
where either negative or neutral members
of a stimulus word pair have just been pre-
sented. In their ABM version of the task,
however, MacLeod et al. either presented all
probes in the loci where the negative words
appeared, in order to induce attentional
vigilance for negative information (attend-
negative training), or in the loci where the
neutral words appeared, in order to induce
attentional avoidance of negative informa-
tion (avoid- negative training). Across several
studies, MacLeod and colleagues showed
that following the delivery of 576 such ABM
trials, participants who received these alter-
native training conditions came to differ as
intended in the pattern of attentional bias
they subsequently displayed on the conven-
tional assessment version of the probe task.
Dandeneau and Baldwin (2004) devel-
oped an ABM variant of the visual search
procedure previously employed to assess
patterns of attentional selectivity associated
with individual differences in emotion regu-
lation. Their attentional training version was
designed to enhance attentional vigilance for
positive information, while inducing atten-
tional avoidance of negative information by
having participants repeatedly search for a
single emotionally positive target image (a
smiling face) in a matrix of emotionally neg-
ative distracter images (frowning faces). Fol-
lowing exposure to 112 trials of this ABM
procedure, participants evidenced a change
in attentional bias consistent with the train-
ing objective, as assessed using the emotional
Stroop task. Once again, this effect has been
replicated across subsequent studies (Dan-
deneau, Baldwin, Baccus, Sakellaropoulo,
& Pruessner, 2007).
Because the attentional impact of ABM
procedures has been demonstrated using
performance- based rather than self- report
measures of attentional selectivity, there
are good grounds for confidence that they
induce genuine attentional change. Further
support for the authenticity of their atten-
tional impact comes from the breadth of
attentional measures that are influenced by
these bias modification procedures. Thus,
for example, ABM tasks alter attentional
selectivity as assessed using eye movement
measures (Wadlinger & Isaacowitz, 2008),
and they also modulate event- related poten-
tial (ERP) indices of attentional selectivity
(Eldar & Bar-Haim, 2010). Using func-
tional magnetic resonance imaging (fMRI),
Browning, Holmes, Murphy, Goodwin, and

512 INTERVENTIONS
Harmer (2010) have demonstrated that the
attentional change elicited by ABM proce-
dures is mediated by altered patterns of acti-
vation in the lateral frontal cortex.
This powerful new capacity to directly
modify selective attentional responding to
emotional information has enabled research-
ers not only to evaluate the hypothesis that
such attentional bias causally contributes to
emotion regulation but also to exploit thera-
peutically the capacity of these ABM pro-
cedures to enhance emotion regulation (cf.
Bar-Haim, 2010; Hertel & Mathews, 2011;
MacLeod & Mathews, 2012). In reviewing
this work, we focus first on laboratory- based
research that has employed single- session
ABM, with the principal aim of illuminat-
ing the functional nature of the associa-
tion between attentional bias and emotion
regulation. We next consider work that has
delivered extended ABM, with the aim of
enhancing emotion regulation in ways that
can reduce emotional vulnerability and dys-
function in real-world settings.
Single-Session ABM Studies:
Determining the Causal
Contribution of Attentional Bias
to Emotion Regulation
Studies employing single- session ABM
procedures have examined the impact of
transiently induced change in attentional
responding to emotional information on
emotional experience within controlled lab-
oratory settings. In some cases, investigators
have studied the effect of such ABM proce-
dures on normal emotional reactions to con-
trived stress. In others, the focus has been
on the capacity of ABM to attenuate dys-
functional emotional symptoms in partici-
pants selected because of their disposition to
experience particular types of emotional dif-
ficulties, such as worry, obsessive concerns,
or social anxiety. The following sections
review the outcomes of these investigations.
Impact of Single‑Session
ABM on Affective Reactivity
to Laboratory‑Based Mood Induction
In two early studies, MacLeod et al. (2002)
employed a single session of the probe-based
ABM task to successfully induce a differen-
tial attentional response to negative verbal
stimuli in students who obtained midrange
scores on a measure of emotional vulnera-
bility. These participants were subsequently
exposed to a stressful anagram task, and
their emotion regulation capability was
determined by assessing the degree to which
this elicited anxious and depressed mood.
While the anagram stressor increased levels
of anxiety and depression across all partici-
pants, the magnitude of this dysphoric emo-
tional response was significantly attenuated
for the participants who had just completed
ABM in the avoid- negative rather than the
attend- negative training condition. Further-
more, the degree to which the ABM proce-
dure influenced the emotional impact of this
stressor was a direct function of the degree
to which it influenced selective attentional
responding to negative stimuli. These find-
ings confirm that selective attentional avoid-
ance of negative information can enhance
down- regulation of dysphoric responses to
stressful experiences, which is a characteris-
tic of good emotion regulation.
Using a single session of their visual search
ABM variant, Dandeneau and Baldwin
(2009) have provided further support for
the idea that attentional selectivity causally
influences emotion regulation. Unselected
workers at an adult education center com-
pleted a single session of this ABM task
either in a condition designed to induce
attentional vigilance for socially positive
information (smiling faces) and avoidance
of socially negative information (frowning
faces), or in a control condition designed to
leave attentional selectivity unaltered. A sub-
sequent attentional probe assessment task
confirmed that participants given the ABM
training, but not those receiving the control
condition, came to display attentional avoid-
ance of information related to social rejec-
tion. The former participants also evidenced
enhanced emotion regulation when exposed
to a variety of stressor tasks. For example,
they felt less subjective rejection than did
control participants following a simulated
interaction in which they were treated coldly,
and this was particularly evident in partici-
pants who initially had scored low in self-
esteem. Participants who had received the
ABM training also reported less rejection-
related thoughts when attempting to com-
plete a subsequent insoluble anagram task.
Regulation of Emotion through Modification of Attention 513
Furthermore, failure on this task exerted a
less negative impact on self- esteem in partici-
pants who received the ABM than was the
case for those in the control condition. Dan-
deneau and Baldwin reasonably concluded
from these findings that selective attentional
responding to emotional information makes
a direct functional contribution to the self-
regulation of emotional experience.
These aforementioned studies indicate
that attentional bias can causally influence
emotion regulation in participants whose
emotional experiences fall within the nor-
mal range. Researchers have gone on to
investigate whether a single session of ABM
can transiently ameliorate dysfunctional
emotion in participants selected on the basis
of preexisting emotional problems.
Impact of Single‑Session ABM
on Negative Thought Intrusions
in Worriers
Hayes, Hirsch, and Mathews (2010)
recruited volunteers who defined themselves
as high worriers, and who scored high on
a conventional worry questionnaire. Half of
these participants were given an ABM proce-
dure that delivered the probe ABM task and
a novel dichotic ABM task, both configured
to induce attentional avoidance of negative
information. The remaining participants
were given control versions of these tasks
without any training contingency. When
attentional bias was subsequently measured,
it was found that participants receiving
ABM differed significantly from the control
participants in terms of attentional bias, and
now demonstrated the intended avoidance of
negative information. A thought- sampling
procedure was then employed to assess neg-
ative thought intrusions before and after a
final worry induction. Although there was
no significant difference between the groups
in terms of negative thought intrusions
before the worry induction, the control par-
ticipants, but not the experimental partici-
pants, evidenced an increase in such intru-
sions following this worry manipulation.
The finding that participants who received
avoid- negative ABM experienced less nega-
tive thought intrusions than did those who
received the no- training control procedure
verifies that attentional bias can make a
casual contribution to worry.
In an extension of this work, using wor-
riers selected in the same manner, Hirsch et
al. (2011) compared the impact of two vari-
ants of ABM. Both employed verbal stimuli,
but one was designed to modify the degree
to which attention is captured by negative
information, while the other was designed
to instead modify the degree to which atten-
tion is held by negative information. These
two procedures altered attentional selectiv-
ity to an equivalent degree, but only the for-
mer ABM variant influenced subsequently
assessed negative thought intrusions. On the
basis of these findings, Hirsch et al. argue
that heightened attentional engagement with
negative information may contribute more
than impaired attentional disengagement
from such information to the evocation of
worry- related negative thought intrusion.
This study illustrates the capacity for care-
fully designed implementations of the ABM
approach to illuminate which aspects of
attentional bias make the greatest contribu-
tions to dysfunctional symptoms reflecting
impaired emotion regulation.
Impact of Single‑Session ABM on
Behavioral Avoidance in Subclinical
Obsessive–Compulsive Disorder
The studies reported so far have assessed
the impact of ABM only on self- report mea-
sures of emotion. However, the modification
of attentional bias to negative information
also has been shown to influence behavioral
indices of dysfunctional emotion. Najmi
and Amir (2010) recruited individuals who
reported excessive concern about germs, dirt,
or contamination, and scored high on a con-
ventional measure of obsessive– compulsive
symptomatology. Half these participants
received a single session of probe-based
ABM, delivered in the avoid- negative train-
ing condition, while the other half received
this task in a control condition that did not
include any training contingencies. The
emotionally negative information presented
in these tasks was words chosen on the basis
of their relevance to contamination- related
concerns. As intended, the former group, but
not the latter, came to exhibit attentional
avoidance of this contamination- related neg-
ative information. A behavioral approach
task was then given, which required par-
ticipants to take as many steps as possible
514 INTERVENTIONS
toward a contamination- related stimulus
(e.g., potting soil mixed with cat hair and
dead crickets). Those who had received
the avoid- negative ABM training exhib-
ited greater levels of behavioral approach
than did those who instead received the
control condition. Moreover, Najmi and
Amir were able to show that the degree to
which this ABM training increased behav-
ioral approach was mediated by the degree
to which it reduced attentional bias to the
negative information. Thus, selective atten-
tional responding to negative information
makes a contribution to not only the regula-
tion of emotion itself but also the regulation
of emotionally relevant patterns of behavior.
Impact of Single‑Session ABM on
Affective, Somatic, and Behavioral
Symptoms of Social Anxiety
Amir, Weber, Beard, Bomyea, and Taylor
(2008) have shown that ABM also influ-
ences emotionally relevant patterns of
behavior in socially anxious people with
a fear of public speaking. Included in the
study were individuals who responded to an
advertisement seeking volunteers who had
difficulty giving speeches, and who scored
high on a questionnaire measure of social
anxiety. They were exposed to a single-
session attentional probe procedure that
presented faces displaying either negative
emotion (disgust) or neutral expressions,
delivered in either the avoid- negative ABM
condition or a no- training control condi-
tion. As intended, the former participants
alone displayed a significant reduction in
attention to the socially relevant negative
information, coming to show less atten-
tion to such negative information than did
control participants. When subsequently
required to give a short speech, the partici-
pants who had received avoid- negative ABM
were rated as performing better than con-
trol participants. They also reported lower
levels of anxiety during speech presentation
than did control participants. The superior
speech performance exhibited by the par-
ticipants who received avoid- negative ABM
was statistically mediated by the emotional
impact of this ABM procedure. This sug-
gests that the induced attentional avoidance
of negative information adaptively regulated
emotional response to the speech stressor,
with beneficial behavioral consequences for
speech performance.
Using a somewhat more complex design,
which involved having participants engage in
physical exercise during the ABM sessions,
Julian, Beard, Schmidt, Powers, and Smits
(2012) recently failed to replicate Amir et al.’s
(2008) finding that ABM designed to reduce
attention to negative information served to
attenuate emotional reactivity to a subse-
quent speech stressor. However, it should be
emphasized that Julian et al.’s (2012) ABM
procedure actually failed to modify selective
attentional responding to negative infor-
mation. Hence, the results of this study do
not challenge the hypothesis that selective
attentional deployment influences emotion
regulation. Other investigators who have
succeeded in modifying this attentional bias
have been able to verify Amir et al.’s (2008)
original conclusion that such attentional
bias modification influences both subjective
and behavioral measures of socially anx-
ious participants’ stress response to a speech
task. Heeren, Lievens, and Philippot et al.
(2011) delivered a range of attentional train-
ing variants to socially anxious participants
and found that an ABM procedure designed
specifically to increase attentional disen-
gagement from faces displaying negative
expressions was most effective in enhancing
independently rated speech performance,
while also reducing self- reported anxiety.
Thus, the pattern of attentional selectivity
that most contributes to the adaptive regula-
tion of social anxiety may involve the ready
disengagement of attention from socially rel-
evant negative information.
The weight of evidence from single- session
ABM studies firmly favors the conclusion
that selective attentional responding to emo-
tional information can make a causal contri-
bution to emotion regulation. Single sessions
of ABM can moderate the intensity of nor-
mal affective reactions to laboratory stress-
ors, and attenuate dysfunctional emotional
symptoms such as excessive worry, elevated
social anxiety, and undue concerns over
contamination within the laboratory set-
ting. Such findings offer empirical support
to the hypothesis that attentional selectiv-
ity contributes to the regulation of emotion.
They also raise the prospect that appropri-
ately designed extensions of single- session
ABM procedures may be capable of enhanc-

Regulation of Emotion through Modification of Attention 515
ing emotion regulation within real-world
settings in ways that may deliver practical
therapeutic benefits. In the next section,
we consider ABM studies that have directly
evaluated this exciting possibility.
Extended-Delivery ABM Studies:
The Therapeutic Enhancement
of Emotion Regulation
in Real-World Settings
While the results of single- session ABM
studies have confirmed that selective atten-
tional responding to emotional information
can influence emotion regulation within the
laboratory, this does not mean that atten-
tional bias necessarily contributes to real-
world emotion regulation. Nor do these
finding permit the conclusion that ABM has
the capacity to enhance emotion regulation
in ways that can reduce dysfunctional emo-
tional experience outside the experimen-
tal setting. Both of these issues have been
addressed, however, by studies designed to
evaluate whether repeated exposure to ABM
procedures can enhance regulation of emo-
tional reactions to naturalistic stressors, and
can improve emotion regulation to an extent
that ameliorates the clinical symptoms of
emotional pathology in the real-world.
Impact of Extended ABM on Affective
Reactivity to Real‑Life Stressors
See, MacLeod, and Bridle (2009) employed
an online procedure to deliver 10-minute
sessions of a probe-based ABM task on each
of 15 consecutive days, to high school grad-
uates approaching the stressor of migrating
overseas to commence tertiary education.
Half received this task in the avoid- negative
training condition, while the other half
received it in a control condition containing
no attentional training contingency. The for-
mer participants, unlike the latter, displayed
a reduction in attention to negative informa-
tion across the duration of the study. Emo-
tional measures taken immediately after par-
ticipants subsequently experienced the stress
of migration were compared with measures
taken prior to the commencement of the
study. As anticipated, this real-world stress
event substantially increased state anxiety.
However, the magnitude of this negative
emotional reaction was attenuated in partic-
ipants who had received the avoid- negative
ABM, compared to control participants.
Furthermore, a measure of trait anxiety,
reflecting general disposition to experience
anxiety symptoms, also was attenuated by
the ABM procedure. The degree to which
ABM reduced attention to negative infor-
mation statistically mediated the degree to
which it reduced trait anxiety, which in turn
mediated its impact on emotional reactivity
to the stressful transition event. Thus, the
reduction of attention to negative informa-
tion through extended exposure to ABM
served to enhance the regulation of emotion
within the naturalistic setting in ways that
decreased negative emotional reactivity to a
real-world stressor.
Similar conclusions are invited by the find-
ings of Dandeneau et al. (2007), who exam-
ined the impact of 5 consecutive days of their
visual search ABM task on self- report and
physiological indices of negative emotional
experience in participants who experienced
stressful real-world situations. Compared
to those who received a no- training con-
trol procedure, students who completed this
extended ABM in the condition designed to
inhibit attention to negative and increase
attention to positive information reacted to
a subsequent school examination with lower
levels of subjective stress and anxiety. When
telemarketers, employed within a stress-
ful work environment, received this 5-day
ABM procedure, they reported an increase
in self- esteem and decrease in subjective
stress, compared to colleagues given a no-
training control procedure. They also evi-
denced a reduction in physiological signs of
stress, were rated as more confident by their
supervisors, and even showed higher levels
of sales performance. Such findings strongly
support the involvement of attentional selec-
tivity in real-world emotion regulation and
demonstrate that attentional bias modifica-
tion procedures can enhance the regulation
of normal emotional experience in ways that
benefit people in stressful real-world situa-
tions.
Impact of Extended ABM
on Pathological Worry
The extended delivery of ABM also has
been shown to attenuate dysfunctional emo-
516 INTERVENTIONS
tional symptoms experienced outside the
laboratory by people who report worry-
ing excessively. Hazen, Vasey, and Schmidt
(2009) recruited participants who exhibited
extreme levels of worry, and across a 2-week
period completed five sessions, each lasting
for 30 minutes, of either probe-based ABM
in the avoid- negative condition or a control
task that contained no training contingency.
The attentional vigilance for negative infor-
mation initially shown by these worriers was
reversed by the avoid- negative ABM. Partici-
pants who received this ABM, unlike con-
trol participants, also displayed a significant
reduction in worry, anxiety, and depression.
Hazen et al. contend, on the basis of their
findings, that the enhancement of emotion
regulation using attentional training may
make a therapeutic contribution to the treat-
ment of generalized anxiety disorder (GAD),
in which pathological worry is the hallmark
symptom.
Subsequent research has lent empiri-
cal weight to this contention. Amir, Beard,
Burns, and Bomyea (2009) directly assessed
whether ABM produced clinically significant
benefits when given to treatment- seeking
individuals meeting diagnostic criteria for
GAD. All patients completed an attentional
probe task twice weekly across a 4-week
period. For one group, this task was given
in the avoid- negative ABM condition, con-
figured to induce attentional avoidance of
negative information, while another group
received a control condition that contained
no training contingency. Clinical symp-
tomatology was assessed before and after
this 4-week period, using both question-
naire measures and diagnostic interview.
Patients who received avoid- negative ABM
procedure displayed the expected decrease
in attention to negative information, which
was not shown by patients in the control
condition, resulting, as intended, in a post-
training group difference in selective atten-
tional responding to negative information.
Across the 4 weeks of the study, patients in
the control condition evidenced no decline in
either questionnaire measures or interview-
based measures of symptomatology that
assessed worry, anxiety, and depression. In
contrast, patients who received the avoid-
negative ABM procedure demonstrated reli-
able reductions in all these symptom mea-
sures. The clinical significance of the change
was such that, after this 4-week period, only
50% of the participants who had received
avoid- negative ABM continued to meet
diagnostic criteria for GAD, compared
with 87% of those in the control condition.
Amir et al. concluded that biased attentional
responding to negative information func-
tionally contributes to the emotional pathol-
ogy observed in GAD. Moreover, on the
basis of their findings, they advocate the use
of ABM as a clinical tool in the treatment of
this emotional disorder.
Impact of Extended ABM on Social
Anxiety Dysfunction
There is compelling evidence that extended
exposure to ABM can enhance emotional
functioning in people experiencing problem-
atic levels of social anxiety. After recruiting
participants who scored high on a question-
naire measure of social anxiety, Li, Tan,
Qian, and Liu (2008) gave them seven con-
secutive daily sessions that presented either
probe-based ABM in the avoid- negative con-
dition, or a control version of this procedure
that contained no training condition. Only
those who received the avoid- negative ABM
came to display reduced attention to nega-
tive information, and only they evidenced
a significant reduction in Social Interaction
Anxiety Scale (SIAS) scores. Li et al.’s find-
ings suggest that the regulation of real-world
social anxiety can be improved by the modi-
fication of attentional bias in nonclinical
participants. Later work carried out with
clinical samples indicates that such ABM
procedures also can therapeutically alleviate
the clinical symptoms of patients with psy-
chological disorders that involve excessive
levels of social anxiety.
Amir, Beard, Taylor, et al. (2009) exam-
ined whether ABM could enhance emo-
tional functioning in patients meeting diag-
nostic criteria for generalized social phobia
(GSP). Patients who received eight sessions
of avoid- negative ABM across a 4-week
period evidenced reduced levels of attention
to socially relevant negative facial expres-
sions, which was not the case for those who
instead received a no- training control ver-
sion of the procedure. Over these 4 weeks,
the former patients, relative to the latter,
also exhibited disproportionate reductions
across all measures of social anxiety, which
Regulation of Emotion through Modification of Attention 517
included the Liebowitz Social Anxiety Scale
(LSAS), the Social Phobia and Anxiety
Inventory (SPAI) and the Sheehan Disability
Scale (SDS). At the end of this period, only
50% of the individuals who had received the
avoid- negative ABM training continued to
meet diagnostic criteria, compared to 86%
of those who received the control condition.
This improvement in social anxiety symp-
toms experienced by the patients given ABM
was maintained at 4-month follow- up.
Carlbring et al. (2012) recently failed to
replicate Amir, Beard, Taylor, et al. (2009)
findings when delivering ABM online to
Internet- recruited volunteers who met diag-
nostic criteria for social anxiety disorder
(SAD). However, it is important to note
that this intended ABM procedure failed to
induce any reduction in selective attention
to negative information. Hence, while Carl-
bring et al.’s study raises questions concern-
ing the most effective methods of bringing
about attentional change, its negative find-
ings do not challenge Amir, Beard, Taylor,
et al.’s (2009) conclusion that attentional
bias causally contributes to impaired emo-
tion regulation in SAD. Moreover, converg-
ing support for the conclusion that ABM can
alleviate such clinical dysfunction has been
independently obtained by other investiga-
tors. For example, Schmidt, Richey, Buck-
ner, and Timpano (2009) evaluated the
impact of avoid- negative ABM on the clini-
cal symptoms of patients diagnosed with
generalized social anxiety disorder (GSAD),
using the same experimental design as Amir,
Beard, Taylor, et al. (2009). Patients who
received eight such ABM sessions across a
4-week period demonstrated reduced levels
of emotional dysfunction compared to par-
ticipants who received the no- training con-
trol condition on all measures of social anxi-
ety, which included the SPAI, the LSAS, and
the Brief Social Phobia Scale. At the end of
this 4-week period only 28% of those who
received the avoid- negative ABM continued
to meet diagnostic criteria for GSAD, com-
pared to 75% of participants in the control
condition. Again, these gains were largely
maintained at 4-month follow- up.
Heeren, Reese, McNally, and Philippot
(2012) have shown that extended exposure
to avoid- negative ABM reduces the negative
emotional symptoms experienced by patients
with social anxiety dysfunction in naturalis-
tic environments and enhances their capacity
to regulate their emotional reaction to exper-
imentally delivered social stress. Patients
diagnosed with GSAD were given four daily
sessions of ABM training in either the avoid-
negative condition or a no- training control
version of the task. Attentional bias to nega-
tive information was significantly attenu-
ated at a posttraining assessment point for
the former participants relative to the latter.
Participants receiving ABM also exhibited
a disproportionate decline in social anxi-
ety, as assessed by the LSAS and the Fear of
Negative Evaluation Scale (FNE). Prior to,
and after, the 4 days of attentional training
participants were exposed to a speech task,
in which they delivered a 2-minute oral pre-
sentation, and the impact of this stressor on
self- report, behavioral, and physiological
measures of emotion was assessed. Partici-
pants who received the avoid- negative ABM
displayed a disproportionate reduction, rela-
tive to control participants, in the degree to
which this stressor evoked negative emotion
at the posttraining assessment point. Those
who had received ABM reported experienc-
ing less distress than did control participants
during the speech task. They also performed
the task with less evidence of negative emo-
tion, as assessed by behavioral ratings and
galvanic skin response (GSR).
Impact of Extended ABM
on Depression
An attentional bias to negative information
is generally considered a more robust char-
acteristic of anxiety than depression, raising
uncertainty concerning whether such atten-
tional selectivity contributes to the regula-
tion of depression. Nevertheless, a few ABM
studies suggest that this is the case, at least
when depression is not severe. For example,
Wells and Beevers (2010) recruited under-
graduate students whose questionnaire
scores indicated mild to moderate levels of
depression. Across a 2-week period they
received four sessions of either probe-based
ABM in the avoid- negative training condi-
tion or a control probe task that contained
no training contingency. Across this period,
the former participants alone displayed a
reduction in attention to negative informa-
tion, and by the end of the 2 weeks they
were displaying significantly lower levels
518 INTERVENTIONS
of attention to negative information than
was the case for control participants. Like-
wise, only the participants who received the
avoid- negative ABM showed a reduction in
depressive symptoms across these 2 weeks,
and at the end of the period their depression
scores were significantly lower than those
of control participants. This ABM-induced
difference in depression levels remained evi-
dent at a follow- up assessment 2 weeks later.
The influence of the training procedure on
depressive symptomatology was statistically
mediated by its impact on attentional bias.
These findings suggest that attentional
selectivity causally contributes to the regula-
tion of depression, as well as anxiety, rais-
ing the possibility that ABM may potentially
yield benefits in the treatment of depressive
dysfunction. It would be premature, how-
ever, to draw conclusions concerning the
responsiveness of clinical depression to this
type of attentional manipulation. Baert, De
Raedt, Schacht, and Koster (2010) reported
finding no therapeutic impact of a novel
probe-based ABM procedure they deliv-
ered, across 10 daily sessions, to students
reporting severe depression, and to patients
receiving concurrent conventional treatment
for clinical depression. It must be noted,
however, that the probe-based procedure
employed by these researchers not only dif-
fered in significant ways from the ABM task
adopted by most other researchers (e.g.,
presenting only single stimuli rather than
stimulus pairs) but also prove ineffective in
altering selective attentional responding to
negative emotional information. We concur
with the authors that their failure to induce
differential attentional selectivity using this
intended bias modification procedure begs
a cautious interpretation of the accompany-
ing failure to influence emotional symptom-
atology in severely depressed students and
clinically depressed patients. It remains to
be seen whether the successful reduction of
attention to negative information can serve
to therapeutically attenuate clinically signif-
icant depression.
Impact of Extended ABM
on Dysfunctional Affect in Children
and Adolescents
Across recent years, growing concern over
emotional dysfunction in childhood and
adolescence has led researchers to recognize
the importance of better understanding the
factors that contribute to emotion regula-
tion in young people and identifying ways
of enhancing this. ABM studies suggest that
selective attentional responding to negative
information causally influences emotion
regulation in children as well as adults, and
provide grounds for optimism that ABM
procedures can be used to enhance emotion
regulation in younger populations. Early
support for the hypothesis that attentional
selectivity contributes to emotion regulation
in children was provided by Eldar, Ricon,
and Bar-Haim (2008). These researchers’
recruited children ages 7–12 years, whose
questionnaire scores placed them midrange
in terms of emotional vulnerability. Across
two sessions, these children completed a
probe-based ABM procedure in either an
avoid- negative or an attend- negative train-
ing condition. Following this, they were
exposed to a stressor, which involved their
being videotaped while trying to solve a dif-
ficult puzzle. Children who had been given
the differing ABM procedures exhibited dis-
crepant emotional reactivity to this stressor.
Those who had received the attend- negative
ABM experienced a pronounced elevation
of negative emotion following exposure to
the stressor, relative to a prestressor base-
line mood measure. In contrast, children
who had received the avoid- negative ABM
showed no elevation of negative emotion
in response to the stressor. Thus, altering
attentional responding to negative informa-
tion influenced the regulation of the emo-
tional reactions to stress exhibited by the
children. In addition to confirming the role
of attentional selectivity in childhood emo-
tion regulation, these findings highlight the
potential value of ABM as a technique for
enhancing such regulation in children with
dysfunctional emotional symptomatology.
More recently, two published randomized
controlled trials further strengthen the con-
clusion that improving emotion regulation
through direct modification of attentional
selectivity can therapeutically alleviate dys-
functional emotional experience in anxious
children. The first of these was carried out
by Bar-Haim, Morag, and Glickman (2011)
on a sample of 10-year-old children who
scored high on a questionnaire instrument
screening for childhood emotional disor-

Regulation of Emotion through Modification of Attention 519
ders. These children were randomly assigned
to receive either a probe-based ABM avoid-
threat training, or a control version of the
task containing no training contingency, on
two occasions separated by 4–6 days. Pre-
training attentional bias was assessed prior
to the first ABM session, while posttrain-
ing attentional bias was assessed 5–8 days
after the second ABM session. At this latter
assessment point, participants were exposed
to the puzzle stressor employed by Eldar et
al. (2008) to ascertain whether ABM influ-
enced regulation of their emotional reactivity
to stress. The avoid- negative ABM training
changed attentional selectivity as intended,
with the children who received this train-
ing displaying reduced attention to negative
information compared to control children.
It also enhanced emotion regulation dur-
ing performance of the stressor task. While
children in the control condition displayed a
significant elevation of negative emotion in
response to the final stressor, no elevation of
negative affect was elicited by this stressor in
children who had undergone avoid- negative
ABM training. Hence, despite their initially
high levels of emotional vulnerability, these
children became more resilient to stress
when they acquired the ability to attention-
ally avoid- negative information, indicat-
ing that this style of attentional selectivity
improves emotion regulation in younger
populations also.
Eldar et al. (2012) recently conducted
the first randomized controlled trial evalu-
ating the impact of ABM on children with
formally diagnosed pediatric emotional dis-
orders. Their sample comprised treatment-
seeking children, ages 8–14 years, recruited
from a hospital- based child anxiety clinic.
All met diagnostic criteria for at least one
anxiety disorder (i.e., separation anxiety
disorder, social phobia, specific phobia, or
GAD), with 75% meeting diagnostic criteria
for more than one such disorder. These clini-
cally anxious children received four weekly
sessions of either probe-based ABM in the
avoid- negative condition or a control ver-
sion of the task containing no training con-
tingency. Children who received the avoid-
negative ABM, but not those who received
the control condition, demonstrated a sig-
nificant decline in both the number of anxi-
ety symptoms recorded at clinical interview
and the rated severity of these symptoms.
Well over twice as many of children who
received avoid- negative ABM, as those who
received the control condition, showed full
remission. Specifically, 33.3% no longer met
diagnostic criteria for any anxiety disorder
after receiving the 4 weeks of avoid- negative
ABM, compared to only 13.3% of those in
the control condition. Hence, for children
who suffer from emotional dysfunction,
as for adults, modification of attentional
selectivity to increase avoidance of negative
information serves to improve emotion regu-
lation, as evidenced by the significant reduc-
tion of dysfunctional emotional experience.
Future Directions for ABM
Approaches to Emotion Regulation
The studies reviewed in this chapter have
demonstrated that ABM procedures can
serve to attenuate dysfunctional emotional
symptoms. It may seem paradoxical that
ABM-induced attentional avoidance of
negative information can be therapeutically
beneficial given that many models implicate
avoidance in the etiology of psychopathol-
ogy. However, such accounts often pertain
to behavioral rather than to cognitive avoid-
ance (see Barlow, 2002). Furthermore, with
respect to cognitive avoidance, it is impor-
tant to distinguish effortful attempts to
suppress negative thoughts from the type
of attentional avoidance induced by ABM.
The former appears to be emotionally coun-
terproductive, probably because effortful
thought suppression commonly fails to pro-
duce the intended pattern of cognitive avoid-
ance and can instead paradoxically increase
the very thoughts one tries to suppress (Weg-
ner, Schneider, Carter, & White, 1987).
In contrast, ABM procedures designed to
reduce attention to negative information
without effort or intention have proven suc-
cessful in inducing attentional avoidance
of such information, with consequent emo-
tional benefits.
The conclusion that ABM procedures
can effectively modify attentional response
to negative information and therapeutically
alleviate dysfunctional emotion has been
bolstered by the outcomes of recent meta-
analyses. One such meta- analysis by Hal-
lion and Ruscio (2011), which did not distin-
guish between bias modification procedures
520 INTERVENTIONS
designed to alter attentional vs. interpretive
selectivity, revealed that cognitive bias mod-
ification exerts a medium- size effect on both
cognitive bias (g = 0.49) and clinical symp-
tomatology (g = 0.39). Other meta- analyses
that have focused specifically on the modifi-
cation of attentional bias (Hakamata et al.,
2010; Beard, Sawyer, & Hofmann, 2012)
have concluded that ABM exerts a large
effect on attention bias (g = 1.06–1.15), and
a medium- to-large effect on clinical symp-
tomatology (g = 0.48–0.77). Meta- analysis
also has identified some clinically relevant
variables that may moderate the magni-
tude of both effects. For example, Beard et
al. report that ABM alters attentional bias
to the greatest degree in participants who
show highest initial levels of negative emo-
tional symptomatology. Bias modification
appears to influence anxiety more than
depression (Hallion & Ruscio, 2011). Haka-
mata et al. (2010) found that ABM has a
greater impact on anxious disposition than
on anxious mood state per se, and that this
emotional impact is nominally greater in
anxiety patients than in nonclinical partici-
pants. This overall pattern lends weight to
the potential value of ABM in the treatment
of emotional dysfunction, particularly in the
case of anxiety disorders.
Having established that attentional avoid-
ance of negative information can success-
fully be induced by ABM procedures, and
serves to increase emotional resilience and
alleviate dysfunction emotional experience,
future research can now build on this firm
foundation in a number of important ways.
We close this chapter by considering several
directions that we believe will prove to be
particularly profitable.
Improving ABM Techniques to Better
Enhance Emotion Regulation
The degree to which emotion is influenced
by ABM procedures is largely determined
by the extent of change in negative atten-
tional bias that they induce (Hakamata
et al., 2010). Therefore, an obvious way
potentially to increase the beneficial impact
of ABM techniques on emotion regulation
will be to improve their capacity to modify
selective attentional responding to nega-
tive information. This endeavor will likely
involve the future development of new CBM
procedures, in ways guided by deeper under-
standing of the mechanisms through which
existing ABM tasks exert their influence.
Additionally, the capacity of existing ABM
tasks to modify attentional selectivity effec-
tively may be further enhanced by refining
the manner in which they are delivered. We
briefly consider, in turn, each of these poten-
tial approaches to the future improvement of
ABM techniques.
One important issue will be to establish
whether ABM tasks exert a highly specific
effect on attentional bias alone, or whether
their emotional benefits reflect change in
more general cognitive control systems, such
as working memory. Consistent with this lat-
ter possibility, Hirsch, Hayes, and Mathews
(2009) reported that their cognitive bias
modification procedure not only reduced the
selective process of negative information but
also improved working memory. Klumpp
and Amir (2010) argued, on the basis of
their findings, that the impact of ABM tasks
on attentional bias may be mediated by a
general improvement in cognitive control.
Whether any such training induces improve-
ment in working memory, directly influences
emotion regulation, or instead facilitates
adaptive change in attentional bias, must
be determined by future research. However,
such considerations suggest that develop-
ing ABM tasks in ways that maximize their
capacity to improve working memory, or
perhaps supplementing the delivery of ABM
tasks with training procedures designed to
directly improve working memory (Owens,
Koster, & Derakshan, 2013), may augment
their capacity to enhance emotion regula-
tion.
The refinement of ABM procedures also
will likely benefit from better understanding
of the particular juncture(s) in the emotion
regulation continuum where ABM exerts
its effect(s). Gross and Thompson (2007)
propose that the influence of selective atten-
tional deployment on emotion regulation
occurs at the emotion generation stage. The
finding that ABM influences emotional
reactivity to stressors is consistent with the
possibility that it affects the generation of
negative emotion. However, ABM also may
exert an influence subsequent to initial emo-
tion generation, at the response- focused
stage of emotional regulation, by operat-
ing to prolong or curtail negative emotional
Regulation of Emotion through Modification of Attention 521
experience. This, too, could contribute to
its observed impact on emotional reactiv-
ity measures, which typically involve the
assessment of emotional experience at least
several minutes after commencing exposure
to an emotion- generating stressor. Indeed,
it may be that modifying certain aspects of
attentional selectivity, such as initial atten-
tional engagement with negative informa-
tion, could influence the emotion- generation
stage, while modifying other aspects of
attentional selectivity, such as the degree
to which negative information holds atten-
tion over time, instead could influence the
longevity of generated emotions (Hirsch
et al., 2011). Determining where alterna-
tive ABM variants exert their influence on
emotion regulation will require fine- grained
assessment of the temporal dynamics of the
resulting change in emotional experience,
and physiological measures such as GSR
and heart rate variability may prove useful
in this regard. Future research of this type
could potentially lead to the development of
new ABM batteries designed either to exert
a broader influence across multiple stages of
emotion regulation or selectively target par-
ticular stages of emotional regulation, per-
haps in an individually tailored manner.
While the creation of new of attentional
training procedures, guided by better under-
standing of the mechanisms through which
ABM influences emotion regulation, will
likely contribute to future progress, it also
seems probable that the efficacy of existing
ABM tasks could be improved by identify-
ing the most effective delivery formats. Most
obviously, increasing the number and length
of ABM sessions may amplify the resulting
attentional change, and it has been suggested
that such change may be more enduring if
ABM sessions are widely distributed across
time rather than grouped in close tempo-
ral proximity (Hertel & Mathews, 2011).
Another interesting possibility is that the
impact of ABM procedures on attentional
bias may be affected by nature of instruc-
tions given to participants. Researchers
commonly have assumed that ABM alters
attention implicitly, and so typically have
not explicitly communicated either the train-
ing contingency or intention to participants
(MacLeod & Mathews, 2012). Consistent
with this assumption, participants often
are unable to subsequently report the train-
ing contingency, or to identify the training
condition to which they have been exposed
(Beard, 2011). However, this represents only
weak evidence that ABM influences atten-
tional selectivity implicitly. Furthermore,
even if attentional change is trained implic-
itly by existing ABM approaches, the pos-
sibility remains that explicit training may
more effectively induce therapeutic change
in attentional bias. Hence, we suggest that
future investigators should empirically con-
trast the impact of implicit and explicit ver-
sions of ABM, which differ with respect
to whether they provide participants train-
ing contingency information and directly
instruct practice in the target pattern of
attentional selectivity. Future research of
this type will identify which approach yields
the greatest emotional benefit, and in doing
so will also illuminate whether implicit or
explicit attentional change more strongly
governs the impact of ABM on emotional
regulation.
Enhancing the power of ABM procedures
must involve not only increasing the size
and longevity of the attentional effects they
evoke but also ensuring good generalization
of this training. Generalization is revealed
by the transfer of training effects beyond
the original training task. A distinction has
been drawn between “far- transfer” and
“near- transfer” where, respectively, train-
ing effects are observed under conditions
that differ either greatly, or only slightly,
from the initial training experience (Her-
tel & Mathews, 2011). Most ABM studies
have confirmed near- transfer of training by
showing that the attentional impact of ABM
can be detected on new stimulus materi-
als not employed in the training task (e.g.,
MacLeod et al., 2002; Hirsch et al., 2011).
However, the practical value of ABM clearly
depends on also ensuring the far- transfer of
training. Given that the effect of ABM can be
detected on emotional assessment tasks very
different in nature from the original training
procedures, it seems clear such far- transfer
can occur (MacLeod & Mathews, 2012).
Further evidence of far- transfer comes from
studies demonstrating that ABM-induced
training effects can be detected across quite
different measures of attentional selectivity
(e.g., Dandeneau & Baldwin, 2004), and
also on measures of interpretive selectivity
(e.g., White, Suway, Pine, Bar-Haim, & Fox,
522 INTERVENTIONS
2011). Nevertheless, occasional transfer fail-
ures (e.g., Bockstaele, Koster, Verschuere,
Crombez, & De Houwer, 2012) underscore
the importance of identifying how best to
optimize far- transfer. It has been proposed
that the prospect of ABM training generaliz-
ing to new contexts and to new tasks might
best be ensured by delivering ABM training
sessions across a variety of differing con-
texts, and varying the task procedures and
parameters employed within them to induce
attentional change (MacLeod, Koster, &
Fox, 2009). As yet, this proposal has not
been formally tested, so it remains to be seen
whether these innovations would indeed
increase such generalization.
While the purpose of ABM in clinical
studies conducted to date has been to induce
general attentional avoidance of negative
information, such a pattern of selectivity
may not be optimal across all contexts. For
example, there will likely be advantages
associated with attending to threat cues in
situations where the risk of genuine danger
is high. Maximal well-being may result from
attentional vigilance to negative informa-
tion in high-risk contexts, and attentional
avoidance of such information in low-risk
contexts. It would therefore be appropriate
for future researchers to investigate whether
ABM can be delivered in ways that induce
situation- specific patterns of attentional
selectivity, such that the optimal attentional
response to negative information becomes
operational within each of these differing
types of context. While undoubtedly ambi-
tious, the viability of this objective is sup-
ported by recent demonstrations that when
alternative patterns of implicit processing
each are trained within a differing situa-
tional context, reinstatement of either train-
ing context favors expression of the spe-
cific pattern of cognition originally trained
within that same context (e.g., D’Angelo,
Milliken, Jimenez, & Lupianez, 2013).
Extending the Application of ABM
to Other Forms of Self‑Regulation
Because this review describes research moti-
vated by the hypothesis that attentional bias
to negative information underpins emo-
tional vulnerability and dysfunction, the
focus has been on ABM procedures designed
specifically to modify attentional response
to negative information. However, the ABM
approach could potentially be employed to
enhance other forms of self- regulation that
plausibly are affected by attentional selec-
tivity. For example, the hypothesis that
heightened positive affectivity reflects an
attentional bias toward positive information
(Taylor, Bomyea, & Amir, 2011), suggests
that ABM designed to modify attentional
responding to positive information could
influence positive emotional reactivity to
happy events. Grafton, Ang, and MacLeod
(2012) recently confirmed this by demon-
strating that participants exposed to an
ABM procedure that increased attention to
positive words, compared to those given an
ABM procedure that reduced attention to
such words, displayed disproportionately
intense positive emotional reactions to a
subsequent success experience.
The potential therapeutic benefits of
ABM need not be restricted to the regula-
tion of emotional experience. In many types
of psychological dysfunction that involve
biased patterns of attentional selectivity, the
primary symptom that could benefit from
better regulation is not emotion. There is
growing evidence that ABM procedures may
prove beneficial in enhancing these other
clinically relevant aspects of self- regulation.
For example, people with chronic pain syn-
drome display an attentional bias toward
pain- related information, and theorists con-
tend that this pattern of attentional selectiv-
ity makes a functional contribution to their
heightened perceptions of pain (Eccleston
& Crombez, 1999). A long- standing aim
of psychological interventions for this con-
dition has been to enhance self- regulation
of pain sensation. Recent work examining
whether ABM can contribute to the regula-
tion of pain experience has yielded promis-
ing outcomes. For example, Sharpe et al.
(2010) gave participants either a conven-
tional relaxation manipulation or a probe-
based ABM procedure designed to induce
attentional avoidance of pain- related infor-
mation, before exposing them to a cold
pressor task designed to induce painful sen-
sation. Those who received the attentional
training reported later onset of pain than did
those given the relaxation procedure. Such
findings should encourage future research-
Regulation of Emotion through Modification of Attention 523
ers to investigate whether the use of ABM
to directly manipulate selective attentional
responding to pain- relevant information can
enhance the regulation of pain sensation in
ways that yield therapeutic benefits for peo-
ple who suffer from this clinical condition.
Another clinical disorder that involves
impaired self- regulation, but in which nega-
tive emotional experience is incidental to the
symptoms of primary concern, is substance
dependency. Addictions are characterized
by biased patterns of attentional selectivity
that favor information related to the abused
substance, and theorists contend that this
bias contributes to associated craving and
consumption (Wiers et al., 2007). This has
led some investigators to examine whether
ABM variants designed to reduce attention
to such information can serve to attenu-
ate craving and/or regulate consumption,
and positive findings have been reported
for both tobacco dependency and alcohol
abuse. After exposure to a single- session
of probe-based ABM, configured to either
increase or decrease attention to smoking
cues, Attwood, O’Sullivan, Leonards, Mack-
intosh, and Munafo (2008) found that male
smokers in the latter group reported reduced
craving for tobacco, and this reduction in
craving was predicted by the magnitude of
the induced attention change. Fadardi and
Cox (2009) gave a sample of problem drink-
ers four weekly sessions of ABM, configured
to reduce attention to alcohol- related infor-
mation. This successfully modified atten-
tional selectivity as intended and led also to
a significant decline in alcohol consumption,
which was maintained at 3-month follow- up.
Thus, it would appear that the modifica-
tion of attentional bias carries the potential
to enhance other forms of self- regulation,
beyond the direct regulation of emotional
experience, so future research could prof-
itably seek to extend the application of the
ABM approach across a wider range of such
conditions. Of course, given that distressing
conditions such as chronic pain and sub-
stance dependency contribute to negative
emotional experience, ABM procedures that
increase the capacity to regulate pain, addic-
tive symptomatology, and other maladaptive
aspects of experience and behavior also will
likely indirectly benefit emotion regulation
itself.
Refining the Clinical Utility of ABM
in the Remediation of Dysfunctional
Emotion Regulation
Given the wealth of evidence that supports
the capacity of ABM approaches to influ-
ence emotion regulation beneficially, it is
no surprise that investigators now are seek-
ing to exploit its therapeutic potential in
the treatment of emotional dysfunction and
other psychological disorders that involve
deficient self- regulation. This approach now
has passed the proof-of- concept stage as a
viable means of therapeutically attenuating
clinically relevant symptoms, warranting
its further development as a practical tool
that can be employed in the clinical setting.
Already, several small-scale studies have
confirmed that ABM configured to induce
attentional avoidance of negative informa-
tion can be employed effectively within con-
ventional psychology clinics to treat patients
suffering from anxiety disorders and/or clin-
ical depression (Brosan, Hoppitt, Shelfer,
Sillence, & Mackintosh, 2011; Beard, Weis-
berg, & Amir, 2011).
The more widespread adoption of ABM
within the clinical setting will partly depend
upon patients’ perceived acceptability of the
approach. Hence, it is reassuring that patient
feedback has generally been favorable. Fol-
lowing delivery of their 12-session ABM
program to socially anxious 10- to 17-year-
olds, Rozenman, Weersing, and Amir (2011)
had recipients and their parents rate the
acceptability of this intervention procedure.
Responses ranged from acceptable to excel-
lent, with mean participant and parent rat-
ings of 4.2 and 4.5, respectively, on a 5-point
scale. Beard (2011) likewise reported that
primary care patients receiving ABM were
generally quite satisfied with the procedure
on average, rating its acceptability at level 3
on 4-point scale. Beard also noted that drop-
out rates from treatment trials delivering
such bias modification have typically been
low, ranging from 0 to 8%, suggesting that
recipients find the procedures acceptable.
Nevertheless, qualitative interviews car-
ried out by Beard and her colleagues (2011)
revealed that some recipients experience
ABM as overly repetitive. Hence, one type
of refinement to ABM that may increase
patient acceptability could involve the intro-

524 INTERVENTIONS
duction of features that sustain interest and
motivation. For example, this might involve
delivering ABM in shorter blocks and pro-
viding performance feedback, so that par-
ticipants can strive to improve across blocks.
Beard (2011) also reports that some patients
express a desire to better understand how
ABM could help with their emotional dif-
ficulties. Therefore, another refinement that
might enhance patient acceptance would be
provision of a rationale that communicates
how this procedure can contribute to the
regulation of negative emotion.
One feature of ABM that Beard’s inter-
view data (2011) identified as being partic-
ularly highly valued by patients is the flex-
ibility and convenience with which it can be
accessed by recipients, due to its computer-
based method of delivery. Recent years have
witnessed the growing use of computer tech-
nology to facilitate the transport of conven-
tional psychological interventions into the
community (Marks & Cavanagh, 2009).
Given its amenability to Internet delivery
(e.g., See et al., 2009), ABM could readily be
integrated into such forms of intervention.
Remotely accessed computerized treatments
that include ABM components already are
beginning emerge. For example, Amir and
Taylor (2012) recently evaluated the effi-
cacy of a home-based treatment that pro-
vided treatment- seeking GAD patients with
both ABM and computerized cognitive-
behavioral therapy (CBT), accessed online
as often as desired across a 6-week period.
Patients who completed this intervention
evidenced reduced attention to negative
information and displayed significant reduc-
tions in both self- reported and clinician-
rated symptoms of anxiety, worry, and
depression. Symptom change was related
to the magnitude of the observed reduction
in attentional bias. At completion of the
6-week intervention, 79% of participants no
longer met diagnostic criteria for GAD. This
study was not designed to distinguish the
contributions made by the CBT and ABM
components of the online package. It will be
important for future investigators to directly
compare the efficacy of CBT and ABM pro-
cedures, and also to determine whether the
combination of these two types of cognitive
intervention can deliver greater therapeutic
benefit than does either approach alone.
Nevertheless, we agree with Amir and Tay-
lor that the incorporation of ABM into com-
puterized, home-based treatments for emo-
tional dysfunction, represents a promising
future method of effectively exploiting its
demonstrated capacity to enhance emotion
regulation.
Closing Comments
The use of ABM procedures to test hypoth-
eses concerning the contributions of atten-
tional selectivity to emotion regulation and
therapeutically enhance such regulation in
people experiencing emotional dysfunction
is still a young area of research. Well over
70% of all the research published in this
field has appeared only within the past 3 or
4 years (MacLeod & Mathews, 2012). Nev-
ertheless, this early work already has clearly
established that biased attentional respond-
ing to negative information does causally
contribute to emotion regulation, affecting
emotional reactivity to stress and influenc-
ing the symptoms of emotional dysfunction.
The research reviewed in this chapter also
has convincingly demonstrated that the use
of ABM procedures to reduce attention to
negative information can improve emotion
regulation. Inducing such attention change
serves to attenuate the degree to which
stress elicits dysphoric emotional responses
and also to alleviate the negative emo-
tional symptoms associated with subclini-
cal or clinical manifestations of emotional
pathology. By drawing upon increasingly
sophisticated contemporary approaches to
attentional bias assessment, designed to
assess progressively more precise dimen-
sions of attention, it should be possible to
develop novel training variants that target
highly specific aspects of selective attention
for modification. Hence, we anticipate that
future ABM research will illuminate the
ways in which particular facets of attention
selectivity functionally contribute to differ-
ent features of emotion regulation. Further-
more, if these evolving ABM technologies
become more widely embedded in the types
of computerized treatment programs now
being used to deliver psychological inter-
ventions, we also can expect that they will
make a growing future contribution to the
effective remediation of emotional dysfunc-
tion.

Regulation of Emotion through Modification of Attention 525
Acknowledgments
Preparation of this chapter was supported
by Australian Research Council Grant No.
DP0879589, and by a grant from the Roma-
nian National Authority for Scientific Research,
CNCS–UEFISCDI, Project No. PNII-ID-
PCCE-2011-2-0045.
References
Amir, N., Beard, C., Burns, M., & Bomyea, J.
(2009). Attention modification program in
individuals with generalized anxiety disor-
der. Journal of Abnormal Psychology, 118(1),
28–33.
Amir, N., Beard, C., Taylor, C. T., Klumpp, H.,
Elias, J., Burns, M., et al. (2009). Attention
training in individuals with generalized social
phobia: A randomized controlled trial. Jour-
nal of Consulting and Clinical Psychology,
77(5), 961–973.
Amir, N., Weber, G., Beard, C., Bomyea, J., &
Taylor, C. T. (2008). The effect of a single-
session attention modification program on
response to a public- speaking challenge
in socially anxious individuals. Journal of
Abnormal Psychology, 117(4), 860–868.
Amir, T., & Taylor, C. (2012). Combining com-
puterized home-based treatments for gener-
alized anxiety disorder: An attention modi-
fication program and cognitive behavioral
therapy. Behavior Therapy, 43, 546–559.
Ashley, V., & Swick, D. (2009). Consequences of
emotional stimuli: Age differences on pure and
mixed blocks of the emotional Stroop. Behav-
ioral and Brain Functions, 5(14).
Attwood, A. S., O’Sullivan, H., Leonards, U.,
Mackintosh, B., & Munafo, M. R. (2008).
Attentional bias training and cue reactivity in
cigarette smokers. Addiction, 103(11), 1875–
1882.
Baert, S., De Raedt, R., Schacht, R., & Koster, E.
H. (2010). Attentional bias training in depres-
sion: Therapeutic effects depend on depression
severity. Journal of Behavior Therapy and
Experimental Psychiatry, 41(3), 265–274.
Bar-Haim, Y. (2010). Attention bias modifica-
tion (ADM): A novel treatment for anxiety
disorders. Journal of Child Psychology and
Psychiatry, 51(8), 859–870.
Bar-Haim, Y., Lamy, D., Pergamin, L.,
Bakermans- Kranenburg, M. J., & van IJzen-
doorn, M. H. (2007). Threat- related atten-
tional bias in anxious and nonanxious indi-
viduals: A meta- analytic study. Psychological
Bulletin, 133, 1–24.
Bar-Haim, Y., Morag, I., & Glickman, S. (2011).
Training anxious children to disengage atten-
tion from threat: A randomized controlled
trial. Journal of Child Psychology and Psy-
chiatry, 52(8), 861–869.
Barlow, D. (2002). Anxiety and its disorders:
The nature and treatment of anxiety and
panic (2nd ed.). New York: Guilford Press.
Beard, C. (2011). Cognitive bias modification as
a treatment for anxiety: Current evidence and
future directions. Expert Review of Neuro-
therapeutics, 11(2), 299–311.
Beard, C., Sawyer, A., & Hofmann, S. (2012).
Efficacy of attention bias modification using
threat and appetitive stimuli: A meta- analytic
review. Behavior Therapy, 43, 724–740.
Beard, C., Weisberg, R. B., & Amir, N. (2011).
Combined cognitive bias modification treat-
ment for social anxiety disorder: A pilot trial.
Depression and Anxiety, 28(11), 981–988.
Bockstaele, V., Koster, E., Verschuere, B., Crom-
bez, G., & de Houwer, J. (2012). Limited
transfer of threat bias following attentional
retraining. Journal of Behavior Therapy and
Experimental Psychiatry, 43, 794–800.
Bockstaele, V., Verschuere, B., Koster, E. H.,
Tibboel, H., De Houwer, J., & Crombez, G.
(2011). Journal of Behavioral Therapy and
Experimental Psychiatry, 42(2), 211–218.
Bradley, B. P., Mogg, K., White, J., Groom, C.,
& de Bono, J. (1999). Attentional bias for
emotional faces in generalized anxiety disor-
der. British Journal of Clinical Psychology,
38, 267–278.
Brosan, L., Hoppitt, L., Shelfer, L., Sillence, A., &
Mackintosh, B. (2011). Cognitive bias modifi-
cation for attention and interpretation reduces
trait and state anxiety in anxious patients
referred to an out- patient service: Results from
a pilot study. Journal of Behavior Therapy and
Experimental Psychiatry, 42(3), 258–264.
Browning, M., Holmes, E. A., Murphy, S. E.,
Goodwin, G. M., & Harmer, C. J. (2010).
Lateral prefrontal cortex mediates the cogni-
tive modification of attentional bias. Biologi-
cal Psychiatry, 67(10), 919–925.
Carlbring, P., Apelstrand, M., Sehlin, H., Amir,
A., Rousseau, A., Hofmann, et al. (2012).
Internet- delivered attention bias modifiction
training in individuals with social anxiety
disorder: A double blind randomized control
trial. BMC Psychatry, 12, 66.
526 INTERVENTIONS
Cisler, J., & Koster, E. H. (2010). Mechanisms
of attentional biases towards threat in anxiety
disorders: An integrative review. Clinical Psy-
chology Review, 30, 203–216.
Dandeneau, S. D., & Baldwin, M. W. (2004).
The inhibition of socially rejecting informa-
tion among people with high versus low self-
esteem: The role of attentional bias and the
effects of bias reduction training. Journal of
Social and Clinical Psychology, 23(4), 584–
602.
Dandeneau, S. D., & Baldwin, M. W. (2009).
The buffering effects of rejection- inhibiting
attentional training on social and performance
threat among adult students. Contemporary
Educational Psychology, 34(1), 42–50.
Dandeneau, S. D., Baldwin, M. W., Baccus, J. R.,
Sakellaropoulo, M., & Pruessner, J. C. (2007).
Cutting stress off at the pass: Reducing vigi-
lance and responsiveness to social threat by
manipulating attention. Journal of Personality
and Social Psychology, 93(4), 651–666.
D’Angelo, M., Milliken, B., Jimenez, L., & Lupi-
anez, J. (2013). Implementing flexibility in
automaticity: Evidence from context- specific
implicit sequence learning. Consciousness and
Cognition, 22(1), 64–81.
Derryberry, D., & Reed, M. A. (2002). Anxiety-
related attentional biases and their regulation
by attentional control. Journal of Abnormal
Psychology, 111(2), 225–236.
Eccleston, C., & Crombez, G. (1999). Pain
demands attention: A cognitive- affective
model of the interruptive function of pain.
Psychological Bulletin, 125(3), 356–366.
Eldar, S., Apter, A., Lotan, D., Edgar, K. P.,
Naim, R., Fox, N. A., & Bar-Haim, Y. (2012).
Attention bias modification treatment for
pediatric anxiety disorders: A randomized
controlled trial. American Journal of Psychia-
try, 169, 213–220.
Eldar, S., & Bar-Haim, Y. (2010). Neural plastic-
ity in response to attention training in anxiety.
Psychological Medicine, 40(4), 667–677.
Eldar, S., Ricon, T., & Bar-Haim, Y. (2008).
Plasticity in attention: Implications for stress
response in children. Behaviour Research and
Therapy, 46(4), 450–461.
Fadardi, J. S., & Cox, W. M. (2009). Reversing
the sequence: Reducing alcohol consumption
by overcoming alcohol attentional bias. Drug
and Alcohol Dependence, 101(3), 137–145.
Grafton, B., Ang, C., & MacLeod, C. (2012).
Always look on the bright side of life: The
attentional basis of positive affectivity. Euro-
pean Journal of Personality, 26, 133–144.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J., & Barrett, L. F. (2011). Emotion
generation and emotion regulation: One or
two depends on your point of view. Emotion
Review, 3, 8–16.
Gross, J. J., & Thompson, R. A. (2007). Emotion
regulation: Conceptual foundations. In J. J.
Gross (Ed.), Handbook of emotion regulation
(pp. 3–24). New York: Guilford Press.
Hakamata, Y., Lissek, S., Bar-Haim, Y., Brit-
ton, J. C., Fox, N. A., Leibenluft, E., et al.
(2010). Attention bias modification treatment:
A meta- analysis toward the establishment of
novel treatment for anxiety. Biological Psy-
chiatry, 68(11), 982–990.
Hallion, L., & Ruscio, A. (2011). A meta- analysis
of the effect of cognitive bias modification on
anxiety and depression. Psychological Bulle-
tin, 6, 940–958.
Hayes, S., Hirsch, C. R., & Mathews, A. (2010).
Facilitating a benign attentional bias reduces
negative thought intrusions. Journal of Abnor-
mal Psychology, 119(1), 235–240.
Hazen, R. A., Vasey, M. W., & Schmidt, N. B.
(2009). Attentional retraining: A randomized
clinical trial for pathological worry. Journal of
Psychiatric Research, 43(6), 627–633.
Heeren, A., Lievens, L., & Philippot, P. (2011).
How does attention training work in social
phobia: Disengagement from threat or re-
engagement to non- threat? Journal of Anxiety
Disorders, 25, 1108–1115.
Heeren, A., Reese, H. E., McNally, R. J., &
Philippot, P. (2012). Attention training toward
and away from threat in social phobia: Effects
on subjective, behavioral, and physiological
measures of anxiety. Behaviour Research and
Therapy, 50, 30–39.
Hertel, P. T., & Mathews, A. (2011). Cognitive
bias modification: Past, perspective, current
findings, and future applications. Perspectives
in Psychological Science, 6(6), 521–536.
Hirsch, C., Hayes, S., & Mathews, A. (2009).
Looking on the bright side: Accessing benign
meanings reduces worry. Journal of Abnormal
Psychology, 118, 44–54.
Hirsch, C., MacLeod, C., Mathews, A., Sandher,
O., Siyani, A., & Hayes, S. (2011). The con-
tribution of attentional bias to worry: Distin-
guishing the roles of selective engagement and
Regulation of Emotion through Modification of Attention 527
disengagement. Journal of Anxiety Disorders,
25, 272–277.
Julian, K., Beard, C., Schmidt, N. B., Powers, M.
B., & Smits, J. A. J. (2012). Attention train-
ing to reduce attention bias and social stressor
reactivity: An attempt to replicate and extend
previous findings. Behavior Research and
Therapy, 50, 350–358.
Klumpp, H., & Amir, N. (2010). Preliminary
study of attention training to threat and neu-
tral faces on anxious reactivity to a social
stressor in social anxiety. Cognitive Therapy
and Research, 34, 263–271.
Kring, A. M., & Werner, K. H. (2004). Emotion
regulation and psychopathology. In P. Philip-
pot & R. S. Feldman (Eds.), The regulation of
emotion (pp. 359–385). Oxford, UK: Psychol-
ogy Press.
Leclerc, C. M., & Kensinger, E. A. (2008). Effects
of age on detection of emotional information.
Psychology and Aging, 23(1), 209–215.
Li, S., Tan, J., Qian, M., & Liu, X. (2008). Con-
tinual training of attentional bias in social
anxiety. Behaviour Research and Therapy,
46(8), 905–912.
MacLeod, C., & Bucks, R. S. (2011). Emotion
regulation and the cognitive- experimental
approach to emotional dysfunction. Emotion
Review, 3(1), 62–73.
MacLeod, C., Koster, E., & Fox, E. (2009).
Whither cognitive bias modification research?:
Commentary on the special section articles.
Journal of Abnormal Psychology, 118, 89–99.
MacLeod, C., & Mathews, A. (2012). Cogni-
tive bias modification approaches to anxiety.
Annual Review of Clinical Psychology, 8,
189–217.
MacLeod, C., Mathews, A., & Tata, P. (1986).
Attentional bias in emotional disorders. Jour-
nal of Abnormal Psychology, 95, 15–20.
MacLeod, C., Soong, L. Y., Rutherford, E. M.,
& Campbell, L. W. (2007). Internet- delivered
assessment and manipulation of anxiety-
linked attentional bias: Validation of a free-
access attentional probe software package.
Behavior Research Methods, 39, 533–538.
MacLeod, C., Rutherford, E., Campbell, L.,
Ebsworthy, G., & Holker, L. (2002). Selec-
tive attention and emotional vulnerability:
Assessing the causal basis of their association
through the experimental manipulation of
attentional bias. Journal of Abnormal Psy-
chology, 111, 107–123.
Mathews, A., & MacLeod, C. (2002). Induced
processing biases have causal effects on anxi-
ety. Cognition and Emotion, 16, 331–354.
Mathews, A., & MacLeod, C. (2005). Cognitive
vulnerability to emotional disorders. Annual
Review of Clinical Psychology, 1, 167–195.
Marks, I., & Cavanagh, K. (2009). Computer-
aided psychological treatments: Evolving
issues. Annual Review of Clinical Psychology,
5, 121–141.
Moses, E. B., & Barlow, D. H. (2006). A new
unified treatment approach for emotional
disorders based on emotion science. Current
Directions in Psychological Science, 15(3),
146–150.
Najmi, S., & Amir, N. (2010). The effect of
attention training on a behavioral test of con-
tamination fears in individuals with subclini-
cal obsessive– compulsive symptoms. Journal
of Abnormal Psychology, 119(1), 136–142.
Nisbett, R. E., & Wilson, T. D. (1977). Telling
more than we can know: Verbal reports on
mental processes. Psychological Review, 84,
231–259.
Owens, M., Koster, E., & Derakshan, N. (2013).
Improving attention control in dysphoria
through cognitive training: Transfer effects on
working memory capacity and filtering effi-
ciency. Psychophysiology, 50(3), 297–307.
Reese, H. E., McNally, R. J., Najmi, S., & Amir,
N. (2010). Attention training for reducing spi-
der fear in spider- fearful individuals. Journal
of Anxiety Disorders, 24, 1–8.
Richards, J. M., & Gross, J. J. (2006). Person-
ality and emotional memory: How regulat-
ing emotion impairs memory for emotional
events. Journal of Research in Personality, 40,
631–651.
Rozenman, M., Weersing, V., & Amir, N. (2011).
A case series of attention modification in clini-
cally anxious youths. Behaviour Research and
Therapy, 49(5), 324–330.
Schmidt, N. B., Richey, J., Buckner, J. D., &
Timpano, K. R. (2009). Attention training for
generalized social anxiety disorder. Journal of
Abnormal Psychology, 118(1), 5–14.
See, J., MacLeod, C., & Bridle, R. (2009). The
reduction of anxiety vulnerability through the
modification of attentional bias: A real-world
study using a home-based cognitive bias modi-
fication procedure. Journal of Abnormal Psy-
chology, 118(1), 65–75.
Sharpe, L., Perry, K. N., Rodgers, P., Dear, B. F.,
Nicholas, M. K., & Refshauge, K. (2010). A
comparison of the effect of attentionl training
528 INTERVENTIONS
and relaxation responses to pain. Pain, 150(3),
469–476.
Taylor, C., Bomyea, J., & Amir, N. (2011). Mal-
leability of attentional bias for positive emo-
tional information and anxiety vulnerability.
Emotion, 11, 127–138.
Thompson, R. A., & Goodman, M. (2010).
Development of emotion regulation: More
than meets the eye. In A. Kring & D. Sloan
(Eds.), Emotion regulation and psychopathol-
ogy (pp. 38–58). New York: Guilford Press.
Troy, A. S., & Mauss, I. B. (2011). Resilience in
the face of stress: Emotion regulation as a pro-
tective factor. In S. M. Southwick, B. T. Litz,
D. Charney, & M. J. Friedman (Eds.), Resil-
ience and mental health: Challenges across the
lifespan (pp. 30–44). New York: Cambridge
University Press.
Wadlinger, H. A., & Isaacowitz, D. M. (2008).
Looking happy: The experimental manipula-
tion of a positive visual attention bias. Emo-
tion, 8(1), 121–126.
Wegner, D. M., Schneider, D. J., Carter S. R.,
& White, T. L. (1987). Paradoxical effects of
thought suppression. Journal of Personality
and Social Psychology, 53, 5–13.
Wells, T. T., & Beevers, C. G. (2010). Biased
attention and dysphoria: Manipulating selec-
tive attention reduces subsequent depressive
symptoms. Cognition and Emotion, 24(4),
719–728.
Werner, K., & Gross, J. J. (2010). Emotion regu-
lation and psychopathology: A conceptual
framework. In A. Kring & D. Sloan (Eds.),
Emotion regulation and psychopathology
(pp. 13–37). New York: Guilford Press.
White, L. K., Helfinstein, S. M., Reeb-
Sutherland, B. C., Degnan, K. A., & Fox, N.
A. (2009). Role of attention in the regulation
of fear and anxiety. Developmental Neurosci-
ence, 31, 309–317.
White, L. K., Suway, J. G., Pine, D. S., Bar-Haim,
Y., & Fox, N. A. (2011). Cascading effects:
The influence of attention bias to threat on
the interpretation of ambiguous information.
Behaviour Research and Therapy, 49(4), 244–
251.
Wiers, R., Bartholow, B., van den Wildenberg,
E., Thush, C., Engels, R., Sher, K., et al.
(2007). Automatic and controlled processes
and the development of addictive behaviors
in adolescents: A review and a model. Phar-
macology Biochemistry and Behavior, 86(2),
263–283.
Williams, M. G., Mathews, A., & MacLeod, C.
(1996). The emotional Stroop task and psy-
chopathology. Psychological Bulletin, 120(1),
3–24.

529
Deficits in emotion regulation skills are
a putative risk and maintaining factor in
various forms of psychopathology (Berking
& Wupperman, 2012; Hofmann, Sawyer,
Fang, & Asnaani, 2012; Werner & Gross,
2010). The defining criteria for many disor-
ders listed in the Diagnostic and Statistical
Manual of Mental Disorders (DSM-5; Amer-
ican Psychiatric Association, 2013) include
undesired emotions, such as sadness, anxi-
ety, or anger, that exceed the intensity and/
or duration of what is considered adaptive.
For other disorders, core symptoms can be
conceptualized as dysfunctional attempts to
avoid or down- regulate undesired emotions.
For example, bingeing in eating disorders
and substance use in alcohol dependence are
often seen as dysfunctional attempts to cope
with states of sadness, anger, boredom, or
other forms of emotional anguish (Berking
et al., 2011; Dingemans, Martijn, Jansen, &
Furth, 2009).
In a similar fashion, the development and
maintenance of depressive thinking can be
conceptualized as dysfunctional emotion
regulation. Appraising a situation as aver-
sive, uncontrollable, and unlikely to change
over time (Teasdale & Barnard, 1993) may
help to reduce the pressure actively to solve
personal problems (because working on
unattainable goals would be pointless) and
provide protection against hurtful disap-
pointments (because hope is an antecedent
of disappointment). In a situation in which
an individual is under high pressure to solve
numerous problems but neither feels able to
do so nor to bear another disappointment, a
depressogenic type of thinking that reduces
the pressure associated with unsolved prob-
lems and/or the pain associated with unful-
filled hopes may become an irresistible temp-
tation. Giving in to this type of thinking may
result in a small and short-lived reduction of
negative affect. In turn, this type of thinking
is reinforced and more likely to occur again
in similar situations in the future. Eventu-
ally, this process may lead into a clinically
significant depression. Adaptive affect reg-
ulation skills present an alternative way of
coping with undesired affective states that
may prevent such depressogenic cognitive
processes or facilitate disengagement from
such processes (Berking & Whitley, 2013).
Given the increasing amount of evidence
favoring such theories across various men-
tal disorders, enhancing emotion regulation
skills can be considered a promising transdi-
agnostic treatment target (Berking, Wupper-
man, et al., 2008; Mennin & Fresco, 2009;
Moses & Barlow, 2006). Others have also
argued that undifferentiated stress responses
(Bogdan & Pizzagalli, 2006), negative mood
states (Van Rijsbergen, Bockting, Berk-
ing, Koeter, & Schene, 2012), motivational
CHAPTER 31
Affect Regulation Training
Matthias Berking
Jeanine Schwarz
530 INTERVENTIONS
impulses (Grawe, 2006), and even appraisal
processes, which are defined through a
strong intrinsic affective component (e.g.,
“feelings” of hopelessness; Gibb, Beevers,
Andover, & Holleran, 2006; Teasdale &
Barnard, 1993), constitute a serious health
risk when the individual is unable to cope
successfully with these phenomena. Based
on the feasibility of applying “emotion” reg-
ulation skills to these other affective states,
we propose that the same arguments that
indicate the importance of emotion regu-
lation skills also apply to undesired affec-
tive states in general (i.e., emotions, stress
responses, moods, and urges; Gross, this
volume). Thus, the potential of targeting
emotion regulation skills in treatment can be
hypothesized to generalize to affect regula-
tion skills in general.
Almost all psychotherapeutic treat-
ments implicitly or explicitly work to
improve affect regulation skills in one way
or another. However, in some treatments
(e.g., person- centered psychotherapy, psy-
chodynamic treatments) general emotion
regulation skills are not explicitly addressed
and directly targeted but are assumed to be
enhanced through the application of strat-
egies considered to stimulate the healing
process in general (e.g., personal growth,
self- understanding, insight into uncon-
scious motives). In other treatments, such as
cognitive- behavioral therapy (CBT), these
efforts often focus on affective states that
are thought to be strongly related to the cli-
ent’s symptoms. For example, clients suffer-
ing from anxiety are primarily taught skills
to cope successfully with anxiety. Although
patients are usually encouraged to apply the
acquired skills to other emotions, specific
attempts to (1) systematically train patients
to apply acquired coping skills in the context
of other challenging affective states or (2) to
teach and practice additional skills that are
not relevant in the context of anxiety but
are relevant for successful coping with other
undesired affective states (e.g., distraction
as a strategy to reduce anger; engagement in
positive activities to reduce dysphoric mood
and feelings of hopelessness) are rare. If these
additional undesired affective states are rel-
evant for the maintenance of the anxiety dis-
order, focusing only on anxiety- managing
skills may not be sufficient to solve the prob-
lem effectively. On such occasions (e.g., if
anger, dysphoric mood, and/or hopelessness
significantly reduce the client’s motivation
and capacity to confront feared stimuli and/
or to draw helpful conclusions from such
exercises or habituate to feared stimuli; e.g.,
Foa, Riggs, Massie, & Yarczower, 1995), a
systematic focus on general affect regulation
skills might be warranted.
Moreover, many patients suffer from more
than one mental disorder (Kessler, Chiu,
Demler, & Walters, 2005). Particularly in
highly comorbid patients, “transaffective”
deficits in adaptive coping skills (e.g., the
inability to accept various undesired affec-
tive states if they cannot be modified) might
be at the core of many of their problems. For
these patients, a systematic focus on affect
regulation skills in general might be a more
efficient way of treatment than a compila-
tion of various disorder- specific manuals.
Minimally, an additional focus on strength-
ening affect regulation skills in addition
to providing disorder- specific treatments
should be considered when there is evidence
that general affect regulation deficits have
contributed to various psychopathological
symptoms. Thus, there is a need for inter-
ventions focusing on general affect regula-
tion skills that may complement disorder-
focused interventions.
Treatments such as emotion regulation
therapy (ERT; Mennin & Fresco, this vol-
ume), the emotion regulation module used
in dialectic behavior therapy (DBT; Neacsiu,
Bohus, & Linehan, this volume), and some
mindfulness- based treatments (Farb, Ander-
son, Irving, & Segal, this volume; Wup-
perman et al., 2012) are based on a similar
conceptualization of mental disorders as
affect regulation training (ART) and, conse-
quently, also address affect regulation skills
in a more general fashion. The main differ-
ence between these treatments and ART is
that ART has from its beginning focused on
communalities across various mental disor-
ders with regard to deficits in general emo-
tion regulation skills. To improve such skills,
ART systematically integrates techniques
from various psychotherapeutic approaches,
such as CBT (e.g., Beck, 1995), compassion-
based therapy (Gilbert, 2011; Weissman &
Weissman, 1996), DBT (Neacsiu et al., this
volume), emotion- focused therapy (EFT;
Greenberg, 2004), ERT (Mennin & Fresco,
this volume), mindfulness- based interven-

Affect Regulation Training 531
tions (Farb et al., this volume), neuropsy-
chotherapeutic translational approaches
(Grawe, 2006), principles used in problem-
solving therapies (D’Zurilla & Nezu, 2010),
and strength- focused interventions (Duck-
worth, Steen, & Seligman, 2005; Grawe,
2002), into a highly standardized and trans-
diagnostically oriented training program.
This program can be utilized (1) as a stand-
alone intervention (when working with
patients suffering from less severe or sub-
threshold mental disorders, with individuals
at risk for developing mental health prob-
lems, or with healthy individuals who sim-
ply desire to enhance their well-being) or (2)
as an adjunctive intervention complement-
ing any empirically validated treatment of
mental disorders whenever a stronger focus
on enhancing general affect regulation skills
is desired. Although ART certainly shares
some theoretical assumptions with many of
the approaches just described and also uses
some of the interventions applied in these
approaches, we believe that the uniqueness
and advantage of ART lies with its compre-
hensive approach of incorporating specific
strategies, interventions, and exercises into
a consistent training program that explic-
itly and exclusively focuses on enhancing
general affect regulation skills— regardless
of the patient’s particular disorder(s) (for
details see Berking & Whitley, 2013).
Inspired by Klaus Grawe’s (2006) trans-
lational ideas on the development of mental
disorders, as well as the lack of transdiagnos-
tic interventions to enhance general affect
regulation skills at the time, we began to
develop ART in 2004. Since then, the train-
ing has become quite popular in the German-
speaking parts of Europe, where it is known
as Training Emotionaler Kompetenzen
(TEK; Berking, 2010; www. tekonline.info).
The training was primarily developed as a
group-based intervention (four to eight par-
ticipants), but it can also be used in individ-
ual therapy. Although the program has only
been evaluated in adults, its application for
adolescents is practicable after adapting the
procedures and materials to the needs and
capacities of this age group. The training,
which takes at least 18 hours to complete,
can be partitioned in various ways (e.g., two
1.5-hour sessions or one 3-hour session per
week over a 6-week period). From our expe-
rience, however, participants should have at
least 4 to 6 weeks time to practice the skills-
building exercises. ART can be delivered by
an ART-trained mental health professional,
such as a licensed psychotherapist with a
master’s degree in medicine or psychology.
Other, nonlicensed mental health profes-
sionals can also be trained to administer
ART, albeit not to individuals suffering from
acute mental disorders. Subsequently, we
outline the theoretical and empirical back-
ground of ART before we provide practical
explanations of how the relevant skills are
delivered to participants. At the end of the
chapter, we review empirical evidence for
the effectiveness of ART and suggest future
directions for our research.
The ART Model of Adaptive
Affect Regulation
The ART model of adaptive affect regu-
lation was developed by synthesizing (1)
established affect regulation theories (e.g.,
Gottman & DeClaire, 1997; Grawe, 2002,
2006; Gross, 1998; Larsen, 2000; Lazarus,
1991; Leahy, 2002; Saarni, 1999; Salovey
& Mayer, 1990), (2) findings from studies
on the association between affect regulation
and psychopathology (Berking & Wupper-
man, 2012), and (3) our extensive clinical
experience. The purpose of this develop-
ment was to provide a normative (i.e., pre-
scriptive) model explaining common failures
in affect regulation and directions for inter-
ventions to enhance affect regulation. In the
resulting model, adaptive affect regulation
is conceptualized as a situation- dependent
interaction between several affect regulation
skills. Figure 31.1 illustrates an overview of
the model. The skills included in the model
are described below.
1. The ability to consciously perceive
and be aware of affective states. The abil-
ity to consciously perceive and be aware of
affective states is the basis of every effortful
attempt to regulate affective states. Effort-
ful and conscious regulation may only play a
small role in the daily routine of regulation
in a healthy individual, because implicit or
highly automatized processes are likely to
dominate the regulation process as long as
these processes are able to maintain the indi-
vidual’s affect in the comfort zone (Koole &
532 INTERVENTIONS
Rothermund, 2011; Gyurak & Etkin, this
volume). However, if undesired affective
states cannot be down- regulated through
implicit processes, conscious information
processing will be activated as a precious
resource reserved for solving problems in the
self- management process (Mauss & Tamir,
this volume). Becoming aware of affective
states may facilitate the use of affect regula-
tion skills requiring cognitive resources (e.g.,
evaluating the degree of undesirability of the
emotion, labeling the emotion, identifying
its causes) and hence foster adaptive regu-
lation. Conversely, the inability to become
aware of one’s emotions impedes the utili-
zation of cognitive problem- solving capaci-
ties, thus intensifying and prolonging the
unwanted affective state (e.g., Lane et al.,
1996; Swart, Kortekaas, & Aleman, 2009).
2. The ability to identify and correctly
label affective states. Identifying and label-
ing affective states refers to matching emo-
tional experiences with the appropriate
semantic categories (e.g., “This feeling I am
experiencing is anger”). The availability of
a differentiated system of cognitive repre-
sentations of affective states and the ability
to correctly assign an experience to a spe-
cific category helps the individual to build
and use knowledge about this state, which
is often helpful for adaptive regulation. For
example, recognizing an emotion as anger
provides information about the nature and
purpose of the emotion (e.g., enhancing
assertive behavior; fighting for one’s rights),
its potential risks (cueing aggression leading
to sanctions through others) and benefits
(getting what one wants, even if one has to
fight for it), as well as information on regu-
lation strategies that are likely to be effec-
tive (potentially effective: distraction, relax-
ation, reappraisal and compassion; likely to
be ineffective: prolonged exposure). Recog-
nizing the emotion facilitates adaptive cog-
FIGURE 31.1. The ART model of adaptive affect regulation.
Affect Regulation Training 533
nitive processing. Thus, this ability should
foster effective affect regulation and hence
mental health and well-being (Feldman-
Barrett, Gross, Christensen, & Benvenuto,
2001).
3. The ability to identify relevant main-
taining factors for current affective states.
Identifying relevant maintaining factors
refers to development of an inner working
model to explain factors that initially cued
the current affective state and, more impor-
tantly, to identify the factors that maintain
the emotional state at that moment. In gen-
eral, psychological treatment for mental
health problems should (a) normalize the
client’s experiences, (b) help to explain the
occurrence of the problem, and (c) pro-
vide direction for successful coping. With
an additional focus on affective states, the
model should include relevant and change-
able factors such as the internal or exter-
nal stimuli or situation cueing the emotion,
appraisal of the stimuli or situation, as well
as desires, beliefs, and goals relevant for the
appraisal. Understanding factors that ini-
tially elicit and maintain an affective state
helps to (a) give meaning to an aversive expe-
rience, thereby making it easier to bear; (b)
clarify whether the emotion can or cannot
be changed (thus enhancing the acceptance
of an emotion when necessary); and (c) iden-
tify targets for effective modification of the
affective state.
4. The ability to modify affective states
actively. To modify affective states actively
is to effectively change the quality, intensity,
and/or duration of an affective state into
a desired direction with the help of strate-
gies that have no unwanted, long-term side
effects. This ability provides one of the most
effective means for preventing the use of
dysfunctional strategies (i.e., strategies that
have unwanted side effects). Moreover, effec-
tive modification skills provide an empirical
basis for the expectation of being able to
modify unwanted affective states whenever
necessary. Such an emotional self- efficacy is
likely to reduce both anxiety about future
aversive events and avoidance tendencies
toward affective states. As a result, this
expectation reduces general anxiety and the
willingness to experience emotionally chal-
lenging situations, thus facilitating further
skills building. Evidence for this hypothesis
comes from studies demonstrating that an
individual’s expectation of successfully alle-
viating negative mood states is significantly
associated with mental health (Catanzaro
& Greenwood, 1994). In this context, it is
noteworthy that ART skills should be under-
stood as categories that include various sub-
skills. This is particularly relevant for the
ability to modify affective states. For exam-
ple, most of the long list of positive activities
used in behavioral therapy for depression
can be seen as modification skills. It is also
noteworthy that the effectiveness of specific
modification skills differs across individuals
and situations, and is likely to be moderated
by numerous individual and contextual fac-
tors (Bonanno, Papa, Lalande, Westphal, &
Coifman, 2004).
5. The ability to accept and tolerate nega-
tive affective states when necessary. Accep-
tance and tolerance of unwanted affective
states is a crucial skill whenever affective
states cannot be changed or when changing
them would involve too high a cost (e.g., in
terms of time and energy). Although an opti-
mistic perspective about one’s abilities to
modify affective states has clear advantages,
acknowledging that affective states are
often very difficult to modify, or that they
sometimes cannot be modified, is equally
advantageous (Hayes, Strosahl, & Wilson,
1999). Modifying undesired affective states
is often difficult— particularly for patients
suffering from mental disorders— and hence
may threaten the need for control. To end
the aversive affective state and/or to restore
a sense of control, the individual may engage
in dysfunctional strategies (e.g., suppres-
sion) that help to avoid the aversive experi-
ence but likely lead to unwanted long-term
outcomes (e.g., Feldner, Zvolensky, Stickle,
Bonn- Miller, & Leen- Feldner, 2006; Lev-
itt, Brown, Orsillo, & Barlow, 2004). Con-
versely, the availability to accept, bear, or
tolerate aversive affective states reduces
this need and hence the risk of engaging in
maladaptive affect regulation (e.g., Wupper-
man, Neumann, & Axelrod, 2008).
6. The ability to approach and confront
situations that may cue negative affective
states. Approaching and confronting situa-
tions that may cue negative affective states
is often necessary to accomplish personally
relevant goals (Hayes et al., 1999). More-

534 INTERVENTIONS
over, confronting these situations provides
an opportunity to improve existing affect
regulation skills and to develop new ones
(arrows 6a and 6b in Figure 31.1). Willingly
encountering occasional situations that cue
negative affective states to attain important
goals when necessary, ensures that one’s
affect regulation skills are practiced on a
regular basis and will be available and effec-
tive in times of emotional anguish. More-
over, as this ability facilitates goal attain-
ment, it helps to satisfy relevant needs and
thus reduces negative affect in the long run.
7. The ability to support oneself compas-
sionately in distressing situations. All of
the skills just described have the potential
to increase emotional suffering in the short
term. For example, consciously perceiving
unwanted affective states is usually associ-
ated with an increase in the perceived inten-
sity of the emotional suffering. Identifying
momentary feelings such as stress, fear,
anger, sadness, dysphoria, shame, and guilt
activates semantic concepts that likely to cue
further negative feelings. Working to under-
stand undesired affective states can lead to
the processing of problems and the acknowl-
edgment of one’s inabilities to solve them,
which is likely to cue further deterioration
of mood. Efforts to modify undesired affec-
tive states are often unsuccessful, or at least
not as successful as expected or desired,
hence cueing disappointment, frustration,
and eventually despair. Attempts to accept
and tolerate an emotion are often associated
with feelings of helplessness and hopeless-
ness, and unrealistic fears of catastrophic
consequences assumed to occur when nega-
tive affective states are no longer suppressed.
Moreover, confronting situations that cue
negative affective states to attain important
goals, by definition, cues negative affective
states. As illustrated by these examples, the
process of applying adaptive affect regula-
tion skills could lead to deterioration of
mood and trigger the use of dysfunctional
strategies. Therefore, self- soothing or self-
encouraging strategies are needed as part
of the regulation process to prevent one’s
mood from deteriorating. Unfortunately,
many individuals with mental disorders
have a tendency to be strongly self- critical
of perceived failures. In the process of affect
regulation, this self- critical tendency is likely
to manifest when individuals perceive that
they have failed to regulate their emotions
successfully. Such a critique usually cues
further (secondary) negative affective states
(see Greenberg, 2004) that impede efforts to
cope with the undesired primary affective
states. Conversely, a compassionate stance
toward oneself can help reduce the risk of
being caught up in such a vicious cycle (Gil-
bert, 2011).
In addition to the list of skills and assump-
tions about why these skills are important
and how they interact, the ART model
includes the hypothesis that modification
and acceptance/tolerance skills are the
only skills in the model that are ultimately
relevant for mental health. All other skills
are not considered relevant by themselves
but only to the extent that they facilitate
the successful application of modification
or acceptance/tolerance. This idea is based
on research on rumination (e.g., Morrow
& Nolen- Hoeksema, 1990) and our clini-
cal observation that many clients obviously
experience their affective states consciously,
analyze them, and work to explain them
but are unable to benefit from these activi-
ties. On the contrary, excessive use of these
strategies seemed to be partly responsible for
maintaining the mental disorder. Empirical
studies on the ART model, reviewed in the
next section, provide further preliminary
support for this assumption.
Empirical Evidence for the ART
Model of Affect Regulation
After we conceptualized the ART model, we
developed and validated the Emotion Regu-
lation Skills Questionnaire (ERSQ; Berking
& Znoj, 2008) to assess the skills included
in the model. This self- report instrument
utilizes a 5-point Likert scale to assess each
skill of the ART model (plus an additional
subscale assessing the ability to use body
sensations to identify experienced affective
states) with three items per scale. Addition-
ally, a total score can be computed as the
average of all items. In several studies, the
ERSQ displayed adequate to good internal
consistencies (Cronbach’s alpha = .82–.94).
Results from exploratory and confirma-
tory factor analyses provide support for the

Affect Regulation Training 535
assumed dimensionality of the measure.
Moreover, sensitivity to change has been
demonstrated in multiple samples of clients
undergoing psychotherapy, and all scales
have demonstrated positive associations with
measures of well-being and mental health,
and negative associations with measures of
psychopathology and ER deficits (Berking,
Ebert, Cuijpers, & Hofmann, 2013; Berking
et al., 2011, 2012; Berking, Meier, & Wup-
perman, 2010; Berking, Orth, Wupperman,
Meier, & Caspar, 2008; Berking, Wupper-
man, et al., 2008; Berking & Znoj, 2008;
Ebert, Christ, & Berking, 2013).
More specifically, all skills in the ART
model have been shown to be cross-
sectionally associated with mental health in
nonclinical samples (Berking et al., 2012;
Berking, Wupperman, et al., 2008; Berking
& Znoj, 2008), and clinical samples and non-
clinical controls report less successful appli-
cation of all skills than do patients treated
for mental disorders (Berking et al., 2011;
Berking, Wupperman, et al., 2008; Berking
et al., 2013). Additionally, an increase in
successful affect regulation during CBT was
shown to be associated with greater symp-
tom reduction (Berking, Wupperman, et
al., 2008). The differences and correlations
reported in these studies were particularly
strong for modification and acceptance/tol-
erance. This finding provides preliminary
support for assumption that these skills
are particularly relevant for mental health.
Moreover, multivariate regression analyses
identified only these two skills areas as mak-
ing a unique contribution when predicting
treatment outcome from changes in affect
regulation skills (e.g., Berking, Wupperman,
et al., 2008). Further research indicated that
affect regulation skills assessed through the
ERSQ predicted subsequent indicators of
psychopathology over a 2-week and a 5-year
period, whereas these indicators did not pre-
dict subsequent success in applying affect
regulation skills (Berking, Orth, et al., 2008;
Berking, Wirtz, Svaldi, & Hofmann, under
review). This finding suggests that deficits
in affect regulation skills are not merely a
symptom of mental health problems but a
relevant antecedent factor.
In two other studies, affect regulation
skills assessed through the ERSQ predicted
relapse during and after inpatient treatment
for alcohol dependency (Berking et al., 2011)
and the reduction of depressive symptoms in
patients treated for major depressive disor-
der (MDD; Radkovsky, McArdle, Bockting,
& Berking, under review). Finally, in both
a clinical and a nonclinical sample, success-
ful modification of undesired affective states
(partly) mediated the relationship between
most ERSQ skills and psychopathology, but
not for acceptance and psychopathology
(Berking et al., 2012), which suggests that
only acceptance and tolerance are relevant
for mental health, regardless of whether
these skills facilitate the modification of
affective states. In summary, a growing
body of evidence supports the hypotheses
that the skills included in the ART model
help maintain or restore mental health and
are thus promising for treatments of mental
health problems.
Brief Description
of the ART Program
Support for the ART model led to the devel-
opment of the ART program, which inte-
grates principles and interventions from
various psychotherapeutic approaches to
foster general affect regulation skills in indi-
viduals likely to benefit from more adap-
tive ways of coping with unwanted affective
states. The following sections provide a brief
description of the ART program (for a more
detailed description, see Berking & Whitley,
2013).
General Structure
ART first introduces participants to the
nature of affective states and their evolution-
ary background, purposes, potential risks,
and benefits, as well as the mechanisms
involved in regulating affective processing.
From our understanding of the mechanisms
involved, seven skills have demonstrated
effectiveness in facilitating adaptive coping
with affective states that are particularly
challenging to manage. These skills include
muscle relaxation, breathing relaxation,
nonjudgmental awareness, acceptance and
tolerance, compassionate self- support, anal-
ysis of affective states, and modification of
affective states. Each skill is practiced in a
long version, then shortened to a version
that can be applied in very short periods of
536 INTERVENTIONS
time (3 seconds to 3 minutes). The shortened
ART skills are then chained together, creat-
ing the ART sequence (see Figure 31.2). This
sequence can be used whenever participants
suffer from any undesired affective state.
ART heavily relies on an intensive train-
ing regimen in which participants practice
the whole ART sequence for 20–30 minutes
daily and additionally engage in at least three
short exercises (for 3 seconds to 3 minutes)
over a period of at least 6 weeks. To sup-
port this practice, participants receive work-
sheets, handouts, and an information book-
let during the training. Additionally, they
receive specific audio files that guide them
through the exercises of the ART sequence,
and a set of 140 text messages is automati-
cally sent to participants throughout the
training. After participants have mastered
the skills, the focus shifts to practicing in
daily life the learned skills to cope success-
fully with challenging situations.
Muscle and Breathing Relaxation
Every skills building module starts with a
short psychoeducation that explains how
different areas of the brain may mutually
activate each other, thus forming neuroaf-
fective vicious cycles that can override
processes responsible for down- regulating
undesired affective states. For example,
when the amygdala is activated by a situa-
tion that is interpreted as a potential threat,
it initiates physiological changes such as
an increase in muscle tension and an accel-
eration of breathing. Because such changes
have occurred in the past when encountering
threatening situations, the muscle tension
and rapid breathing themselves may in turn
have become danger signals to the amyg-
dala (i.e., classical conditioning or the con-
cept of somatic markers; Damasio, 2000).
As a result, a vicious cycle may develop in
which the activation of the amygdala causes
increased muscle tension and rapid breath-
ing, which in turn causes further activation
of the amygdala and even more muscle ten-
sion and further rapid breathing (for details
on how somatic processes are integrated into
information processing relevant for emotion
elicitation and maintenance, see Stemmler,
2004; Teasdale & Barnard, 1993). When
participants realize that they are experienc-
ing stress or negative affective states, they
can break through these vicious cycles by
consciously relaxing their muscles and pur-
posefully calming their breathing. By engag-
ing in these exercises, they can reduce acti-
vation of the sympathetic system, the limbic
system, and the brainstem (Porges, 2007).
After this vicious cycle has been intro-
duced and discussed with participants, we
introduce participants to progressive mus-
cle relaxation techniques (Jacobson, 1964)
combined with a simple exercise to calm
breathing. Studies have consistently shown
the effectiveness of muscle and breathing
relaxation in the treatment of a variety of
mental and physical disorders (e.g., Öst,
1987; Conrad & Roth, 2007). Addition-
ally, with reduced limbic activity through
breathing and muscle relaxation, a shift can
occur that changes the focus from primar-
ily amygdala- driven limbic- and brainstem-
focused responses back to more prefrontal,
cortical responses that are reflective rather
than reactive (Arnsten, 2009). Thus, reduc-
ing psychophysiological arousal with the
help of muscle and breathing relaxation
facilitates the subsequent use of techniques
requiring more cognitive resources.
Nonjudgmental Awareness
When the amygdala detects potential
threats, it alerts the prefrontal cortex (PFC),
FIGURE 31.2. ART skills and ART sequence.
This is the prescriptive model of affect regulation
as it is presented to participants. It includes the
same skills as the ART model of adaptive affect
regulation (Figure 31.1); however, for didactic
purposes, it presents them in a more concrete
fashion, providing a step-by-step guideline for
coping with undesired affective states.
Affect Regulation Training 537
which then focuses attention and con-
scious processing on threat- relevant stimuli
(LeDoux, 2012). Such effortful process-
ing aims to more validly evaluate potential
threats and determine behavioral responses
most likely to be effective. However, if this
processing does not lead to a solution of
the problem, an ongoing focus on aversive
experiences may lead to an ongoing activa-
tion of the amygdala. This activation may
cause the PFC to focus even more attention
on the problem (Amaral, Price, Pitkänen, &
Carmichael, 1992; Gray & McNaughton,
2000; Vuilleumier, Richardson, Armony,
Driver, & Dolan, 2004), thus potentially
resulting in a cyclical reaction of even more
intense processing of problematic stimuli
and further activation in the amygdala. In
attempt to prevent such a negative cycle,
some individuals try to rigidly suppress
“negative” thinking (Ottenbreit & Dob-
son, 2004). However, this strategy is likely
to have a paradoxical effect of increasing
the intensity of negative thinking instead
of reducing it (Wenzlaff & Wegner, 2000).
Thus, instead of fighting negative thinking,
we encourage participants simply to observe
the situation and their affective states with-
out interpreting, judging, or reacting. This
kind of mindfulness- based affect labeling
has been shown to disrupt amygdala activity
(Lieberman et al., 2007) and to be effective
as a reappraisal or distraction in the regula-
tion of negative affect (Lieberman, Inagaki,
Tabibnia, & Crockett, 2011).
When applying this technique as ART
Skill 3, participants start by switching from
regulating their breathing to simply observ-
ing it. Then, they start focusing on their
sensual experiences (sensations in the body,
sounds, odors, and visual perceptions); their
thoughts (“What is the next thought that
comes to mind?”); and their current salient
wishes, desires, and goals (“If everything
was going exactly according to your wishes,
where would you be now? What would you
be doing now? Imagine this perfect scene. . . .
Briefly name the wish or goal expressed in
this image”). At the end of this sequence,
participants are invited to be aware of
their affective states without judging them.
Instead, they are instructed to (1) find a
matter- of-fact label for this emotion (e.g.,
anger), (2) rate its intensity on a scale from
1 to 10, and (3) identify where the emotion
is felt in the body. Finally, participants are
invited to check for additional feelings that
might lie behind the most prominent one and
repeat the same three-step process on them.
This technique of becoming aware of one’s
affective states in nonjudgmental ways aims
to recruit cognitive resources to facilitate
effective affect regulation (Gyurak & Etkin,
this volume; Hariri, Mattay, Tessitore, Fera,
& Weinberger, 2003). Evidence for the effi-
cacy of nonjudgmental awareness includes
studies on the effectiveness of mindfulness-
based interventions (Farb et al., this volume).
Acceptance and Tolerance
of Affective States
Challenging affective states, such as fear or
anger, constitute threats to the goals of feel-
ing good and of being in control (Grawe,
2006). Reponses of the amygdala toward
such threats likely include the activation
of areas responsible for avoidance motiva-
tion (Grawe, 2006). Under the influence
of avoidance goals (Grawe, 2002, 2006),
people may rigidly focus on eliminating the
“threatening” affective states. However, the
goal of getting rid of an undesired emotion
is often difficult to attain. The emotion-
generating system often acts autonomously
of the areas of the brain that initiate con-
sciously controlled behavior (Amaral et al.,
1992; see also the concept of core valuations
outlined by Ochsner & Gross, this volume).
Such a design can be hypothesized to have
provided a significant evolutionary advan-
tage to our ancestors. If negative affective
states could simply be switched off through
conscious control, a strong temptation to
simple down- regulate unpleasant emotions
without going through the trouble of engag-
ing in the behavior the emotion is supposed
to facilitate could interfere with the emo-
tions cueing adaptive behavior. Additionally,
emotional reactions often cause significant
changes in the body that take some time to
dissipate and, as long as they are present,
may impede the ability to make emotional
changes (Teasdale & Barnard, 1993). It can
also been hypothesized that areas such as the
ventromedial PFC and the anterior cingulate
cortex, which are assumed to help in down-
regulation of undesired emotions under the
influence of dorsal or lateral areas of the
PFC, may get “hijacked” by strong emotions
538 INTERVENTIONS
in a way that leads to ruminative processing
of emotion- related information and impedes
effective top-down emotion regulation
(Johnstone & Walter, this volume).
In summary, for various reasons, it is
often very difficult to control affective states
consciously. Thus, rigid attempts to shut
down an undesired affective state instantly
through sheer willpower may fail. Such
unsuccessful attempts at attaining the salient
goal of eliminating an undesired affective
state will likely cue amygdala activation and
increase the salience of the goal (Martin,
Tesser, & McIntosh, 1993; Rothermund,
2003). Because of the resulting increase in
negative arousal, the likelihood of attaining
an increasingly salient goal is significantly
reduced. Thus, a vicious cycle may develop
in which fighting against the emotion only
makes it stronger. To prevent this vicious
cycle, developing the ability to accept and
tolerate negative affective states is impor-
tant (at least as long as such states cannot be
changed). To enhance acceptance and toler-
ance skills, ART first works to help partici-
pants find a helpful definition of acceptance/
tolerance and to understand why these skills
are important. Then, participants develop
their personal, five-step Acceptance and
Tolerance Plan, which includes the follow-
ing steps: (1) Consciously decide to work
on accepting the emotion; (2) strengthen
this decision by finding at least one reason
why acceptance is helpful; (3) work to see
affective states as allies providing impor-
tant information and supporting behavior
that might be used for effective coping with
challenging situations (e.g., for an adap-
tive conceptualization of affective states,
see Cosmides & Tooby, 2000); (4) become
aware of your ability to tolerate negative
affective states for a certain period of time;
and (5) realize that affective states are usu-
ally impermanent experiences that do not
last forever and are likely to fade away (or at
least decrease in intensity).
At the end of the Acceptance and Toler-
ance Plan, participants devise a Personal
Acceptance and Tolerance Statement, such
as the following, to use as self-talk, until
they feel that they can allow affective states
to exist:
“I will work to accept this unpleasant emo-
tion because this is the smartest thing to
do as long as I cannot change it; I know
that this emotion wants to provide me
with very relevant information; it wants to
point out that . . . ; it aims to help me by
enhancing my ability to . . . ; I also know
that I have experienced many unpleas-
ant emotions in the past and have always
been able to get through this in one way or
another; finally, I know that this emotion
will not last forever, I will only have to
tolerate it for so long.”
In the ART sequence, this kind of
acceptance- enhancing self-talk is applied
to affective states that have been perceived
nonjudgmentally at the end of ART Skill
3. Preliminary evidence for the therapeutic
potential for the skills of acceptance and tol-
erance comes from research on the relevance
of avoidance for the development and main-
tenance of mental disorders (e.g., Berking,
Neacsiu, Comtois, & Linehan, 2009), from
studies demonstrating the effectiveness of
mindfulness- based approaches (Farb et al.,
this volume), and from acceptance and com-
mitment therapy, in which acceptance of
negative affective states and willingness to
experience them is one of the primary aims
of therapy (Hayes, Luoma, Bond, Masuda,
& Lillis, 2006).
Compassionate Self‑Support
in Emotionally Challenging Situations
The next vicious cycle starts when someone
who feels stressed or upset begins to self-
blame or self- criticize. That person might
think, “I can’t do anything right. I am a
complete failure. Something is wrong with
me.” These statements are self- inflicted
attacks that threaten the need to feel valued
(Grawe, 2002), cueing additional negative
affective states (e.g., anger, shame, guilt)
associated with further activation of the
amygdala (Longe et al., 2010). The activa-
tion of the amygdala through self- criticism
and additional negative feelings increases
the stress response, likely leading to more
negative feelings, which in turn lead the per-
son to be even more self- critical. Activating
a compassionate attitude toward oneself and
engaging in self- supportive behaviors is an
effective strategy to preclude this vicious
cycle (Gilbert, 2011). ART participants learn
how to provide compassionate self- support
Affect Regulation Training 539
by imagining themselves in a burdensome
situation accompanied by negative feelings.
While reexperiencing these feelings, they
learn to self- assess from the perspective of
a kind and caring (albeit somewhat distant)
inner observer. They are instructed to “let
the feeling of compassion toward themselves
arise from within,” which creates a strong
and warm feeling of self- empathy, accompa-
nied by the desire to help and to end their
suffering (Weissman & Weissman, 1996).
The observing part should then approach
the suffering part of the participant, sup-
porting and comforting that part by nor-
malizing the negative affective states, pro-
viding encouragement, and maybe adding a
physical gesture of compassion (e.g., giving
oneself an imaginary hug). Preliminary evi-
dence for the effectiveness of interventions
that enhance compassionate self- support
has been found in several empirical studies
(Hofman, Grossman, & Hinton, 2011).
Constructive Analysis
of Affective States
Under stress, the amygdala activates pro-
cesses that release stress hormones into the
brain. An excessive release of such hormones
can impair cognitive processing in other
brain areas, including the PFC and the hip-
pocampus (Huether, 1998; LeDoux, 2012),
which play important roles in the analysis
of affective states. The ability to identify
the causes of one’s current feelings correctly
leads to a greater sense of self- mastery and
control over one’s affective states. If through
weakened prefrontal and hippocampal
functioning, the ability to self- analyze one’s
affective states is diminished, a vicious cycle
develops in which the loss of orientation
and control further activates the amygdala,
which in turn perpetuates the cycle. Thus,
maintaining or regaining a sense of mastery
and control over one’s affective states in
stressful situations helps prevent the escala-
tion of negative affect.
In ART Skill 6, participants are trained
to utilize the Analyzing Affective States
Worksheet (depicted in Figure 31.3) to
enhance the ability to identify correctly
the causes of one’s current emotions. The
worksheet is based on an integrative model
of how affective states are cued and main-
tained. It focuses on emotions and feelings
as crucial affective states, but we broadly
define these terms in the training (to avoid
burdening patients with the academic need
for clear classifications). Thus, these terms
include other affective states, such as stress
responses or moods. The general model
of how emotions (i.e., affective states) are
elicited includes external or internal events
that cue the emotion; attention deployment
toward and appraisals of these events; needs,
wishes, and goals that are relevant for these
appraisals; secondary emotions (Greenberg,
2004) cued by appraising the primary emo-
tion in a certain way; and body sensations,
motivational impulses, and behavior associ-
ated with the emotion, all of which provide
feedback into the emotion generating system
and thus affect the subsequent course of the
emotion. To clarify the course of subsequent
action, the worksheet ends with a summary
of the short- and long-term advantages and
disadvantages of the emotion.
ART trainers explain the model and help
participants to apply it to their own affective
states. Eventually, the skill will be included
in the ART sequence in the form of the car-
ing inner observer from ART Skill 5, who
guides the suffering part of the client through
the analysis (“What situation prompted this
feeling?”; ”How did you interpret and evalu-
ate the situation?”; “What was at stake in
this situation?”). Partly based on the find-
ing that insight- oriented psychological
treatments have been shown to be effective
(e.g., Gibbons, Crits- Christoph, Barber,
& Schamberger, 2007) and our own clini-
cal experience, we hypothesize that simply
understanding challenging affective states
with the help of a model normalizes and
hence validates such states; that the impres-
sion of having understood the problem cues
a general feeling of control and hope; and
that offers of specific and promising ways
to cope successfully with undesired states
significantly contributes to the (short-term)
reduction and/or acceptance of undesired
affective states.
Effective Modification
of Affective States
ART Skill 7 aims to empower participants
to actively modify quality, intensity, or dura-
tion of an undesired emotion and restore
their sense of control. This skill, which pro-
540 INTERVENTIONS
vides a step-by-step process to modify affec-
tive states, is based on the general problem-
solving model (D’Zurilla & Nezu, 2010)
and utilizes the Modification of Affective
States Worksheet depicted in Figure 31.4.
Based on the long- and short-term advan-
tages and disadvantages of the emotion sum-
mary from the Analyzing Affective States
Worksheet, the Modifying Affective States
Worksheet first requires participants to set
as a goal how they would rather feel. Then,
for each relevant maintaining factor (i.e.,
each box of the worksheet), participants
brainstorm how changes in these areas could
help cue the target emotion. After partici-
pants have identified as many helpful ideas
as possible for each box, they select the most
promising ideas to plan specific behaviors
that cue the target emotion. Then, they put
the plan into practice and evaluate the out-
come. With regard to the latter, patients are
also taught a plan for how to deal effectively
with unsuccessful modification attempts
(see bottom right-hand corner of Figure
31.4), because such failures often lead to a
complete disengagement from functional
regulation endeavors and the use instead of
impulsive strategies that might effectively
improve one’s mood in the short run but are
associated with negative long-term outcomes
(e.g., engaging in avoidance, substance use,
worry, depressogenic thinking).
After the model is introduced and prac-
ticed with a couple of examples, Skill 7
is included in the ART sequence. At this
point, the compassionate, helping part of
FIGURE 31.3. The Analyzing Affective States Worksheet. To reduce the complexity wherever possible,
the distinction between affect and emotions is not addressed in the training. Instead, the terms emo-
tions and feelings are used in a very broad sense (e.g., with regard to feeling stressed out and relative
to various mood states). Although the worksheet is fairly complex, participants who have been trained
intensively in its use have rated it as one of the most helpful components of ART. Typical explanations
for its popularity include “It gets everything I somehow felt was relevant for how I feel on one sheet of
paper.”
10
1
5
3
7
8
4
6
1211
9
2
Short-term Long-term Short-term Long-term
How do I appraise the primary emotion?
How does this make me feel about my
emotion?
What happened? (facts only!)
How did I feel emotionally & physically at the
time the situation occurred?
Why is this so important to me?
What is at stake?
How and where do I feel the emotion in
my body?
Is the emotion part of an old pattern?
What can I call this pattern?
What caught my attention?
How did I interpret the situation?
What was my evaluation of the situation?
Prompting
Situation
Needs
Goals
Desires
Expectations
Emotion
Impulses to Act
Preceding Emotional Vulnerability
Behavior
Secondary Emotion
Analyzing Emotions
Attention, Interpretation,
& Evaluation
Advantages of Emotion
Disadvantages of Emotion
Emotion in the Body
Old Response Pattern
Affect Regulation Training 541
the self (established in Skill 5) guides the
vulnerable part of the self (also established
in Skill 5) in modifying negative affective
states (“How do you actually want to feel in
this situation?”; “What is your target emo-
tion?”; “How could you change the situa-
tion to achieve your target emotion?”). As
such, ART Skill 7 builds on the other skills
of the ART sequence and utilizes the gen-
eral problem- solving framework to modify
undesired affective states.
Efficacy of ART
Research on the effectiveness of ART is still
in the early stages. However, several stud-
ies have been conducted in a variety of psy-
chotherapeutic settings and early results
are promising. One published study (Berk-
ing, Wupperman, et al., 2008) involved 289
patients from a mental health hospital. The
patients all received 6 weeks of CBT supple-
mented with additional multidisciplinary
treatment as necessary. A randomly selected
subgroup of patients was offered the option
to replace four treatment sessions of CBT per
week with an abbreviated version of ART
during a 3-week period. Results showed that
at the end of the 6-week treatment, patients
who had participated in ART displayed sig-
nificantly greater improvement of affect reg-
ulation skills than patients who had received
CBT only. Additionally, patients participat-
Prompting
Situation
Needs
Goals
Desires
Expectations
Emotion
Behavior
1
Old Response Pattern
Modifying Emotions
Attention, Interpretation,
and Evaluation
2
5
3
How can I change the situation?
How can I improve my physical and emotional
health?
Are other needs, goals, etc. really
more important?
Can I reduce the significance of this
need, goal, etc. to a lower level of
significance?
What would be a more adaptive new pattern?
What can I call this new pattern?
How else can I look at this situation?
4
6
Effective change process:
1. Identify target feeling
2. Brainstorm ideas about how
to activate target feeling
3. Make a plan
4. Put plan into action
5. Success:Rewardyourself
Failure/Partial Success:
1. Reward yourself for trying!
2. Try more of the same
3. Try other strategies
4. Change target or
accept and tolerate
Strategy #3 (Distraction) – Do something pleasurable to distract yourself. The
goal is distraction, NOT avoidance!
Strategy #1 (Use the B
lues) – Identify the helpful actions suggested
by the emotion and then implement them.
Strategy #2 (Opposite Action) – Identify the unhelpful action suggested by
the emotion and then engage in the opposite behavior.
9
Preceding Emotional Vulnerability
Secondary Emotion
Can I change how I think about the
emotion (in order to feel better about the
emotion)?
Emotion in the Body
7
8
How can I foster changes in my body
that would trigger the target emotion?
FIGURE 31.4. The Modifying Affective States Worksheet. When using distraction as a behavior-
oriented modification strategy, it is explained to participants that the same behavior can be considered
(dysfunctional) avoidance or distraction, which can be a very effective modification strategy, depend-
ing on one’s willingness to experience the affective state to be regulated (“As long as we could also face
the undesired state we are free to use distraction”); ART facilitates the adaptive use of distraction by
focusing on experiencing and accepting unwanted affective states in ART Skills 3 and 4 prior to focus-
ing on modification. “Opposite Action”: See Linehan (1993).

542 INTERVENTIONS
ing in ART showed a significantly greater
decrease in symptoms of depression and
negative affect, as well as greater treatment
gains in positive affect.
Similar results were found during a recent,
completely randomized controlled trial with
a sample of over 400 inpatient participants
who met diagnostic criteria for MDD. In
this study, the effectiveness of CBT was sig-
nificantly improved by supplementing tradi-
tional CBT with the ART program (Berking
et al., 2013 ). Additional gains in the CBT
+ ART condition were particularly strong
with regard to the abilities of acceptance,
tolerance, compassionate self- support, and
modification. All these strategies have been
hypothesized to be particularly important
for mental health (see the section on empiri-
cal evidence for the ART model). In another
study (Berking et al., 2010), a group of police
officers participated in ART. Prior to the
training, their affect regulation skills were
significantly lower than those of a control
sample. After participation in the training,
the police officers’ affect regulation skills
had increased to the point that they no lon-
ger differed from the controls. These effects
did not occur in the control condition.
Moreover, in a recent quasi- experimental
study, a combination of ART and CBT was
effective for treating patients with medically
unexplained somatic symptoms (Gottschalk
& Rief, 2012).
Finally, in experimental studies with
clinical and nonclinical samples, we found
evidence for the short-term effectiveness of
specific ART skills. For these studies, we
developed an experimental paradigm in
which health- relevant affective states are
repeatedly induced with the help of a combi-
nation of music and the Velten Mood Induc-
tion Procedure (1968). After each induc-
tion phase, participants are instructed to
use affect regulation skills, such as the ones
included in the ART model. Before and after
each induction and regulation, participants
rate the intensity and acceptability of the
affective state. To control for order effects,
the complete permutation of all possible
combinations of strategies and control con-
ditions is implemented over the total sample.
Using this paradigm in a healthy sample of
undergraduates and a clinically depressed
sample, we found that in both samples the
ART-based audio instructions on how to
cope with an experimentally induced depres-
sive mood were more effective in reducing
the mood’s intensity than the spontane-
ous regulation control condition (Fischer,
Kirchner, Kandl, Hiller, & Berking, 2012;
Kowalsky et al., 2013). Although all these
studies provide significant support for the
assumed efficacy of ART, additional stud-
ies are dearly needed to clarify further the
populations that benefit from ART, and
whether and to what extent these benefits
exceed those of other bona fide treatments.
Future Directions for ART
Several limitations in our basic research on
affect regulation, ART as a clinical inter-
vention, and our studies evaluating the effi-
cacy of ART need to be addressed in future
research. For example, basic research initi-
ated by our group has made extensive use of
self- reports in an area that, at least in part,
is difficult to assess through introspection
(e.g., the ability to identify one’s emotions
correctly). Moreover, the ERSQ asks partici-
pants whether they can successfully apply
certain skills to their feelings or emotions.
Because a depressed patient might refer to
different emotions than would a partici-
pant suffering from an anxiety disorder, the
instrument refers to different affective states
in different participants, and the extent that
these particular difficulties are valid indi-
cators of their general emotion regulation
skills is unclear. Additionally, many assump-
tions in our model (e.g., that the application
of promising affect regulation skills itself
can contribute to short-term mood deterio-
ration) are largely grounded in theory and/
or clinical experience, and need to be inves-
tigated in more detail empirically.
With regard to ART as a clinical inter-
vention, it is noteworthy that in spite of
the obvious advantages of transdiagnostic
interventions (e.g., easier to disseminate in
a cost- effective group setting), the disad-
vantages of potentially neglecting relevant
characteristics of particular disorders have
to be carefully considered. Moreover, from
our clinical work, we developed the impres-
sion that using theories and findings from
the neurosciences to derive health- relevant
affect regulation skills helps to engage par-
ticipants in the systematic training routine.
Affect Regulation Training 543
However, we also learned that finding the
right balance between a clinically help-
ful and a scientifically valid neuroaffective
model of affect regulation is often difficult
to attain. For example, anthropomorphiz-
ing relevant brain areas (e.g., by using the
expression “The amygdala only wants to
protect you”) can be a very effective psycho-
therapeutic intervention, but the approach
is inadequate in the context of pure scien-
tific explanations. Additionally, we have to
acknowledge that remaining up-to-date with
the latest findings in the rapidly developing
field of the neurosciences, while simultane-
ously conducting large randomized clinical
trials, represents a remarkable challenge.
During the time it takes to develop a treat-
ment program, write a treatment manual,
raise the funds for efficacy trials, and con-
duct and publish the results of those trials,
neuroscientific findings and theories used
for developing the treatment program are
likely to be out of date.
With regard to efficacy research, it is of
note that in clinical populations we have
investigated ART exclusively as an adjunc-
tive intervention, which is in line with our
assumption that the characteristics of spe-
cific disorders must also be considered
when offering transdiagnostic interventions
enhancing general emotion regulation skills.
However, the current lack of studies evaluat-
ing ART as a stand-alone intervention also
prevents us from clarifying which disorders
can be treated effectively with interventions
focusing exclusively on enhancing such skills.
We should also note that because of the lack
of multiple assessments and untreated con-
trol conditions, we have not yet been able
to conduct meaningful mediation analyses
clarifying the extent to which ART exerts
positive effects through improving affect
regulation skills as hypothesized. Finally,
it is noteworthy that the current ART pro-
gram teaches a broad range of affect regu-
lation skills, which takes about 18 hours to
complete. From a theoretical and a treat-
ment dissemination perspective, an essential
subsequent step will be to clarify whether all
parts of the program are necessary to obtain
the desired effects.
To overcome these limitations, we have
initiated several research projects. For
example, to address the measurement prob-
lems, we have recently developed and vali-
dated a modified version of the ERSQ called
the Affect Regulation Skills Questionnaire
(ARSQ; Ebert et al., 2013), which assesses
affect regulation skills separately for various
affective states.
1
This measure helps to clar-
ify whether individuals differ in their affect
regulation skills across different affective
states, and it helps to identify which skills
for which affective states are most important
for maintaining or restoring mental health.
Currently we are also limited in our
attempts to use findings from the affective
neurosciences to improve psychotherapeutic
interventions. We acknowledge that we are
unlikely to become cutting- edge experts in
two research fields as vast as the neurosci-
ences and treatment development and evalu-
ation. Thus, we are currently looking for
neuroscience experts who share our inter-
est in translational research. By collaborat-
ing with experts in the neurosciences, we
aim to enhance further the validity of the
translational approach used in ART and
thereby bridge the current gap between basic
research and clinical application.
With regard to the need for more efficacy
trials, in two, large, multicenter trials we are
currently investigating whether ART can
also be used as an effective stand-alone treat-
ment for MDD and/or binge- eating disorder
(BED). In these trials we use experimental,
biological, observer- based, and ambulatory
momentary assessments of affect regula-
tion skills, in addition to retrospective self-
report measures. Moreover, through mul-
tiple assessments and designs that include an
untreated control condition, we will be able
to conduct meaningful mediation analyses.
To clarify whether all parts of the program
are necessary to obtain the desired effects,
we are currently conducting a dismantling
study, in which we compare an ART version
that focuses exclusively on acceptance and
versions that focus exclusively on modifica-
tion or on both acceptance and modifica-
tion. Additionally, we acquired funding for a
large, multicenter trial in which the efficacy
of CBT + ART is compared with traditional
CBT in a sample of patients suffering from
medically unexplained somatic symptoms.
We are also exploring technology enhance-
ments to ART. Text messages have been
used since 2004 to support participants in
their daily skills practice. Since then, numer-
ous innovations have been presented in the

544 INTERVENTIONS
rapidly developing field of computerized
psychological treatment. For example, we
are currently finalizing an interactive online
version of ART, as well as several serious
games that can be used to practice ART
skills in a leisurely fashion. We hope that
these endeavors will help individuals in need
of more effective affect regulation to acquire
and continuously practice the most effective
strategies and thereby overcome their mental
health problems and thereby improve their
well-being and satisfaction with life.
Note
1. An English version of the ARSQ can be
obtained from Matthias Berking.
References
Amaral, D. G., Price J. L., Pitkänen, A., & Car-
michael, S. T. (1992). Anatomical organiza-
tion of the primate amygdaloid complex. In
J. P. Aggleton (Ed.), The amygdala: Neuro-
biological aspects of emotion, memory and
mental dysfunction (pp. 1–66). New York:
Wiley-Liss.
American Psychiatric Association. (2013). Diag-
nostic and statistical manual of mental disor-
ders (5th ed.). Washington, DC: Author.
Arnsten, A. F. T. (2009). Stress signaling path-
ways that impair prefrontal cortex structure
and function. Nature Reviews Neuroscience,
10(6), 410– 422.
Beck, J. S. (1995). Cognitive therapy: Basics and
beyond. New York: Guilford Press.
Berking, M. (2010). Training emotionaler
Kompetenzen (2nd ed.). Berlin/Heidelberg:
Springer- Verlag.
Berking, M., Ebert, D., Cuijpers, P., & Hofmann,
S. (2013). Emotion- regulation skills training
enhances the efficacy of cognitive behavioral
therapy for major depressive disorder. Psycho-
therapy and Psychosomatics, 82, 234–245.
Berking, M., Margraf, M., Ebert, D., Wupper-
mann, P., Hofmann, S., & Junghanns, K.
(2011). Emotion regulation skills as a predic-
tor of relapse during and after treatment of
alcohol dependence. Journal of Consulting
and Clinical Psychology, 79(3), 307–318.
Berking, M., Meier, C., & Wupperman, P.
(2010). Enhancing emotion- regulation skills
in police officers: Results of a pilot controlled
study. Behavior Therapy, 41(3), 329–339.
Berking, M., Neacsiu, A., Comtois, K. A., &
Linehan, M. M. (2009). The impact of expe-
riential avoidance on the reduction of depres-
sion in treatment for borderline personality
disorder. Behaviour Research and Therapy,
47(8), 663–670.
Berking, M., Orth, U., Wupperman, P., Meier,
L., & Caspar, F. (2008). Prospective effects of
emotion regulation on emotional adjustment.
Journal of Counseling Psychology, 55(4),
485–494.
Berking, M., Poppe, C., Luhmann, M., Wup-
permann, P., Jaggi, V., & Seifritz, E. (2012).
Emotion- regulation skills and psychopathol-
ogy: Is the ability to modify one’s negative
emotions the ultimate pathway by which all
other skills affect symptoms of mental disor-
ders? Journal of Behavior Therapy and Exper-
imental Psychiatry, 43, 931–937.
Berking, M., & Whitley, B. (2013). Affect regula-
tion training. New York: Springer.
Berking, M., Wirtz, C., Svaldi, J., & Hofmann,
S. (under review). Emotion regulation skills
predict symptoms of depression over five years
in individuals at risk for depression. Behav-
iour Research and Therapy.
Berking, M., & Wupperman, P. (2012). Emotion
regulation and mental health: Recent findings,
current challenges and future directions. Cur-
rent Opinion in Psychiatry, 25(2), 128–134.
Berking, M., Wupperman, P., Reichardt, A.,
Pejic, T., Dippel, A., & Znoj, H. (2008).
Emotion- regulation skills as a treatment tar-
get in psychotherapy. Behaviour Research and
Therapy, 46(11), 1230–1237.
Berking, M., & Znoj, H. (2008). Entwicklung
und Validierung eines Fragebogens zur stan-
dardisierten Selbsteinschätzung emotionaler
Kompetenzen (SEK-27) [Development and val-
idation of a standardized self- report measure
assessing emotional competencies]. Zeitschrift
für Psychiatrie Psychologie und Psychothera-
pie, 56(2), 141–153.
Bogdan, R., & Pizzagalli, D. A. (2006). Acute
stress reduces reward responsiveness: Impli-
cations for depression. Biological Psychiatry,
60(10), 1147–1154.
Bonanno, G. A., Papa, A., Lalande, K., Westphal,
M., & Coifman, K. (2004). The importance of
being flexible: The ability to both enhance and
suppress emotional expression predicts long-
term adjustment. Psychological Science, 15(7),
482–487.
Catanzaro, S. J., & Greenwood, G. (1994).
Expectancies for negative mood regulation,
coping, and dysphoria among college students.
Affect Regulation Training 545
Journal of Counseling Psychology, 41(1),
34–44.
Conrad, A., & Roth, W. T. (2007). Muscle relax-
ation therapy for anxiety disorders: It works
but how? Journal of Anxiety Disorders, 21,
243–264.
Cosmides, L., & Tooby, J. (2000). Evolutionary
psychology and the emotions. In M. Lewis &
J. M. Haviland- Jones (Eds.), Handbook of
emotions (2nd ed., pp. 91–115). New York:
Guilford Press.
Damasio, A. (2000). The feeling of what hap-
pens: Body and emotion in the making of con-
sciousness. New York: Harcourt Brace.
Dingemans, A. E., Martijn, C., Jansen, A., &
Furth, E. F. (2009). The effect of suppressing
negative emotions on eating behavior in binge
eating disorder. Appetite, 52, 51–57.
Duckworth, A. L., Steen, T. A., & Seligman, M.
E. P. (2005). Positive psychology in clinical
practice. Annual Review of Clinical Psychol-
ogy, 1, 629–651.
D’Zurilla, T. J., & Nezu, A. M. (2010). Problem-
solving therapy. In K. S. Dobson (Ed.), Hand-
book of cognitive behavioral therapies (Vol.
39, pp. 197–226). New York: Guilford Press.
Ebert, D., Christ, O., & Berking, M. (2013). Ent-
wicklung und Validierung eines Fragebogens
zur emotionsspezifischen Selbsteinschätzung
emotionaler Kompetenzen (SEK-ES) [Develop-
ment and validation of a self- report instrument
for the assessment of affect- specific regulation
skills]. Diagnostica, 59(1), 17–32.
Farb, N. A. S., Anderson, A. K., Irving, J. A., &
Segal, Z. V. (this volume, 2014). Mindfuless
interventions and emotion regulation. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 548–568). New York: Guilford
Press.
Feldman- Barrett, L., Gross, J., Christensen, T.
C., & Benvenuto, M. (2001). Knowing what
you’re feeling and knowing what to do about
it: Mapping the relation between emotion dif-
ferentiation and emotion regulation. Cogni-
tion and Emotion, 15(6), 713–724.
Feldner, M. T., Zvolensky, M. J., Stickle, T. R.,
Bonn- Miller, M. O., & Leen- Feldner, E. W.
(2006). Anxiety sensitivity- physical concerns
as a moderator of the emotional consequences
of emotion suppression during biological chal-
lenge: An experimental test using individual
growth curve analysis. Behaviour Research
and Therapy, 44(2), 249–272.
Fischer, A., Kirchner, M., Kandl, M., Hiller, W.,
& Berking, M. (2012, May). Effektivität von
Emotionsregulationsstrategien zur Reduk-
tion dysphorischer Stimmung bei Depression
[Effects of emotion regulation strategies on
the reduction of depressive mood in depressed
patients]. Poster presented at the 30th annual
conference of the Section Clinical Psychology
and Psychotherapy of the German Psychologi-
cal Association, Luxembourg.
Foa, E. B., Riggs, D. S., Massie, E. D., & Yar-
czower, M. (1995). The impact of fear acti-
vation and anger on the efficacy of exposure
treatment for posttraumatic stress disorder.
Behavior Therapy, 26, 487– 499.
Gibb, B. E., Beevers, C. G., Andover, M. S., &
Holleran, K. (2006). The hopelessness theory
of depression: A prospective multi-wave test of
the vulnerability– stress hypothesis. Cognitive
Therapy and Research, 30(6), 763–772.
Gibbons, M. B. C., Crits- Christoph, P., Barber,
J. P., & Schamberger, M. E. (2007). Insight in
psychotherapy: A review of empirical litera-
ture. In L. G. Castonguay & C. E. Hill (Eds.),
Insight in psychotherapy (pp. 143–165). Wash-
ington, DC: American Psychological Associa-
tion.
Gilbert, P. (2011). Compassion focused therapy:
The distinctive features. London: Routledge.
Gottman, J., & DeClaire, J. (1997). The heart of
parenting: Raising an emotionally intelligent
child. New York: Simon & Schuster.
Gottschalk, J.-M., & Rief, W. (2012). Psycho-
therapeutische Ansätze für Patienten mit
somatoformen Störungen [Psychotherapeutic
treatments for patients with somatoform dis-
orders]. Der Nervenarzt, 83, 1115–1127.
Grawe, K. (2002). Psychological therapy. Cam-
bridge, MA: Hogrefe & Huber.
Grawe, K. (2006). Neuropsychotherapy. New
York: Psychology Press.
Gray, J. A., & McNaughton, N. (2000). The
neuropsychology of anxiety: An enquiry into
the functions of the septo- hippocampal system
(2nd ed.). Oxford, UK: Oxford University Press.
Greenberg, L. S. (2004). Emotion- focused ther-
apy. Clinical Psychology and Psychotherapy,
11(1), 1–2.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2(3), 271–299.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
emotion regulation. In J. J. Gross (Ed.),
546 INTERVENTIONS
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera,
F., & Weinberger, D. R. (2003). Neocortical
modulation of the amygdala response to fear-
ful stimuli. Biological Psychiatry, 53, 494–
501.
Hayes, S. C., Luoma, J. B., Bond, F. W., Masuda,
A., & Lillis, J. (2006). Acceptance and com-
mitment therapy: Model, processes and out-
comes. Behaviour Research and Therapy,
44(1), 1–25.
Hayes, S. C., Strosahl, K. D., & Wilson, K. G.
(1999). Acceptance and commitment therapy.
New York: Guilford Press.
Hofmann, S. G., Grossman, P., & Hinton, D.
E. (2011). Loving- kindness and compassion
meditation: Potential for psychological inter-
ventions. Clinical Psychology Review, 31(7),
1126–1132.
Hofmann, S. G., Sawyer, A. T., Fang, A., &
Asnaani, A. (2012). Emotion dysregulation
model of mood and anxiety disorders. Depres-
sion and Anxiety, 29, 409–412.
Huether, G. (1998). Stress and the adaptive self-
organization of neuronal connectivity dur-
ing early childhood. International Journal of
Developmental Neuroscience, 16, 297–306.
Jacobson, E. (1964). Anxiety and tension con-
trol: A physiologic approach. Philadelphia:
Lippincott.
Johnstone, T., & Walter, H. (this volume, 2014).
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 58–75). New York:
Guilford Press.
Kessler, R. C., Chiu, W. T., Demler, O., & Wal-
ters, E. E. (2005). Prevalence, severity, and
comorbidity of 12-month DSM-IV disorders
in the National Comorbidity Survey Replica-
tion. Archives of General Psychiatry, 62(6),
617– 627.
Koole, S. L., & Rothermund, K. (2011). “I feel
better but I don’t know why”: The psychology
of implicit emotion regulation. Cognition and
Emotion, 25(3), 389–399.
Kowalsky, J., Schwarz, J., Tauch, C., Ruppert,
M., Wert, H., & Berking, M. (2013, May).
Effektivität von Emotionsregulationsstrat-
egien—eine experimentelle Untersuchung
[Efficacy of emotion regulation strategies— an
experimental investigation]. Poster presented
at the 31th annual conference of the Section
Clinical Psychology and Psychotherapy of the
German Psychological Association in Trier.
Lane, R. D., Sechrest, L., Reidel, R., Weldon, V.,
Kaszniak, A. W., & Schwartz, G. E. (1996).
Impaired verbal and nonverbal emotion rec-
ognition in alexithymia. Psychosomatic Medi-
cine, 58, 203–210.
Larsen, R. J. (2000). Toward a science of mood
regulation. Psychological Inquiry, 11(3) 129–
141.
Lazarus, R. S. (1991). Emotion and adaptation.
In L. A. Pervin (Ed.), Handbook of person-
ality theory and research (Vol. 21, pp. 609–
637). Oxford, UK: Oxford University Press.
Leahy, R. L. (2002). A model of emotional sche-
mas. Cognitive and Behavioral Practice, 9(3),
177–190.
LeDoux, J. E. (2012). Evolution of human emo-
tion: A view through fear. Progress in Brain
Research, 195, 431–442.
Levitt, J. T., Brown, T. A., Orsillo, S. M., & Bar-
low, D. H. (2004). The effects of acceptance
versus suppression of emotion on subjective
and psychophysiological response to carbon
dioxide challenge in patients with panic disor-
der. Behavior Therapy, 35(4), 747–766.
Lieberman, M. D., Eisenberger, N. I., Crockett,
M. J., Tom, S. M., Pfeifer, J. H., & Way, B.
M. (2007). Putting feelings into words: Affect
labeling disrupts amygdala activity in response
to affective stimuli. Psychological Science,
18(5), 421–428.
Lieberman, M. D., Inagaki, T. K., Tabibnia, G.,
& Crockett, M. J. (2011). Subjective responses
to emotional stimuli during labeling, reap-
praisal, and distraction. Emotion, 11(3), 468–
480.
Linehan, M. M. (1993). Cognitive- behavioral
treatment of borderline personality disorder.
New York: Guilford Press.
Longe, O., Maratos, F. A., Gilbert, P., Evans, G.,
Volker, F., Rockliff, H., et al. (2010). Having a
word with yourself: Neural correlates of self-
criticism and self- reassurance. NeuroImage,
49(2), 1849–1856.
Martin, L. L., Tesser, A., & McIntosh, W. D.
(1993). Wanting but not having: The effects of
unattained goals on thoughts and feelings. In
E. Daniel, M. Wegner, E. James, & W. Pen-
nebaker (Eds.), Handbook of mental control
(pp. 552–572). New York: Prentice Hall.
Mauss, I. B., & Tamir, M. (this volume, 2014).
Emotion goals: How their content, structure,
and operation shape emotion regulation. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 361–375). New York: Guil-
ford Press.
Mennin, D. S., & Fresco, D. M. (2009). Emotion
regulation as an integrative framework for
understanding and treating psychopathology.
Affect Regulation Training 547
In A. M. Kring & D. M. Sloan (Eds.), Emotion
regulation and psychopathology: A transdi-
agnostic approach to etiology and treatment
(pp. 356–379). New York: Guilford Press.
Mennin, D. S., & Fresco, D. M. (this volume,
2014). Emotion regulation therapy. In J. J.
Gross (Ed.), Handbook of emotion regulation
(2nd ed., pp. 469–490). New York: Guilford
Press.
Morrow, J., & Nolen- Hoeksema, S. (1990).
Effects of responses to depression on the reme-
diation of depressive affect. Journal of Person-
ality and Social Psychology, 58(3), 519–527.
Moses, E. B., & Barlow, D. H. (2006). Current
directions in psychological science: Approach
for emotional disorders based on emotion sci-
ence. Psychological Science, 15(3), 146–150.
Neasciu, A. D., Bohus, M., & Linehan, M. (this
volume, 2014). Dialectical behavir therapy:
An intervention for emotion dysregulation.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 491–507). New York:
Guilford Press.
Ochsner, K. N., & Gross, J. J. (this volume,
2014). The neural bases of emotion and emo-
tion regulation: A valuation perspective. In J.
J. Gross (Ed.), Handbook of emotion regula-
tion (2nd ed., pp. 23–42). New York: Guilford
Press.
Öst, L.-G. (1987). Applied relaxation: Descrip-
tion of a coping technique and review of con-
trolled studies. Behaviour Research and Ther-
apy, 25(5), 397– 409.
Ottenbreit, N. D., & Dobson, K. S. (2004).
Avoidance and depression: The construction
of the cognitive behavioral avoidance scale.
Behaviour Research and Therapy, 42, 293–
313.
Porges, S. W. (2007). The polyvagal perspective.
Biological Psychology, 74, 116–143.
Radkovsky, A., McArdle, J., Bockting, C., &
Berking, M. (under review). Emotion regula-
tion skills predict subsequent reduction of
symptom severity in patients suffering from
major depressive disorder: A latent change
analysis. Journal of Consulting and Clinical
Psychology.
Rothermund, K. (2003). Automatic vigilance for
task- related information: Perseverance after
failure and inhibition after success. Memory
and Cognition, 31, 343–352.
Saarni, C. (1999). The development of emo-
tional competence. New York: Guilford Press.
Salovey, P., & Mayer, J. D. (1990). Emotional
intelligence. Imagination, Cognition, and Per-
sonality, 9, 185–211.
Stemmler, G. (2004). Physiological processes dur-
ing emotion. In P. Philippot & R. S. Feldman
(Eds.), The regulation of emotion (pp. 33–70).
Mahwah, NJ: Erlbaum.
Swart, M., Kortekaas, R., & Aleman, A. (2009).
Dealing with feelings: Characterization of
trait alexithymia on emotion regulation strate-
gies and cognitive- emotional processing. PLoS
ONE, 4(6), e5751.
Teasdale, J. D., & Barnard, P. J. (1993). Affect,
cognition and change: Re- modelling depres-
sive thought. Hove, UK: Erlbaum.
Van Rijsbergen, G. D., Bockting, C. L. H.,
Berking, M., Koeter, M. W. J., & Schene,
A. H. (2012). Can a one-item mood scale do
the trick?: Predicting relapse over 5.5-years
in recurrent depression. PloS ONE, 7(10),
e46796.
Velten, E. (1968). A laboratory task for the induc-
tion of mood states. Behavioral Research and
Therapy, 6, 473–482.
Vuilleumier, P., Richardson, M. P., Armony, J.
L., Driver, J., & Dolan, R. J. (2004). Distant
influences of amygdala lesion on visual corti-
cal activation during emotional face process-
ing. Nature Neuroscience, 7(11), 1271–1278.
Weissman, S., & Weissman, R. (1996). Medi-
tation, compassion and lovingkindess. An
approach to Vipassana practice. York Beach,
ME: Weiser.
Wenzlaff, R. M., & Wegner, D. M. (2000).
Thought suppression. Annual Review of Psy-
chology, 51, 59–91.
Werner, K., & Gross, J. J. (2010). Emotion regu-
lation and psychopathology: A conceptual
framework. In A. M. Kring & D. M. Sloan
(Eds.), Emotion regulation and psychopathol-
ogy: A transdiagnostic approach to etiology
and treatment (pp. 13–37). New York: Guil-
ford Press.
Wupperman, P., Marlatt, G. A., Cunningham,
A., Bowen, S., Berking, M., Mulvihill- Rivera,
N., et al. (2012). Mindfulness and modifica-
tion therapy for behavioral dysregulation:
Results from a pilot study targeting alcohol
use and aggression in women. Journal of Clin-
ical Psychology, 67, 1–17.
Wupperman, P., Neumann, C. S., & Axelrod, S.
R. (2008). Do deficits in mindfulness underlie
borderline personality features and core diffi-
culties? Journal of Personality Disorders, 22,
466–482.

548
Mindfulness training (MT) represents the
secular adaptation of Buddhist contem-
plative practices aimed to reduce suffer-
ing and foster well-being. Over the past
three decades, MT interventions have been
increasingly recognized for their ability to
reduce psychological distress across a vari-
ety of clinical disorders (Baer, 2003; Bohl-
meijer, Prenger, Taal, & Cuijpers, 2010;
Grossman, Niemann, Schmidt, & Walach,
2004). They have been particularly effective
in promoting adaptive emotion regulation
in affective disorders such as anxiety and
depression (Hofmann, Sawyer, Witt, & Oh,
2010; Piet & Hougaard, 2011), and appear
to promote self- regulated behavior and posi-
tive emotional states (Brown, Ryan, & Cre-
swell, 2007). Our understanding of mind-
ful emotion regulation is imperfect, but it is
supported by a recent groundswell of exper-
imental and clinical research (for detailed
reviews, see Holzel et al., 2011; Shapiro,
Carlson, Astin, & Freedman, 2006).
This chapter outlines the theoretical foun-
dations of mindfulness, the mechanisms by
which MT appears to support adaptive emo-
tion regulation, and its most prevalent forms
of delivery. We begin by exploring definitions
of mindful emotion regulation, integrating
contributions from Western psychological
research and Buddhist contemplative theory.
From these perspectives, mindfulness can be
viewed as a regulatory strategy that operates
primarily at the level of attention deployment,
subsequently promoting a unique blend of
regulatory techniques. Next, we discuss how
mindfulness is developed through attention
training, reviewing emergent research on the
cognitive and neural mechanisms associated
with MT interventions. Psychologically, the
primary mechanism in MT interventions is
the promotion of present- moment awareness,
which is realized through the cultivation of
interoceptive attention and an attitude of
acceptance toward experience. Neurosci-
entifically, three mechanisms of training
are evident: (1) increased access to sensory
representation; (2) decreased activation of a
network for habitual self- elaboration; and,
in longer term practitioners, (3) increased
attentional stability and decreased reactivity
to emotion provocation. Finally, we review
some of the best- validated MT interventions,
discussing their clinical benefits and limita-
tions, and exploring future directions for
research in this field.
CHAPTER 32
Mindfulness Interventions
and Emotion Regulation
Norman A. S. Farb
Adam K. Anderson
Julie A. Irving
Zindel V. Segal

Mindfulness Interventions and Emotion Regulation 549
What Is Mindful
Emotion Regulation?
Mindfulness has been described as “the
awareness that arises from paying attention
on purpose, in the present moment and non-
judgmentally to things as they are” (Wil-
liams, 2007, p. 47). This definition distin-
guishes mindfulness from regulatory efforts
to improve the hedonic tone of experience,
such as using cognitive reappraisal to make
an image of suffering appear less aversive
(Gross, 2002). Instead, mindfulness pro-
motes exploratory attention to momentary
experience regardless of its unpleasantness.
Such exploration creates opportunities for
insight into one’s mental habits, reducing
automatic efforts to control or manipulate
feeling states. Indeed, the Pali term vipas-
sana, which is the origin of the English
term mindfulness, literally means “insight”
(Bischoff, 1996). To describe how such
insight occurs, we present a mindfulness
theory that is consistent with both Buddhist
and secular models of emotion regulation.
Mindfulness from a Buddhist/
Contemplative Model of Mind
Much of Buddhist theory on mindfulness
is contained in a discourse known as the
Maha Sattpatthana Sutta, which translates
to “the Great Discourse on the Foundations
on Mindfulness” (Silananda, 2002). Within
this history of the Buddha’s teachings, mind-
fulness rests upon four contemplative foun-
dations that represent necessary targets for
attentional focus. These foundations are (1)
the body, (2) feelings, (3) the mind or con-
sciousness, and (4) the Dhammas, or mental
qualities. It is telling that these first three
foci explicitly exclude conceptual elabora-
tion or judgment but instead entail the allo-
cation of attention to concretely perceived
experiences, such as physical sensations,
emotional responses, and thoughts. The
fourth category, the Dhammas, describes
the optimal attitudes and common pitfalls
surrounding mindful attention to objects
in these first three categories. In effect, this
fourth category serves as a set of top-down
regulatory goals for effective mindfulness
practice. It is asserted that it is only through
the correct practice of attention deployment
that the pinnacle of emotion regulation may
be achieved: the complete extinction of suf-
fering.
Mechanistically, the primary tenet of
the Buddhist psychological framework is
that the mind may only hold one object at
a time. Therefore, attention deployment to
the firt three foundations limits conceptual
elaboration that triggers or extends nega-
tive emotion (Silananda, 1998). Thus, in the
face of an emotional challenge, mindfulness
includes the somewhat paradoxical regula-
tory instruction to focus directly upon one’s
negative feelings; by focusing on a feeling
rather than its inciting attentional object, a
person diminishes the elaborative appraisal
that iteratively incites negative feeling. Over
time, it is argued, the habitual tendency
to engage in negative appraisals will itself
diminish, as one internalizes the practice
of nonjudgmental attention. Of course, let-
ting go of past events, particularly aversive
ones, is often easier said than done. Thus,
Buddhist psychology has a rationale for
using meditation formally to train attention.
Mindfulness from a Buddhist perspective
is therefore a practical rather than mystical
regulatory strategy, complete with its own
psychological account of emotion regula-
tion. The phenomenological description of
how attention deployment leads to the atten-
uation of negative emotions provides a rich
set of hypotheses compatible with Western
psychological models of emotion regulation.
Mindfulness from a Psychological
Science Perspective
The idea of attention deployment as emo-
tion regulation is not foreign to scientific
accounts of emotion regulation. For exam-
ple, the process model distinguishes between
multiple strategies for regulation that can
occur before and after emotional awareness,
distinguishing broadly between antecedent-
focused and response- focused regulation
(Gross, this volume). Generally speaking,
the earlier one regulates emotion following
stress perception the better: For example,
the antecedent- focused strategy of cognitive
reappraisal appears to be a healthier regu-
latory strategy than the response- focused
strategy of expressive suppression, leading
to lower stress- related symptoms (Gross
& John, 2003; Moore, Zoellner, & Mol-
lenholt, 2008). However, most research
550 INTERVENTIONS
on antecedent- focused emotion regulation
focuses on appraisal processes, a relatively
late regulation strategy for cognitive change.
Few studies have examined earlier forms of
regulation such as attention deployment, and
those that do tend to focus on distraction
(Gross, this volume), a withdrawal- oriented
strategy often employed in the face of high-
intensity emotional challenge (Sheppes, this
volume).
Mindfulness substantively contributes
to psychological models of emotion regu-
lation by advancing a regulatory model of
approach- oriented attention deployment
(Figure 32.1A). Unlike distraction, mindful-
ness is characterized by acceptance rather
than withdrawal from aversive emotional
experience (Kabat-Zinn, 1982). Through
MT, participants learn nonjudgmental
attention to present- moment sensation,
returning attention their situation rather
than progressing to appraisal processes
and responses (Figure 32.1B). By engaging
cognitive resources in effortful attention
deployment, mindfulness limits the recruit-
ment of habitual secondary appraisals and
reactions. The disruption of automatic reac-
tions and return to sensory attention allows
new aspects of a situation to be perceived,
effectively altering situation construal. This
altered situation perception then permits the
generation of novel responses rather than
mapping experiences into a preexisting con-
ceptual field. The iteration between “top-
down” attentional control and “bottom- up”
sensation forms the basis for how mindful-
ness promotes insight and limits appraisals
of suffering (Teasdale & Chaskalson, 2011).
Since responses following such iteration
are more finely tuned to the idiosyncratic
demands of the situation, they can be more
adaptive and appropriate than habitual reac-
tions (Figure 32.1C). Thus, despite its pri-
mary focus on the deployment of attention,
mindfulness appears to impact additional
stages of the regulatory process: It intro-
duces novel regulatory intentions, facilitates
novel appraisals of emotional experience,
and promotes expression rather than sup-
pression of emotional responses.
What Makes Mindful Emotion
Regulation Unique?
Framing mindfulness within the process
model does not undermine the unique con-
FIGURE 32.1. A process model account of mindful emotion regulation. Mindfulness can be situated
in Gross’s (2002) process model of emotion regulation, acting primarily on attention deployment (Panel
A). Attention redeployment allows new situation perception, without requiring an immediate emo-
tional response, thereby promoting the flexible generation of novel appraisals and responses (Panel B).
The repeated redeployment of attention to sensation serves to interfere with and eventually extinguish
habitual appraisals (Panel C).
Antecedent-Focused
Emotion Regulation
Response-Focused
Emotion Regulation

Mindfulness Interventions and Emotion Regulation 551
tribution of mindfulness to existing mod-
els of emotion regulation. Mindfulness is
unique in promoting meta- awareness of
emotion regulation strategies, detecting and
disrupting rumination, and in creating a tra-
jectory for self- change. We describe each of
these capacities in detail below.
First, mindfulness serves as a meta-
strategy: While MT practices begin with an
emphasis on attention deployment, this atten-
tional focus is designed to cultivate awareness
of regulatory habits. In acknowledging these
habits, a person is then better able to choose
flexibly between regulatory responses rather
than automatically defaulting to particular
strategies. Perhaps because of its adaptive
nature, mindfulness often leads to superior
regulatory effects when compared to exist-
ing forms of regulation mentioned in the
process model, such as expressive suppres-
sion (Liverant, Brown, Barlow, & Roemer,
2008), rumination (Arch & Craske, 2006),
or distraction (Goldin & Gross, 2010). This
is not to disparage the value of any of the
aforementioned techniques, but rather to
suggest that mindfulness may enhance regu-
lation by increasing flexibility in regulatory
strategy selection. By cultivating an attitude
of curiosity in response to an emotional chal-
lenge, mindfulness provides an opportunity
to tailor regulatory responses to address
both personal coping capacity and fluctuat-
ing situational demands.
A second unique contribution of mindful-
ness to emotion regulation is its ability to
disrupt rumination or rehearsal of negative
emotional triggers. By widening attention to
focus on the evolving nature of emotional
experience, mindful attention disrupts cog-
nitive elaboration on negative events. Many
of us have had the experience of ruminating
at length over a perceived slight. By mind-
fully attending to momentary experience
rather than rehearsing an upsetting memory,
negative emotions are permitted to subside.
Consistent with this theory, research sug-
gests that mindfulness is particularly effec-
tive for reducing dysphoric rumination,
outperforming relaxation training control
groups in several cases (Feldman, Gree-
son, & Senville, 2010; Jain et al., 2007). By
attending to negative emotions rather than
treating them as a problem to be solved,
mindfulness reduces suffering by limiting
cognitive elaboration on aversive events.
A third unique contribution of mindful
emotion regulation is its ability to promote
a trajectory of self- change. Mindfulness
requires an intensive investment: As dis-
cussed below, participants in MT interven-
tions are asked to engage in meditative prac-
tices for about an hour a day for 8 weeks
or more, with the explicit goal of increasing
mindfulness throughout daily life. Partici-
pants are asked to take on attitudinal quali-
ties in a trait-like manner. While salutary
MT effects have been observed after train-
ing periods as brief as 20 minutes a day for 3
days (Zeidan, Gordon, Merchant, & Gool-
kasian, 2010), longitudinal benefits of mind-
fulness are more frequently reported follow-
ing training periods of weeks or more. It is
unknown whether formal training regimens
involving reappraisal or suppression gener-
ate similar levels of self- reported change,
although there is reason to believe that that
MT employs unique mechanisms for effect-
ing change, as discussed more fully below.
How Does MT Work?
Attending nonjudgmentally to negative
emotion is often easier said than done.
For this reason, mindfulness is viewed as
a skill that requires training and practice.
Like other traditions in the growing “third
wave” of cognitive psychotherapy tech-
niques (Hayes, 2004), MT is designed to
promote self- awareness of the interpretive
context that drives a person’s maladaptive
reactions. Yet, unlike talk- therapy interven-
tions, MT employs formal meditation prac-
tice to achieve these aims. In mainstream
interventions such as cognitive- behavioral
therapy (CBT), insight is a stepping- stone to
the main therapeutic focus: The recognition
of maladaptive patterns of appraisal allows
their replacement with adaptive alternatives.
In MT, however, increased awareness into
automatic reaction patterns is itself the goal
of the intervention. It is fair to wonder then:
How does awareness alone promote adap-
tive behavior? In this section, we outline
how MT impacts attention, and through
such effects improves the well-being of its
practitioners.
To strengthen attention, MT begins with
concentrative attention practices that often
focus on physical sensations, such as fine-
552 INTERVENTIONS
grained “body scans,” in which individuals
attend to physical sensation from specific
body parts, or feelings of respiration (Kabat-
Zinn, 1990). Such concentrative practices
may already hold some regulatory benefits,
because intense focal attention on a particu-
lar object may help to dissolve self– object
boundaries (Lutz, Slagter, Dunne, & David-
son, 2008), and thereby help to suspend pat-
terns of self- absorbed rumination. However,
mindfulness practices then transition from
focal attention to open monitoring, wide-
spread attention to all sensations, thoughts,
and emotions. Open- monitoring practices
aim to increase awareness of the transi-
tory nature of experience, yielding insight
into one’s own habitual patterns of distrac-
tion and reaction. Encouragingly, short-
term mindfulness interventions appear to
increase the ability to notice changes in the
sensory field and refocus attention appro-
priately (Jha, Krompinger, & Baime, 2007;
Schmertz, Anderson, & Robins, 2009), and
may preserve working memory capacity that
would ordinarily degrade in times of stress
(Jha, Stanley, Kiyonaga, Wong, & Gelfand,
2010).
Of course, the ability to engage in open,
mindful attention is not purely an acquired
skill; we are all mindful of different things
during the day. Nonjudgmental, open
awareness has been shown to arise natu-
ralistically, particularly in conjunction with
positive moods (Fredrickson & Branigan,
2005). The tendency to be mindful may vary
widely from person to person, as attested to
by recently developed mindfulness scales,
which also demonstrate that these trait indi-
ces of mindfulness appear to increase with
meditative practice (Baer et al., 2008; Lau et
al., 2006; Walach, Buchheld, Buttenmuller,
Kleinknecht, & Schmidt, 2006). Conversely,
trait mindfulness may also predict respon-
siveness to MT and the specific attention
tasks therein (Shapiro, Brown, Thoresen, &
Plante, 2011), suggesting a reinforcing cycle
among central components of mindful inten-
tions, attention practice, and attitudes.
Central Components of MT
Mindfulness- based interventions appear to
operate at multiple stages of emotion regu-
lation. At a higher- order, conceptual level,
MT represents an interplay between par-
ticipants’ intentions for self- improvement,
the attentional practices themselves, and the
use of these practices to foster an attitude
of acceptance and nonreactivity to negative
emotion (Shapiro, Carlson, Astin, & Freed-
man, 2006). At a mechanistic level, mind-
fulness can be explained by the effects of the
attentional practices themselves, detailing
how mindful attention alters the preexist-
ing relationship between events and subse-
quent self- attribution, limiting automatic
self- evaluative processing (Frewen, Evans,
Maraj, Dozois, & Partridge, 2008). To pro-
vide a complete account of how mindfulness
impacts each aspect of emotion regulation,
we present intention, attention, perception,
implication, and attitude in a single concep-
tual model (Figure 32.2).
While empirical research has focused on
how mindfulness alters cognition through
attention training, it is important to rec-
ognize that the success of such practices
depends on a participant’s motivation and
expectations for such training. The gen-
eration of a proper intention is critical to
the success of contemporary MT practices
(Kabat-Zinn, 1990; Shapiro & Schwartz,
2000); participants must expect to have
some benefit from the practice to engage
consistently in a novel and intensive practice
of restructuring cognitive habits. Within the
meditative practices themselves, intentions
serve as a foundation for the direction of
attention; by formally setting an intention to
attend to one’s body or breath, a wander-
ing mind becomes indicative of a correctable
loss of attention. Central to holding “right
intention” in mindfulness is the promotion
of participant autonomy, that participants
are ultimately accountable to themselves for
their self- improvement efforts. For example,
in therapeutic contexts, MT emphasizes
daily, self- directed practice rather than rely-
ing on the clinician for directing inquiry
and generating new appraisals. Mindfulness
teachers provide the occasional reminder to
check for mind wandering during these prac-
tices, but with decreasing frequency over the
weeks of a mindfulness course. Instead, par-
ticipants are entrusted with an increasing
responsibility for monitoring and reallocat-
ing attention during meditation. Indeed, it
has been argued that one yardstick of mind-
ful intentions may be participants’ willing-
ness to ascribe personal responsibility for
their reactions to stressful events (Lakey,
Kernis, Heppner, & Lance, 2008).
Mindfulness Interventions and Emotion Regulation 553
The second major factor in MT inter-
ventions is attention. The distinguishing
characteristic of MT is a set of meditative
practices focusing on sensory experiences of
the body. These meditative practices serve
at least three major functions in promoting
adaptive emotion regulation. First, atten-
tion to momentary changes in body sensa-
tion creates a broad awareness of experience
than extends beyond a vigilant or ruminative
focus on stressors. By attending to a broader
set of experiences from moment to moment,
participants learn to reperceive the world
(Shapiro et al., 2006). Reperceiving refers
to the developed capacity to disengage from
“the drama of our personal experience” and
attend to novel sensations and interpreta-
tions (p. 377). Second, this broader atten-
tional focus creates a psychological distance
between dysphoric feelings and broader self-
attributions about efficacy or value. This
psychological distancing is known as decen-
tering, in which self- and situational apprais-
als are experienced as momentary events
rather than immutable facts (Fresco, Segal,
Buis, & Kennedy, 2007). Finally, the novel
perspective afforded by decentering may
serve to disrupt habitual interpretations,
creating a broadened context for appraisal
(Garland, Gaylord, & Fredrickson, 2011).
An increased ability to disengage from rumi-
native self- elaboration in favor of sensory
exploration constitutes a broader context
from which to view the world. From this
broadened context, participants are able to
explore novel interpretations for events and
more skillful means for responding to emo-
tional challenges.
The final major factor in MT is attitude.
Negative emotions naturally arise during
both meditative practice and in everyday life.
However, it is one’s attitude toward these
emotions that determines whether they are
appraised as self- diagnostic. For example,
attention and reflection on negative emotions
can be beneficial when approached with an
attitude of curiosity and openness, leading
to constructive cognitive reappraisal; how-
ever, this same reflective process can be mal-
adaptive when accompanied by an attitude
that negative emotions are unacceptable
and problematic, breeding rumination and
dysphoric self- evaluation (Gotlib & Joor-
mann, 2010). To counter this maladaptive
tendency, nonjudgment is espoused as a core
attitudinal goal in MT, a goal supported by a
constitutive attentional practice. To cultivate
nonjudgment, participants strive to direct
attention away from judging the validity
of experience and toward attending to the
sense of the experience itself. Over time, the
habitual reorienting of attention to sensation
FIGURE 32.2. Broader regulatory consequences of mindfulness. Mindfulness effects extend beyond
attention deployment in modulating stress responses. Different stages of the process model for emotion
regulation are displayed for conventional (top panel) and mindful (bottom panel) stress responses. Con-
ventionally, stressful situations are construed as demanding a regulatory response, prompting attention
to the stressor, dysphoric appraisals, and generation of negative emotional responses. Mindfulness
acts on all four of these phases: Mindful intentions cast stressful situations as opportunities to prac-
tice; mindful attention broadens the field of awareness to include changes in sensation; and mindful
attitudes promote appraisals of engagement and responses of acceptance rather than an obligatory
dysphoric stance.
554 INTERVENTIONS
disengages automatic evaluative responses
to sensations, feelings, and thoughts. In this
fashion, a negative emotion or disparaging
thought about oneself is treated as a momen-
tary experience with no more self- diagnostic
value than an itch upon the skin.
While the relationship between intention,
attentional practices, and attitude can be
seen as a linear progression in the cultiva-
tion of mindful emotion regulation skills,
realistically these components are mutually
reinforcing (Shapiro et al., 2006). Below we
discuss how the interplay among these com-
ponents creates a mechanism for promoting
stress tolerance and well-being.
Psychological Mechanisms
of Mindfulness Training
A recent review of the research literature
links MT to improved attention, body
awareness, emotion regulation, and altered
self- perception (Holzel et al., 2011). How
MT practices generate these positive changes
may, however, benefit from further clarifica-
tion. It may seem strange, for instance, that
repeated attention to body sensations should
provide widespread therapeutic benefits.
How can attention to the breath and other
physical sensations promote a broadening
of attention and reduction of automatic pat-
terns of reactivity? Furthermore, one may
question the importance of attending to emo-
tional appraisals in the first place. Emotions
hold a natural capacity to capture attention
(Ohman, Flykt, & Esteves, 2001); why then
would practice in attending to emotions help
in regulating them? To address these ques-
tions, we expand upon a theoretical model
(Bishop et al., 2004) of how mindful atten-
tion directly supports adaptive emotion reg-
ulation based on two major principles: (1)
attention to the present: broad attention to
present- moment sensation, with an empha-
sis on body sensation rather than cognitive
deliberation; and (2) nonjudgment: the sus-
pension of judging experience to be intrinsi-
cally good or bad in favor of a more general
attitude of acceptance. Together, these prin-
ciples mutually support the gradual process
of reconfiguring attention and cognition,
extinguishing maladaptive patterns of reac-
tivity, and introducing cognitive flexibility
in the response to stress (Figure 32.3).
FIGURE 32.3. The two foundations of mindful attention: Attention to the present and nonjudgment.
Together they support a suspension of self- critical evaluation and are revelatory of reactivity habits.
Over time, such information can yield insights that afford the creation of novel and more adaptive
stress responses, help to dismantle existing patterns of reactivity, and can prevent the formation of
novel conditioned or “knee-jerk” reactions to stress.
Mindfulness Interventions and Emotion Regulation 555
Attention to the Present
The cultivation of momentary awareness is
central to mindfulness practices. Through
repeated exercises in attending to the sensa-
tions of the breath or different regions of the
body, participants are encouraged to develop
interoception, an awareness of momentary
sensations from inside the body. These sen-
sations then act as an access point for under-
standing one’s reactions to the world as
they unfold; rather than conceptually inter-
preting or predicting responses to events,
participants are encouraged to explore
their actual reactions “in real time,” using
interoceptive attention to monitor changes
in body sensation from moment to moment.
While myriad factors determine the efficacy
of a mindfulness intervention, attention to
interoceptive sensation is important for sev-
eral reasons. First, focusing on body sensa-
tion helps to suspend evaluative processes,
disrupting the habitual progression from
sensation to appraisal by redirecting atten-
tional resources from such processes. Sec-
ond, in the absence of habitual evaluation, a
person extends the time course of appraisal,
reducing the need to quickly map events on a
preexisting conceptual field, thereby disem-
powering deeply entrenched dysphoric inter-
pretations. Third, interoception serves as an
indication of physiological stress: By notic-
ing one’s breathing, heartbeat, muscle tone,
or digestion change, one can gain insight into
one’s own emotional appraisals and notice
the kinds of situations that trigger automatic
reactions. Finally, this sensitivity to environ-
mental triggers allows for the revelation of
reactive habits, because intrusive cognitive
failures of interoceptive attention provide an
opportunity to notice where the mind goes
when it wanders. Perhaps because interocep-
tive perceptual details are so distinct from
thoughts and evaluations, these details act
as attentional “anchors” for perception that
make mind wandering more apparent.
Nonjudgment
The cultivation of present- moment attention
is complemented by an intention to refrain
from judgment and cognitive reactivity.
Nonjudgment supports formal meditation
practice, and also works with attention to
the present moment to regulate emotional
challenge adaptively. The formal practice of
mindfulness meditation offers ample oppor-
tunity to cultivate nonjudgment. Mind wan-
dering is inevitable during the attempted
maintenance of interoceptive attention,
prompting from participants a range of
negative reactions, such as self- criticism,
anxiety, frustration, or dejection. Rather
than deny, suppress, or distract attention
from these lapses and subsequent feelings,
participants are encouraged to notice and
accept all experience, including attentional
lapses and feelings of frustration. This atti-
tude of acceptance then allows a more rapid
return to present- moment sensation than
would be expected from digression into self-
recrimination.
The practice of nonjudgment also has
important implications for emotion regula-
tion beyond formal meditation practices.
By focusing on noticing changes in experi-
ence rather than formulating reactions, an
attitude of nonjudgment limits the asso-
ciation between initial appraisals of failure
or challenge and subsequent dysphoric or
avoidant secondary appraisals. This cur-
tailing feature of nonjudgment is critical to
recovery from affective and substance use
disorders, in which inciting mental apprais-
als habitually lead to self- destructive men-
tal and behavioral habits (Scher, Ingram,
& Segal, 2005). Indeed, both dispositional
and treatment- related increases in mindful-
ness predict greater facility in letting go of
negative thoughts (Frewen et al., 2008), and
lower levels of avoidance and rumination
(Kumar, Feldman, & Hayes, 2008). This
development of nonjudgment can be thought
of as two complementary and related capaci-
ties: acceptance of experience and decenter-
ing from attributing self- relevance to experi-
ence, which together support the outcomes
of interoceptive attention described ear-
lier. First, acceptance indicates a willing-
ness to tolerate outcomes even when they
diverge from intentions, limiting the auto-
matic expansion of evaluative reactions. For
example, one study of a mindfulness inter-
vention reported improved quality- of-life,
depression, and fatigue scores in a multiple
sclerosis population, but unaltered ratings of
physical symptoms, such as lower and upper
limb mobility (Grossman et al., 2010), sug-
gesting that the symptom acceptance train-
ing reduced broader secondary appraisals to
556 INTERVENTIONS
produce a training benefit. Second, decenter-
ing involves reducing reliance on conceptual
self- appraisal as the primary determinant
of well-being. Instead, such self- appraisal
is viewed on par with physical sensation, a
momentary experience that does not imply
the existence of a temporally extended,
enduring self. Recent research suggests that
the ability to view one’s emotional reac-
tions from a more objective viewpoint is a
critical determinant of whether reflection on
emotion can be constructive or degenerate
into maladaptive rumination (Kross, Gard,
Deldin, Clifton, & Ayduk, 2012).
Together, attending to the present and
cultivating a nonjudgmental attitude mutu-
ally reinforce one another: Present- moment
attention provides a context in which to
practice nonjudgment, and nonjudgment
enhances the stability of attention. Beyond
supporting each other, these two principles
can account for many of the benefits attrib-
uted to MT. First, mindfulness enables the
extinction of reactive habits by removing
conditioned appraisal responses from their
triggering stimuli and replacing them with
interoceptive attention. For example, MT
has helped chronic pain sufferers to reduce
subjective reports of distress, although the
intensity of pain sensation ratings themselves
did not change (Morone, Greco, & Weiner,
2008; Rosenzweig et al., 2010). Second, by
reducing reactive habits, mindfulness affords
new, creative appraisals, allowing a person
to approach experiences with a sense of
curiosity and exploration. Novel appraisals
then may extend to include previously unat-
tended positive elements, including inter-
personal relationships, enjoyment of daily
activities, or compassion toward oneself and
others (Allen, Bromley, Kuyken, & Sonnen-
berg, 2009; Kuyken et al., 2010). Third, con-
tinued mindfulness practice prevents future
stress conditioning and provides competing
knowledge of constantly changing intero-
ceptive sensations that weakens the need
to turn to conceptual evaluations (Kabat-
Zinn, 1990). The suspension of engagement
in automatic conceptual evaluation during
stress can thereby prevent further condition-
ing of dysphoric cognition. For example,
participants completing MT reported signif-
icant reduction in their experience of daily
hassles and distress compared to a control
group (Williams, Kolar, Reger, & Pearson,
2001). Together, these psychological mecha-
nisms promote and sustain flexibility in the
regulatory response to emotional challenge.
Neural Mechanisms
of Mindfulness Training
The notion that MT cultivates a qualita-
tively distinct approach to emotion regula-
tion is supported by emerging neuroscience
research. Traditionally, emotion regulation
such as reappraisal has been associated with
the prefrontal cortex (PFC), which tends
to be active during the manipulation and
evaluation of information (Ochsner, Bunge,
Gross, & Gabrieli, 2002). The PFC in turn
appears to be component of a broader fron-
toparietal attention network associated with
directing attention to external objects, fil-
tering the endless stream of sensory infor-
mation based on its motivational relevance
(Corbetta & Shulman, 2002). In a sense,
this well- defined attention system seeks to
fix sensory information as a set of objective
facts that can be judged as being good or bad
depending upon their relationship to a per-
son’s goals. In the case of an undesired out-
come, cognitive reappraisal directs attention
toward changing one’s interpretive context
to mitigate the obligatory emotional impact
of contextually derived failure appraisal on
the self.
Mindfulness, however, argues for a quali-
tative shift away from attachment to exter-
nal outcomes. To do so, there is an attempt
to shift attention away from attentional hab-
its that link external sensations to concep-
tual manipulation and elaboration. Instead,
MT teaches individuals to direct attention
internally, to representation of visceral
components of emotion, as supported by a
distinct neural pathway for interoception,
the sensation of the body’s internal state,
including signals from the breath (Farb,
Segal, & Anderson, 2013; Farb et al., 2007),
and heartbeat (Lutz, Greischar, Perlman, &
Davidson, 2009). Through reliance on the
transitory and unelaborated representations
of internal body states, MT may appeal to a
relatively unconditioned system in the sense
that such physical sensations do not have the
same level of association with conceptual
elaboration or regulatory reactivity. Indeed,
one reason that many mindfulness practices
involve interoceptive attention is that such

Mindfulness Interventions and Emotion Regulation 557
practices improve the stability and frequency
with which one perceives the transitory and
reinterpretable nature of human experience
(Baer, Smith, Hopkins, Krietemeyer, &
Toney, 2006; Brown et al., 2007; Ivanovski
& Malhi, 2007; Kabat-Zinn, 1982).
Recent research evidence supports the
idea that MT improves interoceptive access
by decoupling sensory cortices from brain
networks involved in the habitual evaluation
and elaboration of experience. Activation of
cortical midline regions such as the medial
prefrontal cortex (mPFC) appear are asso-
ciated with self- referential evaluation (Farb
et al., 2007; Kelley et al., 2002), depres-
sive rumination (Farb, Anderson, Bloch, &
Segal, 2011), and negative mood (Farb et al.,
2010; Goldin & Gross, 2010). MT appears
to reduce both rumination (Ramel, Goldin,
& Carmona, 2004) and cortical midline
activation (Farb et al., 2010; Goldin, Ramel,
& Gross, 2009); furthermore, mindful
attention can begin to reduce mPFC acti-
vation even in untrained individuals (Farb
et al., 2007). Meditation has been linked
to extensive cortical deactivations (Baeren-
sten et al., 2010; Ives- Deliperi, Solms, &
Meintjes, 2011; Lazar et al., 2000), suggest-
ing its importance for disengaging concep-
tual processing. Following training, par-
ticipants demonstrate a reduced connection
between sensory and evaluative cortices, but
greater connectivity within sensory cortices
and between the brain’s executive control
regions and sensory cortices (Farb et al.,
2007, 2013; Kilpatrick et al., 2011).
A third important neural mechanism by
which MT promotes effective emotion regu-
lation is by enhancing attention control in
more experienced meditators. In one study,
3 months of intensive meditation training
was associated with reduced variability in
low frequency brain signal electroencepha-
lographic (EEG) activity (Lutz, Slagter, et
al., 2009). These brain changes were cor-
related with reduced variability in reaction
time in a focused attention task, suggesting
that this stabilization of neural activity rep-
resents a more stable cognitive workspace in
which to perceive and manipulate sensory
objects. Furthermore, long-term meditators
demonstrated reduced network strength in
the cortical midline default network associ-
ated with conceptual elaboration and mind
wandering but enhanced connectivity within
attention- controlling brain regions (Brewer
et al., 2011), again denoting an improved
ability to resist distraction and to effectively
deploy attention.
In advanced stages, there is evidence
to suggest that mindfulness training may
itself become automatic and promote trait-
like effects. An intriguing neuroimaging
study examined the meditative state across
a range of practice experience (Brefczynski-
Lewis, Lutz, Schaefer, Levinson, & David-
son, 2007). Relatively novice meditators
demonstrated pervasive cortical and limbic
activation relative to controls when entering
a meditative state, typical of engagement in
an effortful and resource intensive cogni-
tive process. However, advanced meditators
(with more than 10,000 hours of practice)
showed little neural change during medita-
tion, suggesting that their default state may
have become something akin to mindful
attention. Thus, the long-term effects of MT
may involve a reconfiguration of the brain’s
information processes to dwell primarily in
unelaborated sensation, in contrast to the
highly automated pattern of cognitive elabo-
ration found in most studies of Westerners
to date.
Therapeutic Applications
of Mindfulness
The first documented clinical effect of MT
was reducing the suffering of patients with
chronic pain (Kabat-Zinn, 1982). Sub-
sequent research has confirmed that the
affective appraisal of pain is altered when
experienced through an open, mindful state
(Perlman, Salomons, Davidson, & Lutz,
2010). Freed from pain- reactive, negative
appraisals about one’s self-worth and quality
of life, cognition can be engaged construc-
tively toward the cultivation of empathy and
compassion (Leary, Tate, Adams, Allen, &
Hancock, 2007).
The reframing of painful or otherwise
upsetting experiences is especially impor-
tant in mood disorders. The effortful down-
regulation of negative emotion is perceived
as more difficult by patients with a mood
disorder (Keightley et al., 2003), for whom
self- compassion seems like a worthwhile but
unachievable strategy (Pauley & McPherson,
2010). An important clinical implication of
558 INTERVENTIONS
these findings is that for patients with mood
disorders, instructions to reappraise cogni-
tively or otherwise regulate negative emotion
may be ineffective. In depression, powerful
and habitual dysphoric interpretations may
be activated during such regulatory efforts,
leading to rumination, in which elaborating
on negative affect ironically perpetuates dys-
phoric mood (Nolen- Hoeksema, 2000; Wat-
kins, Moberly, & Moulds, 2008).
If the engagement of a patient’s cognitive
faculties only serves to exacerbate negative
mood states, one might question how any
behavioral intervention can effect positive
change. Since cognitive elaboration efforts
may automatically trigger negative self-
judgments that cannot be voluntarily over-
ridden, MT provides an alternative to such
elaborative habits. Through the cultiva-
tion of attention to unelaborated, present-
moment sensation, mindfulness presents
patients with a task that does not require
the deployment of cognitive evaluation and
elaboration. Furthermore, by explicitly
practicing nonjudgment, patients can begin
to recognize and disengage from conceptual
judgment in response to their sensory expe-
riences.
Critically, mindful emotion regulation
does not aim to inhibit elaborative process-
ing through thought avoidance, a regulatory
strategy that ironically predicts dysphoric
affect (Ottenbreit & Dobson, 2004). In
MT, attention is instead positively directed
toward present- moment sensation, pro-
viding a nonconceptual and nonthreaten-
ing attentional focus that does not rely on
cortical midline activity. Depression can
be thought of as a combination of both
approach deficits and heightened avoidance
motivation (Trew, 2011); mindfulness tar-
gets both types of motivation, replacing the
tendency to avoid negative experiences with
an exploration of these experiences’ constit-
uent sensations.
As one might expect from such a funda-
mental mechanism of action, mindful emo-
tion regulation strategies can be applied
to a variety of contexts extending beyond
pain and depression. One recent review
catalogued beneficial psychological effects
of mindfulness- based interventions in the
treatment of anxiety disorders, substance
abuse, cancer, heart disease, arthritis, fibro-
myalgia, multiple sclerosis, psoriasis, and
HIV (Chiesa & Serretti, 2010). MT has also
been of benefit in older populations, par-
ticularly in improving the coping skills of
familial caregivers (Oken et al., 2010).
The recent proliferation of MT in clinical
contexts has been supported by the creation
of manualized therapeutic interventions.
Customized interventions now exist that
employ MT techniques to address the needs
of specific clinical populations, such as those
suffering from issues of pain, depression,
and addiction. The standardization of these
interventions has been critical for clinical
research, allowing a consistent application
MT to be evaluated in specific clinical con-
texts. Below, we discuss some of the most
widely researched therapeutic interventions
of mindfulness, detailing their background,
format, and target populations.
Mindfulness‑Based Stress Reduction
Background
Mindfulness- based stress reduction (MBSR)
was developed as an outpatient program
at the University of Massachusetts Medi-
cal School in 1979, based on creator Jon
Kabat-Zinn’s personal experience with
vipassana (insight) meditation, a practice
that originated in the millennia- old Thera-
vada Buddhist tradition. The first published
report on MBSR described reduced pain and
symptoms of negative mood in a group of
51 patients with chronic pain (Kabat-Zinn,
1982). Since that time, MBSR has been stan-
dardized with broad efficacy in reducing
chronic pain and stress.
Format
MBSR is delivered as an 8-week program.
Participants attend weekly group sessions
with between 10 and 30 participants, where
they are introduced to formal mediation
practices, gentle yoga, and psychosocial
education. The program also includes a day
of silent meditation retreat that falls in the
latter half of the course. Group members
are asked to practice yoga and/or medita-
tion for approximately 40 minutes a day for
homework, along with reflection and journ-
aling exercises. Formal meditation practices
Mindfulness Interventions and Emotion Regulation 559
include breath monitoring; body scans (the
progressive direction of attention to differ-
ent parts of the body); and diffuse direction
of attention to sounds, thoughts, feelings,
and bodily sensations. Group sessions gen-
erally last for 2.5 hours and focus on group
meditation practice and discussion of these
practices. However, other commonly dis-
cussed themes include stress and the body,
habitual patterns of reactivity, and creative
ways to respond to stress. Group dialogue
on difficulties in performing practices and
insights gained are also valuable compo-
nents of an MBSR course. During these dia-
logues, instructors model key components
of mindful awareness, such as noticing the
body sensations that accompany different
thoughts and impulses.
As part of the weekly homework, courses
often include diary exercises, which are later
discussed in the group setting. Commonly,
participants spend a week taking notice of
pleasant events, then a week on unpleasant
events, and another week on monitoring
stressful communications. However, com-
mon even to these externalized practices,
there is an emphasis on how these events
occurred from an interoceptive perspective
(i.e., how one felt in one’s body during these
events). There are no hard-and-fast rules for
course compliance, although participants
who are absent are typically contacted by
course instructors to probe commitment to
course participation. Participant retention
in MBSR is generally high, with 80% of
enrolled participants generally completing
the course (Farb et al., 2007; Kabat-Zinn,
Lipworth, & Burney, 1985).
Target Population
In healthy individuals (i.e., those without
a specific medical or psychiatric diagno-
sis), MBSR appears to yield substantial but
nonspecific reductions in reported levels of
stress, in addition to specific reductions in
levels of rumination and anxiety (Chiesa
& Serretti, 2009). Research suggests that
MBSR promotes well-being across a vari-
ety of clinical conditions, including chronic
pain, anxiety, fibromyalgia, cancer, psoria-
sis, and coronary artery disease (Baer, 2003;
Grossman et al., 2004). A third stream of
research suggests that MBSR benefits extend
to health care professionals, improving their
ability to cope with stress (Irving, Dobkin,
& Park, 2009). Finally, adapted forms of the
MBSR program have improved mental func-
tion and reduced stress in primary caregiv-
ers of people with terminal diseases such as
dementia (Oken et al., 2010) and advanced-
stage cancer (Lengacher et al., 2012).
Mindfulness‑Based Cognitive Therapy
Background
Mindfulness- based cognitive therapy
(MBCT) was developed to target the specific
challenges inherent in chronic mood disor-
ders. Major depressive disorder, a leading
cause of disability worldwide (Gelenberg,
2010), has a chronic, cyclical nature due to
high risk of relapse. Presently, maintenance
pharmacotherapy is the most widely imple-
mented approach to reducing relapse risk in
depression (Kupfer et al., 1992). However,
long-term antidepressant use involves prob-
lems with drug tolerance and side effects,
and does not target the dysphoric cognition
that contributes to relapse risk in response
to future stressors. Individuals with a his-
tory of depressive illness, including those
in remission, are prone to increased cogni-
tive reactivity, a pattern of increased nega-
tive self- judgment in response to emotion
challenge. Dysphoric cognitive reactivity
appears to be an important determinant
of depressive relapse, along with genetics,
environment, and social support (Segal et
al., 2006). However, such reactivity may
be modifiable; addressing these patterns of
dysphoric thinking may provide posttreat-
ment protection against relapse (Paykel et
al., 1999).
While Beck’s (1979) formulation of cogni-
tive therapy focused on modifying underly-
ing dysfunctional attitudes and core beliefs,
Ingram and Hollon (1986) argued that pro-
phylactic effects following remission are
dependent on enhancing an individual’s abil-
ity to take a distanced, disidentified perspec-
tive from depressive thoughts (i.e., decenter-
ing). To create a therapy that maximized
long-term prophylactic effects, Segal, Wil-
liams, and Teasdale (2002) expanded on the
existing CBT framework to create MBCT,
which uses mindfulness practices to develop
560 INTERVENTIONS
participant ability to engage in decentered
attention.
Format
The MBCT program involves medium- size
groups (between eight and 13 participants).
The length of sessions ranges between 2 and
2.5 hours in order to accommodate session
content flexibly and any clinical issues that
may arise. Participants are also offered a
full-day “silent retreat” between the sixth
and seventh weeks of the program. Every
session is initiated with a meditation to
ground participants in “being” mode and
help them to transition out of the business
and “doing” mode of their everyday lives.
Meditation practices include the body scan,
gentle yoga, and mindful walking, as well as
sitting meditation practices with foci such
as the breath, sounds, body sensations, and
thoughts. Each session includes a review of
the home practice, in addition to group dis-
cussion of insession mindfulness practices.
MCBT also features the 3-minute breath-
ing space, a condensed version of formal
mindfulness meditation in which partici-
pants broadly inventory mood and quality
of thought, shift attention to the breath,
then return to broad attention on sensation
and cognition. The inclusion of the 3-min-
ute breathing space offers an opportunity to
integrate practice mindfulness in situations
where entrenched patterns of dysphoric cog-
nition make extended meditation difficult
or impossible. It also affords participants a
chance to “check in” with themselves dur-
ing the day, limiting patterns of rumination.
While initially incorporated into MBCT,
the 3-minute breathing space is increasingly
making its way into MBSR and related MT
interventions.
Target Population
MBCT was designed as prophylactic sup-
port in prevention of depressive relapse. In
two large, randomized control trials, MBCT
has been shown to be efficacious for patients
with three or more past episodes of depres-
sion by halving relapse rates better than did
a treatment- as-usual control condition (Ma
& Teasdale, 2004; Teasdale et al., 2000).
In a more recent, large-scale trial, MBCT
was been shown to be as effective as mainte-
nance antidepressant medication in prevent-
ing depressive relapse, with both surpassing
the performance of a placebo control condi-
tion (Segal et al., 2010).
While originally designed to prevent
relapse during remission, MBCT has also
shown efficacy in the treatment of acutely
depressed patients, including those with
treatment- resistant depression (Barnhofer et
al., 2009; Eisendrath, Chartier, & McLane,
2011). MBCT has also been adapted for
children ages 9–13 (Semple, Lee, Rosa, &
Miller, 2010), and for adults over age 65
(Smith, Graham, & Senthinathan, 2007).
MBCT may be appropriate for patients
with other mood disorders, but special
care must be taken to ensure that patients
have adequate capacity to cope with nega-
tive emotions during the meditation process
(Germer, Siegel, & Fulton, 2005).
Mindfulness‑Based
Relapse Prevention
Background
Mindfulness- based relapse prevention
(MBRP), an intervention developed to target
relapse vulnerability in substance use disor-
ders (Marlatt & Gordon, 1985), is modeled
after MBCT to help people recognize inter-
nal triggers to addictive behavior without
responding to them. The course features a
central theme of distinguishing between pri-
mary emotional experience and secondary
appraisals, with an emphasis on the idea that
if the primary experiences can be accepted,
secondary appraisals of coping inadequacy
will lose power to trigger relapse behaviors.
Format
The intervention takes place over eight
weekly sessions and introduces many of
the same formal meditation techniques
described in MBSR and MBCT. The focus
of the group sessions centers on three major
themes: Sessions 1–3 employ MT to provide
awareness of how automatic patterns of
reactivity lead to relapse behavior; Sessions
4–6 focus on implementing mindfulness
techniques in high-risk or trigger situations
to prevent thoughts from triggering relapse
behaviors; Sessions 7 and 8 focus on inte-
grating mindfulness practice into daily life
Mindfulness Interventions and Emotion Regulation 561
to provide continued development of self-
care skills to protect against relapse in the
future.
Similar to the 3-minute breathing space
that is central to MBCT, MBRP introduces
a “SOBER” breathing space (Stop, Observe,
Breathe, Expand, Respond) designed to
allow participants to observe the arousal
of urges and respond skillfully rather than
habitually. By maintaining awareness of the
urge, the participant somewhat ironically
prevents his or her response to the urge,
allowing it to follow a homeostatic time
course and eventually subside. Similar to the
positive and negative events diary in MBSR,
MBRP employs an “awareness of triggers”
diary. By fostering awareness of thoughts,
feelings, sensations, and behaviors during
the arousal of drug cravings, the diary helps
to create recognition of triggering situations.
Target Population
MBRP targets people with a history of sub-
ject use disorders, generally subsequent to
completion of an intensive detoxification
and rehabilitation program. In a recent study
comparing the effects of MBRP to stan-
dard relapse- prevention treatment, MBRP
group participants showed lower rates of
relapse and greater levels of acceptance and
mindful awareness than those in the stan-
dard treatment group (Bowen et al., 2009).
Importantly, subsequent research suggests
that reductions in craving following MBRP
were mediated by increases in acceptance
and mindful awareness (Witkiewitz, Bowen,
Douglas, & Hsu, 2013).
Other Interventions
Incorporating Mindfulness
Many other contemporary therapeutic
interventions incorporate aspects of MT
in a broader clinical protocol. Dialectical
behavior therapy (DBT; see Neacsiu, Bohus,
& Linehan, this volume) is modeled on the
central struggle between acceptance of the
present moment and desire for change. MT
is an important facet of this therapy, help-
ing participants develop radical acceptance
of a problematic situation in order to direct
efforts skillfully toward adaptive change.
Unlike the interventions discussed earlier,
DBT does not require extensive formal med-
itation practice but instead includes a variety
of meditative exercises that may be custom-
ized to a client’s needs and abilities. Accep-
tance and commitment therapy (ACT) also
eschews formal meditation techniques but
places a strong emphasis on viewing expe-
riences with a sense of acceptance or non-
judgment, while focusing efforts on chang-
ing behaviors rather than thoughts, feelings,
and sensations. In both DBT and ACT, there
is much greater emphasis on the adjustment
of behavior than in mindfulness- based inter-
ventions, providing more therapist- led struc-
ture around plans of action.
Contraindications
Despite the promise of MT interventions in
a variety of clinical domains, it is important
to remember that these techniques have only
been validated with specific ailments. In fact,
original MT studies excluded individuals
with active substance abuse, psychotic disor-
ders, eating disorders, obsessive– compulsive
disorder (OCD), and borderline personality
disorder (BPD). The reasons for such exclu-
sion criteria were manifold; individuals with
acute depression often experience compro-
mised attention and concentration, which
may pose significant challenges when learn-
ing an already challenging practice, while
individuals with OCD, BPD, and active sub-
stance abuse might find long formal medi-
tation practices difficult to tolerate due to
intensively distressing intrusive thoughts,
impulses, cravings, or a general tendency
toward states of emotional dysregulation
(Hayes, 2004; Linehan, 1993). Moreover, in
these populations, reliance on emotion regu-
lation strategies such as avoidance may be
deeply ingrained, and the approach- oriented
nature of mindfulness practices may initially
be difficult to tolerate. Effective treatments
such as cognitive therapy for acute depres-
sion or DBT for BPD exist and have a strong
evidence base (Beck, 1979; Linehan, 1993).
Moreover, they are tailored to fit the needs
and tolerances of the individuals enrolled in
such programs. For example, the develop-
ment of mindfulness skills is a significant
component of training in DBT skills. How-
ever, skills practices are shorter and more
varied, building up over the course of sev-
eral months rather than in 8 weeks as in the
formats of MBSR and MBCT.

562 INTERVENTIONS
Clinicians and expert meditation teach-
ers alike have suggested various precau-
tions when treating individuals who have
a significant history of trauma with MBSR
and MBCT. Crane and Williams (2010)
have discussed the importance of preexist-
ing “grounding skills” for those individuals
who may be at-risk of experiencing intrusive
thoughts or being particularly cognitively
reactive. Additionally, they suggest prepar-
ing such individuals for the fact that distress
may arise, in light of evidence that a tendency
toward brooding and cognitive reactivity
is predictive of dropout in MBCT. Patients
planning to engage in this type of intensive
MT require the skills necessary to redirect
their attention should it become drawn into
destabilizing memories or overwhelming
affective states prior to commencing the
MBCT program. A recent review of the lit-
erature examining reports of adverse events
and contraindications for mindfulness- based
approaches found that the topic had been rel-
atively neglected (Dobkin, Irving, & Amar,
2012) and warrants further investigation.
Concluding Remarks
Interest in mindful emotion regulation has
increased rapidly in recent years, especially
as contemplative models of mind have been
increasingly supported by modern psy-
chological science. This has enhanced our
understanding of emotion regulation in two
major ways: (1) It informs research on atten-
tion deployment as a form of emotion regu-
lation, without resorting to distraction from
emotion; and (2) it has begun substantively
to connect scientific inquiry to broader
issues in emotion regulation, such as how
flexibility in regulation techniques serves
as a “meta- regulatory” skill, or how inten-
tion and commitment to a regulatory goal
increase realization of longitudinal improve-
ments in regulatory capacity. Psychologi-
cally, the study of MT provides a window
into the mechanisms of personal growth,
combining elements of attention, intention,
and attitude to form an upward spiral of reg-
ulatory efficacy. Neurobiologically, the study
of mindfulness helps to validate the idea that
sensory attention recruits a distinct intero-
ceptive attention pathway. This pathway
appears to be amenable to voluntary access,
and such access can be facilitated through
attention training. Critically, engagement of
sensory attention seems to disengage brain
networks involved in habitual elaborative
processing, allowing for the extinction of
automatic stress reactions while strengthen-
ing networks for the control of attention.
With elements of mindfulness now being
identified in a variety of other “new wave,”
health- promoting practices such as Tai Chi,
yoga, and, more generally, in psychothera-
peutic practice, the challenge before us is
to justify the spread of these techniques. As
such, it is increasingly necessary to provide
an empirically replicable model of the core
components of mindful emotion regula-
tion. Such a model will distinguish neces-
sary regulatory elements from intervention
elements that are simply customary, and
help to assuage criticisms that mindfulness
involves ushering mysticism into Western,
evidence- based medicine. For example, the
relative contributions of attitude, intention,
and attention are unknown. It may be the
case that daily exercises in setting benefi-
cial self- intentions account for many of the
salutary effects of mindfulness, allowing
one to dispense with much of the medita-
tive practice and instead engage in habits
of self- affirmation. Alternatively, it may be
that attentional improvements are necessary
precursors to the experience of insight and
improvements in well-being. Finally, it may
be the commitment to regulating experience
with an attitude of acceptance that really
pays benefits, with meditative exercises
simply helping to reinforce this commit-
ment. While there is suggestive evidence that
the three core elements of mindfulness—
attitude, intention, and attention— are criti-
cal ingredients, it will be important to find
better ways to measure their relative contri-
butions to advance the science of mindful-
ness and determine its potential for integra-
tion into secular Western life.
References
Allen, M., Bromley, A., Kuyken, W., & Sonnen-
berg, S. J. (2009). Participants’ experiences
of mindfulness- based cognitive therapy: “It
changed me in just about every way possible.”
Behavioral and Cognitive Psychotherapy,
37(4), 413–430.
Mindfulness Interventions and Emotion Regulation 563
Arch, J. J., & Craske, M. G. (2006). Mechanisms
of mindfulness: Emotion regulation follow-
ing a focused breathing induction. Behaviour
Research and Therapy, 44(12), 1849–1858.
Baer, R. A. (2003). Mindfulness training as a
clinical intervention: A conceptual and empir-
ical review. Clinical Psychology Science and
Practice, 10(2), 125–143.
Baer, R. A., Smith, G. T., Hopkins, J., Kriete-
meyer, J., & Toney, L. (2006). Using self-
report assessment methods to explore facets of
mindfulness. Assessment, 13(1), 27–45.
Baer, R. A., Smith, G. T., Lykins, E., Button, D.,
Krietemeyer, J., Sauer, S., et al. (2008). Con-
struct validity of the five facet mindfulness
questionnaire in meditating and nonmeditat-
ing samples. Assessment, 15(3), 329–342.
Baerensten, K. B., Stodkilde- Jorgensen, H.,
Summerlund, B., Hartmann, T., Damsgaard-
Madsen, J., Fosnaes, M., et al. (2010). An
investigation of brain processes support-
ing meditation. Congitive Processing, 11(1),
57–84.
Barnhofer, T., Crane, C., Hargus, E., Amar-
asinghe, M., Winder, R., & Williams, J. M.
(2009). Mindfulness- based cognitive therapy
as a treatment for chronic depression: A pre-
liminary study. Behavioural Research and
Therapy, 47(5), 366–373.
Beck, A. T. (1979). Cognitive therapy of depres-
sion. New York: Guilford Press.
Bischoff, R. (1996). Some notes concerning the
entry “Vipassana” in the Oxford English Dic-
tionary Additions Series, Vol. 2 (Clarendon
Press, Oxford, UK: 1993). International Jour-
nal of Lexicography, 9(1), 35–37.
Bishop, S. R., Lau, M., Shapiro, S., Carlson, L.,
Anderson, N. D., Carmody, J., et al. (2004).
Mindfulness: A proposed operational defin-
tion. Clinical Psychology: Science and Prac-
tice, 11(3), 230–241.
Bohlmeijer, E., Prenger, R., Taal, E., & Cuijpers,
P. (2010). The effects of mindfulness- based
stress reduction therapy on mental health of
adults with a chronic medical disease: A meta-
analysis. Journal of Psychosomatic Research,
68(6), 539–544.
Bowen, S., Chawla, N., Collins, S. E., Wit-
kiewitz, K., Hsu, S., Grow, J., et al. (2009).
Mindfulness- based relapse prevention for
substance use disorders: A pilot efficacy trial.
Substance Abuse, 30(4), 295–305.
Brefczynski- Lewis, J. A., Lutz, A., Schaefer, H.
S., Levinson, D. B., & Davidson, R. J. (2007).
Neural correlates of attentional expertise in
long-term meditation practitioners. Proceed-
ings of the National Academy of Sciences
USA, 104(27), 11483–11488.
Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang,
Y. Y., Weber, J., & Kober, H. (2011). Medita-
tion experience is associated with differences
in default mode network activity and connec-
tivity. Proceedings of the National Academy
of Sciences USA, 108(50), 20254–20259.
Brown, K. W., Ryan, R. M., & Creswell, J. D.
(2007). Mindfulness: Theoretical foundations
and evidence for salutary effects. Psychologi-
cal Inquiry, 18(4), 211–237.
Chiesa, A., & Serretti, A. (2009). Mindfulness-
based stress reduction for stress management
in healthy people: A review and meta- analysis.
Journal of Alternative and Complementary
Medicine, 15(5), 593–600.
Chiesa, A., & Serretti, A. (2010). A systematic
review of neurobiological and clinical features
of mindfulness meditations. Psychological
Medicine, 40(8), 1239–1252.
Corbetta, M., & Shulman, G. L. (2002). Control
of goal- directed and stimulus- driven attention
in the brain. Nature Reviews Neuroscience,
3(3), 201–215.
Crane, C., & Williams, J. M. (2010). Factors
associated with attrition from mindfulness-
based cognitive therapy in patients with a his-
tory of suicidal depression. Mindfulness, 1(1),
10–20.
Dobkin, P. L., Irving, J. A., & Amar, S. (2012).
For whom may participation in a mindfulness-
based stress reduction program be contraindi-
cated? Mindfulness, 3(1), 44–50.
Eisendrath, S., Chartier, M., & McLane, M.
(2011). Adapting mindfulness- based cognitive
therapy for treatment- resistant depression: A
clinical case study. Cognitive and Behavioral
Practice, 18(3), 362–370.
Farb, N. A., Anderson, A. K., Bloch, R. T., &
Segal, Z. V. (2011). Mood- linked responses
in medial prefrontal cortex predict relapse in
patients with recurrent unipolar depression.
Biological Psychiatry, 70(4), 366–372.
Farb, N. A., Anderson, A. K., Mayberg, H.,
Bean, J., McKeon, D., & Segal, Z. V. (2010).
Minding one’s emotions: Mindfulness train-
ing alters the neural expression of sadness.
Emotion, 10(1), 25–33.
Farb, N. A., Segal, Z. V., & Anderson, A. K.
(2013). Mindfulness meditation training alters
cortical representations of interoceptive atten-
tion. Social Cognitive and Affective Neurosci-
ence, 8(1), 15–26.
564 INTERVENTIONS
Farb, N. A., Segal, Z. V., Mayberg, H., Bean, J.,
McKeon, D., Fatima, Z., et al. (2007). Attend-
ing to the present: Mindfulness meditation
reveals distinct neural modes of self- reference.
Social Cognitive and Affective Neuroscience,
2(4), 313–322.
Feldman, G., Greeson, J., & Senville, J. (2010).
Differential effects of mindful breathing,
progressive muscle relaxation, and loving-
kindness meditation on decentering and nega-
tive reactions to repetitive thoughts. Behaviour
Research and Therapy, 48(10), 1002–1011.
Fredrickson, B. L., & Branigan, C. (2005). Posi-
tive emotions broaden the scope of attention
and thought– action repertoires. Cognition
and Emotion, 19(3), 313–332.
Fresco, D. M., Segal, Z. V., Buis, T., & Ken-
nedy, S. (2007). Relationship of posttreatment
decentering and cognitive reactivity to relapse
in major depression. Journal of Consulting
and Clinical Psychology, 75(3), 447–455.
Frewen, P. A., Evans, E. M., Maraj, N., Dozois,
D. J., & Partridge, K. P. (2008). Lettting go:
Mindfulness and negative auotmatic think-
ing. Cognitive Therapy and Research, 32(6),
758–774.
Garland, E. L., Gaylord, S. A., & Fredrickson,
B. L. (2011). Positive reappraisal mediates
the stress- reductive effects of mindfulness:
An upward spiral process. Mindfulness, 2(1),
59– 67.
Gelenberg, A. J. (2010). The prevalence and
impact of depression. Journal of Clinical Psy-
chiatry, 71(3), e06.
Germer, C. K., Siegel, R. D., & Fulton, P. R.
(2005). Mindfulness and psychotherapy. New
York: Guilford Press.
Goldin, P., Ramel, W., & Gross, J. (2009). Mind-
fulness meditation training and self- referential
processing in social anxiety disorder: Behav-
ioral and neural effects. Journal of Cognitive
Psychotherapy, 23(3), 242–257.
Goldin, P. R., & Gross, J. J. (2010). Effects of
mindfulness- based stress reduction (MBSR)
on emotion regulation in social anxiety disor-
der. Emotion,10(1), 83–91.
Gotlib, I. H., & Joormann, J. (2010). Cogni-
tion and depression: Current status and future
directions. Annual Review of Clinical Psy-
chology, 6, 285–312.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39(3), 281–291.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85(2), 348–362.
Grossman, P., Kappos, L., Gensicke, H., D’Souza,
M., Mohr, D. C., Penner, I. K., et al. (2010).
MS quality of life, depression, and fatigue
improve after mindfulness training: A ran-
domized trial. Neurology, 75(13), 1141–1149.
Grossman, P., Niemann, L., Schmidt, S., &
Walach, H. (2004). Mindfulness- based
stress reduction and health benefits. A meta-
analysis. Journal of Psychosomatic Research,
57(1), 35– 43.
Hayes, S. C. (2004). Acceptance and commit-
ment therapy, relational frame theory, and the
third wave of behavioral and cognitive thera-
pies. Behavior Therapy, 35, 639–665.
Hofmann, S. G., Sawyer, A. T., Witt, A. A., &
Oh, D. (2010). The effect of mindfulness-
based therapy on anxiety and depression: A
meta- analytic review. Journal of Consulting
and Clinical Psychology, 78(2), 169–183.
Holzel, B. K., Lazar, S. W., Gard, T., Schuman-
Olivier, Z., Vago, D. R., et al. (2011). How
does mindfulness meditation work?: Propos-
ing mechanisms of action from a conceptual
and neutral perspective. Perspectives on Psy-
chological Science, 6(6), 537–559.
Ingram, R. E., & Hollon, S. D. (1986). Informa-
tion processing approaches to clinical psychol-
ogy. In R. E. Ingram (Ed.), Personality, psy-
chopathology, and psychotherapy series (Vol.
xi, pp. 259–281). San Diego, CA: Academic
Press.
Irving, J. A., Dobkin, P. L., & Park, J. (2009).
Cultivating mindfulness in health care pro-
fessionals: A review of empirical studies of
mindfulness- based stress reduction (MBSR).
Complementary Therapies in Clinical Prac-
tice, 15(2), 61–66.
Ivanovski, B., & Malhi, G. S. (2007). The psy-
chological and neurophysiological concomi-
tants of mindfulness forms of meditation.
Acta Neuropsychiatrica, 19, 76–91.
Ives- Deliperi, V. L., Solms, M., & Meintjes, E.
M. (2011). The neural substrates of mindful-
ness: An fMRI investigation. Journal of Neu-
roscience, 6(3), 231–242.
Jain, S., Shapiro, S. L., Swanick, S., Roesch, S.
C., Mills, P. J., Bell, I., et al. (2007). A ran-
Mindfulness Interventions and Emotion Regulation 565
domized controlled trial of mindfulness medi-
tation versus relaxation training: Effects on
distress, positive states of mind, rumination,
and distraction. Annals of Behavioral Medi-
cine, 33(1), 11–21.
Jha, A. P., Krompinger, J., & Baime, M. J.
(2007). Mindfulness training modifies sub-
systems of attention. Cognitive, Affective, and
Behavioral Neuroscience, 7(2), 109–119.
Jha, A. P., Stanley, E. A., Kiyonaga, A., Wong,
L., & Gelfand, L. (2010). Examining the
protective effects of mindfulness training on
working memory capacity and affective expe-
rience. Emotion, 10(1), 54–64.
Kabat-Zinn, J. (1982). An outpatient program in
behavioral medicine for chronic pain patients
based on the practice of mindfulness medita-
tion: Theoretical considerations and prelimi-
nary results. General Hospital Psychiatry,
4(1), 33 – 47.
Kabat-Zinn, J. (1990). Full catastrophe living:
Using the wisdom of your body and mind to
face stress, pain and illness. New York: Dela-
corte.
Kabat-Zinn, J., Lipworth, L., & Burney, R.
(1985). The clinical use of mindfulness medi-
tation for the self- regulation of chronic pain.
Journal of Behavioral Medicine, 8(2), 163–
190.
Keightley, M. L., Seminowicz, D. A., Bagby, R.
M., Costa, P. T., Fossati, P., & Mayberg, H. S.
(2003). Personality influences limbic– cortical
interactions during sad mood induction. Neu-
roImage, 20(4), 2031–2039.
Kelley, W. M., Macrae, C. N., Wyland, C. L.,
Caglar, S., Inati, S., & Heatherton, T. F.
(2002). Finding the self?: An event- related
fMRI study. Journal of Cognitive Neurosci-
ence, 14(5), 785–794.
Kilpatrick, L. A., Suyenobu, B. Y., Smith, S. R.,
Bueller, J. A., Goodman, T., Creswell, J. D., et
al. (2011). Impact of mindfulness- based stress
reduction training on intrinsic brain connec-
tivity. NeuroImage, 56(1), 290–298.
Kross, E., Gard, D., Deldin, P., Clifton, J., &
Ayduk, O. (2012). “Asking why” from a
distance: Its cognitive and emotional con-
sequences for people with major depressive
disorder. Journal of Abnormal Psychology,
121(3), 559–569.
Kumar, S., Feldman, G., & Hayes, A. (2008).
Changes in mindfulness and emotion regu-
lation in an exposure- based cognitive ther-
apy for depression. Cognitive Therapy and
Research, 32(6), 734–744.
Kupfer, D. J., Frank, E., Perel, J. M., Cornes, C.,
Mallinger, A. G., Thase, M. E., et al. (1992).
Five-year outcomes for maintenance therapies
in recurrent depression. Archives of General
Psychiatry, 49, 769–773.
Kuyken, W., Watkins, E., Holden, E., White, K.,
Taylor, R. S., Byford, S., et al. (2010). How
does mindfulness- based cognitive therapy
work? Behavioural Research and Therapy,
48(11), 1105–1112.
Lakey, C. E., Kernis, M. H., Heppner, W. L., &
Lance, C. E. (2008). Individual differences in
authenticity and mindfulness as predictors of
verbal defensiveness. Journal of Research in
Personality, 42(1), 230–238.
Lau, M. A., Bishop, S. R., Segal, Z. V., Buis, T.,
Anderson, N. D., Carlson, L., et al. (2006).
The Toronto Mindfulness Scale: Development
and validation. Journal of Clinical Psychol-
ogy, 62(12), 1445–1467.
Lazar, S. W., Bush, G., Gollub, R. L., Fricchi-
one, G. L., Khalsa, G., & Benson, H. (2000).
Functional brain mapping of the relaxation
response and meditation. NeuroReport, 11(7),
1581–1585.
Leary, M. R., Tate, E. B., Adams, C. E., Allen,
A. B., & Hancock, J. (2007). Self- compassion
and reactions to unpleasant self- relevant
events: The implications of treating oneself
kindly. Journal of Personality and Social Psy-
chology, 92(5), 887–904.
Lengacher, C. A., Kip, K. E., Barta, M. K.,
Post-White, J., Jacobsen, P., Groer, M., et al.
(2012). A pilot study evaluating the effect of
mindfulness- based stress reduction on psycho-
logical status, physical status, salivary corti-
sol, and interleukin-6 among advanced- stage
cancer patients and their caregivers. Journal of
Holistic Nursing, 30(3), 170 –185.
Linehan, M. (1993). Cognitive- behavioral treat-
ment of borderline personality disorder. New
York: Guilford Press.
Liverant, G. I., Brown, T. A., Barlow, D. H.,
& Roemer, L. (2008). Emotion regulation in
unipolar depression: The effects of acceptance
and suppression of subjective emotional expe-
rience on the intensity and duration of sadness
and negative affect. Behavioural Research and
Therapy, 46(11), 1201–1209.
Lutz, A., Greischar, L. L., Perlman, D. M., &
Davidson, R. J. (2009). BOLD signal in insula
is differentially related to cardiac function
during compassion meditation in experts vs.
novices. NeuroImage, 47(3), 1038–1046.
Lutz, A., Slagter, H. A., Dunne, J. D., & David-
566 INTERVENTIONS
son, R. J. (2008). Attention regulation and
monitoring in meditation. Trends in Cognitive
Sciences, 12(4), 163–169.
Lutz, A., Slagter, H. A., Rawlings, N. B., Fran-
cis, A. D., Greischar, L. L., & Davidson, R. J.
(2009). Mental training enhances attentional
stability: Neural and behavioral evidence.
Journal of Neuroscience, 29(42), 13418–
13427.
Ma, S. H., & Teasdale, J. D. (2004). Mindfulness-
based cognitive therapy for depression: Repli-
cation and exploration of differential relapse
prevention effects. Journal of Consulting and
Clinical Psychology, 72(1), 31– 40.
Marlatt, G. A., & Gordon, J. R. (1985). Relapse
prevention: Maintenance strategies in the
treatment of addictive behaviors. New York:
Guilford Press.
Moore, S. A., Zoellner, L. A., & Mollenholt, N.
(2008). Are expressive suppression and cogni-
tive reappraisal associated with stress- related
symptoms? Behavioural Research and Ther-
apy, 46(9), 993–1000.
Morone, N. E., Greco, C. M., & Weiner, D. K.
(2008). Mindfulness meditation for the treat-
ment of chronic low back pain in older adults:
A randomized controlled pilot study. Pain,
134(3), 310–319.
Neacsiu, A. D., Bohus, M., & Linehan, M. M.
(this volume, 2014). Dialectical behavior ther-
apy: An intervention for emotion dysregula-
tion. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 491–507). New
York: Guilford Press.
Nolen- Hoeksema, S. (2000). The role of rumina-
tion in depressive disorders and mixed anxi-
ety/depressive symptoms. Journal of Abnor-
mal Psychology, 109(3), 504–511.
Ochsner, K. N., Bunge, S. A., Gross, J. J., &
Gabrieli, J. D. (2002). Rethinking feelings:
An fMRI study of the cognitive regulation of
emotion. Journal of Cognitive Neuroscience,
14(8), 1215–1229.
Ohman, A., Flykt, A., & Esteves, F. (2001).
Emotion drives attention: Detecting the snake
in the grass. Journal of Experimental Psychol-
ogy: General, 130(3), 466–478.
Oken, B. S., Fonareva, I., Haas, M., Wahbeh,
H., Lane, J. B., Zajdel, D., et al. (2010). Pilot
controlled trial of mindfulness meditation and
education for dementia caregivers. Journal of
Alternative and Complementary Medicine,
16(10), 1031–1038.
Ottenbreit, N. D., & Dobson, K. S. (2004).
Avoidance and depression: The construction
of the Cognitive- Behavioral Avoidance Scale.
Behavioural Research and Therapy, 42(3),
293–313.
Pauley, G., & McPherson, S. (2010). The expe-
rience and meaning of compassion and self-
compassion for individuals with depression
or anxiety. Psychology and Psychotherapy:
Theory, Research and Practice, 83(Pt. 2),
129–143.
Paykel, E. S., Scott, J., Teasdale, J. D., Johnson,
A. L., Garland, A., Moore, R., et al. (1999).
Prevention of relapse in residual depression by
cognitive therapy: A controlled trial. Archives
of General Psychiatry, 56(9), 829–835.
Perlman, D. M., Salomons, T. V., Davidson, R.
J., & Lutz, A. (2010). Differential effects on
pain intensity and unpleasantness of two med-
itation practices. Emotion, 10(1), 65–71.
Piet, J., & Hougaard, E. (2011). The effect of
mindfulness- based cognitive therapy for pre-
vention of relapse in recurrent major depres-
sive disorder: A systematic review and meta-
analysis. Clinical Psychology Review, 31(6),
1032–1040.
Ramel, W., Goldin, P. R., & Carmona, P. E.
(2004). The effects of mindfulness meditation
on cognitive processes and affect in patients
with past depression. Cognitive Therapy and
Research, 28, 433–455.
Rosenzweig, S., Greeson, J. M., Reibel, D.
K., Green, J. S., Jasser, S. A., & Beasley, D.
(2010). Mindfulness- based stress reduction
for chronic pain conditions: Variation in treat-
ment outcomes and role of home meditation
practice. Journal of Psychosomatic Research,
68(1), 29–36.
Scher, C. D., Ingram, R. E., & Segal, Z. V.
(2005). Cognitive reactivity and vulnerability:
Empirical evaluation of construct activation
and cognitive diatheses in unipolar depression.
Clinical Psychology Review, 25(4), 487–510.
Schmertz, S. K., Anderson, P. L., & Robins, D.
L. (2009). The relation between self- report
mindfulness and performance on tasks of sus-
tained attention. Behavioral Science, 31(1),
60–66.
Segal, Z. V., Bieling, P., Young, T., MacQueen,
G., Cooke, R., Martin, L., et al. (2010). Anti-
depressant monotherapy vs sequential phar-
macotherapy and mindfulness- based cognitive
therapy, or placebo, for relapse prophylaxis in
recurrent depression. Archives of General Psy-
chiatry, 67(12), 1256–1264.
Segal, Z. V., Kennedy, S., Gemar, M., Hood, K.,
Pedersen, R., & Buis, T. (2006). Cognitive
Mindfulness Interventions and Emotion Regulation 567
reactivity to sad mood provocation and the
prediction of depressive relapse. Archives of
General Psychiatry, 63(7), 749–755.
Segal, Z. V., Williams, J. M. G., & Teasdale, J.
D. (2002). Mindfulness- based cognitive ther-
apy for depression: A new approach to pre-
venting relapse. New York: Guilford Press.
Semple, R. J., Lee, J., Rosa, D., & Miller, L. F.
(2010). A randomized trial of mindfulness-
based cognitive therapy for children: Pro-
moting mindful attention to enhance social–
emotional resiliency in children. Journal of
Child and Family Studies, 19, 218–229.
Shapiro, S. L., Brown, K. W., Thoresen, C.,
& Plante, T. G. (2011). The moderation of
mindfulness- based stress reduction effects by
trait mindfulness: Results from a randomized
controlled trial. Journal of Clinical Psychol-
ogy, 67(3), 267–277.
Shapiro, S. L., Carlson, L. E., Astin, J. A., &
Freedman, B. (2006). Mechanisms of mind-
fulness. Journal of Clinical Psychology, 62(3),
373–386.
Shapiro, S. L., & Schwartz, G. E. (2000). Inten-
tional systemic mindfulness: An integrative
model for self- regulation and health. Advances
in Mind–Body Medicine, 16(2), 128–134.
Sheppes, G. (this volume, 2014). Emotion regula-
tion choice: Theory and findings. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp. 126–139). New York: Guilford Press.
Silananda, S. U. (1998). The four foundations of
mindfulness (A summary). Retrieved Novem-
ber 18, 2012, from www.buddhanet.net/bud-
sas/ebud/ebmed063.htm.
Silananda, S. U. (2002). The four foundations of
mindfulness (2nd ed.). Boston: Wisdom.
Smith, A., Graham, L., & Senthinathan, S.
(2007). Mindfulness- based cognitive therapy
for recurring depression in older people: A
qualitative study. Aging and Mental Health,
11(3), 346–357.
Teasdale, J. D., & Chaskalson, M. (2011). How
does mindfulness transform suffering?: II.
The transformation of Dukka. Contempo-
rary Buddhism: An Interdisciplinary Journal,
12(1), 103–124.
Teasdale, J. D., Segal, Z. V., Williams, J. M.,
Ridgeway, V. A., Soulsby, J. M., & Lau, M.
A. (2000). Prevention of relapse/recurrence in
major depression by mindfulness- based cogni-
tive therapy. Journal of Consulting and Clini-
cal Psychology, 68(4), 615–623.
Trew, J. L. (2011). Exploring the roles of approach
and avoidance in depression: An integrative
model. Clinical Psychology Review, 31(7),
1156–1168.
Walach, H., Buchheld, N., Buttenmuller, V.,
Kleinknecht, N., & Schmidt, S. (2006). Mea-
suring mindfulness— The Freiburg Mindful-
ness Inventory (FMI). Personality and Indi-
vidual Differences, 40, 1543–1555.
Watkins, E., Moberly, N. J., & Moulds, M.
L. (2008). Processing mode causally influ-
ences emotional reactivity: Distinct effects of
abstract versus concrete construal on emo-
tional response. Emotion, 8(3), 364–378.
Williams, J. M. G. (2007). The mindful way
through depression: Freeing yourself from
chronic unhappiness. New York: Guilford
Press.
Williams, K. A., Kolar, M. M., Reger, B. E., &
Pearson, J. C. (2001). Evaluation of a wellness-
based mindfulness stress reduction interven-
tion: A controlled trial. American Journal of
Health Promotion, 15(6), 422–432.
Witkiewitz, K., Bowen, S., Douglas, H., & Hsu,
S. H. (2013). Mindfulness- based relapse pre-
vention for substance craving. Addictive
Behaviors, 38(2), 1563–1571.
Zeidan, F., Gordon, N. S., Merchant, J., & Gool-
kasian, P. (2010). The effects of brief mindful-
ness meditation training on experimentally
induced pain. Journal of Pain, 11(3), 199–209.

PART IX
HEALTH IMPLICATIONS

571
Inhibiting the expression of emotions has
long been believed to undermine physi-
cal health, and recent developments in our
ability to map neural– immune interactions
at the genomic level have begun to identify
potential biological bases for such effects.
This chapter reviews basic research linking
emotional inhibition (or suppression; I use
the terms interchangeably) to physical ill-
ness and outlines an emerging theoretical
paradigm in “social genomics” that sheds
light on both the biological mechanisms
of such effects and their potential evolu-
tionary origin. This perspective construes
emotion regulation as one component of a
broader set of biobehavioral adaptations
that have emerged in response to the evo-
lution of a hypersocial “life history strat-
egy” for humans (Darwin, 1872; McDade,
2003; Richerson, Boyd, & Henrich, 2010;
Wilson, 2012). The social– neural– genomic
programs that evolved to mediate this adap-
tation likely held little health cost under
primordial conditions in which trauma and
infection were our primary causes of death
(Finch, 2010). However, in the very differ-
ent social, physical, and cultural environ-
ments that we now inhabit, these biological
programs may functionally connect every-
day “civilized” social behavior to low-level
anticipatory threat reactions that can aggra-
vate many drivers of “modern mortality,”
such as cardiovascular, neurodegenerative,
metabolic, and neoplastic diseases (Finch,
2007; Sterling, 2004).
Emotion Regulation
and Human Health
As far back as the second century A.D., the
Greek physician Galen noted that people
who were emotionally inexpressive seemed
to be at increased risk of cancer (Siegel,
1968). Subsequent epidemiological studies
underscored this observation (Gross, 1989;
Mund & Mitte, 2012) and expanded the
range of health risks associated with emo-
tional inhibition to include cardiovascular
and immune- related diseases (Bell, Jas-
noski, Kagan, & King, 1990; Cohen, Doyle,
Skoner, Rabin, & Gwaltney, 1997; Cohen,
Doyle, Turner, Alper, & Skoner, 2003; Cole,
Kemeny, & Taylor, 1997; Cole, Kemeny,
Taylor, & Visscher, 1996; Denollet, Gidron,
Vrints, & Conraads, 2010; Denollet, Ped-
ersen, Vrints, & Conraads, 2006; Denol-
let et al., 1996; Ironson, O’Cleirigh, Weiss,
Schneiderman, & Costa, 2008; Kagan,
1994; Kagan, Snidman, Julia- Sellers, &
Johnson, 1991; Mund & Mitte, 2012).
Recent experimental studies also suggest
that the active expression of emotions can
mirror the effects of suppression and reduce
CHAPTER 33
Emotion Regulation and Gene Expression
Steven W. Cole

572 HEALTH IMPLICATIONS
the risk of minor illnesses (Booth, 2012;
Frattaroli, 2006; Pennebaker, 1988). The
disease risks associated with emotional inhi-
bition range widely in their specific biology
and in their overall health significance, but
they share in common one basic teleological
puzzle: Why has the human body evolved to
lose vitality when we inhibit the expression
of our emotions?
Surprisingly, mechanistic answers to this
question have emerged more rapidly than
true functional explanations. It is now easer
to understand how emotional inhibition
might affect human health than why that
should be so. Much of our growing mecha-
nistic insight stems from dramatic techni-
cal advances in molecular genetics, and our
growing ability to understand physical dis-
ease in terms of changing patterns of human
gene expression (Cole, 2009; Finch, 2007;
Miller, Chen, & Cole, 2009). The equation
of disease with gene expression dynamics
represents an oversimplification to be sure.
However, construing health and disease
through the lens of molecular genetics has
the significant heuristic advantage of con-
necting these dynamics to theoretical and
teleological perspectives derived from evo-
lutionary biology. Harnessing the tremen-
dous technical infrastructure of molecular
genomics offers opportunities to understand
not only how social inhibition affects health
but also why that connection should have
emerged in the first place. The keys to real-
izing this opportunity lie in understanding
how genes change their behavior in response
to our psychological experiences.
Genetics versus Genomics
As genes have come to be understood as con-
crete DNA sequences rather than abstrac-
tions inferred from inheritance, it has
become increasingly apparent that social
and psychological factors can regulate the
activity or expression of human genes (Cole,
2009). DNA encodes the potential for cellu-
lar behavior, but that potential is only real-
ized if the gene is expressed— if the DNA
is transcribed into RNA (Figure 33.1). It is
RNA and its subsequent translation into
protein that shapes a cell’s structure and
identity, and endows its functional capaci-
ties such as movement, metabolism, and bio-
chemical response to external stimuli (e.g.,
neurotransmission or immune response).
Absent their transcription, DNA genes have
no effect on health or behavioral pheno-
types. With the advent of a sequenced human
genome and the emergence of DNA microar-
ray and sequencing technologies, scientists
can now survey the expression of all human
genes simultaneously and map the specific
subset of genes that is actively expressed in
RNA within a given cell at a given point in
time. One surprising finding from such stud-
ies of functional genomics is that the expres-
sion of a given gene is often more an excep-
tion than the rule. Cells are highly selective
about which genes they express, and our
DNA encodes a great deal more genetic
potential than is realized in RNA and pro-
tein (Djebali et al., 2012). As such, the subset
of genes that is expressed in RNA—the cell’s
transcriptome—can be interpreted as the
product of a cellular decision- making pro-
cess regarding which particular sets of genes
would be most adaptive to express under the
prevailing circumstances. Mechanistically,
these decisions are mediated by protein tran-
scription factors that bind to specific DNA
sequences in the regulatory region (or pro-
moter) of a gene. Most transcription factors
target a large number of genes that contrib-
ute in different and often partially overlap-
ping ways to a common biological process
(e.g., multiple genes encoding receptors, sig-
naling molecules, and transcription factors
that collectively allow a particular cell to
recognize and respond to a neurotransmit-
ter or microbe). As such, transcription fac-
tors are best construed as activating general
gene transcriptional programs or gene mod-
ules rather than acting on specific individual
genes in isolation.
What evolutionary theory adds to this
mechanistic picture is a set of principles for
determining which genes should be coacti-
vated by a given transcription factor (i.e.,
the inclusion– exclusion structure or inten-
sion of a particular gene module) and which
transcription factors should be activated in
response to the stimuli present in the cell’s
environment. In other words, transcription
factors and their “target gene modules”
constitute a biochemical stimulus– response
program, and the development of the whole
system of such programs encoded in a
genome represents the product of evolution-
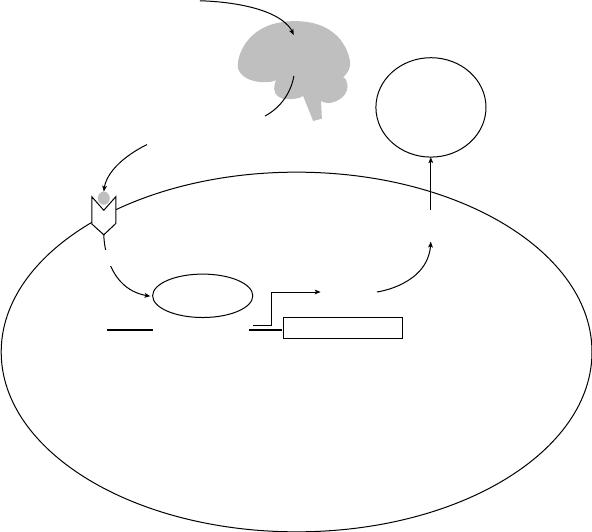
Emotion Regulations and Gene Expression 573
ary trial and error in adapting to changing
environmental conditions. Some “constitu-
tive” transcription factors show steady levels
of activity and act mainly to continue the
expression of genes that confer a cell’s par-
ticular biological identity (e.g., maintaining
the expression of neuron- defining genes in a
particular neuron, so it continues to be a neu-
ron). Other transcription factors are “induc-
ible” and can dramatically up- regulate their
activity from almost total quiescence to
levels 10–100 times above baseline. These
inducible transcription factors act mainly to
alter gene expression in response to chang-
ing environmental conditions as detected by
cellular receptor systems (e.g., increasing the
expression of immune response genes when
a microbe is detected, or changing gene
expression profiles in response to cellular
growth factors, hormones, or neurotrans-
mitters).
For both types of transcription factor,
the mapping of an individual factor onto its
distinctive subset of “target genes” confers
a sort of biological “meaning” to the tran-
scription factor— an intension set of tran-
scriptional responses that presumably have
helped the organism thrive under basal con-
ditions and adapt to emerging challenges.
This connection between the biological
characteristics of coregulated gene modules
and the nature of the upstream transcrip-
tion factors and receptor systems that acti-
vate those gene modules provides a concep-
tual strategy for extracting organismic- level
meaning and teleological design principles
from genomewide transcriptional profiles of
RNA (Cole, 2010). These mappings reflect
FIGURE 33.1. Social signal transduction. Socioenvironmental processes regulate human gene expres-
sion by activating CNS processes that subsequently influence hormone and neurotransmitter activity
in the periphery of the body. Peripheral signaling molecules interact with cellular receptors to activate
transcription factors, which bind to characteristic DNA motifs in gene promoters to initiate (or repress)
gene expression. Only genes that are transcribed into RNA actually impact health and behavioral
phenotypes. Individual differences in promoter DNA sequences (e.g., the [G/C] polymorphism shown
here) can affect the binding of transcription factors and thereby influence genomic sensitivity to socio-
environmental conditions. Adapted from Cole (2009).
Peripheral nervous system
Endocrine system
Receptor
Signal transduction
Transcription
factor
Promoter
Coding region
TGA[G/C]TCA
Gene
DNA
RNA
Protein
Health &
Behavior
Social Environment
Central
nervous system

574 HEALTH IMPLICATIONS
a sort of evolutionarily acquired program-
ming, or a “wisdom of the genome” regard-
ing which genes are most adaptive to express
in a given type of environment.
Accumulating data on the specific recep-
tor systems that activate particular tran-
scription factors also allow a set of more
specific inferences regarding the extracel-
lular physiological signaling pathways that
may trigger observed changes in the cellular
transcriptome. These mappings allow us, for
example, to translate genomewide transcrip-
tional profiles into theories regarding the
specific hormones and neurotransmitters
that may distribute central nervous system
(CNS)-mediated experiences of social or
emotional stimuli into changes in the periph-
eral gene expression dynamics that underlie
health and disease (see Ochsner & Gross;
Proudfit, Dunning, Foti, & Weinberg; John-
stone & Walter; and Gyurak & Etkin, this
volume, for more on the CNS mechanisms
of emotion regulation). These bioinformat-
ics projections essentially reverse the normal
flow of biological information from outside
the body to inside (Figure 33.1), thus pro-
viding a “bottom- up” strategy for detect-
ing the physiological signaling pathways
that may mediate the effects of emotion
regulation on health. Such approaches can
complement a more “top-down” strategy
that surveys known health- relevant periph-
eral neural and endocrine signals, such as
the sympathetic nervous system (SNS) or
hypothalamic– pituitary– adrenal (HPA) axis
(Sapolsky, 1994), to determine their poten-
tial contributions to the health effects of
emotional suppression. So, where might the
top-down effects of emotional suppression
meet up with the bottom- up programming
of the human genome?
Emotion Regulation and Peripheral
Neural Function
Some of the earliest research in human psy-
chophysiology found that people who were
relatively inexpressive of their emotions
nevertheless showed elevated autonomic
responses to stressful or threatening stim-
uli (Buck, 1979; Jones, 1935, 1950, 1960;
Notarius & Levenson, 1979; Weinberger,
Schwartz, & Davidson, 1979). More recent
studies have also noted inhibition- related dif-
ferences in basal levels of SNS activity (Cole,
Kemeny, Fahey, Zack, & Naliboff, 2003;
Kagan, 1994; Kagan, Reznick, & Snidman,
1988), suggesting that dispositional differ-
ences in peripheral autonomic activity might
potentially act on a tonic, daily basis to alter
basal physiological homeostasis. A growing
body of experimental research largely paral-
lels findings from the individual- differences
literature in linking emotional suppression
to increased SNS activity. Asking people
to inhibit the expression or experience of
an emotion in response to emotionally
arousing stimuli can increase SNS activity
(Dan- Glauser & Gross, 2011; Gross, 1998,
2002; Gross & Levenson, 1993, 1997; Har-
ris, 2001; Jackson, Malmstadt, Larson, &
Davidson, 2000; Lissek et al., 2007; Pen-
nebaker & Chew, 1985; Robinson & Dema-
ree, 2009), and actively inducing emotional
expressions in written or spoken form can
reduce inhibition- related SNS responses
(Hughes, Uhlmann, & Pennebaker, 1994;
Pennebaker, Barger, & Tiebout, 1989; Pen-
nebaker, Hughes, & O’Heeron, 1987) and
transiently increase indicators of physi-
cal health (e.g., self- reported illnesses or
objectively reported health care utilization,
vaccine- induced antibody responses, wound
healing, and immune system biological
parameters; Booth, 2012).
Although some inconsistent results exist,
the majority of published research sug-
gests that emotional suppression is associ-
ated with SNS activation. Moreover, in the
one study of individual differences that has
simultaneously assessed psychological inhi-
bition, SNS activity, and biological indi-
cators of disease progression, individual
differences in SNS activity emerged as a
statistically plausible mediator of relation-
ships between psychological inhibition and
HIV-1 viral load (Cole, 2008; Cole et al.,
2001, 2003). A key role for the SNS is also
supported by a growing body of data show-
ing that other manipulations of SNS activity
can aggravate a wide variety of disease pro-
cesses, including the growth and metastasis
of cancer (Cole & Sood, 2012; Sloan et al.,
2010; Thaker et al., 2006). In fact, it was
in studies of disease pathogenesis that the
human genome was first found to be modu-
lated in a broad and systematic way by psy-
chological processes and their effects on the
autonomic nervous system.

Emotion Regulations and Gene Expression 575
Neural Regulation of Human
Gene Expression
The potential for psychosocial regulation
of human gene expression first emerged in
the context of studies analyzing the effect of
social stress and SNS neurotransmitters on
viral genomes such as herpes simplex viruses
(Glaser, Kiecolt- Glaser, Speicher, & Holli-
day, 1985; Jenkins & Baum, 1995; Kupfer
& Summers, 1990; Leib, Nadeau, Rundle,
& Schaffer, 1991; Padgett et al., 1998; Ras-
mussen, Marsh, & Brill, 1957; Schuster,
Chasserot- Golaz, Urier, Beck, & Sergeant,
1991), human immunodeficiency virus (HIV-
1; Capitanio, Mendoza, Lerche, & Mason,
1998; Cole et al., 1997; Cole, Kemeny, Tay-
lor, Visscher, & Fahey, 1996; Sloan et al.,
2007), Epstein– Barr virus (Glaser et al.,
1985; Yang et al., 2010), cytomegalovirus
(Glaser et al., 1985; Prosch et al., 2000), and
the Kaposi’s sarcoma- associated human her-
pesvirus 8 (Chang et al., 2005). As obligate
parasites of human host cells, viruses have
evolved within a microenvironment struc-
tured by our own genome. If social processes
and SNS neurotransmitters can regulate the
expression of viral genes, it stands to reason
that our own complement of roughly 21,000
genes might be modulated as well.
Over the past 5 years, a series of genome-
wide transcriptional profiling studies has
found that extended periods of psycho-
logical or social stress are often associated
with a specific pattern of change in gene
expression within immune cells. Across
several distinct types of adversity such as
social isolation (Cole, Hawkley, Arevalo,
& Cacioppo, 2011; Cole et al., 2007; Cre-
swell et al., 2012), imminent bereavement
(Miller et al., 2008), low socioeconomic
status (SES; Chen, Miller, Kobor, & Cole,
2011; Chen et al., 2009), early life social
deprivation (Miller, Chen, Fok, et al., 2009),
late life social adversity (Cole et al., 2010),
traumatic stress (O’Donovan et al., 2011),
diagnosis with a life- threatening illness
(Antoni et al., 2012; Cohen et al., 2012),
and experimentally imposed social threat
(Cole et al., 2010; Cole, Arevalo, Rugge-
rio, Heckman, & Suomi, 2012; Sloan et al.,
2007, 2010), circulating immune cells (leu-
kocytes) show a conserved transcriptional
response to adversity (CTRA) involving
increased expression of genes involved in
inflammation (e.g., IL1B, IL6, IL8, TNF)
and decreased expression of genes involved
in innate antiviral responses (IFNB and
IFI, MX, and OAS gene families), and the
production of a specific type of antibody
(immunoglobulin G1 [IgG1]) (Cole, 2009,
2010; Irwin & Cole, 2011; Miller, Chen, &
Cole, 2009). Each type of adversity is also
associated with other transcriptional altera-
tions that are relatively unique to that condi-
tion. However, this core CTRA pattern of
proinflammatory and anti- antiviral tran-
scriptome shift has emerged much more con-
sistently than would be expected by chance.
Similar patterns also emerge in response to
experimentally imposed adversity in ani-
mal models of social instability, low social
rank, and social threat or defeat (Cole et al.,
2010, 2012; Irwin & Cole, 2011; Sloan et
al., 2007; Tung et al., 2012).
Given the statistical challenges of mul-
tiple hypothesis testing across roughly
21,000 genes, different “social genomics”
studies rarely find identical sets of differ-
entially expressed genes. Where consistent
patterns do become salient is in subsequent
bioinformatic analyses extracting common
functional themes from the lists of tens to
hundreds of differentially expressed genes in
each study (e.g., Gene Ontology annotations
regarding shared biological functions and
analyses of transcription control pathways
regulating expression of multiple genes)
(Cole, 2010). The recurrence of these core
proinflammatory– anti- antiviral biological
themes across both different adverse envi-
ronments and different mammalian species
suggests that the immune system of social
mammals is programmed to generate a
CTRA response whenever individuals expe-
rience extended periods of stress, threat, or
uncertainty (Antoni et al., 2012; Cole et al.,
2012; Irwin & Cole, 2011). This general
transcriptional program may be expressed
somewhat variably at the level of individual
gene transcripts depending upon specifics
of individual history, genetic background,
and particulars of the current environment
(Cole, 2010). However, the broader bio-
logical principle of increase inflammation
and suppress interferons and IgG1 seems
to be programmed into the basic stimulus–
response logic of the human genome (at least
as expressed in the receptor/transcription
factor logic of leukocytes).
576 HEALTH IMPLICATIONS
A key role for psychological experience in
triggering the CTRA dynamic is suggested
by results from several small randomized
controlled experiments showing that stress-
reducing interventions can reverse CTRA
transcriptional dynamics in human immune
cells (Antoni et al., 2012; Black et al., 2013;
Creswell et al., 2012). A key role for the SNS
in mediating these effects is suggested by
cellular studies showing that CTRA-related
transcriptional dynamics can be mimicked
by pharmacological agents that activate
the beta- adrenergic receptor systems tar-
geted by SNS neurotransmitters and can be
blocked by pharmacological antagonists of
those receptors (Cole et al., 2010; Collado-
Hidalgo, Sung, & Cole, 2006; Hanke, Pow-
ell, Stiner, Bailey, & Sheridan, 2012; Wohleb
et al., 2011). Moreover, the specific pattern
of up- regulated inflammatory gene expres-
sion and down- regulated antiviral- and
antibody- related gene expression observed
in studies of human social adversity maps
very closely to the effects of SNS pharma-
cology in laboratory experimental studies,
whereas other stress- responsive systems
that might potentially mediate such effects,
such as glucocorticoid hormone release from
the HPA axis, activate very different gene
expression programs (Irwin & Cole, 2011).
In fact, several studies have implicated a
selective reduction in glucocorticoid signal-
ing in contributing to the proinflammatory
component of the CTRA profile (Cole et al.,
2007; Hanke et al., 2012; Miller et al., 2008;
Wohleb et al., 2011). These observations do
not rule out a potential effect of glucocorti-
coids in other types of stress- mediated gene
expression dynamics (e.g., responses to more
severe, overwhelming stressors that elicit
defeat/withdrawal responses; Henry, 1992;
Lundberg & Frankenhaeuser, 1980; Sapol-
sky, 1994). However, the general patterns of
transcriptional alteration associated with the
chronic and highly prevalent socioenviron-
mental risk factors for human disease (social
isolation, low SES, etc.) appear to parallel
most closely the profiles of gene expression
induced by beta- adrenergic receptor sig-
naling in experimental cellular and animal
models (Irwin & Cole, 2011).
Why should CTRA-related gene modules
have evolved to become sensitive to beta-
adrenergic signaling from the SNS? CTRA
transcriptional dynamics appear to represent
an evolutionarily adaptive “defensive pro-
gram” that redeploys the immune system’s
transcriptional resources to “allostatically
anticipate” (Sterling, 2004) the changing
patterns of microbial exposure historically
associated with changing life circumstances
(e.g., increased risk of wound- related bacte-
rial infection during periods of acute threat
vs. increased risk of viral contagion during
extended periods of close social contact;
Cole et al., 2011; Irwin & Cole, 2011).
Because antiviral and proinflammatory gene
modules are, to some extent, mutually exclu-
sive (Amit et al., 2009; Negishi et al., 2012;
O’Connell et al., 2004; Shahangian et al.,
2009; Tian et al., 2012), the immune system
must “choose” which gene module to favor
at any given time. The CTRA dynamic sug-
gests that that choice is informed in part by
the broader physiological and environmental
conditions surrounding the individual (i.e.,
organism- level adaptive fitness), as perceived
by the CNS and distributed into peripheral
tissues via the SNS (Cole et al., 2011; Irwin
& Cole, 2011). However, when the CTRA
defensive program is chronically stimu-
lated, the resulting proinflammatory– anti-
antiviral shift in leukocyte transcriptional
equilibrium may promote the complex pat-
tern of “modern mortality” diseases involv-
ing elements of both up- regulated immune
function (e.g., inflammation- related diseases
such as heart disease, neurodegenerative dis-
eases, and some types of cancer) and down-
regulated immune function (e.g., impaired
response to vaccines and viral infections)
(Finch, 2007). The complex pattern of up-
and down- regulated gene modules associ-
ated with the CTRA underscores the fact
that stress is not broadly immunosuppres-
sive, but instead selectively suppresses some
groups of immune response genes (e.g., Type
I interferons and some immunoglobulin
genes) while simultaneously activating oth-
ers (e.g., proinflammatory cytokines) (Irwin
& Cole, 2011).
Beyond the CTRA dynamic in leuko-
cytes, stress biology can also regulate gene
expression in a wide variety of other tissues,
including the CNS (Karelina et al., 2009;
Karssen et al., 2007; Weaver, Meaney, &
Szyf, 2006), peripheral immune system
organs such as the lymph nodes and spleen
(Cole et al., 2010; Sloan et al., 2007), and
diseased tissues such as ovarian cancers and

Emotion Regulations and Gene Expression 577
stroke- wounded brain tissues (Karelina et
al., 2009; Lutgendorf et al., 2009). Given
the much smaller number of social genomics
analyses targeting such solid tissues, and the
relative difficulty in ascertaining the func-
tional significance of specific transcriptional
alterations outside the well- charted terri-
tories of the immune response, it has been
much more difficult to map the specific gene
programs modulated in these tissues and to
deduce their associated teleological rationale
(e.g., are these tissue “defensive programs”
analogous to the leukocyte CTRA, or do
they represent some other type of functional
adaptation specific to the organ system
involved?). However, it is clear that the social
environment surrounding an individual can
modulate broad swaths of gene activity in
a diverse array of bodily tissues via CNS-
mediated interpretations of the environment
as safe/accommodating or threatening/hos-
tile/uncertain.
Emotion Regulation
and Gene Regulation
At the time of this writing, no published
studies have directly examined the effects of
emotional inhibition on human gene expres-
sion. However, coupling the two domains
of research reviewed here suggests a natural
hypothesis regarding how chronic emotional
suppression might influence physical health
via systematic changes in gene expression.
To the extent that (1) emotional suppres-
sion activates the SNS and (2) the SNS acti-
vates gene expression programs such as the
CTRA, chronic emotional suppression may
induce a repetitive or temporally extended
bias in basal gene expression profiles that
simultaneously increases vulnerability to
inflammation- related diseases (e.g., car-
diovascular and neoplastic diseases) and
decreases immune response to vaccines and
viral infections. Assuming this plausible
conjunction holds true empirically, emotion
regulation can be construed as an indirect
form of gene regulation. Moreover, the spe-
cific pattern of gene expression modula-
tions that would be predicted based on this
hypothesis corresponds quite closely to the
profile of specific disease risks that has been
linked to inhibition in empirical epidemio-
logical studies. Beyond providing a plausible
biological mechanism for effects of emo-
tion regulation on human health (Miller,
Chen, & Cole, 2009), however, this equa-
tion of emotion regulation with gene regula-
tion also suggests a broader set of questions
regarding the origins and consequences of
emotion regulation in general physiological
function (i.e., independent of its impacts on
somatic disease).
One distinctive feature of gene transcrip-
tion as an “output” from psychological pro-
cesses is its simultaneous role as an “input”
into the future biological characteristics of
the individual (Cole, 2009). Genes can act
recursively in the sense that they alter the
nature of the molecular machinery that con-
trols their own expression. Figure 33.2 out-
lines some of the developmental implications
of such recursion in allowing environmental
conditions (including psychological percep-
tions and emotional expression) to become
embedded in the molecular biology of the
individual in ways that affect subsequent
perceptual, emotional, and neurobiological
responses to future environmental condi-
tions. For example, if one period of emo-
tional suppression results in altered patterns
of gene expression in the brain, and some
of those regulated genes include molecules
that mediate the perception of social threat
and its transduction into gene expression
(receptors, signal transduction molecules,
transcription factors, etc.), then the individ-
ual’s subsequent psychological response to a
fixed social or emotional stimulus may well
differ from what it would have been other-
wise, purely as a function of that individu-
al’s history of exposures earlier in life. This
recursive feedback dynamic provides one
biological pathway by which early life social
and psychological conditions can become
embedded in an individual’s basic neuro-
biological characteristics and subsequently
influence later patterns of psychological
experience and biological development
(Cole, 2009). Recursive feedback dynamics
also help clarify how particularly acute tran-
sient events can induce persisting alterations
in neurobiological homeostasis (e.g., as in
posttraumatic stress disorder [PTSD]).
Because genes can recursively regulate
their own molecular triggers and produc-
tion machinery, there exists great potential
for nonlinear regulatory dynamics in which
initially small stimuli have quantitatively
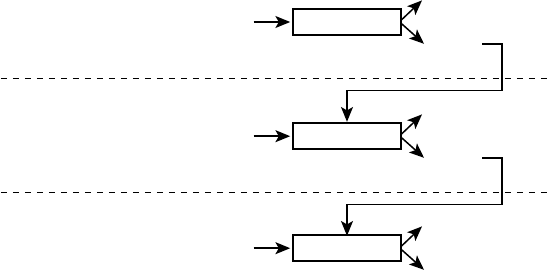
578 HEALTH IMPLICATIONS
or temporally disproportionate effects.
Whether this actually happens or not in any
given situation depends largely on the feed-
back structure of the specific gene networks
involved. To the extent that receptors, signal
transduction molecules, and transcription
factors number among those genes empiri-
cally modulated by a given stimulus, some
form of feedback recursion is likely to occur.
However, recursion alone does not guaran-
tee nonlinear amplification. Whether that
feedback is positive (amplifying), negative
(damping), or nonexistent depends largely
on the empirical pattern of connections
among gene products, or the “wiring dia-
gram” of the human genome as expressed
in a given cell type (i.e., the specific set of
receptors, signaling molecules, and tran-
scription factors expressed in a given cell, or
potentially induced in that cell in response
to environmental stimuli). Outside of the
immune system, the wiring diagram of the
human genome remains almost completely
unknown. However, as studies of CNS
gene expression grow in number, sophis-
tication, and neuroanatomical precision,
new insights will rapidly emerge regarding
the recursive architecture of the genome as
expressed in the brain structures mediating
emotional experience and behavior. Even
in the absence of ideal longitudinal studies
that directly map system dynamics, much
may be learned by simply observing the pat-
tern of changes induced by a single stimulus
event. To the extent that stimulus changes
the expression of genes with known recur-
sive capacity (e.g., genes encoding receptors,
signal transduction molecules, transcription
factors, or epigenetic modulators) the poten-
tial for feedback and nonlinear dynam-
ics becomes substantially greater, and the
specific pattern of up- and down- regulated
recursive molecules can provide some indi-
cations regarding the positive versus nega-
tive feedback elements of the system. As
those wiring diagrams become increasingly
FIGURE 33.2. RNA as a molecular medium of recursive development. Social conditions at one point
in time (Environment
1
) are transduced into changes in behavior (Behavior
1
) and gene expression
(RNA
1
) via CNS perceptual processes that trigger systemic neural and endocrine responses (medi-
ated by Body
1
). Those RNA transcriptional dynamics can alter the molecular characteristics of cells
involved in environmental perception or response, resulting in a functionally altered Body
2
. Body
2
may respond differently to a given environmental challenge than would the previous Body
1
, resulting
in different behavioral (Behavior
2
) and transcriptional responses (RNA
2
). The effect of RNA tran-
scriptional dynamics on persisting cellular protein and functional characteristics provides a molecular
framework for understanding how socioenvironmental conditions in the past may continue to affect
current behavior and health, and how those historical conditions interact with current environments
to shape our future trajectories (e.g., Body
3
, Behavior
3
, RNA
3
). Because gene transcription serves as
both a cause of social behavior (by shaping Body) and a consequence of social behavior (a product of
Environment × Body), RNA serves as the physical medium for a recursive developmental trajectory that
integrates genetic characteristics and historical– environmental regulators to shape individual biologi-
cal and behavioral responses to current environmental conditions. Adapted from Cole (2009).
Body
1Environment
Behavior
RNA
Time 1
1
1
1
Body
2Environment
Behavior
RNA
Time 2
2
2
2
Body
3Environment
Behavior
RNA
Time 3
3
3
3
Emotion Regulations and Gene Expression 579
well mapped, the network structure of such
systems may identify particularly influen-
tial recursive regulators that would provide
highly leveraged targets for interventions to
block the temporally propagating effects of
early life adversity and acute trauma in adult
life. Beyond such clinical implications, there
also emerges a much broader set of questions
regarding how the gene regulation inherent
in emotion regulation might developmen-
tally modify the “behavior factory” of the
CNS, and the role that occasional tactical
suppression may play in laying a molecu-
lar foundation for chronic suppression or
dampened emotional experience.
Given that emotion regulation is an intrin-
sically social adaptation (Darwin, 1872),
how might suppression affect an individual’s
social relationships? A growing body of evi-
dence suggests that chronic emotional sup-
pression may significantly undermine the
number and quality of one’s social connec-
tions to others (Butler et al., 2003; English,
John, Srivastava, & Gross, 2012; Gross,
2002; Gross & John, 2003; Mauss et al.,
2011; Srivastava, Tamir, McGonigal, John,
& Gross, 2009). Experiences of social con-
nection vs. disconnection can also affect
gene expression (Cole et al., 2007, 2011), and
were in fact the context in which the CTRA
gene expression dynamic was first observed.
As such, the health effects of emotional sup-
pression may stem at least in part from their
effects in reducing an individual’s sense of
social connection to others. Indeed, micro-
acts of emotional suppression may kindle a
broader change in other people’s regard for
the individual that comes to assume a tem-
poral scope and situational breadth much
greater than original biological insult cre-
ated by a transient act of suppression. In
this sense, the most significant biological
implications of emotion regulation may
well stem from its impact on an individual’s
social identity and degree of healthy attach-
ment to others. Moreover, individual acts of
emotional suppression can also activate the
SNS in surrounding social partners (Butler
et al., 2003), implying a contagious dimen-
sion extending beyond the suppressor’s own
body. A wide variety of theoretical perspec-
tives note the fundamental role of secure
social bonds in maintaining human health
and well-being (Berkman & Kawachi, 2000;
Bowlby, 1983; Cacioppo & Hawkley, 2009;
Hofer, 1984). To the extent that emotional
suppression undermines one’s sense of true
connection to others and more general faith
in humanity, the social costs of suppression
may well represent its most caustic health
effect.
The bulk of the research on emotion regu-
lation and health has focused on the long-
observed relationship between emotional
suppression and illness. However, it is worth
considering how health and biology might
be affected by the other broad class of emo-
tion regulation strategies involving refram-
ing or reappraisal (Gross, 2002). Much of
the psychophysiological research suggests
that reappraisal has relatively little impact
on SNS activity (Gross, 2002; Gross & Lev-
enson, 1993), and the equation of gene reg-
ulation with SNS activation might thus be
taken to imply that reappraisal- based emo-
tion regulation has relatively little effect on
gene expression and health. A separate lit-
erature on the psychology of stress and the
role of reappraisal- based coping strategies
would also support that hypothesis (Laza-
rus & Folkman, 1984; Sapolsky, 1994).
However, at the level of the CNS, where
gene expression dynamics may also play a
role in establishing the basic machinery of
perception, interpretation, and behavioral
response, it does seem likely that the habit-
ual use of reappraisal strategies may poten-
tially affect the molecular biology of neural
development and plasticity. This might be
particularly true within the brain struc-
tures involved in appraisal itself, the struc-
tures involved in metacognition or executive
function processes that would control the
deployment of reappraisal processes into
the ongoing stream of conscious experience,
and potentially also in the neurobiologi-
cal structures that mediate stress responses
(e.g., involving functional down- regulation
or desensitization of these structures). To
the extent that habitual and adaptive reap-
praisal processes build a different brain at
the neuromolecular level, those biological
developments themselves will likely be struc-
tured at least in part by differential activa-
tion of specific gene modules. Moreover, the
general modularity of brain function implies
that these neurogenomic impacts of habitual
reappraisal (e.g., in down- regulating threat
circuits in the limbic system, up- regulating
analytic or appraisal systems, and possibly

580 HEALTH IMPLICATIONS
reducing the need for intense conscious con-
trolled inhibitory processes) may well spill
over into other domains of experience, thus
altering the activity of any other response
systems that make use of the same func-
tional brain modules. Again, the specific
empirical structure of the gene networks
involved remains to be mapped. However,
we now have available a growing analytic
and technical infrastructure for discovering
the genomic mechanisms of appraisal- based
emotion regulation once empirical transcrip-
tome data become available. These consider-
ations underscore the importance of devel-
oping experimental animal models that can
potentially recapitulate at least some basic
features of the perception/appraisal and sup-
pression/reappraisal systems. More broadly,
to the extent that reappraisal produces a
more fundamentally human and behavior-
ally adaptive CNS, it may well have salutary
effects on peripheral gene expression and
health that extend far beyond a neutral basal
state of physiological homeostasis.
Much remains to be learned about the
molecular mechanisms and biological impli-
cations of human emotion regulation. How-
ever, what we can say from the data already
at hand is that, in a highly social species
such as humans, issues of expressing and
concealing emotional reactions cut deeply
and fundamentally to the core of what it
means to be a participant in the human race.
In that sense, it is perhaps not so surprising
that our conscious and unconscious efforts
to manage the experience and expression
of our emotions may have deep implica-
tions for our health. If the genome is a lens
through which health can be understood, it
is even more surely a molecular blueprint for
helping humans navigate the complexities of
our physical and social worlds (Fox Keller,
2012). In that regard, it comes as no surprise
if the deepest regions of our internal biology,
and the very function of our own individual
human genomes, are inextricably bound
up in our experience of the external social
world around us, the emotional experiences
it evokes in us, and our emotional expres-
sions back to that world. Genomes exist fun-
damentally to help us succeed as humans,
and to the extent that requires the effective
regulation of our emotional experience, the
human genome must carry some molecular
blueprints that help us do that. And to the
extent that we fail to adaptively manage the
experience and expression of our emotions,
the genome also appears to carry molecular
blueprints that may help defend us against
the near-term social– biological costs of that
failure, but only by mortgaging the molecu-
lar underpinnings of our long-term vitality.
Acknowledgments
Preparation of this chapter was supported by
grants from the National Institutes of Health
(Nos. AG028748, AG033590, CA116778) and
benefited greatly from the insightful comments
of James Gross. This review is dedicated to
George Freeman Solomon, a pioneer in the field
of psychoneuroimmunology and a deeply wise,
compassionate, and curious observer of human
nature and its braided strands of emotion, inhibi-
tion, and health.
References
Amit, I., Garber, M., Chevrier, N., Leite, A.
P., Donner, Y., Eisenhaure, T., et al. (2009).
Unbiased reconstruction of a mammalian
transcriptional network mediating pathogen
responses. Science, 326(5950), 257–263.
Antoni, M. H., Lutgendorf, S. K., Blomberg,
B., Stagl, J., Carver, C. S., Lechner, S., et al.
(2012). Transcriptional modulation of human
leukocytes by cognitive- behavioral stress man-
agement in women undergoing treatment for
breast cancer. Biological Psychiatry, 71(4),
366–372.
Appleton, A. A., & Kubzansky, L. D. (this vol-
ume, 2014). Emotion regulation and car-
diovascular disease risk. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 596–612). New York: Guilford Press.
Bell, J. R., Jasnoski, M. L., Kagan, J., & King, D.
S. (1990). Is allergic rhinitis more frequent in
adults with extreme shyness?: A preliminary
study. Psychosomatic Medicine, 52, 517–525.
Berkman, L. F., & Kawachi, I. (2000). Social
epidemiology. New York: Oxford University
Press.
Black, D. S., Cole, S. W., Irwin, M. R., Breen,
E., St. Cyr, N. M., Nazarian, N., et al. (2013).
Yogic meditation reverses NF-kappaB and
IRF-related transcriptome dynamics in leuko-
Emotion Regulations and Gene Expression 581
cytes of family dementia caregivers in a ran-
domized controlled trial. Psychoneuroendo-
crinology, 38(3), 348–355.
Booth, R. J. (2012). Emotional expression and
disclosure. In S. Segerstrom (Ed.), The Oxford
handbook of psychoneuroimmunology (pp.
105–125). New York: Oxford University Press.
Bowlby, J. (1983). Attachment. New York: Basic
Books.
Buck, R. W. (1979). Individual differences in non-
verbal sending accuracy and electrodermal
responding: The externalizing– internalizing
dimension. In R. Rosenthal (Ed.), Skill in non-
verbal communication (pp. 140–170). Cam-
bridge, MA: Oelgeschlager, Gunn & Hain.
Butler, E. A., Egloff, B., Wilhelm, F. H., Smith,
N. C., Erickson, E. A., & Gross, J. J. (2003).
The social consequences of expressive suppres-
sion. Emotion, 3(1), 48– 67.
Cacioppo, J. T., & Hawkley, L. C. (2009). Per-
ceived social isolation and cognition. Trends
in Cognitive Sciences, 13(10), 447–454.
Capitanio, J. P., Mendoza, S. P., Lerche, N. W.,
& Mason, W. A. (1998). Social stress results in
altered glucocorticoid regulation and shorter
survival in simian acquired immune deficiency
syndrome. Proceedings of the National Acad-
emy of Sciences USA, 95(8), 4714–4719.
Chang, M., Brown, H., Collado- Hidalgo, A.,
Arevalo, J., Galic, Z., Symensma, T., et al.
(2005). Beta- adrenoreceptors reactivate Kapo-
si’s sarcoma- related herpesvirus lytic replica-
tion via PKA-dependent control of viral RTA.
Journal of Virology, 72(21), 13538–13547.
Chen, E., & Miller, G. E. (this volume, 2014).
Early-life socioeconomic status, emotion regu-
lation, and the biological mechanisms of dis-
ease across the lifespan. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 586–595). New York: Guilford Press.
Chen, E., Miller, G. E., Kobor, M. S., & Cole,
S. W. (2011). Maternal warmth buffers the
effects of low early-life socioeconomic status
on pro- inflammatory signaling in adulthood.
Molecular Psychiatry, 16, 729–737.
Chen, E., Miller, G. E., Walker, H. A., Arevalo,
J. M., Sung, C. Y., & Cole, S. W. (2009).
Genome-wide transcriptional profiling linked
to social class in asthma. Thorax, 64(1),
38–43.
Cohen, L., Cole, S. W., Sood, A. K., Prinsloo, S.,
Kirschbaum, C., Arevalo, J. M., et al. (2012).
Depressive symptoms and cortisol rhythmicity
predict survival in patients with renal cell car-
cinoma: Role of inflammatory signaling. PLoS
ONE, 7(8), e42324.
Cohen, S., Doyle, W. J., Skoner, D. P., Rabin, B.
S., & Gwaltney, J. M. (1997). Social ties and
susceptibility to the common cold. Journal
of the American Medical Association, 227,
1940–1944.
Cohen, S., Doyle, W. J., Turner, R., Alper, C. M.,
& Skoner, D. P. (2003). Sociability and sus-
ceptibility to the common cold. Psychological
Science, 14(5), 389–395.
Cole, S. W. (2008). Psychosocial influences on
HIV-1 disease progression: Neural, endocrine,
and virologic mechanisms. Psychosomatic
Medicine, 70(5), 562–568.
Cole, S. W. (2009). Social regulation of human
gene expression. Current Directions in Psy-
chological Science, 18(3), 132–137.
Cole, S. W. (2010). Elevating the perspective on
human stress genomics. Psychoneuroendocri-
nology, 35, 955–962.
Cole, S. W., Arevalo, J. M., Ruggerio, A. M.,
Heckman, J. J., & Suomi, S. (2012). Tran-
scriptional modulation of the developing
immune system by early life social adversity.
Proceedings of the National Academy of Sci-
ences USA, 109(50), 20578–20583.
Cole, S. W., Arevalo, J., Takahashi, R., Sloan, E.
K., Lutgendorf, S., Sood, A. K., et al. (2010).
Computational identification of gene– social
environment interaction at the human IL6
locus. Proceedings of the National Academy
of Sciences USA, 107(12), 5681–5686.
Cole, S. W., Hawkley, L. C., Arevalo, J. M.,
& Cacioppo, J. T. (2011). Transcript origin
analysis identifies antigen- presenting cells
as primary targets of socially regulated gene
expression in leukocytes. Proceedings of the
National Academy of Sciences USA, 108(7),
3080–3085.
Cole, S. W., Hawkley, L. C., Arevalo, J. M., Sung,
C. Y., Rose, R. M., & Cacioppo, J. T. (2007).
Social regulation of gene expression in human
leukocytes. Genome Biology, 8(R189), 1–13.
Cole, S. W., Kemeny, M. E., Fahey, J. L., Zack,
J. A., & Naliboff, B. D. (2003). Psychological
risk factors for HIV pathogenesis: Mediation
by the autonomic nervous system. Biological
Psychiatry, 54, 1444–1456.
Cole, S. W., Kemeny, M. E., & Taylor, S. E.
(1997). Social identity and physical health:
Accelerated HIV progression in rejection-
sensitive gay men. Journal of Personality and
Social Psychology, 72, 320–336.
582 HEALTH IMPLICATIONS
Cole, S. W., Kemeny, M. E., Taylor, S. E., &
Visscher, B. R. (1996). Elevated physical
health risk among gay men who conceal their
homosexual identity. Health Psychology, 15,
243–251.
Cole, S. W., Kemeny, M. E., Taylor, S. E., Viss-
cher, B. R., & Fahey, J. L. (1996). Acceler-
ated course of human immunodeficiency virus
infection in gay men who conceal their homo-
sexual identity. Psychosomatic Medicine, 58,
219–231.
Cole, S. W., Naliboff, B. D., Kemeny, M. E.,
Griswold, M. P., Fahey, J. L., & Zack, J. A.
(2001). Impaired response to HAART in
HIV-infected individuals with high auto-
nomic nervous system activity. Proceedings of
the National Academy of Sciences USA, 98,
12695–12700.
Cole, S. W., & Sood, A. K. (2012). Molecular
pathways: Beta- adrenergic signaling in cancer.
Clinical Cancer Research, 18(5), 1201–1206.
Collado- Hidalgo, A., Sung, C., & Cole, S.
(2006). Adrenergic inhibition of innate anti-
viral response: PKA blockade of Type I inter-
feron gene transcription mediates catechol-
amine support for HIV-1 replication. Brain,
Behavior, and Immunity, 20(6), 552–563.
Creswell, J. D., Irwin, M. R., Burklund, L. J.,
Lieberman, M. D., Arevalo, J. M., Ma, J., et
al. (2012). Mindfulness- based stress reduc-
tion training reduces loneliness and pro-
inflammatory gene expression in older adults:
A small randomized controlled trial. Brain,
Behavior, and Immunity, 26(7), 1095–1101.
Dan- Glauser, E. S., & Gross, J. J. (2011). The
temporal dynamics of two response- focused
forms of emotion regulation: Experiential,
expressive, and autonomic consequences. Psy-
chophysiology, 48(9), 1309–1322.
Darwin, C. (1872). The expression of the emo-
tions in man and animals. London: John Mur-
ray.
Denollet, J., Gidron, Y., Vrints, C. J., & Con-
raads, V. M. (2010). Anger, suppressed anger,
and risk of adverse events in patients with
coronary artery disease. American Journal of
Cardiology, 105(11), 1555–1560.
Denollet, J., Pedersen, S. S., Vrints, C. J., & Con-
raads, V. M. (2006). Usefulness of type D per-
sonality in predicting five-year cardiac events
above and beyond concurrent symptoms of
stress in patients with coronary heart disease.
American Journal of Cardiology, 97(7), 970–
973.
Denollet, J., Sys, S. U., Stroobant, N., Rombouts,
H., Gillebert, T. C., & Brutsaert, D. L. (1996).
Personality as an independent predictor of
long-term mortality in patients with coronary
heart disease. Lancet, 347, 417– 421.
Djebali, S., Davis, C. A., Merkel, A., Dobin, A.,
Lassmann, T., Mortazavi, A., et al. (2012).
Landscape of transcription in human cells.
Nature, 489(7414), 101–108.
English, T., John, O. P., Srivastava, S., & Gross,
J. J. (2012). Emotion regulation and peer-
rated social functioning: A 4-year longitudi-
nal study. Journal of Research in Personality,
46(6), 780–784.
Finch, C. E. (2007). The biology of human lon-
gevity: Inflammation, nutrition, and aging in
the evolution of lifespans. Burlington, MA:
Academic Press.
Finch, C. E. (2010). Evolution in health and
medicine Sackler colloquium: Evolution of the
human lifespan and diseases of aging: Roles of
infection, inflammation, and nutrition. Pro-
ceedings of the National Academy of Sciences
USA, 1, 1718–1724.
Fox Keller, E. (2012). Genes, genomes, and
genomics. Biological Theory, 6(2), 132–140.
Frattaroli, J. (2006). Experimental disclosure
and its moderators: A meta- analysis. Psycho-
logical Bulletin, 132(6), 823–865.
Glaser, R., Kiecolt- Glaser, J. K., Speicher, C. E.,
& Holliday, J. E. (1985). Stress, loneliness,
and changes in herpesvirus latency. Journal of
Behavioral Medicine, 8(3), 249–260.
Gross, J. (1989). Emotional expression in can-
cer onset and progression. Social Science and
Medicine, 28, 1239–1248.
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74(1), 224–237.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39(3), 281–291.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships, and
well-being. Journal of Personality and Social
Psychology, 85(2), 348–362.
Gross, J. J., & Levenson, R. W. (1993). Emo-
tional suppression: Physiology, self- report,
and expressive behavior. Journal of Personal-
ity and Social Psychology, 64, 970–986.
Gross, J. J., & Levenson, R. W. (1997). Hiding
Emotion Regulations and Gene Expression 583
feelings: The acute effects of inhibiting nega-
tive and positive emotion. Journal of Abnor-
mal Psychology, 106(1), 95–103.
Gyurak, A., & Etkin, A. (this volume, 2014). A
neurobiological model of implicit and explicit
emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp. 76–90). New York: Guilford Press.
Hanke, M. L., Powell, N. D., Stiner, L. M., Bai-
ley, M. T., & Sheridan, J. F. (2012). Beta adren-
ergic blockade decreases the immunomodula-
tory effects of social disruption stress. Brain,
Behavior, and Immunity, 26(7), 1150–1159.
Harris, C. R. (2001). Cardiovascular responses
of embarrassment and effects of emotional
suppression in a social setting. Journal of Per-
sonality and Social Psychology, 81(5), 886–
897.
Henry, J. P. (1992). Biological basis of the stress
response. Integrative Physiological and
Behavioral Science, 27(1), 66–83.
Hofer, M. A. (1984). Relationships as regulators:
A psychobiologic perspective on bereavement.
Psychosomatic Medicine, 46(3), 183 –197.
Hughes, C. F., Uhlmann, C., & Pennebaker, J.
W. (1994). The body’s response to processing
emotional trauma: Linking verbal text with
autonomic activity. Journal of Personality,
62(4), 565–585.
Ironson, G. H., O’Cleirigh, C., Weiss, A.,
Schneiderman, N., & Costa, P. T., Jr. (2008).
Personality and HIV disease progression: Role
of NEO-PI-R openness, extraversion, and pro-
files of engagement. Psychosomatic Medicine,
70(2), 245–253.
Irwin, M. R., & Cole, S. W. (2011). Reciprocal
regulation of the neural and innate immune
systems. Nature Reviews Immunology, 11(9),
625–632.
Jackson, D. C., Malmstadt, J. R., Larson, C.
L., & Davidson, R. J. (2000). Suppression
and enhancement of emotional responses to
unpleasant pictures. Psychophysiology, 37(4),
515–522.
Jenkins, F. J., & Baum, A. (1995). Stress and
reactivation of latent herpes simplex virus: A
fusion of behavioral medicine and molecu-
lar biology. Annals of Behavioral Medicine,
17(2), 116–123.
Johnstone, T., & Walter, H. (this volume, 2014).
The neural basis of emotion dysregulation. In
J. J. Gross (Ed.), Handbook of emotion regu-
lation (2nd ed., pp.
58–75). New York: Guil-
ford Press.
Jones, H. E. (1935). The galvanic skin response as
related to overt emotional expression. Ameri-
can Journal of Psychology, 47, 241–251.
Jones, H. E. (1950). The study of patterns of
emotional expression. In M. L. Reymert (Ed.),
Feelings and emotions: The Mooseheart Sym-
posium. New York: McGraw-Hill.
Jones, H. E. (1960). The longitudinal method
in the study of personality. In I. Iscoe & H.
W. Stevenson (Eds.), Personality development
in children (pp.
3
–27). Austin: University of
Texas Press.
Kagan, J. (1994). Galen’s prophecy: Tempera-
ment in human nature. New York: Basic
Books.
Kagan, J., Reznick, J. S., & Snidman, N. (1988).
Biological bases of childhood shyness. Sci-
ence, 240, 167–171.
Kagan, J., Snidman, N., Julia-
S
ellers, M., &
Johnson, O. (1991). Temperament and aller-
gic symptoms. Psychosomatic Medicine, 53,
332–340.
Karelina, K., Norman, G. J., Zhang, N., Morris,
J. S., Peng, H., & DeVries, A. C. (2009). Social
isolation alters neuroinflammatory response
to stroke. Proceedings of the National Acad-
emy of Sciences USA, 106(14), 5895–5900.
Karssen, A. M., Her, S., Li, J. Z., Patel, P. D.,
Meng, F., Bunney, W. E., Jr., et al. (2007).
Stress-
i
nduced changes in primate prefrontal
profiles of gene expression. Molecular Psychi-
atry, 12(12), 1089–1102.
Kupfer, S. R., & Summers, W. C. (1990). Identi-
fication of a glucocorticoid-
r
esponsive element
in Epstein–
B
arr virus. Journal of Virology,
64(5), 1984–1990.
Lazarus, R. S., & Folkman, S. (1984). Stress,
appraisal, and coping. New York: Springer.
Leib, D. A., Nadeau, K. C., Rundle, S. A., & Schaf-
fer, P. A. (1991). The promoter of the latency-
a
ssociated transcripts of herpes simplex virus
type 1 contains a functional cAMP-response
element: Role of the latency-
a
ssociated tran-
scripts and cAMP in reactivation of viral
latency. Proceedings of the National Academy
of Sciences USA, 88(1), 48–52.
Lissek, S., Orme, K., McDowell, D. J., Johnson,
L. L., Luckenbaugh, D. A., Baas, J. M., et al.
(2007). Emotion regulation and potentiated
startle across affective picture and threat-
o
f-shock paradigms. Biological Psychology,
76(1–2), 124–133.
Lundberg, U., & Frankenhaeuser, M. (1980).
Pituitary–
adrenal and sympathetic–
adrenal
584 HEALTH IMPLICATIONS
correlates of distress and effort. Journal of
Psychosomatic Research, 24(3–4), 125–130.
Lutgendorf, S. K., Degeest, K., Sung, C. Y., Are-
valo, J. M., Penedo, F., Lucci, J., III, et al.
(2009). Depression, social support, and beta-
a
drenergic transcription control in human
ovarian cancer. Brain, Behavior, and Immu-
nity, 23(2), 176–183.
Mauss, I. B., Shallcross, A. J., Troy, A. S., John,
O. P., Ferrer, E., Wilhelm, F. H., et al. (2011).
Don’t hide your happiness!: Positive emotion
dissociation, social connectedness, and psy-
chological functioning. Journal of Personality
and Social Psychology, 100(4), 738–748.
McDade, T. W. (2003). Life history theory and
the immune system: Steps toward a human
ecological immunology. American Journal
of
Physical Anthropology, 37(Suppl.), 100–
125.
Miller, G., Chen, E., & Cole, S. W. (2009).
Health psychology: Developing biologically
plausible models linking the social world and
physical health. Annual Review of Psychol-
ogy, 60, 501–524.
Miller, G. E., Chen, E., Fok, A. K., Walker, H.,
Lim, A., Nicholls, E. F., et al. (2009). Low
early-life social class leaves a biological resi-
due manifested by decreased glucocorticoid
and increased proinflammatory signaling.
Proceedings of the National Academy of Sci-
ences USA, 106(34), 14716–14721.
Miller, G. E., Chen, E., Sze, J., Marin, T., Are-
valo, J. M., Doll, R., et al. (2008). A func-
tional genomic fingerprint of chronic stress in
humans: Blunted glucocorticoid and increased
NF-kappaB signaling. Biolical Psychiatry,
64(4), 266–272.
Mund, M., & Mitte, K. (2012). The costs of
repression: A meta-
a
nalysis on the relation
between repressive coping and somatic dis-
eases. Health Psychology, 31(5), 640–649.
Negishi, H., Yanai, H., Nakajima, A., Koshiba,
R., Atarashi, K., Matsuda, A., et al. (2012).
Cross-
i
nterference of RLR and TLR signal-
ing pathways modulates antibacterial T cell
responses. Nature Immunology, 13(7), 659–
666.
Notarius, C. I., & Levenson, R. W. (1979).
Expressive tendencies and physiological
response to stress. Journal of Personality and
Social Psychology, 37(7), 1204–1210.
Ochsner, K. N., & Gross, J. J. (this volume, 2014).
The neural bases of emotion and emotion regu-
lation: A valuation perspective. In J. J. Gross
(Ed.), Handbook of emotion regulation (2nd
ed., pp.
23–42). New York: Guilford Press.
O’Connell, R. M., Saha, S. K., Vaidya, S. A.,
Bruhn, K. W., Miranda, G. A., Zarnegar, B.,
et al. (2004). Type I interferon production
enhances susceptibility to Listeria monocy-
togenes infection. Journal of Experimental
Medicine, 200(4), 437–445.
O’Donovan, A., Sun, B., Cole, S., Rempel, H.,
Lenoci, M., Pulliam, L., et al. (2011). Tran-
scriptional control of monocyte gene expres-
sion in post-
t
raumatic stress disorder. Disease
Markers, 30(2–3), 123–132.
Padgett, D. A., Sheridan, J. F., Dorne, J., Bern-
tson, G. G., Candelora, J., & Glaser, R. (1998).
Social stress and the reactivation of latent her-
pes simplex virus type 1. Proceedings of the
National Academy of Sciences USA, 95(12),
7231–7235.
Pennebaker, J. W. (1988). Confession, inhibition,
and disease. In L. Berkowitz (Ed.), Advances
in experimental social psychology (Vol. 22,
pp.
211–242). Orlando, FL: Academic Press.
Pennebaker, J. W., Barger, S. D., & Tiebout,
J. (1989). Disclosure of traumas and health
among Holocaust survivors. Psychosomatic
Medicine, 51(5), 577–589.
Pennebaker, J. W., & Chew, C. H. (1985). Behav-
ioral inhibition and electrodermal activity
during deception. Journal of Personality and
Social Psychology, 49, 1427–1433.
Pennebaker, J. W., Hughes, C. F., & O’Heeron,
R. C. (1987). The psychophysiology of confes-
sion: Linking inhibitory and psychosomatic
processes. Journal of Personality and Social
Psychology, 52(4), 781–793.
Prosch, S., Wendt, C. E. C., Reinke, P., Priemer,
C., Pooert, M., Kruger, D. H., et al. (2000).
A novel link between stress and human cyto-
megalovirus (HCMV) infection: Sympathetic
hyperactivity stimulates HCMV activation.
Virology, 272, 357–365.
Proudfit, G., Dunning J. P., Foti, D., & Wein-
berg, A. (this volume, 2014). Temporal dynam-
ics of emotion regulation. In J. J. Gross (Ed.),
Handbook of emotion regulation (2nd ed.,
pp.
43–57). New York: Guilford Press.
Rasmussen, A. F., Marsh, J. T., & Brill, N. Q.
(1957). Increases susceptibility to hepres sim-
plex in mice subjected to avoidance-
l
earning
stress or restraint. Proceedings of the Society
for Experimental Biology and Medicine, 96,
183–189.
Richerson, P. J., Boyd, R., & Henrich, J. (2010).
Emotion Regulations and Gene Expression 585
Colloquium paper: Gene– culture coevolu-
tion in the age of genomics. Proceedings of
the National Academy of Sciences USA,
107(Suppl. 2), 8985–8992.
Robinson, J. L., & Demaree, H. A. (2009). Expe-
riencing and regulating sadness: Physiological
and cognitive effects. Brain and Cognition,
70(1), 13–20.
Sapolsky, R. M. (1994). Why zebras don’t get
ulcers: A guide to stress, stress-
related dis-
eases, and coping. New York: Freeman.
Schuster, C., Chasserot-
G
olaz, S., Urier, G.,
Beck, G., & Sergeant, A. (1991). Evidence for
a functional glucocorticoid responsive element
in the Epstein–
B
arr virus genome. Molecular
Endocrinology, 5(2), 267–272.
Shahangian, A., Chow, E. K., Tian, X., Kang, J.
R., Ghaffari, A., Liu, S. Y., et al. (2009). Type
I IFNs mediate development of postinfluenza
bacterial pneumonia in mice. Journal of Clini-
cal Investigation, 119(7), 1910–1920.
Siegel, R. E. (1968). Galen’s system of physiol-
ogy and medicine. New York: Karger.
Sloan, E. K., Capitanio, J. P., Tarara, R. P.,
Mendoza, S. P., Mason, W. A., & Cole, S. W.
(2007). Social stress enhances sympathetic
innervation of primate lymph nodes: Mecha-
nisms and implications for viral pathogenesis.
Journal of Neuroscience, 27(33), 8857–8865.
Sloan, E. K., Priceman, S. J., Cox, B. F., Yu, S.,
Pimentel, M. A., Tangkanangnukul, V., et
al. (2010). The sympathetic nervous system
induces a metastatic switch in primary breast
cancer. Cancer Research, 70(18), 7042–7052.
Srivastava, S., Tamir, M., McGonigal, K. M.,
John, O. P., & Gross, J. J. (2009). The social
costs of emotional suppression: A prospective
study of the transition to college. Journal of
Personality and Social Psychology, 96(4),
883 –897.
Sterling, P. (2004). Principles of allostasis: Opti-
mal design, predictive regulation, pathophysi-
ology and rational therapeutics. In J. Schulkin
(Ed.), Allostasis, homeostasis, and the costs of
physiological adaptation (pp.
1
7–64). Cam-
bridge, UK: Cambridge University Press.
Thaker, P. H., Han, L. Y., Kamat, A. A., Arevalo,
J. M., Takahashi, R., Lu, C., et al. (2006).
Chronic stress promotes tumor growth and
angiogenesis in a mouse model of ovarian car-
cinoma. Nature Medicine, 12(8), 939–944.
Tian, X., Xu, F., Lung, W. Y., Meyerson, C.,
Ghaffari, A. A., Cheng, G., et al. (2012). Poly
I:C enhances susceptibility to secondary pul-
monary infections by Gram-
p
ositive bacteria.
PLoS ONE, 7(9), e41879.
Tung, J., Barreiro, L. B., Johnson, Z. P., Han-
sen, K. D., Michopoulos, V., Toufexis, D., et
al. (2012). Social environment is associated
with gene regulatory variation in the rhesus
macaque immune system. Proceedings of the
National Academy of Sciences USA, 109(17),
6490–6495.
Wagner, D. D., & Heatherton, T. F. (this volume,
2014). Emotion and self-
r
egulation failure. In
J. J. Gross (Ed.), Handbook of emotion reg-
ulation (2nd ed., pp.
613–628). New York:
Guilford Press.
Weaver, I. C., Meaney, M. J., & Szyf, M. (2006).
Maternal care effects on the hippocampal
transcriptome and anxiety-
mediated behav-
iors in the offspring that are reversible in
adulthood. Proceedings of the National Acad-
emy of Sciences USA, 103(9), 3480–3485.
Weinberger, D. A., Schwartz, G. E., & Davidson,
R. E. (1979). Low anxious, high anxious, and
repressive coping styles: Psychometric patterns
and physiological responses to stress. Journal
of Abnormal Psychology, 88, 369–380.
Wilson, E. O. (2012). The social conquest of
Earth. New York: Liveright/Norton.
Wohleb, E. S., Hanke, M. L., Corona, A. W.,
Powell, N. D., Stiner, L. M., Bailey, M. T., et al.
(2011). Beta-
a
drenergic receptor antagonism
prevents anxiety-
like behavior and microglial
reactivity induced by repeated social defeat.
Journal of Neuroscience, 31(17), 6277–6288.
Yang, E. V., Webster Marketon, J. I., Chen, M.,
Lo, K. W., Kim, S. J., & Glaser, R. (2010).
Glucocorticoids activate Epstein–
B
arr virus
lytic replication through the upregulation
of immediate early BZLF1 gene expression.
Brain, Behavior, and Immunity, 24(7), 1089–
1096.

586
Health disparities—that is, differences in
disease outcomes by socioeconomic sta-
tus (SES)—remain one of the most press-
ing public health issues in our society. For
example, low-SES individuals— meaning
those who are low in education, income,
or occupational status—are 2.7 times more
likely to have repeated hospitalizations dur-
ing a 1-year period than high-SES individu-
als (National Center for Health Statistics,
2010), and 3.5 times more likely to suf-
fer activity limitations due to disease than
high-SES individuals (Braveman, Cubbin,
Egerter, Williams, & Pamuk, 2010). And by
age 25, those from the lowest SES group are
expected to live 6 fewer years compared to
those in the highest SES group (Braveman et
al., 2010).
This issue has become such a widespread
concern that Healthy People 2010, the
national health objectives from the U.S.
Department of Health and Human Services
(2000), lists eliminating health disparities as
one of two overarching goals. In addition,
the National Institutes of Health (NIH)
ranked the issue of health disparities third
among its top five priorities (Thomson,
Mitchell, & Williams, 2006).
Explanations for why health dispari-
ties are so pervasive have been difficult to
unearth, because commonly suggested fac-
tors, such as access to health care, have
not sufficiently explained existing dispari-
ties (Adler, Boyce, Chesney, Folkman, &
Syme, 1993). In addition, there is growing
consensus that social, and not just biomedi-
cal, determinants of disease are important
to identify (Dankwa- Mullan et al., 2010).
In this chapter, we explore the role that
emotion regulation may play in explaining
health disparities that emerge early in life.
We do this by first providing an overview
of links between childhood SES and disease
outcomes into adulthood. Second, we exam-
ine whether emotion regulation may serve
as one explanation for these associations by
discussing links between emotion regulation
and physiological processes implicated in
disease, as well as meditational evidence for
emotion regulation strategies in relationships
between low-SES and these physiological
processes. Third, we discuss the question of
moderation—that is, whether certain types
of emotion regulation strategies could also
serve as protective buffers for a subgroup of
those who are low in SES. Throughout this
chapter, our premise is that low early life
SES fosters certain emotion regulation strat-
egies that emerge during childhood and have
implications for physiological processes dur-
ing childhood and into adulthood; hence,
we discuss findings that provide potential
CHAPTER 34
Early‑Life Socioeconomic Status,
Emotion Regulation, and the Biological
Mechanisms of Disease across the Lifespan
Edith Chen
Gregory E. Miller

SES, Emotion Regulation, and Mechanisms of Disease 587
explanations for links between childhood
SES and both childhood and adult diseases.
Early Life Environments and Risk
for Disease
We begin by discussing epidemiological evi-
dence that low SES increases risk for disease.
Low SES in childhood confers greater risk
for disease, both throughout childhood and
into adulthood. A number of reviews have
documented that the effects of low SES start
early, and that low SES during childhood
is associated with a number of different
adverse health outcomes, including greater
asthma morbidity, obesity, and injury rates,
and poorer self- and parent- reports of health
(Chen, Matthews, & Boyce, 2002; Good-
man, 1999; Starfield, Riley, Witt, & Rob-
ertson, 2002; Starfield, Robertson, & Wiley,
2002).
In addition, the effects of low SES persist
into adulthood (Miller, Chen, & Parker,
2011). For example, two reviews of the lit-
erature reported that the vast majority of
studies found an increased risk of all-cause
mortality in individuals who grew up in low-
versus high-SES households (Galobardes,
Lynch, & Smith, 2004, 2008). Moreover,
controlling for adult SES did not eliminate
these associations, indicating that some-
thing specific to low SES in childhood con-
fers risk for early mortality. Another review
documented a heightened risk of cardiovas-
cular disease morbidity associated with low
SES in childhood (Galobardes, Shaw, Law-
lor, Lynch, & Smith, 2006). Again, associa-
tions held up after researchers controlled for
adult SES.
These studies are corroborated by quasi-
experimental evidence, such as the viral
challenge paradigm in humans (Cohen,
Doyle, Turner, Alper, & Skoner, 2004), in
which a sample of adults was quarantined
and exposed to rhinoviruses that cause
colds. Participants who came from low-SES
households in childhood were significantly
more likely to become infected with the rhi-
novirus and to develop cold symptoms com-
pared to those who came from high child-
hood SES households. These associations
held even after researchers controlled for
adult SES, suggesting again that experienc-
ing low SES specifically during the child-
hood years increases risk for adverse health
outcomes later in life.
In summary, a large body of epidemiolog-
ical evidence demonstrates that low SES dur-
ing childhood is associated with a variety of
poor health outcomes both during childhood
and into adulthood. In the next section, we
explore the idea that one psychological fac-
tor contributing to this association may be
difficulties with emotion regulation.
The Role of Emotion Regulation as
a Pathway Linking SES and Disease
In this section, we discuss the idea that
emotion regulation may serve as one psy-
chological pathway linking SES and dis-
ease outcomes. To make this argument, we
(1) provide a brief overview of associations
between SES and emotion regulation; (2)
discuss what types of emotion regulation
strategies are relevant to physiological out-
comes; (3) discuss physiological markers rel-
evant to disease; (4) provide an overview of
previous research on links between emotion
regulation and physiological outcomes; and
(5) describe studies that have tested emotion
regulation as a mediator of SES and physiol-
ogy relationships.
SES and Emotion Regulation
Emotion regulation refers to strategies to
increase, decrease, or maintain emotional
responses (Gross, 2001). Gross’s process
model of emotion regulation states that there
are various types of emotion regulation strat-
egies, including antecedent- focused emo-
tion regulation, that is, strategies employed
before emotional responses become fully
activated, and response- focused emotion
regulation, that is, strategies employed after
emotion response tendencies have been acti-
vated (Gross, 1998).
In the context of SES, extensive evidence
documents that low-SES individuals are
more prone to experience negative emotions,
and in turn that these negative emotions
are detrimental for health (for a review, see
Gallo & Matthews, 2003; but note that
the evidence of negative emotions actually
serving as a mediator of the SES–health
relationship is mixed; Matthews & Gallo,
2011). Nonetheless, in this context, effective
588 HEALTH IMPLICATIONS
emotion regulation strategies should reduce
experiences of negative emotions.
Implications of Emotion
Regulation Strategies
for Physiological Responses
Gross (1998) has documented that efforts
that fall under antecedent- focused emotion
regulation, such as reappraisal, have fewer
physiological costs than response- focused
emotion regulation strategies, such as sup-
pression. Hence, in the next sections, we
focus on the role that antecedent- focused
emotion regulation plays in linking SES to
physiological and health outcomes.
Antecedent- focused emotion regulation
involves strategies such as reappraisal—that
is, reevaluating a stressful situation in a way
that seeks to reduce its emotional impact. It
can also include strategies such as situation
selection, situation modification, and atten-
tion deployment; together with reappraisal,
all of these strategies occur temporally before
emotional responses are generated and can
alter behavioral, emotional, and physiologi-
cal response tendencies. Reappraisal is the
strategy that has been studied most fre-
quently with respect to affective, cognitive,
and social consequences (Gross, 2001), so in
the section below we focus below largely on
links between reappraisal and physiological
processes implicated in disease.
Relevant Physiological Markers
for Disease
Conceptualizing pathways to disease on
the biological end entails consideration of
both acute and longer-term physiological
responses. Below we provide a brief over-
view of the types of systems and processes
implicated in chronic diseases—with a focus
on diseases linked to inflammation, such as
cardiovascular disease and asthma, so that
readers will be familiar with the outcomes
we present later on in studies of emotion reg-
ulation and physiological processes. In this
section, we focus on physiological systems
that are capable of being altered by psycho-
social factors (e.g., stress) and hence could
be plausibly linked to variables such as emo-
tion regulation.
Acutely the hypothalamic– pituitary–
adrenal (HPA) axis and the sympathetic
nervous system (SNS) become activated with
many psychosocial stressors, releasing hor-
mones such as cortisol, epinephrine, and nor-
epinephrine (Cannon, 1932; Kemeny, 2003).
These hormones bind to receptors located on
a variety of bodily tissues, exerting effects
on the heart, vasculature, and metabolic
and immune systems. With respect to acute
physiological responses, profiles indicative
of lower disease risk include a reduced mag-
nitude of reactivity of these systems and/or
a quicker recovery time (quicker return to
baseline levels) (Krantz & Manuck, 1984;
Linden, Earle, Gerin, & Christenfeld, 1997;
Linden, Gerin, & Davidson, 2003; McE-
wen, 1998; Schwartz et al., 2003). We note
that much of the literature reviewed below
does not directly measure SNS and HPA
activity, but instead focuses on the responses
of end organs such as the heart and blood
vessels. However, because these organs are
influenced by SNS and HPA activity, and are
the source of eventual manifestations of car-
diovascular disease (CVD), their responses
to acute stressors are relevant here.
Over the long term, with excessive and
prolonged exposure to the hormones men-
tioned earlier, the structure and function of
tissues and organs are thought to be altered,
giving rise to pathogenic processes that drive
CVD, such as obesity, insulin resistance,
systemic inflammation, high blood pressure,
endothelial dysfunction, and platelet activa-
tion (Brotman, Golden, & Wittstein, 2007;
Everson-Rose & Lewis, 2005; Rozanski,
Blumenthal, Davidson, Saab, & Kubzansky,
2005). Hence, we also review links between
emotion regulation strategies and these lon-
ger-term mechanisms.
Longer-term cumulative physiological
risk has sometimes been encapsulated in
concepts such as allostatic load (McEwen,
1998), which is defined as instances when
individuals experience stressors repeatedly
and have more frequent activation of physi-
ological systems over time; or when physi-
ological systems do not show adaptation of
responses after repeated stressors; or when
shutdown mechanisms are delayed or insuf-
ficient, leading to prolonged physiological
responses over time. The strain on these
allostatic systems over years may eventu-
ally cause a breakdown of these systems that
ultimately leads to disease. Empirically, high
levels of obesity, insulin resistance, systemic
SES, Emotion Regulation, and Mechanisms of Disease 589
inflammation, blood pressure, endothelial
dysfunction, and platelet activation all pre-
dict CVD morbidity and mortality (Danesh
et al., 2005; Guh et al., 2009; Lindmark,
Diderholm, Wallentin, & Siegbahn, 2001;
Ridker, Hennedens, Buring, & Rifai, 2000;
Vasan et al., 2001; Yeboah et al., 2009). In
addition, the accumulation of these charac-
teristics, in constellations such as allostatic
load or metabolic syndrome, predicts even
more strongly an increased risk of CVD
later in life (Seeman, Singer, Rowe, Hor-
witz, & McEwen, 1997; Lakka et al., 2002;
Morrison, Friedman, & Gray-McGuire,
2007; Ridker, Buring, Cook, & Rifai, 2003;
National Cholesterol Education Program,
2002).
Emotion Regulation and Physiological
and Disease Outcomes
We focus here on links specifically between
the emotion regulation strategy of reap-
praisal and physiological responses, given
the existing evidence with respect to this
strategy. Physiologically, reappraisals reduce
cardiovascular reactivity to acute stressors.
For example, lower reappraisals of threat
have been associated with reduced blood
pressure reactivity during acute stressors
in both children and adults (El Sheikh &
Harger, 2001; Maier, Waldstein, & Syn-
owski, 2003), and lower ambulatory blood
pressure during daily life social interac-
tions (Chen, Matthews, & Zhou, 2007).
Similarly, individuals high in the ability to
reappraise stressful situations show reduced
vascular reactivity during acutely stress-
ful tasks (Mauss, Cook, Chang, & Gross,
2007), and lower blood pressure and corti-
sol responses to an acute stressor (Salovey,
Stroud, Woolery, & Epel, 2002). Experi-
mental evidence shows that interventions
aimed at changing appraisals in patient pop-
ulations produce increases in benefit find-
ing, as well as decreases in serum cortisol
levels from pre- to postintervention (Cruess
et al., 2000). Finally, consistent with the
idea that underlying positive beliefs about
others shape reappraisals and physiological
responses, those who believe that the world
is fair (high in just world beliefs) reappraise
an acute stressor as less threatening and
show less vascular reactivity to the stressor
(Tomaka & Blascovich, 1994).
Emotion regulation also mitigates longer-
term pathogenic processes implicated in
CVD. For example, better emotion regula-
tion abilities are linked to lower allostatic
load, including higher high- density lipo-
protein (HDL) cholesterol, lower triglycer-
ides, and lower basal systolic blood pres-
sure (Kinnunen, Kokkonen, Kaprio, &
Pulkkinen, 2005). Similarly, reappraisals
have been linked to longer-term markers
of immune processes. For example, HIV-
positive individuals who reported finding
benefit after experiencing a major negative
life event showed slower declines in cluster
of differentiation 4 (CD4) T cell levels over
2–3 years (indicating a slower progression
to the diagnosis of AIDS) (Bower, Kemeny,
Taylor, & Fahey, 1998). Finally, functional
indicators of poor emotion regulation, such
as the experience of high levels of depres-
sion and anger, have been associated with
higher levels of systemic inflammatory
markers that are implicated in CVD, such as
interleukin-6 (IL-6) and C-reactive protein
(CRP) (Kiecolt- Glaser, McGuire, Robles, &
Glaser, 2002; Miller, Maletic, & Raison,
2009).
Finally, emotion regulation strategies can
also alter clinical disease outcomes. Reap-
praisals such as finding benefit after a life-
threatening event predicts a lower likelihood
of having a future heart attack (Affleck, Ten-
nen, Croog, & Levine, 1987). Conversely,
the experience of high levels of negative
affect (arguably an indicator of inadequate
emotion regulation) has robust associations
with CVD-related outcomes (Brosschot,
Gerin, & Thayer, 2006; Everson-Rose &
Lewis, 2005; Krantz & McCeney, 2002;
Kubzansky, Kawachi, Weiss, & Sparrow,
1998). In addition, how effectively one can
manage emotions (emotional intelligence)
is linked to better general indicators of
physical health (better self- reported health
and fewer illnesses; Goldman, Kraemer,
& Salovey, 1996; Schutte, Malouff, Thor-
steinsson, Bhullar, & Rooke, 2007). Finally,
experimental data suggest that interventions
to help individuals process negative emo-
tions effectively, such as through written dis-
closure, produce fewer symptoms and health
center visits in healthy adults, and improve
disease indicators in patient populations
(Smyth, Stone, Hurewitz, & Kaell, 1999;
Smyth, 1998).

590 HEALTH IMPLICATIONS
Emotion Regulation as a Mediator
The previously discussed literature links
emotion regulation strategies to physiologi-
cal, endocrine, and immune processes impli-
cated in chronic diseases such as CVD. But
is there any evidence that emotion regulation
strategies actually mediate the relationship
between SES and these biological processes?
In a series of studies, our research group has
shown evidence for such mediation by focus-
ing on how children and adolescents reap-
praise stressful life situations.
In healthy adolescents, we have shown
that lower SES is associated with greater
cardiovascular reactivity to acute laboratory
stressors. We further showed that low-SES
adolescents were less likely to reappraise
ambiguous life situations (e.g., shopping
with an overly attentive sales clerk nearby)
in benign ways. Finally, we documented
that reappraisals of threat statistically medi-
ated the relationship between low SES and
heightened cardiovascular reactivity (Chen,
Langer, Raphaelson, & Matthews, 2004).
In patient populations, we have docu-
mented similar patterns with disease-
relevant markers. For example, in pediatric
patients with asthma, we have demonstrated
that low SES is associated with greater
asthma inflammation (e.g., greater produc-
tion of cytokines relevant to asthma, higher
eosinophil counts). We further demonstrated
that low SES is associated with being less
likely to reappraise ambiguous life situations
in benign ways in this patient population.
Finally, we documented that reappraisals of
threat statistically mediated the relationship
between low SES and heightened asthma
inflammation (Chen, Fisher, Bacharier, &
Strunk, 2003; Chen et al., 2006).
We further documented that effects of low
SES can be seen at the genomic level. Low
SES children with asthma showed indica-
tions of increased activity of proinflamma-
tory gene networks, and these associations
between low SES and gene expression pat-
terns were no longer significant once reap-
praisals of threat during ambiguous life situ-
ations were statistically controlled (Chen et
al., 2009).
Taken together, this set of studies illus-
trates how low-SES individuals are, on aver-
age, less able to engage in reappraisals of
life situations effectively. Furthermore, reap-
praisals of threat form one pathway explain-
ing why low-SES children show heightened
inflammatory and cardiovascular responses.
In turn, these inflammatory and cardiovas-
cular profiles are predictive of later disease.
The Role of Emotion Regulation
as Buffer
In the previous section we discussed how
emotion regulation strategies serve as one
psychological mediator explaining links
between low SES and detrimental profiles of
physiological processes relevant to disease.
In this section, we turn to the question of
whether emotion regulation could also serve
as a moderator of SES and health outcomes.
Despite the robust associations between low
SES and disease, there remains a subset of
individuals that displays physiologically
healthy profiles despite living under adver-
sity. What can explain this group of individ-
uals? That is, what factors might naturally
protect individuals who grow up in low-SES
environments from the physiological toll
and accumulation of health problems typi-
cally exacted by these environments? Our
research group has articulated a theory
about the psychological characteristics that
may be specifically beneficial to low-SES
individuals (Chen & Miller, 2012). Emotion
regulation plays a key role in this theory;
hence we discuss its role as a buffer for low-
SES individuals in this chapter. In this sec-
tion, we first provide an outline of the shift-
and- persist theory; then we describe the
empirical evidence in support of this theory.
Shift and Persist
The theory begins with the notion that a
lifetime of facing constraints with limited
options leads those living in a low-SES con-
text to place value on the ability to adjust in
response to stressors through emotion regu-
lation strategies such as reappraisals (shift-
ing). At the same time, in this context, suc-
cessful adaptation entails enduring adversity
with strength by finding meaning in difficult
situations and maintaining optimism in the
face of adversity (persisting). We proposed
that this combination of approaches to
dealing with adversity reduces physiologi-
cal responses to stressful situations acutely,
specifically among those who are low in
SES, and over the long term mitigates the
SES, Emotion Regulation, and Mechanisms of Disease 591
progression of pathogenic processes leading
to chronic diseases such as CVD (Chen &
Miller, 2012).
Hence, one of the key components of
this beneficial psychological profile centers
around the ability to regulate one’s emo-
tions through reappraisal strategies. Because
low-SES individuals on average have fewer
opportunities to select or modify their life
situations (alternative forms of emotion regu-
lation; Gross, 1998, 2001), reappraisals rep-
resent a realistic approach to emotion regula-
tion in this group. That is, given the myriad
day-to-day, largely uncontrollable stressors
experienced by many low-SES individuals,
in many instances their best option may be
to control the one thing they can—the self—
rather than engage in what may turn out to
be futile attempts to control their environ-
ment. By controlling the self, they engage in
emotion regulation strategies in which they
accept that a stressor has occurred and try to
change the effect that stressor has on them.
They do this by reappraising the meaning of
an event, so that the implications for their
lives become less negative. And they adjust
their emotional reactions, so that the event
evokes less distress in them. As they come to
see events as having less serious implications
and being less upsetting, the physiological
responses they elicit are mitigated. Hence,
we propose that low-SES individuals uphold
as an ideal the goal of utilizing emotion regu-
lation strategies related to reappraisals when
dealing with stress. The ability to do this suc-
cessfully comprises the “shift” part of our
shift-and- persist model.
Shifting is hypothesized to be neces-
sary but not sufficient for buffering low-
SES individuals from stressors. In addition
to shifting, we hypothesize that it will be
important to persist—that is, to endure
adversity with strength by finding meaning
in life and maintaining optimism about the
future. Finding meaning allows individuals
to understand adversity and to grow from
it. Optimism allows individuals to maintain
hope about the future, and can be essentially
thought of as reappraising the future (as
opposed to shifting, which entails reapprais-
als of events that have already happened).
Thus, reappraisals are an important compo-
nent of both shifting and persisting.
We further postulate that there is some-
thing important about the combination—
that is, it is not sufficient to be able to engage
in emotion regulation to deal with current
adversities; one also needs also to find
broader meaning in life and be able to reap-
praise the future. Hence the label that we
use, “shift and persist,” is intended to con-
note the fact that it is this combination of
characteristics that will be beneficial to low-
SES individuals with respect to their health.
In the next section, we discuss evidence sup-
porting the notion that when low-SES indi-
vidual engage in shift and persist, there are
benefits to the physiological mechanisms
that underlie disease.
Empirical Evidence for Shift
and Persist
In two studies from our research group, we
have documented the benefits of shift and
persist specifically for low-SES individuals.
In the first study, we assessed childhood
SES in a national sample of adults. We mea-
sured cumulative physiological risk via allo-
static load, based on 24 different measures
across seven physiological systems. Shift
and persist was measured using question-
naires probing reappraisal- related coping
styles (shift) and future orientation (persist).
We found a three-way interaction between
childhood SES, shift, and persist in predict-
ing allostatic load in this sample. Breaking
down this three-way interaction revealed
that there was a significant two-way inter-
action between shift and persist in those
from low childhood SES backgrounds, but
no two-way interaction of shift and persist
among those from high childhood SES back-
grounds. The two-way interaction revealed
that those participants with low childhood
SES backgrounds who were high on both
shifting and persisting had the lowest allo-
static load. In contrast, the combination of
shift and persist did not predict allostatic
load among those from high-SES childhood
backgrounds (Chen, Miller, Lachman, Gru-
enewald, & Seeman, 2012).
In a second study, using a clinical sample,
we investigated the effects of shift and per-
sist among children diagnosed with asthma,
using questionnaires that tapped both reap-
praisal styles of coping (for shifting) and
optimism about the future (for persisting).
Among those low in SES, the higher their
shift-and- persist scores, the lower their
asthma inflammation. Also, among low-SES
children, higher shift-and- persist scores pro-

592 HEALTH IMPLICATIONS
spectively predicted less functional impair-
ment (fewer school absences, less rescue
inhaler use) 6 months later, when we con-
trolled for baseline levels. In fact, low-SES
children who scored high on shift and persist
had inflammatory and clinical profiles more
similar to high-SES children with asthma
than to low-SES children who scored low in
shift and persist. Shift and persist was not
related to inflammatory or clinical profiles
in high-SES children with asthma (Chen et
al., 2011).
In summary, we find that low-SES indi-
viduals who are able to engage in emotion
regulation strategies involving reappraisals,
in combination with being optimistic and
future oriented, are the ones who show the
most beneficial physiological profiles and
the least clinical disease impairment. Shift
and persist is only beneficial to those who
are low in SES, not to those who are high
in SES, suggesting that there are context-
specific determinants of what types of strat-
egies will be beneficial physiologically for
whom. In particular, for low-SES individu-
als, engaging in reappraisals and focusing on
the future when encountering current daily
stressors that are largely uncontrollable may
be beneficial. In contrast, for those who are
high in SES, proactive attempts to eliminate
or mitigate stressful situations may be a
more beneficial approach given the greater
resources these individuals tend to have.
In addition, it is important to note that
the combination of shift and persist is criti-
cal to physiological profiles among low-SES
individuals. That is, neither shifting nor per-
sisting alone was predictive of physiological
outcomes; rather, it is only when individuals
combine shifting and persisting that one sees
physiological benefits. This suggests that
emotion regulation strategies of reappraisals
on their own are not sufficient; rather, they
need to be combined with a focus on the
future that emphasizes optimism and mean-
ing in order to derive physiological benefits
among those who are low in SES.
Conclusions
In summary, health disparities are a press-
ing issue in our society, and researchers
have been working to understand what fac-
tors account for such striking differences in
health outcomes across the SES gradient.
One possibility we suggest here is that on
the psychological end, low-SES children may
experience greater difficulties with emotion
regulation. In particular, low-SES children
may find themselves less able to reappraise
stressful situations in positive ways. In turn,
this leads them to be more likely to expe-
rience negative emotions and physiological
costs, both acutely and cumulatively, that
may contribute to risk for disease over the
long term. We note, however, that much of
this work is correlational, and cannot be
used to draw conclusions about causality.
We also document that emotion regula-
tion serves an important function in terms
of buffering low-SES children from detri-
mental physiological profiles. That is, those
low-SES children who are able to engage in
shift and persist (utilizing effective emotion
regulation strategies, such as reappraisals in
combination with persisting with hopes and
finding meaning with respect to one’s future)
exhibit physiological profiles that are more
similar to profiles of high-SES children than
to those of low-SES children who do not
engage in shift and persist. These children
also showed less clinical disease impairment
compared to low-SES children who did not
engage in shift and persist. These findings
suggest that shift and persist serves as a nat-
ural protective factor in low- but not high-
SES children.
Emotion regulation strategies are an
important factor to consider when inves-
tigating individual-level psychological
mechanisms underlying SES disparities in
health. These strategies provide an inter-
face between children and their broader
social environments, and play an important
role in shaping hormonal and inflamma-
tory responses to stress. In turn, these acute
responses appear to have longer-term impli-
cations for the pathogenic processes that
underlie chronic diseases across the lifespan.
References
Adler, N. E., Boyce, W. T., Chesney, M. A., Folk-
man, S., & Syme, S. L. (1993). Socioeconomic
inequalities in health: No easy solution. Jour-
nal of the American Medical Association 269,
3140–3145.
Affleck, G., Tennen, H., Croog, S., & Levine, S.
SES, Emotion Regulation, and Mechanisms of Disease 593
(1987). Causal attribution, perceived benefits,
and morbidity after a heart attack: An 8-year
study. Journal of Consulting and Clinical Psy-
chology, 55, 29–35.
Bower, J. E., Kemeny, M. E., Taylor, S. E.,
& Fahey, J. L. (1998). Cognitive process-
ing, discovery of meaning, CD4 decline, and
AIDS-related mortality among bereaved HIV-
seropositive men. Journal of Consulting and
Clinical Psychology, 66, 979–986.
Braveman, P. A., Cubbin, C., Egerter, S., Wil-
liams, D. R., & Pamuk, E. (2010). Socioeco-
nomic disparities in health in the United States:
What the patterns tell us. American Journal of
Public Health, 100, S186–S196.
Brosschot, J. F., Gerin, W., & Thayer, J. F.
(2006). The perseverative cognition hypothe-
sis: A review of worry, prolonged stress- related
physiological activation, and health. Journal
of Psychosomatic Research, 60, 113–124.
Brotman, D. J., Golden, S. H., & Wittstein, I. S.
(2007). The cardiovascular toll of stress. Lan-
cet, 370, 1089–1100.
Cannon, W. B. (1932). The wisdom of the body.
New York: Norton.
Chen, E., Fisher, E. B., Jr., Bacharier, L. B., &
Strunk, R. C. (2003). Socioeconomic status,
stress, and immune markers in adolescents
with asthma. Psychosomatic Medicine, 65,
984–992.
Chen, E., Hanson, M. D., Paterson, L. Q., Grif-
fin, M. J., Walker, H. A., & Miller, G. E.
(2006). Socioeconomic status and inflamma-
tory processes in childhood asthma: The role
of psychological stress. Journal of Allergy and
Clinical Immunology, 117, 1014–1020.
Chen, E., Langer, D. A., Raphaelson, Y. E., &
Matthews, K. A. (2004). Socioeconomic sta-
tus and health in adolescents: The role of
stress interpretations. Child Development, 75,
1039–1052.
Chen, E., Matthews, K. A., & Boyce, W. T.
(2002). Socioeconomic differences in chil-
dren’s health: How and why do these relation-
ships change with age? Psychological Bulletin,
128, 295–329.
Chen, E., Matthews, K. A., & Zhou, F. (2007).
Interpretations of ambiguous social situations
and cardiovascular responses in adolescents.
Annals of Behavioral Medicine, 34, 26–36.
Chen, E., & Miller, G. E. (2012). “Shift-and-
persist” strategies: Why being low in socio-
economic status isn’t always bad for health.
Perspectives on Psychological Science, 7,
135–158.
Chen, E., Miller, G. E., Lachman, M. E., Gru-
enewald, T. L., & Seeman, T. E. (2012). Pro-
tective factors for adults from low childhood
socioeconomic circumstances: The benefits of
shift-and- persist for allostatic load. Psychoso-
matic Medicine, 74, 178–186.
Chen, E., Miller, G. E., Walker, H. A., Arevalo,
J. M., Sung, C. Y., & Cole, S. W. (2009).
Genome-wide transcriptional profiling linked
to social class in asthma. Thorax, 64, 38–43.
Chen, E., Strunk, R. C., Trethewey, A., Schreier,
H. M., Maharaj, N., & Miller, G. E. (2011).
Resilience in low- socioeconomic- status chil-
dren with asthma: Adaptations to stress. Jour-
nal of Allergy and Clinical Immunology, 128,
970–976.
Cohen, S., Doyle, W. J., Turner, R. B., Alper,
C. M., & Skoner, D. P. (2004). Childhood
socioeconomic status and host resistance to
infectious illness in adulthood. Psychosomatic
Medicine, 66, 553–558.
Cruess, D. G., Antoni, M. H., McGregor, B. A.,
Kilbourn, K. M., Boyers, A. E., Alferi, S. M.,
et al. (2000). Cognitive- behavioral stress man-
agement reduces serum cortisol by enhancing
benefit finding among women being treated
for early stage breast cancer. Psychosomatic
Medicine, 62, 304–308.
Danesh, J., Lewington, S., Thompson, S. G.,
Lowe, G. D., Collins, R., Kostis, J. B., et al.
(2005). Plasma fibrinogen level and the risk of
major cardiovascular diseases and nonvascu-
lar mortality: An individual participant meta-
analysis. Journal of the American Medical
Association, 294, 1799–1809.
Dankwa- Mullan, I., Rhee, K. B., Williams, K.,
Sanchez, I., Sy, F. S., Stinson, N., et al. (2010).
The science of eliminating health disparities:
Summary and analysis of the NIH Summit
recommendations. American Journal of Pub-
lic Health, 100, S12–S18.
El Sheikh, M., & Harger, J. (2001). Appraisals
of marital conflict and children’s adjustment,
health, and physiological reactivity. Develop-
mental Psychology, 37, 875–885.
Everson-Rose, S. A., & Lewis, T. T. (2005).
Psychosocial factors and cardiovascular dis-
eases. Annual Review of Public Health, 26,
469–500.
Gallo, L. C., & Matthews, K. A. (2003). Under-
standing the association between socioeco-
nomic status and physical health: Do negative
emotions play a role? Psychological Bulletin,
129, 10–51.
Galobardes, B., Lynch, J. W., & Smith, G. D.
594 HEALTH IMPLICATIONS
(2004). Childhood socioeconomic circum-
stances and cause- specific mortality in adult-
hood: Systematic review and interpretation.
Epidemiologic Reviews, 26, 7–21.
Galobardes, B., Lynch, J. W., & Smith, G. D.
(2008). Is the association between childhood
socioeconomic circumstances and cause-
specific mortality established?: Update of a
systematic review. Journal of Epidemiology
and Community Health, 62, 387–390.
Galobardes, B., Shaw, M., Lawlor, D. A., Lynch,
J. W., & Smith, G. D. (2006). Indicators of
socioeconomic position (Part 2). Journal of
Epi demiology and Community Health, 60,
95–101.
Goldman, S. L., Kraemer, D. T., & Salovey, P.
(1996). Beliefs about mood moderate the
relationship of stress to illness and symptom
reporting. Journal of Psychosomatic Research,
41, 115–128.
Goodman, E. (1999). The role of socioeconomic
status gradients in explaining differences in
US adolescents’ health. American Journal of
Public Health, 89, 1522–1528.
Gross, J. J. (1998). The emerging field of emotion
regulation: An integrative review. Review of
General Psychology, 2, 271–299.
Gross, J. J. (1998). Antecedent- and response-
focused emotion regulation: Divergent con-
sequences for experience, expression, and
physiology. Journal of Personality and Social
Psychology, 74, 224–237.
Gross, J. J. (2001). Emotion regulation in adult-
hood: Timing is everything. Current Direc-
tions in Psychological Science, 10, 214–219.
Guh, D. P., Zhang, W., Bansback, N., Amarsi,
Z., Birmingham, C. L., & Anis, A. H. (2009).
The incidence of co- morbidities related to obe-
sity and overweight: A systematic review and
meta- analysis. BMC Public Health, 9, 88.
Kemeny, M. E. (2003). The psychobiology of
stress. Current Directions in Psychological
Science, 12, 124–129.
Kiecolt- Glaser, J. K., McGuire, L., Robles, T.
F., & Glaser, R. (2002). Emotions, morbidity,
and mortality: New perspectives from psycho-
neuroimmunology. Annual Review of Psy-
chology, 53, 83 –107.
Kinnunen, M. L., Kokkonen, M., Kaprio, J.,
& Pulkkinen, L. (2005). The associations of
emotion regulation and dysregulation with the
metabolic syndrome factor. Journal of Psycho-
somatic Research, 58, 513–521.
Krantz, D. S., & Manuck, S. B. (1984). Acute
psychophysiologic reactivity and risk of car-
diovascular disease: A review and method-
ological critique. Psychological Bulletin, 96,
435–464.
Krantz, D. S., & McCeney, M. K. (2002). Effects
of psychological and social factors on organic
disease: A critical assessment of research on
coronary heart disease. Annual Review of
Psychology, 53, 341–369.
Kubzansky, L. D., Kawachi, I., Weiss, S. T., &
Sparrow, D. (1998). Anxiety and coronary
heart disease: A synthesis of epidemiological,
psychological, and experimental evidence.
Annals of Behavioral Medicine, 20, 47–58.
Lakka, H. M., Laaksonen, D. E., Lakka, T. A.,
Niskanen, L. K., Kumpusalo, E., Tuomilehto,
J., et al. (2002). The metabolic syndrome and
total and cardiovascular disease mortality in
middle-aged men. Journal of the American
Medical Association, 288, 2709–2716.
Linden, W., Earle, T. L., Gerin, W., & Christen-
feld, N. (1997). Physiological stress reactivity
and recovery: Conceptual siblings separated at
birth? Journal of Psychosomatic Research, 42,
117–135.
Linden, W., Gerin, W., & Davidson, K. (2003).
Cardiovascular reactivity: Status quo and a
research agenda for the new millennium. Psy-
chosomatic Medicine, 65, 5–8.
Lindmark, E., Diderholm, E., Wallentin, L., &
Siegbahn, A. (2001). Relationship between
interleukin 6 and mortality in patients with
unstable coronary artery disease: Effects of an
early invasive or noninvasive strategy. Journal
of the American Medical Association, 286,
2107–2113.
Maier, K. J., Waldstein, S. R., & Synowski, S.
J. (2003). Relation of cognitive appraisal to
cardiovascular reactivity, affect, and task
engagement. Annals of Behavioral Medicine,
26, 32–41.
Matthews, K. A., & Gallo, L. C. (2011). Psy-
chological perspectives on pathways linking
socioeconomic status and physical health.
Annual Review of Psychology, 62, 501–530.
Mauss, I. B., Cook, C. L., Cheng, J. Y., & Gross,
J. J. (2007). Individual differences in cogni-
tive reappraisal: Experiential and physiologi-
cal responses to an anger provocation. Inter-
national Journal of Psychophysiology, 66,
116–124.
McEwen, B. S. (1998). Protective and damaging
effects of stress mediators. New England Jour-
nal of Medicine, 338, 171–179.
Miller, A. H., Maletic, V., & Raison, C. L.
(2009). Inflammation and its discontents: The
SES, Emotion Regulation, and Mechanisms of Disease 595
role of cytokines in the pathophysiology of
major depression. Biological Psychiatry, 65,
732–741.
Miller, G. E., Chen, E., & Parker, K. J. (2011).
Psychological stress in childhood and suscep-
tibility to the chronic diseases of aging: Mov-
ing toward a model of behavioral and biologi-
cal mechanisms. Psychological Bulletin, 137,
959–997.
Morrison, J. A., Friedman, L. A., & Gray-
McGuire, C. (2007). Metabolic syndrome
in childhood predicts adult cardiovascular
disease 25 years later: The Princeton Lipid
Research Clinics Follow-Up Study. Pediatrics,
120, 340–345.
National Center for Health Statistics. (2010).
Health, United States, 2009. Hyattsville, MD:
Author.
National Cholesterol Education Program.
(2002). Third Report of the Expert Panel on
Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults (Adult Treatment
Panel III). Bethesda, MD: National Heart,
Lung, and Blood Institute.
Ridker, P. M., Buring, J. E., Cook, N. R., &
Rifai, N. (2003). C-reactive protein, the meta-
bolic syndrome, and risk of incident cardio-
vascular events. Circulation, 107, 391–397.
Ridker, P. M., Hennekens, C. H., Buring, J. E.,
& Rifai, N. (2000). C-reactive protein and
other markers of inflammation in the predic-
tion of cardiovascular disease in women. New
England Journal of Medicine, 342, 836–843.
Rozanski, A., Blumenthal, J. A., Davidson, K.
W., Saab, P. G., & Kubzansky, L. (2005). The
epidemiology, pathophysiology, and manage-
ment of psychosocial risk factors in cardiac
practice: The emerging field of behavioral car-
diology. Journal of the American College of
Cardiology, 45, 637–651.
Salovey, P., Stroud, L. R., Woolery, A., & Epel,
E. S. (2002). Perceived emotional intelligence,
stress reactivity, and symptom reports: Fur-
ther explorations using the Trait Meta-Mood
Scale. Psychology and Health, 17, 611– 627.
Schutte, N. S., Malouff, J. M., Thorsteinsson,
E. B., Bhullar, N., & Rooke, S. E. (2007). A
meta- analytic investigation of the relationship
between emotional intelligence and health.
Personality and Individual Differences, 42,
921–933.
Schwartz, A. R., Gerin, W., Davidson, K. W.,
Pickering, T. G., Brosschot, J. F., Thayer, J. F.,
et al. (2003). Toward a causal model of car-
diovascular responses to stress and the devel-
opment of cardiovascular disease. Psychoso-
matic Medicine, 65, 22–35.
Seeman, T. E., Singer, B. H., Rowe, J. W., Hor-
witz, R. I., & McEwen, B. S. (1997). Price
of adaptation— allostatic load and its health
consequences: MacArthur studies of success-
ful aging. Archives of Internal Medicine, 157,
2259–2268.
Smyth, J. M. (1998). Written emotional expres-
sion: Effect sizes, outcome types, and mod-
erating variables. Journal of Consulting and
Clinical Psychology, 66, 174–184.
Smyth, J. M., Stone, A. A., Hurewitz, A., &
Kaell, A. (1999). Effects of writing about
stressful experiences on symptom reduction in
patients with asthma or rheumatoid arthritis:
A randomized trial. Journal of the American
Medical Association, 281, 1304–1309.
Starfield, B., Riley, A. W., Witt, W. P., & Robert-
son, J. (2002). Social class gradients in health
during adolescence. Journal of Epidemiology
and Community Health, 56, 354–361.
Starfield, B., Robertson, J., & Riley, A. W. (2002).
Social class gradients and health in childhood.
Ambulatory Pediatrics, 2, 238–246.
Thomson, G. E., Mitchell, F., & Williams, M.
(2006). Examining the Health Disparities
Research Plan of the National Institutes of
Health: Unfinished business. Washington,
DC: National Academies Press.
Tomaka, J., & Blascovich, J. (1994). Effects of
justice beliefs on cognitive appraisal of and
subjective, physiological, and behavioral
responses to potential stress. Journal of Per-
sonality and Social Psychology, 67, 732–740.
U.S. Department of Health and Human Services.
(2000). Healthy People, 2010: Understanding
and improving health. Washington, DC: U.S.
Government Printing Office.
Vasan, R. S., Larson, M. G., Leip, E. P., Evans,
J. C., O’Donnell, C. J., Kannel, W. B., et al.
(2001). Impact of high- normal blood pressure
on the risk of cardiovascular disease. New
England Journal of Medicine, 345, 1291–
1297.
Yeboah, J., Folsom, A. R., Burke, G. L., Johnson,
C., Polak, J. F., Post, W., et al. (2009). Predic-
tive value of brachial flow- mediated dilation
for incident cardiovascular events in a popu-
lation-based study: The multi- ethnic study of
atherosclerosis. Circulation, 120, 502–509.

596
Cardiovascular disease (CVD) is a leading
cause of morbidity and mortality world-
wide (Mendis, Puska, & Norrving, 2011),
and it is increasingly clear that disease pro-
cesses initiate in childhood (Berenson &
Srnivasan, 2005; Lloyd-Jones et al., 2009;
National Heart, Lung, and Blood Institute,
2007). Poor cardiovascular health is defined
as having a combination of unhealthy levels
of lipids, blood pressure, and glucose, being
overweight or obese, smoking, being sed-
entary, and having an unhealthy diet. This
profile is highly prevalent among middle-
aged adults in the United States (Folsom et
al., 2011; Lloyd-Jones et al., 2010) and once
risk factors are elevated, they are difficult to
ameliorate (Lloyd-Jones et al., 2010). With
the growing awareness that major CVD risk
factors such as atherosclerosis and hyperten-
sion are often identifiable many years before
the disease is fully manifest, the Ameri-
can Heart Association recently revised its
national goals for cardiovascular health pro-
motion to emphasize the identification and
prevention of early life risk factors for CVD
(Lloyd-Jones et al., 2010). As such, consid-
ering CVD from a developmental or life
course perspective may provide new insights
into disease etiology and suggest novel ave-
nues for prevention and intervention efforts.
The notion that emotions are inextrica-
bly linked to heart health has been part of
popular discourse for centuries. Terms such
as “broken heart” and “heartsick” invoke
the well- understood sentiment that while
emotions may emanate from within the
psyche, emotions move the heart to behave
and respond in predictable ways. Moreover,
the idea that the control or regulation of
emotions also matter for heart health has
popularly endured as well, as evidenced
in the opening quotation. However, while
emotions and cardiovascular health have
long been recognized as intertwined, the
exact nature of the relationship is not well
understood. Much epidemiological and psy-
chological work describes cardiovascular
risk in association with negative emotions
(for a review, see Suls & Bunde, 2005),
and a growing body of work also describes
the potentially protective effects of posi-
tive emotions on cardiovascular health (for
CHAPTER 35
Emotion Regulation
and Cardiovascular Disease Risk
Allison A. Appleton
Laura D. Kubzansky
My tongue will tell the anger of my heart,
Or else my heart concealing it will break.
—William ShakeSpeare,
The Taming of the Shrew
(Act 4, Scene 3)
Emotion Regulation and Cardiovascular Disease Risk 597
a review, see Boehm & Kubzansky, 2012).
Despite this accumulation of evidence, key
questions remain, including whether or not
emotions truly cause CVD, or whether spe-
cific emotions are a product of the illness.
Additional questions revolve around under-
standing the different roles of positive and
negative emotion in determining CVD risk.
We suggest that examining CVD risk in
relation to emotion regulation will help to
address these issues. Thus, in this review we
consider what the extant literature tells us
about a relationship between emotion regu-
lation and CVD risk, and identify the poten-
tially harmful and health- promoting impact
of various emotion regulation strategies in
this context.
A large literature indicates that both posi-
tive and negative emotions are relevant in
terms of maintaining cardiovascular health
or developing CVD (Boehm & Kubzan-
sky, 2012; Suls & Bunde, 2005). Moreover,
research has increasingly indicated that
positive emotional functioning entails more
than the absence of emotional distress, and
that physical and mental health may depend
in part on our ability to meet environmen-
tal demands (Kubzansky, Park, Peterson,
Vokonas, & Sparrow, 2011). Drawing on
these findings, investigators have begun
to speculate that beyond the effects of any
specific emotion, it is the regulation of emo-
tion that is critical. Emotion regulation is
a higher order feature of emotional func-
tioning that involves the monitoring and
management of emotional experience and
response (Gross, this volume). Emotion
regulation can be conscious or unconscious
and involve both up- and down- regulation
of positive and negative emotions. Emotion
regulation is learned through socialization
and experience over time, with childhood
being an important period of development
as temperament, biology, and social factors
interact to build regulatory skills and strate-
gies that are then used across the life course
(Calkins & Hill, 2007; John & Gross, 2004;
Rothbart, Sheese, & Posner, and Thompson,
this volume). The research findings linking
emotions and CVD risk, the importance of
early life experience in learning to regulate
emotion, and the recent evidence indicating
that deterioration of cardiovascular health
begins in childhood (Berenson & Srnivasan,
2005), have raised the question of whether
emotion regulation may play an important
role in determining CVD risk over the life
course.
In this chapter, we first consider the labo-
ratory and population-based evidence sug-
gesting that emotion regulation may con-
tribute to CVD risk. The literature linking
emotion regulation to CVD has considered
cardiovascular conditions (e.g., cardiovas-
cular disease, hypertension) and the biologi-
cal markers that are considered indicative of
risk of developing CVD in individuals who
are too young to have developed actual dis-
ease. These include indicators of cardiovas-
cular function and risk that are measured in
response to acute stress, generally obtained
from laboratory-based studies (e.g., blood
pressure reactivity), predisease conditions
and markers of risk (e.g., hypertension,
C-reactive protein, metabolic syndrome),
and actual measures of disease (e.g., myo-
cardial infarction, death). The term cardio-
vascular disease refers to a group of dis-
orders of the heart and blood vessels, and
encompasses both coronary heart disease
and cerebrovascular disease (Mendis et al.,
2011), the two cardiovascular disease out-
comes most commonly considered in rela-
tion to emotion. We review work in these
areas, while limiting our discussion to exem-
plar studies that focus on objectively mea-
sured outcomes. Doing so mitigates a com-
mon concern about these associations that
self- report bias inflates or falsely suggests
that emotional factors are causally related
to health (i.e., more distressed individuals
report more symptoms). Also, we primarily
consider prospective studies that examine
longitudinal data in which emotion regula-
tion is assessed initially among individuals
who are disease-free, prior to development
of CVD. These study designs help to miti-
gate another common concern about these
associations: that health conditions actu-
ally drive emotions rather than the reverse.
We also discuss and apply a developmental
perspective to emotion regulation and CVD
risk associations, and consider the evidence
linking childhood emotion regulation to
CVD risk in adulthood. Finally, we close
with recommendations for future work.
Due to space constraints we do not consider
studies of emotion regulation and health risk
behaviors (e.g., smoking) in detail, although
we note that such behaviors are considered

598 HEALTH IMPLICATIONS
potential pathways through which emotion
regulation may influence CVD, and rec-
ommend these as areas for future research.
Also, while we focus on studies of emotion
regulation in relation to CVD etiology (i.e.,
development of disease), we recognize that
emotion regulation is also likely related to
CVD progression and survival. Finally, it is
important to note that while the effects of
emotion regulation are likely involved in the
pathophysiology of many diseases, the evi-
dence to date is strongest for CVD and, as
such, is reviewed specifically in this chapter.
Evidence Linking Emotion
Regulation to Cardiovascular
Function and Disease
Over the past several decades, the study
of emotion regulation and cardiovascular
function and disease has been taken up by
related but traditionally distinct disciplines
of psychology and epidemiology. Psycholog-
ical laboratory-based and epidemiological
population-based studies have documented
associations between emotion regulation
and cardiovascular function and risk. How-
ever, as each discipline has different goals
and generally relies on different methodolo-
gies, the evidence linking emotion regulation
to CVD has developed largely in two differ-
ent literatures, with limited crossover. Lab-
oratory-based studies of emotion regulation
and cardiovascular function tend to have
small sample sizes and focus on identifying
relevant biological alterations occurring in
association with experimentally manipu-
lated use of regulatory strategies. Epidemio-
logical studies tend to be observational and
employ large population-based samples, and
focus more on the diagnostic and health risk
implications of dysregulated emotion. More-
over, epidemiological work includes studies
that explicitly measure emotion regulation
and test associations with cardiovascular
risk (i.e., direct evidence), but these are lim-
ited in number. However, more numerous
are studies of cardiovascular risk that do
not measure emotion regulation directly but
assess aspects of emotional functioning (e.g.,
negative emotions) that may provide indica-
tion of poor emotion regulation. We include
these studies, because they provide relevant
but indirect evidence of the relationships of
interest. In this section, we bring together
important work from each area, integrat-
ing evidence across disciplines in order to
expand the evidence base and move the sci-
ence forward.
It is important to note that whether the use
of an emotion regulation strategy is adap-
tive or maladaptive is context- dependent.
However, based on the empirical findings to
date, we argue that the predominant use of a
particular set of strategies (e.g., suppression,
inhibition) may carry more cardiovascular
health risks than other strategies (e.g., reap-
praisal, disclosure), even though on occasion
the use of such strategies may well be adap-
tive. Thus, we acknowledge that there may
be contexts in which strategies such as reap-
praisal may not always be beneficial, and
strategies such as suppression may be useful.
The larger point is that effective regulation
(regardless of what regulatory strategy is
used in any given situation) versus emotion
dysregulation may have differential effects
on cardiovascular disease risk.
Laboratory‑Based Research
Laboratory-based work has found emotion
regulation strategies to be associated with
identifiable patterns in cardiovascular func-
tion and response to regulatory demands
that have implications for disease risk (see
also Chen & Miller, this volume). The gen-
eral hypothesis guiding work on emotion
regulation in this context is that failing
to manage emotions effectively requires a
certain degree of psychological and physi-
ological exertion, and is essentially a form
of stress exposure. Thus, such exertion is
thought to exact a physiological cost on the
body, which leads to “wear and tear” and
thereby increases vulnerability to disease
over time (Consedine, Magai, & Bonanno,
2002; Pennebaker & Beall, 1986).
Population-based research tends to focus
on clinically relevant risk markers (typi-
cally measured with resting levels) and inci-
dence of disease. In contrast, laboratory-
based work often focuses on indicators of
cardiovascular function and risk that are
measured in response to acute stress, such
as heart rate and blood pressure reactivity,
pulse transmission time, finger pulse ampli-
tude, and cardiac interbeat interval. Stress-
ful or demanding situations activate the
Emotion Regulation and Cardiovascular Disease Risk 599
hypothalamic– pituitary– adrenal (HPA) axis
and the sympathetic nervous system (SNS),
resulting in a cascade of related hormones
that in turn influence a range of end organ
responses, including heart rate and blood
pressure. High reactivity is typically inferred
by higher heart rate and blood pressure lev-
els, greater increases in sympathetic activa-
tion during acute stress, and slower return to
baseline levels of these parameters during a
recovery period, after the acute stress expe-
rience has ended. Reactivity has also been
measured by examining cortisol response to
stress (a marker of HPA activation) (Chida
& Hamer, 2008). Heart rate variability is
another parameter this work has considered;
at rest, lower levels of heart rate variability
have been linked with increased risk of devel-
oping CVD (Thayer & Lane, 2007), while
reduced heart rate variability in response to
stress or slower return to resting levels have
been linked with autonomic dysregulation
(Cohen et al., 2000). Other experimental
work manipulating emotion regulation has
also considered effects on immune- related
markers or alterations in relevant health
conditions.
Much work considering emotion sup-
pression and inhibition in these settings has
found some support for the idea that sup-
pression may alter acute cardiac response
in ways that could impair cardiovascular
health. For example, in two key studies,
participants were instructed to suppress
emotion expression when watching films
designed to elicit diverse emotions (Gross &
Levenson, 1993, 1997). Compared to con-
trols, those who actively suppressed both
positive (amusement) and negative emotion
(disgust, sadness) experienced significantly
increased activation of the autonomic sys-
tem, as measured by a composite variable of
pulse transit time to the finger, finger pulse
amplitude, pulse transit time to the ear, and
figure temperature. Similar patterns of asso-
ciation have been observed across a range
of physiological and health indicators, with
more reactivity or risk- related outcomes
associated with strategies of suppression
and inhibition (Consedine et al., 2002). One
recent study found divergent associations of
emotion suppression and reappraisal with
heart rate variability in response to an emo-
tion regulation task (Denson, Grisham,
& Moulds, 2011). Among 131 women
instructed to suppress, reappraise, or remain
neutral when viewing an anger- eliciting film,
those in the suppression condition exhibited
significantly reduced heart rate variability
compared with baseline, whereas those in
the reappraisal condition showed increased
heart rate variability. These studies suggest
that actively inhibiting or suppressing emo-
tional expression and experience is associ-
ated with deleterious autonomic function-
ing, which over time may take a toll on the
cardiovascular system.
Other laboratory-based work has consid-
ered whether effects of emotion regulation
strategies over a longer period of time may
be associated with beneficial effects on a
variety of other markers of cardiovascular
health. Many studies have found regula-
tory strategies such as emotional disclosure,
or the ability to discuss or disclose emo-
tions verbally or in writing, to have posi-
tive effects on a range of health outcomes,
although fewer studies have directly assessed
outcomes related to cardiovascular health.
In an early experimental study of trauma
disclosure, Pennebaker and Beall (1986)
instructed disease-free subjects to write
in journals over 4 consecutive days about
either a traumatic experience or a neutral-
control event. Compared to controls, those
who wrote about a trauma and related emo-
tions made significantly fewer health center
visits in the 6 months following the disclo-
sure experiment. Such benefits of emotional
disclosure have been replicated across a
variety of samples and with cardiovascular-
related health outcomes. For example, in a
randomized controlled trial of expressive
writing among 179 individuals who recently
experienced a first-time myocardial infarc-
tion, those randomized to the expressive
writing/emotional disclosure condition had
significantly lower blood pressure, reported
fewer cardiac symptoms, had fewer medi-
cal appointments, and used less medica-
tion than controls 5 months postinterven-
tion (Willmott, Harris, Gellaitry, Cooper,
& Horne, 2011). A recent meta- analysis of
146 experimental disclosure studies among
10,994 participants found a positive and
significant effect (small to moderate in size)
of disclosure specifically on cardiovascular
indicators related to CVD, such as lipids and
inflammation (Frattaroli, 2006). Among the
physiological parameters studied, the stron-
600 HEALTH IMPLICATIONS
gest beneficial effect of emotional disclosure
was evident for specific immune function
parameters. For example, experimental dis-
closure was associated with lower levels of
proinflammatory cytokines and C-reactive
protein, which in turn predict reduced risk
of CVD (Danesh et al., 2004; Pearson et al.,
2003).
While these studies generally suggest
potential divergent effects of inhibitive and
expressive forms of regulatory strategies on
cardiovascular function, other studies do
not find such differences, and suggest that
use of either type of strategy requires physi-
ological exertion. For example, in a study of
190 female college students, women were
instructed to suppress emotion, reappraise
emotion, or react naturally (control con-
dition) when discussing an upsetting film
with another participant. Participants in
both the suppression and reappraisal condi-
tions demonstrated larger increases in heart
rate variability compared to controls (But-
ler, Wilhelm, & Gross, 2006). Some stud-
ies linking emotion regulation with altered
cortisol responses in response to stress in
a laboratory setting have described similar
results whereby suppression and reappraisal
are each related to higher cortisol response
(Lam, Dickerson, Zoccola, & Zaldivar,
2009). However, in other studies reap-
praisal is associated with less cortisol reac-
tivity (Chen & Miller, this volume; Salovey,
Stroud, Woolery, & Epel, 2002). Given that
laboratory-based work explicitly consider-
ing potential divergent effects of these strat-
egies on a unified and comparable set of
cardiovascular- relevant outcomes remains
limited, additional work is needed to ascer-
tain whether and how these acute effects
might be informative in regard to long-term
risk. In addition, prospective work would be
useful to determine whether such patterns of
exertion affect health over time.
Population‑Based Work
Direct Evidence
Though capacity to regulate emotions may
influence cardiovascular health, few have
explicitly examined this question in popu-
lation-based research. While highly prom-
ising, evidence is limited to a handful of
studies, because emotion regulation is not
typically assessed in large-scale studies of
the determinants of CVD. Such epidemio-
logical studies generally consider CVD risk
across a set of related outcomes, including
angina (usually ascertained by self- reported
symptoms), myocardial infarction (usually
ascertained by hospital records and diagnos-
tic procedures), and coronary heart disease
death (ascertained by death certificates).
Studies of CVD may also consider hyper-
tension and stroke as relevant outcomes,
although we know of no epidemiological
studies of directly assessed emotion regula-
tion that have considered these conditions.
Incident disease refers to rate of new cases
developing disease rather than the number
of cases in a population at any given time.
Existing results are highly congruent
with those from laboratory and experimen-
tal work, and emerging evidence suggests
that emotion regulation strategies that fre-
quently involve suppression or inhibition
may increase CVD risk, whereas use of
other regulatory strategies may reduce CVD
risk. For example, one prospective study of
1,122 older male participants considered
the relation between self- regulation and the
development of CVD. Self- regulation was
assessed when men were disease-free, using
items from the Minnesota Multiphasic Per-
sonality Inventory assessing one’s ability to
manage impulses, feelings, and behaviors;
emotion regulation was identified as a cen-
tral feature of this measure of self- regulation.
Exemplary items used to construct the Self-
Regulation scale included “I control my
emotion”; “I am not easily angered”; and
“I am usually calm and not easily upset.”
Compared with men who had the lowest
levels of self- regulation, those with the high-
est levels had 62% reduced risk of experi-
encing a nonfatal myocardial infarction or
coronary heart disease (CHD) death over 13
years of follow-up (Kubzansky et al., 2011).
Moreover, the association appeared to be
additive, because each standard deviation
increase in self- regulation was associated
with a 20% reduced risk of incident angina,
nonfatal myocardial infarction, and CHD
death over the follow-up period. Findings
were maintained after adjusting for known
coronary risk factors, as well as main effects
of positive and negative affect.
Another study identified control over
anger as one mechanism linking anger to
Emotion Regulation and Cardiovascular Disease Risk 601
cardiovascular risk (for a review, see Ded-
ert, Calhoun, Watkins, Sherwood, & Beck-
ham, 2010). In a study of Finnish adults (n =
7,933), several dimensions of anger expres-
sion and control were assessed in relation to
incident CVD events (myocardial infarction,
stroke) over 10–15 years of follow-up (Hauk-
kala, Konttinen, Laatikainen, Kawachi, &
Uutela, 2010). Anger control was character-
ized by items related to the extent to which
anger is regulated (e.g., “I control my tem-
per”), and anger expression was character-
ized by how people generally react when
feeling angry (e.g., “I express my anger”).
While experiences of anger per se were not
associated with CVD, participants report-
ing the lowest levels of anger control had
35% significantly higher risk of experienc-
ing a fatal or nonfatal cardiovascular event
in the subsequent 10–15 years compared to
those with the highest levels of anger control
(Haukkala et al., 2010). These findings were
maintained even after researchers took into
account demographic and coronary risk fac-
tors, as well as depressive symptoms. While
not specifically addressing how the anger is
regulated (e.g., suppressed, situation reap-
praised), this study suggests that the occur-
rence of negative emotions such as anger
may not be sufficient to induce risk. Instead,
it is the failure to regulate the emotion effec-
tively that may help explain anger and CVD
risk associations.
Another study of 181 Finnish men and
women observed cross- sectional associa-
tions of adaptive and maladaptive emotion
regulation with metabolic syndrome (Kin-
nunen, Kokkonen, Kaprio, & Pulkkinen,
2005). Metabolic syndrome, which is char-
acterized by hypertension, elevated lipid
levels, central adiposity (a measure of body
fat distribution), and insulin resistance,
is a well- established risk marker for CVD
(Expert Panel on Detection Evaluation and
Treatment of High Blood Cholesterol in
Adults, 2001). In this study, the authors
examined whether use of certain emotion
regulation strategies including mood repair
(e.g., “I am imagining something nice to
keep my mood up”) and mood mainte-
nance (e.g., “I would not want to change
this mood”) were associated with meta-
bolic syndrome at age 42. The authors also
examined whether emotional ambivalence
(a specific form of emotion dysregulation;
“I try to suppress my anger but would like
to let others know how I feel”) was associ-
ated with midlife metabolic syndrome. The
authors found that mood repair and mood
maintenance were significantly associated
with reduced risk of metabolic syndrome,
whereas emotional ambivalence was associ-
ated with higher risk of metabolic syndrome
at age 42. This study suggests that effective
regulation of emotion may protect cardio-
vascular health, and emotion dysregula-
tion may contribute to CVD risk. However,
this study did not account for prior physi-
cal health or other relevant covariates such
as socioeconomic status that could provide
alternative explanations for the associations
observed.
A recent study by our group also observed
divergent associations of different emo-
tion regulation strategies as assessed by the
Emotion Regulation Questionnaire (Gross
& John, 2003) with C-reactive protein
(CRP). CRP, an inflammatory risk marker
for CVD, is associated with atherosclerosis
and incident coronary events (Danesh et al.,
2004; Pearson et al., 2003). CRP is often
used as a predisease marker of CVD by ref-
erencing a diagnostic cut point identified by
the Centers for Disease Control and Preven-
tion (CDC)/American Heart Association
(Pearson et al., 2003). We examined reap-
praisal (i.e., altering how one thinks about
an emotion- eliciting situation in order to
change its emotional impact) and suppres-
sion (i.e., inhibiting emotional expression
in response to an emotion eliciting event).
Among 379 U.S. adults, an increase of one
standard deviation in reappraisal was signif-
icantly associated with 20% lower odds of
having levels of CRP at or above the CDC/
American Heart Association’s high risk cut
point (Appleton, Buka, Loucks, Gilman, &
Kubzansky, 2013). Conversely, a one stan-
dard deviation increase in suppression was
significantly associated with 44% higher
odds of having systemic inflammation levels
indicating high CVD risk. Similarly, some
preliminary analyses by our research group
using the same sample found reappraisal
and suppression also be patterned differen-
tially with 10-year risk of developing CVD
in midlife (Appleton, Loucks, Buka, & Kub-
zansky, unpublished data). This emerging
body of work provides the first direct pop-
ulation-based evidence that emotion regula-
602 HEALTH IMPLICATIONS
tion may contribute significantly to cardio-
vascular health.
Indirect Evidence
While the evidence base explicitly linking
emotion regulation to CVD in the general
population is small, much indirect evidence
comes from studies of poor emotional func-
tioning and negative affective states, which
are characterized in part by dysregulated
emotion (Kubzansky et al., 2011; Taylor,
Lerner, Sage, Lehman, & Seeman, 2004).
Chronic distress is thought to influence car-
diovascular health by way of repeated and
excessive activation of the stress response
and impaired adaptation, which over time
may contribute to damaging the cardio-
vascular system (McEwen, 2003), and also
risk behaviors associated with poor men-
tal health (e.g., smoking) (Everson-Rose &
Lewis, 2005). Work in this area has pri-
marily focused on three negative affective
states in association with CVD risk: anger,
anxiety, and depression (Chida & Steptoe,
2009; Suls & Bunde, 2005). Though epide-
miological studies often consider depression
and anxiety as representing single emotions,
in fact they reflect complex constellations
of chronic elevations of maladaptive cogni-
tions, behaviors, and emotions, of which
dysregulated emotion is one feature (Laza-
rus, 1991). However, given the importance
of dysregulated emotion in these affective
states, we review studies linking them to
CVD, because we speculate that an emo-
tion regulation perspective could add insight
to understanding their observed associa-
tions. Studies reviewed in this section do
not explicitly measure emotion regulation;
we therefore characterize them as providing
indirect evidence that dysregulated emotion
may contribute to CVD.
In a review of 37 studies examining pro-
spective associations of negative emotions
with incident CHD events in initially healthy
individuals, high anger was associated
with 1.5- to threefold higher risk of CHD;
chronic anxiety was associated with 1.5- to
sevenfold higher risk of CHD; and clinically
relevant levels of depression was associated
with more than a 2.5-fold elevated risk, with
gradations in risk evident according to levels
of depressive symptoms (Kubzansky, 2007).
Posttraumatic stress disorder, a severe form
of distress marked by dysregulated emotion
in response to trauma, has also been linked
to incident cardiovascular events in mili-
tary veteran and community-based civilian
populations (for a review, see Dedert et al.,
2010). Taken together, these studies suggest
significantly increased cardiovascular risk
associated with experiencing chronic nega-
tive emotion and poor emotional function-
ing that likely derives in part from maladap-
tive emotion regulation.
Whereas experiencing chronic negative
emotions may indicate dysregulation, posi-
tive emotional functioning may be consid-
ered a marker of effective emotion regula-
tion. A recent review of studies of positive
psychological well-being and cardiovascu-
lar health identified a consistent protective
effect of positive emotions and psychological
attributes related to more frequent occur-
rence of positive emotions (e.g., optimism);
findings also suggested that positive emo-
tions and related factors are associated with
health- related behaviors that reduce risk of
CVD (e.g., increased physical activity) and
more resilient biological function (e.g., less
systemic inflammation) (Boehm & Kub-
zansky, 2012). The evidence of CVD buff-
ering attributable to positive functioning is
most well established for optimism (Boehm
& Kubzansky, 2012; Peterson & Bossio,
2000). For example, in a study of 97,253
participants of the Women’s Health Initia-
tive, women with high levels of dispositional
optimism had significantly lower risk of inci-
dent CHD and CHD mortality compared
to those with low levels of optimism over
8 years of follow-up, even after controlling
for health risk behaviors, obesity, lipids, and
depressive symptoms (Tindle et al., 2009).
Another domain of healthy psychological
functioning that may promote cardiovascu-
lar health is emotional vitality, which can
be defined as feeling energetic or full of pep,
and having a sense of positive well-being and
emotional self- control characterized in part
by effective emotion regulation (Kubzansky
& Thurston, 2007; Rozanski & Kubzansky,
2005). In a study of 6,025 healthy men and
women ages 25–74 years at baseline, those
with the highest levels of emotional vitality
had 19% reduced risk of developing CHD
over 15 years of follow-up compared to those
with the lowest levels of emotional vital-
ity (Kubzansky & Thurston, 2007). Effects

Emotion Regulation and Cardiovascular Disease Risk 603
were maintained after researchers took into
account known coronary risk factors, as
well as depression and psychological prob-
lems. Taken together, these studies suggest
that positive emotional and psychological
functioning protect cardiovascular health,
providing indirect evidence that effective
emotion regulation may increase cardiovas-
cular resilience.
Mechanisms by Which Emotion
Regulation Influences CVD
Emotion regulation may affect CVD risk
through both physiological and behav-
ioral pathways. As noted earlier, dysregu-
lated emotion is associated with increased
likelihood of engaging in deleterious
cardiovascular- related health behaviors such
as smoking, unhealthy diet, and low physical
activity (Kiecolt- Glaser, McGuire, Robles,
& Glaser, 2002). Emotion dysregulation is
also associated with deteriorative biological
functioning in terms of the development of
obesity (Blaine, 2008), high levels of cho-
lesterol and blood pressure, atherosclerosis,
and inflammation (Appleton, Buka, et al.,
2013; Boehm & Kubzansky, 2012). Labo-
ratory-based studies (see earlier sections for
examples) suggest that effects may occur via
heightened or repeated activation of stress–
response systems, including the HPA and
SNS. Such overactivation has been linked to
a variety of potentially damaging biological
alterations, including hemodynamic forces
such as increased turbulence and shear
stress leading to impaired endothelial func-
tion, and flow- related arterial injury leading
to increased atherogenesis, as well as altered
electrical stability of the heart and increased
inflammation (Kubzansky & Kawachi,
2000). For example, heightened HPA and
SNS activity and resultant stress hormones
(e.g., corticosteroids, catecholamines) initi-
ate an inflammatory response characterized
by the production of a number of inflamma-
tory markers, including CRP (Black & Gar-
butt, 2002). Inflammation is of increased
interest in studies of emotion regulation
and CVD, because it is strongly implicated
in the pathophysiology of CVD (Danesh
et al., 2004; Pearson et al., 2003) and has
also been linked with several factors related
to emotion regulation, including depres-
sion (Howren, Lamkin, & Suls, 2009), and
chronic and acute stress (Janicki- Deverts,
Cohen, Matthews, & Cullen, 2008; Steptoe,
Hamer, & Chida, 2007).
Moreover, beyond simply indicating the
absence of deterioration, the ability to regu-
late emotions effectively appears to enhance
cardiovascular health actively. Similar to
mechanisms associated with dysregulation,
effective emotion regulation may promote
cardiovascular health by not only preventing
deteriorative processes but also promoting
restorative health behaviors and biological
functioning. Important to note is that dete-
riorative and restorative processes are not
always on a continuum (for a more detailed
discussion, see Boehm & Kubzansky,
2012). Relevant health- promoting behaviors
include engaging in more opportunities for
rest and restoration (Rozanski & Kubzan-
sky, 2005), frequent consumption of fruits
and vegetables, improved problem solving,
and mobilization of social support (Eisen-
berg, Hofer, & Vaughan, 2007). Whereas
dysregulated emotion may heighten HPA
and SNS activity and initiate a cascade of
physiological risks for CVD, effectively
regulated emotion may prevent such activ-
ity and further enhance healthy cardiovas-
cular functioning, although few studies have
directly tested this hypothesis. In one of
the few empirical tests conducted, a recent
study (reviewed earlier) found support for
this hypothesis by demonstrating that reap-
praisal was associated with lower inflam-
matory risk and suppression was associated
with elevated inflammatory risk (Appleton,
Buka, et al., 2013). Other forms of healthy
psychological functioning that have been
identified as possible markers of adaptive
emotion regulation (e.g., positive well-being,
optimism) have also been linked to relevant
biological markers, such as slower rates of
progression of carotid atherosclerosis and
better endothelial function (Boehm & Kub-
zansky, 2012; Ikeda et al., 2011).
Important to consider is that emotion
regulation strategies are used at different
points during the emotion- generative pro-
cess (Gross, 2001; Gross & John, 2003;
John & Gross, 2004), and strategies can be
employed at any stage. Thus, it may be that
the effect of the emotion regulatory strategy
on physiological activation, inflammation,
and subsequent CVD risk is due in part to

604 HEALTH IMPLICATIONS
the timing of use of each strategy. For exam-
ple, reappraisal is antecedent- focused, which
means that it is employed before the emo-
tion occurs and involves changing cognitive
appraisals about the situation, which then
prevents or reduces the intensity of nega-
tive emotions (Gross, 2001; Gross & John,
2003; John & Gross, 2004). Therefore,
HPA or SNS dysregulation may be avoided,
potentially leading to lower levels of systemic
inflammation, which in turn may lower
CVD risk. On the other hand, suppres-
sion, a response- focused strategy employed
after the emotion has occurred, involves
the modification of behavioral manifesta-
tions of the emotion (Gross, 2001; Gross &
John, 2003; John & Gross, 2004). Though
individuals may appear outwardly tranquil,
chronic negative emotions and associated
HPA and SNS dysregulation may continue
internally unchecked, ultimately contribut-
ing to increased systemic inflammation and
CVD risk. Moreover, suppression has been
found to require significant mental exer-
tion (Consedine et al., 2002; Gross, 2001).
Thus, the act of suppression may further tax
body systems and contribute to elevations in
systemic inflammation and CVD risk. Emo-
tion regulation theory and research suggest
that regulating the emotion earlier in the
emotion- generative process may be more
effective than doing so in the later, response-
focused stage of the process (Gross, 2001).
As such, while the appropriateness of any
given strategy is context dependent, consis-
tent reliance on response- focused (e.g., sup-
pression) versus antecedent- oriented (e.g.,
reappraisal) regulation may differentially
impact biological processes and long-term
CVD risk.
The Way Forward
The confluence of evidence from laboratory-
and population-based research strongly sug-
gests that the association between emotion
regulation and CVD is not spurious. More-
over, risk and protective associations have
been consistently observed across a variety
of emotion regulation indicators, signify-
ing that emotion dysregulation may increase
CVD risk, whereas effective emotion regu-
lation may lower it. While all the evidence
reviewed thus far has been obtained with
adults during their middle to later adult-
hood, a time when CVD is likely to become
manifest, many studies indicate that loss of
cardiovascular health can begin in childhood
and progress over the life course (Berenson
& Srnivasan, 2005). Studies of emotion
regulation and CVD among adults are ill
equipped to answer important etiological
questions as to when these processes emerge
and begin to exert damaging or health-
promoting effects. Given that emotion regu-
lation strategies are learned over time, with
childhood being a major period of develop-
ment (Calkins & Hill, 2007; John & Gross,
2004), a more explicit consideration of these
relationships over the life course may be
highly fruitful. In this section, we argue that
the way forward in emotion regulation and
CVD research is to move toward framing
and evaluating hypotheses from a develop-
mental or life course perspective. Doing so
may not only answer important scientific
questions but also suggest novel avenues for
the prevention of CVD.
Applying a Developmental Perspective
Children are typically born with many of
the requisite components of ideal cardiovas-
cular health. They generally have healthy
blood pressure, lipid, and glucose levels;
they do not smoke and have the potential
for developing an ideal body weight, and
healthy dietary and physical activity prac-
tices. So how does the loss of cardiovascu-
lar health occur, and when does it begin to
become evident? What role does emotion
regulation play in the loss or promotion of
cardiovascular health over time? We believe
the answers to these questions are related
in part to achieving a key socioemotional
developmental milestone during childhood:
attaining emotion regulation skills.
Emotion regulation is not primarily an
inborn trait. Rather, it is a set of strategies
learned through socialization and expe-
rience over time, with childhood being a
key period of development (John & Gross,
2004). Temperament, maturation, and
social experiences shape core emotion regu-
lation competencies during childhood, with
refinements occurring over the lifespan in
accordance with new experiences (Calkins
& Hill, 2007; John & Gross, 2004; Zeman,
Cassano, Perry- Parrish, & Stegall, 2006).
Emotion Regulation and Cardiovascular Disease Risk 605
This developmental or life course perspective
can help us understand how achieving this
developmental milestone specifically influ-
ences CVD risk in adulthood. For example,
children who develop patterns of dysregu-
lated emotion regulation (e.g., impulsivity,
poor attention, depressed mood) generally
continue to exhibit such patterns through
adolescence and adulthood (Brame, Nagin,
& Tremblay, 2001; Caspi, Moffitt, & New-
man, 1996; Dekker et al., 2007). Thus, as
they age, children with such patterns of reg-
ulation are more likely to initiate and main-
tain a range of risk- related health behaviors
(e.g., cigarette smoking, sedentary behav-
ior, poor diet) that over time may contrib-
ute to disease development and progression
(Shonkoff & Phillips, 2000).
Developmental or life course epidemiol-
ogy is the study of chronic disease risk in
terms of the long-term effects of health-
relevant experiences that occur during gesta-
tion, childhood, adolescence, and adulthood
(Ben- Shlomo & Kuh, 2002). Experiences
can be biological, behavioral, environmen-
tal (physical and social), and psychological
in nature. Emotion regulation can be consid-
ered one such factor that may have positive
or negative health implications over time.
From this perspective, emotion regulation
may affect disease risk mainly in two ways.
First, a latency model suggests that both
adverse and health- promoting experiences
during particular sensitive or critical periods
of development will have lasting effects on
health and functioning, with effects emerg-
ing years or decades after the initial experi-
ence (Ben- Shlomo & Kuh, 2002; Shonkoff,
Boyce, & McEwen, 2009). Such a model
implies that early prevention and interven-
tion are crucial. Second, an accumulation
model specifies that the number and dura-
tion of experiences cumulatively add up to
affect health, again, often with a significant
amount of time passing before the effects
manifest as health outcomes (Ben- Shlomo
& Kuh, 2002; Shonkoff et al., 2009). This
perspective suggests multiple windows of
opportunity for prevention and intervention,
because an accumulation model would sug-
gest that positive and negative factors cumu-
latively build to determine disease resilience
and risk, respectively.
Because both latency and accumulation of
risk explanations are plausible and not mutu-
ally exclusive, it is possible that emotion reg-
ulation skills originating during childhood
shape developing brain architecture and
biological systems, as well as contribute to
behaviors that together and independently
influence later cardiovascular risk. Thus,
developing poor emotion regulation skills
during childhood may have lifelong cardio-
vascular consequences by negatively altering
biological systems during sensitive periods
of development and through accumulated
damage over time. For example, persistent
psychological distress (i.e., dysregulated
emotion) is associated with activation of the
HPA axis (Luppino et al., 2010). Prolonged
HPA activity is thought to up- regulate hor-
mones that influence appetite and promote
weight gain (Luppino et al., 2010). As such,
poor childhood emotion regulation may
alter developing metabolic processes during
a sensitive period of development, which in
turn may increase risk of obesity and other
conditions linked with increased suscep-
tibility to developing CVD (e.g., elevated
inflammation, dyslipidemia) later in life.
Conversely, learning effective emotion regu-
lation skills early in life may prevent such
risk and promote resiliency by helping to
buffer such deleterious stress reactivity and
prevent such damage to biological systems.
Also, psychological distress/dysregulated
emotion is associated with behaviors related
to higher risk of obesity that may emerge
even in childhood (and continue through
adolescence and into adulthood), such as
emotional eating, consumption of calorie-
dense foods, and decreased physical activity
(Blaine, 2008), whereas adaptive emotional
functioning is associated with greater like-
lihood of engaging in health- promoting
behaviors (Boehm & Kubzansky, 2012).
While the American Heart Association’s
national goals for cardiovascular health
promotion emphasize that cardiovascular
risk originates early in life and urge study
of early-life antecedents (Lloyd-Jones et al.,
2010), considering emotion regulation and
CVD risk from a developmental perspec-
tive is highly novel. While there is a grow-
ing literature on child emotion regulation
and adult CVD risk, we do not know how
early in life these processes may be evident,
because research examining emotion regula-
tion and biological markers of CVD risk are
scant. We turn now to the emerging empiri-
606 HEALTH IMPLICATIONS
cal evidence linking childhood emotion reg-
ulation to adult CVD risk.
Evidence Linking Child Emotion
Regulation to Adult CVD Risk
Among the limited set of prospective stud-
ies on associations between child emotion
regulation and adult CVD risk, most have
focused on upstream risk markers for CVD,
such as obesity and inflammation, rather
than on objectively measured clinical end-
points of disease (e.g., myocardial infarc-
tion, stroke). This may be due to (1) limited
availability of prospectively assessed infor-
mation on childhood emotional functioning
in cohort studies that were initiated decades
ago, and (2) the relative youth of participants
in the longitudinal cohorts that have col-
lected information on child emotional func-
tioning and adult health, so that actual CVD
outcomes have not yet developed. Moreover,
most studies assess primarily poor emo-
tional functioning, behavior problems, or
psychological disorders. These conditions
can be considered markers of or proxies for
dysregulated emotion, but they do not pro-
vide direct assessments of emotion regula-
tion. As discussed previously in our review
of studies of depression and anxiety with
CVD risk, we do not conflate poor emotion
regulation with psychopathology. Instead,
we acknowledge that child behavior dis-
orders and emotional functioning reflect a
complex set of factors that include problems
with emotion regulation. Thus, these stud-
ies are suggestive, and we encourage future
work to measure child emotion regulation
explicitly in relation to later cardiovascu-
lar risk. That said, findings from life course
studies of child emotional functioning and
behavior problems, and adult CVD risk are
congruent with associations observed in
studies of adults and suggest that cardiovas-
cular risk may have developmental origins in
child emotion regulation.
For example, while the direction of the
relation between emotional functioning and
obesity is debated and is likely to be bidi-
rectional (Atlantis & Baker, 2008; Luppino
et al., 2010), several studies have found that
poor child emotional functioning contrib-
utes to the development of obesity in adult-
hood (Duarte et al., 2010; Goodwin et al.,
2008; Mamun et al., 2009). Mamun et al., in
a prospective study of 2,278 Australian boys
and girls, found that child behavior prob-
lems (a marker of emotional dysfunction) at
ages 5 and 14 years were associated with 4.6
higher risk (95% confidence interval (CI):
2.36, 9.06) of obesity at age 21, once they
controlled for maternal demographic and
lifestyle variables, child dietary patterns, TV
viewing, family meals, and physical activity.
In a study of 2,209 Finnish boys, Duarte
et al. (2010) found that both moderate and
high levels of conduct problems (which may
also mark severely dysregulated emotion)
at age 8 years were associated with two- to
threefold higher risk of overweight and obe-
sity at ages 18–23, compared to low levels of
conduct problems. These associations were
maintained when the researchers controlled
for hyperactivity and sociodemographic
variables.
Similar findings have been observed in
studies of childhood emotional functioning
and systemic inflammation in adulthood.
For example, in a prospective study of 379
U.S. adults, multiple domains of child emo-
tional functioning (directly assessed by a
study psychologist when participants were
age 7) were examined in association with
CRP levels in middle adulthood. Poor child
self- regulation (i.e., unrestrained, impulsive
behavior) and distress proneness (i.e., emo-
tionally labile, easily frustrated) were each
associated with elevated CRP levels 35 years
later, after researchers controlled for demo-
graphics, being born small for gestational
age, child health status, child body mass
index, and child IQ (Appleton et al., 2011).
Associations were robust, because poor
child emotional functioning was associated
with 2.3- to 3.9-fold greater odds of having
CRP levels at or above the CDC/American
Heart Association’s cut point for being at
high-risk for CVD (Pearson et al., 2003).
Moreover, there was evidence that these
associations were mediated by adult weight
status and modified by early life socioeco-
nomic status (Appleton et al., 2011, 2012).
In other words, poor childhood emotional
functioning increased inflammatory risk
in adulthood by way of higher body mass
index, and such associations were more
robust for children growing up in poorer
environments than for those from higher
level socioeconomic environments (see also
Chen and Miller, this volume, for a more

Emotion Regulation and Cardiovascular Disease Risk 607
detailed discussion of the interrelationships
between socioeconomic status and adversity,
emotion regulation, and health). In a study
of 526 male participants of the Dunedin,
New Zealand cohort, Odgers et al. (2007)
examined childhood conduct problem levels
assessed repeatedly from ages 7–26 years in
association with various physical health out-
comes at age 32, including CVD risk factor
clustering (a composite of factors including
overweight, high blood pressure, and dys-
regulated lipid levels, among others) and
inflammation, as measured by CRP. Com-
pared to those with low levels, boys with
persistently high levels of conduct problems
had 2.9 higher odds (95% CI: 1.3–6.2) of
having high-risk CRP in adulthood. Adult-
hood CVD risk factor clustering was also
higher in those with persistent conduct prob-
lems than in better functioning children, but
the association was not significant (Odgers
et al., 2007).
While measures of behavior problems can
be considered markers of emotion dysregu-
lation, they ultimately provide somewhat
limited insight into the broader relationship
between emotion regulation and CVD risk,
since they measure emotional functioning
at only one end of the spectrum rather than
considering the range of functioning. Few
life course studies have considered whether
adaptive or effective emotion regulation in
childhood is associated with reduced CVD
risk in adulthood. This is a critical gap in the
literature. To address this issue, our research
group has conducted two preliminary stud-
ies that examine the potential cardiovascu-
lar benefit of effective emotion regulation in
childhood.
First, in a prospective study of 377 U.S.
adult men and women, we examined asso-
ciations of effective and poor emotion
functioning, assessed by a study psycholo-
gist when participants were age 7, with the
10-year risk of developing CVD in their 40s
(Appleton, Loucks, Buka, Rimm, & Kub-
zansky, 2013). For women, a one standard
deviation increase in child attention regula-
tion (ability to stay focused, considered an
effective regulatory strategy) was marginally
associated with 8% reduced risk (p = .09) of
developing CVD in the next 10 years, and a
one standard deviation increase in distress
proneness (considered poor functioning) was
significantly associated with 31% higher
risk. For men, no association was observed
for attention regulation and 10-year CVD
risk, but each standard deviation increase
in child distress proneness was significantly
associated with 17% higher risk. These
associations were maintained when we con-
trolled for childhood cardiovascular health,
body mass index, chronic conditions, IQ,
socioeconomic status, being born small for
gestational age, and demographic factors.
Next, in another prospective study within
this sample, we considered the role of atten-
tion regulation in association with the devel-
opment of a favorable cardiovascular health
profile at age 42 (Appleton et al., 2013). This
profile is characterized by low blood pres-
sure, low body mass index, healthy lipid
levels, not smoking, and not having diabetes
(Daviglus et al., 2004; Lloyd-Jones, Dyer,
Wang, Daviglus, & Greenland, 2007). A one
standard deviation increase in child atten-
tion regulation was significantly associated
with 40% higher odds of having favorable
cardiovascular health in midlife. Of note,
this association was maintained when we
adjusted for early life cardiovascular health,
socioeconomic status, and potential psy-
chosocial and behavioral pathway variables
from adulthood. These studies suggest that
earlier acquisition of effective emotion regu-
lation may reduce risk of CVD and promote
early development of healthy cardiovascular
functioning.
Summary and Recommendations
for Future Work
Taken together, the evidence from labo-
ratory- and population-based studies in
adulthood and over the life course suggest
that poor emotion regulation may impair
cardiovascular function and increase risk,
whereas effective emotion regulation may
reduce risk and promote cardiovascular
health. Moreover, evidence is accumulating
that CVD may have developmental origins
in child emotion regulation that influence
trajectories of risk or resilience earlier than
was previously considered. However, as is
evident from this examination of the litera-
ture, research in this area is still limited, and
additional work is needed to address critical
remaining issues. For example, studies that
explicitly measure emotion regulation in
608 HEALTH IMPLICATIONS
conjunction with cardiovascular health and
functioning over time (and across a range of
outcomes) will help to identify when these
processes emerge and exert damaging or
health- promoting effects. In addition, stud-
ies that consider not only dysregulation but
also effective emotion regulation in relation
to CVD outcomes would provide clearer
understanding of the spectrum of effects.
Moreover, epidemiological research should
be informed by experimental findings, and
vice versa, to ensure that causal inferences
are accurate and to guide efficient explo-
ration of how effects may occur. Research
on pathways, behavioral and biological,
also remain limited but could facilitate dis-
tinguishing between latency and cumula-
tive effects, thereby indicating key periods
for targeting prevention and intervention
strategies. In fact, the earlier reported find-
ings that effects of emotion regulation were
modified by socioeconomic status are par-
ticularly intriguing, suggesting that effects
of emotion regulation on health may well
be modifiable (Appleton et al., 2012). It may
be that when more resources are available
to children who are somewhat dysregu-
lated, regulatory capacity can be improved,
thereby leading to better health over the life
course (for related discussion of this issue,
see Chen and Miller, this volume).
We suggest that work in this area is excit-
ing and worth pursuing, because it has a
number of important implications, both for
understanding emotion regulation more gen-
erally and for thinking about it as a lifelong
critical asset. Capacity to regulate emotions
may have more far- reaching effects than
have previously been identified, and timing
of acquisition of this capacity may matter for
physical health and perhaps other outcomes
as well. Thus, capacity to regulate emotion is
important not only as an outcome in its own
right, but also as a determinant of health,
which suggests it could play an important
role in health- related prevention strategies.
True prevention of CVD must involve the
prevention of the development of CVD risk
factors in the first place. This “primordial
prevention” has become increasingly more
interesting in public health and medicine
(Lloyd-Jones et al., 2010). While experts
in cardiovascular health largely acknowl-
edge that childhood is a life stage particu-
larly amenable to CVD prevention, efforts
at modifying risk during this time are not
yet well established. In general, public health
and biomedicine have not focused on the
sensitivity of child emotion development to
help safeguard cardiovascular or other forms
of lifelong health, or prioritized emotional
development for resource allocation as a
way to reduce disease burden in adulthood.
Because we believe research in this area will
continue to indicate the central importance
of developing emotion regulatory capacity,
we suggest that greater resources be dedi-
cated to promoting the acquisition of healthy
emotion regulation strategies throughout
life, with a particular emphasis on early life
stages.
However, to our knowledge, programs
designed to promote the development of
healthy emotion regulation skills among
children in the general population are scant.
Work in this area is generally intervention-
focused rather than prevention- oriented.
Thus, efforts target pediatric populations
with identified mental health problems, with
the aim of building or improving emotion
regulation skills. For example, contextual
emotion regulation therapy, a developmen-
tally based treatment approach for pediat-
ric depression, involves learning to manage
emotional distress by identifying contexts
that elicit maladaptive emotional responses,
then incorporating more adaptive responses
that can be used even in challenging situa-
tions (Kovacs & Lopez-Duran, 2012). This
approach is developmentally focused, which
means that therapeutic approaches are
age- appropriate and tailored to the child’s
developmental stage. Moreover, there are a
number of effective school-based programs
designed to prevent anxiety and promote
emotional resilience in children through
cognitive- behavioral therapy and other
forms of psychotherapy (for a review, see Neil
& Christensen, 2009). Such programs might
provide the basis for thinking about preven-
tion strategies that could be implemented
on a larger scale, either in population-based
studies or with high-risk populations. Thus,
prevention programs could consider incor-
porating these and other skills- building
techniques typically used in traditional
psychological treatments for child emotion
dysregulation (e.g., guided practice in using
adaptive strategies, role playing, paren-
tal involvement) in the design of programs

Emotion Regulation and Cardiovascular Disease Risk 609
to build effective emotion regulation skills
and emotional resilience for children at all
levels of functioning in the general popula-
tion (Kovacs & Lopez-Duran, 2012; Zeman
et al., 2006). With future laboratory- and
population-based studies working from a
developmental perspective, we can continue
to build the evidence base in this area and
inform efforts to enhance adaptive emotion
regulation capacities at a population level.
Doing so will not only improve the emo-
tional health of children but may also pro-
tect their health for a lifetime.
References
Appleton, A. A., Buka, S. L., Loucks, E. B., Gil-
man, S. E., & Kubzansky, L. D. (2013). Diver-
gent associations of adaptive and maladaptive
emotion regulation strategies with inflamma-
tion. Health Psychology, 13, 748–756.
Appleton, A. A., Buka, S. L., Loucks, E. B., Mar-
tin, L. T., & Kubzansky, L. D. (2013). A pro-
spective study of positive early life psychoso-
cial factors and favorable cardiovascular risk
in adulthood. Circulation, 12, 905–912.
Appleton, A. A., Buka, S. L., McCormick, M. C.,
Koenen, K. C., Loucks, E. B., Gilman, S. E.,
et al. (2011). Emotional functioning at age 7
years is associated with C-reactive protein in
middle adulthood. Psychosomatic Medicine,
73(4), 295–303.
Appleton, A. A., Buka, S. L., McCormick, M. C.,
Koenen, K. C., Loucks, E. B., & Kubzansky,
L. D. (2012). The association between child-
hood emotional functioning and adulthood
inflammation is modified by early life socio-
economic status. Health Psychology, 31(4),
413–422.
Appleton, A. A., Loucks, E. B., Buka, S. L.,
Rimm, E., & Kubzansky, L. D. (2013). Child-
hood emotional functioning and the develop-
mental origins of cardiovascular disease risk.
Journal of Epidemiology and Community
Health, 67, 405–411.
Atlantis, E., & Baker, M. (2008). Obesity effects
depression: Systematic review of epidemiologi-
cal studies. International Journal of Obesity,
32, 881–891.
Ben- Shlomo, Y., & Kuh, D. (2002). A life course
approach to chronic disease epidemiology:
Conceptual models, empirical challenges and
interdisciplinary perspectives. International
Journal of Epidemiology, 31, 285–293.
Berenson, G. S., & Srnivasan, S. R. (2005). Car-
diovascular risk factors in youth with impli-
cations for aging: The Bogalusa heart study.
Neurobiology of Aging, 26, 303–307.
Black, P. H., & Garbutt, L. D. (2002). Stress,
inflammation and cardiovascular disease.
Journal of Psychosomatic Research, 52, 1–23.
Blaine, B. (2008). Does depression cause obe-
sity?: A meta- analysis of longitudinal studies
of depression and weight control. Journal of
Health Psychology, 13, 1190–1197.
Boehm, J. K., & Kubzansky, L. D. (2012). The
heart’s content: The association between posi-
tive psychological well-being and cardiovas-
cular health. Psychological Bulletin, 138(4),
655–691.
Brame, B., Nagin, D. S., & Tremblay, R. E.
(2001). Developmental trajectories of physical
aggression from school entry to late adoles-
cence. Journal of Child Psychology and Psy-
chiatry, 42(4), 503–512.
Butler, E. A., Wilhelm, F. H., & Gross, J. J.
(2006). Respiratory sinus arrhythmia, emo-
tion and emotion regulation during a social
interaction. Psychophysiology, 43(6), 612–
622.
Calkins, S. D., & Hill, A. (2007). Caregiver
influences on emerging emotion regulation:
Biological and environmental transactions in
early development. In J. J. Gross (Ed.), Hand-
book of emotion regulation (pp. 229–248).
New York: Guilford Press.
Caspi, A., Moffitt, T. E., & Newman, D. L.
(1996). Behavioral observations at age 3 years
predict adult psychiatric disorders: Longitudi-
nal evidence from a birth cohort. Archives of
General Psychiatry, 53, 1033–1039.
Chen, E., & Miller, G. (this volume, 2014).
Early-life socioeconomic status, emotion regu-
lation, and the biological mechanisms of dis-
ease across the lifespan. In J. J. Gross (Ed.),
Hanbook of emotion regulation (2nd ed.,
pp. 586–595). New York: Guilford Press.
Chida, Y., & Hamer, M. (2008). Chronic psy-
chosocial factors and acute physiological
responses to laboratory- induced stress in
healthy populations: A quantitative review of
30 years of investigations. Psychological Bul-
letin, 134(6), 829–885.
Chida, Y., & Steptoe, A. (2009). The associa-
tion of anger and hostility with future coro-
nary heart disease: A meta- analytic review of
prospective evidence. Journal of the American
College of Cardiology, 53(11), 936–946.
Cohen, H., Benjamin, J., Geva, A. B., Matgar,
610 HEALTH IMPLICATIONS
M. A., Kaplan, Z., & Kotler, M. (2000). Auto-
nomic dysregulation in panic disorder and in
post- traumatic stress disorder: Application of
power spectrum analysis of heart rate variabil-
ity at rest and in response to recollection of
trauma or panic attacks. Psychiatry Research,
25, 1–13.
Consedine, N. S., Magai, C., & Bonanno, G. A.
(2002). Moderators of the emotion inhibition-
health relationship: A review and research
agenda. Review of General Psychology, 6(2),
204–228.
Danesh, D., Wheeler, J. G., Hirschfield, G. M.,
Eda, S., Eiriksdottir, G., Rumley, A., et al.
(2004). C-reactive protein and other circulat-
ing markers of inflammation in the prediction
of coronary heart disease. New England Jour-
nal of Medicine, 350(14), 1387–1397.
Daviglus, M. L., Stamler, J., Pirzada, A., Yan,
L. L., Garside, D. B., Liu, K., et al. (2004).
Favorable cardiovascular risk profile in young
women and long-term risk of cardiovascular
and all-cause mortality. Journal of the Ameri-
can Medical Association, 292(13), 1588–
1592.
Dedert, E. A., Calhoun, P. S., Watkins, L. L.,
Sherwood, A., & Beckham, J. C. (2010). Post-
traumatic stress disorder, cardiovascular, and
metabolic disease: A review of the evidence.
Annals of Behavioral Medicine, 39(1), 61–78.
Dekker, M. C., Ferdinand, R. F., van Lang, N.
D., Bongers, I. L., van der Ende, J., & Verhulst,
F. C. (2007). Developmental trajectories of
depressive symptoms from early childhood to
late adolescence: Gender differences and adult
outcome. Journal of Psychology and Psychia-
try and Allied Disciplines, 48(7), 657–666.
Denson, T. F., Grisham, J. R., & Moulds, M. L.
(2011). Cognitive reappraisal increases heart
rate variability in response to an anger provo-
cation. Motivation and Emotion, 35, 14–22.
Duarte, C. S., Sourander, A., Nikolakaros, G.,
Pihlajamaki, H., Helenius, H., Piha, J., et al.
(2010). Child mental health problems and obe-
sity in early adulthood. Journal of Pediatrics,
156(1), 93 –97.
Eisenberg, N., Hofer, C., & Vaughan, J. (2007).
Effortful control and its socioemotional con-
sequences. In J. J. Gross (Ed.), Handbook of
emotion regulation (pp. 287–306). New York:
Guilford Press.
Everson-Rose, S. A., & Lewis, T. T. (2005). Psy-
chosocial factors and cardiovascular disease.
Annual Review of Public Health, 26, 469–
500.
Expert Panel on Detection Evaluation and Treat-
ment of High Blood Cholesterol in Adults.
(2001). Executive Summary of the Third
Report of the National Cholesterol Education
Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel
III). Journal of the American Medical Asso-
ciation, 285(19), 2486–2497.
Folsom, A. R., Yatsuya, H., Nettleton, J. A.,
Lutsey, P. L., Cushman, M., & Rosamond,
W. (2011). Community prevalence of ideal
cardiovascular health, by the American Heart
Association definition, and relationship with
cardiovascular disease incidence. Journal of
the American Journal of Cardiology, 57(16),
1690–1696.
Frattaroli, J. (2006). Experimental disclosure
and its moderators: A meta- analysis. Psycho-
logical Bulletin, 132(6), 823–865.
Goodwin, R. D., Sourander, A., Duarte, C. S.,
Niemela, S., Multimaki, P., Nikolakaros, G.,
et al. (2008). Do mental health problems in
childhood predict chronic physical conditions
among males in early adulthood?: Evidence
from a community-based prospective study.
Psychological Medicine, 39(2), 301–311.
Gross, J. J. (2001). Emotion regulation in adult-
hood: Timing is everything. Current Direc-
tions in Psychological Science, 10(6), 214–219.
Gross, J. J. (this volume, 2014). Emotion regula-
tion: Conceptual and empirical foundations.
In J. J. Gross (Ed.), Handbook of emotion
regulation (2nd ed., pp. 3–20). New York:
Guilford Press.
Gross, J. J., & John, O. P. (2003). Individual dif-
ferences in two emotion regulation processes:
Implications for affect, relationships and well-
being. Journal of Personality and Social Psy-
chology, 85(2), 348–362.
Gross, J. J., & Levenson, R. W. (1993). Emo-
tion suppression: Physiology, self- report, and
expressive behavior. Journal of Personality
and Social Psychology, 64(6), 970–986.
Gross, J. J., & Levenson, R. W. (1997). Hiding
feelings: The acute effects of inhibiting nega-
tive and positive emotion. Journal of Abnor-
mal Psychology, 106(1), 95–103.
Haukkala, A., Konttinen, H., Laatikainen, T.,
Kawachi, I., & Uutela, A. (2010). Hostility,
anger control, and anger expression as predic-
tors of cardiovascular disease. Psychosomatic
Medicine, 72, 556–562.
Howren, M. B., Lamkin, D. M., & Suls, J. (2009).
Associations of depression with C-reactive
Emotion Regulation and Cardiovascular Disease Risk 611
protein, IL-1, and IL-6: A meta- analysis. Psy-
chosomatic Medicine, 71, 171–186.
Ikeda, A., Schwartz, J., Peters, J. L., Fang, S.,
Spiro, A., III, Sparrow, D., et al. (2011). Opti-
mism in relation to inflammation and endo-
thelial dysfunction in older men: The VA Nor-
mative Aging Study. Psychosomatic Medicine,
73(8), 664–671.
Janicki- Deverts, D., Cohen, S., Matthews, K. A.,
& Cullen, M. R. (2008). History of unemploy-
ment predicts future elevations in C-reactive
protein among male participants in the Cor-
onary Artery Risk Development in Young
Adults (CARDIA) study. Annals of Behavioral
Medicine, 36, 176–185.
John, O. P., & Gross, J. J. (2004). Healthy and
unhealthy emotion regulation: Personality
processes, individual differences, and life span
development. Journal of Personality, 72(6),
1301–1334.
Kiecolt- Glaser, J. K., McGuire, L., Robles, T.
F., & Glaser, R. (2002). Emotions, morbidity,
and mortality: New perspectives from psycho-
neuroimmunology. Annual Review of Psy-
chology, 53, 87–107.
Kinnunen, M., Kokkonen, M., Kaprio, J., &
Pulkkinen, L. (2005). The associations of
emotion regulation and dysregulation with the
metabolic syndrome factor. Journal of Psycho-
somatic Research, 58, 513–521.
Kovacs, M., & Lopez-Duran, N. L. (2012). Con-
textual emotion regulation therapy: A devel-
opmentally based intervention for pediatric
depression. Child and Adolescent Psychiatric
Clinics of North America, 21(2), 327–343.
Kubzansky, L. D. (2007). Sick at heart: The
pathophysiology of negative emotions. Cleve-
land Clinic Journal of Medicine, 74(S1), S67–
S72.
Kubzansky, L. D., & Kawachi, I. (2000). Going
to the heart of the matter: Do negative emo-
tions cause coronary heart disease? Journal of
Psychosomatic Research, 48, 323 –337.
Kubzansky, L. D., Park, N., Peterson, C.,
Vokonas, P., & Sparrow, D. (2011). Healthy
psychological functioning and incident cor-
onary heart disease: The importance of self-
regulation. Archives of General Psychiatry,
68(4), 400–408.
Kubzansky, L. D., & Thurston, R. C. (2007).
Emotional vitality and incident coronary heart
disease: Benefits of healthy psychological
functioning. Archives of General Psychiatry,
64(12), 1393–1401.
Lam, S., Dickerson, S. S., Zoccola, P. M., &
Zaldivar, F. (2009). Emotion regulation and
cortisol reactivity to a social- evaluative speech
task. Psychoneuroendocrinology, 34, 1335–
1362.
Lazarus, R. S. (1991). Emotion and adaptation.
New York: Oxford University Press.
Lloyd-Jones, D. M., Adams, R., Carnethon, M.,
De Simone, G., Ferguson, T. B., Flegal, K., et
al. (2009). Heart disease and stroke statistics
2009 update: A report from the American
Heart Association Statistics Committee and
Stroke Statistics Subcommittee. Circulation,
119, e21–e181.
Lloyd-Jones, D. M., Dyer, A. R., Wang, R., Davi-
glus, M. L., & Greenland, P. (2007). Risk fac-
tor burden in middle age and lifetime risks for
cardiovascular and non- cardiovascular death
(Chicago Heart Association Detection Project
in Industry). American Journal of Cardiology,
99, 535–540.
Lloyd-Jones, D. M., Hong, Y., Labarthe, D.,
Mozaffarian, D., Appel, L. J., Van Horn, L., et
al. (2010). Defining and setting national goals
for cardiovascular health promotion and dis-
ease reduction: The American Heart Associa-
tion’s strategic impact goal through 2020 and
beyond. Circulation, 121, 586–613.
Luppino, F. S., de Wit, L. M., Bouvy, P. F., Sti-
jnen, T., Cuijpers, P., Penninx, B. W., & Zit-
man, F. G. (2010). Obesity, overweight, and
depression: A systematic review and meta-
analysis of longitudinal studies. Archives of
General Psychiatry, 67(3), 220–229.
Mamun, A. A., O’Callaghan, M. J., Cramb, S.
M., Najman, J. M., Williams, G. M., & Bor,
W. (2009). Childhood behavioral problems
predict young adults’ BMI and obesity: Evi-
dence from a birth cohort study. Obesity, 17,
761–766.
McEwen, B. S. (2003). Mood disorders and allo-
static load. Biological Psychiatry, 54, 200–
207.
Mendis, S., Puska, P., & Norrving, B. (2011).
Global atlas on cardiovascular disease pre-
vention and control. Geneva: World Health
Organization.
National Heart Lung and Blood Institute.
(2007). Morbidity and mortality: 2007 chart
book on cardiovascular, lung, and blood dis-
eases. Bethesda, MD: Author.
Neil, A. L., & Christensen, H. (2009). Efficacy
and effectiveness of school-based prevention
and early intervention programs for anxiety.
Clinical Psychology Review, 29(3), 208–215.
Odgers, C. L., Caspi, A., Broadbent, J. M.,
612 HEALTH IMPLICATIONS
Dickson, N., Hancox, R. J., Harrington, H.,
et al. (2007). Prediction of differential adult
health burden by conduct problem subtypes
in males. Archives of General Psychiatry, 64,
476–483.
Pearson, T. A., Mensah, G. A., Alexander, R. W.,
Anderson, J. L., Cannon, R .O., Criqui, M.,
et al. (2003). Markers of inflammation and
cardiovascular disease: Application to clini-
cal and public health practice: A statement
for healthcare professionals from the Centers
for Disease Control and Prevention and the
American Heart Association. Circulation,
107, 499–511.
Pennebaker, J. W., & Beall, S. K. (1986). Con-
fronting a traumatic event: Toward an under-
standing of inhibition and disease. Journal of
Abnormal Psychology, 95(3), 274–281.
Peterson, C., & Bossio, L. M. (2000). Optimism
and physical well-being. In E. C. Chang (Ed.),
Optimism and pessimism: Implications for
theory, research, and practice (pp. 127–146).
Washington, DC: American Psychological
Association.
Rothbart, M., Sheese, B. E., & Posner, M. I.
(this volume, 2014). Temperament and emo-
tion regulation. In J. J. Gross (Ed.), Handbook
of emotion regulation (2nd ed., pp. 305–320).
New York: Guilford Press.
Rozanski, A., & Kubzansky, L. D. (2005). Psy-
chologic functioning and physical health: A
paradigm of flexibility. Psychosomatic Medi-
cine, 67(Suppl. 1), S47–S53.
Salovey, P., Stroud, L. R., Woolery, A., & Epel,
E. S. (2002). Perceived emotional intelligence,
stress reactivity, and symptom reports: Fur-
ther explorations using the trait meta-mood
scale. Psychology and Health, 17, 611–627.
Shonkoff, J. P., Boyce, W. T., & McEwen, B.
S. (2009). Neuroscience, molecular biology,
and the childhood roots of health disparities.
Journal of the American Medical Association,
301(21), 2252–2259.
Shonkoff, J. P., & Phillips, D. A. (Eds.). (2000).
From neurons to neighborhoods: The science
of early childhood development. Washington,
DC: National Academy Press.
Steptoe, A., Hamer, M., & Chida, Y. (2007).
The effects of acute psychological stress on
circulating inflammatory factors in humans:
A review and meta- analysis. Brain, Behavior,
and Immunity, 21, 910–912.
Suls, J., & Bunde, J. (2005). Anger, anxiety, and
depression as risk factors for cardiovascular
disease: The problems and implications of
overlapping affective dispositions. Psychologi-
cal Bulletin, 131(2), 260–300.
Taylor, S. E., Lerner, J. S., Sage, R. M., Lehman,
B. J., & Seeman, T. E. (2004). Early envi-
ronment, emotions, responses to stress, and
health. Journal of Personality 72(6), 1365–
1393.
Thayer, J. F., & Lane, R. D. (2007). The role of
vagal function in the risk for cardiovascular
disease and mortality. Biological Psychology,
74(2), 224–242.
Thompson, R. A. (this volume, 2014). Socializa-
tion of emotion and emotion regulation in the
family. In J. J. Gross (Ed.), Handbook of emo-
tion regulation (2nd ed., pp. 173–186). New
York: Guilford Press.
Tindle, H. A., Chang, Y., Kuller, L. H., Man-
son, J. E., Robinson, J. G., Rosal, M. C., et
al. (2009). Optimism, cynical hostility, and
incident coronary heart disease and mortality
in the Women’s Health Initiative. Circulation,
120, 656–662.
Willmott, L., Harris, P., Gellaitry, G., Cooper,
V., & Horne, R. (2011). The effects of expres-
sive writing following first myocardial infarc-
tion: A randomized controlled trial. Health
Psychology, 30(5), 642–650.
Zeman, J., Cassano, M., Perry- Parrish, C., &
Stegall, S. (2006). Emotion regulation in chil-
dren and adolescents. Developmental and
Behavioral Pediatrics, 27(2), 155–168.

613
As most people can attest, conquering vices
and changing bad habits are difficult. Even
when the motivation to change is strong,
self- control failures are common. Subjec-
tively, it often feels as though our capacity
to self- regulate waxes and wanes in the face
of new temptations, changing moods, and
fatigue. Contemporary investigations into
the causes of self- regulation failure have
demonstrated that the ability to self- regulate
can be undermined by a variety of threats
that act by impairing awareness, exhausting
limited resources, or increasing the salience
of temptations. Perhaps the most potent of
these threats is negative affect. When peo-
ple experience emotional distress, be it in
the form of a bad mood, disappointment,
or social rejection, they often find it more
difficult to resist temptations or to sup-
press unwanted impulses and may engage
in various forms of self- defeating behaviors
(for a review, see Baumeister, 1997). How-
ever, the relationship between emotions and
self- control is by no means all one-way; too
much self- regulation over a period of time
can increase emotional reactivity, as well as
impair an individual’s ability to regulate his
or her emotions.
Much like the experience of hunger or
thirst, emotions serve to motivate behavior
and predispose individuals toward certain
actions (e.g., Keltner & Gross, 1999; Lev-
enson, 1994). It has been suggested that one
function of emotion is to indicate whether
individuals are succeeding or failing to meet
their goals (Carver & Scheier, 1990). There-
fore, just as it would be fatally maladaptive
for an organism to have the ability to turn
off thirst, it would be just as detrimental to
be able to completely shut down emotions.
However, there are times when our emo-
tions interfere with our goals or may lead us
to further distress, such as when our mood
causes us to violate social norms (i.e., being
tragically depressed on a first date or over-
joyed at a funeral). Thus, it is often in our
best interests to be able to regulate our affec-
tive states.
In this chapter, we take the view that reg-
ulating emotions relies on much of the same
cognitive and neural machinery as regulating
other responses (e.g., thoughts, behaviors,
impulses) with the understanding that, all
things being equal, emotions are more dif-
ficult to suppress or inhibit. Unlike behav-
iors, thoughts, or cravings, all of which tend
to have a clear target of regulation (e.g., “I
must not grab the cigarette and light it”),
affect is typically more diffuse, with no clear
action to regulate. Indeed, one of the central
findings of research on emotion regulation
is that direct, response- focused attempts to
suppress the outward expression of emotion
are more cognitively taxing and less success-
CHAPTER 36
Emotion and Self‑Regulation Failure
Dylan D. Wagner
Todd F. Heatherton

614 HEALTH IMPLICATIONS
ful than antecedent- focused methods such
as distraction and reappraisal (Richards
& Gross, 2000). Although there may very
well be advantages to considering emotion
regulation as a separate domain (i.e., Gross,
2002), for the present discussion we consider
it fruitful to think of emotion regulation as
another type of self- regulation, thereby ren-
dering it subject to the same vulnerabilities
and threats as other forms of self- control
(see Heatherton & Wagner, 2011).
In the following sections, we examine the
relationship among emotions, emotion regu-
lation, and self- regulation failure. We focus
primarily on intrinsic forms of emotion reg-
ulation, in which individuals regulate their
own internal affective states, rather than
on extrinsic emotion regulation, in which
individuals attempt to influence the emo-
tions of others through affect displays or
other means. Along with an overview of the
mechanisms whereby emotions can derail
self- regulation, we also consider the case of
misregulation, for example, when individu-
als seek out temptations (e.g., food, drugs
or alcohol) as a misguided strategy to repair
their mood or escape from aversive self-
awareness resulting from overindulgence
of these same temptations. In addition, we
review findings that regulation of emotions
exerts a cost on self- regulatory capacity and,
conversely, that engaging in effortful self-
regulation can impair emotion regulation.
Finally, we highlight recent research sug-
gesting that emotional reactivity is increased
when self- regulatory resources are depleted.
The Role of Negative Affect
in Self-Regulation Failure
When dieters, substance abusers, or sexual
offenders are asked to describe their rea-
sons for engaging in harmful behaviors (e.g.,
binge eating, sexual aggression, smoking,
and drug use) they overwhelmingly report
that their actions were triggered by negative
affect (Haedt-Matt & Keel, 2011; Kassel,
Stroud, & Paronis, 2003; Pithers, Kashima,
Cumming, Beal, & Buell, 1988; Sinha,
2007). Among the general population,
negative affect is similarly associated with
impulsive and self- defeating behaviors, such
as alcohol consumption (Witkiewitz & Vil-
larroel, 2009), gambling (Raviv, 1993), risky
sexual behavior (Bousman et al., 2009; Rob-
erts et al., 2012), excessive Internet usage
(LaRose, Lin, & Eastin, 2003), and aggres-
sion (Berkowitz, 1989). Although multiple
emotions can be considered negative or posi-
tive in valence, the extant literature on the
role of affect in self- regulation seldom disso-
ciates them. Here, too, we employ the broad
categories of negative and positive affect,
noting that these necessarily subsume many
different emotion categories.
The role of negative affect in initiating
self- regulation failure has also been stud-
ied in experimental settings using a variety
of affect induction procedures. Generally,
these procedures involve exposure to sad
or aversive stimuli in the form of images,
music, or movie clips. Other commonly used
techniques include writing about negative
life events (i.e., a funeral) or reading a series
of increasingly negative self- referential state-
ments, such as in the Velten (1968) mood
induction procedure. Much of this work has
been conducted within the realm of drug
and alcohol addiction, where negative affect
has long been known to be the most potent
cause of relapse (e.g., Marlatt & J. Gor-
don, 1985). In smokers and other substance
abusers, inducing negative affect in the
laboratory has been shown to increase sub-
stance cravings (Childress et al., 1994; Fox,
Bergquist, Hong, & Sinha, 2007; Tiffany &
Drobes, 1990; Willner & Jones, 1996) and
the intensity of substance use (McKee et al.,
2011) when compared to neutral or positive
mood inductions.
Chronic dieters are another population
that has received considerable attention with
regards to the relationship between nega-
tive affect and self- regulation failure (i.e.,
disinhibited eating). Inducing negative emo-
tional states in dieters increases eating both
in comparison to non- dieters, but also to
dieters in a neutral mood (e.g., Baucom &
Aiken, 1981; Heatherton, Striepe, & Wit-
tenberg, 1998; Herman & Polivy, 1975). For
instance, Heatherton, Herman, and Polivy
(1991) induced negative affect either by giv-
ing participants negative performance feed-
back during a cognitive task or by instruct-
ing them that they would have to prepare a
speech that would be given in the presence
of their peers. In order to measure overeat-
ing, participants were asked to participate
in an unrelated taste-test of various flavors

Emotion and Self‑ Regulation Failure 615
of ice cream, which, unbeknownst to them,
had been weighed prior to the task so that
the amount of ice cream consumed could be
calculated afterwards. In both cases, dieters
ate significantly more ice cream than non-
dysphoric subjects. Moreover, this occurred
even when negative affect was induced by
having participants anticipate a future task
(i.e., public speaking). Similar findings have
been found for social drinkers, in whom fear
of future social evaluations increases alcohol
consumption relative to participants who
were not going to be evaluated (Higgins &
Marlatt, 1975).
Social rejection is another method of
inducing negative affect that has a rich his-
tory of being associated with behavioral
disinhibition, aggression, and violent crimes
(e.g., high school shootings; Leary, Kow-
alski, Smith, & Phillips, 2003). Labora-
tory studies in which subjects are induced
to feel socially excluded have found that
being rejected increases aggression (DeWall,
Twenge, Bushman, Im, & Williams, 2010;
Twenge, Baumeister, Tice, & Stucke, 2001;
Warburton, Williams, & Cairns, 2006)
and reduces the willingness to help others
(Twenge, Baumeister, DeWall, Ciarocco, &
Bartels, 2007). For example, when given the
opportunity to decide how much hot sauce
to administer to a group of people who
had previously been identified as disliking
spicy foods, rejected individuals assigned
four times more hot sauce than nonrejected
peers (Warburton et al., 2006). With respect
to self- regulation failures, social exclusion
has been shown to have the same effects as
other negative affect inductions, leading to
overeating (Baumeister, DeWall, Ciarocco,
& Twenge, 2005; Oaten, Williams, Jones,
& Zadro, 2008) and reduced persistence on
difficult tasks (Baumeister et al., 2005). Thus
it seems that, as with negative affect, social
rejection can lead to self- regulation failure,
although with an added dose of aggression.
Why Does Negative Affect Impair
Self-Control?
So far we have reviewed various lines of
evidence demonstrating that negative affect
can precipitate a variety of maladaptive
behaviors, most of which are indicative of
poor self- control. In the following sections
we consider a number of mechanisms that
have been proposed to explain the disinhib-
iting effects of negative affect. Many of these
mechanisms target specific aspects of self-
regulation, so it is important at this stage to
briefly review some of the core components
involved in theories of self- regulation.
Although the details vary, most models of
self- regulation can be said to deal with three
basic components. The first involves a target
state that is to be attained. This can be a
goal, such as the goal to quit smoking or to
avoid contaminating the palate with cheap
wines, but this may also be a set of standards,
such as rules of conduct (i.e., whenever pos-
sible, avoid drinking and teaching). The
second component involves an awareness of
one’s actions, often referred to as monitor-
ing. In cybernetic models of self- regulation
(e.g., Carver & Scheier, 1981), monitoring
involves comparing current behavior with
the desired goal state and signaling any dis-
crepancy. Monitoring is a particularly vul-
nerable component of self- regulation, as a
failure to monitor ongoing behavior neces-
sarily entails an inability to catch (and there-
fore control) unwanted actions. The final
component is regulation itself. Upon identi-
fying a thought or an emotion that conflicts
with his or her goals, that person must be
capable of implementing a strategy to inhibit
or otherwise disarm the unwanted impulse.
Limited capacity models of self- regulation
(Baumeister & Heatherton, 1996) empha-
size the ways in which this component oper-
ates like a muscle and is therefore subject to
improvement through training and also to
breakdowns through fatigue. Figure 36.1A
depicts a model of self- regulation wherein
monitoring, limited capacity resources, and
goals interact with the strength of impulses
and temptations, ultimately determining
self- regulatory success or failure.
In the following sections we turn to some
of the proposed mechanisms for how nega-
tive affect may impair self- control. As we
shall see, negative affect appears to have
the pernicious ability to operate on each
of the aforementioned components of self-
regulation. Acting like poison tendrils, it
reaches into all aspects of self- control (Fig-
ure 36.1B), interfering with monitoring,
exhausting the capacity to regulate behav-
ior, and increasing the strength of desires
and temptations.

616 HEALTH IMPLICATIONS
How Might Negative Affect Impair
Self‑Control?
Negative Affect Increases
the
Strength of
Temptations
In their dual- system model of self- control,
Metcalf and Mischel (1999) theorize that
negative affect may impair the cool system
involved in executing control, while simul-
taneously increasing the hot system that
responds to temptations and rewards. In sup-
port of this, studies of delay of gratification
have shown that negative affect increases the
frequency with which people accept imme-
diate gratification instead of waiting for
larger, delayed rewards (Mischel, Ebbesen,
& Zeiss, 1973; Seeman & Schwarz, 1974;
Tice, Bratslavsky, & Baumeister, 2001).
Interestingly, studies of nonhuman ani-
mals have revealed similar patterns in the
form of increased reward sensitivity in dis-
tressed animals. For example, the induc-
tion of emotional distress via social isola-
tion increases food and drug consumption
(Campbell Teskey, Kavaliers, & Hirst, 1984;
Ramsey & Van Ree, 1993). This heightened
reward sensitivity is thought to be due to the
release of glucocorticoids during distressing
situations that serve to sensitize the brain’s
reward circuitry to appetitive stimuli (Der-
oche et al., 1995; Piazza & Le Moal, 1996).
Although difficult to directly measure in
humans, research has demonstrated that
when people receive an artificial administra-
tion of glucocorticoids, they subsequently
eat more relative to a placebo group (Tata-
ranni et al., 1996).
In humans, negative affect has been
shown to increase the intensity of smoking
in smokers (McKee et al., 2011), as well as
how much pleasure people report experienc-
ing when smoking (Zinser, Baker, Sherman,
GOALS AND STANDARDS
MONITORING
(working memory,
self-awareness)
CAPACITY
(ego depletion,
strength)
SUCCESS
FAILURE
SELF-REGULATION
TEMPTATIONS
AND DESIRES
(food, drugs, media use, etc.)
GOALS AND STANDARDS
MONITORING
(working memory,
self-awareness)
CAPACITY
(ego depletion,
strength)
SUCCESS
FAILURE
SELF-REGULATION
TEMPTATIONS
AND DESIRES
(food, drugs, media use, etc.)
increases
misregulation
decreases
capacity
decreases
monitoring
increases
impulse strength
failure
increases
negative affect
Negative Affect
A.
B.
FIGURE 36.1. (A) Under normal circumstances, when a person is faced with temptations, successful
self-
r
egulation involves a balance between the strength of impulses and the capacity to monitor and
regulate behavior in the service of goals and standards. (B) Negative affect spreads poison tendrils into
every aspect of self-
r
egulation, amplifying desires, decreasing monitoring, depleting limited capacity,
and encouraging misregulation strategies (e.g., mood repair and escape from aversive self-
a
wareness),
which can relieve negative affect in the short term but often lead to further negative affect upon failure
to meet one’s goals.
Emotion and Self‑ Regulation Failure 617
& Cannon, 1992). Results from a recent
functional neuroimaging study of chronic
dieters offer further evidence that negative
affect may serve to increase the rewarding
properties of appetitive stimuli— in this case
of appetizing foods. Following a negative
or neutral mood induction, chronic diet-
ers were exposed to appetizing food cues
during functional neuroimaging (Wagner,
Boswell, Kelley, & Heatherton, 2012). Rela-
tive to nondieters, dysphoric dieters showed
increased brain activity in the orbitofrontal
cortex, a brain area implicated in represent-
ing the rewarding value of appetizing foods.
Moreover, activity in the orbitofrontal cor-
tex and ventral striatum was correlated with
a measure of how distressing subjects found
the negative mood induction, suggesting that
increases in reward- related brain activity to
food cues were dependent on the strength
of the negative emotional state (Wagner et
al., 2012). Taken together, these findings
from both human and animal studies sug-
gest that negative affect may sensitize people
to rewards, thereby rendering temptations
more difficult to regulate.
Negative Affect Reduces Monitoring
through Cognitive Load
Another mechanism whereby negative affect
may reduce self- control is by impairing the
monitoring component of self- regulation.
Specifically, research demonstrates that
when dieters overeat following a negative
mood induction, they are less aware than
nondieters of the precise amount of food
they have consumed (Heatherton, Polivy,
Herman, & Baumeister, 1993). Reasons for
this reduced awareness vary from a moti-
vated desire to escape from negative affect
(see the next section) to an increase in cogni-
tive load as a result of ruminating over one’s
mood. In this section we focus on the pos-
sibility that experiencing negative affect can
lead to increased working memory load as
people ruminate over their negative mood
and neglect to monitor their behavior (Fig-
ure 36.1B).
As monitoring entails the ability to main-
tain goals and standards in working memory
(Hofmann, Schmeichel, & Baddeley, 2012),
it follows that a concurrent working mem-
ory load (e.g., attempting to regulate mood
or ruminating over one’s performance anxi-
eties) will impair task- relevant monitoring.
Perhaps nowhere has this mechanism been
better studied than in research on the phe-
nomenon of stereotype threat. This work has
shown that reminding women of negative
stereotypes about their gender, or reminding
African Americans of negative stereotypes
about their race, can lead them to underper-
form on math or intelligence tests (Spencer,
Steele, & Quinn, 1999; Steele & Aronson,
1995). Studies demonstrate that this decre-
ment in performance is at least in part due
to a reduction in working memory capacity,
which is thought to result from concurrent
attempts to regulate affect (Johns, Inzlicht,
& Schmader, 2008). This phenomenon has
been shown to “spill over” into nonstereo-
typed domains, as shown in work by Inzli-
cht and Kang (2010), who demonstrate that
inducing stereotype threat in women results
in disinhibited eating of unhealthy foods.
Further evidence that working memory
is impacted by negative affect comes from
studies in which inducing emotional dis-
tress through the anticipation of an upcom-
ing evaluation (e.g., public speaking) leads
to poorer performance on working mem-
ory tests (Schoofs, Preuss, & Wolf, 2008).
Indeed, one imaging study found that ste-
reotype threat leads to increased activity
in brain regions associated with affect (i.e.,
ventral anterior cingulate cortex) but not in
areas that support working memory (Krendl,
Richeson, Kelley, & Heatherton, 2008). In
this case, the authors argued that it is likely
that emotional distress resulting from ste-
reotype threat has downstream effects on
working memory.
Outside of the realm of negative affect,
research on the effects of cognitive load
on self- regulation have shown that cogni-
tive loads impairs a variety of controlled
behaviors, from suppressing thoughts (Weg-
ner & Erber, 1992) to inhibiting food con-
sumption in dieters (Ward & Mann, 2000).
Mann and Ward (2007) proposed an atten-
tional myopia theory of cognitive load,
which posits that cognitive load restricts the
range of attention to targets in the imme-
diate environment, thereby rendering indi-
viduals vulnerable to a variety of external
cues. To the extent that negative affect
increases cognitive load through rumina-
tion or attempts to regulate affect (Johns et
al., 2008), a similar attentional myopia may
618 HEALTH IMPLICATIONS
occur when people are experiencing a nega-
tive emotional state.
Another means by which cognitive load
can bring about self- regulation failure comes
from research on ironic process theory (Weg-
ner, 1994) demonstrating that when mental
capacity is taxed by a concurrent cognitive
load, the likelihood of counterintentional
behavior increases. Within the realm of
emotion regulation, it has been shown that
attempting to change one’s mood (either
from happy to sad or vice versa) while under
a concurrent cognitive load leads to an ironic
increase in the opposite mood state. Specifi-
cally, participants who were instructed to
make themselves feel sad, felt happier if they
regulated their mood while under cognitive
load (Wegner, Erber, & Zanakos, 1993).
With respect to the role of affect in bringing
about self- regulation failure, what this sug-
gests is that to the degree that negative affect
exerts a cognitive load, attempts to regulate
behavior simultaneously, such as stopping
oneself from smoking, may bring about the
exact behavior one is trying to avoid.
Directing people’s attention toward the
self has a long history of being used as a
means to increase monitoring, and thereby
self- regulation. With regard to negative
affect inductions, exposure to mirrors or
to video clips of oneself has been shown to
eliminate the effects of negative mood on
disinhibited behavior. For example, having
dieters watch a video of themselves after a
mood induction eliminated the effects of
negative affect on overeating as compared
to dieters who watched a neutral video
(Heatherton et al., 1993). Similarly, being
forced to sit in front of a mirror has been
shown to eliminate the detrimental effects
of social rejection on a self- regulation task
(Baumeister et al., 2005). It appears then,
that increasing self- awareness tempers the
impact of negative affect on self- regulation
failures by restoring monitoring and increas-
ing the accessibility of behavioral standards
(e.g., Scheier & Carver 1983). However,
the timing of increased self- attention may
be important; for instance, increasing self-
attention during the experience of emotion
has been shown to heighten the intensity of
emotions (Scheier & Carver, 1983; Fenig-
stein, 1979) which in turn may exacerbate
the effects of negative affect on subsequent
self- regulation.
Managing Negative Affect Depletes
Self‑Regulatory
Strength
Over the last decade, considerable evidence
has been gathered in favor of a strength model
of self- regulation (for a recent meta- analysis,
see Hagger, Wood, Stiff, & Chatzisarantis,
2010). This model posits that self- regulation
relies on a common resource that can become
temporarily exhausted by effortful self-
regulation, and that when this resource is
exhausted, further attempts at self- regulation
are likely to fail (Baumeister & Heatherton,
1996). This phenomenon is typically studied
using a sequential task paradigm in which an
initial self- regulatory task is used to deplete
resources, thereby rendering subsequent self-
regulation attempts (even those in different
domains) less likely to succeed. Studies have
shown that tasks as varied as suppressing
thoughts and stereotypes (Gordijn, Hindriks,
Koomen, Dijksterhuis, & Van Knippenberg,
2004; Muraven, Collins, & Nienhaus, 2002),
making decisions (Vohs et al., 2008), and
managing impressions (Richeson & Shelton,
2003) all can lead to self- regulation failure on
subsequent tasks. In terms of relevance to this
chapter, it is interesting to note that one of the
methods most often used for depleting self-
regulatory resources is to have participants
engage in an emotion regulation task (typi-
cally inhibiting emotional responses). For
example, regulating emotional responses to
an emotionally provocative film reduces per-
sistence on difficult tasks (Baumeister, Brat-
slavsky, Muraven, & Tice, 1998), impairs
performance on executive tasks (Schmeichel,
Vohs, & Baumeister, 2003) and leads diet-
ers to break their diets and overeat (Vohs &
Heatherton, 2000). These findings strongly
suggest that emotion regulation exhausts a
common domain- general resource leading
to impairments on subsequent self- control
tasks.
As we have seen in the previous section,
negative affect is often accompanied by
concurrent attempts to regulate that affect.
To the degree that this emotion regulation
strategy is effortful, the strength mode of
self- regulation suggests that, above and
beyond the cost incurred to monitoring (see
previous section), regulating negative affect
would also deplete self-
r
egulatory strength,
increasing the likelihood of self-
r
egulation
failure. Indeed, in the previously mentioned
Emotion and Self‑ Regulation Failure 619
study by Vohs and Heatherton (2000) this
is precisely what was found when dieters
who were asked to inhibit their expression
of sadness while watching an emotionally
evocative video subsequently overindulged
themselves in appetizing ice cream.
Although the bulk of the research on self-
regulatory depletion and emotion examines
how emotion regulation can produce sub-
sequent self- regulation failure on nonemo-
tional tasks, studies shows that this effect
goes both ways, such that engaging in effort-
ful self- regulation also impairs subsequent
attempts at emotion regulation. The first
researchers who looked at this question had
participants engage in a thought suppression
task, followed by an emotion regulation task
that required participants to inhibit express-
ing emotions evoked by an emotional video
(Muraven, Tice, & Baumeister, 1998). They
found that participants whose resources
had been depleted by the thought suppres-
sion task were subsequently less successful at
inhibiting their emotions. In another study,
Schmeichel (2007) extended these findings
to more traditional tasks of executive func-
tion. In this experiment, participants com-
pleted a complex working memory task,
followed by an emotion inhibition task. As
in the Muraven et al. (1998) study, partici-
pants whose self- regulatory resources were
depleted by the complex working memory
task were impaired at suppressing their emo-
tions relative to a nondepleted control group
(Schmeichel, 2007).
More recently, the interplay between self-
regulatory strength and emotional regula-
tion was investigated using functional neu-
roimaging (Wagner & Heatherton, 2013).
In this study, participants were assigned to
either a depletion condition, in which they
completed a difficult attention control task,
or a control condition. Next, all participants
viewed a series of emotional scenes differ-
ing in valence. Compared to control par-
ticipants, those who were depleted exhib-
ited greater neural activity in the amygdala,
a region involved in the perception and
detection of emotion, when viewing nega-
tive emotional scenes. Moreover, depleted
participants exhibited reduced connectiv-
ity between the amygdala and prefrontal
regions implicated in top-down control, sug-
gesting a failure to engage in emotion regu-
lation (Wagner & Heatherton, 2013).
Taken together, the results of the studies
reviewed in this section offer both behav-
ioral and neural evidence that engaging in
self- regulation, be it in the emotional or
the cognitive domain, leads to transient
impairments in subsequent self- regulation
attempts. Thus, with respect to our poison
tendrils model of negative affect, ongoing
attempts to regulate negative affect serve
to reduce self- regulatory capacity, thereby
increasing the likelihood of self- regulation
failure (Figure 36.1B). Moreover, these find-
ings suggest that it is important to consider
the individual’s prior self- regulatory con-
text, as having to engage in other forms of
self- regulation can lead to transient impair-
ments of emotion regulation, which may
exacerbate the effects of negative affect on
self- regulation.
Depleting Self‑Regulatory Strength
Intensifies Emotions and Impulses
Although early models of self- regulation
tacitly assumed that the strength of impulses
and emotions remain essentially unchanged
during self- regulation failure, recent theo-
ries suggest that impulses and emotions
may increase in strength when self- control
is depleted (e.g., Heatherton & Wagner,
2011; Schmeichel, Harmon-Jones, & Har-
mon-Jones, 2010; Vohs et al., submitted).
For instance, the recent study by Vohs and
colleagues demonstrated that when partici-
pants are in a depleted state, they experi-
ence stronger cravings for appetizing food,
as well as rate emotions and pain as being
felt more acutely than do nondepleted par-
ticipants). The results of the brain imaging
study by Wagner and Heatherton (2013)
bear some similarities to these findings.
Although the results of this study were inter-
preted in terms of emotion dysregulation fol-
lowing depletion, it is equally plausible that
self- regulatory depletion served to amplify
people’s experience of affect, resulting in
greater amygdala activity in response to
negative scenes, thus making emotion regu-
lation more difficult.
This relatively new line of research sug-
gests that self- regulatory depletion not only
harms subsequent attempts at self- control
but also increases the strength of emotions
and desires. For the current discussion this
suggests that managing ongoing negative
620 HEALTH IMPLICATIONS
affect may not only reduce self- regulatory
capacity but also lead to a concurrent
increase in the intensity of currently experi-
enced affect, thereby amplifying its deleteri-
ous effects on all aspects of self- regulation,
as indicated by the path between depletion
and negative affect in Figure 36.1B.
Mood Repair and Escaping
Negative Affect Take Precedence
over Long‑Term Goals
One likely explanation for why people turn
to eating, smoking, and drinking in times
of emotional distress is that they believe
such activities can restore their mood (Say-
ette, 1993). Indeed, even in nonhuman ani-
mals, consuming pleasurable foods has been
shown to reduce neuroendocrine markers
of distress (Foster et al., 2009). In humans,
research has shown that merely being
exposed to appetizing stimuli (e.g., food,
drugs, alcohol) can activate positive hedonic
thoughts (Hofmann, van Koningsbrug-
gen, Stroebe, Ramanathan, & Aarts, 2010;
Sayette & Hufford, 1997). For instance, in
dieters, exposure to descriptions of appetiz-
ing foods has been shown to spontaneously
activate more hedonic thoughts about the
pleasurable aspects of eating than descrip-
tions of neutral foods (Papies, Stroebe, &
Aarts, 2007). This attention to the pleasur-
able aspects of temptations can have serious
consequences, especially in cases of alcohol
and substance use in which withdrawal from
the substance elicits negative affect that is
relieved only by further substance use. It
has been suggested that this need to cope
with—and escape from—negative affect
produced by withdrawal is one of the central
motivations underlying addiction (Baker,
Piper, McCarthy, Majeskie, & Fiore, 2004).
Moreover, through prolonged conditioning
of substance use and improved mood, any
experience of negative affect— whether due
to specific triggers or merely the vicissitudes
of daily life—can trigger the desire for drugs
or alcohol (Baker et al., 2004; Childress et
al., 1994).
Given people’s belief that consuming food
and alcohol or engaging in pleasurable activ-
ities will improve their mood, is there any
empirical evidence that people employ this
strategy when experiencing negative affect?
Studies examining a wide range of behav-
iors show that, upon experiencing negative
affect, people report consuming their favor-
ite substance (e.g., food, alcohol, or drugs) or
engaging in their favorite activity (e.g., shop-
ping, television, gambling) precisely because
they believe it will make them feel better (e.g.,
Faber & Christenson, 1996; Rook, 1987;
Sayette, 1993). Thus, when stuck in a nega-
tive emotional state, people will shirk their
long-term goals in order to address the more
immediate need to feel better. One particu-
larly disconcerting example of mood repair
following negative affect comes from a study
in which participants were asked to rate the
personalities of ethnic ingroup and outgroup
members. Participants who were induced to
experience negative affect— as a result of
negative performance feedback on an osten-
sibly unrelated test— subsequently rated the
ethnic outgroup members more negatively
than did control subjects (Fein & Spencer,
1997). Moreover, it was found that this ten-
dency to denigrate outgroup members led to
increased self- esteem in the negative affect
group, suggesting that participants deni-
grated outgroup members in order to make
themselves feel better. Likewise, when peo-
ple experience social rejection, they become
more willing to take illicit drugs or waste
money on goods that are liked by their peers,
but not necessarily by themselves, if doing so
increases their chances at fitting in (Mead,
Baumeister, Stillman, Rawn, & Vohs, 2011).
Other examples involve the effect of nega-
tive affect on spending. In these studies, neg-
ative affect has been shown to increase par-
ticipant’s willingness to pay more for goods,
as well as sell things they already own for
less money (Lerner, Small, & Loewenstein,
2004). These findings were taken to indicate
that people see buying new things or selling
old things as a way of changing their current
state and thereby escape negative affect.
Although attempting to repair one’s mood
through engaging in pleasurable activities
such as eating may seem like a good tem-
porary strategy to alleviate negative affect,
studies show that this form of mood repair
is often a case of misregulation. Unlike other
forms of self- regulation failure, misregu-
lation is not so much a lack of self- control
as the use of self- control in a misguided—
and often futile— attempt to improve one’s
state (Baumeister & Heatherton, 1996). For
instance, studies show that although diet-
ers may binge-eat in an attempt to improve
their mood, they often end up feeling worse

Emotion and Self‑ Regulation Failure 621
after eating than they did before (for a meta-
analysis, see Haedt-Matt & Keel, 2011).
Finally, a study by Tice and colleagues
(2001) elegantly demonstrated that cer-
tain cases of disinhibited behavior are due
entirely to a motivated attempt to improve
affective states. In this study, participants
were induced to experience negative affect,
but in one group they were given a pill
and told that it would freeze their mood.
Whereas participants who did not receive
the mood- freezing pill exhibited typical
signs of self- regulation failure, such as eat-
ing unhealthy foods or procrastinating with
pleasurable activity before a task, those who
took the mood- freezing pill showed no signs
of disinhibition (Tice et al., 2001). Thus,
when participants believed engaging in plea-
surable activities could not improve their
negative emotional state, they ceased trying
to change their mood by indulging in these
activities.
The case of misregulation is particularly
interesting, because it suggests that self-
regulation failure following negative affect
is not always due to a failure in the machin-
ery of self- regulation; rather it reflects a con-
scious shift in priorities as people focus on
improving their immediate affective state
at the expense of their long-term regulatory
goals. Indeed, it has been suggested that
sometimes people actually use self- control
to bring about personal harm, such as when
they smoke or use drugs to fit in and make
friends (Rawn & Vohs, 2011). However,
these short-term strategies often lead to
more negative affect as people move further
and further away from their goals, such as
when dieters overeat to improve their mood
but then finds themselves further from their
goal of weight loss (Heatherton & Polivy,
1992).
Intimately related to the notion of mis-
regulation is research on escape from self-
awareness (Baumeister, 1991; Heatherton &
Baumeister, 1991). In contrast to the mood
repair hypothesis outlined earlier, escape
theory posits that self- awareness is aver-
sive for people who possess negative self-
views (see Higgins, 1987). Unfortunately,
for those with negative self-views, increas-
ing self- awareness does not help them regu-
late, because self- awareness itself can lead to
negative affect as people’s attention becomes
focused on their perceived shortcomings
(Mor & Winquist, 2002). For these people,
escaping self- awareness becomes an effec-
tive strategy for reducing negative affect.
However, attempts to reduce self- awareness
come at the expense of the ability to focus
on long-term goals and an increased vulner-
ability to temptations in the immediate envi-
ronment. For example, dieters who experi-
ence a self- esteem threat are more likely
than nondieters to overeat (Heatherton et
al., 1993). Likewise, following self- esteem
threats, people drink more alcohol (Hull &
Young, 1983), watch more television (Mos-
kalenko & Heine, 2003) and avoid sitting in
front of mirrors (Twenge, Catanese, & Bau-
meister, 2003).
With respect to our model, this suggests
that, in some instances, negative affect inter-
feres with the machinery of self- regulation
(e.g., monitoring or capacity), but in other
circumstances, people chose to indulge in
temptations as a means of repairing their
mood. This form of misregulation may tem-
porarily relieve negative affect, but once the
awareness sets in that people have failed at
their regulatory goals, further negative affect
ensues, thus jeopardizing future attempts
at self- regulation (see also Heatherton and
Polivy’s [1992] spiral model describing the
relationship between negative affect and
chronic dieting).
Are All Negative Emotions
Equally Likely to Cause
Self-Regulation Failure?
In many of the studies we have reviewed,
negative affect refers primarily to any
unpleasurable or aversive emotional state,
thus subsuming a variety of emotional
categories— from frustration to shame
to social rejection. In some cases, nega-
tive affect is assessed through self- reports,
whereas in others the emotional state is
experimentally induced, affording more
control over the specific emotions produced.
That being said, differences between nega-
tive emotion types are seldom reported, with
two exceptions. The first involves research
in chronic dieters demonstrating that nega-
tive affect inductions that specifically target
an individual’s self- esteem (e.g., negative
performance feedback, social rejection) are
more effective at producing disinhibited eat-
ing (Heatherton et al., 1991, 1993, 1998;
Lattimore & Maxwell, 2004) than induc-

622 HEALTH IMPLICATIONS
tions targeting physical distress (e.g., fear of
electric shock; Heatherton et al., 1991; Her-
man & Polivy, 1975).
The second exception involves research on
the consequences of being socially excluded.
As described earlier, social rejection can
lead to poorer task performance, selfishness,
aggression, and increased eating in dieters.
Paradoxically, however, other studies indi-
cate that inducing feelings of social rejection
can increase affiliative behaviors (Maner,
DeWall, Baumeister, & Schaller, 2007; Wil-
liams & Sommer, 1997), as well as improve
memory for social information (Gardner,
Pickett, & Brewer, 2000). One explanation
for this discrepancy is that socially rejected
people are adaptively deploying both pro-
tective and affiliative strategies, such that
when the opportunity for forging new social
bonds appears low (as in some experiments)
they react with selfish and occasionally hos-
tile behaviors, whereas when there is an
opportunity for meaningful social contact,
they instead switch to an affiliative strat-
egy in an attempt to repair their self- esteem
(e.g., Baumeister, Brewer, Tice, & Twenge,
2007; Maner et al., 2007; Smart Richman
& Leary, 2009). Indeed, a recent imaging
study found that interpersonal distress led
to increased mental engagement for posi-
tive social stimuli (as indexed by activity in
medial prefrontal cortical regions associated
with mentalizing) and decreased engagement
for negative social stimuli (Powers, Wagner,
Norris, & Heatherton, 2013). Although
these reactions to social rejection have only
tangential bearing on self- regulation failure,
they do highlight the complexity of the rela-
tionship between negative affect and self-
regulation, demonstrating that reactions to
negative affect can strategically vary accord-
ing to the individual’s goals. As described
in the previous sections, what can appear
to be a failure to maintain self- control may
instead be a strategy to repair mood through
consuming foods or alcohol, or engaging in
pleasurable activities at the expense of other
regulatory goals.
Can Negative Affect Ever Increase
Self-Regulation Success?
Much of the work discussed here has exam-
ined the influence of affect on self- regulation
in “hot” contexts, such as when faced with
the opportunity to indulge in temptations
or pleasurable activities. Emotion can,
however, color behavior in other ways. For
instance, in Schwarz’s (1990) feelings as
information model, an individual’s current
emotional state can be used as information
in making evaluative judgments or resolving
ambiguities. For example, following a nega-
tive mood induction, people tend to judge
their current happiness and life satisfaction
less positively than when in a positive mood
(Schwarz & Clore, 1983). Other research
shows that people in a negative mood tend
to elaborate on information, demonstrat-
ing more accurate performance on tasks
requiring attention to details (reviewed in
Schwarz, 1990). Other theorists have sug-
gested that emotions signal the need for self-
regulation. For instance, Carver and Scheier
(1990) argued that positive emotions signal
that a person is achieving his or her goal
(e.g., successfully dieting), whereas nega-
tive emotions arise when a person is mov-
ing away from his or her goal (e.g., gaining
weight during a diet). From this perspective,
then, negative affect should serve to increase
rather than thwart efforts at self- regulation.
The discrepancy between the preceding the-
ories and the evidence reviewed in this chap-
ter can be resolved if we consider when in
time these effects are likely to take place. In
this chapter we have primarily focused on
the role of negative affect in eliciting self-
regulation failure “in the moment,” when
people are confronted with the immediate
need to regulate themselves, such as when
faced with temptations. Over longer time
frames, outside of these “hot” moments,
negative affect may very well serve to moti-
vate individuals to change their current state
for the better (Heatherton & Polivy, 1992).
The Role of Positive Affect
in Self-Regulation Success
and Failure
In the preceding sections we have focused
solely on the role of negative affect in self-
regulation failure, with scant mention of its
opposite, positive affect. This is largely for
two reasons: First, researchers have often
neglected positive affect in favor of negative
affect (see Ashby, Isen, & Turken, 1999);
second, of the research that does consider
positive affect, there is less evidence that it

Emotion and Self‑ Regulation Failure 623
plays a strong part in self- regulation failures
or successes, at least in the types of contexts
discussed in this chapter. For example, com-
pared to positive moods, negative moods and
events are typically found to be more memo-
rable and are experienced more intensely
(see Baumeister, Bratslavsky, Finkenauer, &
Vohs, 2001). Moreover, in the mood induc-
tion studies reviewed earlier, of those that
have directly compared negative and posi-
tive affect, negative always trump positive
(Willner & Jones, 1996; Tiffanny & Drobes,
1990). Thus, it appears that negative affect
is generally a much more potent force than
positive affect in self- regulation failure.
Does positive affect promote self-
regulation success? There is some research
suggesting that positive affect can serve
to momentarily increase self- regulation
strength. For instance, when people hold
the goal of self- improvement in mind, posi-
tive affect inductions lead to increased
use of self- control (Fishbach & Labroo,
2007). Based on these results, Fishbach and
Labroo suggest that positive affect pushes
people to strive toward a currently acti-
vated goal. If that goal happens to be about
self- improvement, then self- regulation may
follow; however, if the goal is mood regu-
lation, then self- regulation may suffer to
the degree that it is opposed to maintain-
ing a positive mood. Another study show-
ing beneficial effect of positive affect on
self- regulation found that inducing positive
affect restored self- regulation capacity fol-
lowing self- regulatory depletion (Tice, Bau-
meister, Shmueli, & Muraven, 2007). This
finding suggests that the capacity to engage
in self- control is at least partially moderated
by affect. In fact, to the degree that partici-
pants in the Tice et al. study hold the goal to
succeed at the tasks laid out by the experi-
menters, then the results of Fishbach and
Labroo (2007) would suggest that the posi-
tive affect induction reversed the effects of
self- regulatory depletion by pushing subjects
to expend whatever resources they had left
in the service of the goal of succeeding at the
task. It is worth pointing out that although
these studies suggest that positive affect can
increase self- control under certain circum-
stances, it is likely that under a different set
of circumstances (e.g., at a bar), with a dif-
ferent goal in mind (e.g., celebrating with
friends), positive affect may lead to greater
celebratory excess.
Conclusion
Negative affect may very well be the most
potent disinhibitor of restrained behavior.
When people feel worthless, depressed, or
rejected, they are more likely to engage in
a variety of self- defeating behaviors. Fol-
lowing negative affect, dieters overeat, for-
mer smokers smoke, and alcoholics fall into
relapse. More generally, people become more
likely to procrastinate, to be selfish or hos-
tile, and even go so far as to denigrate out-
group members. Negative affect appears to
accomplish this pernicious feat by poisoning
all facets of self- regulation (see Figure 36.1):
sensitizing people to rewards and tempta-
tions, decreasing monitoring of behavior,
reducing self- regulatory capacity, and caus-
ing people to focus on repairing their mood
at the expense of abandoning their goals and
giving in to their impulses and desires.
Although it often appears that our self-
control is entirely at the mercy of our emo-
tions, it is also the case that emotion regu-
lation can be derailed by prior attempts at
self- regulation in the cognitive domain. For
instance, engaging in effortful self- regulation
tasks can exhaust self- regulatory resources,
thereby jeopardizing subsequent emo-
tion regulation attempts. Moreover, recent
research has shown that when people are
cognitively depleted by prior attempts at self-
regulation, emotions and impulses appear to
increase in potency, rendering them a more
forceful adversary to self- control.
The interplay among emotions, emotion
regulation, and self- regulation has been dis-
cussed as though each of the myriad ways
that emotions can hijack self- control work
independently of the others. However, it is
far more likely that the multiple routes to
self- regulation failure are themselves inter-
active. For instance, negative affect can
lead to a desire to escape negative states by
reducing self- awareness and also to engage
in pleasurable activities, even if these activi-
ties conflict with long-term goals. Together,
these may serve to increase the pull of imme-
diately available rewards, because the ability
to monitor behavior is lessened as the focus
shifts to pleasure. Negative affect may also
place a load on working memory indepen-
dent of the desire to escape self- awareness,
thus further reducing the ability to monitor
behavior. Throw in the fact that prior self-
regulatory effort may leave the individual

624 HEALTH IMPLICATIONS
in a depleted state in which both resources
for further self- control are lacking and
the strength of impulses and temptations
is increased, and it is a small miracle that
people are not constantly acting out their
fantasies, drinking, smoking, or indulg-
ing every gastronomic desire. If the studies
reviewed in this chapter paint a dire picture,
it is important to note that emotion is not all
bad: For instance, it has been demonstrated
that experiencing positive emotions can
improve self- regulation by helping to buffer
against the depleting effects of prior tasks
(Tice et al., 2007).
In this chapter we reviewed evidence sug-
gesting that negative emotions are a potent
cause of self- regulation failure and we have
proposed a simple poison tendrils model in
which negative affect invades and disrupts
nearly every aspect of self- regulatory func-
tion. Moreover, results from a variety of
behavioral and neural studies suggest that
the relationship between emotions and self-
regulation is dynamic and interactive, such
that emotion- induced self- regulation fail-
ure serves not only to intensify currently
experienced emotions and desires but also
to increase negative affect as the individual
moves further and further away from his
or her goals. Negative affect is thus a par-
ticularly potent threat to self- regulation,
because it not only reduces the capacity for
control (increased working memory load,
reduced self- awareness and monitoring) but
it may also lead to increases in the strength
of experienced desires and emotions, render-
ing them all the more difficult to resist.
References
Ashby, F. G., Isen, A. M., & Turken, A. U. (1999).
A neuropsychological theory of positive affect
and its influence on cognition. Psychological
Review, 106(3), 529–550.
Baker, T. B., Piper, M. E., McCarthy, D. E.,
Majeskie, M. R., & Fiore, M. C. (2004).
Addiction motivation reformulated: An affec-
tive processing model of negative reinforce-
ment. Psychological Review, 111(1), 33–51.
Baucom, D. H., & Aiken, P. A. (1981). Effect of
depressed mood in eating among obese and
nonobese dieting and nondieting persons.
Journal of Personality and Social Psychology,
41(3), 577–585.
Baumeister, R. F. (1991). Escaping the self: Alco-
holism, spirituality, masochism, and other
flights from the burden of selfhood. New
York: Basic Books.
Baumeister, R. F. (1997). Esteem threat, self-
regulatory breakdown, and emotional distress
as factors in self- defeating behavior. Review of
General Psychology, 1(2), 145–174.
Baumeister, R. F., Bratslavsky, E., Finkenauer,
C., & Vohs, K. D. (2001). Bad is stronger than
good. Review of General Psychology, 5(4),
323–370.
Baumeister, R. F., Bratslavsky, E., Muraven, M.,
& Tice, D. M. (1998). Ego depletion: Is the
active self a limited resource? Journal of Per-
sonality and Social Psychology, 74(5), 1252–
1265.
Baumeister, R. F., Brewer, L. E., Tice, D. M., &
Twenge, J. M. (2007). Thwarting the need to
belong: Understanding the interpersonal and
inner effects of social exclusion. Social and
Personality Psychology Compass, 1(1), 506–
520.
Baumeister, R. F., DeWall, C. N., Ciarocco, N.
J., & Twenge, J. M. (2005). Social exclusion
impairs self- regulation. Journal of Personality
and Social Psychology, 88(4), 589–604.
Baumeister, R. F., & Heatherton, T. (1996). Self-
regulation failure: An overview. Psychological
Inquiry, 7(1), 1–15.
Berkowitz, L. (1989). Frustration– aggression
hypothesis: Examination and reformulation.
Psychological Bulletin, 106(1), 59–73.
Bousman, C. A., Cherner, M., Ake, C., Leten-
dre, S., Atkinson, J. H., Patterson, T. L., et al.
(2009). Negative mood and sexual behavior
among non- monogamous men who have sex
with men in the context of methamphetamine
and HIV. Journal of Affective Disorders,
119(1–3), 84–91.
Campbell Teskey, G., Kavaliers, M., & Hirst, M.
(1984). Social conflict activates opioid analge-
sic and ingestive behaviors in male mice. Life
Sciences, 35(3), 303–315.
Carver, C. S., & Scheier, M. (1981). Attention and
self- regulation: A control- theory approach to
human behavior. New York: Springer- Verlag.
Carver, C. S., & Scheier, M. F. (1990). Origins
and functions of positive and negative affect:
A control- process view. Psychological Review,
97(1), 19–35.
Childress, A. R., Ehrman, R., McLellan, A.
T., MacRae, J., Natale, M., & O’Brien, C.
P. (1994). Can induced moods trigger drug-
related responses in opiate abuse patients?
Emotion and Self‑ Regulation Failure 625
Journal of Substance Abuse Treatment, 11(1),
17–23.
Deroche, V., Marinelli, M., Maccari, S., Le
Moal, M., Simon, H., & Piazza, P. V. (1995).
Stress- induced sensitization and glucocorti-
coids: I. Sensitization of dopamine- dependent
locomotor effects of amphetamine and mor-
phine depends on stress- induced corticos-
terone secretion. Journal of Neuroscience,
15(11), 7181–7188.
DeWall, C. N., Twenge, J. M., Bushman, B.,
Im, C., & Williams, K. (2010). A little accep-
tance goes a long way applying social impact
theory to the rejection– aggression link. Social
Psychological and Personality Science, 1(2),
168–174.
Faber, R. J., & Christenson, G. A. (1996). In the
mood to buy: Differences in the mood states
experienced by compulsive buyers and other
consumers. Psychology and Marketing, 13(8),
803–819.
Fein, S., & Spencer, S. J. (1997). Prejudice as
self-image maintenance: Affirming the self
through derogating others. Journal of Person-
ality and Social Psychology, 73(1), 31– 44.
Fenigstein, A. (1979). Self- consciousness, self-
attention, and social interaction. Journal of Per-
sonality and Social Psychology, 37(1), 75–86.
Fishbach, A., & Labroo, A. A. (2007). Be better
or be merry: How mood affects self- control.
Journal of Personality and Social Psychology,
93(2), 158–173.
Foster, M. T., Warne, J. P., Ginsberg, A. B., Hor-
neman, H. F., Pecoraro, N. C., Akana, S. F., et
al. (2009). Palatable foods, stress, and energy
stores sculpt corticotropin- releasing factor,
adrenocorticotropin, and corticosterone con-
centrations after restraint. Endocrinology,
150(5), 2325–2333.
Fox, H. C., Bergquist, K. L., Hong, K., & Sinha,
R. (2007). Stress- induced and alcohol cue-
induced craving in recently abstinent alcohol-
dependent individuals. Alcoholism: Clinical
and Experimental Research, 31(3), 395–403.
Gardner, W. L., Pickett, C. L., & Brewer, M. B.
(2000). Social exclusion and selective memory:
How the need to belong influences memory for
social events. Personality and Social Psychol-
ogy Bulletin, 26(4), 486–496.
Gordijn, E. H., Hindriks, I., Koomen, W., Dijk-
sterhuis, A., & Van Knippenberg, A. (2004).
Consequences of stereotype suppression
and internal suppression motivation: A self-
regulation approach. Personality and Social
Psychology Bulletin, 30(2), 212–224.
Gross, J. J. (2002). Emotion regulation: Affec-
tive, cognitive, and social consequences. Psy-
chophysiology, 39(3), 281–291.
Haedt-Matt, A. A., & Keel, P. K. (2011). Revisit-
ing the affect regulation model of binge eating:
A meta- analysis of studies using ecological
momentary assessment. Psychological Bulle-
tin, 137(4), 660–681.
Hagger, M. S., Wood, C., Stiff, C., & Chatzisa-
rantis, N. L. D. (2010). Ego depletion and
the strength model of self- control: A meta-
analysis. Psychological Bulletin, 136(4), 495–
525.
Heatherton, T. F., & Baumeister, R. F. (1991).
Binge eating as escape from self- awareness.
Psychological Bulletin, 110(1), 86–108.
Heatherton, T. F., Herman, C. P., & Polivy, J.
(1991). Effects of physical threat and ego
threat on eating behavior. Journal of Person-
ality and Social Psychology, 60(1), 138–143.
Heatherton, T. F., & Polivy, J. (1992). Chronic
dieting and eating disorders: A spiral model.
In J. H. Crowther, D. L. Tennenbaum, S. E.
Hobfoll, & M. A. P. Stephens (Eds.), The eti-
ology of bulimia nervosa: The individual and
familial context (pp. 133–155). Washington,
DC: Hemisphere.
Heatherton, T. F., Polivy, J., Herman, C. P., &
Baumeister, R. F. (1993). Self- awareness, task
failure, and disinhibition: How attentional
focus affects eating. Journal of Personality,
61(1), 49–61.
Heatherton, T. F., Striepe, M., & Wittenberg,
L. (1998). Emotional distress and disinhibited
eating: The role of self. Personality and Social
Psychology Bulletin, 24(3), 301–313.
Heatherton, T. F., & Wagner, D. D. (2011). Cog-
nitive neuroscience of self- regulation failure.
Trends in Cognitive Sciences, 15(3), 132–139.
Herman, C. P., & Polivy, J. (1975). Anxiety,
restraint, and eating behavior. Journal of
Abnormal Psychology, 84(6), 666–672.
Higgins, E. T. (1987). Self- discrepancy: A theory
relating self and affect. Psychological Review,
94(3), 319–340.
Higgins, R. L., & Marlatt, G. A. (1975). Fear
of interpersonal evaluation as a determinant
of alcohol consumption in male social drink-
ers. Journal of Abnormal Psychology, 84(6),
644–651.
Hofmann, W., Schmeichel, B. J., & Baddeley,
A. D. (2012). Executive functions and self-
regulation. Trends in Cognitive Sciences,
16(3), 174–180
Hofmann, W., van Koningsbruggen, G. M.,
626 HEALTH IMPLICATIONS
Stroebe, W., Ramanathan, S., & Aarts,
H. (2010). As pleasure unfolds: Hedonic
responses to tempting food. Psychological Sci-
ence, 21(12), 1863–1870.
Hull, J. G., & Young, R. D. (1983). Self-
consciousness, self- esteem, and success- failure
as determinants of alcohol consumption in
male social drinkers. Journal of Personality
and Social Psychology, 44(6), 1097–1109.
Inzlicht, M., & Kang, S. K. (2010). Stereotype
threat spillover: How coping with threats to
social identity affects aggression, eating, deci-
sion making, and attention. Journal of Person-
ality and Social Psychology, 99(3), 467–481.
Johns, M., Inzlicht, M., & Schmader, T. (2008).
Stereotype threat and executive resource
depletion: Examining the influence of emotion
regulation. Journal of Experimental Psychol-
ogy: General, 137(4), 691–705.
Kassel, J. D., Stroud, L. R., & Paronis, C. A.
(2003). Smoking, stress, and negative affect:
Correlation, causation, and context across
stages of smoking. Psychological Bulletin,
129(2), 270–304.
Keltner, D., & Gross, J. J. (1999). Functional
accounts of emotions. Cognition and Emo-
tion, 13(5), 467–480.
Krendl, A. C., Richeson, J. A., Kelley, W. M., &
Heatherton, T. F. (2008). The negative con-
sequences of threat: A functional magnetic
resonance imaging investigation of the neural
mechanisms underlying women’s underperfor-
mance in math. Psychological Science, 19(2),
168–175.
LaRose, R., Lin, C., & Eastin, M. (2003). Unreg-
ulated Internet usage: Addiction, habit, or
deficient self- regulation? Media Psychology,
5(3), 225–253.
Lattimore, P., & Maxwell, L. (2004). Cognitive
load, stress, and disinhibited eating. Eating
Behaviors, 5(4), 315–324.
Leary, M. R., Kowalski, R. M., Smith, L., &
Phillips, S. (2003). Teasing, rejection, and vio-
lence: Case studies of the school shootings.
Aggressive Behavior, 29(3), 202–214.
Lerner, J. S., Small, D. A., & Loewenstein, G.
(2004). Heart strings and purse strings: Car-
ryover effects of emotions on economic deci-
sions. Psychological Science, 15(5), 337–341.
Levenson, R. W. (1994). Human emotion: A
functional view. In P. Ekman & R. J. Davidson
(Eds.), The nature of emotion: Fundamental
questions (pp. 123–126). New York: Oxford
University Press.
Maner, J. K., DeWall, C. N., Baumeister, R. F.,
& Schaller, M. (2007). Does social exclusion
motivate interpersonal reconnection?: Resolv-
ing the “porcupine problem.” Journal of Per-
sonality and Social Psychology, 92(1), 42–55.
Mann, T., & Ward, A. (2007). Attention, self-
control, and health behaviors. Current Direc-
tions in Psychological Science, 16(5), 280–283.
Marlatt, G. A., & Gordon, J. (1985). Relapse
prevention: Maintenance strategies in the
treatment of addictive behaviors. New York:
Guilford Press.
McKee, S. A., Sinha, R., Weinberger, A. H.,
Sofuoglu, M., Harrison, E. L., Lavery, M., et
al. (2011). Stress decreases the ability to resist
smoking and potentiates smoking intensity
and reward. Journal of Psychopharmacology,
25(4), 490–502.
Mead, N. L., Baumeister, R. F., Stillman, T. F.,
Rawn, C. D., & Vohs, K. D. (2011). Social
exclusion causes people to spend and consume
strategically in the service of affiliation. Jour-
nal of Consumer Research, 37(5), 902–919.
Metcalfe, J., & Mischel, W. (1999). A hot/
cool- system analysis of delay of gratifica-
tion: Dynamics of willpower. Psychological
Review, 106(1), 3–19.
Mischel, W., Ebbesen, E. B., & Zeiss, A. R.
(1973). Selective attention to the self: Situa-
tional and dispositional determinants. Journal
of Personality and Social Psychology, 27(1),
129–142.
Mor, N., & Winquist, J. (2002). Self- focused
attention and negative affect: A meta- analysis.
Psychological Bulletin, 128(4), 638–662.
Moskalenko, S., & Heine, S. J. (2003). Watch-
ing your troubles away: Television viewing as a
stimulus for subjective self- awareness. Person-
ality and Social Psychology Bulletin, 29(1),
76–85.
Muraven, M., Collins, R. L., & Nienhaus, K.
(2002). Self- control and alcohol restraint: An
initial application of the self- control strength
model. Psychology of Addictive Behaviors,
16(2), 113–120.
Muraven, M., Tice, D. M., & Baumeister, R. F.
(1998). Self- control as limited resource: Regu-
latory depletion patterns. Journal of Personal-
ity and Social Psychology, 74(3), 774–789.
Oaten, M., Williams, K. D., Jones, A., & Zadro,
L. (2008). The effects of ostracism on self-
regulation in the socially anxious. Journal of
Social and Clinical Psychology, 27(5), 471–
504.
Papies, E., Stroebe, W., & Aarts, H. (2007). Plea-
sure in the mind: Restrained eating and spon-
Emotion and Self‑ Regulation Failure 627
taneous hedonic thoughts about food. Journal
of Experimental Social Psychology, 43(5),
810–817.
Piazza, P. V., & Le Moal, M. L. (1996). Patho-
physiological basis of vulnerability to drug
abuse: Role of an interaction between stress,
glucocorticoids, and dopaminergic neurons.
Annual Review of Pharmacology and Toxi-
cology, 36, 359–378.
Pithers, W. D., Kashima, K. M., Cumming, G.
F., Beal, L. S., & Buell, M. M. (1988). Relapse
prevention of sexual aggression. Annals of
the New York Academy of Sciences, 528(1),
244–260.
Powers, K. E., Wagner, D. D., Norris, C. J., &
Heatherton, T. F. (2013). Socially excluded
individuals fail to recruit medial prefrontal
cortex for negative social scenes. Social Cog-
nitive and Affective Neuroscience, 8(2), 151–
157.
Ramsey, N. F., & Van Ree, J. M. (1993). Emo-
tional but not physical stress enhances intrave-
nous cocaine self- administration in drug-naive
rats. Brain Research, 608(2), 216–222.
Raviv, M. (1993). Personality characteristics of
sexual addicts and pathological gamblers.
Journal of Gambling Studies, 9(1), 17–30.
Rawn, C. D., & Vohs, K. D. (2011). People use
self- control to risk personal harm: An intra-
interpersonal dilemma. Personality and Social
Psychology Review, 15(3), 267–289.
Richards, J. M., & Gross, J. J. (2000). Emotion
regulation and memory: The cognitive costs of
keeping one’s cool. Journal of Personality and
Social Psychology, 79(3), 410–424.
Richeson, J. A., & Shelton, J. N. (2003). When
prejudice does not pay: Effects of interracial
contact on executive function. Psychological
Science, 14(3), 287–290.
Roberts, M. E., Gibbons, F. X., Gerrard, M.,
Weng, C.-Y., Murry, V. M., Simons, L. G., et
al. (2012). From racial discrimination to risky
sex: Prospective relations involving peers and
parents. Developmental Psychology, 48(1),
89–102.
Rook, D. W. (1987). The buying impulse. Jour-
nal of Consumer Research, 14(2), 189–199.
Sayette, M. A. (1993). An appraisal- disruption
model of alcohol’s effects on stress responses
in social drinkers. Psychological Bulletin,
114(3), 459–476.
Sayette, M. A., & Hufford, M. R. (1997). Effects
of smoking urge on generation of smoking-
related information. Journal of Applied Social
Psychology, 27(16), 1395–1405.
Scheier, M. F., & Carver, C. S. (1983). Self-
directed attention and the comparison of
self with standards. Journal of Experimental
Social Psychology, 19(3), 205–222.
Schmeichel, B. J. (2007). Attention control,
memory updating, and emotion regulation
temporarily reduce the capacity for executive
control. Journal of Experimental Psychology:
General, 136(2), 241–255.
Schmeichel, B. J., Harmon-Jones, C., & Har-
mon-Jones, E. (2010). Exercising self- control
increases approach motivation. Journal of Per-
sonality and Social Psychology, 99(1), 162–173.
Schmeichel, B. J., Vohs, K. D., & Baumeister, R.
F. (2003). Intellectual performance and ego
depletion: Role of the self in logical reason-
ing and other information processing. Journal
of Personality and Social Psychology, 85(1),
33–46.
Schoofs, D., Preuss, D., & Wolf, O. T. (2008).
Psychosocial stress induces working memory
impairments in an n-back paradigm. Psycho-
neuroendocrinology, 33(5), 643–653.
Schwarz, N. (1990). Feelings as information:
Informational and motivational functions
of affective states. In E. T. Higgins & R. M.
Sorrentino (Eds.), Handbook of motivation
and cognition: Foundations of social behav-
ior (Vol. 2, pp. 527–561). New York: Guilford
Press.
Schwarz, N., & Clore, G. L. (1983). Mood,
misattribution, and judgments of well-being:
Informative and directive functions of affec-
tive states. Journal of Personality and Social
Psychology, 45(3), 513–523.
Seeman, G., & Schwarz, J. C. (1974). Affective
state and preference for immediate versus
delayed reward. Journal of Research in Per-
sonality, 7(4), 384–394.
Sinha, R. (2007). The role of stress in addiction
relapse. Current Psychiatry Reports, 9(5),
388–395.
Smart Richman, L., & Leary, M. R. (2009).
Reactions to discrimination, stigmatization,
ostracism, and other forms of interpersonal
rejection: A multimotive model. Psychological
Review, 116(2), 365–383.
Spencer, S. J., Steele, C. M., & Quinn, D. M.
(1999). Stereotype Threat and Women’s Math
Performance. Journal of Experimental Social
Psychology, 35(1), 4 –28.
Steele, C. M., & Aronson, J. (1995). Stereotype
threat and the intellectual test performance
of African Americans. Journal of Personality
and Social Psychology, 69(5), 797–811.
628 HEALTH IMPLICATIONS
Tataranni, P. A., Larson, D. E., Snitker, S., Young,
J. B., Flatt, J. P., & Ravussin, E. (1996). Effects
of glucocorticoids on energy metabolism and
food intake in humans. American Journal of
Physiology: Endocrinology and Metabolism,
271(2), E317–E325.
Tice, D. M., Baumeister, R. F., Shmueli, D., &
Muraven, M. (2007). Restoring the self: Posi-
tive affect helps improve self- regulation fol-
lowing ego depletion. Journal of Experimen-
tal Social Psychology, 43(3), 379–384.
Tice, D. M., Bratslavsky, E., & Baumeister, R.
F. (2001). Emotional distress regulation takes
precedence over impulse control: If you feel
bad, do it! Journal of Personality and Social
Psychology, 80(1), 53 – 67.
Tiffany, S. T., & Drobes, D. J. (1990). Imag-
ery and smoking urges: The manipulation of
affective content. Addictive Behaviors, 15(6),
531–539.
Twenge, J. M., Baumeister, R. F., DeWall, C.
N., Ciarocco, N. J., & Bartels, J. M. (2007).
Social exclusion decreases prosocial behavior.
Journal of Personality and Social Psychology,
92(1), 56–66.
Twenge, J. M., Baumeister, R. F., Tice, D. M.,
& Stucke, T. S. (2001). If you can’t join them,
beat them: Effects of social exclusion on
aggressive behavior. Journal of Personality
and Social Psychology, 81(6), 1058–1069.
Twenge, J. M., Catanese, K. R., & Baumeister,
R. F. (2003). Social exclusion and the decon-
structed state: Time perception, meaning-
lessness, lethargy, lack of emotion, and self-
awareness. Journal of Personality and Social
Psychology, 85(3), 409–423.
Velten, E. (1968). A laboratory task for induc-
tion of mood states. Behaviour Research and
Therapy, 6(4), 473–482.
Vohs, K. D., Baumeister, R. F., Mead, N. L., Hof-
mann, W. J., Ramanathan, S., & Schmeichel,
B. J. (submitted). Engaging in self- control
heightens urges and feelings.
Vohs, K. D., Baumeister, R. F., Schmeichel, B. J.,
Twenge, J. M., Nelson, N. M., & Tice, D. M.
(2008). Making choices impairs subsequent
self- control: A limited- resource account of
decision making, self- regulation, and active
initiative. Journal of Personality and Social
Psychology, 94(5), 883–898.
Vohs, K. D., & Heatherton, T. F. (2000). Self-
regulatory failure: A resource- depletion
approach. Psychological Science, 11(3), 249–
254.
Wagner, D. D., Boswell, R. G., Kelley, W. M.,
& Heatherton, T. F. (2012). Inducing negative
affect increases the reward value of appetizing
foods in dieters. Journal of Cognitive Neuro-
science, 24(7), 1625–1633.
Wagner, D. D., & Heatherton, T. F. (2013). Self-
regulatory depletion increases emotional reac-
tivity in the amygdala. Social Cognitive and
Affective Neuroscience, 8(4), 410–417.
Warburton, W. A., Williams, K. D., & Cairns,
D. R. (2006). When ostracism leads to aggres-
sion: The moderating effects of control depri-
vation. Journal of Experimental Social Psy-
chology, 42(2), 213–220.
Ward, A., & Mann, T. (2000). Don’t mind if I
do: Disinhibited eating under cognitive load.
Journal of Personality and Social Psychology,
78(4), 753–763.
Wegner, D. M. (1994). Ironic processes of men-
tal control. Psychological Review, 101(1),
34–52.
Wegner, D. M., & Erber, R. (1992). The hyper-
accessibility of suppressed thoughts. Journal
of Personality and Social Psychology, 63(6),
903–912.
Wegner, D. M., Erber, R., & Zanakos, S. (1993).
Ironic processes in the mental control of mood
and mood- related thought. Journal of Per-
sonality and Social Psychology, 65(6), 1093–
1104.
Williams, K. D., & Sommer, K. L. (1997). Social
ostracism by coworkers: Does rejection lead
to loafing or compensation? Personality and
Social Psychology Bulletin, 23(7), 693–706.
Willner, P., & Jones, C. (1996). Effects of mood
manipulation on subjective and behavioural
measures of cigarette craving. Behavioural
Pharmacology, 7(4), 355–363.
Witkiewitz, K., & Villarroel, N. A. (2009).
Dynamic association between negative affect
and alcohol lapses following alcohol treat-
ment. Journal of Consulting and Clinical Psy-
chology, 77(4), 633–644.
Zinser, M. C., Baker, T. B., Sherman, J. E., &
Cannon, D. S. (1992). Relation between self-
reported affect and drug urges and cravings in
continuing and withdrawing smokers. Journal
of Abnormal Psychology, 101(4), 617–629.
629
Aaker, J. L., 288
Aarts, H., 244, 350, 354, 356,
362, 366, 367, 620
Abe, K., 435
Abela, J. R., 405
Aber, J. L., 116, 309
Abler, B., 11, 65, 82, 145, 325,
326, 327
Abramovitz, A., 498
Abramson, L. Y., 383
Abu-Lughod, L., 288
Ackerman, B. P., 163
Adams, C. E., 480, 557
Adams, C. M., 95
Adams, G., 285
Adamson, L., 277
Addis, D. R., 104
Addis, M., 497
Adler, N. E., 586
Affleck, G., 589
Aglioti, S. M., 497
Agras, W., 483
Ahadi, S. A., 159, 165, 308, 310
Ahrens, A. H., 383
Aiken, P. A., 116, 117, 614
Ainslie, G., 99
Ainsworth, M. D. S., 226, 229,
237, 273, 277
Akerstedt, A. M., 212
Akiyama, T., 403
Aksan, N., 160, 313
Akutsu, S., 325
Albert, D., 9, 189, 284
Alberts, H. J. E. M., 352
Alcaine, O. M., 84, 403, 470
Aldao, A., 127, 135, 271, 327,
396, 398, 399, 401, 404, 405,
417, 418, 474, 483, 492, 496
Aldridge, J. W., 96, 471
Alea, N., 207
Aleman, A., 141, 148, 532
Alexander, R. D., 225
Algom, D., 132
Alicata, D., 435
Allan, C. L., 69
Allan, N. P., 164, 166
Allen, A. B., 379, 480, 557
Allen, A. H., 81
Allen, J. P., 192, 225, 228
Allen, J. S., 195
Allen, L. B., 496
Allen, M., 556
Allen, N. B., 187, 188, 190, 191,
352
Allman, J. M., 460
Alloy, L. B., 383, 416
Allport, G. W., 252, 306
Almeida, D. M., 209, 210, 211,
212
Alonso-Alonso, M., 355
Alper, C. M., 571, 587
Als, H., 277
Altamirano, L. J., 127
Alvarez, J., 405
Amar, S., 562
Amaral, D. G., 537
Ambady, N., 259
Amiaz, R., 355
Amir, M., 241, 242
Amir, N., 424, 513, 514, 516,
517, 520, 522, 523
Amir, T., 524
Amit, I., 576
Amodio, D. M., 362
An, S. K., 52
Anderson, A. K., 14, 252, 352,
530, 548, 556, 557
Anderson, B., 46
Anderson, C., 325
Anderson, C. L., 370
Anderson, C. S., 497
Anderson, E., 450
Anderson, J. R., 28
Anderson, M. C., 104
Anderson, P. L., 552
Anderson, S. W., 143
Andersson, G., 469
Andover, M. S., 530
Andrade, E. B., 141, 193
Andrade, J., 348
Author Index
630 Author Index
Andrew, C., 66
Andrews-Hanna, J. R., 450, 457,
460
Ang, C., 522
Angstadt, M., 83, 145
Ansell, E., 384
Anstiss, V., 353
Antonenko, O., 11, 341
Antoni, M. H., 575, 576
Antonucci, T. C., 210
Appleton, A. A., 596, 601, 603,
606, 607, 608
Apter, A., 399
Arbuckle, N. L., 260
Arch, J. J., 399, 551
Arellano, C. M., 276
Arevalo, J. M., 575
Ariely, D., 141, 369
Arkowitz, H., 476
Armony, J. L., 537
Arnsten, A. F. T., 230, 536
Arntz, A., 494, 495
Aron, A. R., 28, 33, 227, 273
Aron, E. N., 273
Aronson, E., 381
Aronson, J. A., 98, 141, 617
Asensio, S., 435
Ashby, F. G., 622
Asher, E. R., 226
Ashley, V., 509
Askegaard, S., 346
Aslin, R. N., 121
Asmussen, L., 187
Asnaani, A., 398, 529
Aspinwall, L. G., 291
Astin, J. A., 548, 552
Atlantis, E., 606
Atlas, L. Y., 36, 66
Attwood, A. S., 351, 523
Auerbach, R. P., 405
August, G. J., 431
Auksztulewicz, R., 104
Aupperle, R. L., 471, 473, 476
Austin, S. N., 259
Avenanti, A., 497
Avery, S. N., 81
Avihou-Kanza, N., 239
Avivi, Y. E., 471
Axelrod, S. R., 533
Aycock, J., 213
Ayduk, O., 93, 111, 112, 118,
119, 127, 129, 158, 161, 162,
190, 312, 378, 431, 479, 556
Azuma, H., 285, 294
B
Baars, B. J., 350
Babuscio, T., 439
Baccus, J. R., 384, 511
Bacharier, L. B., 590
Baddeley, A. D., 348, 617
Badiani, A., 429
Badre, D., 28, 33, 472, 477
Baer, R. A., 548, 552, 557, 559
Baerensten, K. B., 557
Baert, S., 422, 424, 518
Baeyens, C., 499, 500
Baeyens, F., 256, 353
Bagby, R. M., 329
Bailey, M. T., 576
Baime, M. J., 552
Baker, C. I., 24
Baker, L., 226
Baker, M., 606
Baker, N., 352
Baker, R., 396, 399
Baker, S. C., 97
Baker, T. B., 432, 616, 620
Bakermans-Kranenburg, M. J.,
63, 229, 510
Bakker, M. P., 164
Baldwin, M. W., 384, 511, 512,
513, 521
Ball, T. M., 398, 399, 400, 401,
402
Balzarotti, S., 325, 326, 327, 330
Banaji, M. R., 254, 352
Band, G. P. H., 53
Bandler, R., 26
Bandstra, N. F., 165
Bandura, A., 361
Banjeree, R., 296
Banks, S. J., 83, 145
Bannister, T., 241
Banton, T., 222
Bar, M., 176, 223, 450, 475
Barbas, H., 61, 459
Barber, B., 188
Barber, J. P., 539
Barbey, A., 448
Barch, D. M., 98, 230, 311, 474
Barden, J., 256
Bargas-Avila, J., 354
Barger, S. D., 574
Bargh, J. A., 31, 77, 254, 255,
288, 366, 367, 377
Bar-Haim, Y., 63, 510, 511, 512,
518, 521
Bariola, E., 189, 190, 191, 322,
328
Barkley, R. A., 98
Barlow, D. H., 14, 62, 134, 135,
370, 377, 393, 394, 395, 396,
397, 401, 406, 417, 424, 461,
469, 470, 473, 479, 481, 496,
498, 499, 508, 519, 529, 533,
551
Barnard, P. J., 529, 530, 536, 537
Barndollar, K., 366
Barnes, R. D., 353
Barnett, W. S., 167
Barnhofer, T., 560
Baron, R. M., 226
Baron, R. S., 227
Barrett, L. F., 5, 12, 13, 14, 24,
26, 27, 28, 29, 176, 223, 224,
252, 289, 447, 448, 449, 450,
451, 452, 453, 454, 455, 456,
459, 460, 461, 508
Barrig, P. S., 309
Barrios, V., 370
Barry, R. A., 167
Barsalou, L. W., 14, 447, 448,
449, 451, 452, 453, 458, 462
Bartels, A., 226
Bartels, J. M., 615
Bartholomew, K., 243
Bartholow, B., 45
Bartley, M., 241
Bassett, H. H., 181
Bastian, B., 385
Bates, J. E., 113, 158, 159, 161,
167, 305, 307, 310, 315
Bateson, G., 296
Baucom, D. H., 116, 117, 614
Baum, A., 575
Baumeister, R. F., 111, 116, 117,
128, 131, 132, 135, 230, 321,
348, 349, 350, 354, 355, 362,
366, 378, 381, 386, 472, 474,
613, 615, 616, 617, 618, 619,
620, 621, 622, 623
Baumgartner, T., 227
Bayles, K., 113
Beach, S. R., 276
Beal, L. S., 614
Beall, S. K., 598, 599
Beard, C., 514, 516, 517, 520,
521, 523, 524
Beauchaine, T. P., 491
Beaulieu-Pelletier, G., 379
Beauregard, M., 64, 84, 311
Author Index 631
Beaver, J. D., 309
Bebko, G. M., 396, 398
Becerra, L., 36, 82
Bechara, A., 101, 102, 141, 143,
223, 230
Beck, A. T., 395, 414, 469, 477,
482, 499, 559, 561
Beck, G., 575
Beck, J. E., 181
Beck, J. S., 530
Becker, E. S., 353
Beckers, T., 353
Beckes, L., 222, 224, 225, 226
Beckham, J. C., 601
Bedny, M., 28
Beer, J. S., 23, 27
Beers, M., 271, 338
Beesdo, K., 82
Beevers, C. G., 424, 474, 517,
530
Bégin, J., 278
Behar, E., 84, 403, 470
Behne, T., 223
Belin, D., 429
Belk, R. W., 346
Bell, J. R., 571
Bell, K. L., 188, 190, 191, 228
Bell, M. A., 316
Bellgowan, P. S., 452
Belloiu, A., 383
Belsky, J., 312
Bemis, J., 229
Benartzi, S., 105
Benca, R. M., 66
Benedict, R., 286
Bennett, S. M., 95, 394
Ben-Shlomo, Y., 605
Bentall, R. P., 418
Bentin, S., 452
Benvenuto, M., 13, 533
Berant, E., 240, 245
Berenson, G. S., 596, 597, 604
Berg, C. A., 204
Berger, A., 311
Berglund, P., 469
Bergman, A. J., 355
Bergquist, K. L., 436, 614
Berking, M., 14, 433, 529, 531,
534, 535, 538, 541, 542, 544
Berkman, E. T., 436, 437
Berkman, L. F., 579
Berkowitz, L., 614
Berlia, N., 293
Berlin, L. J., 241
Berman, M. G., 431
Berman, S., 36
Bernier, A., 229
Berns, G. S., 96, 145
Berntson, G. G., 259
Bernzweig, J., 313
Berridge, K. C., 96, 141, 145,
349, 356, 471
Berry, E. V., 8
Berscheid, E., 222
Bertram, B. C. R., 225
Besser, A., 243
Bessette-Symons, B., 207
Best, J. R., 166, 189
Bettinger, R. L., 222
Bettman, J. R., 134
Betts, J., 195
Bexkens, A., 316
Bhanji, J. P., 66
Bhullar, N., 589
Bickel, W. K., 98, 106
Bigman, Y., 364
Bijttebier, P., 164
Bilkei-Gorzo, A., 61
Billotti, D., 398
Bimson, W. E., 223
Birbaumer, N., 45
Birmingham, W., 204
Birnbaum, G. E., 244, 245
Bischoff, R., 549
Bishop, S. J., 31, 63, 64, 223,
395
Bishop, S. R., 439, 554
Bisighini, R. M., 433
Bixby, W. R., 230
Björntorp, P., 204, 205, 212
Black, D. S., 576
Black, P. H., 603
Blackburn, T. C., 292
Blackford, J. U., 81
Blackstock, E., 11
Blagov, P. S., 328
Blaine, B., 603, 605
Blair, C., 160, 163, 176
Blair, C. B., 161
Blair, K. S., 61, 84
Blanchard, D. C., 96
Blanchard, F. A., 116
Blanchard, R. J., 96
Blanchard-Fields, F., 195, 208,
210, 211, 213
Blankenburg, F., 104
Blascovich, J., 223, 367, 589
Blase, S. L., 486
Blechert, J., 49, 128, 129, 130,
131
Bleckley, M. K., 354
Blehar, M. C., 226, 237, 273,
277
Bliss-Moreau, E., 27, 450, 452
Bloch, L., 6, 267
Bloch, R. T., 557
Block, J., 119, 159, 308, 310, 321
Block, J. H., 159, 321
Blom, J. D., 69
Bluck, S., 207
Blumenthal, J. A., 588
Bocknek, E., 114
Bockstaele, V., 522
Bockting, C. L. H., 529, 535
Boden, M. T., 405
Bodenhausen, G. V., 255, 256,
257, 377
Boehm, J. K., 597, 602, 603, 605
Bogdan, R., 473, 474, 529
Boggio, P. S., 355
Bohlmeijer, E., 548
Böhm, G., 142
Bohner, G., 252
Bohus, M., 14, 491, 495, 530,
561
Boiger, M., 289, 292, 294
Bois, K., 379
Boisseau, C. L., 461
Boissy, A. A., 376
Bokhorst, C., 188
Boland, M., 370
Boldt, L. J., 166
Bolger, N., 36
Bolla, K. I., 436
Bomyea, J., 424, 514, 516, 522
Bonanno, G. A., 127, 128, 135,
245, 363, 368, 369, 371, 398,
415, 533, 598
Bond, F. W., 538
Boney-McCoy, S., 276
Bongers, A., 11, 314
Bonini, N., 141, 146, 147, 148,
149, 150
Bonn-Miller, M. O., 405, 433,
533
Bookheimer, S. Y., 82
Booth, R. J., 572, 574
Bora, E., 435, 437
Borkovec, T. D., 84, 379, 401,
403, 469, 470, 474, 475, 484
Born, L., 198
Bornovalova, M. A., 494
632 Author Index
Borras, C., 36, 82
Borsook, D., 36, 82
Bos, M. G., 316
Bossio, L. M., 602
Bösterling, A., 65
Boswell, J. F., 395
Boswell, R. G., 617
Botvinick, M. M., 79, 98, 103,
132, 311, 474
Bourgouin, P., 311
Bousman, C. A., 614
Bouton, M. E., 34, 61, 473, 480,
481
Bowen, S., 439, 440, 561
Bower, G. H., 140
Bower, J. E., 589
Bowie, B. H., 197
Bowlby, J., 3, 226, 228, 237,
238, 239, 240, 241, 246, 272,
273
Boyce, W. T., 167, 586, 587, 605
Boyd, R., 222, 571
Bradfield, E., Jr., 243
Bradizza, C. M., 433
Bradley, B. P., 309, 395, 422,
474, 478, 510
Bradley, M. M., 5, 44, 45, 46, 47,
132, 270, 310, 450
Braeckman, J., 384
Brame, B., 605
Brammer, M. J., 66, 120
Brandtstaedter, J., 368, 369
Branigan, C., 386, 552
Brasil-Neto, J. P., 70
Bratslavsky, E., 616, 618, 623
Brault, M., 278
Braungart-Rieker, J. M., 161,
165
Braveman, P. A., 586
Braver, T. S., 98, 189, 230, 311,
474
Bray, G. A., 497
Brazelton, T. B., 277
Brefczynski-Lewis, J. A., 458,
557
Brennan, K. A., 238, 243, 276
Brett, M., 63, 223, 395
Brewer, A. A., 449
Brewer, J. A., 439, 440, 557
Brewer, L. E., 622
Brewer, M. B., 222, 256, 622
Brewin, C. R., 353, 355
Bricker, J. B., 440
Bridges, L. J., 161
Bridle, R., 515
Brill, N. Q., 575
Briner, R. B., 6
Briñol, P., 253, 258, 259
Britton, J., 46
Broderick, J. E., 205
Brody, A. L., 435
Bromley, A., 556
Brooks, D., 111
Brooks, J. C. W., 223
Brophy-Herb, H. E., 114
Brosan, L., 523
Brosschot, J. F., 589
Brotman, D. J., 588
Brower, K. J., 496
Brown, C. M., 378
Brown, J. R., 182
Brown, K. W., 548, 552, 557
Brown, L. L., 273
Brown, M. Z., 495
Brown, S. B. R. E., 53
Brown, S. M., 395
Brown, S. P., 222
Brown, T. A., 370, 396, 397,
401, 417, 469, 473, 498, 533,
551
Browning, M., 511
Bruce, G., 351
Bruner, J., 284
Brunstein, J. C., 371
Bryant, D., 166
Bryant, F. B., 378
Bryson, S. E., 161
Bublatzky, F., 45
Büchel, C., 94, 101, 104, 105
Buchheld, N., 552
Buck, R. W., 5, 6, 574
Buckley, M., 188
Buckner, J. D., 517
Buckner, R. L., 104, 450, 457
Bucks, R. S., 511
Buell, M. M., 614
Buhle, J. T., 28, 29
Buhrmester, M. D., 381
Buis, T., 553
Buka, S. L., 601, 603, 607
Bukay, E., 49
Bukowski, W. M., 395
Bulik, C. M., 353
Bulka, D., 194
Bull, R., 213
Bullis, J. R., 396
Bullmore, E., 97
Bullock, B. M., 192
Bunde, J., 596, 597, 602
Bunge, S. A., 83, 120, 144, 189,
229, 244, 262, 288, 311, 361,
366, 398, 472, 556
Burgess, N., 104
Burgess, P. W., 97
Burghy, C. A., 68
Burgos, A. I., 500
Buring, J. E., 589
Burkley, M. A., 253
Burmeister, M., 67
Burnette, J. L., 243, 244
Burney, R., 559
Burns, B. M., 311
Burns, L. R., 355
Burns, M., 516
Burnstein, E., 263
Burrows, C. L., 46
Burton, C., 368, 371
Burton, R., 163
Bush, G., 311
Bush, N. R., 162, 167
Bushman, B., 615
Buss, K., 178
Busschbach, J. J., 494
Butler, A. C., 469
Butler, E. A., 11, 33, 275, 277,
278, 295, 579, 600
Butler, T., 82
Butner, J., 276
Buttenmuller, V., 552
Buttermore, N. E., 376, 379
Buunk, B. P., 382
Buysse, A., 243
C
Cabeza, R., 71, 204, 207
Cacioppo, J. T., 227, 259, 274,
308, 575, 579
Cadet, J. L., 436
Cafferty, T. P., 244
Cahill, S. P., 377
Cairns, D. R., 615
Calhoun, P. S., 601
Calkins, S. D., 176, 178, 188,
190, 191, 309, 597, 604
Call, J., 223
Calu, D., 429
Camerer, C. F., 23, 35, 93, 98,
141, 146, 472
Campbell, L., 511
Campbell Teskey, G., 616
Author Index 633
Campbell-Sills, L., 14, 62, 134,
135, 370, 393, 397, 398, 399,
400, 401, 417, 424, 473, 484
Campo, P., 460
Campos, J. J., 12, 84, 267, 268,
271, 275, 293, 306
Camprodon, J. A., 27, 355
Camras, L., 12, 84
Cancedda, L., 228
Canli, T., 84, 400, 496
Cannon, D. S., 617
Cannon, W. B., 588
Cantor, N., 361
Capitanio, J. P., 575
Caplovitz, B. K., 306
Caporeal, L. R., 222
Caraco, T., 225
Carelli, M. G., 190
Carey, S., 452
Carl, J. R., 396
Carlbring, P., 517
Carlson, E. A., 273
Carlson, G.., 316
Carlson, L. E., 548, 552
Carlson, S. M., 119, 163
Carmichael, S. T., 537
Carmona, P. E., 557
Carnelley, K. B., 243
Carpenter, M., 223
Carrere, S., 272
Carroll, K. M., 433, 434, 436,
438, 439
Carstensen, L. L., 13, 203, 204,
205, 206, 207, 208, 209, 210,
213, 214, 270, 272, 274, 275,
278, 366
Carter, B. L., 351
Carter, C. S., 35, 79, 97, 98, 311,
474
Carter S. R., 519
Carthy, T., 399, 400
Carulli-Rabinowitz, V., 177
Caruso, D. R., 323, 338
Carver, C. S., 159, 251, 321, 323,
328, 330, 331, 332, 334, 340,
349, 350, 361, 362, 364, 366,
369, 377, 378, 471, 613, 615,
618, 622
Casey, B. J., 68, 105, 111, 120,
121, 167, 189, 190, 431
Caspar, F., 535
Caspi, A., 67, 119, 178, 271, 306,
395, 605
Cassano, M. C., 181, 188, 604
Cassidy, J., 173, 182, 226, 238,
240, 244
Catanese, K. R., 621
Catanzaro, S. J., 323, 333, 533
Cato, M. A., 27
Catran, E., 11, 127, 230, 420
Cavanagh, K., 524
Chabris, C. F., 224
Chaiken, S., 93, 252
Chajut, E., 132
Chambers, C. T., 165
Chambers, R., 352
Chang, C.-H., 476
Chang, F., 311
Chang, L. J., 141, 142, 435
Chang, M., 575
Chapman, A. L., 495, 499
Chapman, D., 205
Chapman, J. E., 469
Chappell, K. D., 244
Charles, S. T., 13, 203, 204, 205,
206, 207, 208, 209, 210, 211,
212, 213, 274, 366
Chartier, M., 560
Chartrand, É., 278
Chase, H. W., 434
Chaskalson, M., 550
Chasserot-Golaz, S., 575
Chatterjee, N., 227
Chaturvedi, A., 460
Chatzisarantis, N. L. D., 618
Chawla, N., 439
Chen, E., 572, 575, 577, 586,
587, 589, 590, 591, 592, 598,
600, 606, 608
Chen, K. H., 46
Cheng, C., 368
Cheng, J. Y., 589
Cherek, D. R., 119
Chesney, M. A., 586
Cheung, R. Y. M., 295
Chew, C. H., 574
Chiao, J. Y., 396
Chida, Y., 599, 602, 603
Chiesa, A., 558, 559
Childress, A. R., 430, 433, 614,
620
Chirkov, V., 288
Chiu, W. T., 530
Choate, M. L., 496
Choi, K. S., 460
Chorpita, B. F., 401
Christ, O., 535
Christakou, A., 120
Christenfeld, N., 588
Christensen, A., 270
Christensen, H., 608
Christensen, T. C., 533
Christenson, G. A., 620
Christianson, R., 351
Chugani, H. T., 228
Ciarocco, N. J., 615
Ciarrochi, J., 190
Cicchetti, D., 178
Ciesielski, B. R. G., 133
Cirulli, F., 494
Cisler, J. M., 63, 470, 496, 510
Clark, C. L., 238, 276
Clark, D. A., 395
Clark, L., 431
Clark, M. S., 140
Clarke, R. J., 61, 62
Clasen, P. C., 474, 478
Claudio, V., 422
Claxton, L. J., 163
Clifton, J., 556
Cloak, C., 435
Clore, G. L., 23, 222, 223, 230,
363, 454, 473, 476, 622
Clyburn, A., 115
Coan, J. A., 13, 221, 222, 223,
224, 225, 226, 227, 228, 229,
230, 272, 274, 277, 278
Coats, A. H., 195
Codispoti, M., 45, 46, 132, 310,
450
Coffino, B., 273
Cohen, D., 285
Cohen, H., 599
Cohen, J., 474
Cohen, J. B., 193
Cohen, J. D., 30, 35, 79, 85, 93,
94, 97, 98, 103, 112, 141, 145,
148, 311, 435
Cohen, J. R., 119, 120
Cohen, L., 575
Cohen, N., 400
Cohen, S., 221, 224, 571, 587,
603
Coifman, K., 128, 368, 398, 415,
533
Cole, P. M., 6, 84, 175, 273, 275,
291
Cole, S. W., 190, 571, 572, 573,
574, 575, 576, 577, 578, 579
Coles, M. G., 79, 103
Collado-Hidalgo, A., 576
Collins, A., 23
634 Author Index
Collins, R. L., 382, 618
Collins, W. A., 273
Colombo, J. A., 230
Colvin, P., 352
Compas, B. E., 159, 194, 195
Compton, R. J., 189
Comtois, K. A., 538
Connally, E., 209
Connell, J. P., 161
Conner, O. L., 227
Conner, T., 13
Connolly, C. I., 96
Connolly, T., 386
Connor-Smith, J. K., 159
Conover, K., 96
Conraads, V. M., 571
Conrad, A., 536
Conrey, F. R., 260
Conroy, M., 294
Consedine, N. S., 206, 214, 598,
599, 604
Contreras, J. M., 183
Conway, A. R. A., 354
Cook, C. L., 367, 589
Cook, N. R., 589
Cools, J., 117
Cooney, N. L., 430
Cooney, R. E., 66
Cooper, J. C., 31
Cooper, V., 599
Corbetta, M., 148, 556
Corbin, W., 429
Corcoran, K. A., 34
Cordovil de Sousa Uva, M., 433
Corkin, S., 209
Corr, P. J., 384
Corson, H., 58
Cortina, K. S., 180
Cosmides, L., 491, 538
Costa, P. T., Jr., 571
Côté, S., 271, 338
Covert, M. V., 377
Cowan, C. P., 267, 274
Cowan, P. A., 267, 274
Cowan, R. L., 81
Cox, R. H., 497, 498
Cox, W. M., 52, 351, 523
Coy, K. C., 161, 165
Cozzarelli, C., 241, 242
Craig, A. D., 27, 28, 478
Crandall, C. S., 251
Crane, C., 562
Craske, M. G., 399, 470, 480,
483, 484, 551
Crauthers, D. M., 430
Creswell, J. D., 548, 575, 576
Crişan, L. G., 143
Crisp, V. W., 179
Critchley, H. D., 27, 28, 314
Crits-Christoph, P., 433, 539
Crockenberg, S. C., 161, 309
Crockett, M. J., 11, 396, 537
Crombez, G., 353, 395, 522
Crone, E. A., 61
Croog, S., 589
Crowell, S. E., 229, 491, 494
Cruess, D. G., 589
Csikszentmihalyi, M., 276
Cubbin, C., 586
Cuijpers, P., 469, 535, 548
Cullen, M. R., 603
Culver, C., 114
Cumberland, A., 179
Cumming, G. F., 614
Cummings, E. M., 178, 179
Cunningham, W. A., 23, 27, 251,
252, 260, 261, 262, 454
Curthbert, B. N., 450
Custers, R., 362, 366, 367, 450
Cuthbert, B. N., 44, 45, 132, 310
Cutrona, C. E., 227
D
Dadhkah, A., 296
Dahl, A., 267
Dahl, R. E., 188, 189, 190, 475
Dakof, G. A., 382
Dale, R., 259
Dalgleish, T., 141, 398, 401, 422,
424
Damasio, A. R., 101, 102, 141,
143, 536
Damasio, H., 28, 101, 141, 143
Dandeneau, S. D., 511, 512, 513,
515, 521
D’Andrade, R. G., 284, 289
Danesh, D., 600, 601, 603
Danesh, J., 589
D’Angelo, M., 522
Dan-Glauser, E. S., 7, 574
Dankwa-Mullan, I., 586
Dapretto, M., 432
Darley, J. M., 98
Darwin, C., 571, 579
Daselaar, S. M., 204
Daskalakis, Z. J., 64, 68
Daubman, K. A., 140
Daughters, S. B., 433
Daumann, J., 435, 437
Davachi, L., 27
D’Avanzato, C., 418
David, D., 383
Davidson, B. J., 63
Davidson, K. W., 588
Davidson, M. L., 33, 52, 62, 83,
403
Davidson, R. E., 574
Davidson, R. J., 5, 29, 52, 59, 61,
63, 66, 177, 208, 223, 263,
274, 398, 458, 474, 491, 493,
552, 556, 557, 574
Davies, N. B., 221
Davies, P. T., 178, 179
Daviglus, M. L., 607
Davis, B., 191
Davis, D. E., 243, 245
Davis, J. I., 27, 31
Davis, K. E., 244
Davis, L. S., 225
Davis, M., 60, 63, 471
Davis, S. W., 190, 204
Davis, T., 70
Dawe, S., 93
Dayan, P., 34, 96
de Beurs, E., 497
de Bono, J., 510
De Cesarei, A., 46
de Groot, A. D., 134
De Houwer, J., 77, 243, 254,
256, 353, 522
De Leersnyder, J., 9, 189, 284,
287, 293, 294
De Leonardis, D. M., 207
De Martino, B., 35
De Raedt, R., 422, 424, 518
de Ridder, D. T. D., 332,
349–350
de Rover, M., 46, 53
de Villers-Sidani, E., 86
Deater-Deckard, K., 307
Deaton, A., 205
DeBoer, D. D., 225
Deci, E. L., 361, 369
Decicco, J. M., 52
DeClaire, J., 531
DeCoster, J., 263
Dedert, E. A., 601, 602
Dedovic, K., 223
DeFronzo Dobkin, R., 383
Degnan, K. A., 509
Dekker, M. C., 605
Del Giorno, J. M., 230
Deldin, P., 556
Author Index 635
DeLeone, C. M., 435, 436
Delgado, M. R., 26, 31, 34, 35,
61, 62, 66, 78, 80, 81, 85, 96,
144, 145, 146, 148, 149, 472,
473
Delvaux, E., 284, 295
Demaree, H. A., 11, 189, 354,
574
DeMarree, K. G., 259
Demetrovics, Z., 433
Demler, O., 469, 530
Denham, S. A., 163, 181, 275
Dennis, N. A., 204
Dennis, T. A., 6, 46, 52, 84, 175
Denny, B. T., 27
Denollet, J., 571
Denson, T. F., 599
Derakshan, N., 520
Deroche, V., 616
Derryberry, D., 115, 159, 160,
306, 307, 308, 309, 509
Desimone, R., 28
Désiré, L. L., 376
D’Esposito, M., 472, 477
DeSteno, D. A., 386
Deutsch, R., 257, 262, 263, 352,
353
Devine, P. G., 255
DeVito, E. E., 436, 439
DeVoe, M., 194
DeWall, C. N., 367, 615, 622
Dewar, K. M., 454
Dewitte, M., 243
Dewitte, S., 99
Dhawan, N., 293
di Pellegrino, G., 80, 142
Di Sclafani, V., 435
Di Simplicio, M., 401
Di Tella, C., 49, 129
Diamond, A., 161, 167, 315
Diamond, L. M., 242, 244, 276,
291
Dichiara, G., 437
Dickerson, S. S., 384, 600
Diderholm, E., 589
Dien, J., 44
Diener, E., 205, 285, 286, 363
Diener, M. L., 161, 165
Dietrich, A., 230
Dijksterhuis, A., 80, 366, 618
Dillon, D. G., 129
Dingemans, A. E., 529
Dishion, T. J., 164, 192
Distel, M. A., 494, 495
Ditzen, B., 242
Djebali, S., 572
Dobkin, P. L., 559, 562
Dobson, K. S., 537, 558
Dobzhansky, T., 472
Dodson, J. D., 270
Dolan, R. J., 28, 35, 101, 499,
537
Dolcos, F., 207
Dolev, T., 245
Dollard, J., 471
Donahue, O. M., 497
Donchin, E., 79, 103
Donelan-McCall, N., 182
Donzella, B., 315
Dorris, M., 146
Doss, B. D., 274
Dougherty, D. M., 119
Douglas, H., 561
Douilliez, C., 499
Dovidio, J. F., 255
Downey, G., 118
Downs, D. L., 381
Doyle, W. J., 571, 587
Dozois, D. A., 476, 496
Dozois, D. J., 552
Drabant, E. M., 11, 81, 395
Dragone, D., 142
Drake, R. M., 28
Drevets, W. C., 23, 64, 311
Driver, J., 537
Drobes, D. J., 614, 623
Duarte, C. S., 606
Duchesne, S., 229
Duckworth, A. L., 531
Duclos, S. E., 499
Dudley, A., 195
Dugas, M. J., 395, 475
Duka, T., 351
Dum, R. P., 28
Dumas, J. E., 275
Dunbar, K., 97
Duncan, J., 28, 63, 97, 141, 223,
395
Dundin, L., 499
Dunn, B. D., 398, 401
Dunn, E. W., 198, 259
Dunn, G., 384
Dunne, J. D., 182, 552
Dunning, J. P., 43, 46, 47, 48,
52, 129, 574
Dunton, B. C., 253
Durant, S. M., 226
Durazzo, T. C., 435
Durso, G. R. O., 258
Durston, S., 68
Dutra, L., 438
Duval, S., 377
Dvorak, R. D., 99
Dweck, C. S., 105
Dyer, A. R., 607
Dyson, M. W., 316
D’Zurilla, T. J., 497, 531, 540
E
Eagly, A. H., 252
Earle, T. L., 588
Eastin, M., 614
Eaton, K. L., 179
Ebbesen, E. B., 114, 616
Eberl, C., 353
Ebert, D., 535, 543
Ebmeier, K. P., 69
Ebner-Priemer, U. W., 495
Ebstein, R. P., 229
Ebsworthy, G., 511
Eccles, J. S., 188
Eccleston, C., 522
Eckhardt, C. I., 351
Eddy, K. T., 83, 145
Edelman, G. M., 450
Edge, M. D., 399
Edwards, E. P., 113
Edwards, G., 98
Eftekhari, A., 399
Egerter, S., 586
Eggert, L. D., 243
Eggum, N. D., 158, 164
Egner, T., 31, 61, 78, 79, 80, 81,
316
Ehlert, U., 227
Ehrenberg, M. F., 225
Ehrenreich, J. T., 461
Ehrenthal, J. C., 245
Ehring, T., 65, 83, 127, 396, 399,
418, 419, 420
Ehrlich, A. H., 230
Ehrlich, I., 81
Ehrlich, P. R., 230
Ehrman, R., 433
Eickhoff, S. B., 27, 434
Eid, M., 285, 286, 363
Eidelman, P., 416
Eiden, R. D., 113
Eifert, G. H., 397, 398, 401,
403
Eigsti, I., 111
Eimer, M., 47
Eippert, F., 145
636 Author Index
Eisenberg, N., 114, 115, 119,
157, 158, 159, 160, 161, 162,
163, 164, 165, 166, 168, 179,
181, 313, 603
Eisenberger, N. I., 82, 227, 228,
240
Eisendrath, S., 560
Eiser, J. R., 253
Ekman, P., 294, 325, 491
El Sheikh, M., 589
Eldar, S., 511, 518, 519
Eldreth, D. A., 436
Elgar, M. A., 224
Ellard, K. K., 14, 62, 393, 395,
396, 403, 406, 461, 475
Ellenbogen, M. A., 498
Elliott, A., 288
Elliott, R., 475, 477, 478, 482
Ellis, A. J., 474
Ellis, L. K., 305, 312
Ellsworth, P. C., 5, 291, 292,
363, 383
El-Sheikh, M., 176
Ely, R. J., 116
Ely, T. D., 24, 96
Emery, G., 414, 477, 499
Emery, L., 208
Emery, R. E., 244
Emmons, R. A., 380
Emslie, H., 141
Endler, N. S., 329
Eng, J. S., 14, 192, 321, 325,
326, 343, 399
Engell, A. D., 98
Engle, R. W., 354, 355, 450
English, T. E., 11, 209, 325, 327,
331, 335, 340, 341, 579
Epel, E. S., 589, 600
Epstein, D. H., 429, 433
Epstein, J., 66
Epstein, N. B., 383
Epstein, W., 222
Erath, S. A., 176
Erber, R., 132, 192, 378, 617,
618
Ericson, K. M., 35, 94
Erikson, E. E., 188
Erikson, E. H., 274
Erikson, J., 274
Erk, S., 11, 61, 65, 66, 82, 145
Ernst, T., 435
Ersche, K. D., 437
Erskine, J. A. K., 353
Ersner-Hershfield, H., 274
Erthal, F., 61
Eshleman, A., 251
Eslinger, P. J., 102
Essex, M. J., 164
Esteves, F., 554
Estrada, M. J., 379
Etkin, A., 7, 31, 59, 61, 63, 64,
76, 78, 80, 81, 268, 288, 316,
352, 366, 403, 473, 474, 484,
496, 532, 537, 574
Evans, D., 310
Evans, E. M., 552
Evans, J. St. B. T., 141
Evans, K., 384
Everitt, B. J., 31, 434
Evers, C., 257, 366
Evershed, S., 500
Everson-Rose, S. A., 588, 589,
602
Eves, F., 81
F
Faber, R. J., 620
Fabes, R. A., 114, 161, 165, 179,
181, 313
Fadardi, J. S., 351, 523
Fahey, J. L., 574, 575, 589
Fair, D. A., 311
Fairburn, C., 483
Fairholme, C. P., 461
Falk, E. B., 436
Fan, J., 312, 495
Fan, M., 315
Fang, A., 529
Farabaugh, A. H., 469
Farach, F. J., 396
Farb, N. A. S., 14, 352, 476, 530,
531, 537, 538, 548, 556, 557,
559
Farchione, T. J., 395, 406
Fava, M., 500
Fazio, R. H., 252, 253, 254, 255,
256, 260, 262
Fearnow, M. D., 182
Feeney, J. A., 245, 271
Fehr, E., 35, 148
Fein, G., 435
Fein, S., 620
Feinberg, M., 11, 341
Feinstein, J. S., 309
Feldman, G. C., 416, 551, 555
Feldman, R., 178
Feldman, S. I., 118, 361
Feldman-Barrett, L., 533
Feldner, M. T., 401, 496, 533
Feldon, J., 494
Fendrich, M., 114
Fenigstein, A., 618
Fera, F., 67, 121, 537
Ferdinand, R. F., 164
Ferguson, M. F., 361, 362, 364,
365, 366
Ferguson, M. J., 259
Fernald, A., 269
Ferrari, V., 45, 46
Ferrer, E., 276
Ferri, J., 46, 48, 50, 53
Ferry, A. T., 27, 460
Ferster, C. B., 474, 477, 480, 481
Ferstl, E. C., 27
Festinger, L., 256
Feuerstein, R., 212
Fiebach, C. J., 394
Field, M., 52, 351
Field, N. P., 245, 378
Figner, B., 35, 93, 94, 100, 104
Filkowski, M. M., 460
Finch, C. E., 571, 572, 576
Fine, I., 449
Fine, S. E., 163
Finkel, E. J., 230, 244, 351
Finkenauer, C., 623
Finucane, M. L., 141
Fiore, M. C., 432, 620
Fischer, A., 542
Fischer, E. F., 273
Fischer, S., 65, 83, 127, 418
Fischman, M. W., 429
Fishbach, A., 353, 361, 362, 364,
365, 366, 367, 623
Fisher, A. J., 469
Fisher, E. B., Jr., 590
Fisher, H. E., 273
Fisher, M., 86
Fisher, P., 81, 159, 310
Fisher, R. R., 349
Fissell, K., 79
Fitzgerald, D. A., 96
Fitzgerald, H. E., 496
Fitzgerald, P. B., 64, 68
Fitzsimons, G. M., 230
Flaisch, T., 45
Flanagan, T. J., 396
Fleck, M. S., 204
Fletcher, K., 98
Floerke, V. A., 370
Flombaum, J. I., 312
Flood, M. F., 178
Flor, H., 81
Author Index 637
Florian, V., 229, 241, 242, 244
Florsheim, P., 352
Flykt, A., 554
Flynn, E., 163
Foa, E. B., 134, 377, 530
Focquaert, F., 384
Fok, A. K., 575
Folkman, S., 194, 321, 323, 328,
329, 330, 579, 586
Føllesdal, H., 339
Folsom, A. R., 596
Foltin, R. W., 429
Fong, G. W., 95
Forbes, E. E., 475
Ford, B. Q., 193, 365, 366, 369,
370, 371
Forgas, J. P., 140
Forman, E. M., 179, 352, 469
Forman, H., 189
Förster, G., 348
Forster, J., 478
Forsyth, D. R., 243
Forsyth, J. P., 370, 496
Foster, M. T., 620
Foti, D., 43, 44, 45, 46, 47, 48,
50, 51, 129, 574
Fountain, S., 68
Fourkas, A. D., 497
Fowler, J. S., 434
Fox, E., 522
Fox, H. C., 432, 614
Fox, N. A., 159, 308, 509, 521
Fox, P. T., 27
Fox Keller, E., 580
Fraley, R. C., 229, 238, 245
Francart, B., 499
Franceschini, M. A., 228
Francis, S., 227
Franconeri, S. L., 396
Frank, D., 45
Frankel, C. B., 12, 84
Franken, I. H. A., 52, 53, 347,
354, 434, 495
Frankenburg, F. R., 495
Frankenhaeuser, M., 576
Frankenstein, U. N., 33
Frankforter, T. L., 433
Franklin, S., 350
Franklin, T. R., 435
Franks, M. M., 225
Frattaroli, J., 572, 599
Frazier, P., 229
Frederick, S., 98, 106
Fredrickson, B. L., 117, 275, 363,
386, 552, 553
Freedman, B., 548, 552
Freeman, J. B., 259
Freeman, P., 223
Freer, C., 141
Frenn, K., 188
Fresco, D. M., 14, 396, 406, 469,
470, 473, 475, 479, 483, 484,
496, 529, 530, 553
Freud, A., 366, 367
Freud, S., 3
Freund, A. M., 208, 209
Frewen, P. A., 496, 552, 555
Friedel, E., 67
Friederich, H. C., 245
Friedlmeier, W., 294
Friedman, B. H., 401
Friedman, L. A., 589
Friedman, R. S., 353, 367, 478
Friese, M., 347, 348, 352, 354
Friesen, W. V., 294
Frijda, N. H., 4, 58, 77, 284,
288, 292, 295, 296, 298, 363,
365, 377
Friston, K., 224
Frith, C. D., 28
Frodl, T., 68
Fromme, K., 429
Fromson, P. M., 378
Frost, R. O., 116, 117
Fruzzetti, A. E., 500
Fry, A. F., 98, 111
Fu, L., 436
Fucito, L. M., 433
Fujita, K., 105, 252, 352
Fulton, P. R., 560
Funder, D. C., 119
Fung, H. H., 204, 285, 286,291,
363
Furedy, J. J., 498
Furth, E. F., 529
Fusi, S., 448, 456
Fuster, J. M., 230
G
Gable, P. A., 117, 362
Gable, S. L., 226, 228, 273
Gabrieli, J. D. E., 31, 83, 120,
145, 311, 398, 472, 556
Gagnon, K. T., 223
Gaines, S. O., Jr., 243
Gale, S., 78
Gallagher, H. L., 28
Gallagher, M., 26, 27
Gallistel, C. R., 96
Gallo, L. C., 587
Galloway, G. P., 433
Gallup, G. G., Jr., 384
Galobardes, B., 587
Galvan, A., 121, 189
Gamble, S. A., 433
Gao, W., 204, 311
Garavan, H., 436
Garbutt, L. D., 603
Gard, D., 556
Gardner, C. O., 469
Gardner, W. L., 259, 288, 622
Garland, E. L., 553
Garlow, S. J., 460
Garnefski, N., 127, 195, 323,
331, 332, 368, 416, 417
Garner, M., 395
Garner, P. W., 114, 115
Garon, N., 161, 166
Garvin, E., 363
Gaschke, Y. N., 334
Gasper, K., 363
Gatchalian, K. M., 98
Gatenby, J. C., 95
Gatz, M., 205, 469
Gaunt, R., 93
Gaviria, A. M., 494
Gawronski, B., 253, 255, 256,
257, 262
Gaylord, S. A., 553
Gazzola, V., 227
Gearing-Small, M., 225
Gekoski, M., 177
Gelenberg, A. J., 559
Gelernter, J., 395
Gelfand, L., 552
Gellaitry, G., 599
Gendron, M., 451
Gentzler, A. L., 183
George, M. S., 69
Ger, G., 346
Gerardi-Caulton, G., 311, 312
Gerin, W., 588, 589
Germer, C. K., 560
Gerstorf, D., 212, 213
Getz, S., 189
Ghahremani, D. G., 436
Ghashghaei, H. T., 61
Gianaros, P. J., 27
Gianino, A., 178
Gianotti, L. R., 35
Gibb, B. E., 530
Gibbons, M. B. C., 539
Gidron, Y., 571
638 Author Index
Giedd, J. N., 432
Giesler, R. B., 382
Gilbert, D. G., 430
Gilbert, D. T., 35, 93, 129, 130,
131, 259, 379, 380
Gilbert, P., 480, 530, 534, 538
Gillath, O., 229, 239, 243, 244
Gilligan, C., 119
Gillihan, S. J., 67
Gilliom, M., 181, 182
Gillis, M. M., 35, 66, 145, 472
Gilman, S. E., 601
Gilovich, T., 380
Giordano, L. A., 99
Giorgetta, C., 141, 146, 147, 148,
149, 150
Glaser, R., 575, 589, 603
Glickman, S., 518
Glimcher, P. W., 25, 85, 94, 104,
227
Glover, G., 145
Gnys, M., 430
Goeke-Morey, M. C., 179
Goeleven, E., 422
Gohar, D., 376
Gohm, C. L., 473, 476
Goldberg, R. F., 28
Golden, S. H., 182, 588
Goldin, P. R., 11, 14, 33, 83, 84,
397, 398, 400, 402, 403, 406,
496, 551, 557
Goldman, R., 475
Goldman, S. L., 589
Goldsmith, D., 285
Goldsmith, H. H., 177, 306
Goldstein, C. R., 394
Goldstein, M., 316
Goldstein, R. Z., 51, 230, 433,
434
Goldstone, R. L., 453
Goldwyn, R., 276
Golland, P., 452
Golland, Y., 452
Gollwitzer, P. M., 255, 361, 366,
367, 377
Golub, S. A., 380
Gonzaga, G. C., 226, 273
Goodkind, M. S., 269, 277, 400
Goodman, D. F., 449
Goodman, E., 587
Goodman, M., 175, 177, 508
Goodwin, G. M., 511
Goodwin, R. D., 178, 606
Goolkasian, G. A., 116
Goolkasian, P., 551
Gordijn, E. H., 618
Gordon, J. R., 560, 614
Gordon, N. S., 551
Gore, J. C., 95
Goren, D., 208
Gorman, J. M., 31
Gormley, B., 241
Gotlib, I. H., 66, 134, 378, 416,
417, 418, 419, 422, 423, 431,
475, 553
Gottman, J. M., 179, 180, 181,
206, 270, 271, 272, 274, 277,
278, 279, 531
Gottman, J. S., 272, 279
Gottschalk, J.-M., 542
Goubert, L., 243
Gouzoulis-Mayfrank, E., 437
Graff, J., 270
Graham, B. M., 14, 81, 351, 508,
522
Graham, L., 560
Granqvist, P., 239
Grässman, R., 371
Grasso, D. J., 45
Gratton, G., 79, 103
Gratz, K. L., 275, 323, 333, 337,
338, 495
Grawe, K., 530, 531, 537, 538
Gray, J. A., 307, 308, 354, 471,
537
Gray, J. R., 115, 189, 367
Gray, M. A., 27, 28
Graybiel, A. M., 96
Gray-McGuire, C., 589
Graziano, W. G., 314
Greco, C. M., 556
Grecucci, A., 140, 141, 142, 146,
147, 148, 149, 150, 190
Green, J. D., 243
Green, L., 98, 111
Green, M. J., 418
Greenbaum, C. W., 178
Greenberg, L. S., 475, 530, 534,
539
Greene, J. D., 98
Greenland, P., 607
Greenwald, A. G., 253, 254, 366
Greenwald, M. K., 270
Greenwood, G., 533
Greeson, J., 551
Grégoire, J., 121
Greicius, M. D., 403
Greischar, L. L., 458, 556
Greiveldinger, L. L., 376
Grich Stevens, J., 229
Griffin, K. M., 350
Griggs, C., 188
Grisham, J. R., 384, 599
Grolnick, W. S., 161
Groom, C., 510
Gross, J. J., 3, 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 23, 24, 28,
30, 31, 33, 43, 45, 47, 48, 49,
58, 59, 60, 65, 76, 77, 78, 83,
84, 94, 105, 106, 118, 120,
121, 127, 128, 129, 130, 131,
133, 134, 136, 142, 145, 146,
157, 158, 187, 189, 194, 195,
196, 210, 252, 257, 268, 269,
270, 271, 274, 275, 276, 277,
287, 288, 290, 295, 311, 314,
321, 322, 323, 324, 325, 326,
327, 328, 329, 330, 334, 335,
337, 341, 350, 351, 354, 361,
365, 366, 367, 368, 382, 394,
395, 397, 398, 399, 400, 405,
415, 416, 417, 418, 420, 423,
432, 434, 441, 447, 451, 456,
457, 472, 474, 475, 476, 477,
480, 492, 496, 508, 510, 520,
529, 531, 533, 537, 549, 550,
551, 556, 557, 571, 574, 579,
587, 588, 589, 591, 597, 599,
600, 601, 603, 604, 613, 614
Gross, R. E., 460
Grossman, P., 539, 548, 555, 559
Grossmann, I., 291
Gruber, J., 84, 127, 341, 370,
414, 416, 417, 420
Gruenewald, T. L., 591
Grühn, D., 205, 206, 209, 213,
214
Grunewald, T. L., 384
Grunhaus, L., 355
Gruzelier, J., 81
Gschwendner, T., 253, 352
Guest, D. E., 384
Guh, D. P., 589
Gujar, N., 492, 497
Gullone, E., 189, 195, 322, 323,
328
Günaydin, G., 240
Gunderson, J. G., 495
Gunnar, M. R., 176, 178, 188,
315
Gupta, S., 128
Gurwitz, V., 239
Guth, W., 146
Guthrie, I. K., 161, 165
Gutiérrez-Zotes, J. A., 494
Author Index 639
Gwaltney, J. M., 571
Gyurak, A., 7, 59, 76, 78, 268,
269, 271, 276, 277, 288, 352,
366, 400, 532, 537, 574
H
Haaga, D. A. F., 383
Haase, C. M., 6, 267
Häcker, F., 45
Hackett, M. L., 497
Haedt-Matt, A. A., 614, 621
Hagan, R., 395
Hagemann, T., 277
Haggard, P., 403
Hagger, M. S., 618
Hagtvet, K. A., 339
Haidt, J., 76, 363
Hajcak, G., 10, 44, 45, 46, 47,
48, 49, 50, 51, 52, 53, 129,
130
Hakamata, Y., 520
Hakimi, S., 83, 400, 496
Hakkaart-van Roijen, L., 494
Halberstadt, A. G., 179
Hale, C. R., 451
Halford, G. S., 230
Hall, E. E., 230
Hallion, L. S., 421, 519, 520
Halperin, E., 14, 15
Hamann, S. B., 11, 24,66, 96,
225
Hamby, S. H., 276
Hamer, M., 242, 599, 603
Hammond, G., 421
Hampshire, A., 424
Hamre, B. K., 163
Han, H. A., 105, 352
Hancock, J., 480, 557
Hänel, M., 274
Haney, M., 429, 432
Hanke, M. L., 576
Hannon, P. A., 244
Hansenne, M., 8
Harber, K. D., 224
Hardaway, C. R., 164
Hardin, C. D., 255
Hare, T. A., 35, 79, 81, 85, 93,
94, 100, 101, 104, 121, 167
Harger, J., 589
Hariri, A. R., 67, 82, 100, 120,
121, 395, 537
Harlan, E. T., 119, 161, 309
Harlé, K. M., 141, 148
Harley, R., 500
Harlow, H. F., 229
Harman, C., 306, 308
Harmer, C. J., 512
Harmon-Jones, C., 347, 362, 619
Harmon-Jones, E., 117, 347, 362,
619
Harned, M. S., 492, 494
Harold, G. T., 179
Harris, C. R., 11, 574
Harris, J. D., 165
Harris, P., 599
Harrison, A., 496
Harrison, N. A., 27, 28
Hart, C. L., 52, 429, 433, 435,
437
Hart, D., 378
Hart, S. L., 209
Harter, S., 381
Hartley, C. A., 23, 33
Hartman, C. A., 164
Harvey, A. G., 84, 127, 341, 416,
417, 461
Hasher, L., 422
Hashmi, N., 224
Haslam, N., 461
Hasselmo, K., 224
Hatfield, B. E., 163
Hatzenbuehler, M. L., 188, 404
Haukkala, A., 601
Hauser, M., 27
Hauser, S. T., 192
Havermans, R. C., 353
Havighurst, R. J., 273
Hawkley, L. C., 274, 575, 579
Haydon, K. C., 273
Hayes, A. M., 481, 555
Hayes, J. P., 11
Hayes, S. C., 378, 474, 475, 478,
479, 481, 482, 498, 513, 520,
533, 538, 551, 561
Hazan, C., 240
Hazen, R. A., 516
Hazlett, E. A., 494
Head, J., 241
Heatherton, T. F., 116, 119, 135,
230, 362, 366, 613, 614, 615,
617, 618, 619, 620, 621, 622
Heaven, P. C., 190
Heavin, S., 352
Hebb, D. O., 228
Heckman, J. J., 575
Heekeren, H. R., 104
Heeren, A., 514, 517
Heering, S., 398
Heffner, M., 397, 398, 403
Heilman, R. M., 143, 149, 150
Heim, C., 176
Heimberg, R. G., 84, 396, 400,
469, 473, 496
Heimpel, S. A., 363
Heine, S. J., 285, 286, 289, 621
Heinrichs, M., 227
Heinz, A., 67, 145, 436
Heinz, S. P., 128
Heishman, S. J., 433
Heissler, J., 11, 65, 314
Hektner, J. M., 276
Helfinstein, S. M., 509
Heller, A. S., 66, 263, 496
Hellmuth, J. C., 271
Henik, A., 400
Hennekens, C. H., 589
Henrich, J., 571
Henry, B., 271
Henry, J. D., 206, 210
Henry, J. P., 576
Heppner, W. L., 552
Herbert, C., 45, 257
Herman, C. P., 116, 614, 617,
622
Hermann, A. D., 81, 253
Hermans, D., 353, 424
Hernandez, A., 494
Hernández-López, M., 440
Herpertz, S. C., 494
Herrmann, L. L., 69
Herrmann, M. J., 52
Hershberger, W. A., 97
Hershey, K. L., 159, 165, 308,
310
Hertel, P. T., 422, 512, 521
Herwig, U., 65, 82
Heslenfeld, D. J., 61, 223
Hess, R. D., 294
Hess, T. M., 208, 214
Hester, R., 436
Hickcox, M., 430
Hicklin, D., 227
Hicks, A. M., 242, 244, 276
Higashi, M., 225
Higgins, E. T., 348, 377, 380,
471, 473, 476, 621
Higgins, R. L., 615
Hilgard, J., 45
Hilgetag, C. C., 61
Hill, A. L., 165, 597, 604
Hill, C. L., 398
Hill, K. R., 222
Hillegaart, V., 96
640 Author Index
Hiller, W., 542
Hillyard, S. A., 48
Hilmert, C. J., 228
Hilt, L. M., 383
Hilton, J. L., 253, 256
Hindriks, I., 618
Hinton, D. E., 539
Hinton, P. S., 497
Hiraoka, R., 352
Hirsch, C. R., 513, 520, 521
Hirsch, J., 31, 61, 78, 79, 316
Hirschberger, G., 239
Hirst, M., 616
Hobdy, J., 241
Hoberman, H. M., 221
Hoch, S. J., 99
Hochschild, A. R., 9
Hochschild, J. L., 284, 285
Hoeft, F., 80, 403, 473
Hoek, H. W., 69
Hoeksma, J. B., 189
Hofer, C., 119, 157, 162, 163,
164, 603
Hofer, M. A., 229, 579
Hoffman, J. M., 24, 96
Hofmann, S. G., 11, 370, 395,
397, 398, 417, 520, 535, 529,
539, 548
Hofmann, W., 253, 346, 347,
348, 349, 350, 351, 352, 353,
354, 355, 617, 620
Hofstede, G. H., 295
Hogarth, L., 434
Holaway, R. M., 469, 473
Holdzheimer, P. E., 460
Holker, L., 511
Holland, P. C., 26, 27
Holland, R. W., 353
Holleran, K., 530
Hollerman, J. R., 145
Holley, S. R., 6, 267, 270, 275
Holliday, J. E., 575
Hollon, S. D., 559
Holloway, J., 396
Holmberg, D., 241, 242
Holmes, A., 47, 67
Holmes, E. A., 511
Holmes, J. G., 243
Holt-Lunstad, J., 222
Holtzheimer, P. E., 62, 65, 70
Holzel, B. K., 439, 458, 548,
554
Hommer, D., 95
Hong, K. A., 432, 614
Hong, Y., 291
Honorado, E., 162
Hood, J., 495
Hooley, J. M., 178, 495
Hooven, C., 179, 180
Hopkins, J., 557
Hopp, H., 368
Hoppitt, L., 523
Horesh, N., 241, 399
Horne, R., 599
Horodynski, M. A., 114
Horwitz, R. I., 589
Hosein, V. L., 433
Hoshino-Browne, E., 285
Hosie, J. A., 206, 210
Hot, P., 46
Houben, K., 353
Houck, G. M., 113
House, A. O., 497
Houser, D., 143, 146, 150
Houston, A. I., 221
Hovland, C. I., 253
Howard, A., 255
Howland, M., 229
Howren, M. B., 603
Hrapczynski, K. M., 383
Hsu, S. H., 561
Hu, P., 492
Huang, C., 224
Hubbard, J. A., 180
Huber, O., 13, 27
Hübner, R., 128
Huether, G., 539
Huettel, S. A., 71
Hufford, M. R., 620
Hughes, A. E., 229
Hughes, B. L., 31, 33, 52, 62,
83, 403
Hughes, C. F., 574
Hughes, E. K., 189, 195, 322
Huizenga, H. M., 316
Hull, J. G., 621
Hunter, J. J., 242
Hunter, M. A., 225
Huot, J. R., 209
Hupka, R. B., 500
Huppert, F. A., 205
Huppert, J. D., 377
Hurewitz, A., 589
Hurwitz, B. E., 498
Husarek, S. J., 161
Huss, M., 114
Hutcherson, C. A., 94, 105
Hutchinson, K. A., 500
Huygens, K., 395
Hyman, S. E., 145
I
Ikeda, A., 603
Ikemoto, S., 96
Illingworth, K. S. S., 380
Im, C., 615
Imada, T., 297
Imperato, A., 437
Impett, E. A., 226
Inagaki, T. K., 11, 396, 537
Ingram, R. E., 555, 559
Inzlicht, M., 11, 617
Ippolito, M. F., 114
Ironson, G. H., 571
Irvine, W. B., 352
Irving, J. A., 14, 352, 530, 548,
559, 562
Irwin, M. R., 575, 576
Isaacowitz, D. M., 13, 206, 208,
213, 274, 366, 511
Isen, A. M., 140, 622
Israel, S., 243
Ito, T. A., 308
Ivanov, I., 431, 432
Ivanovski, B., 557
Iverson, K. M., 500
Ives-Deliperi, V. L., 557
Izard, C. E., 163, 499
J
Jackson, A. L., 222
Jackson, D. C., 59, 208, 398, 574
Jackson, J. R., 253
Jackson, R. J., 395
Jacob, G. A., 495
Jacobo, M., 500
Jacobs, S. E., 326, 354, 420
Jacobsen, T., 114
Jacobson, E., 536
Jacobson, N. S., 497
Jaffe, F. K., 429
Jaffe, J. H., 429
Jäger, T., 206
Jahng, S., 494
Jahromi, L. B., 114
Jain, S., 551
James, W., 77
Jamieson, J. P., 11
Janicki-Deverts, D., 603
Janis, I. L., 253
Jankowiak, W. R., 273
Jansen, A. T. M., 351, 529
Jarcho, J. M., 27, 36, 82
Author Index 641
Jarmolowicz, D. P., 98
Jarvis, W. B. G., 258
Jasnoski, M. L., 571
Jaynes, J., 379
Jenkins, F. J., 575
Jensen, R. A., 430
Jerga, C., 245, 246
Jha, A. P., 552
Jimenez, L., 522
Jin, R., 469
Jochem, R. A., 181
Jog, M. S., 96
John, O. P., 8, 11, 14, 65, 127,
192, 268, 271, 275, 321, 322,
323, 324, 325, 326, 327, 328,
330, 331, 333, 334, 335, 337,
339, 340, 341, 343, 354, 368,
399, 415, 416, 420, 549, 579,
597, 601, 603, 604
Johns, M. J., 11, 617
Johnson, B. D., 129, 255
Johnson, C., 255
Johnson, E. J., 134
Johnson, K. L., 259
Johnson, M. C., 178
Johnson, M. H., 309
Johnson, M. K., 27, 207, 260
Johnson, O., 571
Johnson, R. E., 141, 476
Johnson, S. L., 230, 367, 414,
415, 416, 419
Johnson, S. M., 272, 279
Johnston, L., 353
Johnston, W. A., 128
Johnstone, T., 4, 24, 29, 52, 58,
59, 61, 62, 65, 84, 432, 458,
474, 538, 574
Joiner, T. E., Jr., 379, 495, 497
Jones, A., 615
Jones, B. T., 351, 429, 430
Jones, C. R., 251, 255, 256, 454,
614, 623
Jones, D. L., 349
Jones, H. E., 574
Jones, L. B., 311
Jonides, J. J., 28, 30, 141, 148
Joormann, J., 14, 134, 378, 379,
397, 413, 416, 417, 418, 419,
420, 421, 422, 423, 553
Joseph, D. L., 339
Jou, R. L., 26
Joudy, R., 378
Julian, K., 514
Juliano, L. M., 433
Julia-Sellers, M., 571
Julien, D., 278
Junghofer, M., 45
Jurivich, D. A., 204
K
Kabat-Zinn, J., 378, 478, 479,
550, 552, 556, 557, 558, 559
Kable, J. W., 85, 94, 104, 227
Kacelnik, A., 221
Kaell, A., 589
Kagan, J., 63, 159, 273, 308,
381, 571, 574
Kahn, R. L., 274
Kahn, R. S., 141, 148
Kahneman, D., 95, 97, 98, 136,
144
Kalin, N. H., 29, 52, 61, 208,
474
Kalisch, R., 23, 28, 33, 61, 78,
79, 81, 83, 131, 479
Kamholz, B. W., 417
Kamphuis, J. H., 196
Kandel, E. R., 31, 61, 79, 316
Kandl, M., 542
Kane, M. J., 354, 355
Kang, S. K., 617
Kanske, P., 11, 65, 309, 314, 316
Kanter, J. W., 480, 481
Kaplan, H. I., 6
Kappas, A., 12, 298
Kaprio, J., 589, 601
Karasawa, M., 285, 288, 363
Karau, S. J., 230
Karelina, K., 576, 577
Karg, K., 67
Karpinski, A., 253, 256
Karremans, J. C., 223, 244
Karssen, A. M., 576
Kashdan, T. B., 127, 134, 368,
370, 394, 405, 414
Kashima, K. M., 614
Kashiwagi, K., 294
Kassel, J. A., 430
Kassel, J. D., 614
Kassinove, H., 499
Kastner, S., 28
Katz, L. F., 179, 180
Kaufman, M., 145
Kavaliers, M., 616
Kavanagh, D. J., 348, 350, 356
Kawachi, I., 579, 589, 601, 603
Kawakami, K., 255
Kazdin, A. E., 486
Keay, K. A., 26
Keedwell, P. A., 66
Keel, P. K., 614, 621
Keener, A. D., 66
Keightley, M. L., 557
Keil, A., 45, 47, 48, 209, 367
Kelley, H. H., 253
Kelley, M. E., 460
Kelley, W. M., 557, 617
Kelsey, R. M., 223, 367
Keltner, D., 76, 77, 127, 224,
325, 341, 363, 613
Kemeny, M. E., 384, 571, 574,
575, 588, 589
Kemps, E., 351
Kendler, K. S., 461, 469
Kennedy, C., 230
Kennedy, Q., 208
Kennedy, S., 553
Kenny, D. A., 226
Kensinger, E. A., 207, 209, 510
Kentish, J., 422
Keough, M. E., 395
Kernis, M. H., 552
Kerns, J. G., 103
Kerns, K. A., 183
Kessler, E., 210
Kessler, H., 325, 326, 327
Kessler, R. C., 413, 430, 469,
530
Kety, S. S., 230
Khantzian, E. J., 430, 432
Kidd, C., 121
Kidd, T., 242
Kiecolt-Glaser, J. K., 227, 575,
589, 603
Kieras, J. E., 314
Kilpatrick, L. A., 557
Kilts, C. D., 24, 96
Kiluk, B. D., 439
Kim, B., 93, 113
Kim, D., 165
Kim, H., 285, 287
Kim, J.-H., 46, 416
Kim, M., 118
Kim, S. H., 11, 66, 166, 167,
225, 416
Kim, Y.-H., 11, 288, 295
Kindt, M., 395
King, D. S., 571
King, R. A., 294
Kinnunen, M. L., 589, 601
Kirby, K. N., 99
Kirchner, M., 542
Kirkland, T., 251, 454
642 Author Index
Kirsch, P., 394
Kirschbaum, C., 227
Kirson, D., 500
Kisley, M. A., 46
Kissler, J., 45
Kitayama, S., 284, 285, 286,
287, 288, 290, 292, 294, 297,
363
Kivnick, H., 274
Kiyonaga, A., 552
Kleczar, A., 61
Klein, D. N., 46, 316
Kleinknecht, N., 552
Klenk, M. M., 473, 484
Kliegel, M., 206
Kliewer, W., 182
Klipker, K., 187
Klonsky, E., 321, 337
Klumpp, H., 520
Knaack, A., 112, 163, 164, 165,
167
Knetsch, J. L., 144
Knierim, K., 403
Knight, R. T., 141
Knill, D. C., 224
Knobloch, L. K., 325
Knoch, D., 35, 148
Knowles, R., 418
Knudsen, E. I., 351
Knutson, B., 66, 95, 96, 145,
285, 363
Kobak, R. R., 238, 241
Kobau, R., 205
Kobayashi, T., 46
Kober, H., 14, 27, 29, 33, 52,
120, 121, 176, 428, 429, 433,
434, 435, 436, 437, 439, 450,
452
Kobor, M. S., 575
Koch, H. E., 213
Kochanska, G., 112, 119, 160,
161, 163, 164, 165, 166, 167,
182, 308, 309, 310, 313
Koenigs, M., 78, 142
Koerner, K., 495
Koestner, R., 379
Koeter, M. W. J., 529
Koffarnus, M. N., 98
Kokkonen, M., 589, 601
Kolar, M. M., 556
Kolarz, C. M., 205
Koningsbruggen, G. M., 350
Konttinen, H., 601
Koob, G. F., 430, 437
Kool, W., 132
Koole, S. L., 3, 61, 127, 128,
132, 133, 288, 351, 362, 367,
368, 496, 531
Koomen, W., 618
Koons, C. R., 500
Kordts, R., 353
Korfine, L., 495
Kornadt, H., 293
Kortekaas, R., 532
Koster, E. H. W., 63, 243, 395,
422, 424, 510, 518, 520, 522
Kotabe, H., 346, 349
Kotter, R., 456
Kotter-Grühn, D., 205
Kotz, S. A., 309, 316
Koutsouleris, N., 68
Kovacs, M., 608, 609
Kowalski, R. M., 615
Kowalsky, J., 542
Kozak, M. J., 134
Kraaij, V., 195, 323, 331, 332,
368, 416, 417
Kraemer, D. T., 589
Kraemer, H., 483
Kramer, J. H., 269, 400
Krantz, D. S., 588, 589
Kraus, M. W., 224
Krause, J., 224
Kravitz, D. J., 24
Krebs, J. R., 221
Kreifelts, B., 207
Krendl, A. C., 209, 617
Krietemeyer, J., 557
Kring, A. M., 321, 325, 460,
461, 496, 500, 508
Krivoshekova, Y. S., 206
Kroger, J. K., 97
Krompinger, J., 49, 552
Kross, E. F., 52, 127, 129, 240,
378, 433, 434, 439, 479, 556
Krueger, R. F., 119, 461, 469,
484
Kruesi, M. J. P., 114
Krug, M. K., 35
Kruglanski, A. W., 350, 353,
356, 365, 367
Kübler, A., 257
Kubota, Y., 96
Kubzansky, L. D., 588, 589, 596,
597, 600, 601, 602, 603, 605,
607
Kudadjie-Gyamfi, E., 206
Kugler, T., 386
Kuh, D., 605
Kuhl, J., 242
Kuipers, P., 284
Kujawa, A., 46
Kumar, S., 293, 555
Kumashiro, M. A., 244
Kummel, P., 241
Kun, B., 433
Kunda, Z., 350, 382
Kunzmann, U., 205, 206, 209,
214, 269
Kuo, J. R., 495, 499
Kuperminc, G., 228
Kupersmidt, J., 166
Kupfer, D. J., 559
Kupfer, S. R., 575
Kuppens, P., 11, 417
Kupperbusch, C. S., 269
Kurth, F., 27
Kurzban, R., 230
Kuyken, W., 556
Kwang, T., 382
L
La Greca, A. M., 228
Laatikainen, T., 601
Labad, A., 494
LaBar, K. S., 71, 95, 129
Labouvie-Vief, G., 194, 213, 242
Labroo, A. A., 623
Lachman, M. E., 591
Ladouceur, C. D., 23, 191
LaFreniere, P. J., 275
Lagattuta, K. H., 167
Laible, D. J., 165, 182
Laibson, D. I., 35, 85, 93, 94,
99, 112
Laird, A. R., 27, 64, 97, 434
Laird, J. D., 499
Lakey, C. E., 552
Lakka, H. M., 589
Lalande, K., 128, 368, 398, 415,
533
Lam, D., 419
Lam, S., 600
Lamb, M. E., 177, 306
Lambrecht, L., 207
Lamkin, D. M., 603
Lampman-Petraitis, C., 187
Lamy, D, 63, 510
Lanaj, K., 476
Lancaster, K., 352
Author Index 643
Lance, C. E., 552
Lancee, W. J., 242
Landy, F., 339
Lane, R. D., 31, 532, 599
Lane, S. D., 119
Laneri, M., 98
Lang, F. R., 210
Lang, P. J., 5, 6, 44, 45, 47, 132,
270, 310, 450, 471
Langer, D. A., 590
Langer, S. K., 225
Langeslag, S. J. E., 46
Langston, C. A., 226
Lanius, R. A., 496
Lansford, J. E., 210
Laptook, R. S., 316
Larkin, G. R., 208
LaRose, R., 614
Larose, S., 229
Larsen, H., 313
Larsen, R. J., 8, 341, 363, 531
Larson, C. L., 59, 398, 574
Larson, E. B., 9
Larson, J., 331
Larson, R., 187, 190
LaTaillade, J. J., 383
Lattimore, P., 621
Lau, H. C., 403
Lau, M. A., 552
Laugesen, N., 395
Laurenceau, J.-P., 471
Laurent, H., 278
Lavy, S., 244
Lawlor, D. A., 587
Lawrence, A. D., 63, 223, 395
Lawrence, A. J., 431
Lawrence, J. W., 377
Lawson, C., 421
Lawton, M. P., 210
Layton, J. B., 222
Lazar, S. W., 557
Lazarus, A. A., 498
Lazarus, R. S., 3, 4, 6, 194, 321,
323, 328, 329, 330, 531, 579,
602
Le, H., 253
Le Moal, M. L., 430, 432, 616
Leahy, R. L., 531
Leary, M. R., 376, 378, 379, 381,
384, 385, 480, 557, 615, 622
LeBel, E. P., 256, 262
Lebra, T. S., 285
Leclerc, C. M., 207, 510
Lecours, S., 379
Lecuyer-Maus, E. A., 113
LeDoux, J. E., 26, 27, 29, 31, 34,
61, 62, 77, 78, 80, 81, 95, 96,
145, 306, 307, 309, 472, 473,
537, 539
Lee, A. Y., 288, 289
Lee, B. O., 52
Lee, C. H., 52
Lee, D. S., 416
Lee, E. A., 11, 52, 295
Lee, G. P., 101
Lee, H., 263
Lee, J., 560
Lee, K., 315
Lee, T. L., 11
Lee-Chai, A., 366, 377
Leen-Feldner, E. W., 533
Leerkes, E. M. J., 161, 309
Legerstee, J., 195, 417
Legrand, D., 452
Lehle, C., 128
Lehman, B. J., 82, 602
Lehman, D. R., 285
Lehrer, J., 111
Leib, D. A., 575
Leibowitz, R. Q., 433
Leith, K. P., 386
Leitten, C. L., 223, 367
Lejuez, C. W., 143, 495
Leland, D. S., 121
Lemche, E., 242
Lemery, K. S., 164
Lemogne, C., 67
LeMoult, J., 422
Lengacher, C. A., 559
Lengua, L. J., 162, 164, 310
Lenton, A. P., 500
Leonard, K. E., 113
Leonards, U., 351, 523
Leotti, L. A., 36
Lerche, N. W., 575
Lerew, D. R., 395
Lerner, J. S., 141, 602, 620
Leroy, V., 121
Lessard, D. A., 223
Leu, J., 285
Leung, A. K.-Y., 285
Leutgeb, V., 52, 395
Levenson, R. W., 4, 5, 6, 10, 11,
24, 28, 76, 77, 83, 117, 206,
211, 213, 267, 268, 269, 270,
271, 272, 274, 275, 276, 277,
278, 321, 397, 400, 574, 579,
599, 613
Leventhal, H., 23, 26
Levesque, J., 64, 68, 84, 311
Levin, F. R., 430
Levine, B., 97
Levine, D., 495
Levine, L. J., 208
Levine, S., 589
Levinson, D. B., 557
Levinthal, D. J., 28
Levitt, J. T., 397, 398, 399, 403,
498, 533
Levy, B. J., 104
Levy, D. J., 25, 355
Levy, R. I., 292, 298
Lewinsohn, P. M., 480, 481, 497
Lewis, C. C., 289
Lewis, M. D., 176
Lewis, T. T., 588, 589, 602
Li, C. S. R., 430, 432, 433, 436
Li, S., 516
Li, X., 99
Liberman, N., 105
Liberzon, I., 46, 64, 395
Lieberman, M. D., 11, 27, 28, 35,
36, 79, 82, 93, 119, 120, 228,
255, 396, 432, 436, 537
Liebowitz, M., 396
Lievens, L., 514
Lillis, J., 538
Limberger, M. F., 495
Lin, C., 614
Linden, D. E. J., 66
Linden, W., 588
Lindenberger, U., 187, 192, 212,
214, 366
Lindenmeyer, J., 353
Lindmark, E., 589
Lindquist, K. A., 27, 28,
224,448, 451, 452, 453, 456,
459, 460
Lindquist, M. A., 33, 36, 52, 62,
83, 403
Lindsey, S., 254
Linehan, M. M., 14, 136, 378,
470, 475, 477, 478, 479, 491,
492, 494, 495, 496, 497, 498,
499, 500, 502, 530, 538, 541,
561
Lin-Stein, T., 241
Lipina, S. J., 230
Lipkus, I., 243
Lipworth, L., 559
Lissek, S., 81, 475, 574
Liston, C., 68
644 Author Index
Litt, M. D., 430
Littel, M., 52, 53, 434
Little, T. D., 205
Liu, E. H., 46
Liu, H., 435, 437
Liu, X., 516
Liverant, G. I., 417, 473, 551
Livneh, U., 81
Liwag, M., 284
Llera, S., 470, 475, 481
Lloyd-Jones, D. M., 596, 605,
607, 608
Lo, B. C. Y., 352
Lobbestael, J., 495
Löckenhoff, C. E., 209
Lockwood, P, 382
Loewenstein, G. F., 35, 85, 93,
94, 98, 99, 112, 141, 369, 620
Logan, G. D., 161
Lohr, J. M., 475
Löken, L. S., 227
Loman, M. M., 176
Lomore, C. D., 241
London, E. D., 436
Long, J. D., 188
Longe, O., 538
Lonigan, C. J., 164, 166
Loo, C. K., 69
Lopes, P. N., 271, 338, 339
Lopez, A. D., 62
Lopez, F. G., 241
Lopez-Duran, N. L., 608, 609
Loucks, E. B., 601, 607
Lovibond, P. F., 80
Lowery, B. S., 255
Loxton, N. J., 93
Lozano, A. M., 70
Lu, Q., 315
Lubaroff, D. M., 227
Luciano, M. C., 440
Luck, S., 44
Luerssen, A., 111, 190, 431
Luhmann, M., 349
Lujan, J. L., 460
Lukon, J. L., 181
Luna, B., 190
Lundberg, U., 576
Lundh, L.-G., 11
Lunkenheimer, E. S., 180
Luo, X., 436
Luoma, J. B., 538
Luong, G., 210
Lupianez, J., 522
Lupien, S. J., 176
Luppino, F. S., 605, 606
Lüscher, C., 437
Luterek, J. A., 396
Lutgendorf, S. K., 577
Lutz, A., 458, 552, 556, 557
Lutz, C., 284
Luu, P., 311
Lynch, J. W., 587
Lynch, T. R., 498, 499
Lyubomirsky, S., 127, 382, 414,
415, 470
Lyvers, M., 496
M
Ma, S. H., 560
MacArthur, R. H., 221, 224
Maccari, S., 432
MacDonald, A. W., 97, 98
MacGregor, D. G., 141
MacKay-Soroka, S., 182
Mackey, S., 227
Mackie, D. M., 258
Mackintosh, B., 351, 523
MacLean, P. D., 6
MacLeod, C., 14, 62, 63, 351,
395, 421, 422, 508, 509, 510,
511, 512, 515, 521, 522, 524
MacNamara, A., 44, 45, 46, 48,
50, 51, 53, 129, 130
MacPherson, L., 433
Macrae, C. N., 377
Madden, D. J., 71
Maddux, W. W., 256
Madey, S. E., 380
Magai, C., 206, 214, 598
Magen, E., 105, 121
Maguire, E. A., 104
Mahoney, A. E., 395
Mahoney, M. J., 351
Maier, K. J., 589
Maier, M. E., 80
Main, A., 267
Main, M., 229, 276
Majeskie, M. R., 432, 620
Major, B., 241
Makkar, S. R., 384
Malach, R., 452
Malatesta, C. Z., 114
Malenka, R. C., 437
Maletic, V., 589
Malhi, G. S., 557
Maliken, A. C., 179
Malkin, C., 177
Maller, J., 64
Malmaud, J., 85
Malmstadt, J. R., 59, 398, 574
Malone, T. W., 224
Malouff, J. M., 589
Mamun, A. A., 606
Manber, T., 83, 400, 402, 496
Manber-Ball, T., 84, 400, 402
Mandai, O., 46
Maner, J. K., 622
Mangelsdorf, S. C., 161, 178
Mangold, R., 230
Mann, T., 353, 617
Mansell, W., 461
Mäntylä, T., 190
Manuck, S. B., 588
Manwell, L. A., 363
Maraj, N., 552
Marco-Pallarés, J., 103
Marcovitch, S., 167
Maresh, E. L., 13, 221
Marinetti, C., 284
Marissen, M. E., 495
Mark Eddy, J., 188
Markman, H. J., 274, 276
Markon, K. E., 469, 484
Marks, I., 524
Markus, H. R., 284, 285, 286,
292, 293, 380
Marlatt, G. A., 432, 439, 560,
614, 615
Marr, D., 456
Marrone, G. F., 433
Marsh, J. T., 575
Marshall, R. D., 379
Marshall-Berenz, E. C., 433
Martell, C. R., 497, 499
Martijn, C., 529
Martin, A., 452
Martin, L. L., 10, 379, 538
Martin, L. N., 85, 144, 146, 148,
149
Martin, R. A., 270
Martin, S. E., 6, 84, 175
Martinez-Raga, J., 355
Marvin, C. B., 435
Marziali, E., 495
Marzolf, D., 161
Masicampo, E. J., 350
Mason, W. A., 575
Massie, E. D., 530
Master, S. L., 240
Masters, J. C., 7
Masuda, A., 538
Masuda, T., 285, 290, 294
Matarazzo, O., 196
Author Index 645
Mather, M., 207, 208, 274
Mathers, C. D., 62
Mathews, A., 62, 395, 421, 422,
508, 509, 510, 511, 512, 513,
520, 521, 524
Mathias, C. W., 119
Matochik, J. A., 436
Matsumoto, D., 294, 295, 297,
325
Matsuzaka, Y., 403
Mattay, V. S., 67, 121, 537
Matthews, K. A., 587, 589, 590,
603
Maunder, R. G., 242
Mauss, I. B., 4, 9, 11, 14, 24, 29,
76, 127, 189, 257, 276, 288,
295, 331, 332, 341, 361, 366,
367, 368, 370, 382, 406, 508,
509, 532, 579, 589
Maxwell, L., 621
May, C. P., 422
May, J., 348
May, M. G., 449
Mayberg, H. S., 62, 65, 70
Mayer, J. D., 13, 323, 334, 338,
531
Mayo, R., 263
Mayr, U., 205
Mazziotta, J. C., 82
Mbirkou, S., 257
McArdle, J., 535
McCandliss, B. D., 312, 315
McCarter, L., 4, 24, 76, 276
McCarthy, D. E., 432, 620
McCeney, M. K., 275, 277, 589
McClelland, J. L., 97, 452
McClernon, F. J., 430
McClure, E. B., 83
McClure, S. M., 35, 85, 93, 94,
96, 99, 100, 103, 104, 106,
112, 113, 120, 121, 145
McComb, K., 226
McConnell, A. R., 257, 258, 259,
378
McCrae, R. R., 306
McCulloch, K. C., 367
McDade, T. W., 571
McEvoy, P. M., 395
McEwen, B. S., 176, 588, 589,
602, 605
McGee, R. O., 271
McGhee, D. E., 253, 366
McGlone, F., 47
McGonigal, K. M., 11, 327
McGowan, P. O., 229
McGrath, P. J., 165
McGuire, J. T., 132
McGuire, L., 589, 603
McIntosh, W. D., 538
McIntyre, M. C., 33
McKay, G., 221, 224
McKee, S. A., 614, 616
McKenzie, G., 416
McLane, M., 560
McLaughlin, K. A., 188, 189,
396, 399, 404
McMillan, K. M., 97
McMurrich, S., 416
McNally, L., 222
McNally, R. J., 117, 517
McNaughton, N., 307, 308, 471,
537
McNulty, J. K., 271
McPherson, R., 206
McPherson, S., 558
McRae, K., 11, 33, 83, 133, 196,
197, 203, 204, 326, 341, 354,
397, 416, 420, 432
Mead, M., 296
Mead, N. L., 620
Meaney, M. J., 576
Mearns, J., 323, 333
Medvec, V. H., 380
Medway, F. J., 244
Meehl, P. E., 66
Meier, C., 535
Meier, L., 535
Meintjes, E. M., 557
Meiran, N., 11, 127, 129, 230,
420
Meisenzahl, E. M., 68
Mele, A., 348
Melzer, D., 205
Mendes, W. B., 11
Mende-Siedlecki, P., 212, 434, 439
Mendis, S., 596, 597
Mendoza, S. P., 575
Mendoza-Denton, R., 93
Mennin, D. S., 14, 188, 379, 396,
398, 399, 401, 404, 406, 469,
470, 473, 474, 475, 483, 484,
496, 529, 530
Menon, V., 80, 403, 460, 473
Mensink, W., 350, 356
Mentzel, H.-J., 81
Merchant, J., 551
Merckelbach, H., 395
Mesquita, B., 9, 28, 189, 284,
285, 287, 288, 289, 292, 293,
294, 295, 296, 298, 363
Metalsky, F. I., 383
Metcalfe, J., 119, 616
Metevia, L., 98
Metts, S., 325
Meuleman, L., 495
Meyer, K., 35, 148
Meyer, S. C., 174, 180, 181
Meyerhoff, D. J., 435
Miao, F., 286
Michelena, P., 225
Miclea, M., 143
Mienaltowski, A., 210
Miers, A., 188
Mikels, J. A., 207, 208, 209, 274
Mikolajczak, M., 8, 121
Mikulincer, M., 114, 182, 189,
226, 229, 237, 238, 239, 240,
241, 242, 243, 244, 245, 246,
273, 274, 328
Milad, M. R., 61, 78, 79, 81
Miles, E., 10, 321, 361, 368
Miles, L. K., 213
Millan, M. J., 461
Miller, A. H., 589
Miller, B. L., 269, 277, 400
Miller, E. K., 30, 97, 113, 141,
148, 435
Miller, E. M., 93, 187, 188
Miller, G. E., 369, 572, 575, 576,
577, 586, 587, 590, 591, 598,
600, 606, 608
Miller, L. F., 560
Miller, N. E., 471
Miller, P. A., 182
Miller, P. C., 230
Miller, P. H., 166, 189
Miller, P. J., 291
Miller, R. S., 384
Milliken, B., 522
Milne, A. B., 206, 210, 377
Milner, B., 104
Miltner, W. H. R., 81
Mineka, S., 395, 473
Minnick, M. R., 11, 295
Mintz, J., 291
Mintz, T. M., 163
Minzenberg, M. J., 495
Miranda, R., 379
Mischel, W., 3, 35, 52, 93, 105,
111, 112, 114, 115, 116, 117,
119, 158, 161, 162, 271, 309,
310, 312, 352, 361, 431, 433,
616
Mishkin, M., 24
Mitchell, C., 365
646 Author Index
Mitchell, D. G. V., 61, 142, 148
Mitchell, F., 586
Mitchell, J. P., 27, 28
Mitchell, M. A., 395
Mitte, K., 571
Miu, A. C., 143
Miyake, A., 127
Miyake, K., 285
Miyamoto, Y., 292
Mobbs, D., 28, 30, 78, 82, 225
Moberly, N. J., 558
Mocaiber, I., 51, 53
Moeller, F. G., 119
Moffitt, T. E., 67, 119, 271, 431,
605
Mogenson, G. J., 349
Mogg, K., 309, 395, 422, 474,
478, 510
Mohammadi, B., 103
Mohanty, A., 189
Moll, H., 222, 223
Mollenholt, N., 11, 405, 549
Moneta, G., 187
Monin, B., 9
Monk, C. S., 68, 82
Monson, C. M., 230
Monsour, M., 167
Montag, C., 394
Montague, P. R., 23, 34, 96, 98,
145
Monterosso, J., 436
Montesinos, F., 440
Monti, D., 496
Monti, J. M., 496
Mooney, D. K., 430
Moore, B. S., 115, 117
Moore, C., 165, 228
Moore, L. J., 223
Moore, M. T., 469, 473
Moore, S. A., 11, 405, 549
Moors, A., 77, 254
Mor, N., 621
Morag, I., 518
Moran, E. K., 460
Morasco, B. J., 430
Moratti, S., 46, 47
Morelli, G. A., 285
Moretti, L., 142
Moriarty, D. G., 205
Morling, B., 292
Morone, N. E., 556
Morrell, B., 382
Morris, A. S., 159, 162, 174, 181,
187, 190, 191, 321, 322
Morrison, I., 227
Morrison, J. A., 589
Morrow, J., 134, 534
Morse, P., 430
Morton, K., 431
Moscovitch, M., 209
Moser, J. S., 49
Moses, E. B., 508, 529
Moses, L. J., 163
Moskalenko, S., 621
Moskowitz, G. B., 255
Moss, C., 226
Moulds, M. L., 415, 420, 558,
599
Moyal, N., 400
Moylan, S. J., 140
Mroczek, D. K., 205, 211, 212
Mueller, E. T., 98
Muhlberger, A., 31
Mulkens, S., 352
Müller, V., 212
Munafo, M. R., 351, 395, 523
Mund, M., 571
Munoz, R. F., 14
Munro, S., 167
Münte, T. F., 103
Muraven, M., 117, 128, 131,
132, 135, 230,472, 474, 618,
619, 623
Murnighan, J. K., 146
Murphy, B. C., 114, 161, 181
Murphy, G. L., 451, 453
Murphy, S. E., 511
Murphy, V., 398
Murray, C. J. L., 62
Murray, E. A., 23, 27, 33
Murray, K. T., 119, 161, 165, 309
Murray, S. L., 277
Murrell, A. R., 472, 481, 482
Mushiake, H., 403
Myers, S. S., 174, 190, 321
Myerson, J., 98, 111
Myerson, R. B., 146
Myers-Schulz, B., 78
N
Naaman, S., 230
Nachmias, M., 178, 183
Nachmias, O., 239
Nadeau, K. C., 575
Nagin, D. S., 605
Naidu, N. V. R., 293
Naidu, R. K., 293
Najmi, S., 513, 514
Naliboff, B. D., 574
Namkoong, K., 52
Nathan, P. J., 83, 96, 145
Naumann, L. P., 326
Neacsiu, A. D., 14, 491, 496,
500, 530, 538, 561
Nearing, K. I., 35, 61, 62, 78, 80,
81, 145, 148
Neely, J. H., 28
Negel, L., 384
Negishi, H., 576
Neil, A. L., 608
Nelis, D., 271
Nelis, S., 416
Nelson, B., 263
Nelson, E. E., 68
Nelson, S. N., 192
Nesse, R. M., 363
Nesselroade, J. R., 205, 276
Nestor, L. J., 436
Neufeld, R. J., 496
Neumann, C. S., 533
Neumann, R., 31
New, A. S., 400, 402, 495
Newcorn, J., 431
Newman, D. A., 339
Newman, D. L., 605
Newman, M. G., 469, 470, 475,
481
Newton, E. K., 173
Neyer, F. J., 274
Nezlek, J. B., 11, 417
Nezu, A. M., 497, 531, 540
Nich, C., 433, 439
Niedenthal, P. M., 377, 448
Niedtfeld, I., 494, 495
Niemann, L., 548
Nienhaus, K., 618
Nieuwenhuis, S., 49, 53, 61, 129
Nikitin, J., 208
Nisbett, R. E., 289, 509
Nitschke, J. B., 63, 81, 82
Nocera, C. C., 367
Nolan, R. P., 242
Nolen-Hoeksema, S., 127, 134,
135, 188, 271, 327, 331, 337,
396, 399, 404, 405, 414, 415,
417, 419, 470, 474, 480, 484,
492, 534, 558
Nordgren, L. F., 354
Nordin, S., 45
Nordling, J. K., 166
Norem, J. K., 380
Norman, D. A., 307
Norrholm, S. D., 81
Author Index 647
Norris, C. J., 622
Norrving, B., 596
Northoff, G., 452
Notarius, C. I., 574
Novin, S., 296
Nowicka, A., 45
Nunes, E. V., 430
Nunes, P. M., 494
Nurius, P., 380
Nurmikko, T. J., 223
Nyland, J. E., 355
Nyman, M., 313
Nystrom, L. E., 79, 98, 141
O
Oaten, M., 615
O’Boyle, C. G., 10, 309
Obradović, J., 167
O’Brien, C. P., 433
O’Brien, L, 251
Ochsner, K. N., 5, 11, 12, 13,
23, 24, 27, 28, 29, 30, 31, 33,
35, 43, 51, 52, 58, 59, 60, 62,
76, 78, 83, 120, 121, 130, 131,
133, 142, 144, 146, 148, 176,
189, 212, 269, 278, 311, 314,
396, 398, 403, 416, 432, 433,
434, 451, 472, 537, 556, 574
O’Cleirigh, C., 571
O’Connell, K. A., 433
O’Connell, R. M., 576
O’Connor, C., 500
Odgers, C. L., 607
O’Doherty, J. P., 23, 473
O’Donoghue, T., 98
O’Donovan, A., 575
Oh, D., 548
O’Hearn, R. E., 244
O’Heeron, R. C., 574
Öhman, A., 471, 554
O’Keefe, J., 104
Oken, B. S., 558, 559
Olatunji, B. O., 395, 470, 475,
496
Olausson, H., 227
Oldehinkel, A. J., 164
Olds, J., 96
O’Leary, K. C., 230
Olino, T. M., 316
Olofsson, J., 45, 46
Olson, M. A., 253, 256
Olson, S. L., 113
Olsson, A., 27, 28, 30, 34
Olvet, D. M., 44, 45, 46, 129
Ong, A. D., 212, 213
Ongur, D., 27, 460
Oosterlaan, J., 189
Opitz, P. C., 13, 187, 197
Oppenheim, D., 285
Orbach, I., 229
Ordaz, S., 190
Ordóñez, L. D., 386
Ormel, J., 164
Orr, I., 244
Orsel, B., 106
Orsillo, S. M., 370, 397, 398,
475, 478, 479, 498, 533
Orth, U., 535
Ortony, A., 23, 29, 454
Öst, L.-G., 536
O’Sullivan, H., 351, 523
Ottenbreit, N. D., 537, 558
Otter-Henderson, K. A., 242,
244
Otto, P. E., 134
Owen, A. M., 97
Owens, M., 396, 520
P
Paas, N. D., 433
Packard, M. G., 26
Packer, D. J., 260, 261
Padberg, F., 69
Padgett, D. A., 575
Palau, C., 435
Palmeri, H., 121
Pamuk, E., 586
Panfile, T. M., 165
Panksepp, J., 96
Papa, A., 128, 368, 398, 415,
533
Papageorgiou, C., 414, 415
Papies, E. K., 352, 354, 356, 458,
620
Paquette, V., 64, 84, 395
Paradise, M. J., 378
Parekh, N., 222
Paret, C., 83
Park, I. J. K., 295
Park, J., 559
Park, L. E., 243
Park, N., 597
Parke, S., 227
Parker, J. D. A., 329, 330
Parker, K. J., 587
Parker, L. E., 331
Parkinson, B., 6, 8, 135, 293,
368
Paronis, C. A., 614
Parritz, R. H., 178
Parrott, W. G., 9, 363
Partridge, K. P., 552
Parvaz, M., 51, 53
Pascual-Leone, A., 27, 35, 148,
355
Pashler, H., 128
Passingham, R. E., 403
Pasupathi, M., 205
Paty, J. A., 430
Pauley, G., 558
Pauli, P., 31, 257
Paulus, M. P., 121, 309, 398,
471, 473, 476
Paxton, J. L., 230
Paykel, E. S., 559
Payne, B. K., 253
Payne, J. W., 134
Paz, R., 81
Peake, P. K., 112, 310
Pearson, J. C., 556
Pearson, T. A., 600, 601, 603
Pedersen, N. L., 469
Pedersen, S. S., 571
Pejic, T., 81
Pennebaker, J. W., 572, 574, 598,
599
Pentland, A., 224
Peraza, D. M., 31, 61, 79, 316
Pereg, D., 229
Perez, C. R., 11, 295
Pérez-Edgar, K., 309
Pergamin, L., 63, 510
Perkins, K. A., 433
Perlman, D. M., 458, 556, 557
Perlman, G., 68
Perry-Parrish, C., 188, 197, 604
Perugini, M., 353
Perunovic, M., 243
Pessiglione, M., 96
Pessoa, L., 23, 28, 31, 33, 77
Peters, E., 141
Peters, J., 94, 101, 104, 105
Peterson, C., 597, 602
Peterson, R., 145
Petrican, R., 209
Petrocelli, J. V., 378
Petty, R. E., 253, 256, 258, 259,
271
Pezawas, L., 67, 395
Pfefferbaum, A., 435
Pfeifer, J. H., 190, 432
648 Author Index
Pfister, H.-R., 142
Phan, K. L., 46, 83, 96, 145
Phelps, E. A., 23, 26, 29, 33, 34,
35, 61, 62, 66, 78, 80, 81, 95,
96, 141, 145, 472
Philiastides, M. G., 104
Philibert, R. A., 167
Philippe, F. L., 379
Philippot, P., 499, 500, 514, 517
Phillips, A. G., 377, 378
Phillips, D. A., 605
Phillips, L. H., 206, 210, 211, 213
Phillips, M. L., 23, 33, 66, 141
Phillips, R. G., 96
Phillips, S., 230, 615
Pianka, E. R., 221, 224
Piazza, J. R., 205, 210, 212
Piazza, P. V., 616
Piazza J. R., 206
Pickett, C. L., 622
Piguet, O., 209
Pillutla, M. M., 146
Pine, A., 101
Pine, D. S., 521
Pineles, S. L., 395
Pinulas, A., 313
Piper, M. E., 432, 620
Pithers, W. D., 614
Pitkänen, A., 537
Pizarro, D. A., 208
Pizzagalli, D. A., 63, 473,
474–475, 529
Plante, T. G., 552
Plassmann, H., 94
Platek, S. M., 384
Ploghaus, A., 36, 82
Pluess, M., 312
Poch, C., 460
Poldrack, R. A., 28, 70
Pole, N., 81
Polich, J., 45, 46
Polivy, J., 116, 614, 617, 621, 622
Polk, T. A., 28
Pollack, P. R., 115, 117
Pollak, S. D., 176, 177
Ponesse, J. S., 161
Porat, R., 15
Porges, S. W., 474, 536
Posner, M. I., 63, 93, 115, 161,
167, 228, 305, 306, 307, 308,
309, 310, 311, 312, 313, 314,
315, 399, 478, 597
Potenza, M. N., 434, 436, 438,
439
Pott, M., 285
Pouget, A., 224
Poulin, R., 450
Pourtois, G., 351
Powell, N. D., 576
Power, M. J., 422
Powers, K. E., 622
Powers, M. B., 514
Powers, S., 278
Prater, K. E., 80, 403, 473
Pratt, F. J., 116
Prenger, R., 548
Prescott, T. J., 253
Preston, K. L., 433
Preuss, D., 617
Price, E. L., 241
Price, J. L., 27, 460, 537
Priel, B., 243
Prinstein, M. J., 228
Prochaska, J. J., 117
Proffitt, D. R., 222, 224
Prosch, S., 575
Proudfit, G., 43, 574
Proudfoot, J. G., 384
Pruessner, J. C., 242, 243, 511
Pryce, C. R., 494
Przeworski, A., 469
Pulkkinen, L., 589, 601
Pulliam, H. R., 225
Puska, P., 596
Putnam, K. M., 63, 66
Putnam, S. P., 305
Pyke, G. H., 225
Q
Qian, M., 516
Quack, D., 396, 399
Quinn, D. M., 617
Quirin, M., 242, 243
Quirk, G. J., 23, 27, 34, 61, 81
Quoidbach, J., 8
R
Rabin, B. S., 571
Rachman, S., 475, 484
Racine, C. A., 230
Radke-Yarrow, M., 294
Radkovsky, A., 535
Radu, P. T., 106, 128
Radwan, K., 230
Raes, F., 164, 416, 424
Raffa, S. D., 370
Rahman, J., 106
Raichle, M. E., 311, 313, 314
Raikes, A., 180, 181
Raikes, H. A., 182
Raison, C. L., 589
Ramanathan, S., 350, 620
Ramel, W., 11, 33, 83, 397, 557
Ramsawh, H. J., 398
Ramsden, S. R., 180
Ramsey, N. F., 616
Rando, K., 435
Rangel, A., 23, 24, 25, 27, 33,
34, 35, 85, 93, 94, 98
Raphaelson, Y. E., 590
Raskin, P. M., 241
Rasmussen, A. F., 575
Rauch, S. L., 63
Rauers, A., 198
Rauss, K., 351
Raver, C. C., 160
Raviv, M., 614
Rawn, C. D., 259, 620, 621
Ray, R. D., 11, 84, 326, 354,
398, 403, 416, 420, 480
Ray, R. R., 27, 28
Ray, W. J., 494
Raye, C. L., 27
Razza, R. P., 163
Read, D., 106
Reading, S., 30
Ready, R. E., 212
Rebucal, K. A., 213
Reddish, M., 452
Redick, T. S., 355
Redish, A. D., 96
Reeb-Sutherland, B. C., 509
Reed, A. E., 209
Reed, M. A., 307, 309, 509
Reese, H. E., 517
Reger, B. E., 556
Reichardt, L. F., 228
Reidler, J. S., 450
Reijntjes, A., 196
Reinhold, E., 68
Reis, H. T., 226
Reisenzein, R., 422
Reiser, M., 68, 162
Reitz, S., 495
Rempel, J. K., 252
Remy, F., 33
Renner, B., 45
Repetti, R. L., 177, 278
Rettek, S., 293
Reuter, M., 395
Reuter-Lorenz, P., 208
Author Index 649
Reynolds, B., 134, 177
Reynolds, C. A., 205
Reynolds, S., 6
Reznick, J. S., 574
Rhoades, G. K., 274
Rhoades, H., 119
Rhodes, E., 363
Rholes, W. S., 229
Riccardi, C. J., 395
Richards, J. M., 8, 11, 33, 129,
268, 277, 295, 324, 327, 334,
416, 510, 614
Richards, M. H., 187
Richardson, M. P., 537
Richerson, P. J., 222, 571
Richeson, J. A., 617, 618
Richey, J., 517
Richter, D., 206
Richter, W., 33
Richters, J. E., 178
Ricon, T., 518
Ridderinkhof, K. R., 61, 102
Ridker, P. M., 589
Riediger, M., 187, 192, 193, 198,
212, 214, 366
Rief, W., 542
Rieffe, C., 296
Rieskamp, J., 134
Rifai, N., 589
Riggs, D. S., 530
Riley, A. W., 587
Riley, H., 433
Rilling, J. K., 36, 98, 141
Rimm, E., 607
Rinck, M., 353
Ringo Ho, M. H., 405
Rips, L. J., 452
Riskind, J. H., 242
Ritchey, M., 129
Rizvi, S. L., 492, 496, 502
Roales-Nieto, J. G., 440
Robbins, S. J., 433
Robbins, T. W., 28, 434
Roberts, G., 224
Roberts, M. E., 614
Roberts, N. A., 223, 277
Robertson, E. R., 31
Robertson, J., 587
Robichaud, M., 475
Robins, D. L., 552
Robinson, J. L., 574
Robinson, L. R., 174, 190, 321
Robinson, M. D., 189
Robinson, T. E., 96, 145, 471
Robles, T. F., 177, 589, 603
Rochester, M., 182
Röcke, C., 205
Rockstroh, B., 367
Rodriguez, C., 93, 113
Rodriguez, M. L., 35, 105, 111,
112, 113, 116, 309
Roemer, L., 275, 323, 333, 337,
338, 370, 379, 396, 398, 399,
406, 417, 475, 478, 479, 551
Rogers, R. D., 403
Rogers, S. J., 225
Rogoff, B., 285
Rogosch, F., 178
Rohlfing, T., 435
Rolls, E. T., 25, 27, 64
Romero, F. J., 435
Romero, M. J., 435
Rook, D. W., 620
Rooke, S. E., 589
Rosa, D., 560
Rosaldo, M. Z., 288, 296
Roseman, I., 293
Rosen, Z., 132
Rosenbloom, M., 435
Rosenthal, M. Z., 495, 498, 499,
501
Rosenzweig, S., 556
Roskos-Ewoldsen, D. R., 262
Ross, L., 382
Ross, M., 208
Roth, W. T., 536
Rothbart, M. K., 10, 115, 158,
159, 160, 161, 165, 167, 228,
305, 306, 307, 308, 309, 310,
311, 312, 313, 314, 315, 317,
399, 478, 597
Rothbaum, F. M., 194, 285, 290,
292, 380
Rothermund, K., 367, 368, 369,
532, 538
Rothstein,, J. D., 212
Rottenberg, J., 127, 134, 367,
368, 394, 414, 474
Rounsaville, B. J., 434
Rovee-Collier, C., 177
Rowe, J. W., 274, 589
Roy, M., 460
Rozanski, A., 588, 602, 603
Rozenman, M., 523
Rubia, K., 120
Ruby, P., 452
Rucker, L., 396
Rueda, M. R., 305, 310, 311,
312, 315
Ruef, A. M., 276
Ruff, H. A., 161
Ruggerio, A. M., 575
Rule, N. O., 259
Rumbaugh, D. M., 315
Rundle, S. A., 575
Ruocco, A. C., 495
Ruppert, J., 448
Rusbult, C. E., 243, 244
Ruscio, A. M., 421, 469, 519,
520
Rush, A. J., 414, 499
Rush, J., 477
Russell, D. W., 227
Russell, J. A., 27, 251, 450
Rusting, C. L., 363
Rutherford, E., 511
Ruxton, G., 224
Ryan, D. H., 497
Ryan, E., 365
Ryan, R. M., 361, 369, 548
Rydell, R. J., 257, 258
Rydstrom, A., 49, 129
S
Saab, P. G., 588
Saarni, C., 173, 175, 188, 333,
334, 337, 339, 531
Sabatinelli, D., 45, 47
Saccomanno, L., 315
Saddoris, M. P., 27
Sadock, B. J., 6
Safran, M. A., 205
Safren, S., 500
Sage, R. M., 602
Saito, Y., 46
Sakellaropoulo, M., 511
Saleem, K. S., 24
Salerno, J., 363
Salinas, E., 224
Salisch, M. V., 194
Salomons, T. V., 557
Salovey, P., 13, 271, 323, 334,
337, 338, 386, 531, 589, 600
Salters, K., 370
Salters-Pedneault, K., 396, 398,
399
Salthouse, T. A., 274
Saltzman, H., 159
Salzman, C. D., 448, 456
Samanez-Larkin, G. R., 207
Samii, A., 103
Samson, A. C., 10, 13, 27
Sanchez, A., 435
650 Author Index
Sanfey, A. G., 98, 140, 141, 142,
146, 147, 148, 149, 190
Sanislow, C. A., 461
Santucci, A. K., 118
Sapir-Lavid, Y., 239
Sapolsky, R. M., 574, 576, 579
Sarinopoulos, I., 82
Sassenberg, K., 255
Satpute, A. B., 27, 456, 460
Sauer-Zavala, S. E., 396
Saugar, C., 46
Savani, K., 293
Savino, N. S., 370
Savitz, J., 64
Sawchuk, C., 475
Sawyer, A. T., 398, 520, 529, 548
Saxbe, D., 82, 278
Saxe, R., 27
Sayette, M. A., 350, 620
Sayfan, L., 167
Sayialel, S., 226
Sbarra, D. A., 244
Schaal, B., 255
Schachar, R. J., 161
Schacht, R., 518
Schachter, S., 382
Schacter, D. L., 35, 104
Schaefer, A., 500
Schaefer, H. S., 66, 223, 274, 557
Schäfer, A., 52, 395
Schaffer, P. A., 575
Schaller, M., 622
Schamberger, M. E., 539
Schardt, D. M., 67
Scharfe, E., 243
Schatzberg, A. F., 80, 403, 473,
474, 484
Schauenburg, H., 245
Scheibe, S., 13, 128, 130, 208,
209, 211, 399, 423
Scheier, M. F., 251, 321, 323,
328, 330, 334, 340, 349, 350,
361, 362, 364, 366, 369, 377,
378, 613, 615, 618, 622
Schene, A. H., 529
Scher, C. D., 555
Scherer, K. R., 4, 5, 23, 24, 26,
28, 29, 59, 383
Schienle, A., 52, 395
Schiller, D., 473
Schimmack, U., 209, 309
Schipper, E. M., 189
Schmader, T., 11, 617
Schmälzle, R., 45
Schmeichel, B. J., 189, 347, 348,
354, 617, 618, 619
Schmertz, S. K., 552
Schmidhempel, P, 221
Schmidt, J. A., 276
Schmidt, N. B., 395, 514, 516,
517
Schmidt, S., 81, 548, 552
Schmiedek, F., 187, 192, 214,
366
Schmitt, M., 253, 352
Schmittberger, R., 146
Schmittner, J., 433
Schnall, S., 224, 230
Schneider, D. J., 519
Schneiderman, N., 571
Schnülle, J., 65, 83, 127, 418
Schnur, J., 383
Schoenbaum, G., 23, 27, 34
Schoenmakers, T., 351
Schonberg, M. A., 181
Schönfelder, S., 11, 65, 314
Schönfeldt-Lecuona, C., 70
Schoofs, D., 617
Schoofs, H., 416
Schooler, J. W., 35, 369, 478
Schooler, T. Y., 254
Schorr, A., 4, 24, 59
Schotte, D. E., 117
Schredl, M., 495
Schreier, J., 363
Schroeder, D. G., 363
Schroeder, S. A., 346
Schryer, E., 208
Schul, Y., 263
Schultheiss, O. C., 371
Schultz, D., 163
Schultz, W., 34, 96, 145
Schulz, M. S., 191
Schulz, R., 369
Schulze, L., 495
Schupp, H. T., 45
Schuster, C., 575
Schut, H., 350, 356
Schutte, N. S., 433, 589
Schutter, D. J. L. G., 69
Schwartz, A. R., 588
Schwartz, C. E., 63
Schwartz, G. E., 552, 574
Schwartz, J. E., 205, 433, 500
Schwartz, J. L. K., 253, 366
Schwartz, S., 351
Schwartzman, A. E., 498
Schwarz, B., 146
Schwarz, J., 14, 529
Schwarz, J. C., 113, 115, 116,
117, 616
Schwarz, N., 117, 223, 252, 622
Schweiger Gallo, I. S., 367
Schweizer, S., 127, 271, 327, 396,
401, 417, 424, 492
Schyns, P. G., 453
Scissors, L. E., 244
Scott, W. A., 263
Scoville, W. B., 104
Seay, R. B., 210
Sebanc, A. M., 315
See, J., 515, 524
Seeley, J. R., 497
Seeman, G., 113, 115, 117, 616
Seeman, T. E., 589, 591, 602
Segal, Z. V., 14, 352, 475, 478,
479, 480, 498, 530, 548, 553,
555, 556, 557, 559, 560
Seibt, B., 257
Seider, B. H., 6, 206, 267
Seivert, N. H., 128, 369
Selby, E. A., 495
Selcuk, E., 240
Seligman, M. E. P., 531
Semegon, A. B., 207
Semple, R. J., 560
Sen, S., 67
Senthinathan, S., 560
Senville, J., 551
Seppala, E., 286
Sepulcre, J., 450
Sequeira, H., 45, 46
Sergeant, A., 575
Serketich, W. J., 275
Serretti, A., 558, 559
Sethi, A., 116, 309, 313
Seymour, B., 101
Shackman, A. J., 63
Shafran, R., 461
Shah, J. Y., 353
Shaham, Y., 429
Shahangian, A., 576
Shallcross, A. J., 341, 368, 370,
406, 420
Shallcross, S. L., 229
Shallice, T., 97, 307
Shamaskin, A. M., 209
Shamosh, N. A., 100
Shapiro, D. A., 495, 500
Shapiro, J. R., 161
Shapiro, S. L., 548, 552, 553,
554
Author Index 651
Sharot, T., 35
Sharpe, L., 522
Shaver, P. R., 113, 182, 189, 226,
229, 237, 238, 239, 240, 241,
242, 243, 244, 245, 246, 273,
274, 276, 328, 500
Shaw, B. F., 414, 477, 499
Shaw, D. S., 164, 181, 475
Shaw, M., 587
Shedden, K., 67
Sheeber, L., 187, 188, 191
Sheeran, P., 10, 321, 361, 367
Sheese, B. E., 115, 167, 305, 309,
312, 313, 399, 597
Shefrin, H., 93
Sheldon, K., 288
Shelfer, L., 523
Shelton, J. N., 618
Shenk, C., 500
Shepard, B., 114
Shepard, S., 161
Shepperd, J. A., 380
Sheppes, G., 6, 11, 13, 47, 48, 49,
126, 127, 128, 129, 130, 131,
132, 133, 136, 230, 268, 287,
362, 399, 420, 423, 472, 550
Sher, K. J., 494
Sheridan, J. F., 576
Sherman, A. M., 210
Sherman, J. E., 616
Sherwood, A., 601
Sherwood, C. C., 230
Shields, A. M., 180
Shields, S. A., 187, 188
Shiffman, S., 349, 428, 430, 433,
434
Shiffrin, R. M., 93
Shih, M. C., 355
Shin, L. M., 63, 64, 81, 395
Shiner, R. L., 305, 306
Shiner, T., 35, 101
Shiota, L., 341
Shiota, M. N., 11, 206, 211, 213,
274, 277
Shipstead, Z., 355
Shizgal, P., 96
Shmueli, D., 117, 623
Shoda, Y., 35, 105, 111, 112,
116, 118, 309, 310, 312
Shohamy, D., 460
Shonkoff, J. P., 605
Shorck, N. J., 395
Shortt, J. W., 188, 191
Shrestha, S., 291
Shulman, G. L., 148, 556
Shweder, R. A., 285
Siegbahn, A., 589
Siegel, E. H., 450
Siegel, R. D., 560
Siegel, R. E., 571
Siegfried, W. R., 225
Siegle, G. J., 395
Siegler, R. S., 134
Siemer, M., 6, 14, 134, 378, 382,
397, 413, 418, 419, 422
Siever, L. J., 495
Silananda, S. U., 549
Silk, J. S., 174, 187, 188, 189,
190, 196, 321
Sillence, A., 523
Silva, K. M., 164
Silva, P. A., 271
Silver, R., 435
Silverman, I. W., 114
Silvers, J. A., 29, 196, 432
Silvia, P. J., 377, 378
Sim, L., 189
Simmons, A., 309
Simmons, W. K., 448, 451, 452
Simon, H., 432
Simons, G., 293
Simons, R. F., 45, 49
Simpson, J. A., 229, 273
Sinclair, S., 255
Singer, B. H., 589
Singer, D. H., 204
Singer, J. E., 382
Singh, K. D., 223
Singleton, E. G., 433
Sinha, R., 430, 432, 433, 436,
614
Skinner, E. A., 194, 195
Skoner, D. P., 571, 587
Slagter, H. A., 552, 557
Slav, K., 243
Slessor, G., 213
Sliwinski, M. J., 211, 213
Sloan, D. M., 417, 461, 496,
500
Sloan, D. S., 321
Sloan, E. K., 575, 576
Sloman, S. A., 257
Slotema, C. W., 69
Slovic, P., 141
Slovik, L. F., 243
Small, B. J., 225
Small, D. A., 141, 620
Smallwood, J., 478
Smart, L., 353, 355
Smart Richman, L., 622
Smeets, M., 352
Smeets, T., 242
Smeetsa, P. A. M., 350
Smets, J., 416
Smider, N. A., 164
Smith, A., 560
Smith, B., 416
Smith, C. A., 383
Smith, C. F., 497
Smith, C. L., 162
Smith, E. A., 221
Smith, E. E., 28, 30, 141, 148,
435
Smith, E. R., 260, 263, 378
Smith, G. D., 587
Smith, G. T., 557
Smith, I. M., 161
Smith, J., 205
Smith, L., 615
Smith, M. R., 28
Smith, P. K., 80
Smith, T. B., 222
Smith-Shine, J. N., 188
Smits, J. A. J., 514
Smyth, J. M., 211, 589
Snidman, N., 571, 574
Snyder, C. R. R., 63, 93
Snyder, D. K., 276
Snyder, S. S., 194, 380
Snyder, W., 93
Soeteman, D. I., 494
Sofuoglu, M., 434
Sokol-Hessner, P., 141, 144, 146,
148, 149, 150, 472
Sokoloff, L., 230
Soler, J., 500
Solms, M., 557
Solomon, A., 416
Solomon, B., 52
Solomon, R. C., 284
Somerville, L. H., 63
Sommer, I. E. C., 69
Sommer, K. L., 622
Sonnenberg, S. J., 556
Sood, A. K., 574
Sorensen, E., 191
Sorg, S. F., 435
Soto, C. J., 326
Soto, J. A., 11, 295, 296
Soucy, N., 229
Sparrow, D., 589, 597
Speer, A. M., 69
652 Author Index
Speicher, C. E., 575
Speilberg, J. M., 403
Spencer, S. J., 617, 620
Spinrad, T. L., 119, 157, 158,
160, 161, 162, 163, 164, 165,
168, 179
Spira, A. P., 401
Spivey, M. J., 259
Sporns, O., 450, 456
Sprich, S., 500
Springer, C., 384
Srivastava, S., 11, 14, 327, 335,
337, 341, 579
Srnivasan, S. R., 596, 597, 604
Sroufe, L. A., 273
Srull, T. K., 367
St. Jacques, P. L., 207, 208
Stafford, T., 253
Stalnaker, T. A., 27
Stanley, E. A., 552
Stanley, S. M., 274
Stansbury, K., 114
Stansfeld, S., 241
Starfield, B., 587
Stark, R., 81, 223
Stasiewicz, P. R., 433
Staudinger, M. R., 11, 66, 145,
146, 148, 149, 150
Staudinger, U. M., 210
Stawski, R. S., 211, 212
Steele, C. M., 617
Steen, T. A., 531
Stefanucci, J. K., 222, 223, 224,
230
Stegall, S., 188, 604
Steger, M. F., 370
Stegge, H., 196
Stein, M. B., 309, 395, 398, 471
Stein, N. L., 284
Steinberg, L., 174, 187, 189, 190,
321, 432
Steiner, M., 198
Steinhauser, M., 128
Stein-Seroussi, A., 382
Stemmler, G., 11, 536
Stenberg, C., 293, 306
Stenger, V. A., 97
Stepper, S., 10, 499
Steptoe, A., 242, 602, 603
Sterling, P., 571, 576
Stern, Y., 212
Sternberg, R. J., 274
Stettler, N. M., 179
Stewart, J., 498
Stickle, T. R., 533
Stieglitz, R. D., 495
Stiff, C., 618
Stifter, C. A., 114, 161
Stiglmayr, C. E., 495
Stiles, W. B., 500
Stillman, T. F., 620
Stiner, L. M., 576
Stockburger, J., 45
Stokes, M. B., 253
Stone, A. A., 205, 589
Stone, M., 188
Stoolmiller, M., 188
Story, T. N., 206
Stouthamer-Loeber, M., 119
Strachman, A., 226, 273
Strack, F., 10, 256, 257, 263,
347, 353, 499
Strain, L. M., 258
Strange, B. A., 46
Straube, T., 79, 81, 82
Strauman, T. J., 473
Straus, M. A., 276
Streubel, B., 209
Strick, P. L., 28
Striepe, M., 614
Stroebe, W., 350, 354, 356, 620
Stroop, J. R., 79
Strosahl, K. D., 378, 474, 533
Strotz, R., 94, 104
Stroud, L. R., 187, 188, 589,
600, 614
Strunk, R. C., 590
Stucke, T. S., 615
Sturm, V. E., 270
Stuss, D. T., 97, 141
Suárez, L. M., 394
Sugarman, D. B., 276
Suh, E. M., 205
Sulik, M. J., 119, 157, 166
Sullivan, E. V., 435
Sullivan, K. A., 496
Sullivan, S., 274, 496
Suls, J., 382, 596, 597, 602, 603
Sumer, N., 241
Summers, W. C., 575
Sumter, S., 188
Sundin, E. C., 245
Sung, C., 576
Sunstein, C. R., 351
Suomi, S., 575
Supavadeeprasit, S., 190
Suri, G., 13, 128, 130, 399, 423
Sütterlin, S., 257
Sutton, R. E., 9, 145
Sutton, R. I., 9
Suvak, M. K., 460
Suway, J. G., 521
Svaldi, J., 535
Swann, W. B., Jr., 363, 381, 382
Swanson, C., 272
Swart, M., 532
Sweeny, K., 380
Swick, D., 509
Syme, S. L., 586
Synowski, S. J., 589
Szasz, P. L., 11
Szczurek, L., 9
Sze, J. A., 276
Szentagotai, A., 11
Szyf, M., 576
T
Taal, E., 548
Tabibnia, G., 11, 396, 435, 537
Tacikowski, P., 45
Taffe, J., 323, 328
Tafrate, R. C., 499
Tai, S., 418
Taioli, E., 432
Takacs, T. A., 241
Tamang, B. L., 291
Tambor, E. S., 381
Tamir, M., 3, 9, 11, 14, 15, 127,
192, 193, 223, 230, 297, 327,
335, 337, 361, 363, 364, 365,
366, 369, 370, 371, 532
Tan, J., 516
Tan, P. Z., 402
Tancer, M. E., 96
Tang, C. Y., 495
Tang, Y.-Y., 314, 315
Tangney, J. P., 377, 378, 384
Tanji, J., 403
Tannenbaum, D., 242
Tannock, R., 161
Tantleff-Dunn, S., 353
Tarter, R. E., 431, 432
Tata, P., 62, 395, 422, 510
Tate, E. B., 480, 557
Taylor, C. T., 514, 516, 517, 522,
524
Taylor, G. J., 13
Taylor, J., 145
Taylor, K., 243
Taylor, S. E., 46, 82, 83, 228,
382, 571, 575, 589, 602
Tchanturia, K., 496
Teachman, B. A., 205, 213
Author Index 653
Teasdale, J. D., 370, 414, 475,
479, 498, 529, 530, 536, 537,
550, 559, 560
Tebartz van Elst, L., 495
Teerds, J., 195, 417
Telch, M. J., 196
Tellegen, A., 251
Templeton, J., 188
Tennen, H., 589
Tenney, N., 395
Terdal, S. K., 381
Terschure, E., 284
Terwogt, M. M., 196
Tesman, J. R., 114
Tesser, A., 132, 538
Tessitore, A., 67, 121, 537
Testa, A., 118
Teti, L. O., 273
Thaker, P. H., 574
Thaler, R. H., 93, 99, 105, 106,
144, 351
Thapa, K., 293
Thayer, J. F., 401, 589, 599
Thewissen, R., 352
Thibaut, J. P., 453
Thiruchselvam, R., 10, 45, 48,
49, 51, 129, 130
Thomas, J., 167, 418
Thomas, K. M., 312
Thomas, P. W., 396
Thomas, S., 256, 396
Thomas, T. R., 497
Thompson, E., 252
Thompson, R. A., 5, 7, 12, 76,
77, 115, 118, 127, 128, 133,
173, 174, 175, 176, 177, 178,
180, 181, 182, 190, 191, 272,
322, 361, 368, 371, 399, 492,
496, 508, 520, 597
Thompson-Holland, J., 395
Thompson-Schill, S. L., 28, 33
Thomsen, A. H., 159
Thomson, G. E., 586
Thorberg, F. A., 496
Thoresen, C. E., 351, 552
Thorne, A., 188
Thorsteinsson, E. B., 589
Thurston, R. C., 602
Tian, X., 576
Tice, D. M., 111, 116, 117, 135,
230, 321, 615, 616, 618, 619,
621, 622, 623, 624
Tiebout, J., 574
Tiffany, S. T., 351, 352, 614, 623
Tiggemann, M., 351
Timpano, K. R., 395, 517
Tindle, H. A., 602
Tjebkes, T. L., 161
Tobin, R. M., 314
Todd, R. M., 252, 260
Tolin, D. F., 470
Toll, B. A., 433
Tomaka, J., 223, 367, 589
Tomarken, A. J., 66
Tomasello, M., 222, 223
Tomasi, D., 434
Tomich, P. L., 183
Tomko, R. L., 494
Toner, K., 208
Toney, L., 557
Tonge, B., 195
Tononi, G., 450
Tooby, J., 491, 538
Tormala, Z. L., 258
Torpey, D., 46
Toth, S., 178
Tottenham, N., 68, 167
Totterdell, P., 6, 135, 368
Toufexis, D. J., 63
Tout, K., 315
Townsend, A. L., 225
Trabasso, T., 284
Tracey, I., 33
Tracy, J. L., 384
Tran, S., 273
Tranel, D., 28, 141, 142
Treasure, J., 496
Tremblay, R. E., 605
Trew, J. L., 558
Treyer, V., 35, 148
Tricamo, M., 431
Trommsdorff, G., 293, 294
Tronick, E. Z., 178, 273, 277,
278
Trope, Y., 93, 105
Trötschel, R., 366
Troy, A. S., 127, 341, 368, 370,
406, 420, 508, 509
Trull, T. J., 494, 495
Tsai, A., 292
Tsai, J. L., 9, 206, 209, 285, 286,
287, 295, 363, 364
Tucker, A. M., 212
Tugade, M. M., 450
Tull, M. T., 396, 399, 495
Tung, J., 575
Turk, C. L., 396, 399, 469, 473,
496
Turk-Charles, S., 207
Turken, A. U., 622
Turner, R. B., 571, 587
Turza, A. C., 429
Tuschen-Caffier, B., 65, 83, 127,
418
Tversky, A., 136, 144
Twenge, J. M., 615, 621, 622
Tyler, E., 351
Tzur, G., 311
U
Uchida, Y., 289
Uchino, B. N., 204, 212, 227
Uher, R., 67
Uhlmann, C., 574
Ullsperger, M., 61
Underhill, L. G., 225
Underwood, B., 115
Ungerleider, L. G., 28
Updegraff, J. A., 398
Urgesi, C., 497
Urier, G., 575
Urry, H. L., 6, 13, 29, 33, 48, 52,
61, 128, 187, 210, 268, 287,
416, 420, 474
Ursache, A., 160, 164
Uutela, A., 601
Uyeji, L., 229
V
Vaillant, G., 367
Vainiger, D., 355
Vaitl, D., 81
Valiente, C., 160, 164, 168, 179
Van Bavel, J. J., 260, 261
Van Damme, S., 395
van den Bos, W., 94, 99
van den Hout, M., 395
van den Kommer, T., 195, 417
van den Wildenberg, W. P., 316
Van der Laan, L. N., 349
van der Molen, M. W., 316
van der Pligt, J., 354
Van Dillen, L. F., 61, 128, 132,
223, 347, 348, 349, 351, 352,
362
van Dulmen, M. M., 315
Van Gucht, D., 353
van Harreveld, F., 354
van IJzendoorn, M. H., 63, 229,
510
Van Knippenberg, A., 353, 618
654 Author Index
van Koningsbruggen, G. M.,
620
Van Lange, P. A., 223
van Oppen, P., 469
Van Overwalle, F., 452
Van Pelt, J., 188
Van Ree, J. M., 616
van Reekum, C. M., 29, 52, 61,
71, 263, 474
Van Rijsbergen, G. D., 529
van Steenbergen, H., 53
van Straten, A., 469
Van Strien, J. W., 46
van Veen, V., 35
Vansteenwegen, D., 353
Van’t Wout, M., 141, 142, 146,
148, 149, 150
VanYperen, N. W., 382
Vasan, R. S., 589
Vasey, M. W., 164, 516
Vaughan, J., 603
Veissier, I. I., 376
Veling, H., 353
Velten, E., 614
Verdejo-García, A., 431, 433
Verette, J., 243
Verhasselt, S., 500
Verheul, R., 494
Verhulst, F. C., 164
Vernon, M. L., 245
Veroff, J., 378
Versace, F., 52
Verschuere, B., 395, 522
Verstraeten, K., 164
Vervliet, B., 480
Viborg, G., 11
Vidmar, M., 166
Viergevera, M. A., 350
Vierthaler, J., 245
Vigil, S. A., 399
Villamil, E., 205, 210
Villarroel, N. A., 614
Vincenzo Piazza, P., 432
Vine, S. J., 223
Vinogradov, S., 86
Virmani, E. A., 180, 181
Visscher, B. R., 571, 575
Voegler-Lee, M., 166
Voelker, P. M., 167, 309, 312,
313
Vogelgesang, J., 195
Vohs, K. D., 116, 135, 230, 347,
348, 349, 350, 356, 618, 619,
620, 621, 623
Vokonas, P., 597
Volkow, N. D., 145, 230, 433,
434, 436, 437
Volokhov, R. N., 189, 354
von Cramon, D. Y., 27
Vrints, C. J., 571
Vuilleumier, P., 45, 47, 537
Vujanovic, A. A., 433
W
Wachs, T. D., 310
Wade, A. R., 449
Wadlinger, H. A., 511
Wadsworth, M. E., 159
Wager, T. D., 26, 27, 29, 30, 33,
36, 52, 62, 64, 83, 403, 452,
460, 496
Waggoner, A. S., 260
Wagner, A. D., 28, 33
Wagner, D. D., 119, 613, 614,
617, 619, 622
Wagner, G. G., 187, 192, 212,
214, 366
Wagner, J., 274
Walach, H., 548, 552
Waldinger, R. J., 191
Waldstein, S. R., 589
Walker, C. D., 498
Walker, D. L., 63
Walker, M. P., 492
Wall, S., 226, 237, 273, 277
Walle, E. A., 267
Wallentin, L., 589
Walter, H., 11, 58, 61, 65, 66,
82, 145, 432, 538, 574
Walters, E. E., 469, 530
Wang, G. J., 434
Wang, P. S., 413
Wang, R., 607
Wang, T. S., 119
Wang, X. T., 99
Wang, Y., 162
Wang, Z., 307
Wang Erber, M., 192
Warburton, W. A., 615
Ward, A. S., 353, 429, 617
Wardle, J., 116
Warlop, L., 99
Wasel, W., 255
Washburn, D. A., 315
Waters, E., 226, 237, 239, 273,
277
Waters, H. S., 239
Waters, S. F., 180, 181, 182, 183
Watkins, E. R., 127, 134, 415,
461, 470, 479, 558
Watkins, L. L., 601
Watson, D., 251, 461, 469, 484
Watson, J. C., 475
Wayment, H. A., 245
Weaver, I. C. G., 228, 229, 576
Webb, T. L., 10, 321, 341, 361,
367, 368
Weber, G., 514
Wechsler, R. L., 230
Weersing, V., 523
Wegner, D. M., 353, 378, 519,
537, 617, 618
Weierich, M. R., 209
Weimer, B. L., 183
Weinberg, A., 43, 44, 45, 46, 51,
53, 321, 337, 574
Weinberger, D. A., 367, 574
Weinberger, D. R., 67, 121, 537
Weiner, B., 382
Weiner, D. K., 556
Weintraub, J. K., 323, 330
Weisberg, R. B., 523
Weiss, A., 571
Weiss, F., 437
Weiss, N. H., 396, 399, 405
Weiss, S. T., 589
Weiss, T., 81
Weissman, R., 530, 539
Weissman, S., 530, 539
Weisz, J. R., 194, 285, 292, 380
Wells, A., 414, 415
Wells, T. T., 424, 474, 517
Welsh, R., 46
Wendelken, C., 229, 244
Wentzel, M., 165
Wenzlaff, R. M., 537
Werlinich, C. A., 383
Werner, K., 84, 400, 508, 529
Werner, K. H., 84, 400, 496,
508, 529
Werner-Seidler, A., 420
Wertheim, E. H., 113, 116
Wessa, M., 11, 65, 81, 314
Westbrook, C., 433, 434, 439
Westen, D., 8, 328
Westenberg, P., 188
Westlund, K. N., 26
Westlye, L. T., 204
Westphal, M., 128, 135, 368,
369, 398, 415, 533
Westra, H., 476
Wewerka, S., 188
Whalen, P. J., 96, 296
Author Index 655
Wheeler, L., 382
Whisman, M. A., 276
White, D., 450
White, J., 119, 510
White, L. K., 509, 521
White, T. L., 519
Whitley, B., 529, 531, 535
Whitmer, A. J., 127, 475
Whitney, G. A., 243
Whittington, E. J., 363
Wicker, B., 95
Wicklund, R. A., 377
Wiech, K., 28
Wiers, R. W., 351, 352, 353, 354,
523
Wiersema, J. R., 395
Wierzbicka, A., 284
Wieser, M. J., 31
Wilamowska, Z. A., 401
Wilbarger, J. L., 141, 349
Wildgruber, D., 207
Wile, D. B., 272, 279
Wilhelm, F. H., 4, 24, 76, 257,
276, 366, 368, 406, 420, 480,
600
Wilkinson, R. B., 245
Willer, R., 11, 341
Willhelm, F. H., 341
Williams, B. R., 161
Williams, C. J., 253
Williams, D. R., 586
Williams, H., 49, 129
Williams, J. B. W., 500
Williams, J. M. G., 424, 475,
498, 549, 559, 562
Williams, K. A., 556, 615
Williams, K. D., 230, 615, 622
Williams, L. E., 31, 288, 367
Williams, M. G., 509, 586
Williams, N. L., 242
Williams, P., 141
Williams, R., 422
Williams, R. M., 479
Williams, S. C. R., 66
Williamson, D. A., 497
Williamson, P. C., 460
Williford, A. P., 309
Willis, W. D., 26
Willmott, L., 599
Willner, P., 614, 623
Willoughby, M. T., 161, 166
Wilson, G., 483
Wilson, H. R., 208
Wilson, K. G., 378, 472, 474,
481, 482, 533
Wilson, M. N., 164
Wilson, M. R., 223
Wilson, S., 187
Wilson, T. D., 129, 130, 131,
254, 255, 256, 258, 350, 379,
380, 509
Wilson, W. H., 230
Wilson-Mendenhall, C. D., 14,
447, 448, 451, 453, 454, 458
Winecoff, A., 71
Winkielman, P., 141, 349
Winkler, I., 45
Winquist, J., 621
Wirth, R. J., 161
Wirtz, C., 535
Wisco, B. E., 127, 414, 470
Wise, R. A., 96, 145
Wise, S., 277
Witkiewitz, K., 432, 440, 561,
614
Witt, A. A., 548
Witt, W. P., 587
Wittenberg, L., 614
Wittman, M., 121
Wittstein, I. S., 588
Wohleb, E. S., 576
Woitach, M. J., 178
Wojnowicz, M. T., 259, 260
Wolf, O. T., 617
Wolfe, C. D., 316
Wolgast, M., 11, 398
Wolitzky-Taylor, K. B., 395
Woltering, S., 176
Wong, G., 419
Wong, L., 552
Wong, M. M., 496
Wood, C., 618
Wood, J. V., 363
Wood, P. K., 494
Wood, S., 46
Woody, S., 475, 484
Woolery, A., 589, 600
Woolley, A. W., 224
Worthington, E. L., Jr., 243
Wrase, J., 145
Wright, C. I., 63
Wrosch, C., 369
Wrzus, C., 187, 192, 212, 274
Wundt, W., 450
Wupperman, P., 529, 530, 531,
533, 535, 541
Wurm, L. H., 213
Wyatt, T., 181
Wyer, R. S., 367
Wynder, E. L., 432
X
Xiao, E., 146, 150
Xu, F., 454
Y
Yamamura, N., 225
Yan, P., 436
Yang, E. V., 575
Yang, Y., 315
Yao, X., 9
Yap, M. B., 187, 188, 189, 191
Yarczower, M., 530
Yárnoz-Yaben, S., 243
Yeboah, J., 589
Yen, N. S., 46
Yen, Y., 118
Yeomans, J. S., 96
Yerkes, R. M., 270
Yeung, D., 286
Yeung, N., 103
Yi, R., 106
Yiend, J., 401
Yim, C. Y., 349
Yirmiya, N., 178
Yoo, S., 492
Yoon, J. E., 166
Young, L., 27
Young, R. D., 621
Young, R. M., 496
Younger, J., 227, 228
Youngstrom, E., 163
Youngstrom, E. A., 163
Yu, B. H., 416
Yuan, J. W., 275, 276, 278
Yurgelun-Todd, D., 189
Z
Zack, J. A., 574
Zack, M. M., 205
Zacks, R. T., 422
Zadro, L., 615
Zahn-Waxler, C., 273, 294
Zajonc, R. B., 6, 140
Zaki, J., 27, 28
Zaldivar, F., 600
Zanakos, S., 378, 618
Zanarini, M. C., 495
Zangen, A., 355
Zanna, M. P., 252
Zayas, V., 240
656 Author Index
Zeidan, F., 551
Zeiss, A. R., 114, 616
Zeki, S., 226
Zelazo, P. D., 23, 252, 260, 261,
262
Zell, A. L., 321
Zeman, J., 181, 188, 189, 197,
604, 609
Zentner, M., 305
Zgierska, A., 439
Zhang, F., 242
Zhang, L., 111
Zhou, F., 589
Zhou, Q., 158, 160, 162, 166
Ziaie, H., 10, 309
Ziegenhain, U., 114
Zikopoulous, B., 459
Zilcha-Mano, S., 239
Zilles, K., 27
Zimmer-Gembeck, M. J., 194, 195
Zinser, M. C., 616
Znoj, H., 534, 535
Zoccola, P. M., 600
Zoellner, L. A., 11, 399, 405,
549
Zucker, R. A., 496
Zuckerman, M., 307
Zulfiqar, U., 204
Zvolensky, M. J., 401, 433, 533
Zysset, S., 27
657
Academic outcomes, 164–165,
431, 586
Acceptance
affect regulation training and,
536f, 537–538
anxiety disorders and, 397,
398, 403
ART model of adaptive affect
regulation and, 532f, 533
mindfulness training and,
554f, 555–556
Acceptance and commitment
therapy (ACT), 479, 481,
561
Acceptance-based interventions,
352, 378, 475
Adaptation
adaptive hopelessness, 532f
ART model of adaptive affect
regulation and, 532f
emotion regulation choice and,
129
normative emotional
functioning and, 472–473
overview, 195–197
self-awareness and, 385
Adjustment outcomes,
326–327
Adjustment problems, 163–164
Adolescence. See also
Developmental factors
attentional bias modification
approach and, 518–519
cardiovascular disease (CVD)
and, 606–607
development of emotional
regulation skills and,
189–192
emotion regulation strategies
and, 193–197
motivational processes and,
192–193
overview, 187–189, 197–198,
368
Adult attachment, 237, 240–247
Affect, 6, 305–306
Affect regulation training (ART).
See also ART model of
adaptive affect regulation
affect regulation training and,
530–531
efficacy of, 541–542
future directions, 542–544
overview, 529–531, 535–541,
536f, 540f, 541f
Affective processes, 5–6
Affective processing model, 32f,
432–433
Affective state, 115–117, 119,
347t. See also Negative
affect
Aggression, 119, 307
Aging. See also Developmental
factors
antecedent-focused emotion
regulation and, 207–210
avoiding negative emotions,
212
cardiovascular disease (CVD)
and, 606–607
cognitive functioning and,
207–210
emotion regulation in couples
and, 274–275
emotional experience and,
205–206
future directions, 213–214
negative events and, 210–212
overview, 203–204
physical costs of emotion
regulation, 212
physiological system and,
204–205
responding to negative events,
210–212
socioemotional selectivity
theory (SST) and, 206
Subject Index
658 Subject Index
Alcohol use. See Substance use
disorders
Amygdala. See also Neural
systems
affect regulation training and,
537, 538–539, 543
aging and, 207–208
anhedonia and, 66
anxiety and, 63
borderline personality disorder
(BPD), 494–495
delay discounting and, 96–97
delay of gratification and,
120
emotion regulation and
dysregulation and, 60f
genetic factors and, 67
perception–valuation–
activation (PVA) sequence
and, 32f
reactivity and, 176
research findings and, 80
self-regulation failure and,
619
temperament and, 316
valuation and, 26–27
Analyzing emotions, 536f, 539,
540f
Anger. See also Perceptions
cardiovascular disease (CVD)
and, 601
cultural regulation of emotions
and, 295
decision making and, 149f
effortful control and,
159–160
reactivity and, 176–177
situated conceptualizations
and, 451, 460–461
social decision making and,
146–147
temperament and, 307
Antecedent-focused emotion
regulation, 207–210, 297,
549–550, 587–588
Anterior cingulate cortex (ACC).
See also Dorsal anterior
cingulate cortex (dACC);
Neural systems
attention and, 314
depression and, 65
emotion regulation and
dysregulation and, 60f, 61
executive attention and, 311
overview, 78
research findings and, 80–82
situated conceptualizations
and, 460
substance use disorders and,
435, 436
temperament and, 316
Anticipatory regulation, 48–50,
79f, 81–82
Antisocial behavior, 310
Anxiety. See also Anxiety
disorders
attentional bias modification
approach and, 514–515,
515–517, 518
attribution theory and, 383
decision making and, 150
effortful control and, 162
emotion dysregulation and,
62–66
implicit–explicit processes
and, 84
mindfulness-based stress
reduction (MBSR) and,
558–559
reflecting on the past and
future, 379–381
temperament and, 307, 309
Anxiety disorders. See also
Anxiety; Mood disorders
attentional bias modification
approach and, 519
conceptual framework for,
394–397
co-occuring disorders and,
469–471
emotion regulation strategies
and, 397–399, 401–402
future directions, 406–407
implicit–explicit processes and,
80, 82, 84
maladaptive emotion
regulation and, 404–406,
407
motivational awareness skills
training and, 476
neurobiological differences
and, 402–403
overview, 393–394
pathways to ineffective
emotion regulation in,
399–404
relationship to emotion
regulation, 396–397
Anxious attachment. See also
Attachment theory/styles;
Insecure attachment
death of a close partner,
245–246
overview, 241, 242–243
responding to a partner’s
hurtful behaviors and,
243–244
separations and breakups,
244–245
Appraisals. See also Reappraisals
adult attachment and, 242
affect regulation training and,
529
aging and, 209
anxiety disorders and, 398
awareness of other people’s
perceptions and evaluations,
384
cultural regulation of emotions
and, 292–294
DBT model of emotion
regulation, 492t, 493t
mindfulness training and, 553,
553f, 554f
mood disorders and, 421f
overview, 4, 10
process model of emotion
regulation, 7–8, 7f, 323–
328, 324f, 326f, 327f, 328f
social regulation of emotion
and, 224
valuation and, 27
Approach system, 307–308, 310
ART model of adaptive affect
regulation, 531–534, 532f,
534–535. See also Affect
regulation training (ART)
Assessment
attentional bias and, 510
coping-with-stress approach
and, 328–333
decision making and, 143–144
delay of gratification and,
112–113
effortful control and, 167
emotion regulation in couples
and, 275–278
emotional competence
approach and, 333–339,
335t, 336f
executive attention and,
311–312
Subject Index 659
implicit–explicit processes
and, 83–84
temperament and, 315–316
Association–propositional
evaluation (APE) model,
255–257
Associative system, 256, 257–259
Attachment theory/styles. See
also Adult attachment;
Insecure attachment; Secure
attachment
delay of gratification and,
113–114
emotion regulation and,
239–246
overview, 237–239, 238–239,
246–247, 272–273
parent–child attachment
security, 182–183
social regulation of emotion
and, 229
Attention. See also Attentional
functioning
attention allocation, 351–352
biases and, 62, 395, 421f.
See also Attentional bias
modification approach
focusing, 158, 161
late positive potential (LPP)
and, 45, 47–48, 47f
mindfulness training and,
551–552, 553f
process model of emotion
regulation, 7–8, 323–328,
324f, 326f, 327f, 328f
regulatory skills training,
477–478
selectivity, 508–509
shifting, 158, 161, 162
situation selection and, 289,
290–292
temperament and, 308–315
Attention Networks Task (ANT),
312, 314
Attentional bias modification
approach
clinical applications, 523–524
extended-delivery studies of,
515–519
future directions, 519–524
impact of, 512–519
overview, 510–512
single-session studies of,
512–515
Attentional deployment
cultural regulation of emotions
and, 290–292
DBT model of emotion
regulation, 492t, 493t, 498
overview, 10
perception–valuation–
activation (PVA) sequence
and, 31, 32f, 33
process model of emotion
regulation, 7f, 323–328,
324f, 326f, 327f, 328f
Attentional functioning
aging and, 208
DBT model of emotion
regulation, 492t
effortful control and, 310–315
mood disorders and, 421f
regulatory skills training, 478
Attention-deficit/hyperactivity dis-
order (ADHD), 98, 431–432
Attitude models
associative-propositional
evaluation (APE) model,
255–257
dual-attitudes model, 254–255
iterative reprocessing model
(IR), 259–262, 261f
meta-cognitive model (MCM),
259
mindfulness training and, 553,
553f
MODE (Motivation and
Opportunity as DEtermin-
ants) model, 253–254
models of, 253–262, 261f
overview, 251–253, 262–263
systems of evaluation model
(SEM), 257–259
Automatic emotion regulation,
268, 367–368
Avoidance
affect regulation training and,
537
attachment and, 238
avoidance training, 353
borderline personality disorder
(BPD), 495–496
individual differences and,
240–241
self-awareness and, 378
Avoidant attachment, 240–246.
See also Attachment theory/
styles; Insecure attachment
B
Balloon Analogue Risk Task
(BART), 143–144
Behavior. See also Goal-directed
behavior
cultural regulation of emotions
and, 294–297
DBT model of emotion
regulation, 492t
decision making and, 149f
desire and, 349–350
effortful control and, 166
overview, 4–5
substance use disorders and,
431–432
temperament and, 306–308
Behavioral approach system
(BAS), 354
Beliefs, 36, 384–385
Bidirectional influences, 176,
269, 314
Binge-eating disorder (BED), 543
Biological-experiential change,
492t, 493t, 498–499
Biology change, 492t, 493t
Bipolar disorder, 413–415, 416,
423–425. See also Mood
disorders
Borderline personality disorder
(BPD)
DBT model of emotion
regulation and, 496–500
delay of gratification and,
118–119
dialectical behavior therapy
and, 491
mindfulness training and, 561
overview, 494–496
Brain. See Neural systems
C
Cardiovascular disease (CVD).
See also Health factors
developmental perspective,
604–607
future directions, 607–609
linking emotion regulation to,
598–603
mechanisms by which emotion
regulation influences,
603–604
660 Subject Index
Cardiovascular disease (cont.)
overview, 204–205, 596–598,
607–609
socioeconomic status (SES)
and, 588, 589, 591
Caregiver factors, 113–115,
238. See also Family
factors; Parenting factors;
Socialization
Catch Yourself Reacting (CYR)
form of self-monitoring,
477
Categorization, 449–450, 449f,
451–453, 461n–462n
Central nervous system, 204,
574, 576, 578–580
Childhood, 312–313, 518–519,
606–607
Child-rearing practices, 290,
291–292. See also Parenting
factors
Choice, emotion regulation. See
Emotion regulatory choices
Choice delay tasks, 112–113,
115, 117
Cognition
decision making and, 141–142
desire and, 347t, 348
emotion regulation choice and,
129, 131
overview, 93–94
self-awareness and, 380
temperament and, 306–308
Cognitive bias modification for
attention (CBM-A), 511.
See also Attentional bias
modification approach
Cognitive biases, 421f
Cognitive change. See also
Reappraisals
DBT model of emotion
regulation, 492t, 493t, 498
overview, 10
perception–valuation–
activation (PVA) sequence
and, 31, 32f, 33
process model of emotion
regulation, 7–8, 7f, 323–
328, 324f, 326f, 327f, 328f
situated conceptualizations
and, 457–458
Cognitive control, 97–98, 189,
421f, 422–423, 423–424
Cognitive dissonance, 256, 381
Cognitive Emotion Regulation
Questionnaire (CERQ),
331–333
Cognitive functioning, 11,
207–210
Cognitive load, 352, 617–618
Cognitive reappraisals. See also
Reappraisals
anxiety disorders and, 397,
398, 400–401, 402–403
late positive potential (LPP)
and, 48–52
mindfulness and, 549–550,
556–557
prefrontal cortex (PFC) and,
59–60
process model of emotion
regulation, 324
Cognitive-behavioral therapy
(CBT)
affect regulation training
and, 530, 535, 541–542,
543–544
attentional bias modification
approach and, 524
attribution theory and, 383
co-occuring disorders and,
469–471
desire regulation and, 353
emotion regulation therapy
and, 475
motivational interviewing
(MI), 476
regulatory skills training,
477–478
situated conceptualizations
and, 458–459
substance use disorders and,
438–439, 439–440
Compassionate self-support,
534, 536f, 538–539. See
also ART model of adaptive
affect regulation
Compassion-based therapy,
530–531
Conscience development, 307,
313, 367
Conserved transcriptional
response to adversity
(CTRA), 575–577, 579
Contextual learning, 471,
472–473, 474–475, 485f
Co-occuring disorders, 469–471,
477–484
COPE Inventory, 330–331, 332
Coping, 8, 242, 244–245, 369
Coping-with-stress approach,
322–323, 323f, 328–333
Coronary heart disease
(CHD), 600. See also
Cardiovascular disease
(CVD)
Cravings, 350, 433–434, 561,
616f
Criticism, 243–244
Cultural factors, 284–298,
325–326, 326f, 327f
D
Deactivation, 238, 240–241
Decision making
emotion and, 133–134,
140–142, 142–147
intertemporal choice and,
104–106
overview, 147–150, 149t
social decision making,
146–147
valuation perspective and, 35
Defense, 241, 307–308,
367–368
Delay discounting
cognitive control, 97–98
intertemporal choice and,
98–101, 99f
multiple interacting systems,
101–104, 103f
neural systems involved in,
94–101, 95f, 99f
overview, 93–94, 106
valuation and, 94–97, 95f
Delay of gratification
affective state and, 115–117
caregiver emotion and
responsivity and, 113–115
emotion regulation and,
118–121
overview, 111–113, 121–122
substance use disorders and,
431
Depression. See also Mood
disorders
affect regulation training and,
535, 543
aging and, 205
attention and, 314
Subject Index 661
attentional bias modification
approach and, 517–518
cognitive control and,
422–423
co-occuring disorders and,
469–471
cortical brain stimulation as a
treatment for, 68–70
decision making and, 150
delay of gratification and, 119
dysfunctional emotional
functioning and, 473–474
effortful control and, 162, 164
emotion dysregulation and,
62–66, 68
exposure techniques and, 481
implicit–explicit processes
and, 80
maladaptive emotion
regulation and, 405
mindfulness-based cognitive
therapy (MBCT) and,
559–560
overview, 413–415
reappraisals and, 416–417
rumination and, 415–416
self-awareness and, 377
situated conceptualizations
and, 460–461
Desire
characteristics of, 347–350,
347t
desire regulation, 346–347,
350–353, 353–355. See also
Desire
emotion goals and, 363–364
individual differences and,
353–355
mindfulness training and, 561
overview, 346–347, 355–356
self-regulation failure and, 616f
substance use disorders and,
433–434
Developmental factors. See also
Adolescence; Aging
effortful control and, 160–166
emotion regulation and, 174
emotion regulation and
dysregulation and, 66–68
emotion regulation in couples
and, 272–275
overview, 175
socioemotional development,
161–166
Developmental perspective, 174–
176, 272–275, 604–606
Dialectical behavior therapy
(DBT)
affect regulation training and,
530–531
DBT model of emotion
regulation, 491–494, 492f,
493t
evidence for, 500
future research, 500–502
mindfulness training and, 561
overview, 475, 491, 496–500
Difficulties in Emotion
Regulation Scale (DERS),
337–338
Diffusion tension imaging (DTI),
435
Disease. See Health factors
Disengagement, 242, 309,
399–400
Distancing, 477, 479
Distraction, 313–314, 378, 419,
421f
Distress tolerance, 433, 497–498
Dopamine system, 62, 95–96,
101
Dorsal anterior cingulate cortex
(dACC). See also Anterior
cingulate cortex (ACC);
Neural systems
anxiety disorders and,
402–403
delay discounting and, 95f, 98,
101, 103f
depression and, 65
emotion regulation and
dysregulation and, 60f
perception–valuation–
activation (PVA) sequence
and, 32f
social regulation of emotion
and, 227
substance use disorders and,
436
Dorsolateral prefrontal cortex
(dlPFC). See also Neural
systems; Prefrontal cortex
(PFC)
decision making and, 150
delay discounting and, 97–98,
101, 101–104, 102–104,
103f
delay of gratification and, 120
emotion regulation and
dysregulation and, 60f
implicit–explicit processes and,
84, 85–86
late positive potential (LPP)
and, 46
overview, 78
perception–valuation–
activation (PVA) sequence
and, 32f
social baseline theory (SBT)
and, 222–223
substance use disorders and,
434, 436, 439
Dorsomedial prefrontal cortex
(dmPFC), 27, 60f. See also
Neural systems; Prefrontal
cortex (PFC)
Drug use, 52–53, 429–430. See
also Substance use disorders
E
Effortful control. See also
Temperament
assessment and, 316
conceptual issues, 157–160
developmental factors,
160–166
effortful activational control,
162
executive attention and,
310–315
future directions, 166–168
overview, 157, 158–159, 168,
268
temperament and, 305
Electroencephalogram (EEG)
emotion regulation choice and,
129
event-related potentials (ERPs)
and, 44, 44f
late positive potential (LPP)
and, 45–46, 53–54
mindfulness training and,
557
Emotion dysregulation, 62–66,
493–496
Emotion evaluation, 175
Emotion goals. See also Goal-
directed behavior
content of, 363–364
future directions, 370–371

662 Subject Index
Emotion goals (cont.)
operation of, 366–368
overview, 362, 362f
structure of, 364–366, 364f
well-being and, 368–370
Emotion monitoring, 175
Emotion Regulation IAT
(ER-IAT), 366–367
Emotion regulation overview, 3,
6–8, 7f, 77–78, 174–176,
268–271, 394, 508
component processes and
definition of, 76–78
in couples, 267–275, 278–279
delay of gratification and,
118–121
future research, 12–15
goals, 8–9, 9f, 13–14
intertemporal choice and,
104–106
outcomes, 8, 10–12
valuation perspective on,
29–33, 32f
Emotion Regulation
Questionnaire (ERQ)
cardiovascular disease (CVD)
and, 601–602
overview, 275, 325, 341
process model of emotion
regulation, 326–327
Trait Meta-Mood Scales
(TMMS) and, 334–336,
335t, 336f
Emotion Regulation Skills
Questionnaire (ERSQ),
534–535, 535, 542, 543
Emotion regulation strategies
adolescent development of
emotional regulation skills
and, 193–197, 198
anxiety disorders and,
397–404
mood disorders and, 415–421,
421f
overview, 8, 9–10
Emotion regulation therapy
(ERT)
affect regulation training and,
530, 530–531
clinical applications,
475–484
motivational awareness skills
training, 476–477
overview, 469–471, 484–486,
485f
regulatory skills training,
477–484
research findings and,
483–484
Emotion regulatory choices
conceptualizing, 128–130
emotional, cognitive, and
motivational determinants
of, 130–131
future directions, 135–136
implications, 133–135
importance of, 126–128
overview, 126
underlying mechanisms of,
131–133
Emotional competence approach,
323, 323f, 333–339, 335t,
336f
Emotional intelligence, 338–339,
433
Emotional processing, 132, 493t
Emotion-coaching, 179–180, 191
Emotion-focused coping, 194
Emotion-focused therapy (EFT),
530–531
Emotion-motivation, 306–308.
See also Motivation
Emotion-related socialization
behaviors (ERSBs), 114
Emotions
delay of gratification and,
115–116
desire as, 347–348, 347t, 349
overview, 3–6, 4f, 5f, 306, 394
self-regulation failure and,
619–620
separating from emotion
regulation, 12–13
as situated conceptualizations,
453–459, 455f
temperament and, 306–308
valuation perspective on,
29–33, 32f
Empathy, 162, 165–166, 307
Environmental factors, 121, 577,
578f, 586–592
Evaluation
awareness of other people’s
perceptions and evaluations,
384–385
emotion goals and, 369–370
iterative reprocessing model
(IR) and, 260–262, 261f
overview, 251–253
self-evaluation, 381–384
Event-related potentials (ERPs).
See also Late positive
potential (LPP)
attentional bias modification
approach and, 511–512
emotion regulation choice and,
129
future research, 53–54
overview, 43, 44, 44f
Everyday Temptations Study
(TSC), 354, 355, 356
Evolutionary theory, 572–573
Executive functioning
desire and, 355
effortful control and, 158,
160, 166
social cognition and, 163
substance use disorders and,
431–432
Expectancies, 36, 384–385
Exposure techniques, 480–483,
485f
Expression/action change, 492t,
493t, 499
Expressive suppression, 10, 33,
324, 417. See also Response
modulation; Suppression
External factors, 191–192, 197
Extinction, 79f, 80–81
F
Failure, self-regulation. See Self-
regulation failure
Family factors. See also
Caregiver factors; Parenting
factors; Socialization
emotional climate of family,
176–180, 178–180
influences on emotion self-
regulatory strategies,
180 –182, 190–191
overview, 173–174, 183
parent–child attachment
security, 182–183
Fear
exposure techniques and,
480–483
situated conceptualizations
and, 451, 454, 459, 460–
461, 462n
temperament and, 307
Fear of Negative Evaluation Scale
(FNE), 517
Subject Index 663
Flanker task, 102–103, 189, 312
Flexibility, 368, 477–478, 554
Functional analysis, 438–439
Functional magnetic resonance
imaging (fMRI)
adult attachment and, 242–243
anhedonia and, 66
attentional bias modification
approach and, 511–512
decision making and, 141, 145
delay discounting and, 94, 96,
100–101
depression and, 64–65
implicit–explicit processes
and, 80
late positive potential (LPP)
and, 45–46, 52, 53–54
overview, 43, 70
social baseline and, 223
social decision making and,
146–147
substance use disorders and,
436
Functionalist approach, 174–176
G
Gender differences, 325–326,
326f
Generalized anxiety disorder
(GAD). See also Anxiety
disorders
attentional bias modification
approach and, 516, 519, 524
co-occuring disorders and,
469–471
dysfunctional emotional
functioning and, 473–474
emotion regulation strategies
and, 401–402
implicit–explicit processes and,
80, 82, 84
maladaptive emotion
regulation and, 406
motivational awareness skills
training and, 476
neurobiological differences
and, 402–403
overview, 396
relationship to emotion
regulation, 396–397
Generalized Expectancies for
Negative Mood Regulation
Scale (NMR), 333–334
Genetic factors
anxiety disorders and,
394–395
emotion regulation and
dysregulation and, 66–68
gene regulation, 577–580, 578f
genetics versus genomics,
572–574, 573f
neural regulation of gene
expression, 575–577
overview, 175
peripheral neural function, 574
Goal framework, 129, 362, 362f,
370–371, 377. See also
Emotion goals
Goal-directed behavior. See also
Behavior; Emotion goals
effortful control and, 162
individual differences and,
322–323
overview, 23–24, 361
situated conceptualizations
and, 462n
Gratification delay. See Delay of
gratification
Guilt, 165, 286, 307, 349, 384
H
Habituation
cultural regulation of emotions
and, 295
individual differences and,
340–341
mood disorders and, 417
overview, 79f, 80–81
situated conceptualizations
and, 457–458
Health factors. See also
Cardiovascular disease
(CVD)
genetics versus genomics,
572–574, 573f
neural regulation of gene
expression, 575–577
overview, 571–572
peripheral neural function,
574
socioeconomic status (SES)
and, 586–592
Heart rate variability (HRV),
401–402, 454–455, 474
Hedonism, 355, 363–364
Hormonal factors, 188, 588
Hypothalamic–pituitary–adrenal
(HPA) axis
adult attachment and, 242
cardiovascular disease (CVD)
and, 598–599, 603–604,
605
risk for disease and, 588
social regulation of emotion
and, 227
I
Illness, 460–461. See also Health
factors
Implicit Association Test (IAT),
253, 255, 366–367
Implicit attitudes, 254–255
Implicit emotion regulation, 268,
297
Implicit–explicit process
future research, 84–86
incidental regulation, 82–83
instructed regulation, 83–84
overview, 76, 77–78, 86, 174
research findings and, 78–84,
79f
Impulse control. See also Self-
control
anxiety disorders and, 398
delay of gratification and, 119
effortful control and, 159–160
overview, 4–5, 93
self-regulation failure and,
619–620
substance use disorders and,
431–432
Incidental regulation, 82–83
Individual differences
attachment and, 240–246
Cognitive Emotion Regulation
Questionnaire (CERQ),
331–333
COPE Inventory, 330–331
decision making and, 143–146
delay of gratification and,
115–116
desire and, 353–355
emotion regulation choice and,
135–136
emotion regulation strategies
and, 399, 400–401
emotional competence approach,
333–339, 335t, 336f
future directions, 339–341
664 Subject Index
Individual differences (cont.)
future research, 342f
late positive potential (LPP)
and, 46
overview, 321–323, 323f,
339–341, 342f
process model of emotion
regulation, 323–328, 324f,
326f, 327f, 328f
Ways of Coping
Questionnaire, 329–330
Inhibitory control, 162, 163, 316.
See also Suppression
Insecure attachment, 243–
244. See also Anxious
attachment; Attachment
theory/styles; Avoidant
attachment
Integrative perspective, 182–183,
340
Intensity of emotions, 205–206,
399–400, 469
Internal factors, 191–192, 197
International Affective Picture
System (IAPS), 44, 196–197,
209–210
Interpretations, 421–422, 421f
Intertemporal choices, 93,
98–101, 99f, 104–106. See
also Delay discounting
Interventions, 14, 68–70, 179,
315, 352–353. See also
Treatment
Intrapersonal processes, 297–298
Intrinsic processes, 174
Intuition, 355
Iowa Gambling Task (IGT),
143–144
Iterative reprocessing model (IR),
259–262, 261f, 268–269
L
Late positive potential (LPP). See
also Event-related potentials
(ERPs)
clinical applications, 52–53
emotion regulation and,
47–53, 47f, 130
future research, 53–54
overview, 43, 44–46, 44f
Late-life dyads, 274–275. See
also Aging; Romantic
relationships
Lifespan development, 206,
604–606. See also Aging;
Developmental factors
Loss, 244–245, 245–246
Loss aversion, 144–145, 149f
M
Major depressive disorder. See
Depression
Medial prefrontal cortex (mPFC),
27–28, 78, 226. See also
Neural systems; Prefrontal
cortex (PFC)
Mediating mechanisms, 226–
228, 590
Meditation, 378, 479
Memory
adolescent development of
emotional regulation skills
and, 189
aging and, 208–209
associative–propositional
evaluation (APE) model and,
256
attentional bias modification
approach and, 520
biases and, 421f
delay discounting and, 104
mood disorders and, 423
reflecting on the past and
future, 379–381
sensory input and, 450–451
situated conceptualizations
and, 453
Mindfulness, 549–551, 550f
Mindfulness training (MT),
548, 551–562, 553f, 554f,
562
Mindfulness-based cognitive
therapy (MBCT), 478,
559–560
Mindfulness-based interventions
DBT model of emotion
regulation, 497–498, 502
desire regulation and, 352
emotion regulation therapy
and, 475
overview, 548
regulatory skills training, 478,
479
self-awareness and, 378
substance use disorders and,
434, 439–440
Mindfulness-based relapse
prevention (MBRP),
439–440, 560–562
Mindfulness-based stress
reduction (MBSR), 478,
479, 558–559
Modal model of emotion, 5, 5f,
76–77
MODE (Motivation and
Opportunity as
DEterminants) model,
253–254
Mood disorders. See also
Anxiety disorders;
Depression
adaptive strategies and,
419– 421
attentional bias modification
approach and, 512–513
cognitive biases, 421–422,
421f
cognitive control and,
422–423
cognitive processes and,
421–423, 421f
emotion regulation strategies
and, 415–421, 421f
future directions, 423–425
mood-incongruent recall, 420
overview, 413–415, 423–425
reappraisals and, 416–417, 420
rumination and, 415–416
suppression and, 417
Mood regulation, 8
Moods, 420, 421f, 529–530,
620–621
Moral development, 162, 307
Motivation
cultural differences in,
288–289
desire and, 347t, 348, 349
dysfunctional emotional
functioning and, 473
emotion regulation choice and,
129
emotion regulation therapy
and, 485f
goals, 131
MODE (Motivation
and Opportunity as
DEterminants) model, 254
normative emotional
functioning and, 471–472
overview, 192–193, 471
temperament and, 306–308
Subject Index 665
Motivational awareness skills
training, 476–477. See also
Emotion regulation therapy
Motivational interviewing (MI),
476
N
Negative affect. See also
Affective state
decision making and, 141
delay of gratification and,
115–117
self-regulation failure and,
614–622, 616f, 623–624
Negative emotions
adolescent development of
emotional regulation skills
and, 189
aging and, 205–206
drug use as emotion
regulation, 429–430
emotion regulation and, 174
emotion regulation in couples
and, 272
emotional climate of family, 179
rumination and, 415–416
self-awareness and, 385–386
temperament and, 309
Negative events, 210–212, 421f
Neural systems. See also
Amygdala; Anterior
cingulate cortex (ACC);
Dorsal anterior cingulate
cortex (dACC); Dorsolateral
prefrontal cortex (dlPFC);
Prefrontal cortex (PFC);
Situated conceptualizations;
Ventrolateral prefrontal
cortex (vlPFC);
Ventromedial prefrontal
cortex (vmPFC)
affect regulation training and,
543
aging and, 204, 207–208
anxiety disorders and,
402–403
borderline personality disorder
(BPD), 494–495
cortical brain stimulation as
a treatment for depression,
68–70
DBT model of emotion
regulation, 501
decision making and, 148–
150, 149t
delay discounting and,
94–101, 95f, 99f
delay of gratification and,
119–121
desire and, 349–350
emotion regulation and
dysregulation and, 58–59
machine metaphor of brain
function, 447–449, 447f
mindfulness training and,
556–557
neural regulation of gene
expression, 575–577
overview, 70–71, 78, 175
perception–valuation–
activation (PVA) sequence
and, 31–33, 32f
peripheral neural function, 574
reactivity and, 176
research findings and, 78–84,
79f
social baseline theory (SBT)
and, 222–223
social regulation of emotion
and, 226–229
substance use disorders and,
434–438
valuation and, 25–28, 26f
Neuroanatomical bases,
148–150, 149t
Neurobiology, 399, 402–403
Neuroimaging studies. See
Electroencephalogram
(EEG); Functional magnetic
resonance imaging
(fMRI); Positron emission
tomography (PET)
Neuroticism, 309, 469
Nonjudgmental awareness,
536–537, 536f, 555–556
Norepinephrine, 62, 471–472
Nucleus accumbens (NAcc), 60f,
95–97, 101
O
Obsessive–compulsive disorder
(OCD), 396, 513–514, 561.
See also Anxiety disorders
Online reappraisals, 420,
423–424
Online regulation, 47–48, 47f
Orbitofrontal cortex (OFC)
decision making and, 145, 148,
149f
depression and, 64
desire and, 349–350
dysfunctional emotional
functioning and, 473
normative emotional
functioning and, 472
substance use disorders and,
435, 436
valuation and, 27
P
Panic disorder (PD), 395–397,
402–403. See also Anxiety
disorders
Parent–child relationships,
173, 177–178, 181–182,
190–191, 272–273. See also
Family factors; Parenting
factors
Parenting factors. See also
Caregiver factors; Family
factors; Socialization
adolescent development of
emotional regulation skills
and, 190–191
cultural regulation of emotions
and, 290, 291–292
delay of gratification and,
113–115
effortful control and, 165
emotion regulation in couples
and, 272–273
emotional reactivity and,
176–180
influences on emotion self-
regulatory strategies,
180 –182
parental interventions to
manage emotions, 177–178
parent–child attachment
security, 182–183
situated conceptualizations
and, 453–454
Peer relationships, 165–166
Perceptions. See also Situated
conceptualizations
awareness of other people’s
perceptions and evaluations,
384–385
self-evaluation and, 381–384
666 Subject Index
Perceptions (cont.)
sensory input and, 449–451,
449f
social regulation of emotion
and, 224
Perception–valuation–activation
(PVA) sequence
applications of, 33–36
emotion as a type of, 29–30
overview, 23–29, 25f, 26f,
36–37
Personality, 306, 310, 321, 355.
See also Temperament
Physiology, 4–5, 10–11, 204–
205, 274–275
Positive emotions. See also
Affective state
aging and, 205–206
delay of gratification and, 117
drug use as emotion
regulation, 429–430
emotion regulation and, 174
self-awareness and, 385–386
self-regulation failure and,
622–623
Positron emission tomography
(PET), 60, 64, 81, 436
Posterior–anterior shift in aging
(PASA), 204, 207–208
Posttraumatic stress disorder
(PTSD). See also Anxiety
disorders
genetic factors and, 577
implicit–explicit processes
and, 81
maladaptive emotion
regulation and, 405
neurobiological differences
and, 402–403
overview, 64, 396
relationship of to emotion
regulation, 396–397
situated conceptualizations
and, 459, 460–461
Prediction error, 145–146
Predictive control, 380–381, 457
Prefrontal cortex (PFC). See also
Dorsolateral prefrontal
cortex (dlPFC); Neural
systems
adolescent development and,
189
affect regulation training and,
537, 537–538
anhedonia and, 66
anxiety disorders and, 400,
402–403
attention and, 314
depression and, 64–65
effects of drug use on,
437–438
emotion regulation and, 59–62
genetic factors and, 67
implicit–explicit processes and,
81, 84, 85
late positive potential (LPP)
and, 46, 51–52
overview, 70–71, 78
social baseline theory (SBT)
and, 230n
social regulation of emotion
and, 226
substance use disorders and,
429, 429f, 434–438
valuation and, 27–28
Process model of emotion
regulation
effortful control and, 167–168
individual differences and,
323–328, 324f, 326f, 327f,
328f
mindfulness and, 550–551,
550f, 551f
overview, 7–8, 7f, 322
situated conceptualizations
and, 458
Prosocial behavior, 162,
165–166, 310
Psychopathology
adolescence and, 188–189
affect regulation training and,
529–530
dialectical behavior therapy
and, 500–501
overview, 14, 188
situated conceptualizations
and, 459–461
substance use disorders and,
430
R
Reactivity
aging and, 204–205
cardiovascular disease (CVD)
and, 599
delay of gratification and, 121
emotion regulation in couples
and, 278
family influences on, 176–180
overview, 306
research findings and, 80
Reappraisals. See also
Appraisals; Cognitive
change; Cognitive
reappraisals
adolescent development of
emotional regulation skills
and, 194
anxiety disorders and, 398,
401–402, 402–403
attention and, 314
cultural regulation of emotions
and, 296–297
decision making and, 149f
desire regulation and, 352–353
emotion regulation choice and,
129, 132–133, 135
emotion regulation strategies
and, 399
late positive potential (LPP)
and, 48–52
mindfulness and, 549–550
mood disorders and, 416–417,
418–419, 420, 423–424
overview, 10–12, 79f
regulatory skills training, 480
situated conceptualizations
and, 461
social decision making and, 146
Reflection, 379–381, 384,
385–386
Reframing, 477, 480
Regulatory processes, 6–7,
13–14, 142–147
Regulatory skills training, 477–
484, 485f. See also Emotion
regulation therapy
Rejection, 243–244
Relapse prevention model, 432–
433, 438. See also Treatment
Relaxation techniques, 536, 536f
Repetitive transcranial magnetic
stimulation (rTMS), 68–69,
100–101
Response modulation. See also
Expressive suppression
overview, 10
perception–valuation–
activation (PVA) sequence
and, 31, 32f, 33
process model of emotion
regulation, 7–8, 7f, 323–
328, 324f, 326f, 327f, 328f
Subject Index 667
Response-focused emotion
regulation, 194, 587–588
Revised Conflict Tactics Scales,
276
Reward system, 471–472, 485f
Rewards, 145–146, 149f,
616–617
Risk factors
adult attachment and, 240
anxiety disorders and,
404–406
cardiovascular disease (CVD),
596, 598–603
health factors and, 587
socialization and, 183
substance use disorders and,
429–434
Risk-seeking behavior, 143–144,
149f, 432
Romantic relationships, 243–
246, 272–275
Rumination
adult attachment and, 242,
243
emotion regulation choice and,
127
mindfulness-based stress
reduction (MBSR) and, 559
mood disorders and, 415–416,
418–419, 421f
reflecting on the past and
future, 379–381
Ruminative coping, 337
S
Safety seeking, 471–472
School-related outcomes,
164–165, 431, 586
Secondary attachment strategies,
238, 240. See also
Attachment theory/styles
Secure attachment, 238,
243–244
Security system, 471–472, 485f
Self-awareness
overview, 376–377, 385–386
personal standards and,
377–378
reflecting on the past and
future, 379–381
self-evaluation, 381–384
self-regulation failure and,
616f
Self-control, 93, 98. See also
Impulse control
Self-discrepancy theory, 377–378
Self-evaluation, 381–385. See
also Evaluation
Self-inflicted injuries, 495–496
Selfishness, 147, 149f
Self-medication hypothesis, 430,
432–433. See also Substance
use disorders
Self-monitoring, 477, 616f,
617– 618
Self-regulation. See also Self-
regulation failure
delay of gratification and,
115–116
effortful control and, 157,
159–160
family influences on, 180–182
normative emotional
functioning and, 472
overview, 158, 306
positive affect and, 622–623
Self-regulation failure. See also
Self-regulation
overview, 613–614, 623–624
positive affect and, 622–623
role of negative affect in,
614–622, 616f
Sensory input, 449–451, 449f
Serotonin, 62, 67. See also
Genetic factors
Shame, 289–290, 307, 349, 384
Sheehan Disability Scale (SDS),
517
Shifting, 158, 161, 162,
590–592
Simulation, 449–450, 449f
Situated conceptualizations. See
also Neural systems
emotions and emotion
regulation as, 453–459,
455f
machine metaphor of brain
function, 447–448, 447f
overview, 447–449, 451–453,
461
psychopathology and,
459–461
sensory input and, 449–451,
449f
Situation modification
DBT model of emotion
regulation, 492t, 493t
overview, 9–10
perception–valuation–
activation (PVA) sequence
and, 31
process model of emotion
regulation, 7–8, 7f, 323–
328, 324f, 326f, 327f, 328f
Situation selection
cultural regulation of emotions
and, 288–290
DBT model of emotion
regulation, 492t, 493t, 497
perception–valuation–
activation (PVA) sequence
and, 31
process model of emotion
regulation, 7–8, 7f,
323–328, 324f, 326f, 327f,
328f
Social anxiety disorder
(SAD). See also Anxiety
disorders
attentional bias modification
approach and, 514–515,
516–517
maladaptive emotion
regulation and, 406
neurobiological differences
and, 402–403
overview, 395–396
relationship of to emotion
regulation, 396–397
Social baseline theory (SBT),
221, 222–230
Social emotions, 384–385
Social factors, 11, 162–166,
165–166, 190–191,
223–229
Social Interaction Anxiety Scale
(SIAS), 516
Social Phobia and Anxiety
Inventory (SPAI), 517
Social regulation of emotion,
221, 223–229, 229–230,
287–297
Socialization
cardiovascular disease (CVD)
and, 604–605
emotional reactivity and,
176–180
influences on emotion self-
regulatory strategies,
180 –182
overview, 173–174, 183
parent–child attachment
security, 182–183
668 Subject Index
Socioeconomic status (SES)
emotion regulation as buffer,
590–592
influences on emotion self-
regulatory strategies, 181
overview, 586–587, 592
risk for disease and,
587–590
Socioemotional development,
161–166. See also
Developmental factors
Socioemotional selectivity theory
(SST)
aging and, 206, 207–210
attention and working memory
and, 208
future directions, 213–214
overview, 204
Specific emotion regulation
process approach, 322–323,
323f
Steady-state visual evoked
potentials (ssVEP), 48,
53
Strange Situation, 113–114,
273
Strategies in emotion regulation.
See Emotion regulation
strategies
Strength and vulnerability
integration (SAVI) model
aging and, 206–207
avoiding negative emotions,
212
future directions, 213–214
overview, 204
physical costs of emotion
regulation, 212
responding to negative events,
210–212
Stress, 241–243, 554f, 588
Stress response
affect regulation training and,
529–530
aging and, 210–212
attentional bias modification
approach and, 515
mindfulness-based stress
reduction (MBSR) and,
558–559
reactivity and, 176–180
situated conceptualizations
and, 459
Stroop task, 102–103, 189, 316,
509
Substance use disorders. See also
Alcohol use; Drug use
drug use as emotion
regulation, 429–430
emotion (dys)regulation as a
causal factor in, 430–434
mindfulness training and, 561
overview, 428–429, 429f, 440
prefrontal cortex (PFC) and,
434–438
treatment and, 438–440
Suicidal behavior, 119, 495–496
Suppression. See also Expressive
suppression
anxiety disorders and,
397–398, 399–400
cardiovascular disease (CVD)
and, 599
cultural regulation of emotions
and, 295
desire regulation and, 353, 355
mood disorders and, 417,
418–419
overview, 10–12, 571
Sympathetic nervous system
cardiovascular disease (CVD)
and, 599, 603–604
genetic factors and, 578–579
neural regulation of gene
expression, 575–577
risk for disease and, 588
T
Technology, 543–544
Temperament. See also
Personality
assessment and, 315–316
attention and, 308–315
cardiovascular disease (CVD)
and, 604–605
delay of gratification and,
115–116
effortful control and, 158–159,
166, 310–315
emotion regulation strategies
and, 399
emotion–motivation and,
306–308
future directions, 316–317
overview, 305–306
traits, 306
Temporoparietal junction (TPJ),
148, 149t
Temptation, 348–349, 616–617,
616f
Theory of mind (ToM), 163
Thoughts, 379–381, 385–386,
513
Threat avoidance/detection/
perception, 62, 63, 176–
177, 239, 310, 471–472,
480–483
Tolerance, 532f, 533, 536f,
537–538
Training Emotionaler
Kompetenzen (TEK), 531.
See also Affect regulation
training (ART)
Trait Meta-Mood Scales
(TMMS), 334–336, 335t,
336f
Transcranial direct current
stimulation (tDCS), 69–70,
355
Transcranial magnetic
stimulation (TMS), 355
Treatment. See also Interventions
cortical brain stimulation as
a treatment for depression,
68–70
emotion regulation choice and,
134–135
emotion regulation in couples
and, 272
emotion regulation therapy,
475–484
mindfulness training and,
557–562
mood disorders and, 424
self-awareness and, 378
situated conceptualizations
and, 458–459
substance use disorders and,
438–440
Two-system model of
discounting, 99–100,
101–104, 103f. See also
Delay discounting
V
Validity, 278
Valuation
decision making and, 145–146
delay discounting and, 94–97,
95f
overview, 23–29, 25f, 26f
Subject Index 669
Valuation perspective
applications of, 33–36
on emotion and emotion
regulation, 29–33, 32f
overview, 36–37
Ventral portions of the anterior
cingulate cortex (vACC),
78, 82
Ventral striatum, 26–27, 32f,
66, 95f
Ventrolateral prefrontal cortex
(vlPFC). See also Neural
systems
anxiety and, 63–64, 64
delay of gratification and, 120
depression and, 64
emotion regulation and
dysregulation and, 60f, 61
implicit–explicit processes
and, 85–86
overview, 78
perception–valuation–
activation (PVA) sequence
and, 32f
social regulation of emotion
and, 227
substance use disorders and,
435, 439
valuation and, 28
Ventromedial prefrontal cortex
(vmPFC). See also Neural
systems
affect regulation training and,
537–538
anxiety and, 64
delay discounting and, 95f,
101–102
depression and, 65
dysfunctional emotional
functioning and, 473
emotion regulation and
dysregulation and, 60f
genetic factors and, 67
normative emotional
functioning and,
471–472
overview, 70
social regulation of emotion
and, 227–228
valuation and, 27
Vulnerability factors, 497, 508
W
Ways of Coping Questionnaire,
329–330
Well-being, 205–206, 368–370
Working memory, 208, 520,
616f. See also Memory
Working memory capacity
(WMC), 354–355, 356
Worry, 84, 513, 515–516
